-
据统计[1],2021年,全球葡萄种植面积达到734×104 hm2,葡萄酒产量超过2 500×107 L。葡萄酒生产过程中产生的有机废水主要来源于加工设备的清洗和残液的排出,这些废水中含有大量由醇、糖和有机酸组成的溶解性有机物、微量营养素、多酚类化合物[2-5]。受葡萄生长周期的影响,在9—11月,葡萄酒酿制高峰期所产生的废水量及废水中的有机物浓度增加明显[6]。综上,葡萄酒生产废水总体呈酸性,色度和有机物浓度高,可生化性好,但水质、水量季节性波动明显。
厌氧消化作为一种能高效降解有机质的技术,被广泛用于葡萄酒生产废水的预处理过程。厌氧序批式生物膜反应器(AnSBBR)兼备生物膜法、序批式反应器的优点。生物膜法通过载体富集微生物,从而增加了反应器中生物质的浓度和多样性,提高了系统的抗冲击能力[7-9]。序批式工艺不仅自动化程度高,还存在周期性循环和完全混合的特性,从而提高了抗冲击能力[5]。目前,AnSBBR优异的处理性能和抗冲击能力已在生物柴油[7]、甘油[8]和金属加工[9]等多种废水的处理研究中得以证实,但其对葡萄酒生产废水的处理负荷和消化性能尚不明确。
通常,较低的有机负荷(OLR)虽利于反应器的运行稳定,但增加了运行成本;而高OLR虽有助于有机物利用,但易导致系统酸化。故通过逐步提高OLR探索AnSBBR处理葡萄酒生产废水所能承受的最大OLR尤为重要。目前,厌氧反应器在不同OLR下处理葡萄酒生产废水的研究主要是OLR对消化性能的影响。然而,微生物对环境变化敏感,高OLR下导致反应器运行失败的原因是破坏了体系中菌群结构的平衡[10]。因此,有必要深入探究OLR变化对菌群结构的影响。
本研究采用AnSBBR处理模拟葡萄酒生产废水,通过监测AnSBBR各OLR运行期间出水水质、甲烷产量和产甲烷活性等来评估AnSBBR处理葡萄酒生产废水的性能,利用高通量测序对生物膜体系中群落结构的演替规律进行解析,确定AnSBBR处理葡萄酒生产废水的最佳工况条件,旨在为推动AnSBBR的工程化应用提供依据。
-
实验所用生物膜填料和活性污泥取自西安市第四污水厂缺氧池,生物膜载体为K3填料(直径25 mm,比表面积500 m2·m−3),填料上挂膜量为2 507.6 mg·m−2,反应器中生物填充率为35%。使用4 L活性污泥(5 677.5 mg·L−1)用于挂膜。进水用实际葡萄酒水稀释配制,葡萄酒COD为(220 450±2 100) mg·L−1,总磷为(26.1±1.3) mg·L−1,总氮为(1 479±37) mg·L−1,乙酸为(1 409.6±13.8) mg·L−1,多糖为(2 524.84±65.37) mg·L−1,蛋白质为(17 899.47±203.31) mg·L−1,pH为3.99±0.2,投加碳酸氢钠控制进水pH为7.3~7.5。
-
实验所用AnSBBR有效容积为10 L,φ270 mm×360 mm。采用蠕动泵间歇进水、推流泵搅拌,控制温度为(35±1) ℃,进出水和搅拌采用可编程控制器(PLC)控制,产生的沼气流入湿式气体流量计。
AnSBBR运行过程分为驯化启动、强化和稳定3个阶段,实验期间进水COD、水力停留时间和有机负荷见表1。反应器采取低负荷启动,起始COD为(1±0.1) g·L−1,通过缩短HRT提高有机负荷以完成对生物膜的驯化培养,处理后的废水用于灌溉时,需满足农田灌溉标准(GB 5084-2021)中旱作物对COD排放的要求 (COD<200 mg·L−1) 。强化阶段,根据BEZERRA等 [7]的方法提升OLR。强化和稳定阶段的运行周期为4 h (包括进水5 min、反应230 min、出水5 min) ,进水体积为2 L。
-
产甲烷速率测试能够评估厌氧污泥的产甲烷潜力,分别在3种负荷(1.2、5.4、9.6 g·(L·d)−1))下和接种污泥中进行。生物膜填料用PBS清洗3次后,加入250 mL厌氧瓶中,再加入150 mL营养液。氮吹5 min以排出瓶中空气,将厌氧瓶置于35 ℃水浴摇床,用排水集气法测定甲烷,并配制2 mol·L−1 NaOH溶液,吸收H2S和CO2。最后利用Gompertz模型方程[11]对产甲烷过程进行拟合分析,模型方程见式(1)。
式中:Pt为t时刻累积甲烷产量,mL·g−1(以TS计);Pmax为最大甲烷产量,mL·g−1(以TS计);Rmax为最大甲烷产率,mL·(g·h)−1(以TS计);e=2.718 3;λ为停滞时间,h。
-
1) 理化指标分析。采用哈希(DRB-200)消解仪消解COD;采用雷磁PHS-3C测定pH;采用湿式气体流量计(LMF-1)计量沼气;采用标准方法分析TS、VS;联合滴定法测定总碱度(TA)、部分碱度(PA),pH滴定终点分别3.8和5.75,碳酸氢盐碱度(BA)为1.2PA[12];采用FID-气相色谱仪(SP-3420A)测定VFAs;采用TCD-气相色谱仪(SP-3420A)测定沼气组分;参照LI等[13]的热提法提取胞外聚合物(EPS)并分别用苯酚硫酸法和福林酚法测定多糖(PS)和蛋白质(PN);参照赵阳等[14]的方法测定辅酶F420浓度;参照ZHAO等[15]的方法测定INT-ETS;参照李韧等[16]的方法测定生物膜量。
2) 高通量测序。取接种和反应器3种负荷(OLR为1.2、5.4和9.6 g·(L·d)−1)的生物膜污泥,运用高通量测序技术对微生物群落结构进行分析。细菌和古菌用带有barcode特异性引物515FmodF(5′-GTGYCAGCMGCCGCGGTAA-3′)和806RmodR(5′-GGACTACNVGGGTWTCTAAT-3′),针对全菌16S rRNA基因进行PCR扩增并利用Illumina公司的Miseq PE300平台进行测序。使用IBM SPSS25.0软件对数据进行显著性分析,采用t检验,显著性水平为P< 0.05差异显著。
-
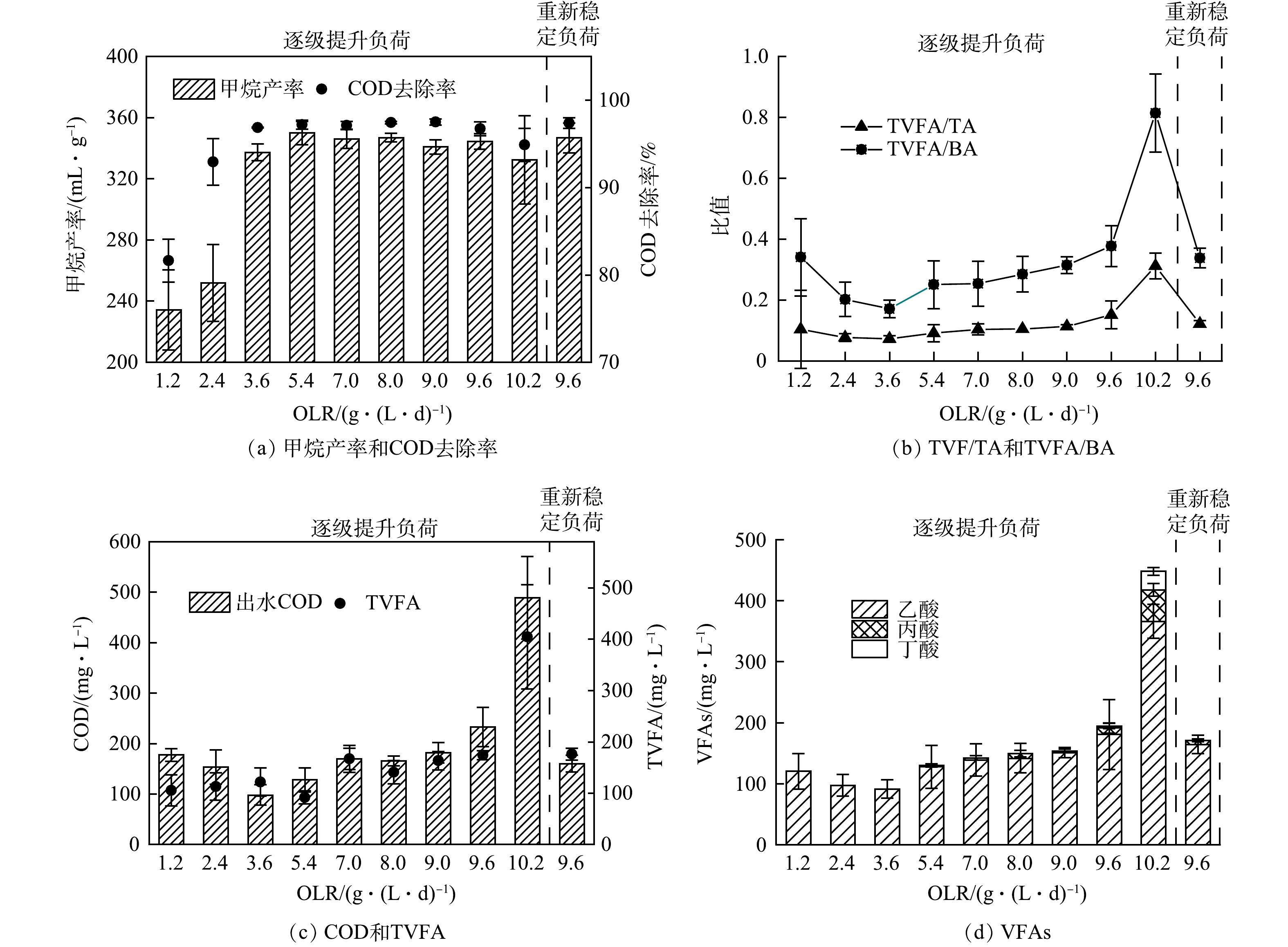
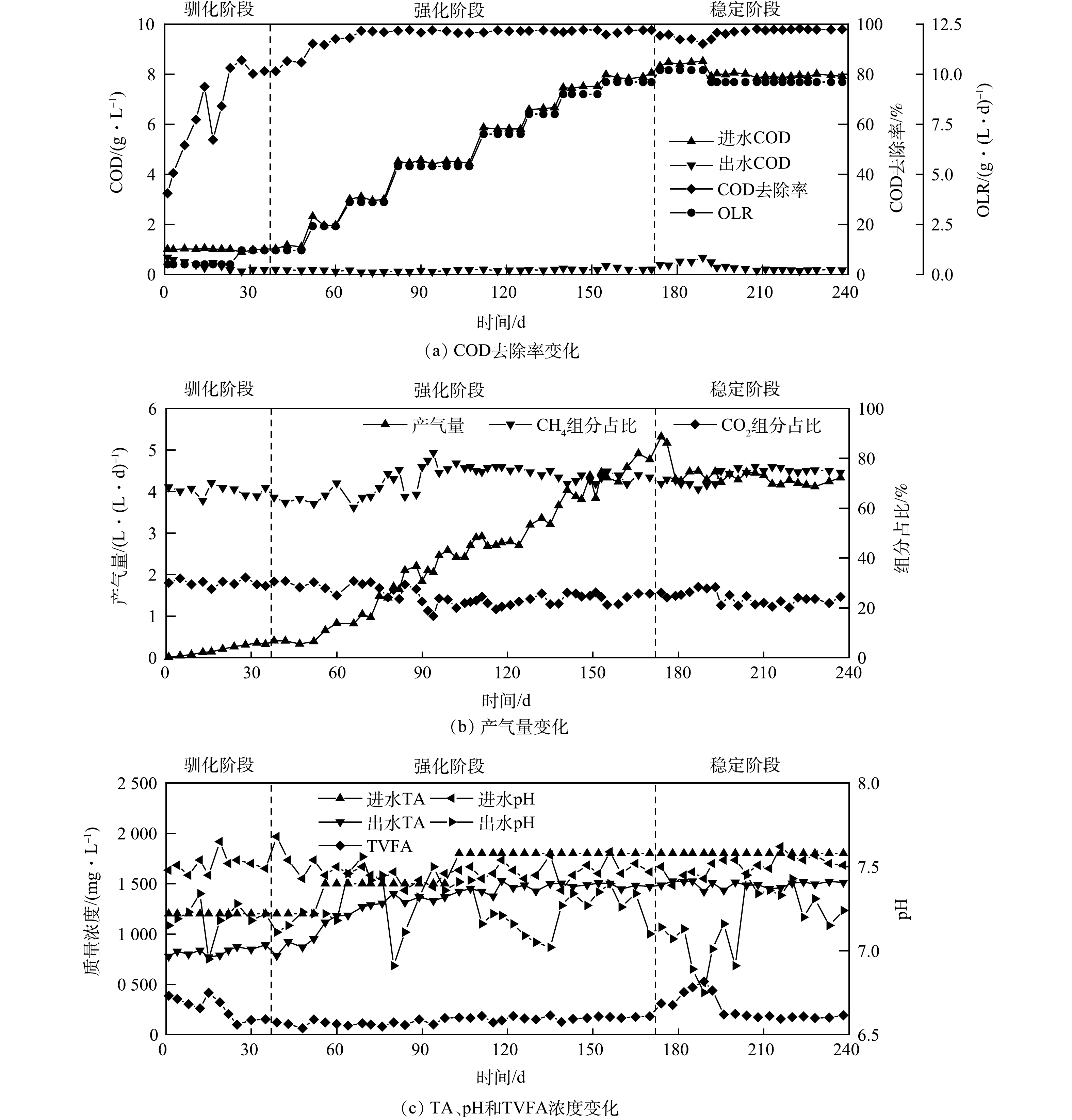
1) AnSBBR的运行特性。反应器连续运行的效果见图1。反应器经37 d驯化培养后,COD去除率由(52.2±17.4)%增至(75±12.2)%,出水COD降至(171±30) mg·L−1,满足了农田灌溉标准(GB 5084-2021)中旱作物对COD值的要求,表示AnSBBR启动成功。强化运行阶段(OLR为1.2~9.6 g·(L·d)−1),COD去除率(图1(a))由81.6%增至97.7%,沼气产量(图1(b))由0.37 L·(L·d)−1增至4.6 L·(L·d)−1。pH在6.8~7.8内,TVFA为66~193 mg·L−1,总碱度为780~1 520 mg·L−1;稳定运行阶段(OLR为9.6~10.2 g·(L·d)−1),当OLR为10.2 g·(L·d)−1时,出水COD最大,达到675 mg·L−1,COD去除率降至94.9%,甲烷产率降至(332.4±28.7) mL·g−1,TVFA为532 mg·L−1。随后OLR降至9.6 g·(L·d)−1,运行16 d后,反应器各项运行参数重新恢复正常。可以看出,AnSBBR处理OLR为1.2~9.6 g·(L·d)−1的葡萄酒生产废水时,具备优异的处理性能。
2) OLR对AnSBBR性能及稳定性的影响。有机物降解及甲烷产量是评价厌氧反应器消化效率的重要指标[11]。OLR对反应器性能及稳定性的影响见图2。OLR超过2.4 g·(L·d)−1后,COD去除率维持在较高的水平(>96%)(图2(a))。当OLR为1.2~5.4 g·(L·d)−1时,甲烷产率随OLR的提升而增加,OLR超过5.4 g·(L·d)−1后甲烷产率随OLR的提升而减少。当OLR为5.4 g·(L·d)−1时,甲烷产率最大,达到 (349.9±7.6) mL·g−1。对比稳定阶段前后结果,可以看出,重新恢复后的反应器在COD去除率和甲烷产率方面表现更好。此外,与其他厌氧反应器对比,可以看出,AnSBBR在出水COD和甲烷产率方面更具优势[3-4,17-18] (表2) 。
系统的缓冲能力是评估反应器运行稳定性的关键指标,其变化受消化过程中碱度和VFAs的共同影响。当OLR为10.2 g·(L·d)−1时,pH降至7.0以下,但总碱度波动较小(图1(c)),TVFA/TA<0.4(图2(b))。这是因为碳酸氢盐碱度被消耗转化为挥发性脂肪酸碱度,但后者不能提供缓冲,从而出现pH降低但总碱度变化小的情况[12]。这说明,TVFA/TA无法准确表示体系缓冲能力的变化情况,故再引入TVFA/BA。通常,在高OLR下,TVFA/BA<0.4时,系统处于稳定状态[19]。当OLR为1.2~9.6 g·(L·d)−1)时,比值低于0.4,反应器缓冲能力良好(图2(b));当OLR为10.2 g·(L·d)−1)时,比值增至(0.81±0.13),反应器酸化风险激增。此时,乙酸增至(366.18±27.79) mg·L−1,丙酸为 (51.63±10.35) mg·L−1,丁酸为(30.44±6.52) mg·L−1 (图2(d)),且体系中VFAs呈现出继续增加的趋势,表明反应器无法适应OLR为10.2 g·(L·d)−1的运行条件。
-
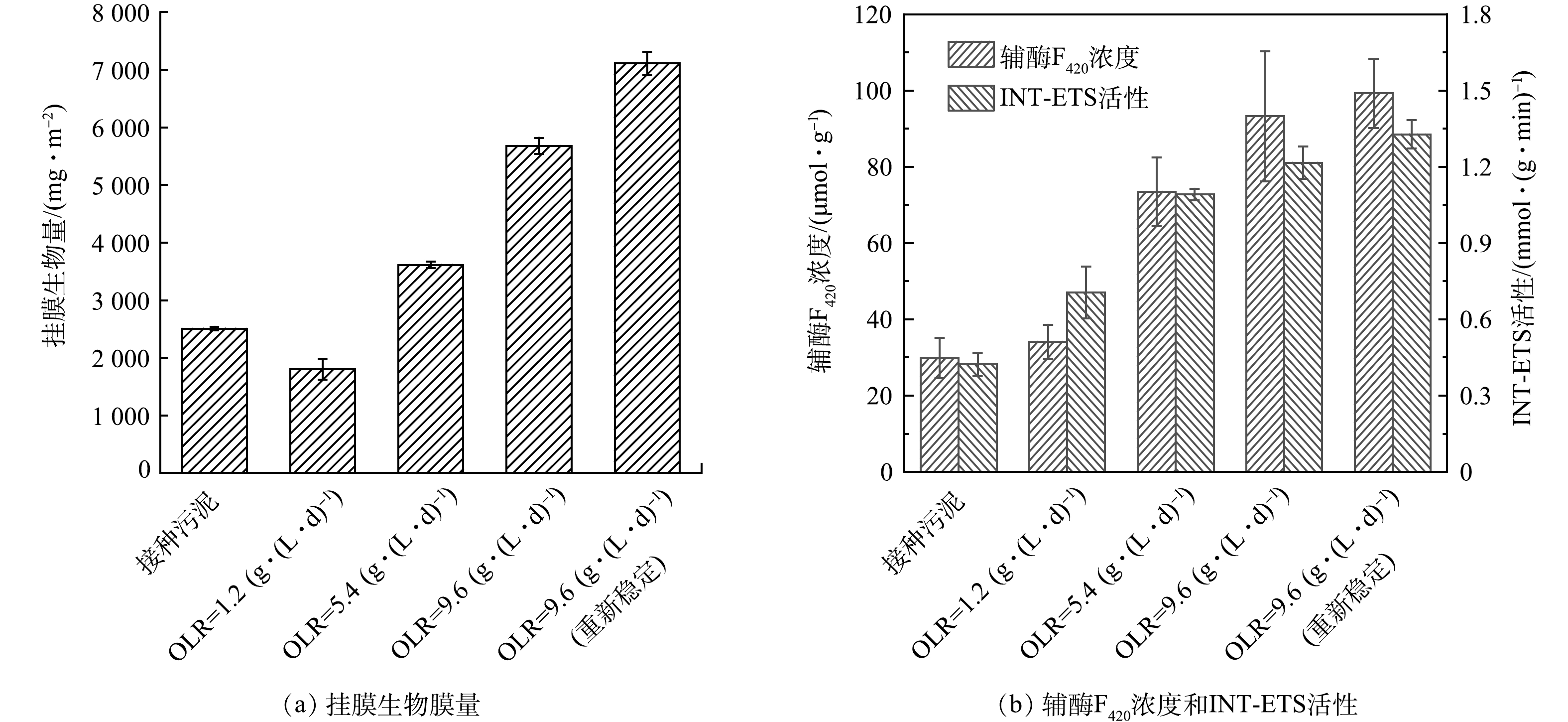
1) 生物膜量。微生物利用有机物代谢并在填料表面聚集形成生物膜,并通过脱落更新来稳定生物量[20]。OLR对生物量的影响见图3。与接种污泥相比,当OLR为1.2 g·(L·d)−1)时,生物量为1 802.8 mg·m−2,减少了28.0%(图3(a)),这是部分原有微生物无法适应新环境导致的死亡脱落。在OLR从1.2 g·(L·d)−1增至9.6 g·(L·d)−1后,生物膜量由1 802.8 mg·m−2增至7 108.8 mg·m−2。这不仅得益于废水中有机物易被微生物代谢利用,还因序批式工艺存在“吸收-储存-利用”的特性,促进了微生物的增殖[21]。
辅酶F420是产甲烷菌特有的一种低电位电子载体。REYNOLDS等[22]认为,辅酶F420由氢营养型产甲烷菌分泌,其浓度可反映产甲烷菌的数量或产甲烷活性。INT-ETS活性指微生物的电子传递速率,反映厌氧微生物的活性[15]。辅酶F420浓度和INT-ETS活性随负荷的提升而增加(图3(b)),在OLR从1.2 g·(L·d)−1增至5.4 g·(L·d)−1期间,辅酶F420浓度和INT-ETS活性增加最为明显,分别增加了115.2%和102.9%。
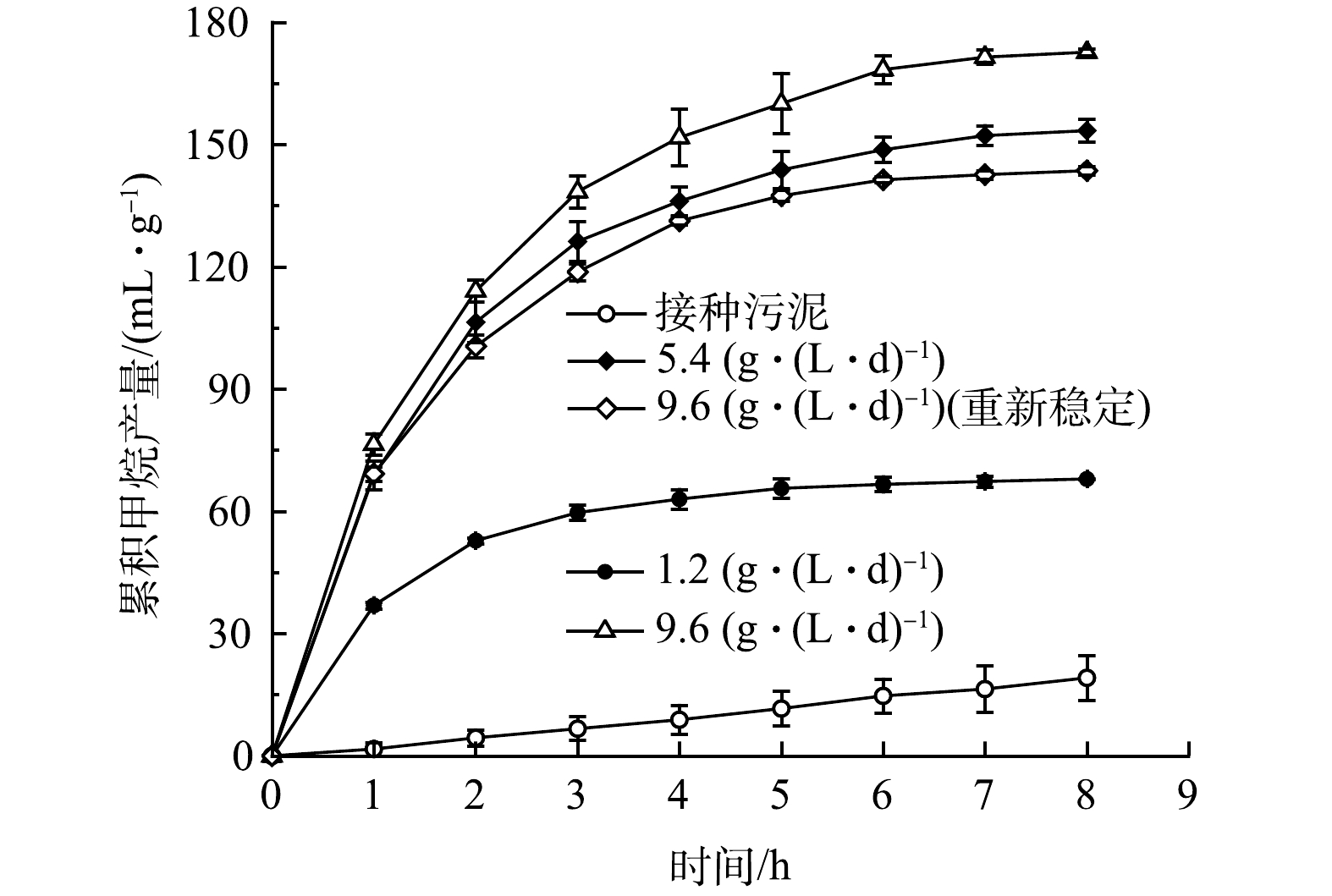
图4反映了OLR对产甲烷速率的影响。使用Gompertz模型对结果进行拟合,结果见表3。针对特定的废水,接种污泥对其降解的能力不足,甲烷产量和产甲烷速率分别为19.16 mL·g−1和2.83 mL·(g·h)−1。驯化后甲烷产量显著增加,达到67.95 mL·g−1,产甲烷速率为34.44 mL·(g·h)−1。当OLR为9.6 g·(L·d)−1时,产甲烷速率最大,为59.36 mL·(g·h)−1,累计甲烷产量达到了172.68 mL·g−1。可以看出,生物膜的产甲烷速率随OLR的提升而提高,在OLR从1.2 g·(L·d)−1增至5.4 g·(L·d)−1期间,产甲烷速率提高较明显,产甲烷速率和甲烷产量分别提高了68.0%和125.8%。
总体来看,生物膜量、辅酶F420浓度、INT-ETS活性和产甲烷速率随OLR的提升呈现不同程度的增加,但是在较高OLR下,增加速率明显减缓。这说明,逐级提升OLR的运行策略可促进相关功能菌的富集,并加快微生物对基质的利用速率。但由于产甲烷菌属对OLR的适应范围存在差异,过高的OLR对部分产甲烷菌生长和代谢产生抑制[10],导致后续OLR提升过程对生物膜体系的促进效果削弱。
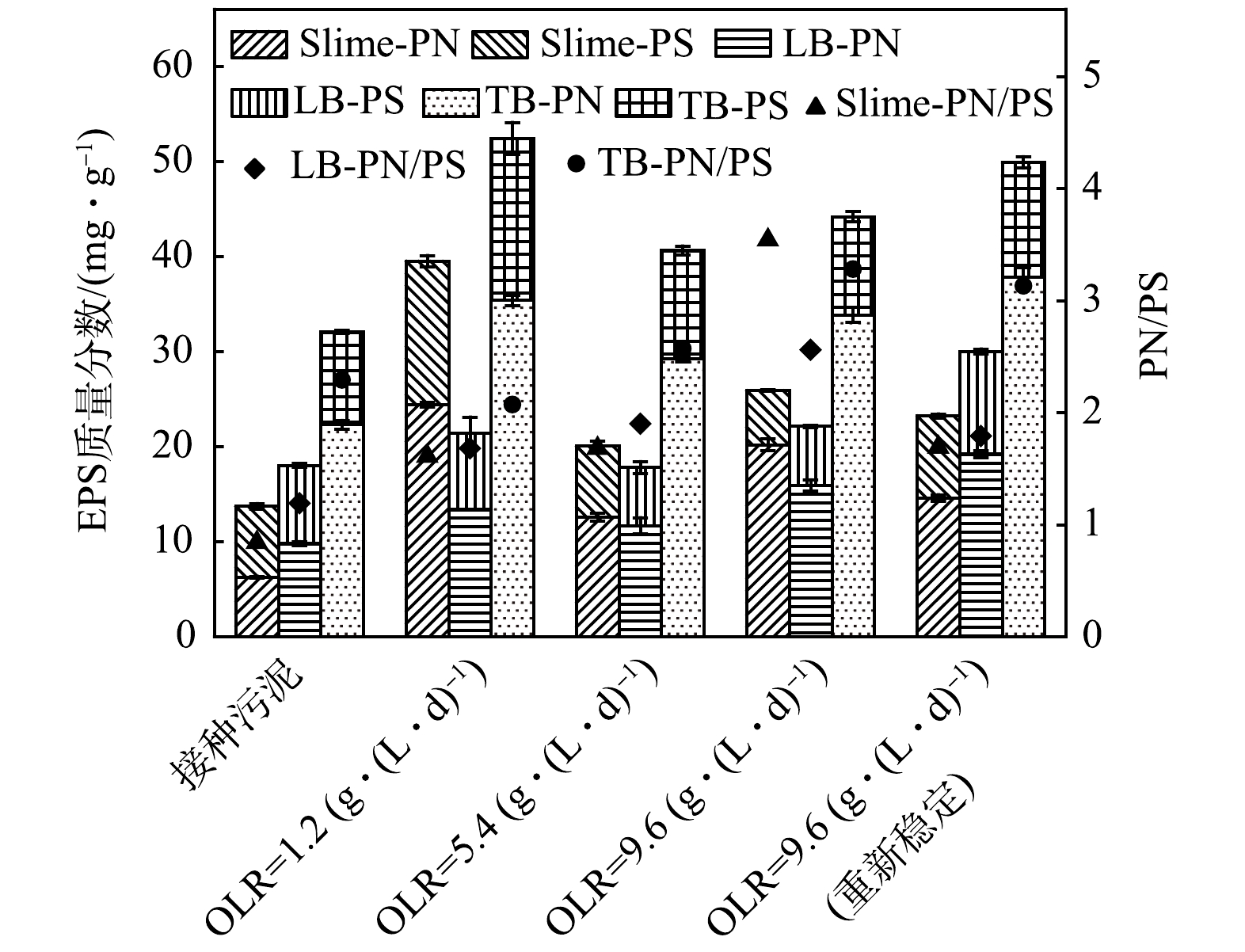
2) EPS特性。生物膜体系中胞外聚合物是实现微生物与载体、微生物与微生物之间黏附的关键,通过细胞分泌、脱落和外部吸附等途径形成,主要由蛋白质和多糖组成[23]。EPS空间结构包括溶解态EPS(Slime-EPS)、松散型EPS(LB-EPS)和紧密型EPS(TB-EPS)。驯化成功后的Slime-EPS和TB-EPS浓度相较于接种污泥明显增加,Slime-PN和Slime-PS浓度分别提高了291.0%和103.0%,TB-PN和TB-PS浓度分别提高了58.5%和75.1%(图5)。当原有微生物因无法适应新环境死亡脱落后被吸附在生物膜表面,从而使EPS最外层的Slime-EPS浓度明显增加。TB-EPS是与细胞紧密结合的聚合物,当反应器成功启动时,TB-EPS浓度的增加,有利于微生物聚集体的黏附聚集以及维持聚集体空间结构和功能的完整。随后,微生物代谢增殖消耗EPS表面吸附的过量营养物质使得OLR为5.4 g·(L·d)−1时EPS浓度降低。
EPS存在于污泥表面,当环境发生改变时,其组成成分更易受到影响[24]。各层EPS中PN/PS比值随负荷的提升而增加,当OLR为9.6 g·(L·d)−1时,Slime-PN/PS、LB-PN/PS和TB-PN/PS比值分别达到了3.54、2.56和3.28。PN具有疏水性,在逐级提升负荷期间,较高的PN/PS比值可以有效保证污泥的稳定性。重新稳定后,各层EPS中PN/PS的比值变小,分别为1.69、1.79和3.13。PN和PS携带相反的电荷基团,经更高负荷冲击后,为避免过高VFA浓度可能导致的生物膜解体,EPS组分中PN/PS比值变小可以有效减少聚集体间的静电排斥力,从而使生物膜更加密实,以应对高负荷环境对生物膜体系的影响[25]。
-
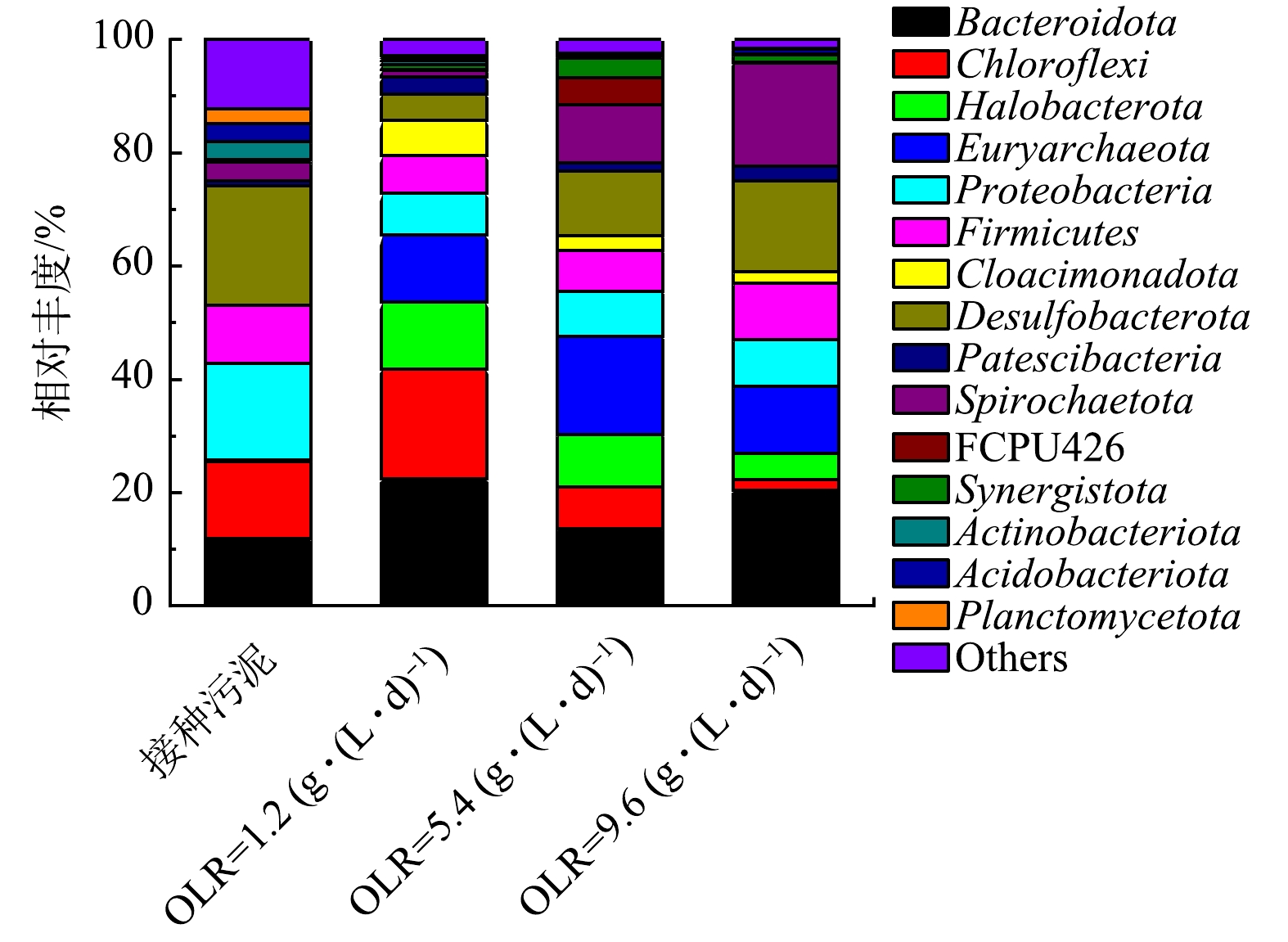
1) 门水平群落特性。门水平上菌群分布结果如图6所示。经驯化后,优势菌(相对丰度>7%)从Desulfobacterota (21.1%)、Proteobacteria (17.1%)、Chloroflexi (13.7%)、Bacteroidota (11.8%)和Firmicutes (10.3%)演替为Bacteroidota (22.4%)、Chloroflexi (19.5%)、Halobacterota (11.8%)、Euryarchaeota (11.7%)和Proteobacteria (7.4%),这表明进水基质组分将显著影响微生物群落结构。在OLR从1.2 g·(L·d)−1增至9.6 g·(L·d)−1期间,优势菌Proteobacteria (P>0.05)、Desulfobacterota (P<0.05)和Spirochaetota (P<0.05)丰度随OLR提升而增加。Halobacterota (P<0.05)和Chloroflexi (P>0.05)丰度随OLR提升而减少。Proteobacteria、Chloroflexi、Spirochaetota和Bacteroidota是中温厌氧体系中常见的水解细菌,可将蛋白质和碳水化合物降解为VFAs或乙醇等物质[15]。Firmicutes和Euryarchaeota丰度呈现先增后减的趋势,而Bacteroidota丰度则是先减后增。这说明,在低OLR下,Firmicutes更易表现出高聚集性;在高OLR下,Bacteroidota生物活性更高。在酿造工序中会添加SO2抑制细菌,SO2氧化后以硫酸盐的形式存在于废水中,Desulfobacterota可将硫酸盐转化为硫化氢。
Halobacterota和Euryarchaeota均为产甲烷古菌门[15]。当OLR为1.2、5.4和9.6 g·(L·d)−1时,菌群中古菌门整体相对丰度分别约为22.5%、26.7%和16.5%。这表明,在OLR从1.2 g·(L·d)−1增至5.4 g·(L·d)−1期间,产甲烷古菌得到了富集,增强了AnSBBR的消化性能和稳定性。在负荷进一步提升后,丰度明显下降,说明高负荷环境对部分产甲烷古菌存在抑制效果。
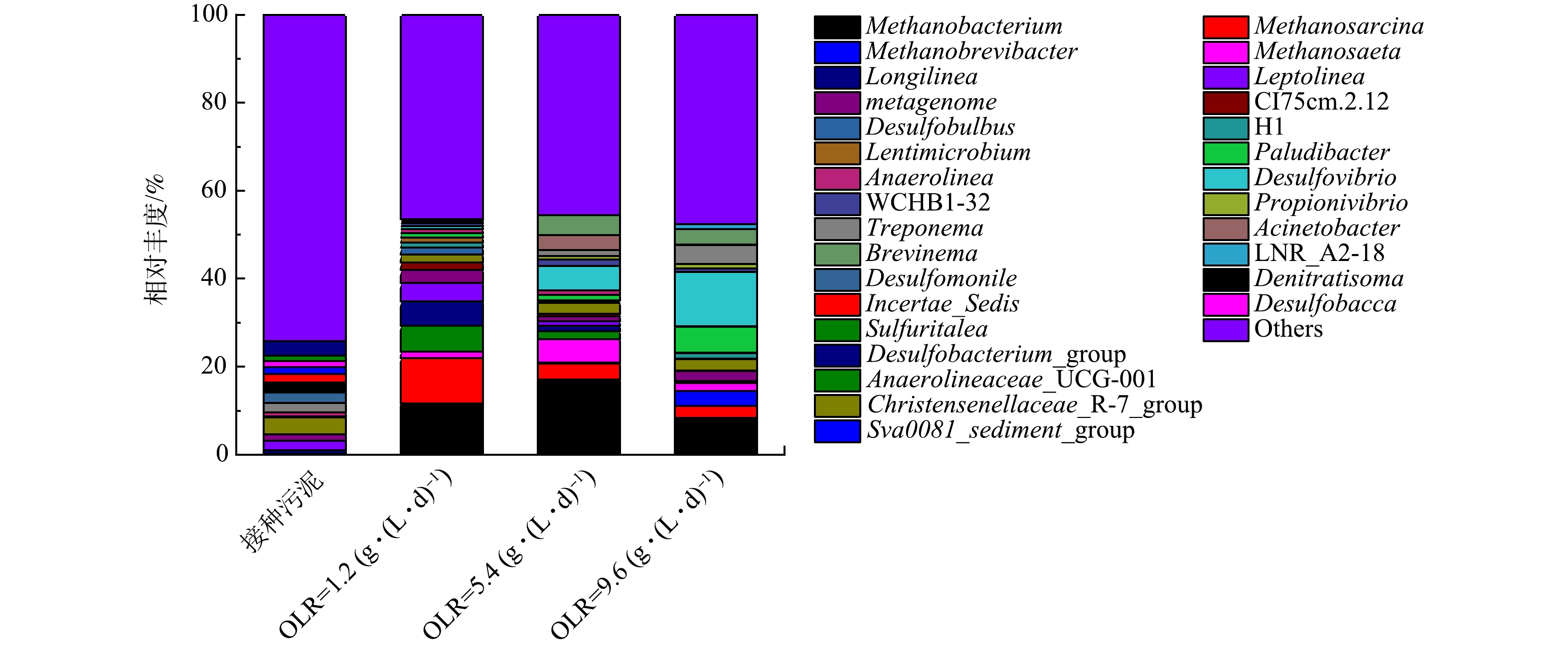
2) 属水平群落特性。接种污泥经驯化后,Methanobacterium (11.7%)、Methanosarcina (10.3%)、Anaerolineaceae_UCG-001 (5.9%)、Longilinea (5.5%)和Leptolinea (4.3%)成为主要的菌属(图7)。Methanobacterium是利用H2/CO2产生CH4的嗜氢产甲烷菌,Methanosarcina是可同时利用乙酸、H2/CO2和甲基三种底物的产甲烷菌。因二者均属r策略菌,具备世代时间短、比生长速率高的特性,在驯化后丰度明显增加[26]。Anaerolineaceae_UCG-001、Longilinea和Leptolinea可转化多糖等碳水化合物为乙酸,葡萄酒生产废水中丰富的有机物促进了菌属的增殖[10,13]。
在OLR从1.2 g·(L·d)−1增至9.6 g·(L·d)−1期间,主要存在4种古菌属和8种细菌属 (相对丰度>3%)。Methanosarcina、Anaerolineaceae_UCG-001和Longilinea丰度随OLR提升而减少 (P>0.05);Methanobrevibacter与Paludibacter (P>0.05),Desulfovibrio与Treponema (P<0.05)丰度随OLR提升而增加;Methanobacterium、Acinetobacter和Brevinema丰度呈现先增后减的趋势,Methanosaeta丰度变化与之相反。
在OLR从1.2 g·(L·d)−1增至5.4 g·(L·d)−1后, Methanobacterium丰度由11.7%增至17.1%,Methanosaeta丰度由1.4%增至5.3%,Desulfovibrio丰度由0.8%增至5.6%,Brevinema丰度由0.01%增至4.6%,Methanosarcina丰度由10.3%降至3.7%,Anaerolineaceae_UCG-001丰度由5.9%降至1.7%,Longilinea丰度由5.5%降至1.3%。研究[10]显示,专性嗜乙酸产甲烷菌Methanosaeta在较低的OLR下对乙酸的利用率更高,从而抑制了Methanosarcina的生长。Desulfovibrio是典型的乙醇氧化菌,能够代谢包括乙醇在内的多种碳水化合物,随进水有机物浓度的增加而逐渐富集。
在OLR从5.4 g·(L·d)−1增至9.6 g·(L·d)−1后,Desulfovibrio丰度由5.6%增至12.4%,Paludibacter丰度由1.3%增至5.8%,Methanobrevibacter丰度由0.02%增至3.3%,Treponema丰度从1.3%增至4.3%,Methanobacterium丰度由17.1%降至8.3%,Methanosaeta丰度由5.3%降至1.8%。Desulfovibrio进一步富集后,与Methanosaeta和Methanosarcina竞争基质中的乙酸,又因Methanosarcina可利用多种基质代谢,故受到的影响明显小于Methanosaeta[10,13]。Paludibacter为产酸菌,可利用多种单糖和二糖产生乙酸、丙酸,Treponema参与同型产乙酸过程[12]。Methanobrevibacter可通过产生氧化型NAD+载体来提高产甲烷活性,并且在高OLR下生物活性更高[27]。
当OLR为1.2、5.4和9.6 g·(L·d)−1时,菌群中整体古菌属相对丰度分别约为23.4%、26.3%和16.2%;当OLR为5.4 g·(L·d)−1时,产甲烷古菌整体丰度最大并且存在Methanosphaerula(0.2%)等其他产甲烷菌。产甲烷过程作为厌氧消化的限速步骤,产甲烷菌的丰度越大、种类越多,越有利于提高生物膜体系的消化性能和应对潜在环境变化的能力。
-
1) 在满足出水COD低于200 mg·L−1的前提下,AnSBBR可有效处理OLR为1.2~9.6 g·(L·d)−1的葡萄酒生产废水。OLR为5.4 g·(L·d)−1时,运行性能最佳,COD去除率为(97.2±0.5)%,甲烷产量和甲烷产率分别为20.2 L·d−1和(349.9±7.6) mL·g−1。
2) 与OLR为1.2 g·(L·d)−1相比,OLR为9.6 g·(L·d)−1时,产甲烷速率提高69.3%,生物膜量增加294.3%,辅酶F420浓度增加190.8%以及INT-ETS活性提高88.4%。这说明,逐级提升OLR的运行策略可促进厌氧生物膜体系的生长。
3) 高通量测序显示,群落中主要细菌为Desulfovibrio、Brevinema、Treponema、Longilinea、Paludibacter和Leptolinea,主要古菌为Methanobacterium、Methanobrevibacter,Methanosaeta和Methanosarcina,其相对丰度随负荷的提升发生变化。当OLR为5.4 g·(L·d)−1时,产甲烷菌整体丰度最大且多样性更高;当OLR为9.6 g·(L·d)−1时,培养出特定的菌属(Methanobrevibacter)来适应环境。
厌氧序批式生物膜反应器处理葡萄酒生产废水性能及菌群演替分析
Performance on treating winery wastewater by AnSBBR and analysis of the microbial community succession
-
摘要: 为探究厌氧序批式生物膜法处理葡萄酒生产废水的可行性,采用厌氧序批式生物膜反应器(AnSBBR)预处理模拟葡萄酒生产废水,通过逐级增加进水浓度来提升有机负荷并对AnSBBR在不同负荷下的运行特性及菌群演替规律。结果表明: 在35 ℃、HRT为20 h、周期为4 h的运行工况下,当OLR为1.2~9.6 g·(L·d)−1时,AnSBBR均能保证出水COD值低于200 mg·L−1,当OLR为5.4 g·(L·d)−1时,运行性能最佳,COD去除率为(97.2±0.5)%,甲烷产率为(349.9±7.6) mL·g−1;与OLR为1.2 g·(L·d)−1相比,OLR为9.6 g·(L·d)−1时产甲烷速率提高69.3%,生物量增加294.3%,辅酶F420浓度增加190.8%,电子传递活性(INT-ETS)提高88.4%;当OLR增至10.2 g·(L·d)−1后,反应器中VFA浓度持续增加,丙酸和丁酸积累,缓冲能力明显下降,系统无法适应该负荷条件;高通量测序显示,群落中主要的细菌为Desulfovibrio、Brevinema、Treponema、Longilinea、Paludibacter和Leptolinea,主要的古菌为Methanobacterium、Methanobrevibacter,Methanosaeta和Methanosarcina;受进水基质组分和负荷的影响,细菌和古菌中丰度占比最大的分别为Desulfovibrio(12.4%)和Methanobacterium(17.1%);当OLR为5.4 g·(L·d)−1时,产甲烷菌多样性更高且在门和属水平上整体丰度最大,分别为26.7%和26.3%;当OLR为9.6 g·(L·d)−1时,培养出特定的菌属(Methanobrevibacter)以适应环境。由此可以看出,逐级提升OLR的运行策略可促进生物膜系统的增殖和代谢活性。本研究确定了AnSBBR处理葡萄酒生产废水的最大及最佳运行负荷,该结果可为推动AnSBBR的工程化应用提供依据。
-
关键词:
- 葡萄酒生产废水 /
- 厌氧序批式生物膜反应器 /
- 有机负荷 /
- 胞外聚合物 /
- 群落结构
Abstract: In order to explore the feasibility of treating winery wastewater by anaerobic sequencing biofilm batch reactor (AnSBBR), a laboratory-scale AnSBBR was used to pretreat the simulated winery wastewater. The organic loading rate was achieved by the gradual increase of the influent concentration, and its operational characteristics and microbial community composition at different loading rates were studied. The results showed that at 35 ℃, HRT of 20 h and operation period of 4 h, the effluent COD of AnSBBR was lower than 200 mg·L−1 when the OLR was 1.2~9.6 g·(L·d)−1. When the OLR was 5.4 g·(L·d)−1, the COD removal rate was 97.2±0.5%, the methane yield was 349.9±7.6 mL·g−1. Compared with the OLR of 1.2 g·(L·d)−1, the methanogenic rate increased by 69.3%, the biofilm biomass increased by 294.3%, the content of coenzyme F420 increased by 190.8%, and the activity of INT-ETS increased by 88.4% at the OLR of 9.6 g·(L·d)−1. When the OLR reached 10.2 g·(L·d)−1, the volatile fatty acids (VFA) concentration in AnSBBR continued to increase, the propionic acid and butyric acid gradually accumulated, and the buffer capacity significantly decreased, indicating that the AnSBBR system could note adopt the OLR of 10.2 g·(L·d)−1. Illumina Miseq sequencing showed that the dominant bacteria were Desulfovibrio, Brevinema, Treponema, Longilinea, Paludibacter, and Leptolinea, the dominant archaea were Methanobacterium, Methanobrevibacter, Methanosaeta, and Methanosarcina. The relative abundances of Desulfovibrio(12.4%)and Methanobacterium(17.1%) were the largest because the microbial community structure was affected by the characteristics of the influent matrix. When the OLR was 5.4 g·(L·d)−1, the diversity of archaea was higher, and the abundances were the largest at the level of phylum and genus with 26.7% and 26.3%, respectively. At the OLR of 9.6 g·(L·d)−1, the relative abundance of methanobacterium and methanosarcina were reduced and specific archaea (Methanobrevibacter) was cultivated to adapt to the environment. The results found that the operation strategy of increasing OLR step by step promotes proliferation and metabolic activity of the biofilm system. And the maximum and optimal organic loading rate of AnSBBR for the treatment of winery wastewater were determined in this study, then promoted the engineering application of AnSBBR. -

-
表 1 AnSBBR运行参数
Table 1. Operation parameters of AnSBBR
阶段 时间/
dCOD/
(g·L−1)水力停留
时间/h运行
周期/h有机负荷/
(g·(L·d)−1)驯化 1~25 1.0 40 8 0.6 驯化 26~37 1.0 20 4 1.2 强化 38~172 1.0~8.0 20 4 1.2~9.6 稳定 173~238 8.0~8.5 20 4 9.6~10.2 表 2 不同类型反应器在中温下处理葡萄酒生产废水的性能对比
Table 2. Comparison of reactor performance under the mesophilic condition between this study and many other studies
表 3 不同OLR下Gompertz模型产甲烷拟合结果
Table 3. Results of Gompertz methane production under different OLRs
有机负荷/
(g·(L·d)−1)最大甲烷产量/
(mL·g−1)最大甲烷产率/
(mL·(g·h)−1)拟合系数R2 实测产量/
(mL·g−1)接种污泥 26.54±6.19 2.83±0.32 0.99 19.16±5.46 1.2 65.47±1.42 34.44±5.35 0.98 67.95±0.17 5.4 147.96±3.78 57.10±8.02 0.98 153.43±2.80 9.6 167.34±4.88 59.36±8.74 0.98 172.68±0.87 9.61) 138.96±4.00 58.31±9.90 0.97 143.58±1.04 注:1) 为重新稳定的负荷。 -
[1] International Organisation of Vine and Wine (OIV). OIV statistical report on world vitiviniculture [DB/OL]. (2021-07-16) [2022-12-28]. https: //www. oiv. int. [2] VITAL-JACOME M A, BUITRÓN G. Thermophilic anaerobic digestion of winery effluents in a two-stage process and the effect of the feeding frequency on methane production[J]. Chemosphere, 2021, 272: 129865. doi: 10.1016/j.chemosphere.2021.129865 [3] KEYSER M, WITTHUHN R C, RONQUEST L C, et al. Treatment of winery effluent with upflow anaerobic sludge blanket (UASB): Granular sludges enriched with Enterobacter sakazakii[J]. Biotechnology Letters, 2003, 25(22): 1893-1898. doi: 10.1023/B:BILE.0000003978.72266.96 [4] RUIZ C, TORRIJOS M, SOUSBIE P, et al. Treatment of winery wastewater by an anaerobic sequencing batch reactor[J]. Water Science and Technology, 2002, 45(10): 219-224. doi: 10.2166/wst.2002.0336 [5] IOANNOU L A, PUMA G L, FATTA-KASSINOS D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review[J]. Journal of Hazardous Materials, 2015, 286: 343-368. doi: 10.1016/j.jhazmat.2014.12.043 [6] LIANG X Y, RENGASAMY P C, SMERNIK R, et al. Does the high potassium content in recycled winery wastewater used for irrigation pose risks to soil structural stability?[J]. Agricultural Water Management, 2021, 243: 106422. doi: 10.1016/j.agwat.2020.106422 [7] BEZERRA R A, RODRIGUES J A, RATUSZNEI S M, et al. Effect of organic load on the performance and methane production of an AnSBBR treating effluent from biodiesel production[J]. Applied Biochemistry and Biotechnology, 2011, 165(1): 347-368. doi: 10.1007/s12010-011-9255-6 [8] LOVATO G, MONCAYO BRAVO I S, RATUSZNEI S M, et al. The effect of organic load and feed strategy on biohydrogen production in an AnSBBR treating glycerin-based wastewater[J]. Journal of Environmental Management, 2015, 154: 128-137. [9] CARVALHINHA P P, FLÔRES A, RODRIGUES J A D, et al. AnSBBR applied to the treatment of metalworking fluid wastewater: effect of organic and shock load[J]. Applied Biochemistry and Biotechnology, 2010, 162(6): 1708-1724. doi: 10.1007/s12010-010-8952-x [10] VÁSQUEZ J, NAKASAKI K. Effects of shock loading versus stepwise acclimation on microbial consortia during the anaerobic digestion of glycerol[J]. Biomass and Bioenergy, 2016, 86: 129-135. doi: 10.1016/j.biombioe.2016.02.001 [11] 熊荣波, 柳丽, 孟艳, 李屹, 等. 含固率和接种比对菜籽饼中温厌氧消化特性的影响[J]. 环境科学研究, 2022, 35(1): 230-237. doi: 10.13198/j.issn.1001-6929.2021.08.20 [12] LI D, CHEN L, LIU X F, et al. Instability mechanisms and early warning indicators for mesophilic anaerobic digestion of vegetable waste[J]. Bioresource Technology, 2017, 245: 90-97. doi: 10.1016/j.biortech.2017.07.098 [13] LI J Z, YAN H, CHEN Q Y, et al. Performance of anaerobic sludge and the microbial social behaviors induced by quorum sensing in a UASB after a shock loading[J]. Bioresource Technology, 2021, 330: 124972. doi: 10.1016/j.biortech.2021.124972 [14] 赵阳, 李秀芬, 堵国成, 等. 钴及其配合物对产甲烷关键酶的影响[J]. 水资源保护, 2008, 24(2): 82-85. doi: 10.3969/j.issn.1004-6933.2008.02.022 [15] ZHAO Z Q, ZHANG Y B, YU Q L, et al. Communities stimulated with ethanol to perform direct inter species electron transfer for syntrophic metabolism of propionate and butyrate[J]. Water Research, 2016, 102: 475-484. doi: 10.1016/j.watres.2016.07.005 [16] 李韧, 于莉芳, 张兴秀, 等. 硝化生物膜系统对低温的适应特性: MBBR和IFAS[J]. 环境科学, 2020, 41(8): 3691-3698. [17] BASSET N, SANTOS E, DOSTA J, et al. Start-up and operation of an AnMBR for winery wastewater treatment[J]. Ecological Engineering, 2016, 86: 279-289. doi: 10.1016/j.ecoleng.2015.11.003 [18] CHAI S L, GUO J, CHAI Y, et al. Anaerobic treatment of winery wastewater in moving bed biofilm reactors[J]. Desalination and Water Treatment, 2014, 52(10): 1841-1849. [19] LI L, PENG X Y, WANG X M, et al. Anaerobic digestion of food waste: A review focusing on process stability[J]. Bioresource Technology, 2018, 248: 20-28. doi: 10.1016/j.biortech.2017.07.012 [20] YUAN K, LI S, ZHONG F. Treatment of coking wastewater in biofilmbased bioaugmentation process: Biofilm formation and microbial community analysis[J]. Journal of Hazardous Materials, 2020, 400(13): 123117. [21] 柴立, 薛旭东, 邓彦, 等. 冲击负荷对厌氧序批式反应器的影响及其恢复重建过程[J]. 环境污染与防治, 2013, 35(9): 54-57. doi: 10.3969/j.issn.1001-3865.2013.09.011 [22] REYNOLDS P J, COLLERAN E. Evaluation and improvement of methods for coenzyme F420 analysis in anaerobic sludges[J]. Journal of Microbiological Methods, 1987, 7(2/3): 115-130. [23] WILÉN B M, JIN B, LANT P. The influence of key chemical constituents in activated sludge on surface and flocculating properties[J]. Water Research, 2003, 37(9): 2127-2139. doi: 10.1016/S0043-1354(02)00629-2 [24] THOMAS S, DERLON N, DUEHOLM M S, et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis[J]. Water Research, 2019, 151(15): 1-7. [25] YOU G X, HOU J, WANG P F, et al. Effects of CeO2 nanoparticles on sludge aggregation and the role of extracellular polymeric substances: Explanation based on extended DLVO[J]. Environmental Research, 2016, 151: 698-705. doi: 10.1016/j.envres.2016.08.023 [26] KONG D W, ZHANG K Q, LIANG J F, et al. Methanogenic community during the anaerobic digestion of different substrates and organic loading rates[J]. MicrobiologyOpen, 2019, 8(5): e00709. doi: 10.1002/mbo3.709 [27] HUANG W H, WANG Z Y, ZHOU Y, et al. The role of hydrogenotrophic methanogens in an acidogenic reactor[J]. Chemosphere, 2015, 140: 40-46. doi: 10.1016/j.chemosphere.2014.10.047 -



 下载:
下载: