-
偶氮染料、蒽醌染料和三苯甲烷类染料是使用量较多的染料,而结晶紫(crystal violet,CV)是应用较为广泛的三苯甲烷类染料,具有消毒杀菌效果好和染色效果好等优点,因而应用范围涉及医学消毒和纺织染色等领域[1]。但结晶紫可被氧化反应而产生具有致突变、致畸和致癌作用的有毒代谢物,对动物和人类健康产生不利影响。结晶紫分子的结构复杂,分子质量较大,导致该类废水可生化性差,难以生物处理。徐圣东等[2]利用猴头菌初提纯漆酶对结晶紫进行降解,在反应24 h时,结晶紫的降解率仅为27.3%。混凝法是目前应用较为传统的处理工艺,而聚丙烯酰胺是混凝法中常用的助凝剂[3]。陈新[4]将自制阴离子聚丙烯酰胺用于结晶紫脱色,当混凝剂投加量为30 mg·L−1,初始pH为9,结晶紫浓度为20 mg·L−1最佳条件下,最高结晶紫脱色率仅为57.5%,说明混凝难以去除结晶紫。
高级氧化技术可以产生强氧化性的自由基,在难降解废水中的研究已经受到了广泛的关注。臭氧和过硫酸盐高级氧化技术是目前的研究热点。但也存在臭氧耗电量大、过硫酸盐会引入硫酸根离子等问题[5]。四氧化三铁表面的Fe(Ⅱ)和Fe(Ⅲ)之间的转换可以在八面体结构相同的位置进行,因此被认为是臭氧和过硫酸盐的有效催化剂,可以减少臭氧和过硫酸盐的使用[6-7]。四氧化三铁本身具有的磁性也能使得它易被磁力分离,因此易于被回收利用。目前关于臭氧/过硫酸盐[8-9]、臭氧/催化剂[10-11]和过硫酸盐/催化剂[12-13]工艺的研究已有许多报道,但关于臭氧/过硫酸盐/催化剂工艺的研究并不多,同时该工艺中引入了较多的变量,因此变量间的交互效应是需要重点考虑的问题。
本研究通过对比臭氧/过硫酸盐/四氧化三铁(O3/PDS/Fe3O4)与其他3种子体系的结晶紫降解能力,考察臭氧流量、过硫酸盐浓度和四氧化三铁浓度和pH对结晶紫降解的影响;基于响应面法建立各操作条件与结晶紫降解率的多元二次回归模型,分析各因素之间交互效应的程度,并对O3/PDS/Fe3O4工艺降解结晶紫的操作参数进行优化,确定最优工艺条件;利用SEM、Raman和FT-IR表征Fe3O4反应前后的变化,使用EPR技术直接鉴定降解工艺过程中产生的自由基,探索O3/PDS/Fe3O4工艺的催化反应机理,以期为结晶紫废水的深度处理提供理论参考。
-
过硫酸钠(Na2S2O8 CAS: 7775-27-1)、结晶紫(C25H30N3Cl CAS号: 548-62-9)、氢氧化钠(NaOH CAS: 1310-73-2)、5,5-二甲基-1-吡咯啉-N-氧化物(C6H11NO CAS号: 222-011-1)购自麦克林,200目四氧化三铁粉(Fe3O4 CAS号: 1317-61-9)由清河县安迪金属材料有限公司制造,实验用水为超纯水。SW 004-10G型臭氧发生器,购自青岛维斯特电子净化设备有限公司;P901型酸度计,购自上海佑科仪器仪表有限公司;T2600型紫外-可见分光光度计,购自上海佑科仪器仪表有限公司;Milli-Q Integral 5型高纯水超纯水一体化系统,法国默克密理博公司;LZB-4型玻璃转子流量计,购自常州双环热工仪表有限公司;SN-MS-10L型磁力搅拌器,购自上海尚仪仪器设备有限公司;EMX nano型电子顺磁共振波谱仪(EPR),购自德国布鲁克(北京)科技有限公司。
-
实验以自制玻璃钢圆柱反应器,先将适量Fe3O4颗粒加入1.9 L的结晶紫溶液,开启磁力搅拌器,转速为300 r·min−1。废水pH值的调节使用1.0 mol·L−1的H2SO4和NaOH溶液进行粗调,使用0.1 mol·L−1的H2SO4和NaOH溶液进行微调。然后加入0.1 L适当浓度PDS溶液,定容到2 L,保证结晶紫溶液质量浓度为20 mg·L−1。打开臭氧发生器,调节适当流量通入臭氧,反应时间设定为60 min。每隔一段时间取样,经磁力除去Fe3O4粉末后注入样品瓶中,加入硫代硫酸钠(浓度为0.4 mol·L−1)终止反应,测定处理后结晶紫质量浓度。
-
采用上海佑科仪器仪表有限公司的T2600型紫外-可见分光光度计测定结晶紫浓度,对结晶紫溶液进行全波长扫描,选择587 nm为结晶紫检测波长,测得的标准曲线为Y=0.104 99X+0.009 57 (R2=0.997 59) 。根据标准曲线计算出实验样品相应的结晶紫浓度。拉曼光谱(Raman)仪器型号:HORIBA Scientific Lab RAM HR Evolution,日本Horiba Lab RAM HR Evolution,激光器波长532 nm,测试波数为50~4 000 cm−1。采用德国布鲁克(北京)科技有限公司的ENSOR型傅里叶红外光谱(FT-IR)分析仪测定反应前后的四氧化三铁的红外光谱,在干燥环境中,样品与KBr研磨充分后压片。测定波数区间为500~4 000 cm−1。扫描电镜为美国-FeI-Quanta 250 FEG,能谱仪为英国牛津-INCA-X-MAX50。使用型号为Anton Paar(安东帕) Sur PASS3的固体表面Zeta电位分析仪测定Fe3O4的Zeta电位。采用购自德国布鲁克(北京)科技有限公司的EMX nano型电子顺磁共振波谱仪(EPR)测定自由基,使用5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为捕获剂,DMPO浓度为0.1 mol·L−1。
-
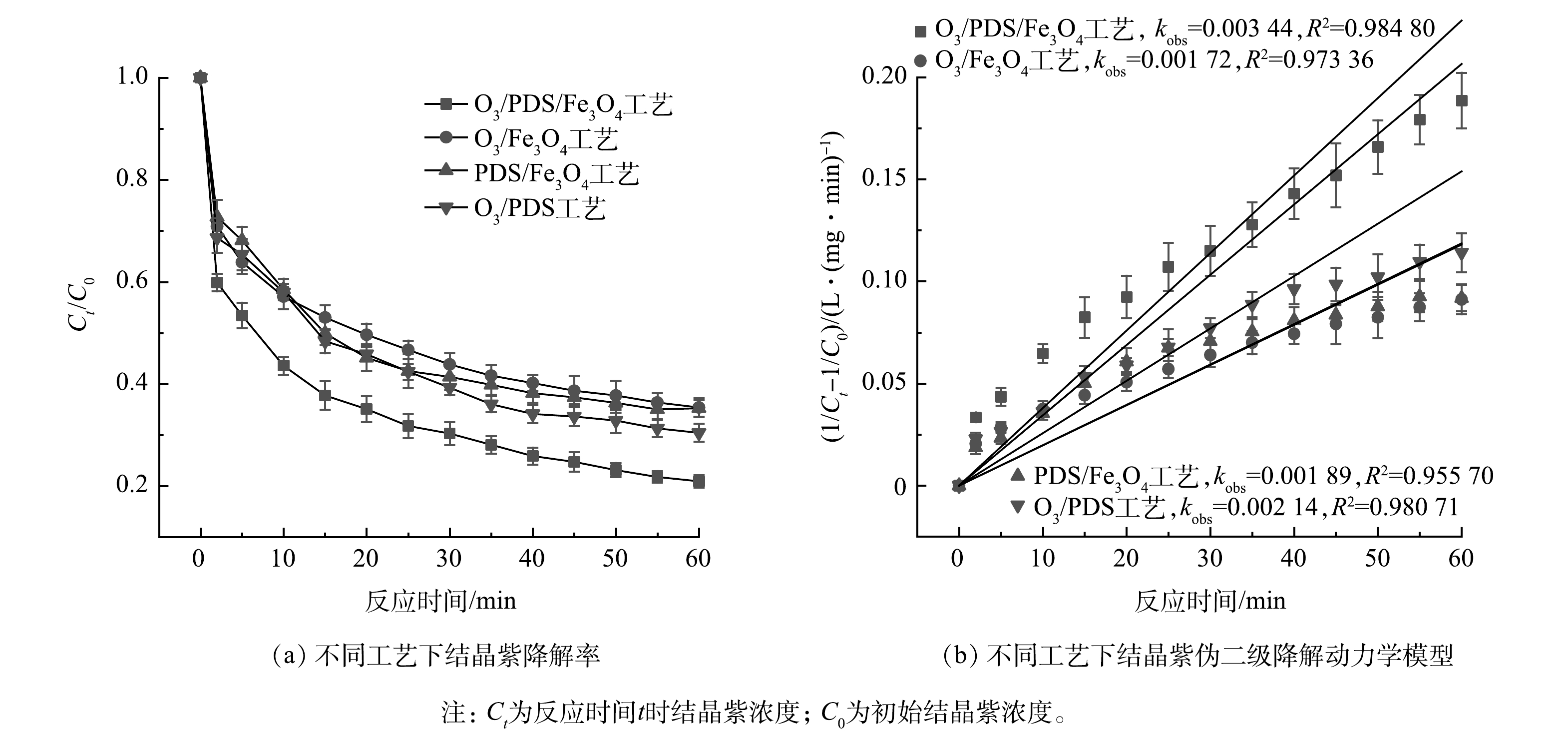
由表1可知,在结晶紫的降解遵循伪二级动力学模型(R2>0.95)。如图1所示,O3/PDS/Fe3O4工艺的降解结晶紫的伪二级速率常数(kobs)分别是O3/Fe3O4工艺、PDS/Fe3O4工艺和O3/PDS工艺的降解结晶紫kobss的1.99倍、1.82倍和1.61倍。反应60 min后,O3/PDS/Fe3O4工艺、O3/Fe3O4工艺、PDS/Fe3O4工艺和O3/PDS工艺对结晶紫的降解率分别为79.05%、63.60%、60.75%和67.60%。可以看出,相比于其他体系,O3/PDS/Fe3O4工艺具有更强的结晶紫降解能力,O3、PDS和Fe3O4会共同促进结晶紫的降解。
-
1)臭氧流量的影响。臭氧剂量由流量和气体浓度确定, 臭氧平均气体质量浓度为4.27 mg·L−1。如图2所示,结晶紫在不同臭氧流量下降解过程均符合伪二级动力学(R2>0.95)。当臭氧流量由0.0 L·min−1增至1.0 L·min−1后,结晶紫降解的伪二级速率常数kobs从0.001 63提高到0.008 74 L·(mg·min)−1,在反应时间为60 min时,结晶紫的降解率会迅速由60.75%增至89.65%。这可能是因为随着臭氧流量的增加,臭氧在水中快速溶解,大量臭氧被分解为·OH,激活PDS,产生更多的SO4·–。但是当臭氧流量继续从1.0 L·min−1增至1.2 L·min−1时,结晶紫降解的kobs从0.008 74 L·(mg·min)−1仅增至0.009 95 L·(mg·min)−1,反应时间为60 min时,结晶紫降解率从89.65%仅增至90.70%。这是因为当水中的臭氧浓度超过溶解度时,不会随臭氧流量的增加而增加。臭氧过多时会增加能耗,这是不经济的,因此,本实验选取0.6 L·min−1为后续实验的臭氧流量。
2)过硫酸盐浓度的影响。如图3所示,结晶紫在不同PDS浓度下降解过程均符合伪二级动力学模型(R2>0.96)。当PDS浓度在0~1.0 mmol·L−1区间内增加时,反应时间为60 min,结晶紫的降解率从63.60%迅速增至85.05%,结晶紫降解的kobs从0.001 74 L·(mg·min)−1增至0.004 92 L·(mg·min)−1。这是因为较多的PDS可被Fe3O4表面的Fe(Ⅱ)催化产生SO4·–。另一方面,O3溶解在PDS中可以迅速生成更多的·OH,同时·OH会活化S2O82−,生成SO4·–[14-15]。但是,PDS在达到过量浓度后,会出现污染物降解率降低现象,这是因为过量的S2O82−与SO4·–反应,形成具有较低反应性的S2O8·–自由基。但在本实验中没有发现这种现象。出于经济成本考虑,本实验选取0.4 mmol·L−1为后续实验的PDS浓度。
3)四氧化三铁浓度的影响。如图4所示,结晶紫在不同Fe3O4浓度下降解过程均符合伪二级动力学模型(R2>0.96)。当Fe3O4浓度从0增至4 mmol·L−1时,kobs从0.001 97提高到0.003 98 L·(mg·min)−1,反应时间为60 min时,结晶紫降解率从67.60%提高到81.8%。因此随着Fe3O4浓度的增加,表面的反应活性位点增加,这促进了自由基的产生,从而提高结晶紫降解率[16]。当Fe3O4浓度继续从4 mmol·L−1增至8 mmol·L−1时,kobs从0.003 98 L·(mg·min)−1降至0.002 66 L·(mg·min)−1,反应时间为60 min时,结晶紫降解率反而从81.8%降至73.95%。这是因为Fe3O4浓度过高会产生大量的·OH,它们相互淬灭,使得臭氧无效分解逐渐增加[17]。此外,过高的Fe3O4浓度可能导致团聚导致Fe3O4比表面积的减少,影响臭氧分解。因此,选择2 mmol·L−1为后续实验的Fe3O4浓度。
4)初始pH的影响。自然水体和污水厂出水的pH多介于3~11之间,本研究探索了结晶紫在此pH范围内的降解情况。如图5所示,在O3/PDS/Fe3O4工艺中,结晶紫在不同初始pH下降解过程均符合伪二级动力学模型(R2>0.97)。可以看出,当工艺初始pH从3增至4时,结晶紫降解的kobs分别从0.002 61 L·(mg·min)−1降至0.002 02 L·(mg·min)−1,结晶紫降解率从75.80%降至68.50%。测定Fe3O4在不同pH下的Zeta电位,得出四氧化三铁pHpzc约为6.5,当pH<pHpzc时,Fe3O4具有较多的正表面电荷。因此,酸性pH=3条件更有利于阴离子S2O82−分子和Fe3O4之间的静电吸引,产生更多的自由基[18]。初始pH从4增至6.8时结晶紫降解率68.50%增至79.05%,结晶紫降解的kobs也从0.002 06 L·(mg·min)−1增至0.003 44 L·(mg·min)−1。在pH为6.8时,O3/PDS/Fe3O4工艺具有较高的降解率。这是因为,此时的pH接近Fe3O4本身的pHpzc,羟基的质子化或脱质子化不占主导地位,对O3催化活性较强,能产生更多的·OH [19-21]。当初始pH从9增至11时,结晶紫降解的kobs分别从0.002 02 L·(mg·min)−1提高到0.002 50 L·(mg·min)−1,结晶紫降解率从67.35%增至75.05%。这是因为结晶紫有2个酸解离常数,在碱性条件下,氧化剂对结晶紫阳离子结构的反应性更强,更有利于降解[22]。另一方面,氢氧根加速了臭氧分子和过硫酸盐的分解,产生更多的自由基[23]。实验结果表明,此工艺在较宽的pH区间(3~11)内均有较高的结晶紫降解能力。
-
1)实验设计。采用Design-Expert 8.0软件,以臭氧流量X1、过硫酸盐浓度X2、四氧化三铁浓度X3和反应时间X4为自变量因素,并以1、0和+1代表3种水平。以结晶紫降解率为响应值,记为变量Y。各因素和水平见表2。采用Box-Behnken Design设计4因素3水平实验,共进行29次实验,利用紫外可见分光光度计测定结晶紫的浓度,并计算其降解率。实验设计方案及结果见表3。
2) 回归模型及方差分析。本研究共设计29组实验,其中实验序号为1、7、9、16、17的实验是评估纯误差的重复实验,实验按随机顺序进行,实验方案及结果如表3所示。对表3中数据进行多元回归拟合,可以得到臭氧流量X1、过硫酸盐浓度X2、四氧化三铁浓度X3和反应时间X4与结晶紫降解率(Y)之间的多项式回归模型(式(1))。
式中:X1为臭氧流量,L·min−1;X2为过硫酸盐浓度,mmol·L−1;X3为四氧化三铁浓度,mmol·L−1;X4为反应时间,min。
为了进一步对实验结果进行系统分析,釆用Design-Expert 8.0统计软件进行方差分析并检查拟合模型的显著性,方差分析结果见表3。P值和F值代表变量的显著水平和模型方程统计显著性。F值越大,P值越小,说明该模型的显著性就越强。由表4可以看出,多项式模型的F值为197.53,且P值<0.000 1,说明拟合方程的回归性和显著性均较好。方差分析中的拟合系数R2反映模型的拟合程度,模型的有效性是由R2和R2Adj决定的[24]。模型的拟合系数R2为0.990 3,表明有99.03%的响应值可以由此方程来解释,仅有0.97%的响应值不可由此方程来解释,模型的可靠性和参考价值较高[25]。模型的R2Adj和R2Pred值分别为0.986 4和0.971 9,两者差值为0.014 5(<0.2),说明该模型的可信度、精密度和适应性较高。失拟检验项为0.202 0(>0.05),表明模型残差是由随机误差产生,模型可对O3/PDS/Fe3O4工艺过程中的结晶紫的降解率进行相关预测和分析。变异系数CV可以用来度量数据的变异性,通常小于10%为可接受范围,模型的CV为0.74%,说明模型的再现性是合理的[26]。模型的信噪比为56.840(>4),表明模型有很强的信号,而噪声较弱。
3个线性项(X1、X2和X4)、交互项X1X4和二次项X12的P均小于0.000 1。线性项X3和交互项X1X2的P分别为0.000 5和0.000 9。说明4个因素对结晶紫降解率的影响都非常显著。由F值可以看出,因素按其重要程度排列为臭氧流量(F=886.73)>反应时间(F=628.37)>过硫酸盐浓度(F=232.72)>四氧化三铁浓度(F=17.22),臭氧流量和反应时间之间具有最显著的交互效应。原因可能是,随着反应时间的增加,水中溶解臭氧浓度不断提高,可以产生较多的氧化性自由基。此外,臭氧流量和过硫酸盐之间也具有明显的交互效应。臭氧流量和过硫酸盐浓度之间存在交互效应,是因为臭氧能促使过硫酸盐分解,产生SO4·–,从而促进结晶紫的降解。
3)交互效应分析。为了更直观地说明实验因素以及臭氧流量、过硫酸盐浓度、四氧化三铁浓度和反应时间两两因素交互效应对结晶紫降解率的影响,根据所建立的回归模型绘制了响应曲面图和等高线图。曲面坡度反映各因素间交互效应对响应值结晶紫降解率的影响,呈正相关关系。响应面坡度陡峭则表明,该因素在一定范围内对结晶紫降解率影响较大;响应面坡度平缓表明,该因素在一定范围内对结晶紫降解率影响较小。由图6和图7可知,2组响应面形状均呈上凸状且具有一定坡度,说明臭氧流量、过硫酸盐浓度和反应时间对结晶紫降解率有显著影响。臭氧流量和反应时间的响应曲面较臭氧流量和过硫酸盐的响应面坡度更陡峭,因此交互效应更为显著。这和方差分析的结果是一致的。
4)残差分析。图8(a)显示了预测值和实际值的拟合情况。可以看出,模型预测值与实验实际值各点非常近似,呈45°直线分布,说明此模型可用于预测响应值[27]。图8(b)为外学生化残差的正态概率图。可以看出,图像所有残差点基本呈直线状紧密分布,残差分布可以被认为是正态分布的,这同样证实了该模型能够较好地预测响应值[28]。图8(c)为外学生化残差和预测值图。可以看出,所有外学生化残差表现为随机散点,这表明预测结果和实验实际值之间的差值较小,回归模型拟合程度较高,可以证明模型的准确性和可靠性。图8(d)为外学生化残差和实验顺序图。可以看出,外学生化残差未显示任何特定趋势,实验数据点无规律地分布在0附近,显示出残差的平均值接近0,这表明实验条件的假设是独立的。
5)结果优化及验证。在上述实验结果分析和模型拟合的基础上,对操作参数进行进一步优化。为了获得最优条件,将结晶紫降解率的期望目标设为最大值,而将其他操作参数设为在此范围内,可以得到降解结晶紫的各因子的最优运行条件:臭氧流量为1.000 L·min−1,过硫酸盐浓度为0.968 mmol·L−1,四氧化三铁浓度为2.158 mmol·L−1,反应时间为41.702 min。根据预测模型,求出优化降解率为89.099%。为了验证多项式回归模型的准确性和实用性,利用上述拟合的反应条件,进行3次平行实验,结晶紫降解率平均值为88.10%,与模型预测值的相对偏差仅为−1.12%,该值在可接受的误差范围内。这说明模型具有良好的拟合性和显著性,预测模型可以较好地预测实验结果。
-
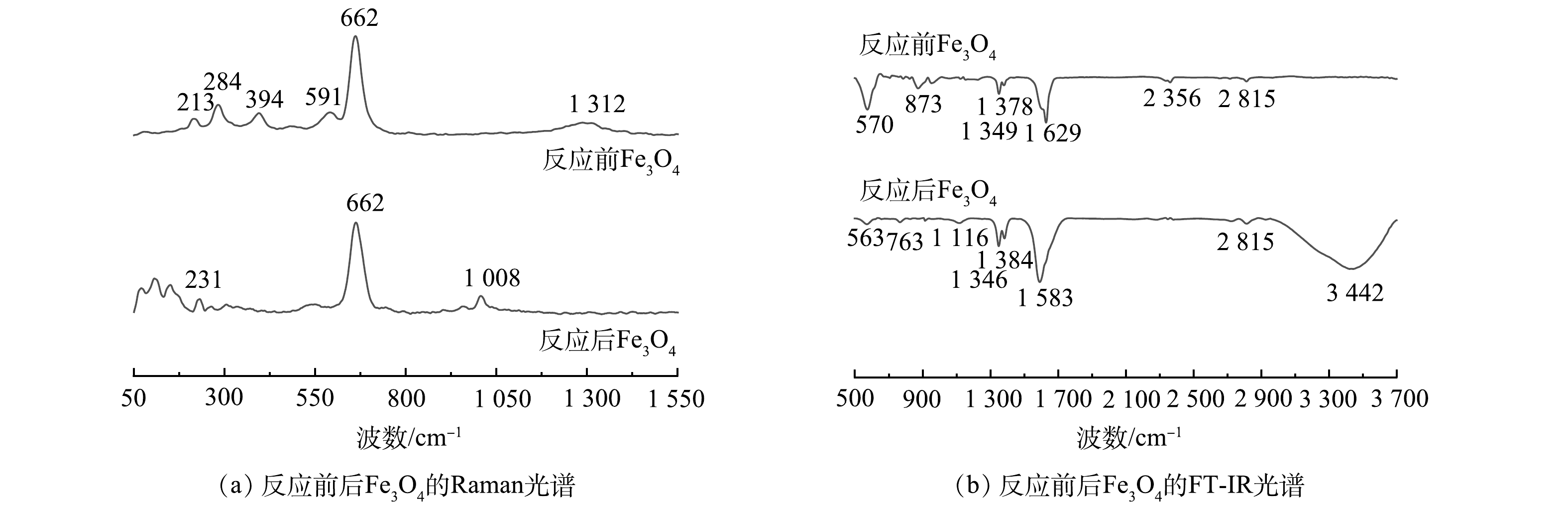
图9为扫描电镜观察到的Fe3O4表面形貌及尺寸。反应前Fe3O4颗粒呈沟壑状存在,尺寸约为75 μm。增加电镜放大倍数后,可观察到Fe3O4颗粒表面有疏松团絮状的纳米级至微米级小颗粒。表面的小颗粒呈无序的密集分布,使得Fe3O4颗粒拥有较大的比表面积。在反应后,可以明显看出,Fe3O4的表面粘连的小颗粒逐渐消失,表面变得更加光滑,粒径略微减小到60 μm左右。使用X射线能谱(EDS)来进一步确定反应前后Fe3O4的元素组成。EDS数据结果表明,反应后Fe3O4的Fe元素质量分数从48.24%降至35.31%,这可能是由于Fe3O4在PDS营造的酸性环境中会溶出部分Fe2+造成的。反应后Fe3O4的O元素质量分数从34.05%增至37.59%,这可能是Fe3O4被氧化为γ-Fe2O3或α-Fe2O3所致。硫元素的质量分数从0.39%增至1.09%,原因可能是Fe3O4表面产生了少量的施威特曼石(Fe8O8(OH)6SO4)。
图10(a)为反应前后Fe3O4中的Raman光谱。213 cm−1和231 cm−1左右处的拉曼峰代表γ-Fe2O3和α-Fe2O3的A1g模式振动,284 cm−1的拉曼峰代表α-Fe2O3的E1g模式弯曲振动峰[29-30]。1 313 cm−1的峰代表α-Fe2O3和γ-Fe2O3的A1g峰,这是由声子二次散射振动引起的。这2处α-Fe2O3峰的消失可能是O3或PDS将Fe(Ⅲ)还原为Fe(Ⅱ)造成的。394 cm−1和591 cm−1的拉曼峰代表Fe3O4的T2g对称振动,可以看出,反应后属于Fe3O4的拉曼峰部分消失,这是由于Fe3O4表面Fe(Ⅱ)在电子转移过程中转变为Fe(Ⅲ),使得Fe3O4大量转化为γ-Fe2O3和α-Fe2O3。在662 cm−1处的拉曼峰代表Fe3O4的Fe—O拉曼活性的A1g对称模式呼吸振动,是Fe3+在四面体位置(Fe3+—O2−)的对称拉伸[31-32]。
图10(b)为反应前后Fe3O4中FT-IR光谱。3 442 cm−1的红外峰为化学吸附水的O—H拉伸振动,此处的红外峰反应后O—H基团强度明显提高[33]。1 629 cm−1和1 583 cm−1的吸收峰为化学吸附水的O—H弯曲振动,反应后O—H基团强度也得到提高,原因可能是,反应过程中有少量水分吸附在Fe3O4表面。2 356 cm−1的吸收峰代表FeOOH的Fe—OH键,与未反应Fe3O4相比,反应后Fe3O4的FeOOH峰的强度消失。原因可能是,臭氧通过静电吸附与水竞争,取代这些表面羟基,产生了·OH,使得FeOOH转化成了更为稳定的γ-Fe2O3,说明表面羟基是O3/PDS/Fe3O4过程中的活性位点。763 cm−1和873 cm−1的吸收峰代表Fe—OH的弯曲振动,可以看出,反应后此处峰强下降,并向低波长方向移动,与上述结果一致。位于1 346~1 384 cm−1的红外峰为γ-Fe2O3的Fe—O峰收缩振动产生的谱带,可以看出,反应后峰强提高,可能是Fe3O4或FeOOH被氧化而形成的。563 cm−1和570 cm−1的吸收峰为Fe3O4的Fe—O伸缩振动,可以看出,反应后的Fe—O峰变弱。由此推断,有部分Fe3O4通过电子转移过程催化臭氧和过硫酸盐,从而被氧化生成了γ-Fe2O3,这与拉曼光谱结果[34]一致。1 116 cm−1处产生的新峰属于施威特曼石Fe8O8(OH)6SO4的S—O键伸缩振动峰,Fe8O8(OH)6SO4会在酸性溶液且存在大量SO42−和Fe3+的溶液中生成,这与EDS的结果一致。
采用EPR技术直接识别SO4·−和·OH,以5,5-二甲基1-吡咯啉-N-氧化物(DMPO)为主要自旋捕集剂。由图11可以看出,在O3/PDS/Fe3O4工艺降解结晶紫的过程中,DMPO与体系中的·OH和SO4·−反应,产生强度比为1∶2∶2∶1的DMPO-·OH峰和1∶1∶1∶1∶1∶1的DMPO-SO4·−加合物的峰,因此可以证明在O3/PDS/Fe3O4过程中SO4·−和·OH的存在。
-
1) 在O3/PDS/Fe3O4工艺过程中,较高的臭氧流量、过硫酸盐浓度和四氧化三铁浓度可以提高结晶紫的降解率,在较宽的pH区间(3~11)内均有较高的结晶紫降解性能。
2) 采用Box-Behnken响应面设计,建立回归模型(R2=0.990 3)来预测结晶紫的降解效率,模型的R2Adj和R2Pred值分别为0.986 4和0.971 9,两者差值为0.014 5(<0.2),模型失拟检验项为0.202 0(>0.05),模型的变异系数CV为0.74%(<10%),信噪比为56.840(>4),都在可接受范围内。
3) Box-Behnken Design模型的残差符合随机分布和正态分布的规律,说明模型具有合理的再现性和良好的预测性。操作参数按重要程度排列为臭氧流量(F=886.73)>反应时间(F=628.37)>过硫酸盐浓度(F=232.72)>四氧化三铁浓度(F=17.22);臭氧流量和反应时间的交互效应对结晶紫降解的影响最显著,其次是臭氧流量和过硫酸盐的交互效应。
4) Box-Behnken Design模型预测最佳条件:臭氧流量1.000 L·min−1,过硫酸盐浓度0.968 mmol·L−1,四氧化三铁浓度2.158 mmol·L−1,反应时间41.702 min。根据预测模型,求出优化降解率为89.099%。在上述反应条件下,3次实验的结晶紫降解率平均值为88.10%,与模型预测值的相对偏差仅为-1.12%。
5) SEM-EDS、Raman和FT-IR表征结果表明,反应后Fe3O4的表面变得更加光滑,铁元素质量分数从48.24%降至35.31%,而氧和硫元素质量分数分别从34.05%和0.39%增至37.59%和1.09%,Fe3O4在催化过程中部分转变为Fe2O3,此外,Fe3O4表面的羟基基团的存在也对臭氧和过硫酸盐催化有重要影响。采用EPR技术鉴定出臭氧/过硫酸盐/四氧化三铁工艺过程中存在SO4·−和·OH。
基于响应曲面法优化的臭氧/过硫酸盐/四氧化三铁工艺对结晶紫的降解
Optimization of crystal violet degradation in ozone/persulfate/ferroferric oxide system by response surface methodology
-
摘要: 为有效去除水中结晶紫,利用臭氧/过硫酸盐/四氧化三铁工艺对结晶紫的氧化效果进行研究,设计单因素实验探索臭氧流量、过硫酸盐浓度、四氧化三铁浓度和pH对结晶紫降解的影响,依据响应曲面法的Box-Behnken Design(BBD)实验设计原理,探究臭氧流量、过硫酸盐浓度、四氧化三铁浓度和反应时间对降解效果的影响,并优化工艺参数;使用SEM-EDS、FT-IR和Raman表征了反应前后的四氧化三铁,并用EPR技术直接鉴定出工艺过程中的活性氧。结果表明:此工艺在较宽的pH区间(3~11)都具有较高的结晶紫降解能力,臭氧流量、过硫酸盐浓度和四氧化三铁浓度与结晶紫的降解率成正比;臭氧流量1.000 L·min−1,过硫酸盐浓度0.968 mmol·L−1,四氧化三铁浓度2.158 mmol·L−1,反应时间41.702 min为预测的最佳工艺条件;在最佳工艺条件下得到的实际降解率与预测降解率相对偏差仅为−1.12%;催化反应后Fe3O4粒径减小,表面变得更加光滑;反应后的Fe3O4的铁元素质量分数由48.24%降至35.31%,而氧和硫元素质量分数由34.05%和0.39%分别增至37.59%和1.09%;臭氧/过硫酸盐/四氧化三铁工艺过程中存在SO4·–和·OH。由此可知,BBD优化模型预测与实际处理效果基本一致。该研究成果为可为难降解的结晶紫废水的深度处理提供参考。Abstract: In order to effectively remove crystalline violet from water, the ozone/persulfate/ferroferric oxide process was used to investigate the effects of ozone flow rate, persulfate concentration, ferroferric oxide concentration and pH on the degradation of crystalline violet, then the Box-Behnken Design (BBD) experimental design principle of response surface method was used to determine the effects of ozone flow rate, persulfate concentration, ferroferric oxide concentration and reaction time on the degradation of crystalline violet and optimize the process parameters. The tetroxide before and after the reaction was characterized by SEM-EDS, FT-IR and Raman, and the reactive oxygen species in the process was identified directly by EPR technique. The results showed that this process had a good ability on crystalline violet degradation over a wide pH range (3~11), and the ozone flow rate, persulfate concentration and tetroxide concentration were proportional to the degradation rate of crystalline violet. The model predicted that the optimum process conditions were following: ozone flow rate of 1.000 L·min−1, persulfate concentration of 0.968 mmol·L−1, ferroferric oxide concentration of 2.158 mmol·L−1, and reaction time of 41.702 min. The relative deviation of the actual degradation rate from the predicted degradation rate under the optimum conditions was only −1.12%. After the catalytic reaction, the particle size of Fe3O4 decreased and its surface became smoother, the mass fraction of Fe3O4 decreased from 48.24% to 35.31%, while the mass fractions of oxygen and sulfur increased from 34.05% and 0.39% to 37.59% and 1.09%, respectively. SO4·– and ·OH occurred in the ozone/persulfate/ferroferric oxide process. It can be seen that the prediction of BBD optimization model is basically consistent with the actual treatment effect; this study can provide a reference for the deep treatment of refractory crystalline violet wastewater.
-
Key words:
- response surface /
- ozone /
- persulfate /
- ferroferric oxide /
- crystal violet
-

-
表 1 不同工艺过程中结晶紫降解的伪一级动力学和伪二级动力学
Table 1. Pseudo-first-order kinetics and pseudo-second-order kinetics for crystal violet degradation of in different systems
反应体系 伪一级动力学 伪二级动力学 拟合方程 拟合系数 拟合方程 拟合系数 O3/PDS/Fe3O4工艺 −ln(Ct/C0)=0.032 65t 0.929 37 $1/C_t- 1/C_0=0.003 \;44t$ 0.984 80 O3/Fe3O4工艺 −ln(Ct/C0)=0.021 23t 0.934 23 $1/C_t- 1/C_0=0.001\; 73t$ 0.973 36 PDS/Fe3O4工艺 −ln(Ct/C0)=0.023 00t 0.914 68 $1/C_t- 1/C_0=0.001 \;89t$ 0.955 70 O3/PDS工艺 −ln(Ct/C0)=0.023 98t 0.940 85 $1/C_t- 1/C_0=0.002 \;14t$ 0.980 71 表 2 实验因素和水平
Table 2. Experimental factors and levels
水平 因素 臭氧流量X1/(L·min−1) 过硫酸盐浓度X2/(mmol·L−1) 四氧化三铁浓度X3/(mmol·L−1) 反应时间X4/min −1 0.6 0.4 2 20 0 0.8 0.7 3 40 1 1.0 1.0 4 60 表 3 实验设计及结果
Table 3. Experimental design and results
序号 臭氧流量/
(L·min−1)过硫酸
盐浓度/
(mmol·L−1)四氧化
三铁浓度/
(mmol·L−1)反应时
间/min实际
值/%预测
值/%1 0.8 0.7 3 40 78.25 77.76 2 0.8 0.4 3 60 79.80 80.40 3 1.0 1.0 3 40 88.15 88.63 4 0.6 0.7 3 60 80.90 81.81 5 0.8 0.4 4 40 75.50 75.41 6 0.8 1.0 2 40 82.15 82.03 7 0.8 0.7 3 40 78.15 77.76 8 0.6 1.0 3 40 80.2 80.76 9 0.8 0.7 3 40 78.10 77.76 10 0.8 1.0 4 40 80.6 80.62 11 0.6 0.7 4 40 75.95 75.34 12 1.0 0.7 4 40 85.85 85.50 13 0.8 0.7 4 20 72.55 72.77 14 0.8 0.4 3 20 71.25 71.84 15 1.0 0.7 3 20 83.3 83.42 16 0.8 0.7 3 40 77.25 77.76 17 0.8 0.7 3 40 78.05 77.76 18 0.6 0.7 3 20 69.55 70.28 19 1.0 0.7 3 60 88.70 89.00 20 1.0 0.7 2 40 86.85 86.92 21 0.8 1.0 3 60 85.85 85.61 22 0.8 0.7 2 60 82.95 82.75 23 0.6 0.4 3 40 73.80 73.26 24 0.8 0.4 2 40 76.75 76.83 25 1.0 0.4 3 40 86.35 85.72 26 0.8 0.7 2 20 73.55 74.19 27 0.8 1.0 3 20 77.75 77.05 28 0.8 0.7 4 60 81.10 81.33 29 0.6 0.7 2 40 77.80 76.75 表 4 方差分析
Table 4. Analysis of variance
方差
来源平方
和自由
度均方 F值 P值 显著性 模型 711.54 8 88.94 254.35 <0.000 1 非常显著 X1 310.08 1 310.08 886.73 <0.000 1 非常显著 X2 81.38 1 81.38 232.72 <0.000 1 非常显著 X3 6.02 1 6.02 17.22 0.000 5 非常显著 X4 219.74 1 219.74 628.37 <0.000 1 非常显著 X1X2 5.29 1 5.29 15.13 0.000 9 非常显著 X1X4 8.85 1 8.85 25.31 <0.000 1 非常显著 X12 78.41 1 78.41 224.23 <0.000 1 非常显著 X22 6.42 1 6.42 18.35 0.000 4 非常显著 残差 6.99 20 0.35 失拟项 6.34 16 0.40 2.43 0.202 0 不显著 纯误差 0.65 4 0.16 总离差 718.54 28 -
[1] 徐圣东, 周金洋, 王丽, 等. 猴头菌和金针菇漆酶对不同染料的降解[J]. 菌物学报, 2021, 40(6): 1525-1537. [2] 陈智超, 陈坤, 杨承峰, 等. 磁混凝工艺在山东某污水处理厂提标改造中的应用[J]. 工业水处理, 2023, 43(3): 181-185. [3] 陈新. 阴离子聚丙烯酰胺P(AM-IA-AMPS)的制备及应用研究[D]. 重庆: 重庆大学, 2019. [4] 刘汝鹏, 宋依辉, 陈飞勇, 等. 水动力传质在臭氧氧化水处理工艺的研究进展[J]. 净水技术, 2023, 42(2): 14-22. [5] GUO H, LI Z, XIANG L, et al. Efficient removal of antibiotic thiamphenicol by pulsed discharge plasma coupled with complex catalysis using nanocomposites[J]. Journal of Hazardous Materials, 2021, 403: 123673. [6] HU L, WANG P, LIU G, et al. Catalytic degradation of p-nitrophenol by magnetically recoverable Fe3O4 as a persulfate activator under microwave irradiation[J]. Chemosphere, 2020, 240: 124971-124977. [7] 冯华良, 毛文龙, 王晓君, 等. 不同臭氧催化氧化体系处理老龄垃圾渗滤液的效果及能耗分析[J]. 环境工程学报, 2020, 14(10): 2689-2700. [8] 刘汝鹏, 张震, 宋依辉, 等. 基于臭氧的复合工艺处理医药废水研究进展[J]. 工业水处理, 2022, 42(5): 41-49. [9] 刘东坡, 陈伟锐, 王静, 等. 铁锌共掺杂MCM-41构建双酸性中心及其催化臭氧化布洛芬[J]. 环境工程学报, 2022, 16(9): 2850-2861. [10] 刘汝鹏, 张震, 孙翠珍, 等. 非均相催化臭氧化水中药物与个人护理品的研究进展[J]. 精细化工, 2022, 39(3): 469-479. [11] 张震, 刘汝鹏, 孙翠珍, 等. 铁基材料协同活化过硫酸盐研究进展[J]. 环境科学与技术, 2022, 45(9): 169-180. [12] 谭凤训, 陈永凯, 王榕, 等. g-C3N4/PDS光催化降解阿特拉津的效能及机理研究[J]. 中国给水排水, 2023, 39(1): 91-98. [13] AMR S, AZIZ H A, ADLAN M N. Optimization of stabilized leachate treatment using ozone/persulfate in the advanced oxidation process[J]. Waste Management, 2013, 33(6): 1434-1441. [14] WEN G, QIANG C, FENG Y, et al. Bromate formation during the oxidation of bromide-containing water by ozone/peroxymonosulfate process: Influencing factors and mechanisms[J]. Chemical Engineering Journal, 2018, 352: 316-324. [15] YIN R, GUO W, ZHOU X, et al. Enhanced sulfamethoxazole ozonation based on magnetic Fe3O4 nanoparticles by noble-metal-free catalysis: Catalytic performance and degradation mechanism[J]. RSC Advances, 2016, 6(23): 19265-19270. [16] QI F, CHU W, XU B. Ozonation of phenacetin in associated with a magnetic catalyst CuFe2O4: The reaction and transformation[J]. Chemical Engineering Journal, 2015, 262: 552-562. [17] ZHENG H, DU J, ZHONG H, et al. Enhanced persulfate activation by sulfur-modified Fe3O4 composites for atrazine degradation: Performance and mechanism[J]. Process Safety and Environmental Protection, 2023, 170: 1052-1065. [18] BAI Z, YANG Q, WANG J. Catalytic ozonation of sulfamethazine using Ce0.1Fe 0.9OOH as catalyst: Mineralization and catalytic mechanisms[J]. Chemical Engineering Journal, 2016, 300: 169-176. [19] BAI Z Y, YANG Q, WANG J L. Fe3O4/multi-walled carbon nanotubes as an efficient catalyst for catalytic ozonation of p-hydroxybenzoic acid[J]. International Journal of Environmental Science and Technology, 2015, 13: 483-492. [20] 刘静, 杨璐冰, 李晨, 等. ML-WO3/TiO2异质结的制备及其对罗丹明B的光催化降解[J]. 精细化工, 2022, 39(12): 2456-2466. [21] ABDI M, BALAGABRI M, KARIMI H, et al. Degradation of crystal violet (CV) from aqueous solutions using ozone, peroxone, electroperoxone, and electrolysis processes: A comparison study[J]. Applied Water Science, 2020, 10(7): 1-10. [22] CASTRO J, PAZ S, MENA N, et al. Evaluation of heterogeneous catalytic ozonation process for diclofenac degradation in solutions synthetically prepared[J]. Environmental Science and Pollution Research, 2019, 26(5): 4488-4497. [23] GUO W, REN N, WANG X, et al. Optimization of culture conditions for hydrogen production by Ethanoligenens harbinense B49 using response surface methodology[J]. Bioresource Technology, 2009, 100(3): 1192-1196. [24] GRCIC I, VUJEVIC D, AEPCIC J, et al. Minimization of organic content in simulated industrial wastewater by Fenton type processes: A case study[J]. Journal of Hazardous Materials, 2009, 170(2): 954-961. [25] ZINATIZADEH A, MOHAMED A R, ABDULLAH A Z, et al. Process modeling and analysis of palm oil mill effluent treatment in an up-flow anaerobic sludge fixed film bioreactor using response surface methodology (RSM)[J]. Water Research, 2006, 40(17): 3193-3208. [26] MICHAELIS M, LEOPOLD C S. A measurement system analysis with design of experiments: Investigation of the adhesion performance of a pressure sensitive adhesive with the probe tack test[J]. International Journal of Pharmaceutics, 2015, 496(2): 448-456. [27] KHATAEE A R, FATHINIA R, ABER R, et al. Optimization of photocatalytic treatment of dye solution on supported TiO2 nanoparticles by central composite design: Intermediates identification[J]. Journal of Hazardous Materials, 2010, 181(1/2/3): 886-897. [28] CHRISTIE T C, SUNDARAMURTHY J, KALAIVANI M. Electrospun α-Fe2O3 nanorods as a stable, high capacity anode material for Li-ion batteries[J]. Journal of Materials Chemistry, 2012, 22: 12198-12204. [29] TRPKOV D, PANIAN M, KOPANJA L, et al. Hydrothermal synthesis, morphology, magnetic properties and self-assembly of hierarchical α-Fe2O3 (hematite) mushroom-, cube- and sphere-like superstructures[J]. Applied Surface Science, 2018, 457: 427-438. [30] KACZMARCZYK J, ZASADA F, JANAS J, et al. Thermodynamic stability, redox properties, and reactivity of Mn3O4, Fe3O4, and Co3O4 model catalysts for N2O decomposition: Resolving the origins of steady turnover[J]. ACS Catalysis, 2016, 6(2): 1235-1246. [31] LIN X, WANG X, ZHOU Q, et al. Magnetically recyclable MoS2/Fe3O4 hybrid composite as visible light responsive photocatalyst with enhanced photocatalytic performance[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1673-1682. [32] XIAO F, WANG Y, XIE X, et al. Preparation of Fe/C-Mt composite catalyst and ofloxacin removal by peroxymonosulfate activation[J]. Separation and Purification Technology, 2022, 298: 121548. [33] KREHULA S, MUSIC S, SKOKO Z, et al. The influence of Zn-dopant on the precipitation of α-FeOOH in highly alkaline media[J]. Journal of Alloys and Compounds, 2006, 420(1): 260-268. -



 下载:
下载: