-
碘代消毒副产物是在水中含碘离子或含碘化合物的条件下,利用次氯酸钠,二氧化氯等消毒剂对饮用水或者污水进行消毒,与水中其他的化合物经过一系列复杂的反应生成的一类含碘的副产物。碘代消毒副产物的种类包括碘代三卤甲烷,碘代卤乙酸,碘代卤乙腈,碘代乙酰胺等[1-3]。碘代消毒副产物虽然在水中含量较低,但是大量的研究表明,其具有比常规的氯代和溴代消毒副产物更高的遗传毒性和细胞毒性[1]。我国的沿海地区和部分内陆地区水源中含有较高含量的碘离子,特别是北京、河南和河北部分浅层水中碘离子的含量高达1 000 µg·L−1,具有较高的碘代消毒副产物生成风险[4-5]。目前,针对碘代消毒副产物的控制研究大多集中在利用传统的混凝、沉淀,臭氧以及膜过滤等手段去除其前驱物,而对于碘代消毒副产物本身的降解研究较少[6]。
高级氧化技术是一种处理难降解有机废水的有效手段。芬顿氧化技术是应用最广泛和成熟的高级氧化技术,在处理印染、医药、石化等行业废水有重要的应用。但是传统的均相芬顿氧化技术还存在着pH适用范围窄、双氧水利用效率低以及存在铁泥二次污染等问题。多相芬顿催化氧化是近年来快速发展的一种改进型高级氧化技术,其目的在于克服均相芬顿技术的弊端。多相芬顿催化氧化主要是将自由的金属离子固相化,形成金属、金属氧化物、金属负载型以及金属离子掺杂型固体催化剂[7-11]。相比于传统的均相催化氧化技术,多相芬顿催化技术具有pH响应范围宽,不产生二次污染以及活性组分易分离的优点。但是,固液界面的存在使得多相芬顿催化氧化反应受到催化剂结构形貌、表面性质等自身特性影响,还受到污染物特性,氧化剂种类和浓度等反应条件的影响,其催化机理在当前尚未形成统一的认识,这极大增加了催化剂结构设计以及活性调控的难度[12-13]。这些催化剂的设计与调控大多依赖于催化剂内金属组分高价态与低价态之间的转换。因此,还存在着氧化剂利用率低,中性条件下催化效果不理想以及催化剂稳定性差等不足[14]。
双反应中心(dual-reactor centers,DRCs)催化剂是指通过在催化剂表面进行电子调控,构建具有贫富电子微区的双反应中心(DRCs),使得富电子中心可以为O2,H2O2等氧化剂提供电子,发生还原反应,生成活性氧物种 (包括·OH,O2·−等)。而缺电子中心则可以快速捕获体系中的污染物等作为电子供体,实现污染物多途径降解,并且两个中心之间通过化学键桥实现电子高效转移[15]。利用双反应中心原理构建的催化剂实现了突破了传统芬顿反应利用金属离子高低价态转换来实现H2O2的氧化还原,有效突破了反应的速率限制步骤,提高了H2O2的利用率,也大大提高了催化剂的稳定性[16]。有研究表明,利用阳离子-用键是构建高效双反应中心催化剂关键。比如,通过在CuAlO2催化剂表面嫁接CN(C3N4)有机配体,形成了C-O-Cu键桥,CN的引入一方面减少了CuAlO2的氧空穴的数量,另一方面大大的加速了电子在以C和Cu为贫富中心之间的传递,从而使得催化剂对双酚A的降解效率提高了25倍以上。但嫁接有机配体的方式存在有机配体脱落的风险,如何优化催化剂的合成方法,快速制备高效的双反应中心催化剂具有重要的意义。
之前的研究发现,氧空位的存在可以在一定程度上影响芬顿反应的发生,其对电子的转移以及污染物的捕获都有重要的意义。ZHAN等人将氧化钴掺杂到氧化锌(ZnO)纤锌矿晶格中,成功地在催化剂表面构建了富含未配对电子的氧空位富电子中心和Co(III)贫电子中心,催化效率提高了17倍以上。也有研究表明,铜系类芬顿催化剂在催化芬顿反应时,比铁系类芬顿催化剂具有更高的反应速率和更宽的pH适用范围。这是因为Cu (II) 被过氧化氢催化还原的速率(1.0×104 mol·s−1)要远高于Fe(III)(74 mol·s−1)。但是大部分的铜基的催化剂在污染物或者酸的作用下(0.5~10 mg·L−1),容易发生泄露,高于美国饮用水标准1.3 mg·L−1,从而造成一定的环境影响。因此,如何在保持铜元素高效催化效率的同时,提高铜元素的稳定性对于催化剂的设计和开发就显得尤为重要。
基于此,本文通过水热合成的方法在ZnO纤锌矿晶格中嵌入氧化铜(CuO),从而得到一种全新的双中心反应催化剂。通过XPS,EPR,FTIR等手段对催化剂的表面性质进行表征。并利用水中典型的碘代消毒副产物碘乙腈进行降解研究,重点研究了碘乙腈降解效果,影响因素以及矿化效果,同时根据EPR,XPS的分析结果推测了碘乙腈的降解机制。
-
双氧水(国药集团化学试剂陕西有限公司,H2O2,30%)、氢氧化钠(AR,96%)、碳酸氢钠、磷酸氢二钠合二水(Na2HPO4·2H2O,AR,99.0%~100.5%)、磷酸二氢钾(KH2PO4,GR,99.5%~100.5%)、对苯醌(麦克林,AR,97%)、叔丁醇 (CP,98%) 、无水硫酸钠(AR,99%)、甲基叔丁基醚(沃凯,MTBE)、2.5水合硝酸铜(98.0%~102.0%,Cu(NO3)2·2.5H2O,Alfa Aesar)、六水合硝酸锌(Zn(NO3)2·6H2O,98%,Alfa Aesar)、超纯水、氨水(AR,25%~28%)、碘乙腈(麦克林,98%,IAN),分子式C2H2IN。所有化学试剂至少是分析纯,实验用水为超纯水。
-
按照设定的比例称取一定量的六水合硝酸锌和三水合硝酸铜溶于200 mL超纯水中,搅拌30 min,向其中加入氢氧化钠溶液,用氨水调节pH至碱性,150 ℃下反应8 h。反应完成后,将产物过滤并清洗3遍,随后将所得固体烘干后置于马弗炉中550 ℃条件下煅烧,制得Cu-ZnO催化剂。
-
采用透射电子显微镜(TEM,日本JEOL 公司JEM-2 100 型)观察催化剂的微观结构特征及构造;采用X-射线粉末衍射仪(XRD,Philips PW3 040 /60 型)来观察催化剂的晶型和物相;采用X 射线光电子能谱(XPS,Thermo Scientific K-Alpha+)对金属元素价态和表面元素组成和含量信息进行收集,X射线源:单色化AlKa源(Mono AlKa)能量:1 486.6 eV,电压:15 kV,束流:15 mA,分析器扫描模式:CAE。采用傅立叶变换红外光谱仪(BRUKER VERTEX70型)来分析制备的催化剂表面官能团。通过电子顺磁共振波谱仪(德国布鲁克公司ESP300E型)测试固体催化剂的EPR信号。
-
取一定量的催化剂加入到预先配好的碘乙腈溶液中,充分搅拌后,立即加入过氧化氢溶液,反应时间为240 min。分别在0、10、30、60、120、240 min取样,并过0.22 μm水系滤膜过滤。碘乙腈溶液初始浓度为Co,反应后浓度为Ce,用Ce/Co表示碘乙腈的降解程度。过滤后的样品加入3 g无水硫酸钠,拧紧瓶盖后振荡,直至无水硫酸钠基本溶解,继而用移液管加入2 mL MTBE,拧紧瓶盖后,振荡1 min、静止5 min后,吸取上层有机液,装入2 mL的进样瓶,使用气相色谱-质谱联用仪GC-MS(岛津,GC2 010-TQ8040)分析。
-
IAN的浓度通过GC-MS分析测定。色谱柱为SH-Rxi-5Sil MS(L:30 m,ID:0.25 mm,DF:0.25 μm);GC参数:柱箱及进样口温度分别是40 ℃和260 ℃,不分流进样,进样量1 µL,载气为氦气;升温程序为:40 ℃保持3 min,随后以20 ℃·min−1的升温速率升至100 ℃,最后以40 ℃·min−1的升温速率升至240 ℃保持3 min,总程序时间为12.50 min,离子源及接口温度分别是220 ℃和260 ℃;采用外标法进行定量分析,得到标准曲线相关系数大于0.99;在此方法下:IAN的保留时间为5.860 min,目标离子m/z为167,参考离子m/z分别为40和127。I−、IO3−采用离子色谱1100分析;色谱柱型号IC(AS-16,150 mm×4 mm)。TOC测试采用过总有机碳分析仪测定。Zn和Cu离子采用离子色谱质谱联用仪(ICP-MS)测定。
-
Cu-ZnO的透射电镜图和元素能谱图如图1所示。由图1(a)~(b)可以看出,Cu-ZnO催化剂为纳米棒状结构。由图1(c)~(d)中可以看到明显的晶格条纹,说明该催化剂具备铜掺杂的氧化锌晶型。由图1(e)~(h)可以发现,催化剂主要元素为锌、铜和氧元素,并且铜元素均匀的分布在氧化锌纳米棒上。这说明铜和锌形成了较好的键联。由于锌的电负性为1.65 eV,铜的电负性为1.9 eV。因此,在催化剂的表面可能形成铜富电子中心和锌贫电子中心[17]。两者通过氧原子进行连接,并传递电子,捕获周围的污染物或者氧化剂发生氧化还原反应。
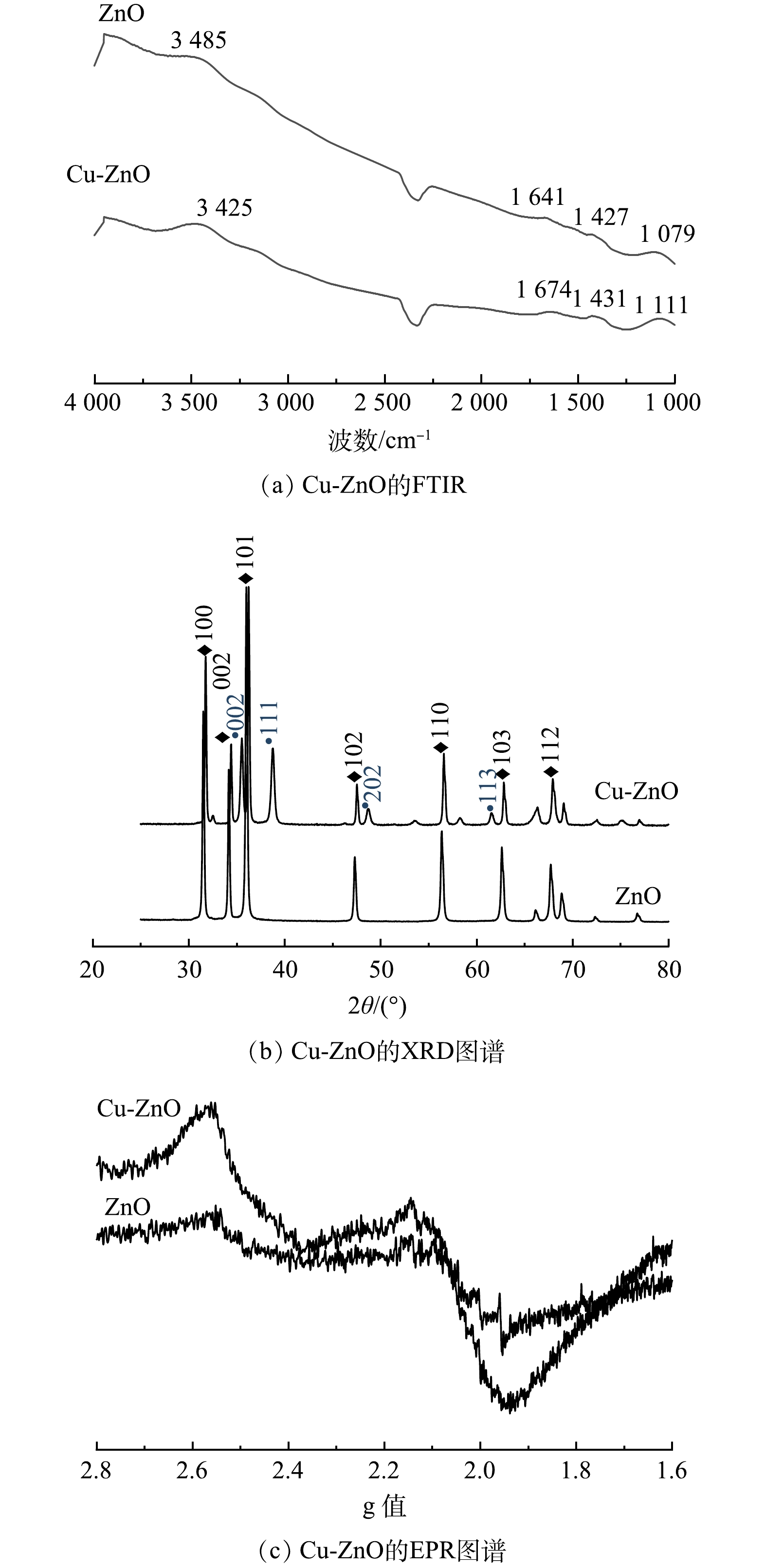
图2为催化剂的FT-IR、XRD以及EPR图谱。由图2(a)可以看出,ZnO和Cu-ZnO表面均存在羟基官能团。对于ZnO来说,3 485 cm−1和1 427 cm−1分别归属于催化剂表面羟基的伸缩振动峰和面内振动峰。而Cu-ZnO的特征峰有明显的红移,羟基的伸缩振动峰和面内振动峰分别出现在3 525 cm−1和1 431 cm−1。这说明在Cu-ZnO中,由于铜元素的加入,影响了氧化锌表面的电荷分布,从而影响了催化剂表面附着羟基的振动[18]。XRD图谱(图2(b))中2θ为31.73º、36.20º、56.52º分别对应ZnO的(100)、(101)以及(110)晶面。2θ为35.47º、38.74º、48.82º分别对应CuO的(002)、(111)以及(202)晶面。这说明合成的催化剂基本保留了氧化锌六方纤锌矿的晶型结构。由于铜离子和锌离子的原子半径接近,因此,铜离子可以很好地掺杂到氧化锌的晶型结构中[19]。如图2(c)所示,在引入Cu之后,EPR的自由电子信号显著增强,说明催化剂表面电子发生重排,出现极化性的电子高低密度区,有利于后续反应中与环境介质交换电子,发生氧化还原反应[20]。
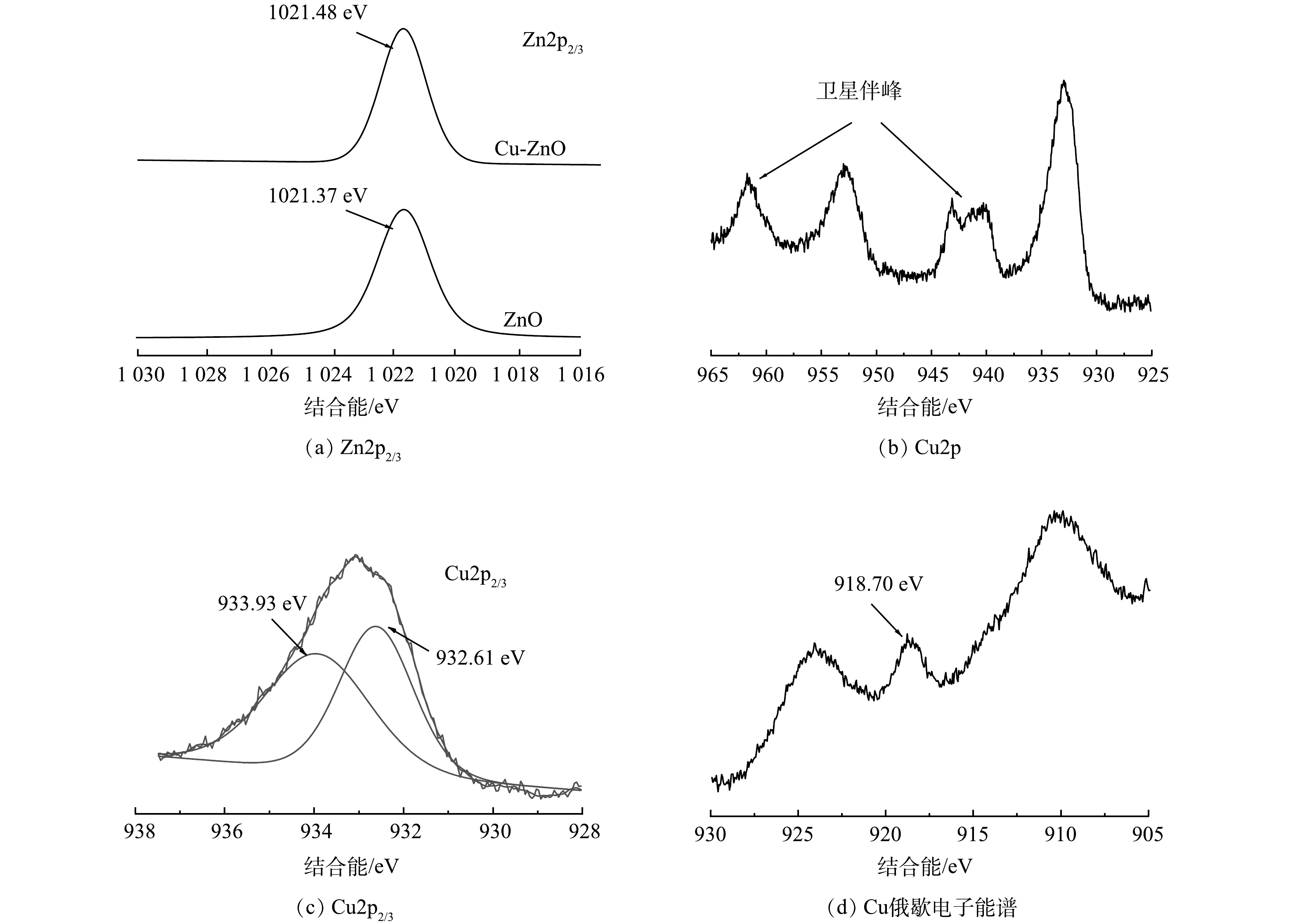
Cu-ZnO催化剂表面Cu和Zn的XPS表征结果如图3(a)所示。可见,1021.48 eV对应的是Zn的2p3/2光电子峰,相比于纯的ZnO对比,Zn的电子结合能增加了0.11 eV。这是因为铜原子的电负性要比锌原子大,铜加入后Zn有给电子的倾向,从而使得锌对于剩余电子束缚能力增强,结合能增大。如图3(b)所示,Cu-ZnO催化剂中铜元素的光电子能谱在942~945 eV和962 eV附近有2个较强的卫星峰,说明Cu主要以Cu(II)形态存在[21]。使用Avantage软件对Cu2p2/3图谱进行分峰处理(图3(c))。结果表明,可能存在2种形态,932.61 eV对应的是Cu0或者Cu+,933.91 eV对应的是Cu2+。通过对Cu LMM俄歇电子能谱的分析得知(图3(d)),Cu的峰值在918.70 eV左右,归属于Cu0[22]。
-
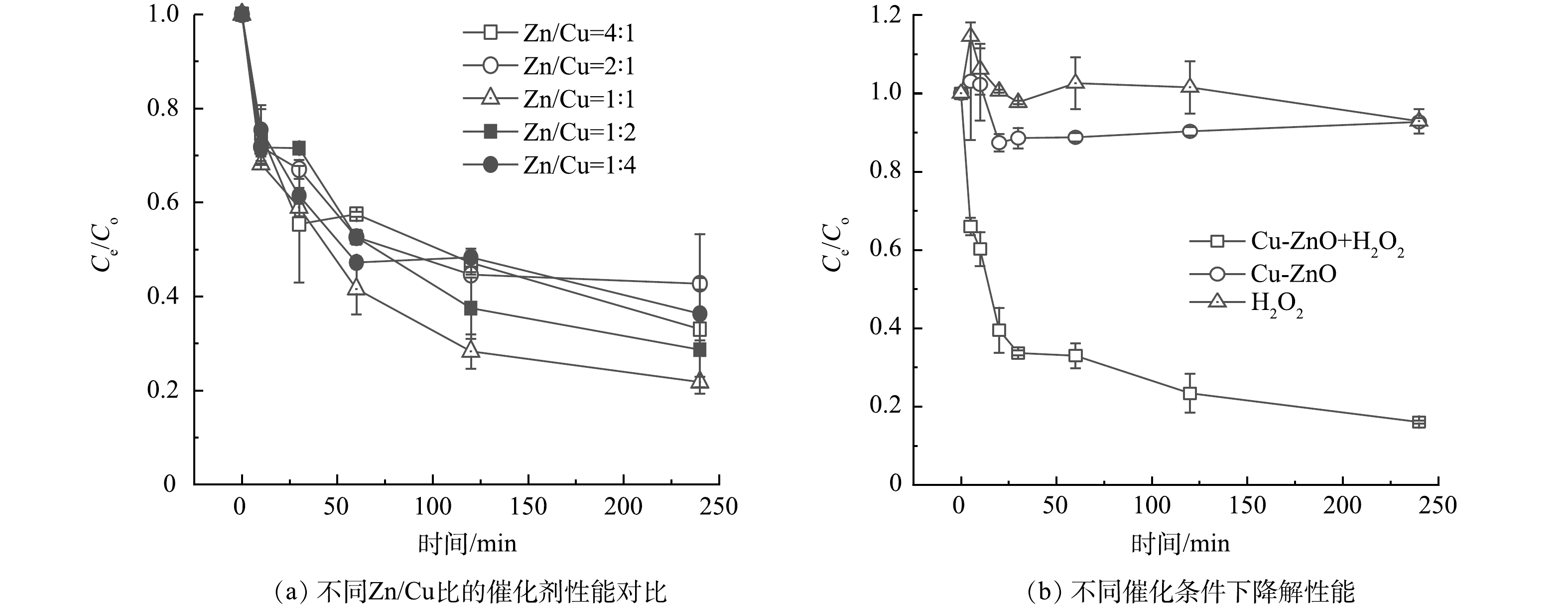
为了研究不同Cu/Zn配比条件下催化剂的降解效果,本研究合成了Zn/Cu原子比例为4∶1、2∶1、1∶1、1∶2、1∶4的5种催化剂,结果如图4(a)所示。所有的催化剂都表现出了对碘乙腈良好的降解效果,当Cu/Zn为1∶1的时候,降解效果最佳。图4(b)反映了在单独双氧水(10mmol·L−1),单独Cu-ZnO催化剂(1 g·L−1)以及催化剂+双氧水(1 g·L−1+10 mmol·L−1)3个条件下碘乙腈的降解效果。单独双氧水和单独催化剂对碘乙腈的降解效果有限,去除率小于20%。在催化剂投加量为1 g·L−1,H2O2投加量为10 mmol·L−1时,碘乙腈的去除率为84%。这说明合成的Cu-ZnO催化剂可能诱导H2O2产生·OH、O2·−等活性物质,从而降解水中的污染物。
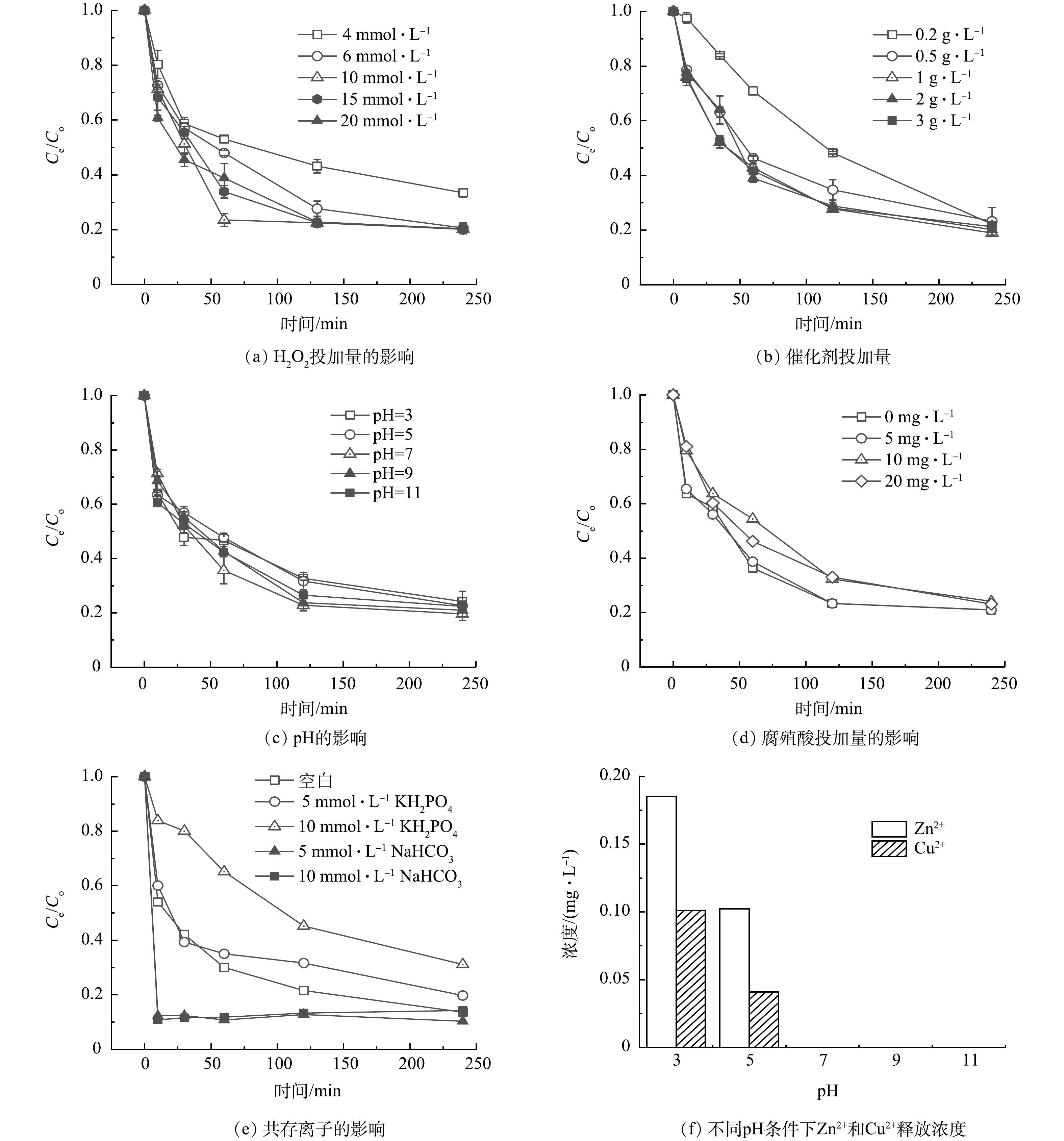
为了优化合成的Cu-ZnO催化剂降解碘乙腈的反应条件,研究了不同H2O2投加量,催化剂投加量,pH对碘乙腈降解效果得影响,同时研究了环境中的腐殖酸和共存离子的影响,实验结果如图5(a)~(e)所示。可以看出,随着双氧水投加量的增加,碘乙腈的降解速率有明显的提升,但是当双氧水的浓度增加到6 mmol·L−1以上时,碘乙腈的去除率达到82%,并且不再上升。同时,当催化剂的浓度为10 mmol·L−1时,再次提高双氧水的浓度对碘乙腈的降解率和速率不再提升(图5(a))。图5(b)反映了催化剂投加量对碘乙腈去除效果的影响。可见,随着催化剂的投加量的增加,碘乙腈的去除率和降解速率也有所增加。当催化剂的投加量达到1 g·L−1时,碘乙腈去除率和降解速率增加不再明显。因此,本次研究确定双氧水和催化剂的最佳投加量分别为10 mmol·L−1和1 g·L−1。图5(c)反映了不同pH条件下,催化剂对碘乙腈的降解效果,与大部分常规多相芬顿催化剂反应不同的是,在本次研究中当溶液的pH为酸性时(pH=3和5),碘乙腈的去除率较pH为中性或者碱性的时候低。一般地认为,在pH为酸性时,更有利于H2O2的还原和·OH的生成,从而更有利于污染物的降解[23]。而本次合成的Cu-ZnO双反应中心催化剂利用铜和锌之间电负性的差异,在催化剂内部构建了多个具有正负极的微型原电池,从而实现了电子的快速转移。相比于传统的均相芬顿或者多相芬顿技术,基于双反应中心催化理论设计的类芬顿催化剂本身对pH的依赖性较小。另一方面,对于污染物碘乙腈来说,在酸性条件下具有较好的稳定性。当溶液pH上升,碘乙腈也更容易水解,在一定程度上促进了碘乙腈的降解[24]。
图5(d)和图5(e)反映了腐殖酸(HA)、磷酸根(PO43-)以及碳酸氢根(HCO3−)对碘乙腈去除率的影响。由图5(d)可知,当HA的浓度小于5 mmol·L−1时,碘乙腈的去除率不受影响。当HA的浓度高于10 mmol·L−1时,碘乙腈的去除率降低。这可能是由于腐殖酸会吸附在催化剂的表面,从而减少了污染物和催化剂表面的接触。从图5(e)可以看出,磷酸根离子对碘乙腈的降解有抑制作用,而碳酸氢根对于碘乙腈的降解有促进的作用。当反应体系中磷酸根的浓度分别为5 mmol·L−1和10 mmol·L−1时,碘乙腈的去除率下降了分别为6.19%和18.11%。而当溶液中有碳酸氢根存在时,碘乙腈的降解速率大大加快了,10 min内碘乙腈的去除率达到了80%。有研究表明,HCO3−由于对·OH有淬灭作用,因此,对大多数的多相芬顿催化剂有抑制作用。但在本次研究中,碳酸氢根对碘乙腈的降解基本没有抑制作用,反而有促进作用。这可能是由于碳酸氢根可以吸附到催化剂的表面,从而为电子的转移起到架桥的作用,从而加快了电子的转移速率,具体的理论解释需要进一步的实验研究证明。
图5(f)反映了在不同pH条件下催化剂的Zn2+和Cu2+的释放情况。结果表明,在pH为酸性的时候,催化剂会释放出少量的Zn2+和Cu2+,其质量浓度小于0.2 mg·L−1,小于《城镇污水处理厂污染物排放标准》(GB 18918-2002)》的规定值(Zn≤1.0 mg·L−1,Cu≤0.5 mg·L−1)。当pH为中性时,几乎观察不到溶液当中的Zn和Cu。这表明催化剂具有良好的化学稳定性和较为广阔的应用前景。
-
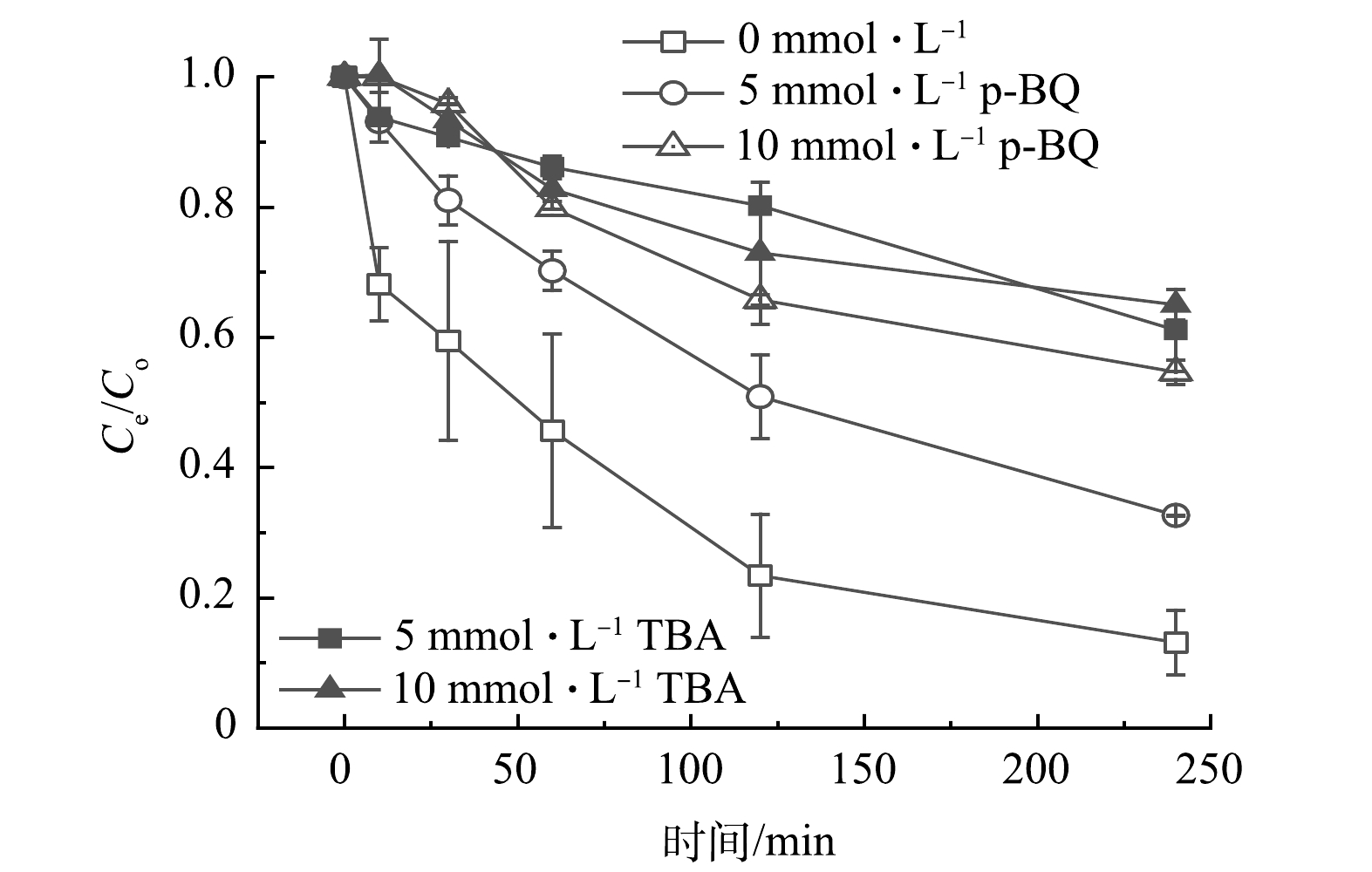
为深入探究自由基的产生类型以及对碘乙腈降解效果的影响,本次研究采用了叔丁醇(TBA)和对苯醌(p-BQ)2种淬灭剂分别对体系中可能产生的·OH和O2·−进行淬灭,结果如图6所示。2种淬灭剂对碘乙腈的降解有明显的抑制作用,尤其是叔丁醇。当叔丁醇的投加量为5 mmol·L−1时,碘乙腈的去除率降低了64%,再次提高叔丁醇的投加量,对碘乙腈降解的抑制作用不再增强。而p-BQ的投加量为5 mmol·L−1和10 mmol·L−1时,碘乙腈的去除率由91%下降到33%和22%。这说明·OH和O2·−都对碘乙腈的降解有重要的作用,并且·OH的影响更大。
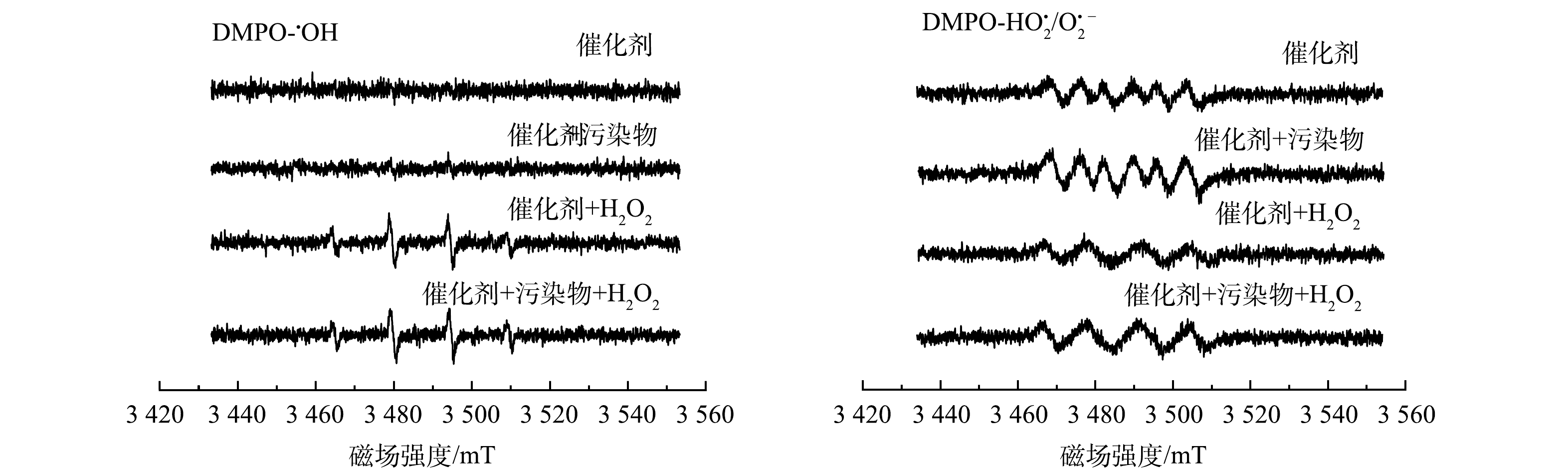
为进一步探究自由基的产生机理,采用ESR技术考察了在不同条件下HO2/O2·−的生成情况,结果如图7(a)和图7(b)所示。可以发现,在只有催化剂的存在的情况下,在体系中可以捕捉到HO2/O2·−的信号,但没有检测到·OH。这是因为催化剂本身富电子区就能活化氧气产生HO2/O2·−。在加入污染物后,可以看到·OH的信号明显增强。这说明污染物加入后,可以作为催化剂表明双中心反应的电子供体,提高了催化剂内部的电子传递速率,从而促进·OH的产生[24]。产生的·OH可以进一步对碘乙腈进行氧化,提高体系的催化效率[16]。
图8反映了催化反应过程中I-和IO3−的生成量变化趋势。由图8(a)可知,随着IAN的降解,反应体系中I-和IO3−的质量浓度逐步上升。有研究表明,当水中的碘化物降解时,会释放出I-或者含碘的降解产物。当体系中有氧化剂存在时,碘离子可以被氧化生成氢碘酸 (HOI)。氧化生成的HOI在水中不稳定,会继续发生以下2种路径:一方面会继续发生歧化反应生成碘离子和碘酸根,生成的碘酸根离子是无毒的,且化学性质稳定,对环境的影响较小,被认为是水中碘元素演变的理想归宿;另一方面,当水中有天然有机物(NOM)存在时,HOI可以继续生成含碘的副产物,这也是水中含碘消毒副产物的主要来源[25]。在本研究中发现,虽然随着IAN的降解I-和IO3−的质量浓度都在上升,但生成的I-和IO3−的数量要小于IAN的降解量。这说明虽然在碘乙腈降解的过程中,碘乙腈并没有完全矿化,还生成了其他含碘的降解产物。为了更深入研究IAN矿化程度,本研究检测了在降解过程中TOC的变化规律,实验结果如图8(b)所示。理论计算的TOC浓度(TOC理论)根据反应后体系中剩余的IAN的浓度理论计算而来,计算公式如式(1)所示。TOC实测为体系中实测的TOC浓度。结果表明,随着IAN的不断降解,反应后体系中TOC的浓度也在逐渐降低,这说明有部分的IAN已经被完全矿化。同时,本研究也观察到实测的TOC浓度要大于体系中IAN所产生的TOC浓度。这说明,被降解的IAN并没有全部矿化生成二氧化碳和水,仍然存在部分有机中间产物。这部分中间产物可能包括碘乙酰胺,碘乙酸以及含碘甲烷等[26]。
式中:C理论为理论计算的TOC的浓度,mg·L−1,Ce为反应后碘乙腈的浓度,mg·L−1,M1为碳元素的摩尔质量,g·mol−1,M2为碘乙腈的摩尔质量,g·mol−1。
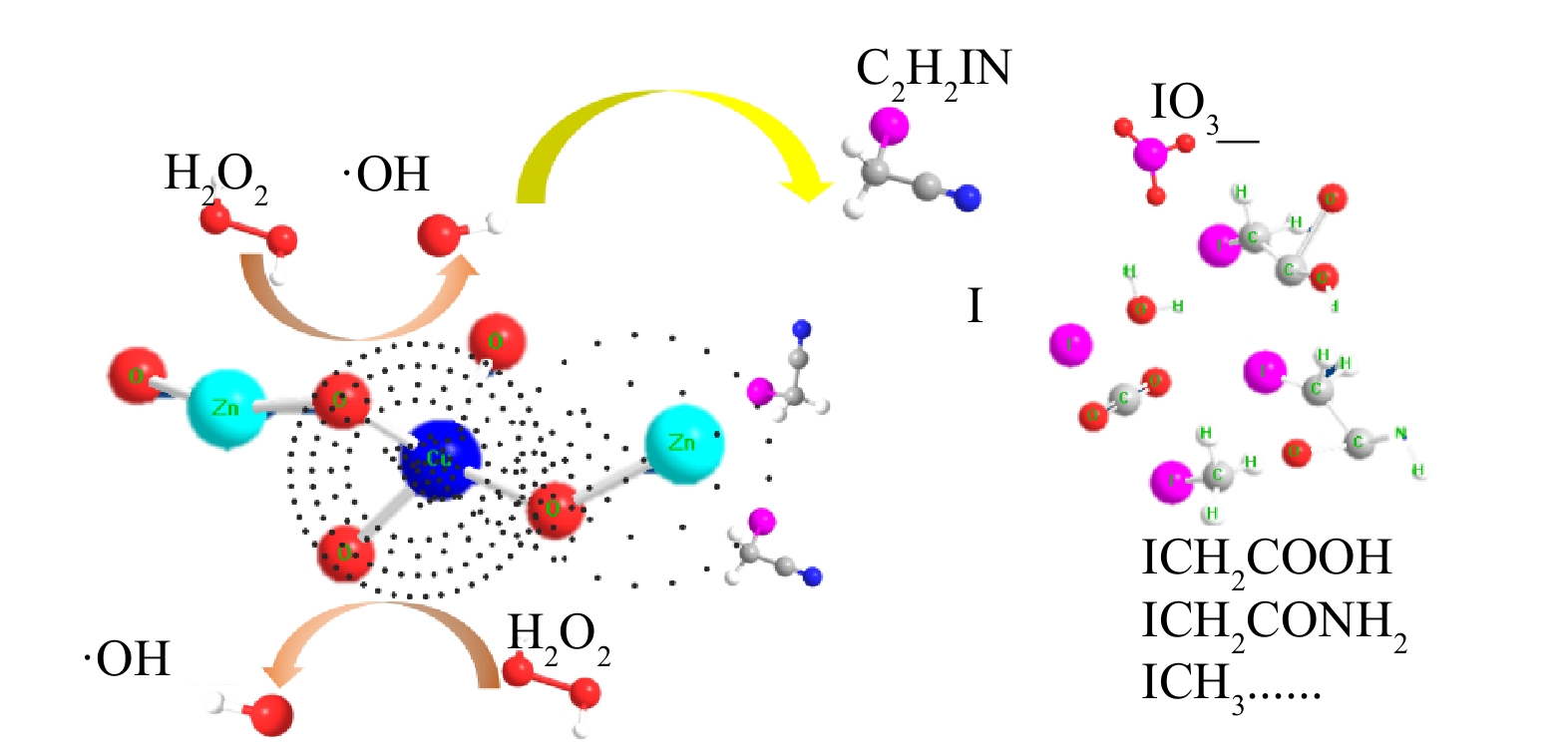
综上所述,推断了采用Cu-ZnO降解碘乙腈的双中心反应机制(图9)。首先,由于在催化剂内部锌和铜的电负性差异,形成了以铜富电子中心和以锌贫电子中心,2个中心通过氧原子键联,电子经Cu-O-Zn键桥传递。在H2O2存在的条件下,H2O2分子在富电子中心得电子还原产生·OH,从而氧化降解水中的碘乙腈。同时,碘乙腈可以作为电子供体围绕在贫电子锌中心周围,一方面,可以为催化反应提供电子从而自身发生氧化降解;另一方面,也在一定程度上阻隔H2O2的无效分解。多数碘乙腈在·OH的作用下矿化成CO2和H2O,碘离子最终降解成I-和IO3-;部分未能被矿化的碘乙腈可能生成碘乙酸、碘乙酰胺以及碘仿等副产物。
-
1)通过水热合成的Cu-ZnO双反应中心纳米催化剂成功的将Cu掺杂到ZnO的晶格中,其具有良好的催化活性,能够有效地去除水中的碘乙腈。
2)当Cu-ZnO投加量为1 g·L−1,双氧水投加量为10 mmol·L−1时,Cu-ZnO对碘乙腈的去除率可达91%以上。
3) pH和低浓度的HA对催化降解效果影响较小,高浓度的HA和磷酸氢根有抑制作用,碳酸根浓度大于0.3 mmol·L−1时对碘乙腈的去除速率和去除率均有促进作用。
4)·OH和HO2/O2·−是降解碘乙腈主要的活性物种,降解产物包括I-、IO3−、CO2、水等产物。
Cu-ZnO双反应中心类芬顿催化剂降解水中碘乙腈性能与机制
Catalytic oxidation of iodoacetonitrile by Cu-ZnO dual-reaction center catalyst in water and its mechanism
-
摘要: 多相芬顿催化虽然克服了均相芬顿pH适用范围窄,铁泥二次污染等问题,但还面临着稳定性差,H2O2利用率低的问题,从而限制了其实际应用。本文利用锌和铜的电负性差异制备了一种双反应中心催化剂Cu-ZnO,通过在催化剂表面构建贫富电子中心,从而诱导H2O2产生·OH降解污染物。采用TEM、EDS、XRD、FTIR、XPS以及EPR对催化剂进行表征,并研究了催化剂投加量、H2O2投加量、pH以及共存离子对碘乙腈降解效果的影响。结果表明,在催化剂投加量为1 g·L-1,双氧水投加量为10 mmol·L-1时,Cu-ZnO对碘乙腈的去除率在91%。大部分共存离子对催化剂的降解效果影响较小,且在酸性条件下催化剂稳定性较好,中性和碱性条件下金属离子基本无释放。催化剂诱导产生的•OH和HO2•/O2•−是碘乙腈(IAN)降解的主要活性物种,降解产物包括CO2,H2O,I-和IO3- 等。Abstract: The practical application of heterogeneous Fenton catalysis was limited by the instability and inefficiency of H2O2, even though which overcomes the problems such as narrow pH range and secondary pollution of iron sludge compared to homogeneous Fenton. In this study, a dual-reaction center catalyst Cu-ZnO was developed by taking advantage of the electronegativity difference between zinc and copper, and the rich and poor electron centers were constructed on the surface of the catalyst which could induce H2O2 and produce •OH for pollutants degradation. The catalysts were characterized by TEM, EDS, XRD, FTIR, XPS and EPR, and the effects of dosage of catalyst and H2O2, pH and coexisting ions on the degradation of iodoacetonitrile(IAN) were investigated. The results showed that 91% IAN was removed when the dosages of catalyst and H2O2 were 1 g·L-1 and 10 mM, respectively. Most coexisting ions had slight effects on IAN degradation by Cu-ZnO which showed a better stability under acidic conditions, and no metal ions released at neutral and basic pHs. •OH and HO2•/O2•− induced by catalyst are the main active species for IAN degradation, the degradation products included CO2, H2O, I-, IO3- and other organic products.
-
Key words:
- heterogeneous Fenton /
- dual-reaction center /
- iodoacetonitrile /
- H2O2
-

-
-
[1] LI J, JIANG J, PANG S Y, et al. Oxidation of iodide and hypoiodous acid by non-chlorinated water treatment oxidants and formation of iodinated organic compounds: A review [J]. Chemical Engineering Journal, 2020, 386. [2] LIU S, LI Z, DONG H, et al. Formation of iodo-trihalomethanes, iodo-acetic acids, and iodo-acetamides during chloramination of iodide-containing waters: Factors influencing formation and reaction pathways[J]. Journal of Hazardous Materials, 2017, 321: 28-36. doi: 10.1016/j.jhazmat.2016.08.071 [3] SHIN J, LEE Y, VON GUNTEN U. Kinetics of the reaction between hydrogen peroxide and aqueous iodine: Implications for technical and natural aquatic systems[J]. Water Research, 2020, 179: 115852. doi: 10.1016/j.watres.2020.115852 [4] 钱永, 张兆吉, 费宇红, 等. 华北平原饮用地下水碘分布及碘盐分区供应探讨[J]. 生态与农村环境学报, 2014, 30(1): 9-14. doi: 10.3969/j.issn.1673-4831.2014.01.002 [5] 于志恒, 陈崇义, 谭凤珠. 中国高碘地方性甲状腺肿的发现历程和分布概况[J]. 中华预防医学杂志, . 2001, (5): 64-5. [6] 张杰. 碘代消毒副产物生成机理和预测模型的研究[D]. 合肥: 中国科学技术大学, 2018. [7] 宋思扬, 吴丹, 赵焕新, 等. Co-FeOOH/g-C3N4的制备及其在非均相光芬顿反应中的催化性能[J]. 环境工程学报, 2020, 14(12): 3262-3269. doi: 10.12030/j.cjee.201912147 [8] BARRETO-RODRIGUES M, SILVA F T, PAIVA T C B. Optimization of Brazilian TNT industry wastewater treatment using combined zero-valent iron and fenton processes[J]. Journal of Hazardous Materials, 2009, 168(2): 1065-1069. [9] YANG J, ZENG D, ZHANG Q, et al. Single Mn atom anchored on N-doped porous carbon as highly efficient Fenton-like catalyst for the degradation of organic contaminants[J]. Applied Catalysis B: Environmental, 2020, 279. [10] GHANBARLOU H, NASERNEJAD B, NIKBAKHT FINI M, et al. Synthesis of an iron-graphene based particle electrode for pesticide removal in three-dimensional heterogeneous electro-Fenton water treatment system[J]. Chemical Engineering Journal, 2020, 395: 12505-12519. [11] 李孟宣, 盛光遥, 何岸飞. 金属催化剂在宽泛pH进行Fenton反应的进展[J]. 工业水处理, 2021, 41(2): 20-25. [12] 白青青, 吴小宁, 王倩, 等. Fenton反应中拓展pH的研究进展[J]. 化学通报, 2018, 81(3): 217-222. doi: 10.14159/j.cnki.0441-3776.2018.03.004 [13] 吕来, 胡春. 多相芬顿催化水处理技术与原理[J]. 化学进展, 2017, 29(9): 981-999. [14] ZHANG H, ZHOU C, ZENG H, et al. Can Cu2ZnSnS4 nanoparticles be used as heterogeneous catalysts for sulfadiazine degradation?[J]. Journal of Hazardous Materials, 2020, 395: 122613. doi: 10.1016/j.jhazmat.2020.122613 [15] 张帆, 宋阳, 胡春, 等. 铁钛共掺杂氧化铝诱发表面双反应中心催化臭氧化去除水中污染物[J]. 环境科学, 2021, 42(5): 2360-2369. doi: 10.13227/j.hjkx.202009099 [16] LYU L, YAN D, YU G, et al. Efficient destruction of pollutants in water by a dual-reaction-center Fenton-like process over carbon nitride compounds-complexed Cu(II)-CuAlO2[J]. Environmental Science & Technology, 2018, 52(7): 4294-4304. [17] LYU L, ZHANG L, HU C. Galvanic-like cells produced by negative charge nonuniformity of lattice oxygen on d-TiCuAl–SiO2 nanospheres for enhancement of Fenton-catalytic efficiency[J]. Environmental Science:Nano, 2016, 3(6): 1483-1492. doi: 10.1039/C6EN00290K [18] HUANG R, GU X, SUN W, et al. In situ synthesis of Cu+ self-doped CuWO4/g-C3N4 heterogeneous Fenton-like catalysts: The key role of Cu+ in enhancing catalytic performance[J]. Separation and Purification Technology, 2020, 250: 117174-117183. doi: 10.1016/j.seppur.2020.117174 [19] BABU B, ASWANI T, RAO G T, et al. Room temperature ferromagnetism and optical properties of Cu2+ doped ZnO nanopowder by ultrasound assisted solid state reaction technique[J]. Journal of Magnetism and Magnetic Materials, 2014, 355: 76-80. doi: 10.1016/j.jmmm.2013.11.038 [20] 陈鸿博, 于腊佳, 袁友珠, 等. 与甲醇合成铜基催化剂研究有关的ZnO和ZnO-Al2O3体系的ESR及XPS表征[J]. 分子催化, 1994, 8(1): 58-62. [21] LIU Y, TAN N, GUO J, et al. Catalytic activation of O2 by Al(0)-CNTs-Cu2O composite for Fenton-like degradation of sulfamerazine antibiotic at wide pH range[J]. Journal of Hazardous Materials, 2020, 396: 122751. doi: 10.1016/j.jhazmat.2020.122751 [22] LI H, CHENG R, LIU Z, et al. Waste control by waste: Fenton-like oxidation of phenol over Cu modified ZSM-5 from coal gangue[J]. Science of the Total Environment, 2019, 683: 638-647. doi: 10.1016/j.scitotenv.2019.05.242 [23] HUANG X, ZHU N, MAO F, et al. Enhanced heterogeneous photo-Fenton catalytic degradation of tetracycline over yCeO2/Fh composites: Performance, degradation pathways, Fe2+ regeneration and mechanism[J]. Chemical Engineering Journal, 2020, 392: 123636-123649. doi: 10.1016/j.cej.2019.123636 [24] WANG L, YAN D, LYU L, et al. Notable light-free catalytic activity for pollutant destruction over flower-like BiOI microspheres by a dual-reaction-center Fenton-like process[J]. Journal of Colloid and Interface Science, 2018, 527: 251-259. doi: 10.1016/j.jcis.2018.05.055 [25] DONG H, QIANG Z, RICHARDSON S D. Formation of iodinated disinfection byproducts (I-DBPs) in drinking Water: Emerging concerns and current issues[J]. Accounts of Chemical Research, 2019, 52(4): 896-905. doi: 10.1021/acs.accounts.8b00641 [26] DUIRK S E, LINDELL C, CORNELISON C C, et al. Formation of toxic iodinated disinfection by-products from compounds used in medical imaging[J]. Environmental Science & Technology, 2011, 45(16): 6845-6854. -




 下载:
下载: