-
随着社会经济的快速发展,城市生活垃圾产生量也大幅增加[1]。由于技术的局限,过去生活垃圾的处置方式主要是填埋处理。生活垃圾在填埋发酵过程中会发生一系列生物化学反应,产生大量的垃圾渗滤液[2]。渗滤液中污染物种类繁多,具有较大的环境威胁,如果得不到妥善处置,将会严重污染周边土壤及地下水。为进一步提高渗滤液处理后的出水水质,满足污染控制标准,一些成熟的膜处理技术(如纳滤NF和反渗透RO)作为生化单元后的深度处理单元,越来越多地应用于渗滤液处理的工程实践中[3-6]。但是,上述膜处理技术本质上仅是一种物理分离过程,不可避免地产生10%~30%的膜过滤浓缩液[7-8]。膜浓缩液成分复杂,含有大量的难降解有机物(腐殖质络合体、芳香族化合物、卤代烃)、重金属、无机盐离子等,处理难度较大[9-10]。因此,寻找一种经济高效的渗滤液浓缩液处理方案成为当前亟需解决的一个难题。
目前,浓缩液的处理技术主要包含减量处理(蒸发)、全量处理(高级氧化、回喷焚烧)等[11-15]。回灌方式在过去10 a的浓缩液处理中比较普遍[16-17],但目前多数地区已禁止浓缩液回灌。蒸发技术对浓缩液的减量效果显著(70%~90%),但该技术存在设备易腐蚀、易结垢、存在明显的二次污染(恶臭、蒸发母液)等问题。焚烧法处理浓缩液,初期投资较大、焚烧过程控制复杂,且运行中容易导致系统腐蚀、炉渣结渣、结焦[18]。因此,更多的学者着手研究环境友好的高级氧化技术处理垃圾浓缩液。
由于高级氧化技术对难降解有机物的去除效果较好[19-21],且设备简单高效、适用范围广,可以作为预处理或深度处理单元与其他水处理技术联用,因此,受到研究人员的广泛关注。高级氧化技术的核心是在热量[22]、UV辐射[23-24]、超声[25]、电等[26-27]能量场的作用下,通过一系列物理化学过程产生大量的高活性自由基(·OH、·Cl、·SO4-等)。在自由基的氧化作用下,大分子有机物通过断链和开环等反应被分解成低毒、易生物降解的小分子,甚至被完全矿化为CO2和H2O[28-30]。作为一种成熟的高级氧化技术,传统Fenton技术(Fe2+/H2O2体系)利用Fe2+与H2O2在酸性条件下反应生成羟基自由基降解有机物,但其存在pH适用范围窄、铁泥产生量较大和H2O2利用效率低等缺s点。UV-Fenton催化氧化法结合了UV/H2O2和H2O2/Fe2+ 2种技术的优势,兼具紫外线的光解活性和羟基自由基的强氧化能力,可在一定程度上克服上述单一技术的缺陷[31]。UV-Fenton催化氧化技术被应用至不同类型合成废水或一些实际废水,但将其应用于膜浓缩液中COD深度处理比较少见。
本课题组前期对比研究了UV-Fenton催化氧化技术与传统Fenton法、UV/H2O2法对浓缩液中COD的去除效果,对UV-Fenton工艺的实验条件进行了系统优化,此外,比较了EM (effective microorganisms)菌单级好氧法与EM菌AO法处理浓缩液的脱氮效果,优化了EM菌AO法组合MBR膜法的工艺参数。在前期研究的基础上,本研究以具有代表性的深圳市下坪填埋场垃圾渗滤液膜浓缩液作为实验对象,采用UV-Fenton催化氧化+EM高效生物脱氮(AO法+MBR膜法)组合工艺开展连续120 d的中试实验,综合考察组合工艺在中试尺度上对难降解有机物以及总氮、氨氮的去除效果。
-
本文涉及的中试实验所用浓缩液来自深圳市下坪生活垃圾填埋场渗滤液处理系统的NF单元,COD为900~1 100 mg·L−1,TN为60~90 mg·L−1,NH3-N为15~20 mg·L−1。UV-Fenton催化氧化单元所用的催化剂硫酸铁(工业级)购买自巩义市圣泉净水材料厂,氧化剂双氧水(H2O2,50%)购买自广州市布思化工有限公司。EM高效生物脱氮单元所需的EM复合功能菌剂购买自广州甘度生物技术有限公司,后经进一步驯化培养所得。常规污染指标检测所需的全部化学药剂(分析纯)都购买自国药试剂公司。
-
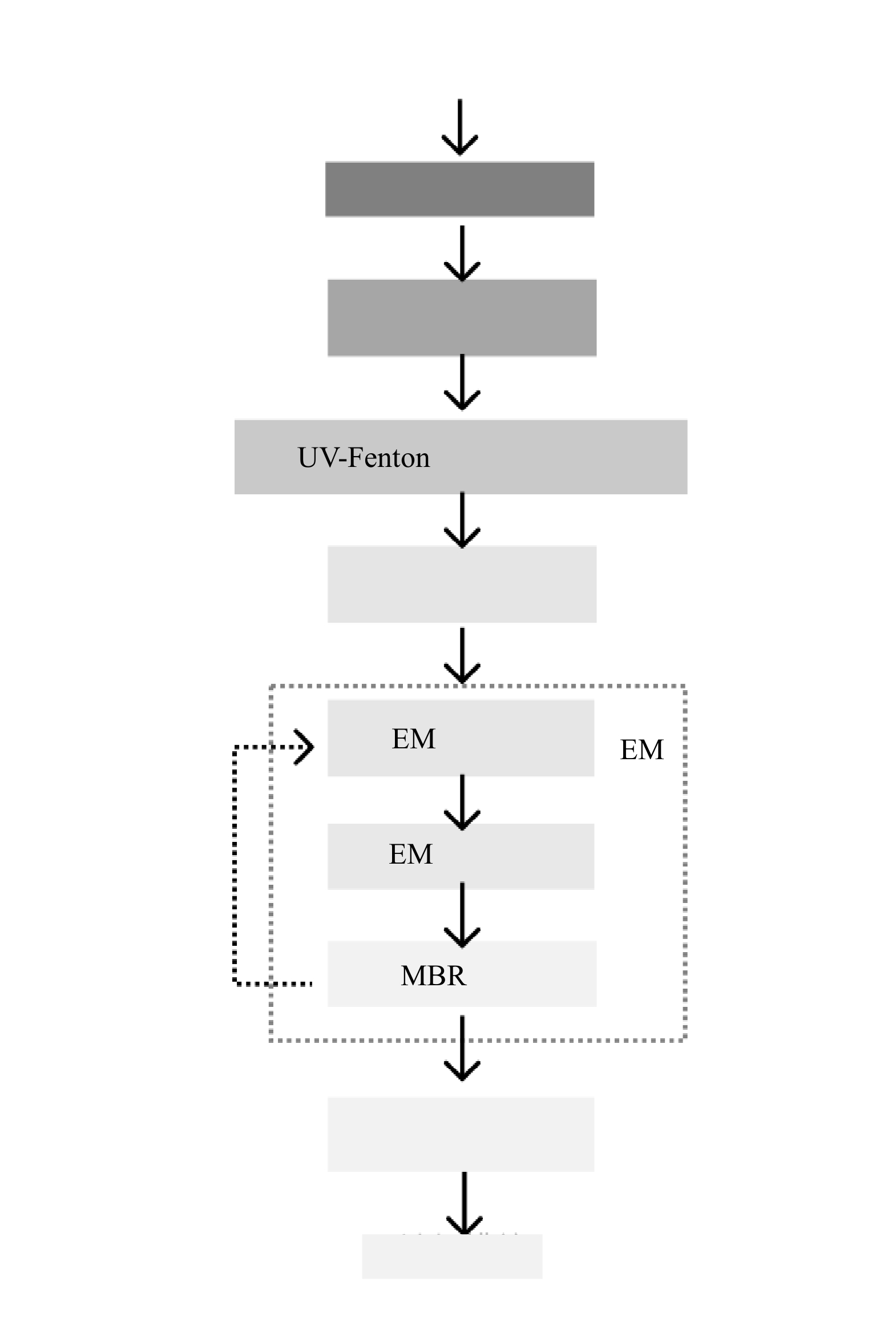
中试处理规模为1 t·d−1,实验装置如图1所示。整个装置由集水池、pH调节池、UV-Fenton催化氧化反应器、中间水池、EM菌高效生物脱氮一体化反应器和清水池组成。
-
1)菌种驯化及培养。微生物的生长速度以及菌种数量在一定程度上影响着生化系统处理废水的有效性,因此,菌群的前期培养和驯化是保证生化单元良好脱氮效果的重要前提。本文对EM菌的驯化和培养步骤如下:在装有2/3清水的培养桶,投加一定比例的培养液(包含葡萄糖、氯化铵和磷酸二氢钾,其中BOD:N:P的比例为100:5:1),曝气的同时搅拌均匀;投加适量EM菌剂至上述培养桶中,曝气12 h,停止6 h后继续曝气,如此往复进行;每24 h添加1次营养液,间隔相同时间取样,使用显微镜观察菌种数量及菌种状态;等到菌种数量达到设定值后逐渐添加待处理浓缩液,直至完全置换掉培养桶中的清水,继续培养3~5 d后终止培养。
2)组合工艺中试实验。本研究采用的组合工艺流程图如图2所示。具体工艺流程为:垃圾渗滤液膜浓缩液由提升泵进入集水池,后进入调节池进行pH调节,然后进入UV-Fenton反应器进行深度降解或矿化;UV-Fenton反应器出水经氧化剂分解器处理后可去除残留氧化剂,然后沉淀去除铁泥;UV-Fenton反应器出水进入EM菌反应器,即EM菌AO法组合MBR膜反应器。首先进入缺氧池(A池)进行硝化作用,然后进入好氧池(O池)去除部分COD和进行反硝化作用,最后进入MBR膜池分离菌泥后进入清水池,实现达标排放。基于前期的实验研究,本研究确定UV-Fenton催化氧化单元的最佳运行条件为:H2O2投加方式为连续流投加且H2O2投加质量浓度为15.3 g·L−1,FeSO4·7H2O的质量浓度为2.0 g·L−1,体系pH为3.0,反应温度控制在25 oC。EM菌生物脱氮的最佳运行条件为:进水pH为7.0~8.0,停留时间为20 h,缺氧段溶解氧含量低于0.5 mg·L−1,好氧段溶解氧为2.0 mg·L−1,反应温度为25 oC。浸入式MBR选用亲水改性的聚四氟乙烯中空纤维膜组件(SPMW-301AD5, Poreflon SUMITOMO),平均孔径为0.2 μm。曝气方式为鼓风池底曝气。MBR池中反应器温度保持在25~28 ℃,DO控制为3~6 mg·L−1,污泥(MLSS)质量浓度维持在9 000~12 000 mg·L−1。MBR膜池采用间歇抽吸式出水方式(运行10 min,暂停2 min),实验期间反应器以取样形式排泥。
-
主要常规指标的检测方法参照《水和废水监测分析方法(第四版)》[32]。其中,化学需氧量(COD)采用重铬酸钾法测定,总氮(TN)采用碱性过硫酸钾消解紫外分光光度法(HJ 636-2012),氨氮(NH3-N)的测定采用纳氏试剂比色(HJ 535-2009)法。
采用气相色谱-质谱(GC-MS)法对浓缩液中难降解有机物尤其是痕量环境激素邻苯二甲酸酯类(PAEs)进行定性、定量分析,以研究组合工艺尤其是UV-Fenton催化氧化单元对难降解有机物的处理能力。具体方法如下:用丁酮溶剂对试样中的PAEs进行超声波提取及定容后,用气相色谱/质谱联用仪(GCMS-QP2010 Ultra, Shimadzu, Japan)进行测定。采用全扫描的总离子流色谱图(TIC)和质谱图(MS)进行定性,选择离子检测和外标法进行定量。主要参数选择如下:色谱柱采用HP-5MS石英毛细管柱;离子源选择EI源,离子源温度为230 ℃,电离能量为70 eV;进样口温度为280 ℃,载气流速为1.0 mL·min−1。其中,邻苯二甲酸二丁酯DBP保留时间为9.6 min,定性离子为150、205、223,定量离子为149。邻苯二甲酸二辛酯DEHP保留时间为13.0 min,定性离子为150、167、279,定量离子为149。该方法的检出限为0.1~1 μg·L−1,加标回收率为77.8%~109.9%,相对标准偏差RSD为1.8%~7.2%。
紫外-可见(Ultraviolet-visible, UV-vis)光谱采用岛津UV-2550紫外可见分光光度计测定,光度模式为Abs,扫描速度为中速,扫描范围为200~700 nm,光谱带宽为2 nm,扫描步长为l nm。水样测试前需先用孔径为0.45 μm的滤膜过滤,再稀释20倍。采用日本HITACHI公司的F-7000荧光光谱分析仪采集样品的三维激发-发射(three dimensional excitation and emission, 3D EEMs)光谱,激发光源为150 W氙弧灯,PMT电压为700 V,信噪比大于110。激发波长为220~600 nm,发射波长为250~650 nm,两者步长皆设定为5 nm,扫描速度为2 400 nm·min−1。采用去离子水作为空白,在测试前先用0.45 μm滤膜对水样进行过滤预处理。腐殖化指数(humification index, HIX)被用来指示浓缩液中溶解性有机物(dissolved organic matter, DOM)的腐殖化程度、来源等,在激发波长为254 nm时,发射波长435~480 nm的区域积分值除以发射波长300~345 nm的区域积分值为HIX值。在激发波长370 nm处,发射波长450 nm的荧光强度值除以发射波长500 nm的荧光强度值为荧光因子(fluorescent index, FI)值,其常用来指示DOM的来源。自生源指标(biological index, BIX)为激发波长为310 nm时发射波长在380 nm和430 nm处的荧光强度比值,其可反映DOM的自生源特征以及生物可利用性。
-
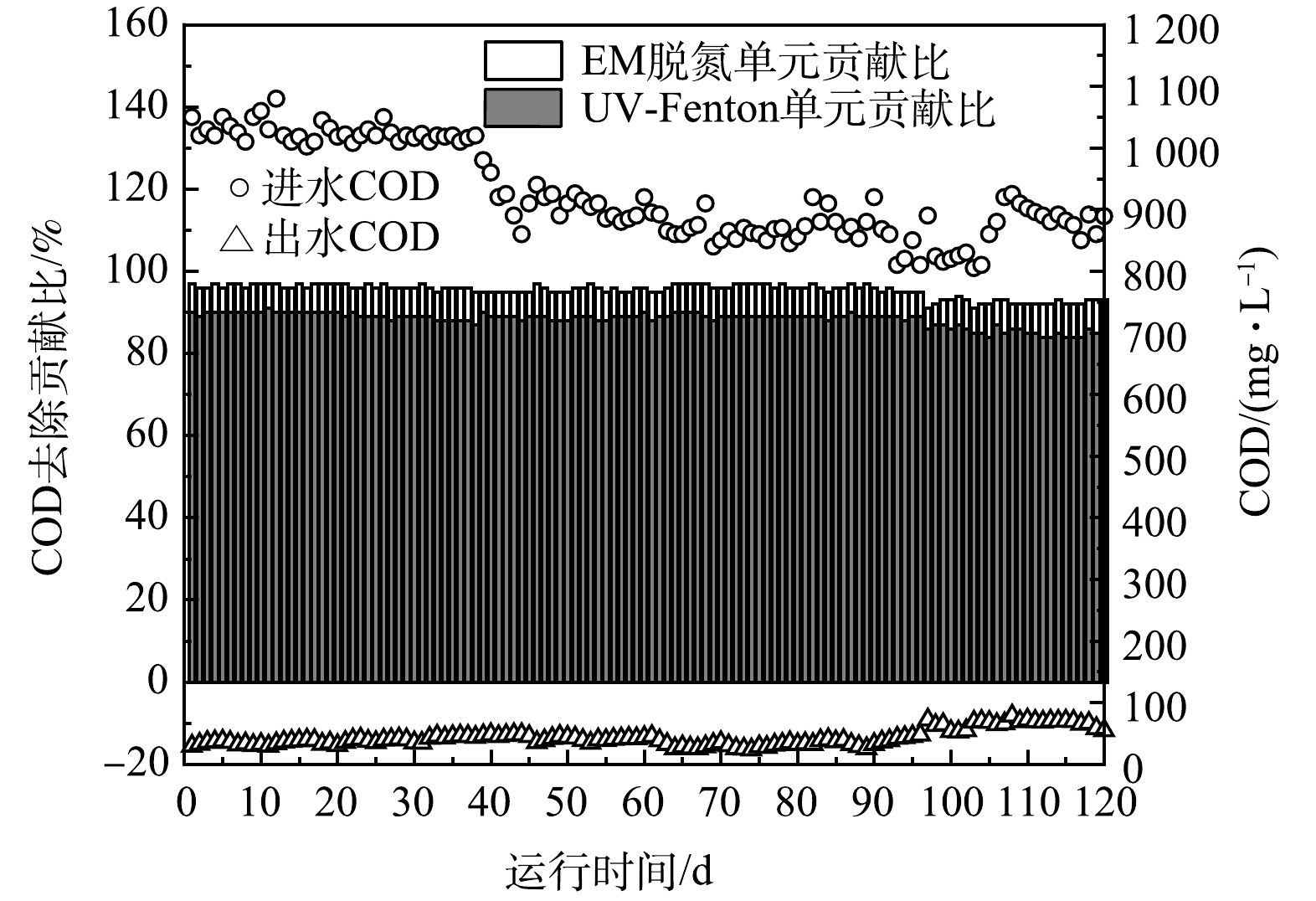
1) COD的去除效果。本文考察了组合工艺对浓缩液中COD的去除效果。由图3可知,在连续120 d的中试实验过程中,进水COD在1 050~850 mg·L−1波动,呈现缓慢下降的趋势。在整个中试周期内,组合工艺对浓缩液中COD的去除率为91.7%~95.2%,出水COD均明显低于100 mg·L−1,且去除效果稳定,完全符合《生活垃圾填埋场污染控制标准》(GB 16889-2008)相关排放限值要求。此外,组合工艺UV-Fenton氧化单元对COD的去除贡献率约为90%,EM高效生物脱氮单元对COD的去除贡献则较低。以上结果表明,UV-Fenton单元对浓缩液中难降解有机污染物的去除发挥重要作用,可为后续EM菌高效生物脱氮单元提供良好的进水条件。对于UV-Fenton催化氧化体系,小于300 nm的UV光照条件下产生的光电子可以与Fenton氧化剂H2O2反应生成·OH,自由基的产生效率明显高于传统的Fenton体系。尤其是在pH<5的体系中,Fe3+水解生成的Fe(OH)2+可在UV光照条件下分解产生Fe2+和·OH,从而强化Haber-Wesis循环和·OH产量。此外,光解反应也能使三价铁羧酸盐Fe3+(RHCO2)去羧基化生成Fe2+,Fe2+的增加在一定程度上能够促进H2O2的催化分解,进而加强对有机物的降解。因此,使用UV-Fenton体系处理浓缩液中的难降解有机物时,即使在进水COD波动的情况下,仍可表现出更高的去除效率和稳定性。
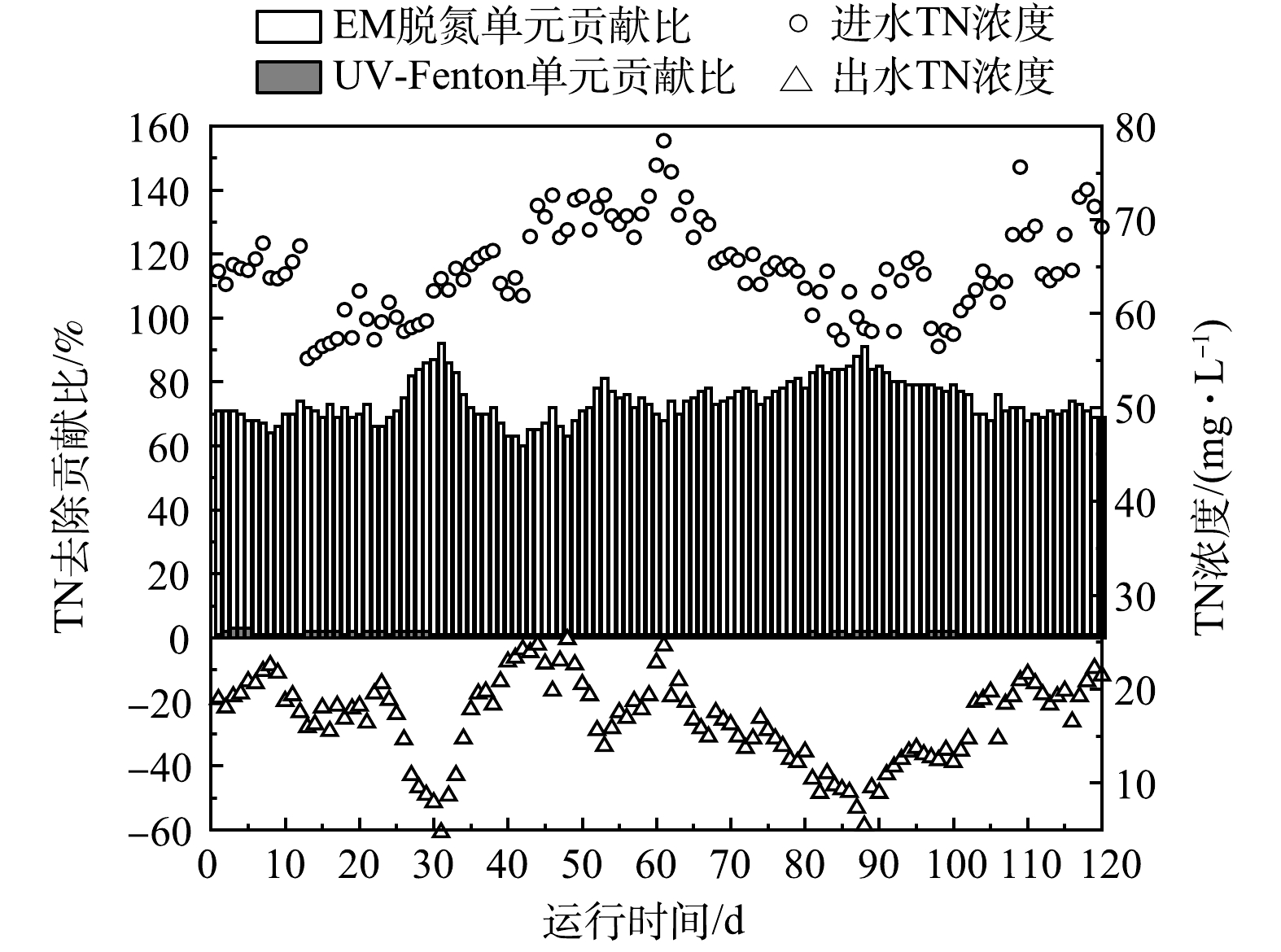
2)对TN的去除。图4反映了组合工艺对浓缩液中TN的去除效果。在120 d的中试周期内,浓缩液中的TN质量浓度(56~80 mg·L−1)存在较显著的不规则波动,这可能与上游渗滤液处理站工艺条件变化有关。由图4可见,UV-Fenton催化氧化单元对浓缩液中的TN几乎没有削减作用。这可能是因为UV-Fenton技术可有效转化硝态氮和亚硝态氮为氨氮,但不会改变浓缩液中的TN含量。另外,由图4可知,EM菌高效脱氮工艺对浓缩液中TN的去除发挥主要作用,去除贡献率约为80%~90%。硝化是整个生物脱氮过程的限速步骤,因而提高硝化速度是提高整个生物脱氮速度的关键。而本文中使用的EM有效微生物菌群可以显著提高缺氧阶段的硝化速度。此外,相对于传统的生物脱氮工艺,经EM菌群强化后,脱氮反应器中的硝化菌尤其是亚硝化菌对于浓缩液重金属离子、酚、硫脲及其衍生物、游离氨等的抗性显著增强,从而增强生化脱氮工艺的稳定性。总体来说,浓缩液在经过组合工艺处理后,出水TN含量在10~25 mg·L−1,完全满足《生活垃圾填埋场污染控制标准》(GB 16889-2008)TN低于40 mg·L−1的排放要求。
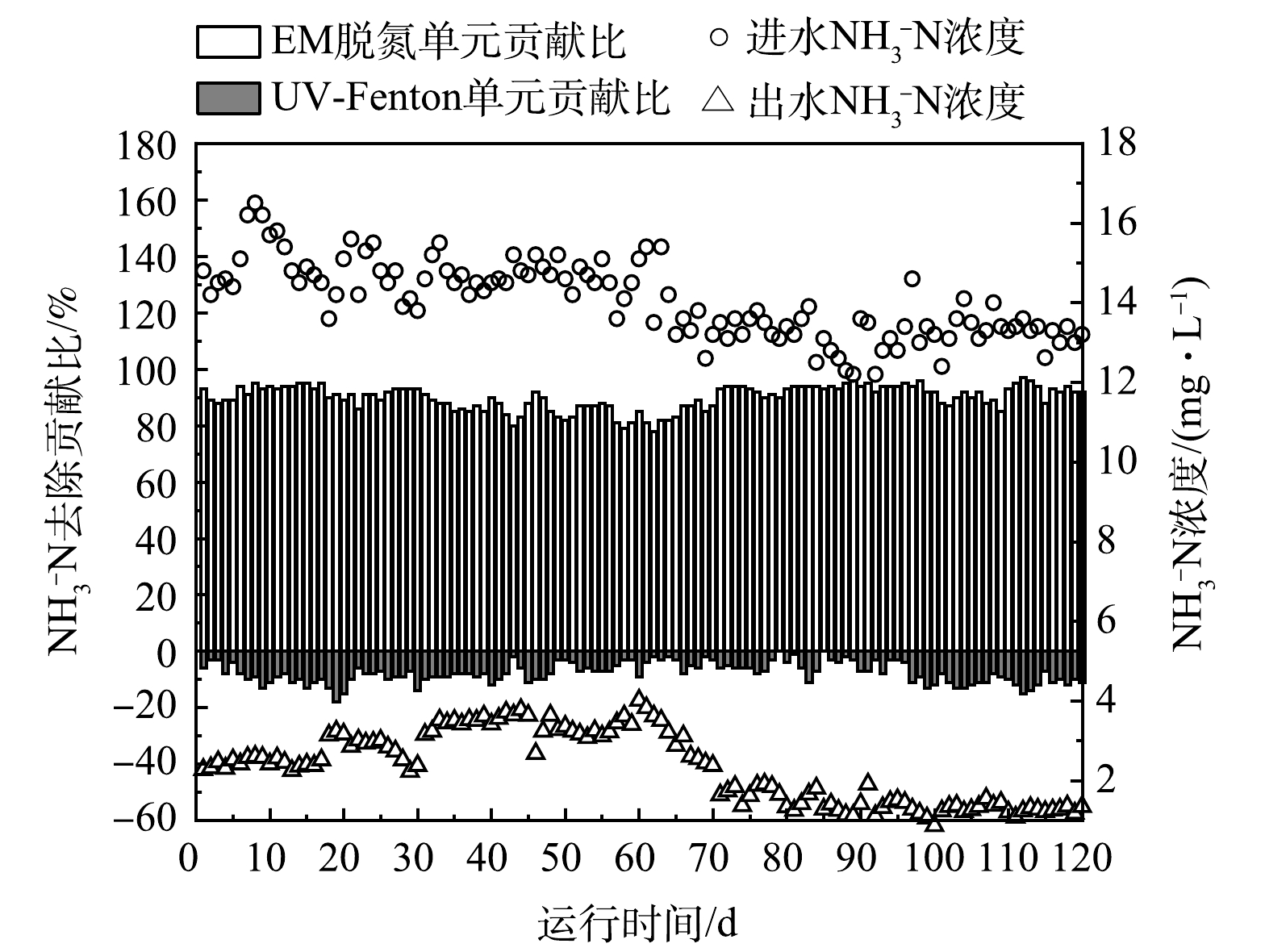
3) 对NH3-N的去除。本文在考察组合工艺对TN脱除效果的同时也考察了NH3-N的脱除效果。如图5所示,在120 d的中试周期内,浓缩液进水的NH3-N(12~16 mg·L−1)同样在一定范围内波动。值得注意的是,UV-Fenton催化氧化单元对NH3-N表现出10%~20%的负去除率,这可能是因为UV-Fenton反应产生的大量羟基自由基将硝态氮和亚硝态氮转化为NH3-N所致。相反地,EM菌高效生物脱氮单元对浓缩液的NH3-N展现优异的去除效果,去除率可高达80%~95%,证实了EM菌在生物脱氮过程中的优势作用。在120 d的中试实验周期内,浓缩液经组合工艺处理后,出水NH3-N为1~4 mg·L−1,完全满足《生活垃圾填埋场污染控制标准》(GB 16889-2008)中NH3-N低于25 mg·L−1的排放要求。
4)对其他污染指标的去除。《生活垃圾填埋场污染控制标准》(GB16889-2008)中除了对COD、TN、NH3-N有要求外,其他指标要求包括总磷、粪大肠菌群菌群数、色度、悬浮物、总汞等。浓缩液经过组合工艺处理后,出水以上指标均可达标。相关工程实践证实,UV-Fenton催化氧化工艺可以将次亚磷质量浓度从10 g·L−1削减到100 mg·L−1以内,去除率达到99%以上,对亚磷的氧化达标是UV-Fenton催化氧化工艺的重要优势之一。工艺出水总磷质量浓度为2.6 mg·L−1,低于《生活垃圾填埋场污染控制标准》(GB 16889-2008)要求的3 mg·L−1。出水粪大肠菌群菌群数低于20个·L−1,远低于《生活垃圾填埋场污染控制标准》(GB 16889-2008)要求的10 000个·L−1,这主要可以归结为紫外光对微生物的高效杀灭作用。工艺出水色度澄清透明,悬浮物SS质量浓度为10 mg·L−1,可达到排放标准关于色度和SS指标的要求。由于组合工艺前端的UV-Fenton单元可以将浓缩液中的重金属螯合物彻底分解,金属汞被释放成离子态后最终以沉淀物的形式去除,使得出水总汞质量浓度低于0.000 1 mg·L−1,显著低于排放限值。浓缩液原液可生化性较差,组合工艺的UV-Fenton单元将浓缩液中的大分子难降解有机物降解为易降解的小分子有机物,甚至完全矿化为CO2和H2O,可以显著提高浓缩液的可生化性。即使经过了EM菌高效生物脱氮单元,出水的B/C仍然接近0.3,满足排放标准。
-
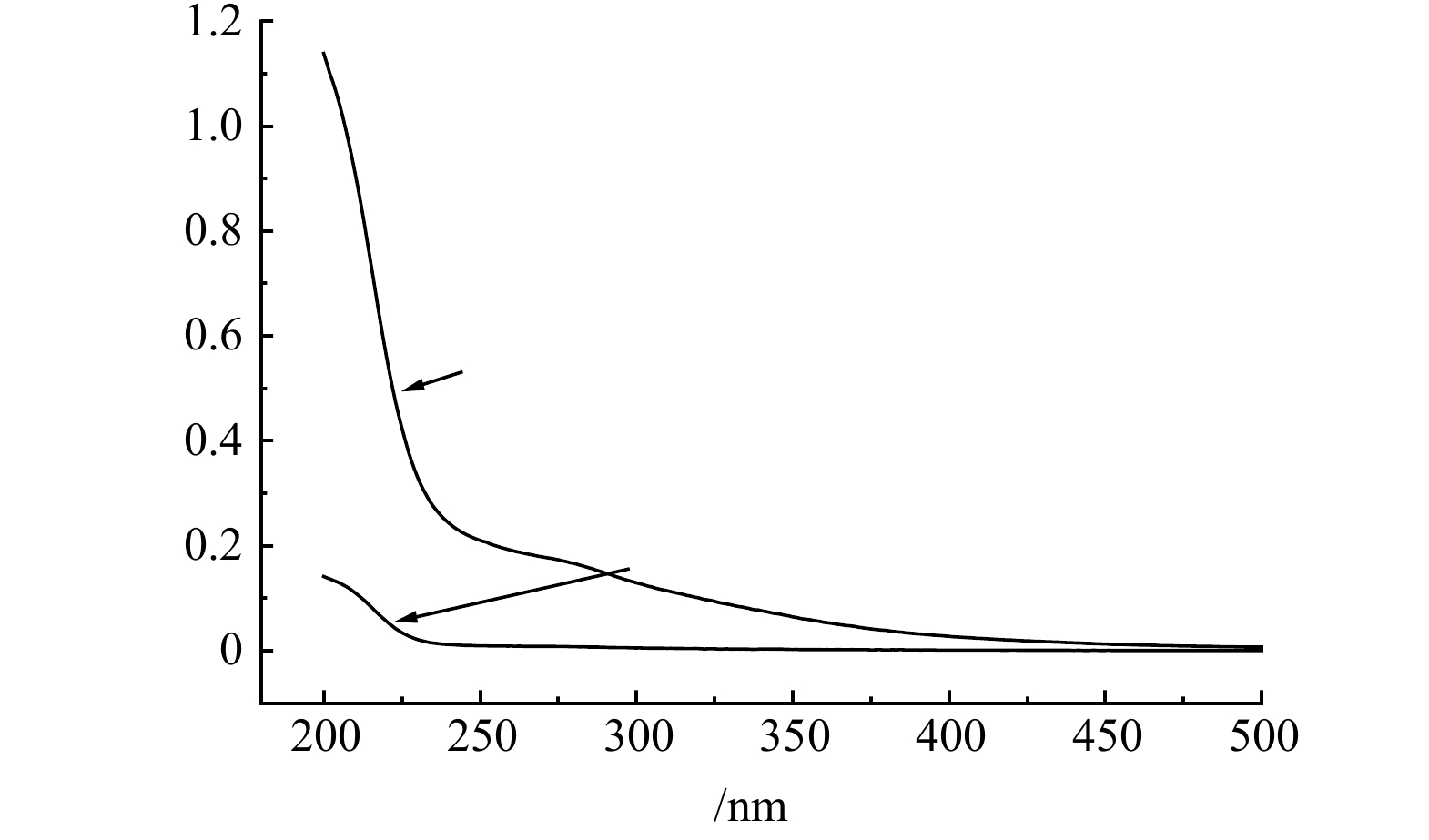
1)紫外-可见(UV-Vis)光谱分析。UV-Vis光谱吸光度与浓缩液中溶解性有机物(DOM)的种类、浓度、芳香性和复杂程度有关[33],通过UV-Vis光谱检测结果可以推测DOM的官能团以及取代基的位置、种类和数目等[34]。由图6可知,浓缩液原液的UV-Vis光谱在紫外光区吸收较强,且明显高于可见光区吸收强度,说明浓缩液中含有较多的芳香性及含共轭键的有机物[35]。在UV-vis光谱中没有出现明显的特征吸收峰,可能是因为浓缩液中有机物结构复杂,多种官能团相互干扰,导致大量的发色基团的吸收峰发生重叠[33]。对于浓缩液原液的UV-vis光谱来说,210~250 nm处的强吸收说明浓缩液中的DOM存在不饱和共轭双键(共轭二烯烃、α、β不饱和醛、不饱和酮)[36-37];250~290 nm处的中等强度吸收表明共轭体系中可能存在芳香杂环化合物(吡咯、吲哚和噻唑)[38],其中260 nm处的弱吸收可以归结为有机羧酸、酚类及多环烃类物质的π-π*电子跃迁[39];290~350 nm的吸收峰可能表明DOM含有羰基等共轭官能团[40]。经过组合工艺处理后,水样UV-vis光谱的吸光度在整个紫外光区(200~400 nm)显著下降,可能是因为DOM中的发色基团(-C=C-C=C-、C=O、苯环)、助色团(如-Cl、-OH、-OR)被消除,导致共轭效应或超共轭效应减弱[41]。在强氧化自由基的诱导作用下,UV-Fenton体系中发生剧烈的环加成反应使得共轭键有机物的芳香性受到了较大程度的破坏,分子量聚合度大幅降低,腐殖质之类的大分子有机物被降解形成小分子物质。综上,虽然浓缩液经过组合工艺处理后依然存在少量的可溶解性有机物,但是其芳香性和复杂化程度明显降低。
此外,还可利用UV-vis光谱中特定波长的吸光度以及不同吸光强度的比值表示不同类有机物的结构特性[42-46]。由表1可知,浓缩液经组合工艺处理后,UV254和E280显著减小。这意味着浓缩液中的腐殖质类大分子有机物得以降解,芳香性也明显降低。经组合工艺处理后,浓缩液的E240/E420由12.22增至22.60,E300/E400由4.75增至6.86,说明处理后大量腐殖质向非腐殖质转化,分子质量聚合度有所降低。E253/E203由0.19降至0.06,表明组合工艺处理后着浓缩液DOM的羰基、羧基、羟基、酯类、胺基等取代基含量明显降低,脂肪链取代基增多,这与有机物芳香性的降低相一致。通过对比发现,出水的E250/E365高于浓缩液原液,表明DOM的主要成分由高相对分子质量物质转变为低相对分子质量,自生源有机物浓度升高。
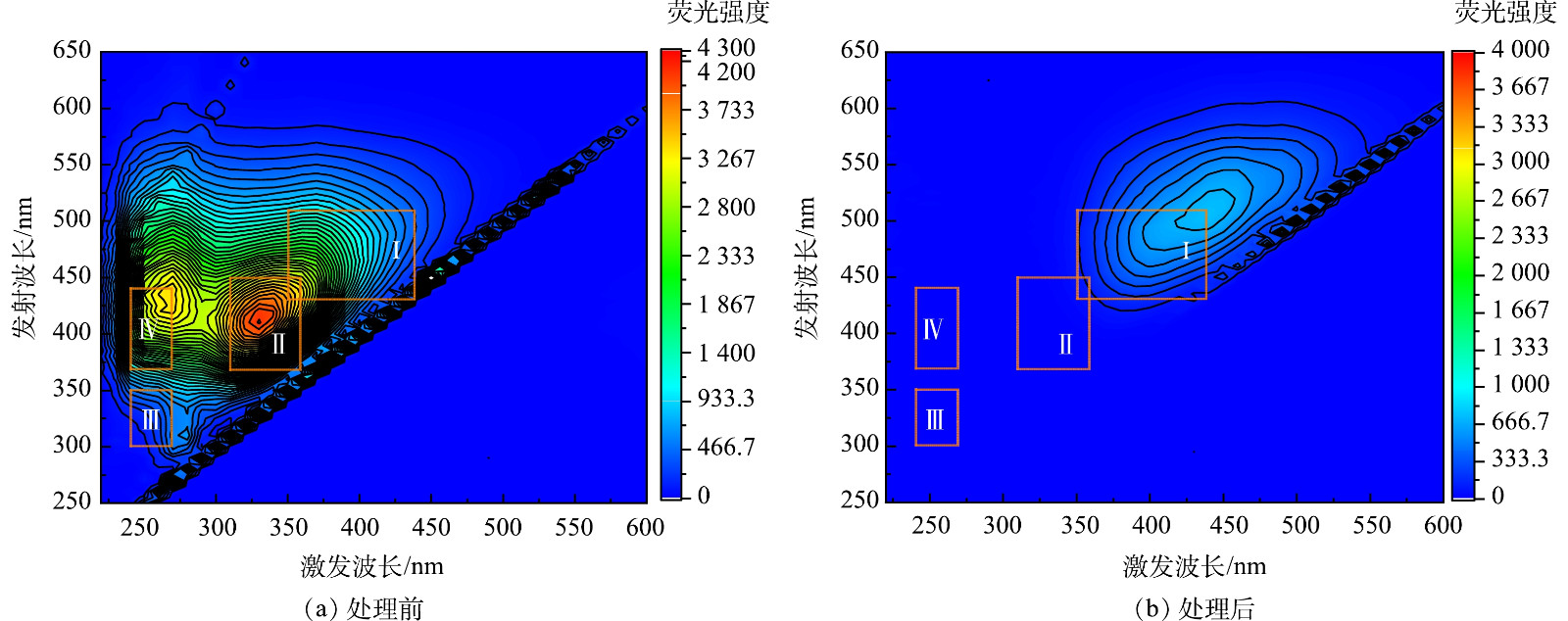
2)三维激发-发射光谱(3D-EEMs)分析。浓缩液中的DOM种类繁多,主要由腐殖酸、富里酸、多环芳烃类等含共轭双键芳香烃或含双键、羧基等基团的物质组成,故可通过3D-EEMs光谱中特征峰位置和荧光强度研究浓缩液中的DOM组成及降解转化规律[47-49],本研究的相关结果见图7。水中对应的DOM激发/发射(λex/λem)荧光峰的位置大致分为4个区[50-52]:I为类腐殖酸类物质(λex/λem为(350~440)nm/(430~510)nm);II为可见区类富里酸类物质(λex/λem为(310~360)nm/(370~450)nm);III为类蛋白类物质(λex/λem为(240~270)nm/(300~350)nm);IV为紫外区类富里酸类物质(λex/λem为(240~270)nm/(370~440)nm)。浓缩液原液中含有较高浓度的类富里酸、类蛋白物质以及类腐殖质,尤其以分子质量大、相对稳定的可见光区的类富里酸含量居多,进一步证实浓缩液中存在大量的难降解有机物。浓缩液原液的腐殖化指数HIX高达5.63,表示该水样腐殖化程度较高[53];生物源指数BIX为0.21,表示DOM的自源性(即生物可利用度)较低,反映出浓缩液的可生化性低[54];荧光指数FI(f450/500)为0.87,意味着浓缩液中的有机质为陆源和生物源输入[55]。因此,渗滤液膜浓缩液呈现出典型的难降解废水的特征。经过处理后,原有4类物质的荧光特征峰都显著减弱,表明浓缩液中有机物分子缩合程度大幅降低,腐殖质与芳香类物质等难降解大分子有机物转变为没有荧光特性的小分子物质,这充分证实了组合工艺尤其是UV-Fenton单元处理难降解大分子的有效性。此外,处理后出水荧光光谱的可见区富里酸荧光峰峰位发生了显著蓝移,可能与羰基、羧基、羟基和胺基等不饱和取代基的减少有关,这与UV-vis光谱中E253/E203的变化趋势一致。
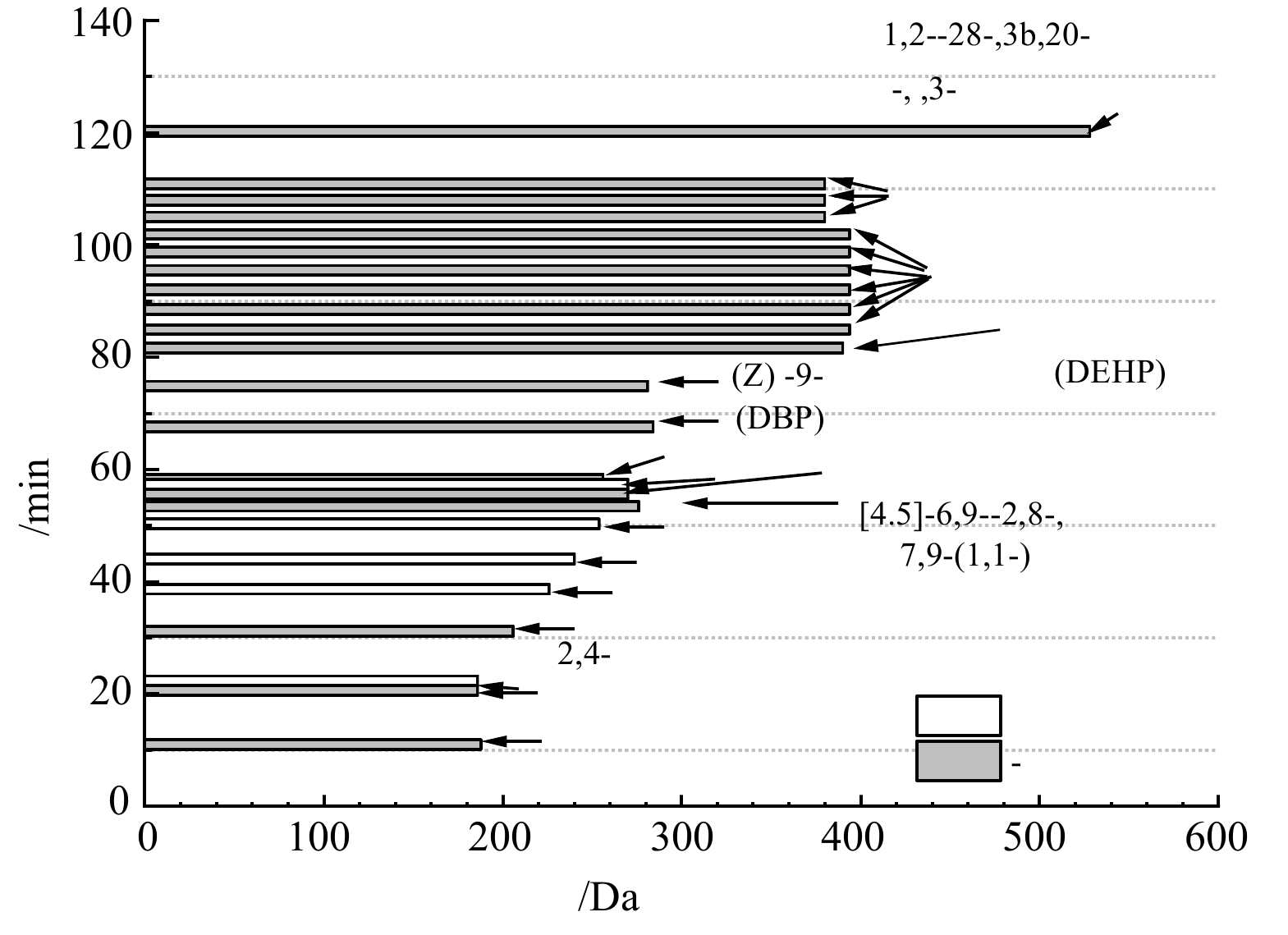
3) GC-MS分析。为分析组合工艺处理膜浓缩液前后水质中所含难降解有机物成分变化,利用GC-MS对处理前后水样进行了对比分析。由于缺乏标准参考物质,GC-MS不能有效分析出每一种有机物底物,因此,本文主要针对相对含量较高以及匹配度高于60%的物质进行定性分析。由图8可知,膜浓缩液原水中总共含有19种有机污染物,且其中大部分为大分子有机物,其中所含的邻苯二甲酸被美国环境保护部列为优先控制污染物。经过组合工艺处理后的出水仅含有5种有机物,其中全部为小分子有机物,这主要归因于组合工艺中UV-Fenton氧化单元对膜浓缩液中难降解有机物的深度处理。
邻苯二甲酸酯(PAEs)是生态环境中普遍存在的一种环境激素类污染物,该类物质水溶性低、辛醇-水分配系数高,在垃圾填埋场厌氧条件下难以完全降解,导致渗滤液可生化性降低。如图8所示,下坪填埋场浓缩液中含有邻苯二甲酸二丁酯(DBP,360 µg·L−1)和邻苯二甲酸二辛酯(DEHP,457 µg·L−1)。DBP和DEHP是PAEs类物质中最不可生化降解的2类物质,在自然界的半衰期可达数十年。本研究在上述中试条件下考察了组合工艺对垃圾渗滤液膜浓缩液中上述2种典型环境激素类物质的降解和矿化效果。由于环境激素类物质在生化单元无法被降解,其消除主要依赖前端的UV-Fenton技术。由图9可知,经过60 min的 UV-Fenton催化氧化处理后,浓缩液中DBP和DEHP的质量浓度分别为5.3 µg·L−1和25.8 µg·L−1。反应120 min时DBP和DEHP均无法被检测出,一方面是因为目标污染物浓度低于GC-MS的检测限;另一方面可能因为浓缩液中的DBP和DEHP被氧化开环后生成其他中间产物,甚至被完全降解或矿化。
-
1) V-vis光谱、3D-EEMs光谱及GC-MS结果证实,浓缩液中含有多种难降解的大分子有机污染物,腐殖化程度高,可生化性差。UV-Fenton催化氧化单元作为生化系统的前处理单元,主要用来降解渗滤液浓缩液中的大分子有机物,提高可生化性。UV光与Fenton体系(Fe3+/H2O2)的协同作用可以显著提升·OH的产生效率和浓度,在强氧化自由基的诱导作用下,体系中发生剧烈的环加成反应使得浓缩液中的共轭有机物的芳香性结构被破坏,腐殖质类大分子有机物被降解成小分子物质,有机物的分子量聚合度也大幅降低。
2) EM菌强化的AO+MBR工艺则可以实现浓缩液中氨氮及总氮的高效脱除。经UV-Fenton工艺处理后,EM菌生物脱氮单元则可以有效去除浓缩液中80%~90%的TN以及NH3-N,脱氮效果良好。
3)在整个中试期间,组合工艺高效稳定运行,出水的COD、TN、NH3-N等指标都可以完全达到《生活垃圾填埋场污染控制标准》(GB 16889-2008)的排放要求,从而在中试尺度下验证了UV-Fenton催化氧化+EM菌高效生物脱氮组合工艺处理浓缩液的可行性。
紫外光芬顿+EM菌高效脱氮组合工艺在垃圾渗滤液膜浓缩液处理的中试应用
Pilot-scale application of the combined process of UV-Fenton with EM micro-flora based biochemical denitrification in the treatment of landfill leachate membrane concentrate
-
摘要: 针对垃圾渗滤液膜浓缩液中有机物成分复杂、难以降解的特点,采用UV-Fenton催化氧化+EM菌(有效微生物菌群)高效生物脱氮组合工艺开展连续120 d的中试实验,考察了组合工艺中各处理单元对浓缩液中COD、TN和NH3-N的去除效果。结果表明,组合工艺运行稳定,对COD、TN和NH3-N的去除率分别达到95.2%、90%和95%,出水各项指标均能满足《生活垃圾填埋场污染控制标准》(GB 16889-2008)排放要求。结合紫外可见光谱、三维激发-发射光谱和气相色谱-质谱实验结果,UV-Fenton催化氧化可使浓缩液中含有共轭键有机物的芳香结构在较大程度上被破坏,分子质量聚合度大幅降低,从而将腐殖质以及可见光区富里酸等难降解的大分子有机物降解为小分子,从而提高可生化性。后续的EM菌高效生物脱氮单元基于硝化反硝化,可以进一步高效去除NH3-N和TN。Abstract: With regards to the complex composition and difficult degradation of organic compounds in landfill leachate membrane concentrate, a pilot-scale test was carried out continuously in 120 days by using the combined process of UV-Fenton catalytic oxidation with highly efficient microorganism (EM) based biological denitrification. The removal efficiency of COD, TN and NH3-N of leachate membrane concentrate by each treatment unit of the combined process were investigated. The results illustrated that the combined process performed stably, and the corresponding removal rate of COD, TN and NH3-N reached 95.2%, 90% and 95%, respectively. Meanwhile, each effluent indicator could meet the discharge requirements in Domestic Waste Pollution Control Standard (GB 16889-2008). On the basis of UV-vis spectra, three-dimensional fluorescence spectra and GC-MS analysis, the UV-Fenton unit could greatly damage the aromatic structure of the conjugated organic compounds in the leachate concentrate, and significantly reduced polymerization degree of the molecular weight, thus degraded the refractory macromolecules such as humus-like substance or fulvic acids in visible light region into small molecules and improved the biodegradability of leachate concentrate. The subsequent EM based biological denitrification unit could further and efficiently remove NH3-N and TN.
-

-
表 1 浓缩液处理前后的特征吸光度及比值变化
Table 1. Comparison of the values and ratios of the specific absorbances of leachate concentrate before and after treatment
水样 E254 E280 E240/E420 E253/E203 E250/E365 E300/E400 原液 0.20 0.17 12.22 0.19 4.25 4.75 出水 0.01 0.01 22.60 0.06 6.92 6.86 -
[1] MIAO L, Yang G, TAO T, PENG Y. Recent advances in nitrogen removal from landfill leachate using biological treatments: A review[J]. Environmental Management, 2019, 235: 178-185. [2] TENG C Y, ZHOU K G, PENG C H, et al. Characterization and treatment of landfill leachate: A review[J]. Water Research, 2021, 203: 117525. doi: 10.1016/j.watres.2021.117525 [3] 陈媛媛, 孙琳, 陈建飞. 反渗透膜在垃圾渗滤液处理中的应用[J]. 工程技术研究, 2021, 6(11): 126-127. doi: 10.3969/j.issn.1671-3818.2021.11.059 [4] 李丛林. 论膜技术在垃圾渗滤液处理中的应用[J]. 资源节约与环保, 2020, 11: 115-116. doi: 10.3969/j.issn.1673-2251.2020.05.099 [5] SHAO L M, DENG Y T, QIU J J, et al. DOM chemodiversity pierced performance of each tandem unit along a full-scale “MBR+NF” process for mature landfill leachate treatment[J]. Water Research, 2021, 195(17): 117000. [6] AMARAL M C S, MORAVIA W G, LANGE L C, et al. Nanofiltration as post-treatment of MBR treating landfill leachate[J]. Desalination & Water Treatment Science & Engineering, 2015, 53: 1482-1491. [7] LI H K and LIU H. Treatment and recovery methods for leachate concentrate from landfill and incineration: A state-of-the-art review[J]. Journal of Cleaner Production, 2021, 329: 129720. doi: 10.1016/j.jclepro.2021.129720 [8] KEYIKOGLUeyikoglu R, KARATAS O, REZANIA H, et al. A review on treatment of membrane concentrates generated from landfill leachate treatment processes[J]. Separation and Purification Technology, 2021, 259: 118182. doi: 10.1016/j.seppur.2020.118182 [9] CHEN L, LI F Q, HE F D, et al. Membrane distillation combined with electrocoagulation and electrooxidation for the treatment of landfill leachate concentrate[J]. Separation and Purification Technology, 2022, 291: 120936. doi: 10.1016/j.seppur.2022.120936 [10] LIU D, YUAN Y, WEI Y Q, et al. Removal of refractory organics and heavy metals in landfill leachate concentrate by peroxi-coagulation process[J]. Journal of Environmental Sciences, 2022, 116: 43-51. doi: 10.1016/j.jes.2021.07.006 [11] ZHANG H, JI Z H, ZENG Y X, et al. Solidification/stabilization of landfill leachate concentrate contaminants using solid alkali-activated geopolymers with a high liquid solid ratio and fixing rate[J]. Chemosphere, 2022, 288(2): 132495. [12] GUVENC S Y. Optimization of COD removal from leachate nanofiltration concentrate using H2O2/Fe2+/heat- activated persulfate oxidation processes[J]. Process Safety and Environmental Protection, 2019, 126: 7-17. doi: 10.1016/j.psep.2019.03.034 [13] BENYOUCEF F, MAKAN A, El GHMARI A, et al. Optimized evaporation technique for leachate treatment: small scale implementation[J]. Journal of Environmental Management, 2016, 170: 131-135. [14] TALALAJ I A. Mineral and organic compounds in leachate from landfill with concentrate recirculation[J]. Environmental Science & Pollution Research, 2015, 22: 2622-2633. [15] 王子龙. 垃圾渗沥液纳滤浓缩液处理工艺路线探讨[J]. 福建建设科技, 2020, 4: 58-60. doi: 10.3969/j.issn.1006-3943.2020.04.017 [16] 邱中平, 李明星, 刘洋, 等. 好氧生物反应器填埋场的渗滤液回灌量研究[J]. 西南交通大学学报, 2019, 54(1): 168-172. [17] TALALAJ I A, BIEDKA P. Impact of concentrated leachate recirculation on effectiveness of leachate treatment by reverse osmosis[J]. Ecological Engineering, 2015, 85: 185-192. doi: 10.1016/j.ecoleng.2015.10.002 [18] 吴子涵, 任旭, 肖玉, 等. 上海市某垃圾焚烧厂渗滤液膜浓缩液回喷焚烧后的固相物质转化特性[J]. 环境工程学报, 2019, 13(8): 1949-1958. doi: 10.12030/j.cjee.201812128 [19] REHMAN F, SAYED M, KHAN J A, et al. Oxidative removal of brilliant green by UV/S2O82-, UV/HSO5- and UV/H2O2 processes in aqueous media: A comparative study[J]. Journal of Hazardous Materials, 2018, 357: 506-514. doi: 10.1016/j.jhazmat.2018.06.012 [20] LIN K Y A, ZHANG Z Y. Degradation of Bisphenol A using peroxymonosulfate activated by one-step prepared sulfur-doped carbon nitride as a metal-free heterogeneous catalyst[J]. Chemical Engineering Journal. 2017, 313: 1320-1327. [21] AO X, LIU W, SUN W, et al. Medium pressure UV-activated peroxymonosulfate for ciprofloxacin degradation: Kinetics, mechanism, and genotoxicity[J]. Chemical Engineering Journal, 2018, 345: 87-97. doi: 10.1016/j.cej.2018.03.133 [22] WALDEMER R H, TRATNYEK P G, JOHNSON R L, NURMI J T. Oxidation of chlorinated ethenes by heat-activated persulfate: kinetics and products[J]. Environmental Science & Technology, 2007, 41: 1010-1015. [23] DE L A, HE X, DIONYSION D D, et al. Effects of bromide on the degradation of organic contaminants with UV and Fe2+ activated persulfate[J]. Chemical Engineering Journal, 2017, 318: 206-213. doi: 10.1016/j.cej.2016.06.066 [24] SU S, CAO C, ZHAO Y, DIONYSION D D. Efficient transformation and elimination of roxarsone and its metabolites by a new a-FeOOH@GCA activating persulfate system under UV irradiation with subsequent As(V) recovery[J]. Applied Catalysis B:Environmental, 2019, 245: 207-219. doi: 10.1016/j.apcatb.2018.12.050 [25] SONGLIN W, NING Z, SI W, QI Z, ZHI Y. Modeling the oxidation kinetics of sono-activated persulfate’s process on the degradation of humic acid[J]. Ultrasonics Sonochemistry, 2015, 23: 128-134. doi: 10.1016/j.ultsonch.2014.10.026 [26] ARELLANO M, SANROMAN M A, PAZOS M. Electro-assisted activation of peroxymonosulfate by iron-based minerals for the degradation of 1-butyl-1-methylpyrrolidinium chloride[J]. Separation & Purification Technology, 2019, 208: 34-41. [27] DU X, ZHANG K, XIE B, ZHAO J, et al. Peroxymonosulfate-assisted electro-oxidation/ coagulation coupled with ceramic membrane for manganese and phosphorus removal in surface water[J]. Chemical Engineering Journal, 2019, 365: 334-343. doi: 10.1016/j.cej.2019.02.028 [28] LEE M, MERLE T, RENTSCH D, et al. Abatement of polychoro-1, 3-butadienes in aqueous solution by ozone, UV-photolysis, and advanced oxidation processes (O3/H2O2 and UV/H2O2)[J]. Environmental Science & Technology, 2017, 51(1): 497-505. [29] CHEN L, CAI T, CHENG C, et al. Degradation of acetamiprid in UV/H2O2 and UV/persulfate systems: A comparative study[J]. Chemical Engineering Journal, 2018, 351: 1137-1146. doi: 10.1016/j.cej.2018.06.107 [30] MOREIRAF C, BOAVENTURA R A R, BRILLASRILLAS E, et al. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters[J]. Applied Catalysis B:Environment, 2017, 202: 217-261. doi: 10.1016/j.apcatb.2016.08.037 [31] ZHANG M, DONG H, ZHAO L, et al. A review on Fenton process for organic wastewater treatment based on optimization perspective[J]. Science of the Total Environment, 2019, 670: 110-121. doi: 10.1016/j.scitotenv.2019.03.180 [32] 魏复盛. 水和废水监测分析方法(第4版)[M]. 北京: 中国环境科学出版社, 2002. [33] BRIDGEMAN J, BIEROZA M, BAKERAKER A. The application of fluorescence spectroscopy to organic matter characterisation in drinking water treatment[J]. Reviews in Environmental Science & Biotechnology, 2011, 10: 277. [34] KAVURMACI S S, BEKBOLET M. Specific UV-vis absorbance changes of humic acid in the presence of clay particles during photocatalytic oxidation[J]. Desalination & Water Treatment, 2014, 52: 1903-1910. [35] KE W, LI W, GONG X J, LI Y, et al. Spectral study of dissolved organic matter in biosolid during the composting process using inorganic bulking agent: UV-vis, GPC, FTIR and EEM[J]. International Biodeterioration & Biodegradation, 2013, 85: 617-623. [36] WIN M S, TIAN Z, ZHAO H, XIAO K, et al. Atmospheric HULIS and its ability to mediate the reactive oxygen species (ROS): A review[J]. Journal of Environmental Science, 2018, 71: 13-31. doi: 10.1016/j.jes.2017.12.004 [37] SOOMRO G S, QU C, REN N, et al. Efficient removal of refractory organics in landfill leachate concentrates by electrocoagulation in tandem with simultaneous electro-oxidation and in-situ peroxone[J]. Environmental Research, 2020, 183: 109249. doi: 10.1016/j.envres.2020.109249 [38] LIU X, NOVAK J T, HE Z. Removal of landfill leachate ultraviolet quenching substances by electricity induced humic acid precipitation and electrooxidation in a membrane electrochemical reactor[J]. Science of Total Environment, 2019, 689: 571-579. doi: 10.1016/j.scitotenv.2019.06.329 [39] HE P J, XUE J F, SHAO L M, et al. Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill[J], Water Research, 2006, 40(7): 1465-1473. [40] LONG Y Y, XU J, SHEN D S, et al. Effective removal of contaminants in landfill leachate membrane concentrates by coagulation[J]. Chemosphere, 2017, 167: 512-519. doi: 10.1016/j.chemosphere.2016.10.016 [41] SONIA R G B, JORGE N, WAGNER J B. Origin of dissolved organic carbon studied by UV-vis spectroscopy[J]. Acta Hydrochim. Hydrobiol, 2003, 31(6): 513-518. doi: 10.1002/aheh.200300510 [42] 林星杰, 杨慧芬, 宋存义. UV254在水质监测中应用的研究[J]. 能源与环境, 2006(10): 22-24. [43] 刘国强. 垃圾渗滤液中DOM 特性分析及去除性能研究[D]. 重庆: 重庆大学, 2007. [44] MARTA F, GUSTAVO G, JOSE M. The usefulness of UV-visible and fluorescence spectroscopies to study the chemical nature of humic substances from soils and composts[J]. Organic Geochemistry, 2006, 27: 1949-1959. [45] KANG K H, SHIN H S, PARK H. Characterization of humic substances present in landfill leachates with different landfill ages and its implications[J]. Water Research, 2002, 36(16): 4023-4032. doi: 10.1016/S0043-1354(02)00114-8 [46] HE P J, XUE J F, SHAO L M, et al. Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill[J]. Water Research, 2006, 40(7): 1465-1473. doi: 10.1016/j.watres.2006.01.048 [47] 何小松, 席北斗, 魏自民, 等. 堆放垃圾渗滤液水溶性有机物的荧光特性[J]. 中国环境科学, 2010, 30(6): 752-757. [48] ZHANG Q Q, TIAN B H, ZHANG X, et al. Investigation on characteristics of leachate and concentrated leachate in three landfill leachate treatment plants[J]. Waste Management, 2013, 33(11): 2277-2286. doi: 10.1016/j.wasman.2013.07.021 [49] HUO S, BEIDOU X I, HAICHAN Y U, et al. Characteristics of dissolved organic matter (DOM) in leachate with different landfill ages[J]. Journal of Environmental Sciences, 2008, 20(4): 492-498. doi: 10.1016/S1001-0742(08)62085-9 [50] CHEN W M, HE C, ZHUO X C, et al. Comprehensive evaluation of dissolved organic matter molecular transformation in municipal solid waste incineration leachate[J]. Chemical Engineering Journal, 2020, 400: 126003-126010. doi: 10.1016/j.cej.2020.126003 [51] CHEN W M, WANG F, HE C, et al. Molecular-level comparison study on microwave irradiation-activated persulfate and hydrogen peroxide processes for the treatment of refractory organics in mature landfill leachate[J]. Journal of Hazardous Materials, 2020, 397: 122785. doi: 10.1016/j.jhazmat.2020.122785 [52] WANG F, GU Z P, HU Y S, et al. Split dosing of H2O2 for enhancing recalcitrant organics removal from landfill leachate in the Fe0/H2O2 process: Degradation efficiency and mechanism[J]. Separation and Purification Technology, 2021, 278: 119564. doi: 10.1016/j.seppur.2021.119564 [53] OHNO T, CHOROVER J, OMOIKE A, et al. Molecular weight and humification index as predictors of adsorption for plant and manure derived dissolved organic matter to Goethite[J]. European Journal of Soil Science, 2007, 58(1): 125-132. doi: 10.1111/j.1365-2389.2006.00817.x [54] OHNO T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter[J]. Environmental Science & Technology, 2002, 36(4): 742-746. [55] JOHNSON M S, COUTO E G, ABDO M, et al. Fluorescence index as an indicator of dissolved organic carbon quality in hydrologic flowpaths of forested tropical watersheds[J]. Biogeochemistry, 2011, 105(1/2/3): 149-157. -




 下载:
下载: