-
生物发酵法生产长链二元酸(C10~C18)是石化领域的新工艺。中国是全球最大的生物发酵法长链二元酸生产基地,投产和在建的二元酸装置超过30套[1]。二元酸生产废水是新型石化工艺伴生高浓度点源废水的代表。二元酸生产废水中残留硫酸、二元酸盐以及培养基成分,表现出较强酸性,含有高浓度的总磷、硫酸盐和COD,对综合污水厂进水总磷、COD负荷冲击极大,无法满足达标排放要求。另外,高浓度的硫酸盐在厌氧处理单元会生成H2S,建有二元酸装置的石化企业都承受着巨大的综合污染负荷冲击[2-3]。因此,采用简单高效的预处理手段,大幅度削减二元酸生产废水的总磷、硫酸盐和COD负荷,从而保障综合污水厂安全运行和稳定达标,是石化企业工艺路线升级发展过程中亟待解决的问题。
已有研究的二元酸生产废水处理技术多以高COD为主要去除目标[3-6]。“中和沉淀+序批式活性污泥法(sequencing batch reactor, SBR)”工艺对COD和BOD5去除率可达90%,但高硫酸盐导致厌氧处理过程H2S溢出严重[3]。全好氧工艺可避免厌氧环境下的H2S产生与溢出难题,通过添加脂肪酶来促进生物降解,可将COD去除率稳定在95%[4]。但是生物处理技术对高浓度总磷和硫酸盐的去除基本无效。采用高级氧化或生物-高级氧化组合工艺虽然可以进一步提高COD去除率,但对总磷和硫酸盐的去除效果甚微[5-6]。二元酸生产废水预处理的关键是大幅度削减硫酸盐和总磷负荷,化学沉淀法是快速高效、操作简便且容易实现工程化的预处理手段[7-8]。钡盐被广泛用于化学沉淀法中以去除硫酸盐,但药剂成本高,难以大规模应用,而且对总磷的去除率低。铁盐和铝盐是常用的除磷剂,对低浓度总磷的去除更有效,但对高浓度总磷和硫酸盐的去除效果却很有限[9-11]。钙盐更适合于高浓度总磷的去除[12],可应用于二元酸生产废水的预处理,通过生成磷酸钙(Ca3(PO4)2)、羟基磷酸钙(Ca10(PO4)6(OH)2)等沉淀去除高浓度总磷,并在静电吸附作用下去除有机酸阴离子,对总磷和COD的去除率分别为97%和41%。由于受限于硫酸钙的高溶解度(pKsp=3.70),因此,钙盐对高浓度硫酸盐的去除效果并不理想[2]。
钙矾石(分子式Ca6Al2(SO4)3(OH)12·26H2O)简称AFt,是Al3+、Ca2+与SO42−结合形成的一种不溶性针状晶体(pKsp=43.13)。钙矾石沉淀法不仅可以高效去除硫酸盐,而且游离Ca2+能与磷酸盐形成羟基磷灰石沉淀,实现对硫酸盐和总磷的同步脱除[12-13]。以水合铝盐及石灰乳为脱除剂,对模拟工业废水中高浓度的硫酸盐(1 720 mg·L−1)进行去除,去除率达到94%[14]。以石灰乳和氯化铝为脱除剂,在pH为11,SO42−和Al3+摩尔比为1.7时,对煤矿酸性矿井水中高浓度硫酸盐(1 804 mg·L−1)进行去除,去除率高达95%[15]。以水合氯化铝及氧化钙为脱除剂,对烟气脱硫废水中高浓度的硫酸盐(2 086 mg·L−1)进行去除,去除率高达92%,沉淀产物为针状纳米钙矾石结晶[16]。钙矾石法同步去除真实工业废水中高浓度总磷、硫酸盐和有机污染负荷的研究鲜有报道。
本研究利用钙矾石沉淀法同步去除二元酸废水中高浓度总磷、硫酸盐和有机污染负荷,设计正交实验并确定最优工艺参数条件,探索预处理过程中多污染物同步去除机理,旨在解决已投产二元酸装置石化企业的污水处理问题,实现对高浓度点源二元酸废水的源头控制,以期为投产二元酸装置石化企业或化工园区的污水处理系统安全运行和稳定达标提供技术支撑。
-
水样取自华东某石化企业新建1 000 t·a−1 二元酸生产装置集水池。该废水的pH为4.5,总磷为272 mg·L−1,硫酸盐为10 360 mg·L−1,COD为6 201 mg·L−1,总溶解性有机碳(dissolved organic carbon,DOC)为2 441 mg·L−1,BOD5为3 485 mg·L−1。
实验药剂包括氢氧化钠(NaOH)、盐酸(HCl)、聚合氯化铝(PAC,碱化度80%,有效铝含量28%)、氯化钙(CaCl2)、氢氧化钙(Ca(OH)2)等,均为工业级。
-
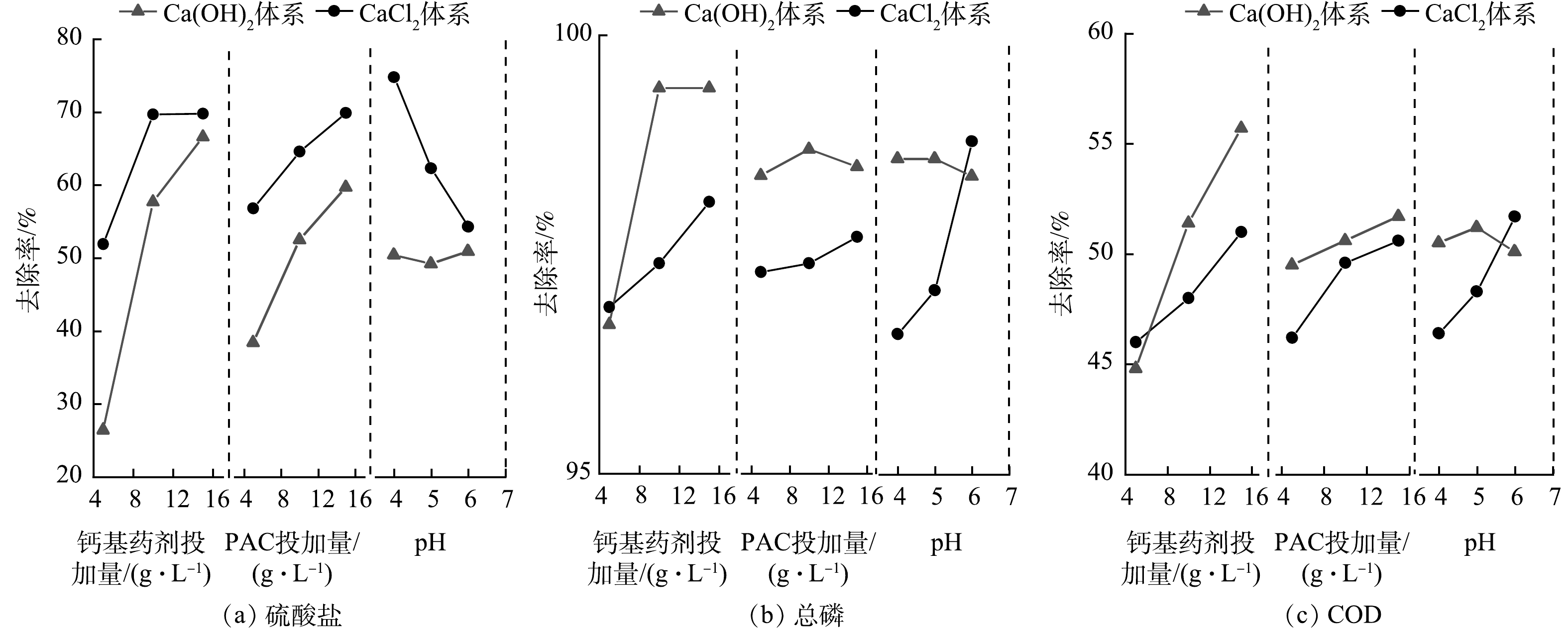
钙矾石沉淀法的处理效率与钙剂、铝剂的投加量和反应体系的pH 3个因素密切相关。因此,设计3因素3水平L9(33)正交实验(表1和表2),考察CaCl2或Ca(OH)2投加量、PAC投加量及反应体系初始pH这3个因素在不同水平下对总磷、硫酸盐和COD去除效率的影响。通过前期预实验,设置了各因素水平的范围及增量(表1)[17],因素A、B、C分别表示Ca(OH)2或CaCl2投加量(A1和A2分别代表Ca(OH)2和CaCl2)、PAC投加量和反应体系初始pH,水平1、2、3对应不同的投加量和反应体系pH。
取若干份100 mL水样于250 mL烧杯中,投加一定量的钙剂和铝剂,利用NaOH(1 mol·L−1)和HCl(1 mol·L−1)调节反应体系初始pH,先以300 r·min−1快速搅拌30 s,再以100 r·min−1慢速搅拌5 min,静置20 min后取上清液过滤并测量总磷、硫酸盐及COD。
利用极差分析确定钙矾石沉淀法的最佳工艺参数条件,讨论各因素对总磷、硫酸盐以及COD去除率影响的大小和主次。极差越大,表示其对应的因素水平变化对实验结果的影响越大。
-
称取105 ℃下烘干研磨后的沉淀副产物1.0 g若干份,置于150 mL烧杯中,加入100 mL纯水,利用NaOH和HCl调节溶液pH,pH梯度设置为2、3、4、5、6、7、8、9、10、11、12、13,以100 r·min−1慢速搅拌1、2和4 h。测试在不同pH条件下,不同浸润时间沉淀副产物PO43−溶出浓度。
-
水质指标分析可以表征水质污染程度、评价预处理效果。水质指标包括pH、总磷、硫酸盐、COD、DOC、BOD5和总溶解固体(total dissolved solids,TDS)。pH采用梅特勒-托利多FE28型pH仪测定。总磷测定参照《水质 总磷的测定 钼酸铵分光光度法》,硫酸盐测定参照《无机化工产品中硫酸盐测定通用方法》,均采用美国哈希DR1900型便携式分光光度计测定。COD使用承德华通CTL-12型COD速测仪测定。DOC采用日本岛津TOC-L CPH CN 200 TOC分析仪测定,水样测定前用0.45 μm滤膜过滤并酸化。BOD5使用美国哈希BODTrakII型BOD测定仪测定。TDS参照重量法测定。Ca2+和Al3+采用美国珀金埃尔默ICP-AES测定,测定前需对水样进行过滤(0.45 μm滤膜)酸化消解预处理。SO42−和PO43−均采用美国 Thermo Scientific公司的 Dionex Aquion 型离子色谱测定。
有机污染组成分析可以评价预处理效果、推测污染物去除机理。有机组成分析采用美国Thermo-Finnigan Trace DSQ型四级杆气相色谱-质谱分析仪进行。仪器条件:采用HP-5MS色谱柱,进样分流比5∶1,进样温度50 ℃,接口温度300 ℃。色谱柱升温程序:50 ℃恒温,2 min;10 ℃·min −1升至100 ℃,恒温2 min;10 ℃·min −1升至200 ℃,恒温2 min;10 ℃·min −1升至280 ℃。MS数据在全扫描(m/z 50~550)模式下获取,溶剂延迟5.00 min。利用Qualitative Analysis B.07.00软件和Agilent NIST14数据库进行化合物鉴定。
采用光学显微镜(BK1201,重庆光电)观察沉淀物的形貌特征。利用X射线荧光光谱法(X Ray Fluorescence, XRF)对沉淀副产物的元素组成进行分析,仪器为日本Rigaku公司ZSX-100E;利用X射线衍射(X-ray diffraction, XRD)对沉淀副产物的晶格结构进行分析,仪器为日本岛津公司XRD-6000。样品预处理方法:将沉淀产物于105 ℃下烘干,研磨后压片送样检测。
在进行沉淀物副产物分析过程中,待天平预热稳定后,记下陶瓷舟质量m1后归零,称取约1 g沉淀副产物于陶瓷舟中,记下质量m2。向陶瓷舟中滴加1 mol·L−1 HCl,直到没有气泡产生,将无机碳充分除去。用纯水冲洗样品及陶瓷舟,直至用 pH 试纸检验外壁呈中性,放入105 ℃烘箱中干燥,平衡称重记录m3后上机测量。沉淀副产物有机碳质量分数计算方法见式(1)。
式中:wTOC为沉淀副产物有机碳质量分数;wTOCM为TOC仪测得的有机碳质量分数;m1为陶瓷舟质量,g;m2为沉淀副产物质量,g;m3为去除无机碳后副产物质量,g。
-
钙矾石法同步脱硫除磷预处理正交实验结果见表3。
硫酸盐、总磷和COD去除率的极差分析结果分别见表4、表5及表6。kj1、kj2、kj3分别表示A1(A2)、B、C 3个因素在水平1、2、3下的平均去除率,反映各因素在不同水平下对去除率的影响[18-19]。
Ca(OH)2-PAC体系对总磷、硫酸盐和COD去除率的影响因素均为Ca(OH)2>PAC>pH。Ca(OH)2本身为碱性且对总磷去除能力强,对硫酸盐也有一定去除效果,带正电的Ca(OH)2絮体可以通过静电吸附作用去除游离的有机酸阴离子进而降低COD[2],此外,PAC水解生成的多核羟基铝盐对污染物也有一定的吸附去除能力。在Ca(OH)2-PAC体系中,Ca(OH)2对3类污染物的去除均占据主导作用且呈正相关关系,随着Ca(OH)2投加量的增加,污染物去除效果均显著提高(图1)。在15 g·L−1 Ca(OH)2、20 g·L−1 PAC、初始pH=6.0的最优水平组合下,对总磷、硫酸盐和COD的去除率均达到最高值,分别为99.2%、76.8%和61.7%。
CaCl2-PAC体系对总磷、硫酸盐和COD去除率的影响因素均为pH>CaCl2>PAC,pH的升高与硫酸盐的去除率呈负相关关系(图1(a)),与COD和总磷的去除率呈正相关关系(图1(b)和图1(c))。初始pH对CaCl2-PAC体系的影响远大于Ca(OH)2-PAC体系,由于CaCl2不会向水体中引入OH−,故反应初始pH直接影响了水体中Ca2+的赋存状态,进而影响对硫酸盐的去除。CaCl2所提供的游离Ca2+与PO43−缓慢反应生成Ca3(PO4)2,随着pH的升高,OH−能加速Ca2+和PO43−生成Ca10(PO4)6(OH)2的过程,从而提高总磷去除率。高碱化度的PAC水解产物不仅有Ala可溶性单体铝,还预制了低聚态羟基铝盐络合物(Alb)和高聚态羟基铝盐络合物(Alc),在酸性条件下,Alb和Alc结构相对稳定,对污染物有较强的吸附去除作用。因此,低pH条件下,COD的去除归功于PAC水解生成的羟基铝盐络合物及Ca3(PO4)2对污染物的吸附作用;随着pH的上升,加速了多核羟基铝盐络合物的生成,多核羟基铝盐络合物具有更大的比表面积,通过吸附-混凝-共沉淀作用强化去除有机污染物。综上,在CaCl2-PAC体系中,初始pH对3类污染物的去除均占主导作用。在15 g·L−1 CaCl2、20 g·L−1 PAC、初始pH=4.0的最优水平组合下,CaCl2-PAC体系对总磷、硫酸盐和COD的去除率分别为98.9%、88.2%和60.2%。
在最优水平组合下,CaCl2-PAC体系对COD和总磷去除率略低于Ca(OH)2-PAC体系,但具有更高的硫酸盐去除率且无需调节反应初始pH。考虑到二元酸废水是酸性的强缓冲体系,避免调节pH能有效降低预处理成本。根据多评价指标正交实验综合平衡原则,优选CaCl2-PAC体系开展后续实验。
-
在15 g·L−1 CaCl2、20 g·L−1 PAC、初始pH=4.0的最优工艺条件下,考察了CaCl2-PAC预处理药剂体系对二元酸废水的污染物综合去除效果。预处理前后的主要污染指标变化情况见表7。CaCl2-PAC体系对总磷和硫酸盐的去除率分别高达98.9%和88.2%,对DOC、COD和BOD5等有机污染负荷的去除率均在60.0%以上,总磷、硫酸盐和有机污染负荷得以同步高效去除。而且预处理药剂的引入并没有造成水体TDS的增加(降低8.70%),说明该沉淀反应进行得比较彻底,能够一定程度上降低盐含量对后续综合污水处理厂生化系统性能的影响。二元酸废水的生物降解性在预处理后略有下降,BOD5/COD从0.56降至0.44,但仍属于易生物降解的有机废水。
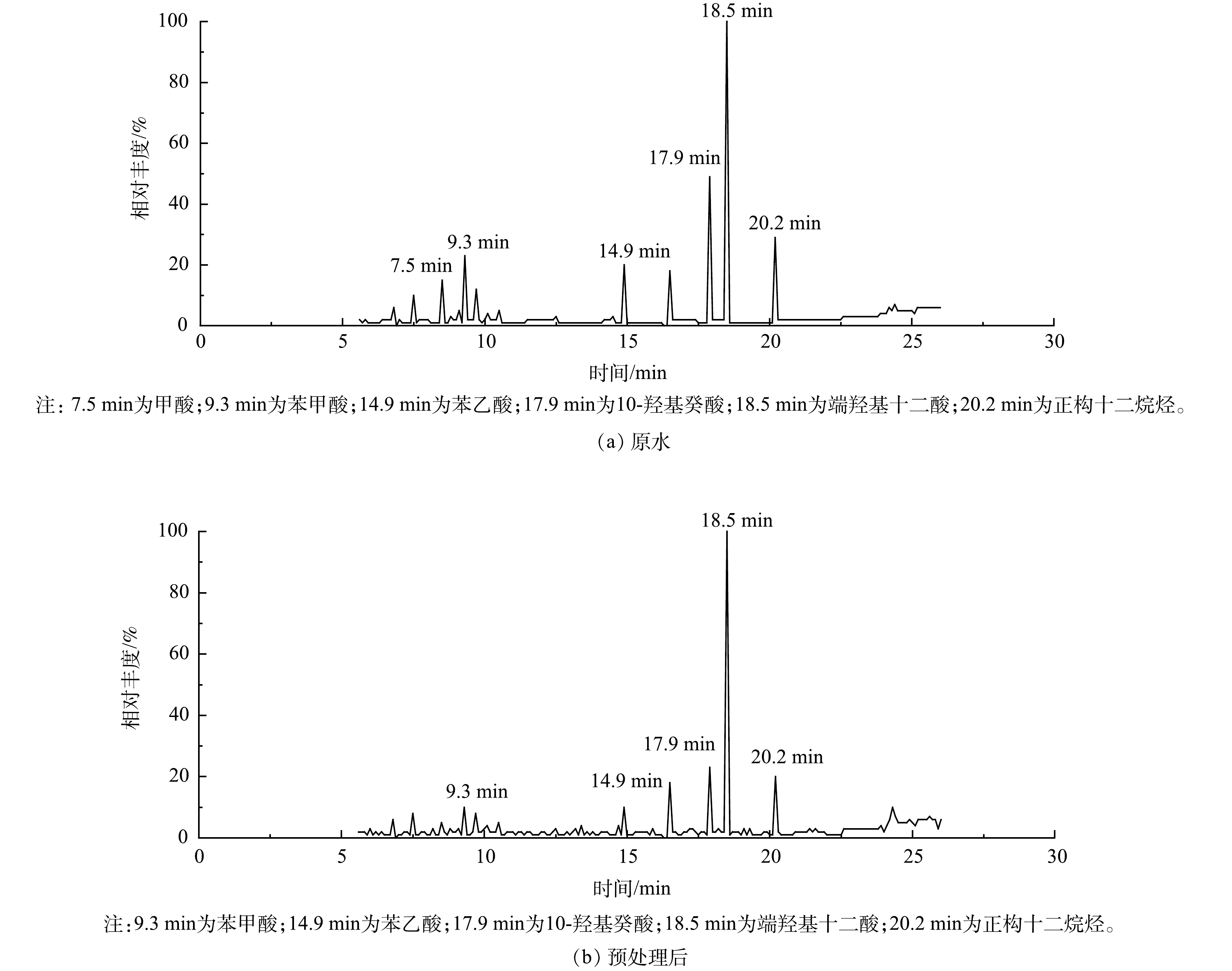
结合处理效果评估结果,推测生物降解性降低的原因主要包括2个方面:一方面,预处理药剂给水体带来的过剩Ca2+和Cl−会影响微生物代谢活性[20-23];另一方面,预处理过程去除了部分易生物降解的小分子有机酸类。GC-MS分析结果显示,二元酸废水中含有大量的有机酸类物质,包括甲酸、苯甲酸、己二酸、苯乙酸、发酵中间产物10-羟基癸酸、11-氧代十二酸和端羟基十二酸等(图2(a)),有机酸类占比达90.0%以上。从预处理前后的有机污染组成变化(表8)可以看出,烷烃和醇类被彻底去除,部分易生物降解的小分子有机酸类也被去除(图2(b))。但预处理后的有机污染负荷总体仍以小分子有机酸类为主(相对丰度91.4%),因而保持了较好的生物降解性;同时部分有机污染物的去除造成胺类(5.30%)和酯类(3.30%)的相对丰度有所增加。总体上,CaCl2-PAC预处理药剂体系是削减二元酸废水综合污染负荷有效的手段,对后续综合污水处理系统达标运行的保障有显著作用。
-
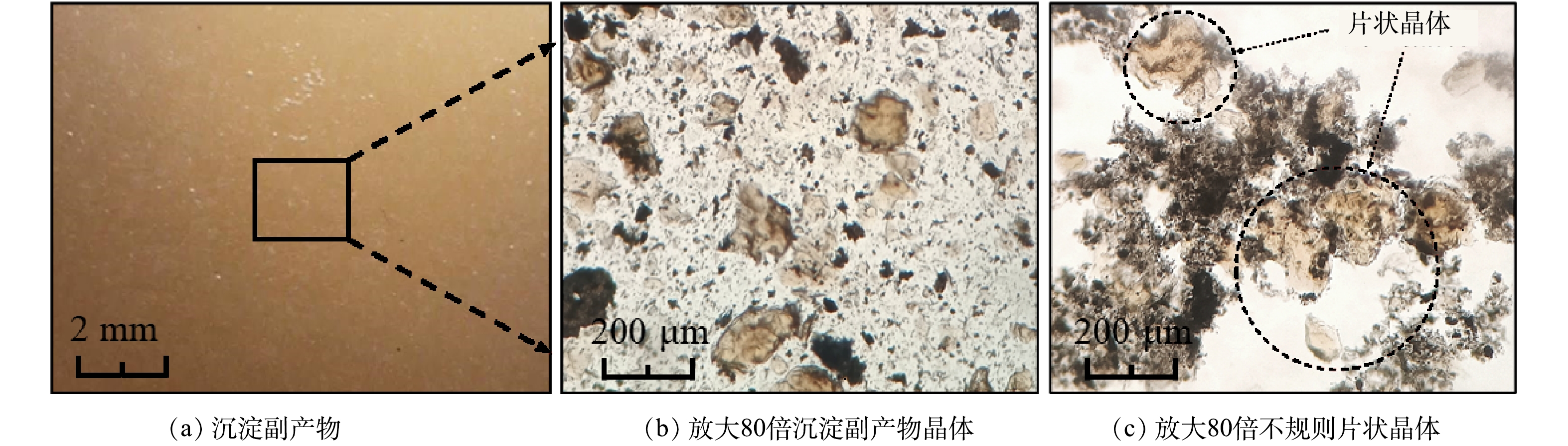
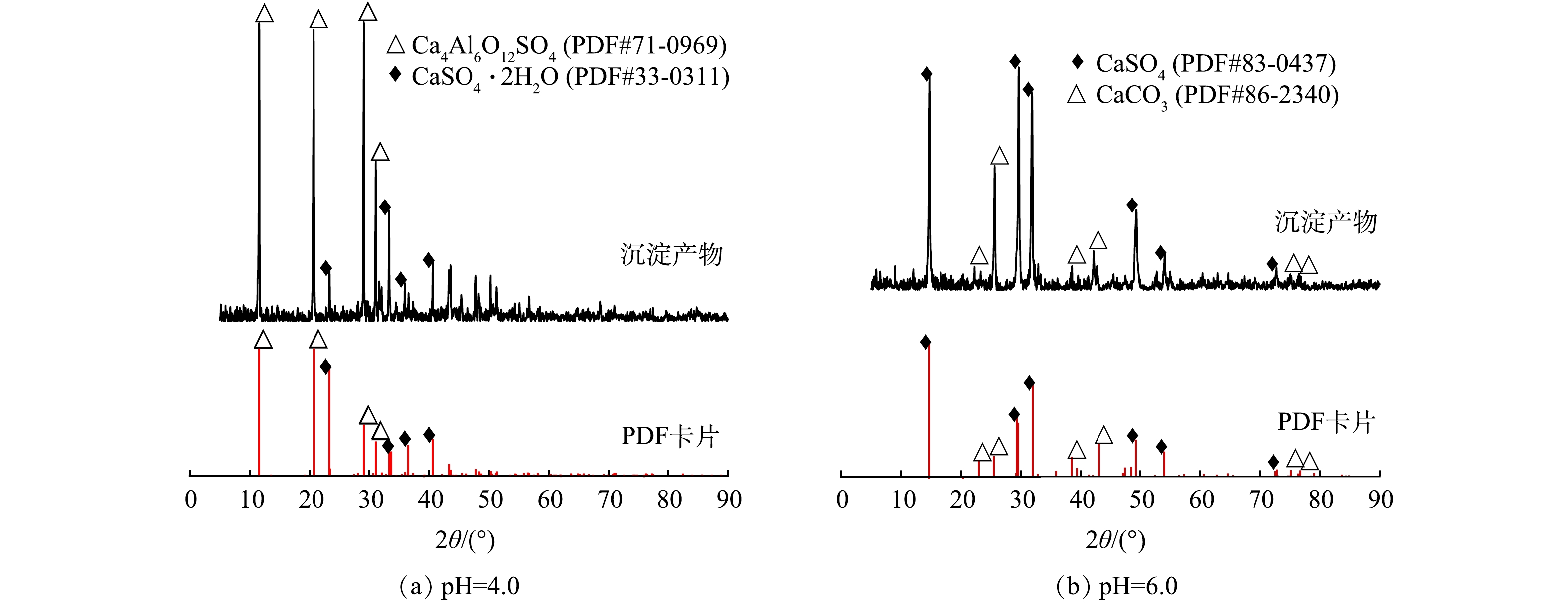
基于沉淀副产物形貌特征(图3)和XRD分析结果(图4),探究CaCl2-PAC体系对硫酸盐的去除机理。当初始pH=4.0时,CaCl2-PAC体系的沉淀副产物以单硫酸盐型硫铝酸钙(Ca4Al6O12SO4, AFm)和CaSO4·2H2O为主(图4(a)),并未检测到AFt的晶格结构。AFm是六角薄板状或不规则玫瑰片状晶体,通常呈片状,其性质与AFt相似,在一定条件下两者可以互相转化,通常将AFm视为AFt的中间产物。预处理沉淀副产物中存在形貌呈不规则片状或薄板状结构的晶体(图3(b)和图3(c)),推测其为AFm晶体,在沉淀过程中生成并与AFt相互转化[24-25]。但也有可能是因为AFt的晶格结构较不稳定,XRD制样过程中AFt晶格结构发生变化,失去结晶水生成AFm[26]。当初始pH=4.0时,硫酸盐的高去除率得益于难溶性硫酸盐的协同共沉淀作用。PAC向体系中提供了足量的Ala(Al3+和Al(OH)2+等)促进了AFm的生成,其水解产物中多核羟基铝盐络合物巨大的比表面积和吸附能力进一步强化共沉淀作用,进而提高污染物去除率。以往研究报道碱性条件下的Ca2+和硫酸盐更容易生成AFt,硫酸盐去除率随着pH的升高而提高[26]。但本研究对二元酸废水预处理得出了不同的实验结果,低pH更有利于硫酸盐的去除。初始pH=6.0条件下沉淀副产物的XRD分析结果表明,沉淀副产物主要由CaSO4以及CaCO3构成,并未检测到AFm和AFt晶体(图4(b))。由此判断,本研究中硫酸盐的去除率随初始pH升高而降低的实验现象应与二元酸废水体系离子组成和碱度密切相关。
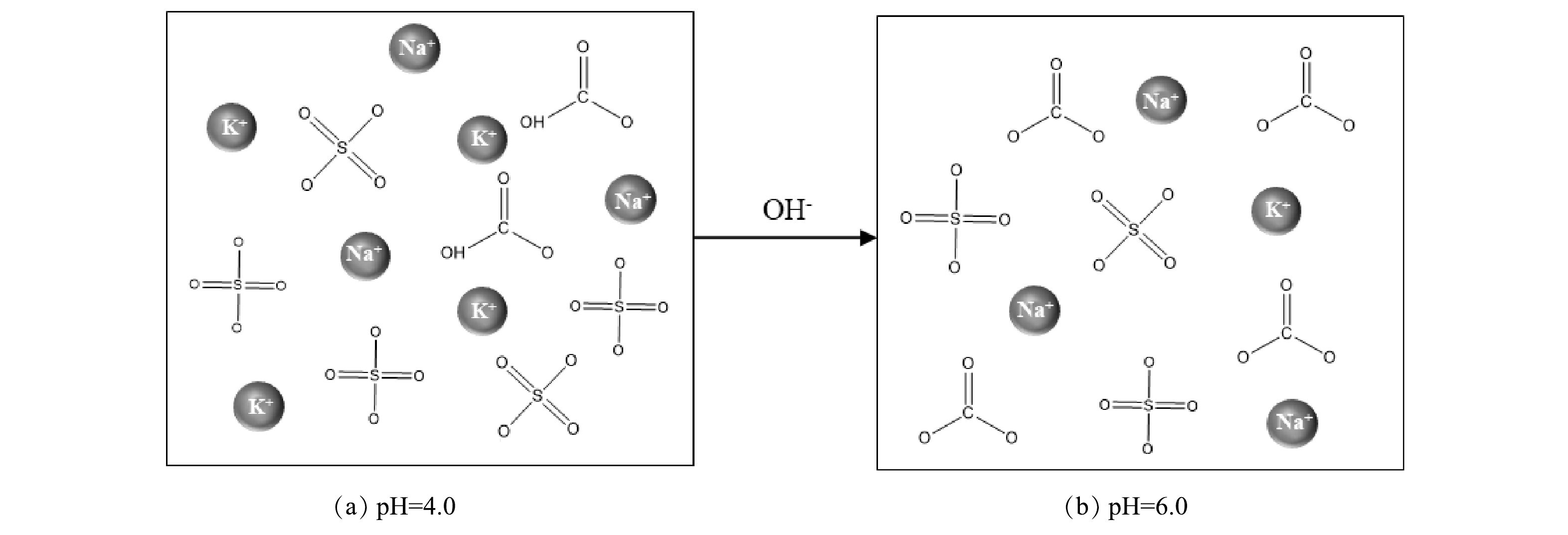
体系的碱度是影响硫酸盐去除效果的关键,生物发酵法生产二元酸需要投加大量NaHCO3以保持发酵罐内的碱度,使得二元酸废水具有极强的缓冲能力。CaCl2-PAC体系利用氢氧化钠调节反应初始pH,该过程中HCO3−与OH−不断反应生成CO32−(图5)。当pH=6.0时,水体存在大量游离的CO32−,CaCO3溶度积(Ksp=2.8×10−9)远小于CaSO4(Ksp=5.02×10−6)和Ca(OH)2(Ksp=4.93×10−5)。投加CaCl2后,游离的Ca2+迅速与CO32−反应,生成CaCO3沉淀,而CaCO3沉淀在体系中不参与AFt、AFm的生成[25]。此外,高pH促进了部分Ca(OH)2的生成,使Ca2+的有效浓度进一步降低,导致体系AFm的生成效率下降,进而降低硫酸盐去除率[27-28]。
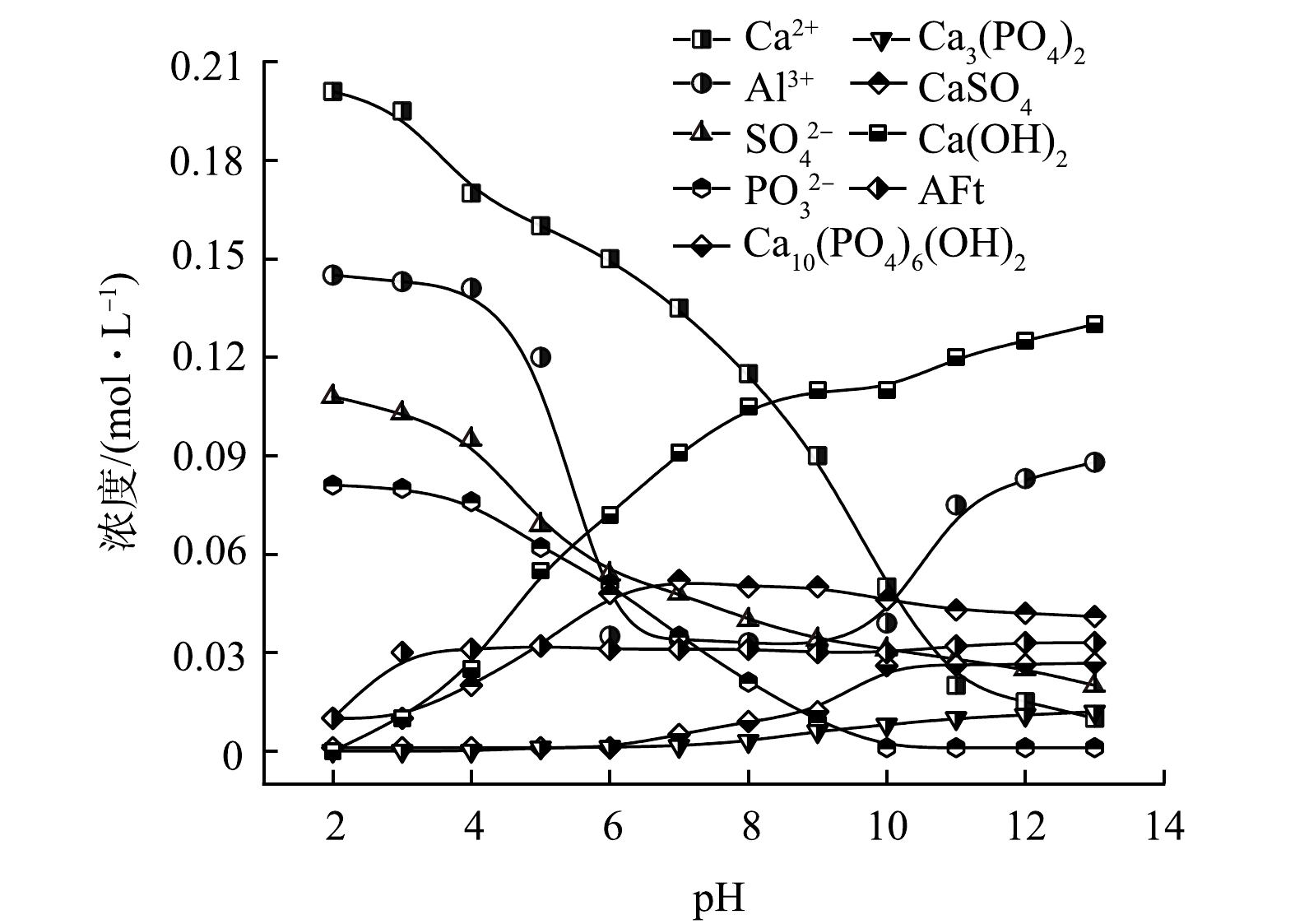
结合实验现象和测量结果,绘制了CaCl2-PAC体系沉淀物的解离平衡关系图,探究了体系中Ca2+和Al3+、沉淀物与水体pH的关系。当水体pH<4时,在高浓度Ca2+和SO42−的驱动下,缓慢生成CaSO4,同时水体中PO43−浓度略微下降,说明生成了微量的Ca3(PO4)2沉淀(图6);当4<pH<6时,体系中OH−浓度升高,PAC水解生成的Ala与Ca2+和SO42−生成AFm沉淀,同时缓慢生成Ca3(PO4)2和CaSO4,此时水体中Al3+、Ca2+、SO42−和PO43−浓度均有明显下降;当7<pH<9时,在OH−的驱动下,Ca2+与PO43−、OH−生成Ca(OH)2和Ca10(PO4)6(OH)2,同时也伴随着部分AFt的生成,Ala/Alb/Alc水解生成分子质量更大的多核羟基铝盐络合物和无定形Al(OH)3沉淀,进而被分离去除,Al3+浓度下降明显;当10<pH<13时,Ca2+与OH−持续生成Ca(OH)2,Ca10(PO4)6(OH)2、AFt和AFm等沉淀在碱性条件下相对稳定,但水体中多核羟基铝盐络合物以及无定形Al(OH)3沉淀重新溶解转化为Ala,Al3+浓度上升。
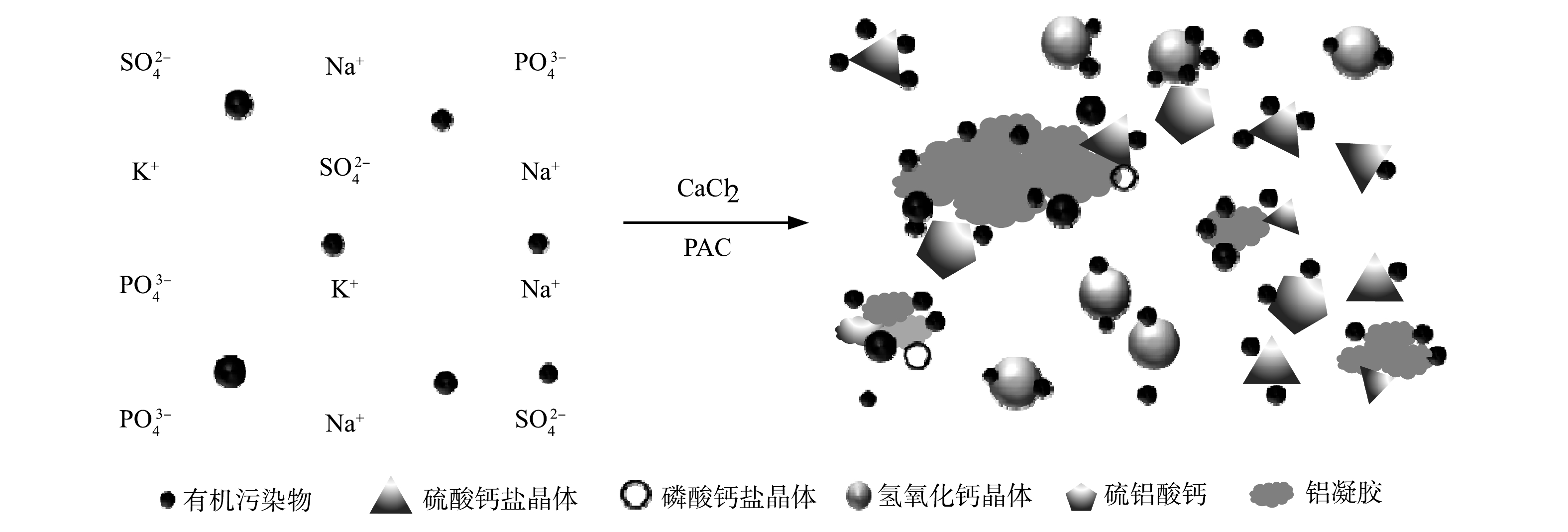
结合以上分析和实验现象,可以推测CaCl2-PAC预处理药剂体系对总磷、硫酸盐和有机污染负荷的同步去除机理。在反应体系pH=4.0时,CaCl2水解提供Ca2+,PAC水解生成Ala,包括Al3+、Al(OH)4−和Al(OH)2+[29],Ca2+、Ala与PO43−和SO42−生成不溶性的沉淀微晶体AFm、CaSO4、Ca3(PO4)2等,实现对总磷和硫酸盐的同步去除。这些具有大比表面积的晶体在生成过程中还能通过吸附和沉淀作用去除部分小分子有机酸类、胺类和醇类物质。高碱化度的PAC在酸性条件下会水解生成部分Alb及Alc,促进对污染物的吸附和黏结架桥作用。Alb和Alc继续水解,生成分子质量更大的多核羟基铝盐络合物,进一步促进上述吸附和沉淀作用(图7),最终通过吸附-混凝-共沉淀作用,强化对总磷、硫酸盐和有机污染负荷的同步高效去除。
-
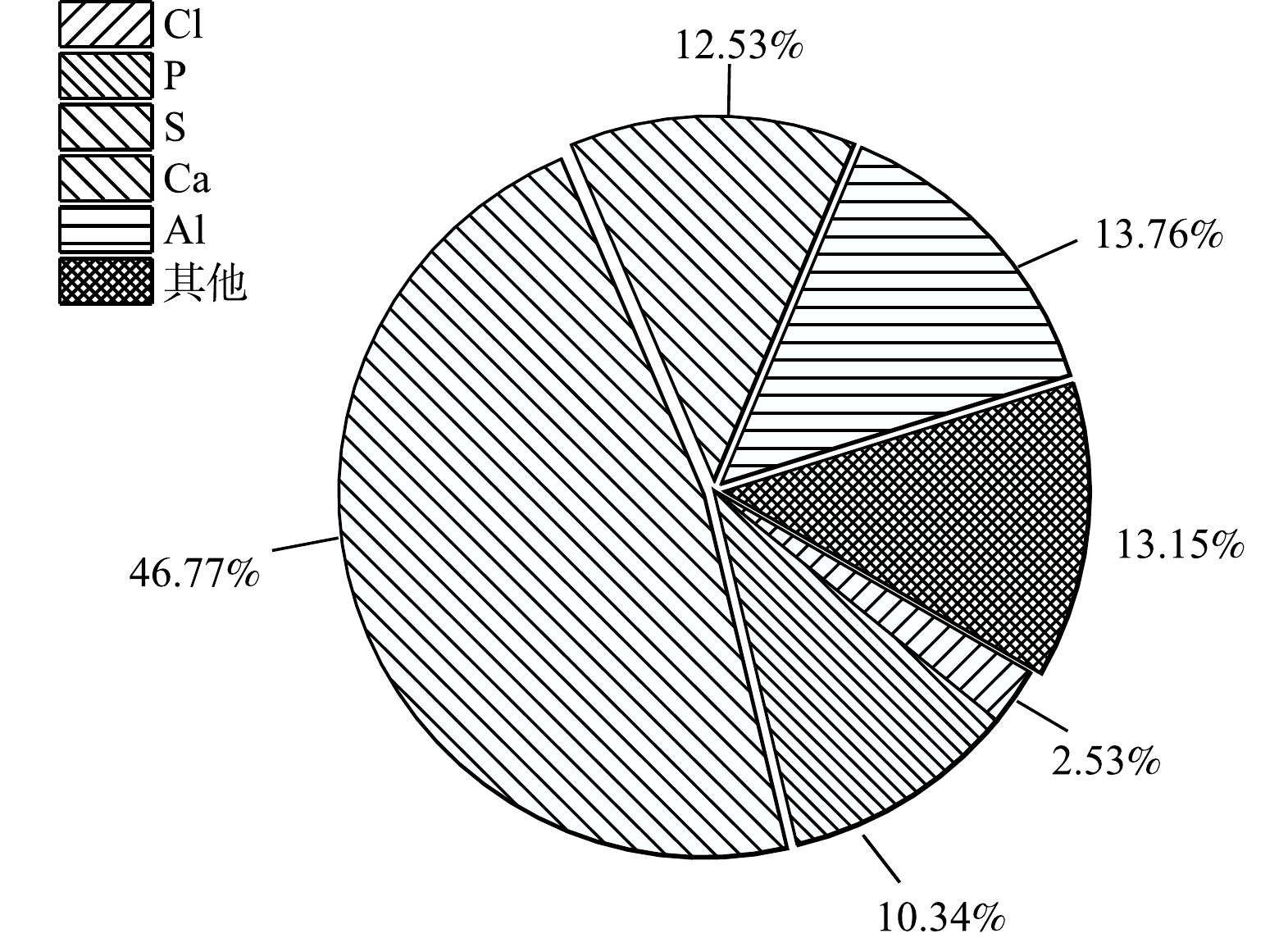
使用CaCl2-PAC药剂体系预处理二元酸废水,在最优实验条件下,废水沉淀副产物产率约为18.8 kg·t−1。副产物主要由AFm、CaSO4·2H2O和Ca3(SO4)2等构成,其主要元素组成为S、P、Ca、Al和Cl,其中以S元素占比最高,达46.8%,Ca、Al、P元素的占比分别为12.5%、13.8%和10.3%(图8)。固相碳含量分析结果表明,副产物TOC相对含量为10.6%,证明钙矾石沉淀法能够通过吸附-共沉淀作用大幅削减二元酸废水有机污染负荷。预处理生成的副产物中含有大量的AFm和CaSO4。AFm-CaO-CaSO4复合膨胀剂是一类应用广泛的混凝土膨胀剂,因此,该预处理工艺产生的副产物,经灼烧预处理去除有机污染物后,可改造利用为混凝土膨胀剂[17]。
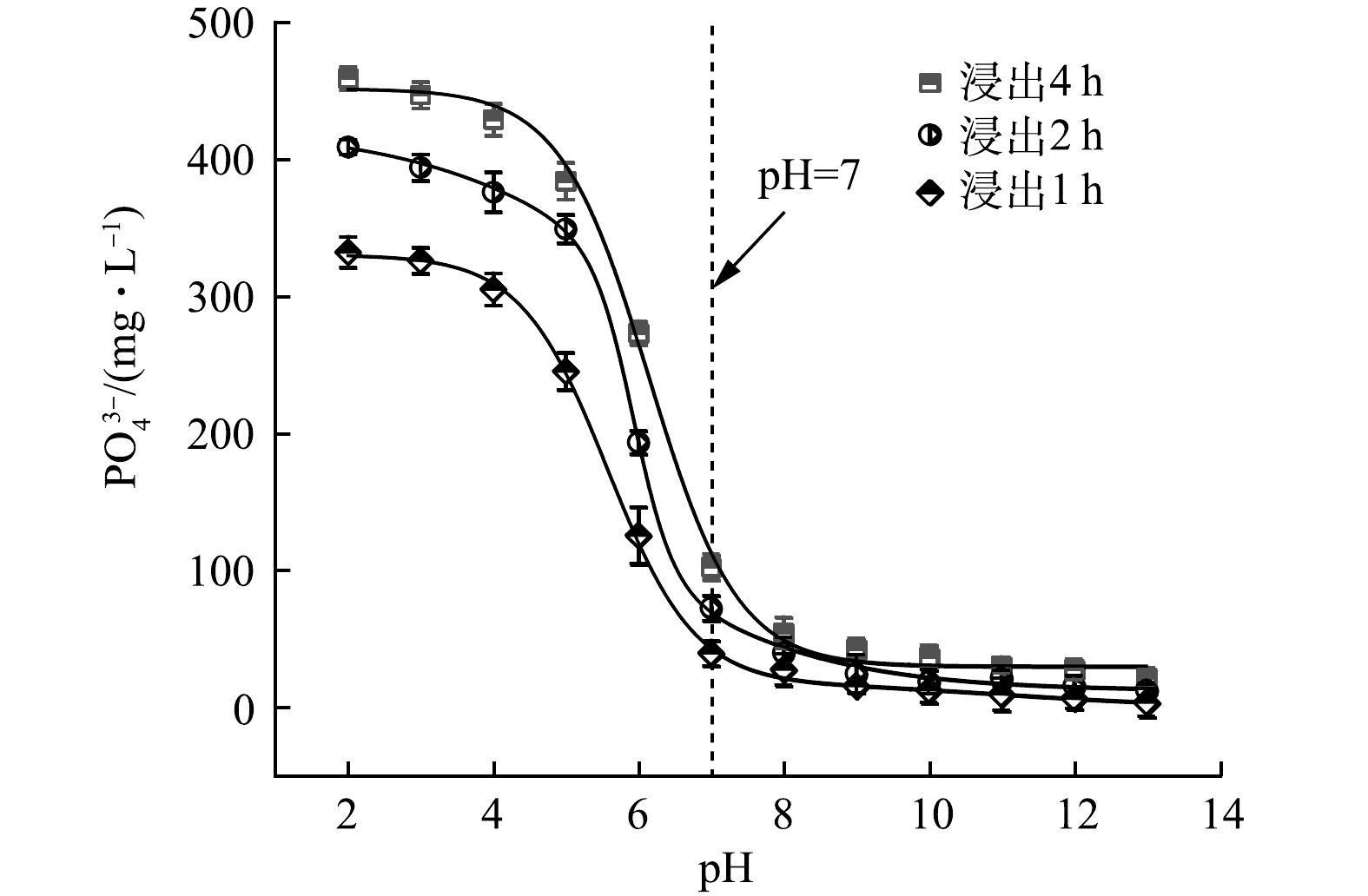
沉淀副产物PO43−溶出实验结果表明,在碱性及中性条件下,Ca3(PO4)2和Ca10(PO4)6(OH)2相对稳定,几乎没有PO43−溶出。pH<7时,PO43−溶出量随pH的下降而显著增加;pH<3时,PO43−溶出量趋于稳定。当pH=2.0,浸出时间为4 h时,PO43−溶出量高达462 mg·L−1(图9)。由于二元酸废水体系离子浓度高,形成沉淀物的驱动力更大,因此,在酸性条件下,依旧可以生成Ca3(PO4)2和Ca10(PO4)6(OH)2。而沉淀副产物PO43−溶出实验在纯水中进行,在酸性条件下,Ca3(PO4)2和Ca10(PO4)6(OH)2溶解度均显著增大,能持续向水体中释放PO43−。植物生长过程中根部分泌的柠檬酸等酸性物质会增大沉淀副产物中Ca3(PO4)2和Ca10(PO4)6(OH)2的溶解度,加速PO43−溶出的同时能有效改善土壤pH,有利于植物对PO43−等营养成分的吸收,故沉淀副产物具有一定的缓释磷肥价值[30]。
沉淀物中残留一定量的有机污染物,该部分有机污染物来自生物发酵副产物,主要是低分子质量的有机酸类(二元酸、甲酸、乙酸等),属于易生物利用的有机质,作为缓释磷肥再利用时不易对土壤造成二次污染。部分有机酸类物质还能通过形成配位体的方式与土壤中重金属相结合,提高重金属的生物有效性[31],当受纳土壤要求较为严格时,同样可以通过灼烧去除有机酸。综上,当沉淀物作为混凝土膨化剂再利用时,可以通过灼烧去除沉淀物中吸附的小分子有机酸;当作为缓释磷肥再利用时,也可以通过灼烧去除小分子有机酸,Ca3(PO4)2和Ca10(PO4)6(OH)2等沉淀受热不易分解,灼烧并不会影响其作为缓释磷肥的价值。这也表明,钙矾石法不仅可以实现二元酸废水的预处理,解决这类石化废水的达标处理问题,而且产物便于利用,可为相关石化企业就地解决废水处理问题提供参考。
-
1)从高浓度二元酸废水源头控制的现实难题出发,采用正交实验优选出的基于CaCl2-PAC体系的钙矾石法预处理工艺,在15 g·L−1 CaCl2、20 g·L−1 PAC、初始pH=4.0的最优条件下,对二元酸废水的总磷、硫酸盐及COD去除率分别高达98.9%、88.2%和60.2%。
2) CaCl2电离出的Ca2+,与PO43−形成Ca3(PO4)2和Ca10(PO4)6(OH)2沉淀,并与PAC水解产物Ala和SO42−形成AFm,实现对总磷和硫酸盐的高效去除;并在多核羟基铝盐络合物和沉淀物的吸附-混凝-共沉淀作用下,去除部分小分子有机酸类、醇类和胺类物质,同步实现有机污染负荷的大幅度削减,且预处理后废水可生化性较强,有利于后续生化降解。
3)废水预处理沉淀副产物产量约为18.8 kg·t−1,TOC相对含量为10.6%,其主要成分为AFm、CaSO4·2H2O和Ca3(PO4)2,沉淀副产物经处理后具有再利用于混凝土膨化剂以及植物缓释磷肥的潜力。
4)钙矾石法预处理工艺对二元酸废水的污染物综合去除效果显著,可以为投产二元酸装置石化企业或化工园区的综合污水处理厂安全运行和稳定达标提供技术支撑。
基于钙矾石沉淀法的长链二元酸生产废水预处理工艺
Pretreatment process of long chain dicarboxylic acid wastewater based on an ettringite precipitation method
-
摘要: 为避免长链二元酸生产废水中高浓度的总磷和硫酸盐对石化企业污水处理系统造成冲击,利用钙矾石沉淀法同步去除高浓度的总磷和硫酸盐,设计3因素3水平正交实验,探究了钙盐投加量、铝盐投加量和pH对污染物去除效果的影响,并优化工艺参数;利用GC-MS探究预处理前后废水有机污染组分的变化;利用XRD和XRF分析沉淀副产物的物质组成,并通过离子溶出实验探究沉淀副产物的可再利用潜力。结果表明:CaCl2-PAC体系对污染物的去除效果优于Ca(OH)2-PAC体系;CaCl2-PAC体系中各因素对污染物去除效果的影响顺序为pH> CaCl2投加量>PAC投加量;在15 g·L−1 CaCl2、20 g·L−1 PAC以及初始pH=4.0的最优工艺条件下,对总磷、硫酸盐及COD的去除率分别高达98.9%、88.2%和60.2%;Ca2+与PO43−生成Ca3(PO4)2和Ca10(PO4)6(OH)2沉淀,同时,PAC水解产物Ala和Ca2+及SO42−形成单硫型硫酸铝钙(AFm)沉淀,实现对总磷和硫酸盐的高效同步去除,并通过吸附-混凝-共沉淀的综合作用大幅削减有机污染负荷;二元酸废水中有机污染物以小分子酸为主,预处理后生物降解性略有下降,但仍属于易生物降解的有机废水;沉淀副产物具有再利用于混凝土膨化剂以及植物缓释磷肥的潜力。钙矾石沉淀法预处理工艺简单、效果稳定、成本低,该工艺可为投产二元酸装置石化企业污水处理系统的稳定达标运行提供有效保障。Abstract: To avoid the impact of high concentrations of total phosphorus (TP) and sulfate in dicarboxylic acid wastewater on the wastewater treatment plant (WWTP) of petrochemical enterprises, an ettringite precipitation method was used to remove high concentration of TP and sulfate simultaneously, the orthogonal experiments with three factors and three levels were designed, the effects of calcium salt dosage, aluminum salt dosage and pH on pollutant removal were investigated, and the process parameters were optimized; the changes in organic pollution components of wastewater before and after pretreatment were studied by using GC-MS; the material composition of precipitation byproducts was analyzed by using XRD and XRF, and the potential for reuse of precipitation byproducts was explored by using ion dissolution experiments. The results show that the removal effect of pollutants by CaCl2-PAC system was better than that by Ca(OH)2-PAC system. In the CaCl2-PAC system, the influence order of each factor on the pollutant removal effect was pH > CaCl2 dosage > PAC dosage. Under the optimal process conditions of 15 g·L−1 CaCl2, 20 g·L−1 PAC, and initial pH=4.0, the removal rates of TP, sulfate, and COD were as high as 98.9%, 88.2%, and 60.2%, respectively. Ca3(PO4)2 and Ca10(PO4)6(OH)2 precipitation occurred between Ca2+and PO43−, at the same time, a single sulfur aluminum calcium sulfate (AFm) precipitate occurred among PAC hydrolysis product Ala forms, Ca2+and SO42−, the simultaneous removal of TP and sulfate was achieved, and the organic pollution load was largely reduced through adsorption-coagulation-coprecipitation. The organic pollutants in dicarboxylic acid wastewater are mainly small molecule acids, and its biodegradability decreased slightly after pre-treatment, but it still belonged to easily biodegradable organic wastewater. The byproducts have a potential for being reused as concrete expansion agents and plant slow-release phosphate fertilizers. The pretreatment process is simple, stable and low in cost, which can provide an effective guarantee for the stable and standard operation of WWTP of petrochemical enterprises with the dicarboxylic acid production device.
-

-
表 1 正交实验表
Table 1. Orthogonal experimental table
实验 因素A1(A2) 因素B 因素C 实验1 1 1 1 实验2 1 2 3 实验3 1 3 2 实验4 2 1 3 实验5 2 2 2 实验6 2 3 1 实验7 3 1 2 实验8 3 2 1 实验9 3 3 3 表 2 各因素水平表
Table 2. Factor level table of orthogonal experiment
水平 因素A1(A2) 因素B 因素C 水平1 5.0 10 4.0 水平2 10 15 5.0 水平3 15 20 6.0 表 3 正交实验结果
Table 3. Orthogonal experiment results
实验
序号因素 硫酸盐
去除率/%总磷
去除率/%COD
去除率/%A1 B C 1 1 1 1 19.7 96.4 49.1 2 1 2 3 25.5 96.9 50.5 3 1 3 2 33.8 96.8 52.8 4 2 1 3 43.1 99.3 56.1 5 2 2 2 61.4 99.4 57.4 6 2 3 1 68.6 99.7 58.6 7 3 1 2 52.4 99.4 61.4 8 3 2 1 70.5 99.7 61.9 9 3 3 3 76.7 99.1 61.7 实验
序号因素 硫酸盐
去除率/%总磷
去除率/%COD
去除率/%A2 B C 1 1 1 1 57.2 96.0 47.2 2 1 2 3 42.1 98.1 56.1 3 1 3 2 56.4 96.9 54.4 4 2 1 3 53.2 98.6 55.2 5 2 2 2 70.3 97.0 54.3 6 2 3 1 85.6 96.6 53.6 7 3 1 2 60.1 97.3 54.1 8 3 2 1 81.5 97.2 56.5 9 3 3 3 67.7 99.7 61.2 表 4 硫酸盐去除率极差分析
Table 4. Orthogonal experimental results of sulfate removals
因素 kj1 kj2 kj3 极差R 因素 kj1 kj2 kj3 极差R A1 26.4 57.7 66.6 40.2 A2 51.9 69.7 69.8 17.8 B 38.4 52.5 59.7 21.3 B 56.8 64.6 69.9 13.1 C 50.4 49.2 50.9 1.70 C 74.8 62.3 54.3 20.4 主次顺序 A1>B>C 主次顺序 C>A2>B 表 5 总磷去除率极差分析
Table 5. Orthogonal experimental results of total phosphorus removals
因素 kj1 kj2 kj3 极差R 因素 kj1 kj2 kj3 极差R A1 96.7 99.4 99.4 2.70 A2 96.9 97.4 98.1 1.10 B 98.4 98.7 98.5 0.30 B 97.3 97.4 97.7 0.30 C 98.6 98.6 98.4 0.20 C 96.6 97.1 98.8 1.70 主次顺序 A1>B>C 主次顺序 C>A2>B 表 6 COD去除率极差分析
Table 6. Orthogonal experimental results of COD removals
因素 kj1 kj2 kj3 极差R 因素 kj1 kj2 kj3 极差R A1 44.8 51.4 55.7 10.9 A2 46.5 48.4 51.4 4.90 B 49.5 50.6 51.7 2.20 B 46.2 49.6 50.6 4.40 C 50.5 51.2 50.1 1.10 C 46.4 48.3 51.7 5.30 主次顺序 A1>B>C 主次顺序 C>A2>B 表 7 预处理前后主要污染指标变化
Table 7. Changes in main pollution indexes before and after pretreatment
序号 污染指标 预处理前/(mg·L−1) 预处理后/(mg·L−1) 去除率/% 1 总磷 272 2.9 98.9 2 硫酸盐 10 360 1 224 88.2 3 COD 6 201 2 470 60.2 4 BOD5 3 485 1 080 69.0 5 BOD5/COD 0.56 0.44 — 6 DOC 2 441 951 61.5 7 TDS 19 197 17 513 8.70 表 8 预处理前后有机污染组成变化
Table 8. Changes in organic pollution compositions before and after pretreatment
水样 有机物类型 碳数/个 污染物种类 相对分子质量 相对丰度/% 预处理前的水样 有机酸类 6~12 11 116~230 92.9 酯类 6~10 3 296~390 3.40 醇类 8 1 122 0.40 烷烃类 11 1 156 1.10 胺类 8 2 135 2.20 预处理后的水样 有机酸类 6~12 9 122~230 91.4 酯类 6~10 3 142~234 3.30 胺类 8 1 135 5.30 -
[1] 张全, 文志琼, 张霖, 等. 长链二元酸发酵菌种创制和工艺研究进展[J]. 生物工程学报, 2022, 38(12): 4420-4431. [2] 陈霖, 曹越, 张仁忠, 等. 某石化企业长链二元酸生产废水的预处理工艺及现场应用[J]. 环境工程学报, 2022, 16(2): 666-673. [3] 杨健, 黄伟星, 王士芬, 等. 十三碳二元酸发酵有机废水处理研究[J]. 环境污染与防治, 1999, 21(1): 15-18. [4] 车树刚, 马娜娜, 傅英旬, 等. 生物酶法处理二元酸废水[J]. 环境科技, 2019, 32(4): 36-40. [5] 许莉, 王明毓, 蔡永益. 电解法处理十三碳二元酸有机废水的研究[J]. 流体机械, 2007, 12(10): 1-4. [6] 于永辉, 刘守新, 李作臣, 等. 二元酸废水的生物-光催化氧化组合处理技术[J]. 工业水处理, 2004, 12(2): 23-25. [7] SUKALYAN S, ARKA P. Selective removal of phosphorus from wastewater combined with its recovery as a solid-phase fertilizer[J]. Water Research, 2011, 11: 3318-330. [8] ZHANG Z B, LI Y, WEI L L. Effect of ferric chloride on the properties of biological sludge in co-precipitation phosphorus removal process[J]. Chinese Journal of Chemical Engineering, 2013, 21(5): 564-568. doi: 10.1016/S1004-9541(13)60511-X [9] BARCA C, GERENTE C, MEYER D. Phosphate removal from synthetic and real wastewater using steel slags produced in Europe[J]. Water Research, 2012, 46: 2376-2384. doi: 10.1016/j.watres.2012.02.012 [10] 珍珠, 范瑞江. 两级石灰沉淀法在高浓度含氟含磷污水处理中的应用[J]. 化肥设计, 2015, 53(6): 34-37. [11] 史正学. 浅谈硫酸根的去除方法[J]. 盐业与化工, 2015, 44(7): 27-29. [12] NORAPAT P, SIWAT S, YOTHIN C, et al. Sulfate removal from lignite coal mine drainage in Thailand using ettringite precipitation[J]. Chemosphere, 2021, 285: 131357. doi: 10.1016/j.chemosphere.2021.131357 [13] WEI X D, ZHEN Z, LU J, et al. Sulfate removal from wastewater using ettringite precipitation: Magnesium ion inhibition and process optimization[J]. Journal of Environmental Management, 2017, 196: 518-526. doi: 10.1016/j.jenvman.2017.03.054 [14] 王海鹰, 彭小玉, 王云燕, 等. 采用复盐法脱除工业废水中的硫酸根[J]. 中南大学学报(自然科学版), 2010, 41(2): 434-439. [15] 胡文容. 铝盐沉淀法去除酸性矿井水中SO42-的试验研究[J]. 煤矿环境保护, 1996, 12(5): 18-20. [16] 王玉东, 赵丹, 董延茂, 等. 钙矾石沉淀法去除镁剂脱硫废水中硫酸根离子研究[J]. 工业水处理, 2015, 35(6): 54-57. [17] 袁辉洲, 柯水洲, 涂家勇, 等. pH对聚合铝形态分布与混凝效果的影响[J]. 工业水处理, 2016, 36(4): 50-53. [18] DONG R, XIAO B, FANG Y. The theoretical analysis of orthogonal test designs[J]. Journal of Anhui Institute of Architecture, 2004, 12(14): 145-149. [19] HUI C, JIAN M W, TAO P, et al. Study on the orthogonal yest of al-substituted α-Ni(OH)2 prepared by complexation-precipitation method[J]. Electrochemistry, 2002, 9(12): 122-129. [20] 周鹏. 盐度冲击对活性污泥系统性能影响的研究[J]. 环境科学与技术, 2011, 34(5): 65-68. [21] KINCANNON D F, GAUDY A F. Response of biological wastetreatment systems to changes in salt concentration[J]. Biotechnology and Bioengineering, 1968, 10(12): 483-496. [22] BUMRTT W E. The effect of salinity variations on theactivated sludge process[J]. Water and Sewage Works, 1974, 121(65): 37-38. [23] UTGUR A. KARGI F. Salt inhibition on biological nutrientremocal from saline wastewater in a sequencing batch reactor[J]. Enzyme and Microbial Technology, 2004, 34(71): 313-318. [24] BLACK L, BREEN C, YARWOOD J, et al. In situ raman analysis of hydrating C3A and C4AF pastes in presence and absence of sulfate[J]. Advances in Applied Ceramics, 2006, 5(4): 209-216. [25] BARNETT S J, ADAM C D, JACKSON A R W. Solid solutions between ettringite, Ca6Al2(SO4)3(OH)12·26H2O, and thaumasite, Ca3SiSO4CO3(OH)6·12H2O[J]. Journal of Materials Science, 2000, 35(16): 4109-4114. doi: 10.1023/A:1004898623884 [26] CODY A M, LEE H, CODY R D, et al. The effects of chemical environment on the nucleation, growth, and stability of ettringite[Ca3Al(OH)6]2(SO4)3·26H2O[J]. Cement Concrete Research, 2004, 34(5): 869-881. doi: 10.1016/j.cemconres.2003.10.023 [27] POELLMANN H, KUZEL H J, WENDA R. Solid solution of ettringites part I: Incorporation of OH− and CO32– in 3CaO·A12O3·32H2O[J]. Cement Concrete Research, 1990, 20(6): 941-947. doi: 10.1016/0008-8846(90)90057-5 [28] 张文生, 张金山, 叶家元等. 合成条件对钙矾石形貌的影响[J]. 硅酸盐学报, 2017, 9(5): 631-638. [29] 王趁义, 毕树平. 环境水体中聚合铝形态的分析测试技术研究进展[J]. 分析科学学报, 2004, 20(3): 317-321. [30] 刘翠, 牟凤利, 王吉秀, 等. 低分子量有机酸对植物吸收和累积重金属的影响综述[J]. 江苏农业科学, 2021, 49(8): 38-43. [31] 束良佐. 生长介质和局部供磷对白羽扇豆排根形成和柠檬酸分泌的影响[D]. 北京: 中国农业大学, 2005. -



 下载:
下载: