-
镉(Cd)具有致畸和致癌作用,是对环境和人体危害最大的重金属之一[1]。环境中Cd主要来源于开采金属、电镀、农药、化肥及防腐剂等行业的排放[2]。国内外研究表明,Cd具有器官毒性,会通过空气、水、食物进入人体,长期接触可能导致癌症以及骨骼、神经、泌尿、生殖系统病变[3]。2022年3月,生态环境部发布了《关于进一步加强重金属污染防控的意见》,对包括Cd在内的5种重金属污染物排放量实施总量控制,进一步提升绿色发展。目前,处理Cd(Ⅱ)的方法有吸附法、电渗析法[4]、微生物法[5]、沉淀过滤[6]等多种,因吸附法具有简单、高效、经济等优点被广泛应用[7]。
氧化石墨烯(graphene oxide, 简称GO)是一种近年来备受关注的新型吸附材料,其表面分布众多烃基、羟基,并拥有巨大的比表面积和优异的力学性能[8]。传统GO制备方法是将石墨、高锰酸钾和98%的浓硫酸混合氧化2 h,具有一定危险性。使用电解氧化法制备GO与传统方法制备出的GO性能相似,并且减少了强氧化剂的使用,缩短了制备时间[9]。但以上2种方法制备出的GO易团聚,分散在水中较难分离。近年来,国内外学者利用各种材料对GO进行功能化改性,或通过自组装法构建一种基于石墨烯的3D宏观结构材料来解决此类问题[10-11]。GO复合材料不仅能够去除有毒重金属,如Cr(Ⅵ)[12]、Hg(Ⅱ)[13]、Pb(Ⅱ)[14]、Zn(Ⅱ)[15]、Cu(Ⅱ)[16]和放射性污染物U(Ⅵ)[17]等,还可去除多种有机物和染料,如亚甲基蓝[18]、罗丹明B[19]、刚果红[20]等。MADADRANG等[14]成功将乙二胺四乙酸(EDTA)合成在GO的表面,以此增加材料表面的螯合基团,提高Pb(Ⅱ)的吸附容量。磁性纳米铁具有顺磁性,可以被磁铁吸引,WANG等[21]制备出磁性GO,不仅可以使吸附材料重复使用,并且增强了对Cr(Ⅵ)的吸附性能。绿色吸附材料是近年来研究的热点,廉价材料受到极大的关注[22]。利用淀粉可降低材料的制备成本,BHAT等[23]制备出马铃薯双淀粉磷酸盐用于吸附铅和铜,去除率可达78.1%和58.5%,但这种复合材料存在制备复杂、难分离的缺点。淀粉由直链淀粉和支链淀粉组成,含有许多糖苷基和羟基,并且具有一定的还原性[24]。利用这种还原性将GO组装为3D结构,同时使用磁性纳米铁降低GO的团聚,并提高吸附容量。目前,国内外对于利用淀粉特性制备的淀粉/三维磁性氧化石墨烯(starch/3D magnetic graphene oxide,简称SMGO)鲜有报道。
本研究使用电解氧化法制备GO,并利用改性淀粉的还原性与磁性纳米铁一步水热合成SMGO。通过扫描电镜(scanning electron microscope,SEM)、能谱仪(energy dispersive spectroscopy,EDS)、傅里叶变换红外光谱(Fourier transform infrared spectrometer,FTIR)、X射线光电能谱(X-ray photoelectron spectroscopy,XPS)、磁强计(vibrating sample magnetometer,VSM)等方法对材料进行表征分析;考察了不同因素对Cd(Ⅱ)在SMGO上吸附的影响,采用正交实验探讨了SMGO对Cd(Ⅱ)的最佳吸附条件;采用吸附热力学和动力学研究SMGO的吸附能力和吸附特性,以期为Cd(Ⅱ)污染废水处理的实际应用提供科学依据与技术支撑。
-
本实验所用试剂均为分析纯,其中,石墨纸(0.2 mm)购置廊坊运存保温材料有限公司,浓硫酸(98%)购置广州广试试剂科技有限公司,淀粉、氯化铁(FeCl3)、氯化亚铁(FeCl2)、过氧化氢(H2O2)、氢氧化钠(NaOH)、盐酸(HCl)、柠檬酸钠(C6H5Na3O7)、硝酸镉(Cd(NO3)2)等购置阿拉丁试剂(上海)有限公司。实验过程中使用电阻率为18.2 MΩ的超纯水。
实验所用仪器有:水热反应釜(SYF-280,上海申生科技有限公司)、恒温磁力搅拌器(IKA RCT,德国IKA公司)、超声波清洗机(YM-120ST,深圳市洁盟清洗设备有限公司)、电热鼓风干燥箱(DHG-9920,上海一恒科学仪器有限公司)、pH计(PHSJ-3F,上海仪电科学仪器股份有限公司)、小型移动电源(HY3005ET,东莞华仪仪表科技有限公司)等。
-
首先使用电化学法制备GO。将石墨纸裁成合适尺寸的薄片,浸入浓硫酸中插层15~20 min,之后浸入稀硫酸中电解剥离3~5 min,用抽滤装置回收稀硫酸,剩余部分用超纯水和H2O2洗涤至中性,超声后用冷冻干燥器干燥24 h,得到GO。
以共沉淀法制备的磁性纳米铁为磁源。称取0.63 g FeCl2和1.62 g FeCl3溶于100 mL超纯水,用NaOH溶液调节溶液pH=10,加热至60 ℃,期间不断搅拌,冷却沉淀后,用磁铁分离并洗涤至中性,得到磁性纳米铁。
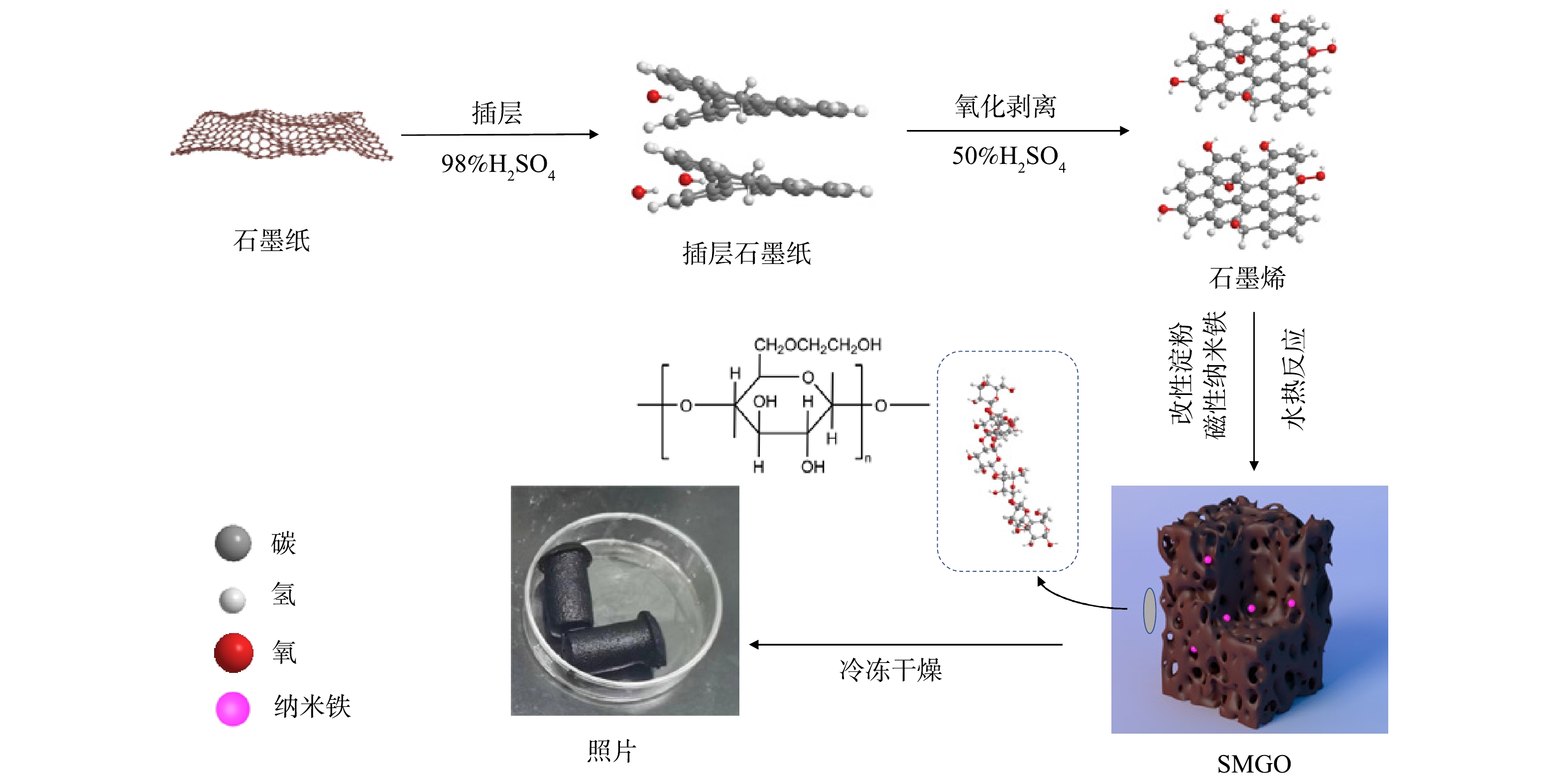
以淀粉为有机连接配体,通过水热法合成SMGO。将淀粉加热至80 ℃进行改性,同时称取1.5 g GO超声分散20 min,加入磁性纳米铁、柠檬酸钠和改性淀粉,放入水热反应釜中在180 ℃下反应8 h,冷冻干燥后得到SMGO。SMGO具体制备见图1。
-
1)溶液pH对SMGO吸附Cd(Ⅱ)的影响。取20 mL溶液pH分别为2、3、4、5、6、7、8、9和10的Cd(Ⅱ)溶液于锥形瓶中,投加20 mg SMGO吸附材料,置于308.15 K的恒温气浴振荡箱中以120 r·min−1的速度振荡1 h,使用磁铁分离后取样,采用电感耦合等离子体发射光谱仪测定Cd(Ⅱ) 质量浓度。
2)吸附时间对SMGO吸附Cd(Ⅱ)的影响。称取10 mL质量浓度为100 mg·L−1的Cd(Ⅱ)溶液于锥形瓶中,投加10 mg SMGO吸附材料,置于308.15 K的恒温气浴振荡箱中以120 r·min−1的速度振荡,分别于10、30、50、70、90、120、180和240 min使用磁铁分离后取样,采用电感耦合等离子体发射光谱仪测定Cd(Ⅱ) 质量浓度。在t时刻的吸附容量根据式(1)进行计算。
式中:qt是
t时刻Cd(Ⅱ)的吸附质量,mg·g−1;C0是Cd(Ⅱ)溶液初始质量浓度,mg·L−1;Ct是Cd(Ⅱ)溶液t时刻的质量浓度,mg·L−1;V是吸附溶液体积,L;m是吸附剂的质量,g。 3)多因素影响下SMGO吸附Cd(Ⅱ)的正交实验。以SMGO为吸附剂吸附水中的Cd(Ⅱ)。基于溶液pH、吸附时间的单因素实验结果,并结合相关文献,确定对去除率有明显影响的4个因子(溶液pH、吸附时间、反应温度和转速)作为实验影响因素,选用L9(34)型正交表进行实验设计,每个因素设置3个水平,共9组处理。实验设计详见表1。9组实验中的不同质量浓度Cd(Ⅱ)溶液均用Cd(NO3)2和超纯水配置,不同pH溶液使用0.36 mol·L−1的HCl溶液和NaOH溶液配置,反应温度和转速使用恒温气浴箱进行调节。使用磁铁分离后取样,采用电感耦合等离子体发射光谱仪测定Cd(Ⅱ) 质量浓度。实验过程中的吸附实验结果同样列于表1中。
4)吸附热力学。取20 mL质量浓度分别为10、50、100、125、150、200和300 mg·L−1的Cd(Ⅱ)溶液于锥形瓶中,投加20 mg SMGO吸附材料,置于308.15 K的恒温气浴振荡箱中以120 r·min−1的速度振荡1 h,使用磁铁分离后取样,采用电感耦合等离子体发射光谱仪测定Cd(Ⅱ) 质量浓度。吸附等温线模型采用Langmuir模型(式(2))[25]和Freundlich模型(式(4))[26] 进行拟合,并分别转换为Langmuir线性表达式(式(3))和Freundlich线性表达式(式(5))。
式中:C0是初始时刻Cd(Ⅱ)的质量浓度,mg·L−1;Ce是平衡时刻Cd(Ⅱ)的质量浓度,mg·L−1;qe是平衡吸附容量,mg·g−1;qm是最大吸附容量,mg·g−1;KL是Langmuir常数,L·mg−1;KF和n是Freundlich常数。
5)吸附动力学。称取10 mL质量浓度为100 mg·L−1的Cd(Ⅱ)溶液于锥形瓶中,投加10 mg吸附材料,根据吸附时间进行采样,通过磁铁分离后采用电感耦合等离子体发射光谱仪测定剩余溶液Cd(Ⅱ) 质量浓度。吸附动力学模型采用准一级动力学方程(式(6))[27]和准二级动力学方程(式(8))[28],并分别转换为准一级动力学线性方程(式(7))和准二级动力学线性方程(式(9))进行拟合。
式中:qt为t时刻的吸附量,mg·g−1;qe为平衡吸附量,mg·g−1;k1为一级反应速率常数, s−1;k2为二级反应速率常数, L·(mol·s)−1 。
6)SMGO再生实验。取20 mL质量浓度为100 mg·L−1的Cd(Ⅱ)溶液于锥形瓶中,投加20 mg SMGO吸附材料,置于308.15 K的恒温气浴振荡箱中以120 r·min−1的速度振荡,使用磁铁将SMGO分离,采用超声波再生法洗涤至中性,冻干后重复进行吸附实验,采用电感耦合等离子体发射光谱仪测定Cd(Ⅱ) 质量浓度。
-
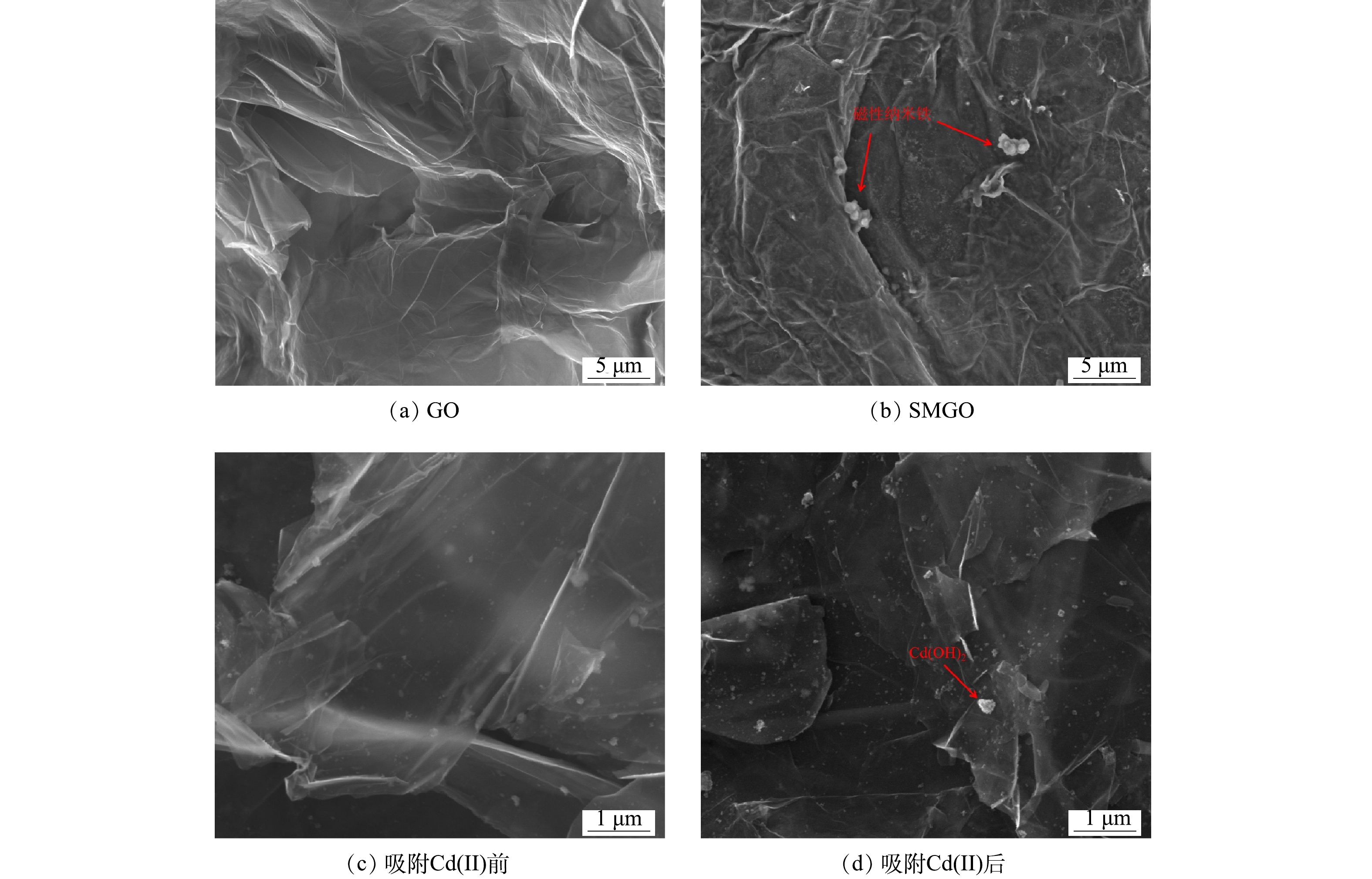
使用SEM和EDS对GO和SMGO进行表征分析,结果如图2所示。由图2(a)可以看出,制备的GO具有明显褶皱的片层结构,且片层长度超过10 µm,呈层状堆叠。这说明通过电化学法剥离的GO保留了良好的片层结构。SMGO的电镜照片显示出相似的片层结构(图2(b)),同时可以清楚看到圆形颗粒附着在SMGO片层之中。经EDS分析,SMGO中元素包括75.76% C、22.33% O、0.59% Na、1.08% S和0.25% Fe。吸附后的材料出现了Cd峰,占比为0.44%,同时氧元素占比降至17.75%。SMGO中含有的铁元素表明分散在SMGO表面的类球型颗粒为磁性纳米铁(图2(c)),其在SMGO中占据一部分空间位点,可减少GO之间重叠和团聚作用。对比图2(c)和图2(d)可以看出,吸附后的SMGO中包含Cd(OH)2颗粒。结合吸附前后的EDS分析,SMGO材料可以成功吸附Cd(Ⅱ),具有作为吸附重金属材料的能力。
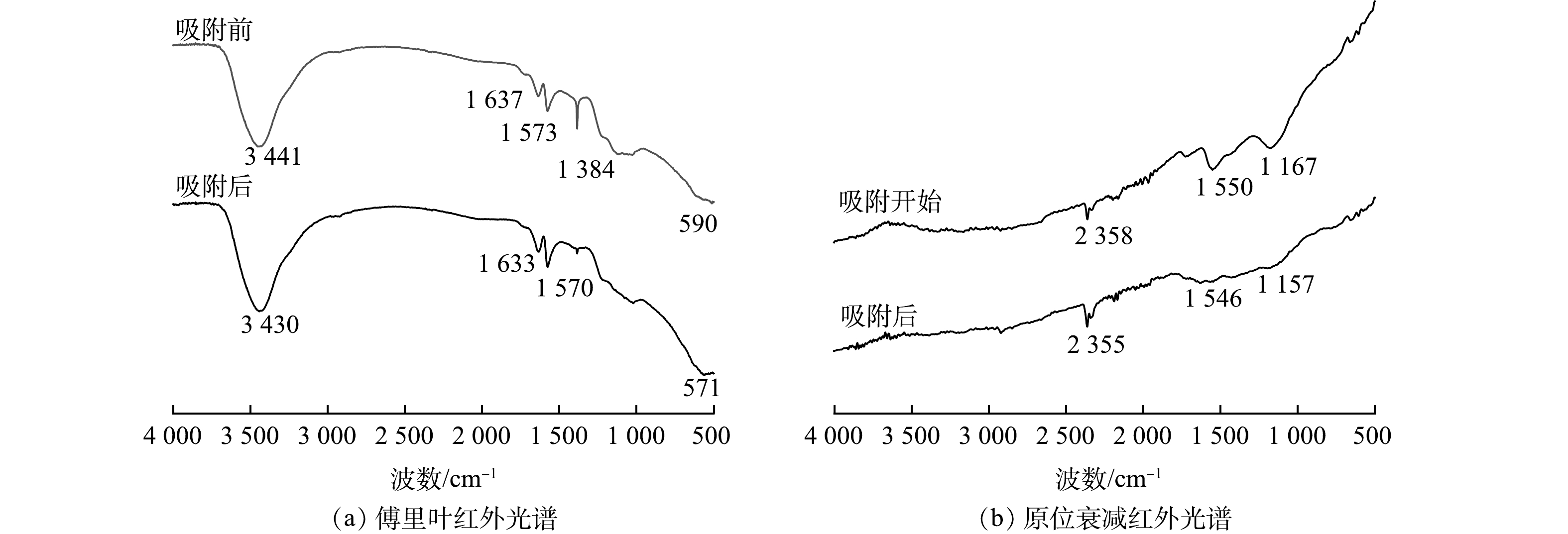
使用FTIR对GO和SMGO进行分析,图谱如图3所示。可以看出,GO和SMGO均有4~5个尖锐吸收峰,位于3 450 cm−1处的峰源自O—H的伸缩振动,可能是由吸附在表面的水分子氢键引起,SMGO相对强度较高,淀粉颗粒的负载使—OH增加,两者共同表现为在3 450 cm−1处吸收峰增强[29]。587 cm−1处的峰源属于Fe3O4,是由Fe—O—H振动引起的,说明磁性纳米铁成功负载在GO上[30]。1 735 cm−1处有C=O特征峰,是由于制备GO片层中有部分缺陷,在断层处的碳原子会与氧原子形成双键结构。1 623 cm−1处的特征峰源自C=C的伸缩振动,通常是由于共轭大π键造成的,说明GO片层之间成功剥离,形成多而少层的GO。1 409 cm−1处是C—OH形变振动特征峰,1 222 cm−1处特征峰源自C—O伸缩振动,GO的氧化通常是由边缘开始,故羟基、羧基等含氧基团多分布于GO的边缘,说明GO的氧化程度较高[31]。对比GO和SMGO可以看出,SMGO的红外光谱发生了蓝移,可能是由于共轭体系的共平面性被破坏,产生了空间障碍。此外,C—O振动吸收峰增强,C=C振动吸收峰减弱,表明成功地将淀粉引入到GO中。
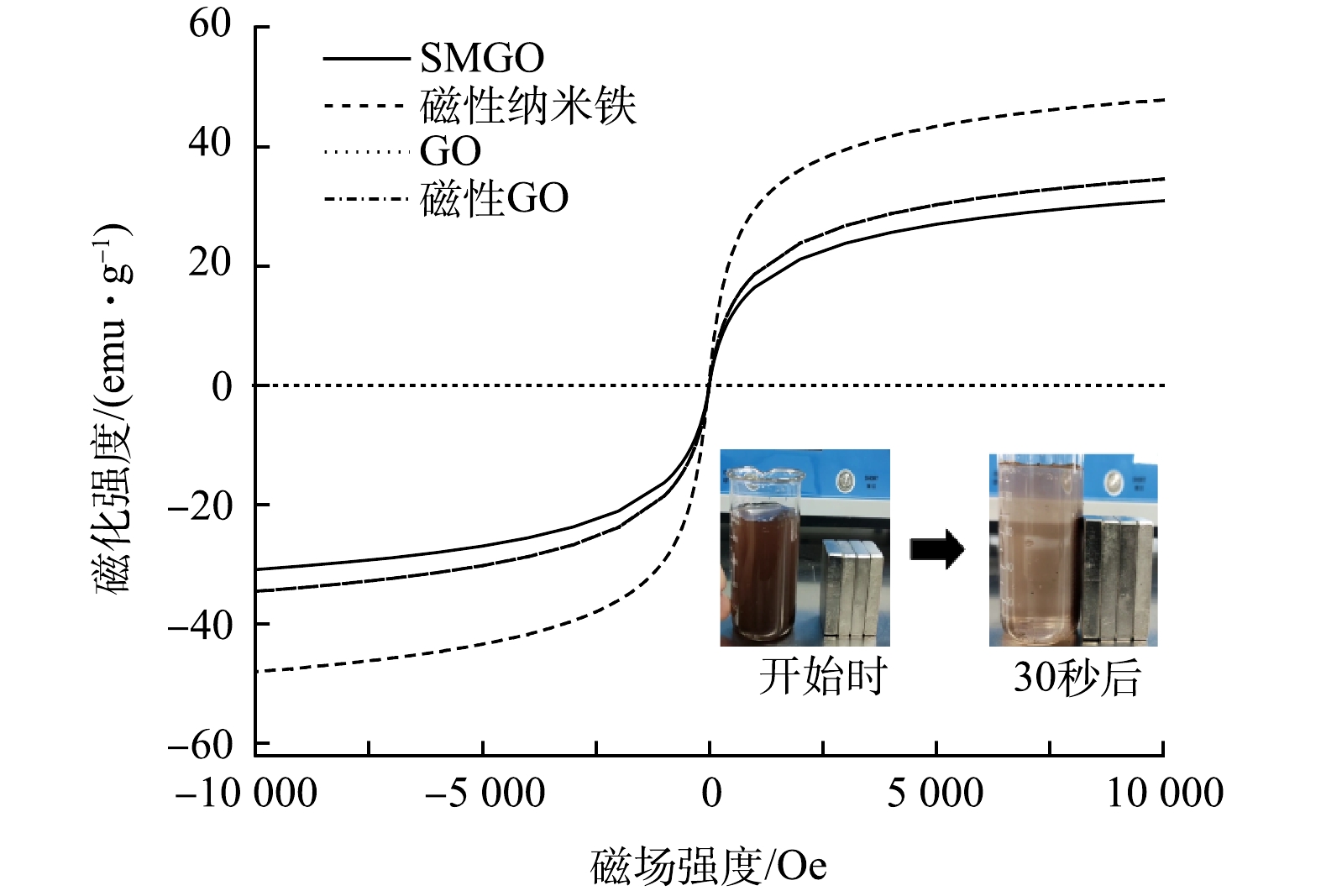
使用VSM对材料进行磁性测试,得到磁滞回线如图4所示。由图4可知,GO并无磁性,而制备出的磁性纳米铁、磁性GO和SMGO均具有铁磁材料的特征,磁性纳米铁的饱和磁化强度为47.76 emu·g−1,磁性GO的饱和磁化强度为34.54 emu·g−1,SMGO饱和磁化强度为31.85 emu·g−1;三者剩余磁化强度接近0,使用磁铁均可使磁性纳米铁、磁性GO和SMGO分离。此外,SMGO的饱和磁化强度与其他磁性材料相较略低,这可能与非磁性成分的复合有关[32-33]。通过在烧杯旁放置磁铁,测试了SMGO的磁分离性,实验过程中黑色物质在30 s内被磁铁吸引,说明SMGO具有高磁灵敏度。结合SEM和EDS观察发现,磁性纳米铁已成功附着在GO片层之间。这些结果表明SMGO成功制备。
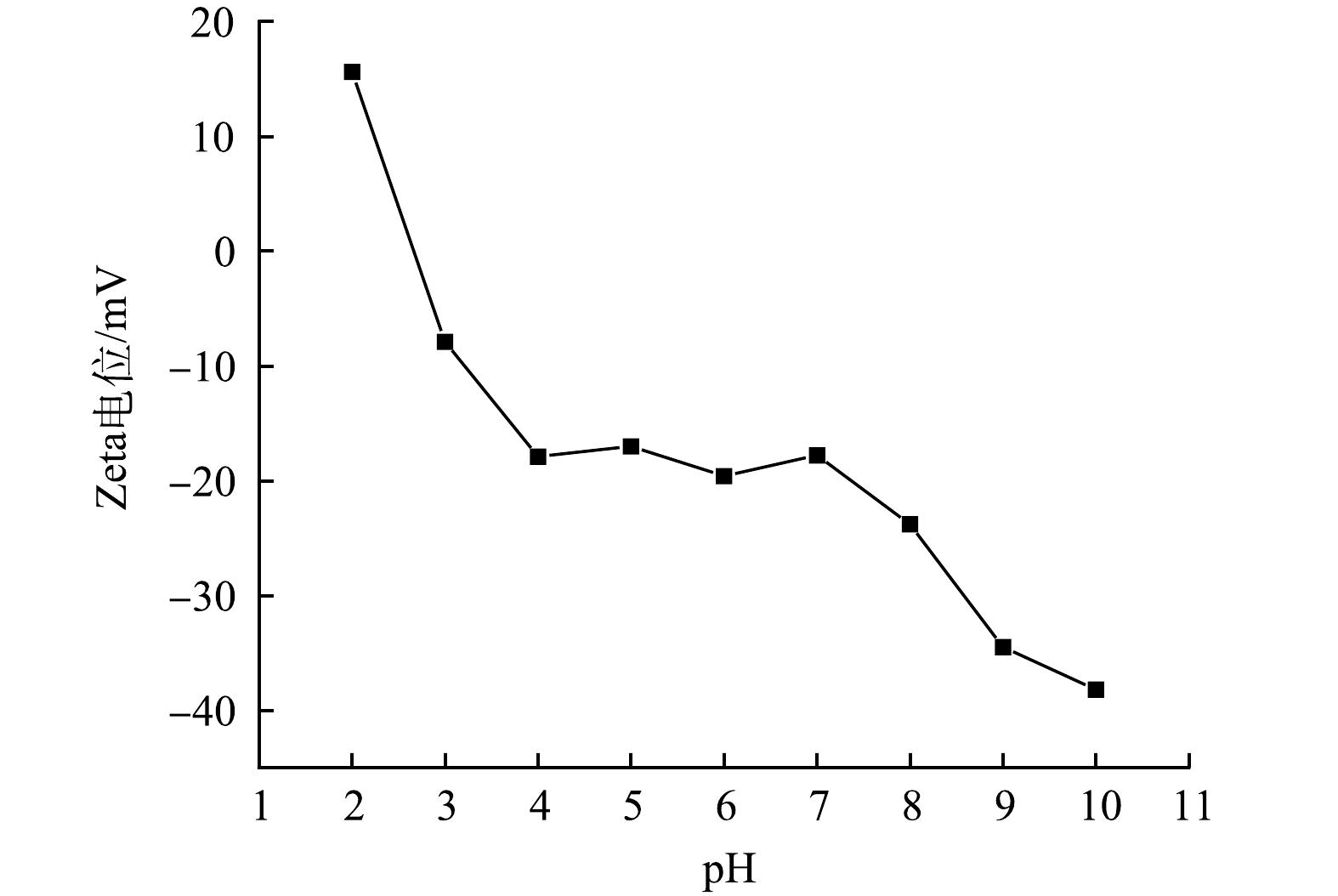
对不同pH下SMGO的Zeta电位进行了表征,结果如图5所示。由图5可知,当pH=2时,SMGO水溶液的Zeta电位为15.6 mV。说明SMGO表面带有正电荷;随着pH增加,Zeta电位逐渐降低,pH=2.5为SMGO的等电点。当pH>2.5时,SMGO水溶液的Zeta电位由正转负,此时SMGO表面带负电荷。随着pH继续增加,SMGO表面所带的负电荷逐渐增多,pH=10时Zeta电位达到-38.2 mV,有利于对带有正电荷物质进行吸附。
-
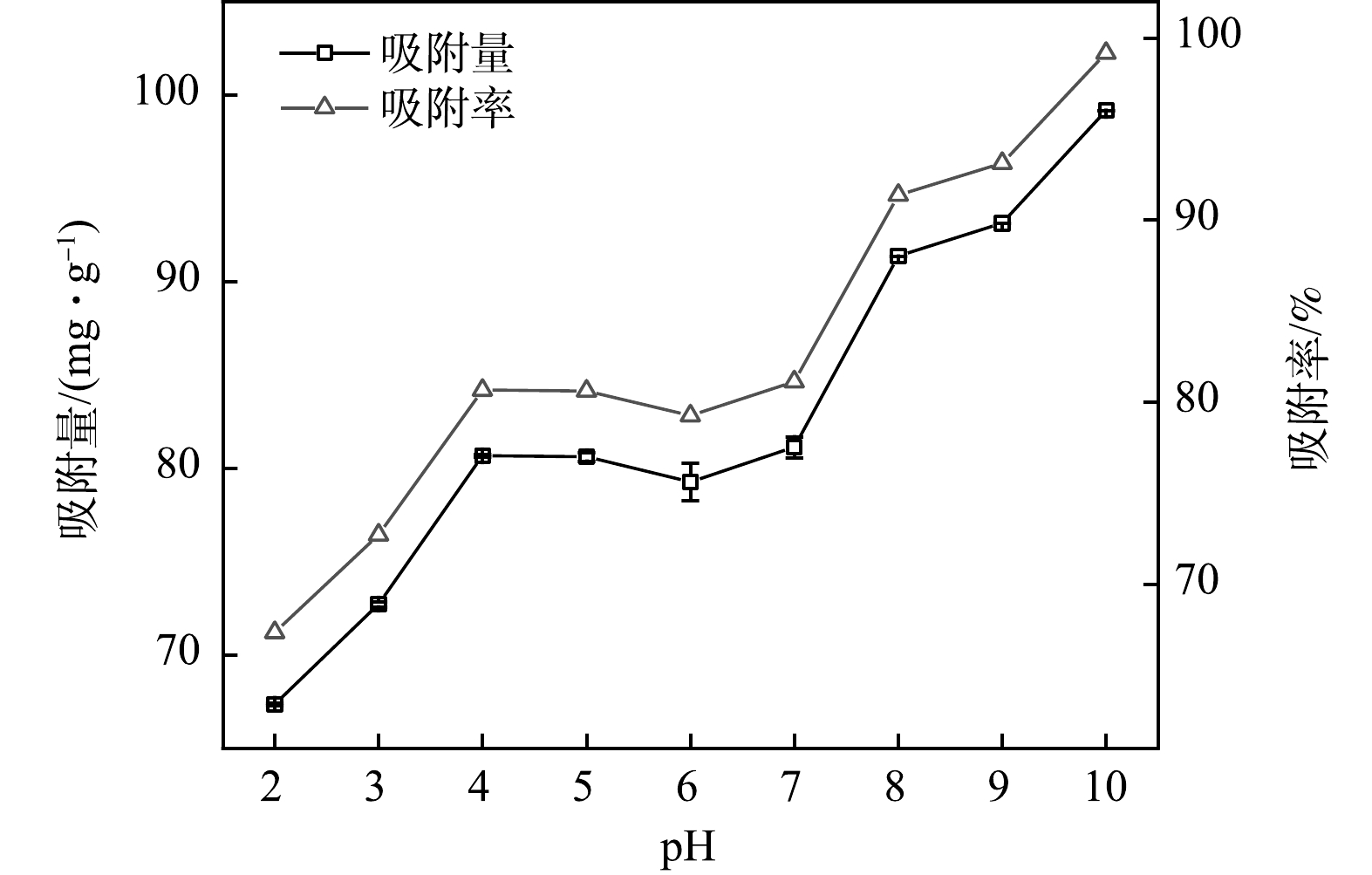
溶液pH、吸附时间是影响吸附剂吸附性能的重要因素[34-35]。溶液pH不仅影响吸附剂表面的电荷数量,而且还影响吸附剂表面功能基团的解离度[36]。由图6可知,SMGO对Cd(Ⅱ)的吸附率随pH的升高而增加。SMGO在pH < 4时对Cd(Ⅱ)的吸附量较小,与在pH较低时,SMGO表面的负电荷较少有关。随着pH的增加,溶液中的氢质子减少,SMGO表面的负电荷增多,吸附容量由67.37 mg·g−1增加至80.66 mg·g−1,吸附率由67%升至80%。pH在4~7时,吸附量和吸附率增长变缓,吸附量增加至81.12 mg·g−1,吸附率升为81% ,此时SMGO表面逐步发生去质子化反应,去质子化位点更容易保留金属离子,有利于Cd(Ⅱ)的吸附[37]。当pH大于7后,SMGO表面负电荷增加,吸附量和吸附率迅速上升;同时溶液中H+浓度较低,减少了与Cd(Ⅱ)的竞争吸附[38]。
吸附平衡时间是吸附剂实际应用过程中需考虑的重要因素[39]。地表水环境pH通常在6~9,在pH=6时,吸附时间对SMGO吸附Cd(Ⅱ)的影响如图7所示。由图7可以看出,在0~50 min内,SMGO对Cd(Ⅱ)的吸附量迅速增加,于50 min后吸附量逐渐减缓,并在60 min后达到吸附平衡。这是由于最初的SMGO有关Cd(Ⅱ)的吸附位点较多,吸附速率较快;随时间推移,SMGO表面的有效吸附位点逐渐减少,最终接近平衡状态。SMGO的吸附率从10 min时的25%迅速提升至30 min时的45%,并在50 min后增长速度减缓。这时因为SMGO在吸附初期有较多微孔,有利于吸附质通过微孔进入内部,随着吸附的进行,有效位点逐步减少,吸附率趋于平稳。
反应温度和转速也是研究吸附剂吸附性能的重要因素。一般认为,在0~150 r·min−1转速下吸附剂对污染物的影响较大,转速越高,流体动力学越强,其传质能力越强;但转速过高,污染物扩散速率降低,不利于SMGO的传质吸附[34]。此外,反应温度的变化引发了GO内部结构的变化,低温促进了GO的平面效应,而升温则抑制其反应[35]。
利用上述制备SMGO对模拟含Cd(Ⅱ)的废水进行吸附,在单因素实验结果的基础上,根据正交设计原理,并结合前人研究设计四因素三水平实验[40]。本文选择溶液pH、吸附时间、反应温度和转速4个因子,取3个水平,以SMGO对Cd(Ⅱ)的吸附率为考察指标,采用L9(34)正交实验探讨SMGO对Cd(Ⅱ)的最佳吸附条件。不同因子影响SMGO吸附Cd(Ⅱ)的强弱可根据其显著性得出,其最适条件可通过比较各因素的平均值k得出[41]。由表1可知,溶液pH、吸附时间、反应温度和转速4种因素均对SMGO吸附水中Cd(Ⅱ)的效果具有一定影响,其影响程度存在差异性。与其他处理相比,实验6的效果最好,对水中Cd(Ⅱ)的去除率达到88%,吸附容量为88.38 mg·g−1。实验4和实验7处理效果相对较差,去除率分别为69%和70%,吸附容量分别为69.46 mg·g−1和69.53 mg·g−1。各因子综合评分的极值为R2>R3>R4>R1,即SMGO吸附水中Cd(Ⅱ)的实验因素影响程度依次为:pH>反应温度>吸附时间>转速。结合方差分析结果(表2)可知,转速的离差平方和在4种影响因素中最小,可将其作为误差列进行分析。溶液pH对SMGO吸附水中Cd(Ⅱ)综合效果的影响达到显著水平(P<0.05)。溶液 pH 往往通过影响吸附剂表面电荷及被吸附物质的离子化程度和存在形态来影响吸附过程[36]。实验过程中溶液pH对SMGO吸附水中Cd(Ⅱ)的影响最大,对Cd(Ⅱ)去除率的综合评分的平均值随着pH的升高而增大。当溶液 pH 升高时,吸附点位的增加以及静电作用使得SMGO更容易吸附重金属离子[42],因此,本实验条件下较高的pH 更利于SMGO对Cd(Ⅱ)的去除。反应温度、吸附时间和转速对吸附结果的影响不显著(P>0.05)。一般而言,在未达到吸附平衡时,温度的升高使得吸附剂表面可利用的活性位点增多或者使吸附质的扩散速率增高,从而使吸附量增加;一旦达到了吸附平衡,吸附率会降低[43]。过大的转速影响液体剪切力,可能导致吸附能力降低[44]。吸附发生的初始时刻,吸附剂表明有大量的吸附位点可供吸附,有利于吸附质通过孔进入微粒内部,随着吸附的进行可利用位点逐渐减少[45]。综合分析可知,利用SMGO吸附水中Cd(Ⅱ)的最佳影响因素组合为k13、k23、k31、k42,即最佳工艺条件为:pH=10,反应温度25 ℃,吸附时间60 min,转速120 r·min−1。
为了更直观反应转速、溶液pH、反应温度和吸附时间4个因子对SMGO吸附水中Cd(Ⅱ)的影响,通过绘制单因素效应曲线图进行分析,并结合效应曲线直观判断SMGO的最佳吸附条件。影响SMGO吸附因素的效应曲线如表3所示。由表3可知,转速120 r·min−1、pH=10、温度25 ℃是SMGO吸附水中Cd(Ⅱ)的最佳实验条件。在最佳实验条件下进行SMGO吸附Cd(Ⅱ)实验,最佳吸附时间60 min后溶液中Cd(Ⅱ)的质量浓度为6.89 mg·L−1。根据式(1)计算得出SMGO吸附水中Cd(Ⅱ)量可达93.11 mg·g−1,SMGO对Cd(Ⅱ)的吸附率达到93%。
由表4可知,与传统吸附材料(活性炭等)和其他石墨烯吸附剂相比,SMGO对溶液中Cd(Ⅱ)表现出优异的吸附性能,吸附量远高于活性炭,约为其他GO吸附剂的2~4倍。XIE等[46]制备的新型氮化碳复合吸附材料对Cd(Ⅱ)有较好的吸附效果,但制备过程需要500 ℃以上的高温。SMGO制备过程相对简单,淀粉等原料亦廉价易得。SMGO通过利用改性淀粉的弱还原性增强了SMGO材料的稳定性,在提高吸附容量和控制成本的同时,简化了GO复合材料的制备过程,由此进一步表明SMGO具有较大的实际应用潜力。
-
为深入了解SMGO对Cd(Ⅱ)的吸附过程,采用准一级动力学(式(6))和准二级动力学模型(式(8))对实验数据进行拟合,结果如图8和表5所示。由图8和表5可知,与准一级动力学模型(R2=0.847)相比,准二级动力学模型(R2=0.999)更符合SMGO对Cd(Ⅱ)的吸附过程。准一级动力学模型表示吸附剂对吸附质的结合位点较少,吸附主要受扩散作用控制;准二级动力学模型通常与化学反应控制整体吸附动力学有关,吸附/解吸过程中涉及电子的转移,吸附剂表面有多个用于吸附的交换位点[52]。化学键的形成是影响准二级动力学吸附作用的主要因子[53],因此,SMGO对Cd(Ⅱ)的吸附过程主要以化学吸附为主,吸附剂表面有多种Cd(Ⅱ)结合位点,主要来源于磁性纳米铁和淀粉。准二级动力学模型拟合得到的平衡吸附容量为61.501 mg·g−1,接近实际值(qe,exp)59.84 mg·g−1。而准一级动力学方程模拟得到的平衡吸附量则与实际值存在较大偏差。
-
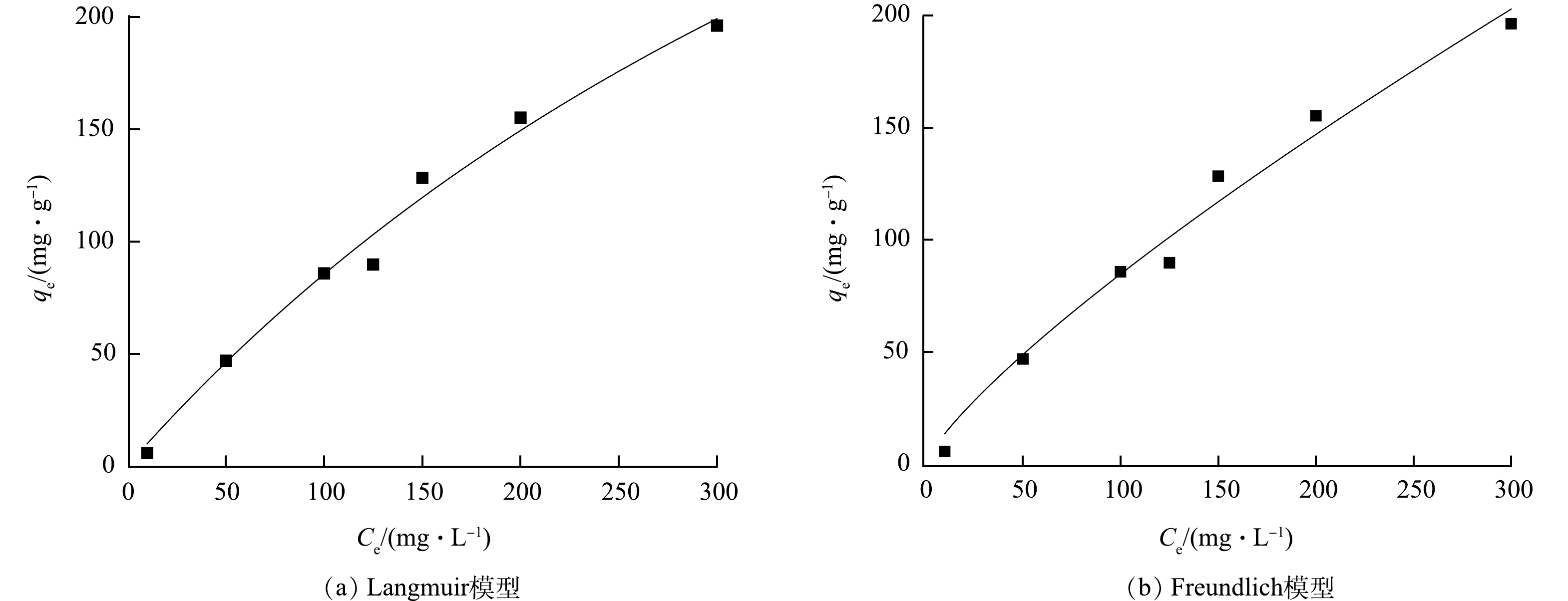
为进一步探讨SMGO的吸附机理,使用Langmuir等温线(式(3))和Freundlich等温线模型(式(4))对吸附等温线进行拟合,结果如图9和表6所示。Langmuir模型表示吸附质在吸附剂表面形成单分子层,并且含有氧或氮等高静电官能团;Freundlich模型适用于吸附质被不均匀表面吸附,吸附剂含有强官能团和弱官能团的组合[54]。由图9和表6可以看出,SMGO对Cd(Ⅱ)的吸附的Langmuir可决系数(R2=0.984 9)大于Freundlich(R2=0.979 1),同时,由Langmuir等温线模型计算得出的最大单层吸附容量(598.7±155.4) mg·g−1更接近实验数值,由此表明SMGO对Cd(Ⅱ)的吸附更符合Langmuir等温线模型,属于单分子层的吸附。这与前人的研究结果一致[55]。SMGO表面有较多高静电官能团,以单层吸附为主,同时SMGO具有的大比表面积增加了对Cd(Ⅱ)的吸附位点。
-
再生性能是吸附剂可大规模应于实际水处理工艺中的重要因素之一。若一次性吸附剂使用后被遗弃,则其吸附的有毒重金属可能会长期存在并再次污染环境,因此绿色吸附剂除了具有较好的吸附能力外,还应具有优异的循环再生能力[56]。由图10可知,随着循环次数的增加,吸附剂SMGO对Cd(Ⅱ)的吸附能力略有下降,可能与不完全解吸有关[57]。经过5次循环吸附后,SMGO在循环使用时对Cd(Ⅱ)的吸附容量变化较小,Cd(Ⅱ)的去除率由80%降至75%,仍保持了较高的去除率。由此可以看出,吸附剂SMGO具有良好的循环吸附性能和稳定性能,在实际水处理工程中可提高应用周期,降低规模化应用的成本,在去除环境水体中的Cd(Ⅱ)方面具有很大的应用潜力。
对重金属的吸附去除日渐趋向全过程无害化处理,包括吸附材料的再生以及废液的资源化处理。超声波再生是处理吸附材料的有效途径之一,相较于传统处理方法,其具有能耗小、可回收、对材料结构影响小的优点[58]。本研究中磁铁可将烧杯中的SMGO在1 min内分离,再结合超声波再生法从SMGO中解吸出Cd(Ⅱ)。产生的废液中含有较高浓度的Cd(Ⅱ),可使用浓盐酸回流混合液,将其重结晶后得到CdCl2,产品在重结晶后进行干燥,回收氯化镉。废盐酸用滤压式电解槽电解,对产生的氯气进行回收,实现资源重复利用。
-
对吸附Cd(Ⅱ)前后的SMGO样品进行了FTIR和XPS分析,结果如图11所示。由图11(a)和图12(b)可以看出,SMGO上存在较多的羟基和羧基等含氧官能,吸附Cd(Ⅱ)后,3 441 cm−1处的峰移动至3 430 cm−1,1 637 cm−1处的峰移动至1 633 cm−1,可能与Cd(Ⅱ)和SMGO表面羟基(—OH)之间的相互作用有关,电荷的转移导致吸收峰的变化。由图11(b)可以看出,SMGO对Cd(Ⅱ)吸附导致1 167 cm−1和1 550 cm−1处峰强度降低, C—O伸缩振动减弱,而1 300~1 500 cm−1处峰强度增加,氢氧弯曲振动增强。上述结果表明,C=O、O—C=O和C—O等相关基团中的孤对电子向Cd(Ⅱ)提供了配位,镉离子与含氧官能团之间形成了金属配体键,从而易于产生表面络合[59]。由图12(a)可知,SMGO主要含有C、O、Fe主要元素,结合能分别为284.1、532.2和710.7 eV,对应C1s、O1s和Fe2p。SMGO在吸附后结合能为411、405和403 eV处出现了明显的Cd 3d特征峰。吸附后O1s分峰图(图12(c))显示,M—OH、M—O、M—OH2分别占比为70.20%、12.14%、17.66%。结合Cd 3d分峰图(图12(d))可知,水溶液中镉离子主要以Cd(OH)+、Cd(OH)2等形式存在,SMGO中含氧基团与其络合生成C—O—Cd(OH)、O—C—O—Cd(OH)等物质,部分通过静电作用在表面形成分子层。
pH影响了溶液中离子的存在形式,对SMGO的吸附性能产生显著影响。根据Zeta电位分析结果(图5),在pH<2.5的情况下,SMGO中的羟基、羧基发生质子化反应,使其表面带有正电荷。SMGO表面含氧基团以COOH、COOH2+、C—OH等形式存在,游离H+会减少SMGO的吸附位点,溶液中Cd2+、Cd(NO3)+、Cd(OH)+被SMGO表面永久负电荷点位所吸附;随着pH升高并超过等电点,SMGO发生去质子化反应,表面逐渐转为负电,羟基、羧基等含氧基团成为主要吸附点位,部分镉离子被吸附后形成络合物。同时,SMGO中的—COOH和磁性纳米铁中的—FeOH拥有一定的离子交换能力,可进行离子交换反应,共同促进SMGO对Cd(Ⅱ)的吸附[60]。
为了进一步证明重金属吸附在SMGO表面,通过SEM和EDS分析研究了SMGO吸附前后的形貌和组成(图2(c)、图2(d))。可以看出,Cd(Ⅱ)被SMGO成功吸附,并均匀分布在其表面。综上所述,SMGO对Cd(Ⅱ)的吸附主要依靠静电吸附和络合反应,并体现出优异的吸附性能。
-
1)采用GO作为载体,成功将磁性纳米铁和淀粉引入,制备出SMGO。使用改性淀粉替代了有毒还原剂的使用,并将更多的羟基和羧基引入到SMGO材料之中,增强了其吸附性能;磁性纳米铁的负载,使其在兼顾吸附量的同时被易分离再生。
2) 4种因子对SMGO吸附Cd(Ⅱ)的影响依次为pH>温度>时间>转速;SMGO吸附Cd(Ⅱ)的最佳吸附条件为:转速120 r·min−1、pH=10、温度25 ℃、时间60 min,该条件下SMGO对Cd(Ⅱ)的吸附率可达93%。
3) SMGO对Cd(Ⅱ)的吸附更符合准二级动力学模型和Langmuir等温线模型,表明该吸附过程为单分子层化学吸附过程。由上述2个模型计算得到SMGO对Cd(Ⅱ)的平衡吸附量和最大吸附量分别为61.501 mg·g−1和598.7 mg·g−1。经过5次吸附-解吸以后,Cd(Ⅱ)的去除率仍可达到75%。SMGO对Cd(Ⅱ)的吸附能力远高于其他GO材料,具有很高的应用价值。
4) SMGO中的C=O、O—C=O和C—O等官能团在吸附Cd(Ⅱ)过程中起主要作用,溶液pH会影响Cd(Ⅱ)的存在形式和SMGO的表面电荷,高pH有利于SMGO对Cd(Ⅱ)的吸附,同时静电吸附、络合反应是SMGO吸附Cd(Ⅱ)的主要途径。
淀粉/三维磁性氧化石墨烯的制备及其对Cd(Ⅱ)的吸附性能和机理
Preparation of starch/3D magnetic graphene oxide and its adsorption performance and mechanism to Cd(Ⅱ)
-
摘要: 采用电化学法和水热合成法成功制备了淀粉/三维磁性氧化石墨烯(SMGO),通过红外光谱、扫描电镜和磁性分析证实了SMGO的成功制备。通过正交实验研究了pH、吸附时间、反应温度和转速对SMGO吸附性能的影响,采用吸附动力学和热力学分析并结合红外光谱、Zeta电位和光电能谱等手段探讨了SMGO的材料特性及吸附机理。结果表明:SMGO具有超顺磁性,其最大吸附量可达(598.7±155.4) mg·g−1;pH对吸附效果的影响最大;转速120 r·min−1、pH=10、温度25 ℃、时间60 min为最佳吸附条件;SMGO对Cd(Ⅱ)的吸附符合准二级动力学模型和Langmuir等温线模型,主要为单分子层化学吸附过程;经过5次循环再生后SMGO对Cd(Ⅱ)的去除率仍可达到75%,是一种极具潜力的Cd(Ⅱ)绿色吸附剂。实验证明在吸附过程中SMGO吸附剂与Cd(Ⅱ)之间主要存在静电吸附和络合作用。以上研究结果可为SMGO应用于含Cd(Ⅱ)废水处理提供参考。Abstract: Starch/3D magnetic graphene oxide composite (SMGO) was successfully prepared by electrochemical and hydrothermal synthesis, and the successful preparation of SMGO was confirmed by infrared spectrum, scanning electron microscope and vibrating sample magnetometer. The orthogonal experiments were conducted to study the effects of pH, adsorption time, reaction temperature and rotation speed on the adsorption properties of SMGO. The materials characteristic and adsorption mechanism of SMGO were discussed by adsorption kinetics, thermodynamics, infrared spectrum, Zeta potential and photoelectric energy spectrum. The results show that SMGO had superparamagnetic property, and its maximum adsorption capacity could reach (598.7±155.4) mg·g−1, pH had the greatest influence on the adsorption effect. The optimum adsorption conditions were following: rotation speed of 120 r·min−1, pH10, 25 ℃ and 60 min. The adsorption of Cd(Ⅱ) by SMGO conformed to the quasi-secondary kinetic model and the Langmuir isotherm model, was dominated by the monolayer chemisorption process; The removal rate of Cd(Ⅱ) by SMGO could still maintain 75% after 5 cycles of regeneration. SMGO is a type of green adsorbent with high-performance. The experiments proved that electrostatic adsorption and complexation between SMGO adsorbent and Cd(Ⅱ) occurred in the adsorption process between SMGO and Cd(Ⅱ). The results can provide a reference for the application of SMGO in the treatment of wastewater containing Cd(Ⅱ).
-
Key words:
- graphene oxide /
- green adsorbent /
- Cd(Ⅱ) removal /
- magnetic material
-

-
表 1 正交试实验设计和结果
Table 1. Orthogonal experiment design and the results
名称 因素 吸附容量/
(mg·g−1)吸附
率/%(1)转速 (2)pH (3)反应
温度/ ℃(4)吸附
时间/min实验1 40 4 25 30 70.95 71 实验2 40 7 35 60 81.39 81 实验3 40 10 45 90 85.65 86 实验4 80 4 35 90 69.46 69 实验5 80 7 45 30 72.22 72 实验6 80 10 25 60 88.38 88 实验7 120 4 45 60 69.53 70 实验8 120 7 25 90 83.53 84 实验9 120 10 35 30 85.24 85 K1 237.99 209.94 242.86 228.40 — — K2 230.06 237.14 236.08 239.31 — — K3 238.30 259.27 227.40 238.63 — — k1 79.33 69.98 80.95 76.13 — — k2 76.69 79.05 78.69 79.77 — — k3 79.43 86.42 75.80 79.54 — — R 2.74 16.44 5.15 3.63 — — 注:K1~K3为每个因素各水平下的指标总和;k1~k3 为每个因素各水平下的均值;R表示极值;“—”表示无数据。 表 2 SMGO吸附水中Cd(Ⅱ)效果的综合评分方差分析
Table 2. Comprehensive evaluation of variance analysis for Cd(Ⅱ) adsorption on SMGO in the water
方差来源 离差平方和 自由度 均方差 F P 显著性 转速 14.542 2 7.271 1 0.5 — pH 407.003 2 203.502 27.988 0.034 显著 温度 40.040 2 20.02 2.753 0.283 — 时间 24.853 2 12.427 1.709 0.369 — 误差 14.542 2 — — — — 注:“—”表示无数据;P<0.05时为显著。 表 3 不同影响因素下的去除率
Table 3. Removal rate at different effect factors
不同转速下的去除率/% 不同pH下的去除率/% 不同温度下的去除率/% 不同时间下的去除率/% 40 r·min−1 80 r·min−1 120 r·min−1 4 7 10 25 ℃ 35 ℃ 45 ℃ 30 min 60 min 90 min 79.33 76.69 79.43 69.98 79.05 86.42 80.95 78.69 75.8 76.13 79.77 79.54 表 4 SMGO与其他含石墨烯吸附剂对Cd(Ⅱ)吸附性能对比
Table 4. Comparison of Cd(Ⅱ) adsorption properties of SMGO with other graphene-based adsorbents
表 5 SMGO吸附动力学模型参数
Table 5. Adsorption kinetics models parameters of SMGO
C0/(mg·g−1) qe,exp/(mg·g−1) 准一级动力学 准二级动力学 K1 qe/(mg·g−1) R12 K2 qe/(mg·g−1) R22 100 59.84 0.012 9±0.002 19.609±5.611 0.847 0.002±0.000 2 61.501±0.671 0.999 表 6 吸附等温线模型参数
Table 6. Adsorption isotherms models parameters
温度/K Langmuir模型 Freundlich模型 qm/(mg·g−1) KL R2 Kf 1/n R2 308 598.7±155.4 0.001 6±0.000 6 0.984 9 2.18±0.77 0.79±0.06 0.979 1 -
[1] BOSU S, RAJAMOHAN N, RAJASIMMAN M. Enhanced remediation of lead (Ⅱ) and cadmium (Ⅱ) ions from aqueous media using porous magnetic nanocomposites: A comprehensive review on applications and mechanism[J]. Environmental Research, 2022, 213: 113720. doi: 10.1016/j.envres.2022.113720 [2] WEI B, YANG L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China[J]. Microchemical Journal, 2010, 94(2): 99-107. doi: 10.1016/j.microc.2009.09.014 [3] RAFATI RAHIMZADEH M, RAFATI RAHIMZADEH M, KAZEMI S, et al. Cadmium toxicity and treatment: An update[J]. Caspian Journal of Internal Medicine, 2017, 8(3): 135-145. [4] JAKOBSEN M. Electrodialytic removal of cadmium from wastewater sludge[J]. Journal of Hazardous Materials, 2004, 106(2-3): 127-132. doi: 10.1016/j.jhazmat.2003.10.005 [5] 刘红娟, 张慧, 党志, 等. 一株耐镉细菌的分离及其富集Cd的机理[J]. 环境工程学报, 2009, 3(2): 367-371. [6] 滕云, 游少鸿, 陈梦华, 等. 香蒲根际过滤对水中镉的去除[J]. 环境工程学报, 2017, 11(3): 1545-1548. [7] SHEN C, ZHAO Y, LI W, et al. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual[J]. Chemical Engineering Journal, 2019, 372: 1019-1027. doi: 10.1016/j.cej.2019.04.219 [8] AHMAD S Z N, WAN SALLEH W N, ISMAIL A F, et al. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms[J]. Chemosphere, 2020, 248: 126008. doi: 10.1016/j.chemosphere.2020.126008 [9] PEI S, WEI Q, HUANG K, et al. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation[J]. Nature Communications, 2018, 9(1): 1-9. doi: 10.1038/s41467-017-02088-w [10] ABU-NADA A, MCKAY G, ABDALA A. Recent advances in applications of hybrid graphene materials for metals removal from wastewater[J]. Nanomaterials, 2020, 595(10): 1-31. [11] ZHANG Z, XIAO F, GUO Y, et al. One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities[J]. ACS Applied Materials & Interfaces, 2013, 5(6): 2227-2233. [12] SINGH S, ANIL A G, KHASNABIS S, et al. Sustainable removal of Cr(Ⅵ) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics[J]. Environmental Research, 2022, 203: 111891. doi: 10.1016/j.envres.2021.111891 [13] TANG J, HUANG Y, GONG Y, et al. Preparation of a novel graphene oxide/Fe-Mn composite and its application for aqueous Hg(II) removal[J]. Journal of Hazardous Materials, 2016, 316: 151-158. doi: 10.1016/j.jhazmat.2016.05.028 [14] MADADRANG C J, KIM H Y, GAO G, et al. Adsorption behavior of EDTA-graphene oxide for Pb (II) removal[J]. ACS Applied Materials & Interfaces, 2012, 4(3): 1186-1193. [15] HOU W, XINGZHONG Y, YAN W, et al. Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution[J]. Applied Surface Science, 2013, 279: 432-440. doi: 10.1016/j.apsusc.2013.04.133 [16] LI X, TANG X, FANG Y. Using graphene oxide as a superior adsorbent for the highly efficient immobilization of Cu(Ⅱ) from aqueous solution[J]. Journal of Molecular Liquids, 2014, 199: 237-243. doi: 10.1016/j.molliq.2014.09.020 [17] SUN Y, YANG S, CHEN Y, et al. Adsorption and desorption of U(Ⅵ) on functionalized graphene oxides: A combined experimental and theoretical study[J]. Environmental Science & Technology, 2015, 49(7): 4255-4262. [18] SARKAR A K, BEDIAKO J K, CHOI J, et al. Functionalized magnetic biopolymeric graphene oxide with outstanding performance in water purification[J]. NPG Asia Materials, 2019, 4(11): 1-10. [19] CHANG S, ZHANG Q, LU Y, et al. High-efficiency and selective adsorption of organic pollutants by magnetic CoFe2O4/graphene oxide adsorbents: Experimental and molecular dynamics simulation study[J]. Separation and Purification Technology, 2020, 238: 116400. doi: 10.1016/j.seppur.2019.116400 [20] RAMALINGAM B, PARANDHAMAN T, CHOUDHARY P, et al. Biomaterial functionalized graphene-magnetite nanocomposite: A novel approach for simultaneous removal of anionic dyes and heavy-metal ions[J]. ACS sustainable chemistry & engineering, 2018, 6(5): 6328-6341. [21] WANG Y, ZHAO D, FENG S, et al. Ammonium thiocyanate functionalized graphene oxide-supported nanoscale zero-valent iron for adsorption and reduction of Cr(Ⅵ)[J]. Journal of Colloid and Interface Science, 2020, 580: 345-353. doi: 10.1016/j.jcis.2020.07.016 [22] GUPTA A D, RAWAT K P, BHADAURIA V, et al. Recent trends in the application of modified starch in the adsorption of heavy metals from water: A review[J]. Carbohydr Polym, 2021, 269: 1-66. [23] BHAT M A, CHISTI H, SHAH S A. Removal of heavy metal ions from water by cross-linked potato di-starch phosphate polymer[J]. Separation Science and Technology, 2015, 50(12): 1741-1747. doi: 10.1080/01496395.2014.978469 [24] 谢冬冬, 侯英, 黄贵臣, 等. QCM-D研究淀粉和油酸钠与磁铁矿的吸附机理[J]. 中南大学学报(自然科学版), 2019, 50(7): 1514-1520. [25] 李明恩, 冯庆革, 林海英, 等. 氨基功能氧化石墨烯的制备(DH-GO)及其对废水中Cr(Ⅵ)的去除效果[J]. 环境工程学报, 2022, 16(3): 926-936. [26] 莫京倚, 张卫民, 陈家鸿, 等. 2种不同碱度钢渣及其负载HAP吸附镉的比较[J]. 环境工程学报, 2019, 13(8): 1800-1808. [27] 杨月红, 舒敦涛, 宁平. 微波场诱导改性磷石膏吸附Cu2+, Zn2+, Pb2+和Cd2+的动力学与热力学研究[J]. 中南大学学报(自然科学版), 2013, 44(5): 2157-2164. [28] 肖海梅, 蔡蕾, 张朝晖, 等. 磁性氧化石墨烯/MIL-101(Cr)表面金属离子印迹聚合物制备及其对Cu(Ⅱ)和Pb(Ⅱ)选择性吸附[J]. 应用化学, 2020, 37(9): 1076-1086. [29] CUI L, WANG Y, GAO L, et al. EDTA functionalized magnetic graphene oxide for removal of Pb(Ⅱ), Hg(Ⅱ) and Cu(Ⅱ) in water treatment: Adsorption mechanism and separation property[J]. Chemical Engineering Journal, 2015, 281: 1-10. doi: 10.1016/j.cej.2015.06.043 [30] SAMUEL M S, SHAH S S, BHATTACHARYA J, et al. Adsorption of Pb(II) from aqueous solution using a magnetic chitosan/graphene oxide composite and its toxicity studies[J]. International Journal of Biological Macromolecules, 2018, 115: 1142-1150. doi: 10.1016/j.ijbiomac.2018.04.185 [31] ZHANG Y, WU L, DENG H, et al. Modified graphene oxide composite aerogels for enhanced adsorption behavior to heavy metal ions[J]. Journal of Environmental Chemical Engineering, 2021, 106008(5): 1-10. [32] ZHU J, WEI S, GU H, et al. One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal[J]. Environmental Science & Technology, 2012, 46(2): 977-985. [33] AI L, ZHANG C, CHEN Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite[J]. Journal of Hazardous Materials, 2011, 192(3): 1515-1524. doi: 10.1016/j.jhazmat.2011.06.068 [34] ZHOU W, ZHANG W, CAI Y. Enzyme-enhanced adsorption of laccase immobilized graphene oxide for micro-pollutant removal[J]. Separation and Purification Technology, 2022, 294: 1-10. [35] REN W, CHANG H, MAO T, et al. Planarity effect of polychlorinated biphenyls adsorption by graphene nanomaterials: The influence of graphene characteristics, solution pH and temperature[J]. Chemical Engineering Journal, 2019, 362: 160-168. doi: 10.1016/j.cej.2019.01.027 [36] LI X, ZHOU H, WU W, et al. Studies of heavy metal ion adsorption on chitosan/sulfydryl-functionalized graphene oxide composites[J]. Journal of Colloid and Interface Science, 2015, 448: 389-397. doi: 10.1016/j.jcis.2015.02.039 [37] LI J, ZHANG S, CHEN C, et al. Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles[J]. ACS Applied Materials & Interfaces, 2012, 4(9): 4991-5000. [38] HUONG L M, THINH D B, TU T H, et al. Ice segregation induced self-assembly of graphene oxide into graphene-based aerogel for enhanced adsorption of heavy metal ions and phenolic compounds in aqueous media[J]. Surfaces and Interfaces, 2021, 26: 101309. doi: 10.1016/j.surfin.2021.101309 [39] SITKO R, TUREK E, ZAWISZA B, et al. Adsorption of divalent metal ions from aqueous solutions using graphene oxide[J]. Dalton Transactions, 2013, 42(16): 5682-5689. doi: 10.1039/c3dt33097d [40] 刘国, 吴茜, 李君. 乙二胺四乙酸插层水滑石吸附Cd(Ⅱ)的影响因素研究[J]. 环境工程, 2015, 33(7): 41-45. [41] 罗冬, 谢翼飞, 谭周亮, 等. NaOH改性玉米秸秆对石油类污染物的吸附研究[J]. 环境科学与技术, 2014, 37(1): 28-32. [42] FU W, HUANG Z. Magnetic dithiocarbamate functionalized reduced graphene oxide for the removal of Cu(II), Cd(II), Pb(II), and Hg(II) ions from aqueous solution: Synthesis, adsorption, and regeneration[J]. Chemosphere, 2018, 209: 449-456. doi: 10.1016/j.chemosphere.2018.06.087 [43] GAO Y, LI Y, ZHANG L, et al. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide[J]. Journal of Colloid and Interface Science, 2012, 368(1): 540-546. doi: 10.1016/j.jcis.2011.11.015 [44] 杜文琪, 曹玮, 周航, 等. 磁性生物炭对重金属污染废水处理条件优化及机理[J]. 环境科学学报, 2018, 38(2): 492-500. [45] 刘伟, 杨琦, 李博, 等. 磁性石墨烯吸附水中Cr(Ⅵ)研究[J]. 环境科学, 2015, 36(2): 537-544. [46] XIE H, ZHANG J, WANG D, et al. Construction of three-dimensional g-C3N4/attapulgite hybrids for Cd(II) adsorption and the reutilization of waste adsorbent[J]. Applied Surface Science, 2020, 504: 144456. doi: 10.1016/j.apsusc.2019.144456 [47] 邓清, 李春阳, 邓志华, 等. 活性炭对含Zn2+和Cd2+的重金属废水吸附净化效果研究[J]. 化工新型材料, 2019, 47(4): 204-207. [48] LIU J, DU H, YUAN S, et al. Alkaline deoxygenated graphene oxide as adsorbent for cadmium ions removal from aqueous solutions[J]. Water Science and Technology, 2015, 71(11): 1611-1619. doi: 10.2166/wst.2015.124 [49] 李仕友, 熊凡, 王亮, 等. 氧化石墨烯/SiO2复合材料对Cd(Ⅱ)的吸附[J]. 复合材料学报, 2017, 34(6): 1205-1211. [50] 唐振平, 谢严兴, 毕玉玺, 等. 磁性介孔二氧化钛/氧化石墨烯复合材料的制备及其对 Cd(Ⅱ)的吸附[J]. 科学技术与工程, 2019, 19(35): 388-394. doi: 10.3969/j.issn.1671-1815.2019.35.059 [51] HUANG D, WU J, WANG L, et al. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water[J]. Chemical Engineering Journal, 2019, 358: 1399-1409. doi: 10.1016/j.cej.2018.10.138 [52] PLAZINSKI W, RUDZINSKI W, PLAZINSKA A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review[J]. Advances in Colloid and Interface Science, 2009, 152(1): 1-13. [53] 杨秀敏, 王文, 谢琼丹. 改性膨润土对废水中Cd(Ⅱ)的吸附特征及吸附动力学研究[J]. 过程工程学报, 2022, 22(11): 1512-1520. [54] DIAGBOYA P N, MMAKO H K, DIKIO E D, et al. Synthesis of amine and thiol dual functionalized graphene oxide for aqueous sequestration of lead[J]. Journal of Environmental Chemical Engineering, 2019, 7(6): 1-7. [55] DENG X, LÜ L, LI H, et al. The adsorption properties of Pb(Ⅱ) and Cd(Ⅱ) on functionalized graphene prepared by electrolysis method[J]. Journal of Hazardous Materials, 2010, 183(1-3): 923-930. doi: 10.1016/j.jhazmat.2010.07.117 [56] JAIN A, KUMARI S, AGARWAL S, et al. Water purification via novel nano-adsorbents and their regeneration strategies[J]. Process Safety and Environmental Protection, 2021, 152: 441-454. doi: 10.1016/j.psep.2021.06.031 [57] JORGE GONÇALVES F, ALVES GURGEL L V, CATONE SOARES L, et al. Application of pyridine-modified chitosan derivative for simultaneous adsorption of Cu(Ⅱ) and oxyanions of Cr(Ⅵ) from aqueous solution[J]. Journal of Environmental Management, 2021, 282: 1-18. [58] 刘晓咏, 欧阳平. 吸附材料超声波再生的研究进展[J]. 材料导报, 2016, 30(11): 110-115. [59] LI H, DONG X, DA SILVA E B, et al. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications[J]. Chemosphere, 2017, 178: 466-478. doi: 10.1016/j.chemosphere.2017.03.072 [60] ZOU Y, WANG X, KHAN A, et al. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review[J]. Environmental Science & Technology, 2016, 50(14): 7290-7304. -



 下载:
下载: