-
氟是自然界的微量元素之一,氟在自然界中以萤石(CaF2)、冰晶石、氟磷灰石等形式存在,这些矿物埋藏在地下,随着地下水的侵蚀与沉积,F−被溶出并富集至地下水中,使得水中的F−浓度偏高。我国高氟水分布十分广泛,尤其集中在北方地区,比如辽宁省中部,山东省西南部,围绕渤海地区以及广袤的西北地区都有大面积的高氟地下水区域,这些含氟地下水不仅给当地居民的饮水带来诸多不便,而且会给当地的基础建设和经济发展带来阻碍,根据资料显示:氟与人体的身体健康密切相关,人体每天需要摄取的氟含量大约为0.1 mg,大约90%摄入的氟会随身体新陈代谢排除体外,留下的大部分氟会存在于身体的骨骼和牙齿之中,人体摄入氟的最主要途径是饮食,这也是地方性氟中毒的主要原因,因此需要确保饮用水氟安全,过量摄入氟化物会导致人体骨骼、牙齿、肝脏、肾脏、大脑等的多器官毒性[1],目前仍缺乏有效的治疗手段,除氟技术可以有效解决饮用水中氟含量超标的问题。
目前国内主要的除氟方法有吸附法[2]、混凝沉淀法[3]、离子交换法[4]、反渗透[5]等,吸附法由于其方法便捷、经济高效等原因是目前饮用水除氟的主要方式。目前主要的吸附剂有铝基金属材料、天然矿石材料等, SANINI等[6]利用CeO2改性活性氧化铝来去除水中氟离子; AYALEW 等[7]比较了高岭土和石灰石吸附剂对地下水中的除氟效果;LAONAPAKUL等[8]通过煅烧高岭土/羟基磷灰石复合材料来吸附除氟;THIRUNAVUKKARASU等[9]对生物吸附做了系统性探讨,阐述了生物吸附未来前景和发展潜力;AMIN等[10]利用白腐菌杏鲍菇来吸附水溶液中的氟;韩晓峰等[11]采用浸渍法制备了负载镧镁的活性氧化铝吸附剂,该吸附剂在3 h内可将10 mg·L−1的含氟溶液中的氟离子去除95%左右;BAKHTA等[12]利用通过金属浸渍改性活性炭可提高其吸附性能,是一种很有前途的水处理材料。目前研究报道的大多数吸附剂存在以下缺点:1)吸附量不足,吸附效率不能满足人们的需求。2)抗干扰离子能力弱,选择性差。3) pH适宜范围窄,尤其是生物吸附剂,水相pH对吸附剂吸附性能有很大影响,一般pH越低,除氟性能越好4)材料获取困难,制作过程复杂。5)铝基金属吸附材料会有Al3+溶出的问题,不仅会造成2次污染,而且长期饮用含高铝离子的水会给人体带来危害。因此选择一种高效、安全的饮用水除氟剂是吸附法的关键。
MgO作为我国储量很大的金属氧化物,具有高吸附能力、强亲和力、热稳定性,是一种十分安全并具有发展潜力的吸附除氟材料。刘理华等[13]研究了棒状改性氧化镁及其吸附性能,发现经过改性后的氧化镁有良好的吸附活性;王慧玲等[14]利用酸改性活性氧化镁,改性后吸附容量有所提高。本实验为了提高活性氧化镁的吸附容量和吸附效率,扩充活性氧化镁适宜pH的范围,增强活性氧化镁与其他离子共存时的吸附能力,提高其选择性和除氟稳定性,以活性氧化镁作为多孔性载体,通过铁盐,钙盐浸渍使其附着在多孔性载体上或者内表面,再通过焙烧制得改性吸附剂,探讨其最佳制备条件,并通过扫描电镜,X射线粉末衍射仪,傅里叶变换红外光谱仪对吸附剂进行表征。
-
氧化镁,柠檬酸三钠,冰乙酸,氯化钠,氯化铁,氯化钙,氢氧化钠,氟化钠,碳酸钠,硝酸钠,硫酸钠,磷酸钠,盐酸均为分析纯。
CJJ78-1磁力搅拌器;PF-2-01氟离子选择电极;232参比电极;Multi3620 pH计;PX223ZH/E电子分析天平;DHG-9013A恒温鼓风干燥箱;SX2-2.5-10箱式高温电阻炉;HWS-26恒温水浴锅;D8 advance X射线粉末衍射仪;MERLIN compact 扫描电子显微镜;is5 傅里叶变换红外光谱仪。
-
将氧化镁在420 ℃下灼烧1.5 h得到活性氧化镁,将活性氧化镁与金属盐浸渍液按照1 g:20 mL的比例混合,并置于磁力搅拌器上搅拌2 h,静置,待出现明显固液分离,用吸管将上清液吸出,将剩余含水的粘稠物质放入烘箱在105 ℃下烘干12 h,将干燥固体取出研磨成粉末,放入马弗炉内,在一定温度下高温焙烧2 h,制得改性活性氧化镁吸附剂。
对单一含氟水和模拟含氟饮用水用改性活性氧化镁吸附除氟,用盐酸和氢氧化钠溶液调节pH,单一含氟水中只含有氟离子,模拟含氟饮用水中杂质离子有Cl−、CO32−、SO42−、NO3−、HPO42−、Na+。在实验过程中,改变吸附时间、吸附剂投加量、pH等实验因素以进行不同条件下的吸附实验对比,吸附后,使用电极法测试滤液的氟离子浓度,计算除氟率和氟吸附量,每组实验重复3次,取平均值作为最终结果。
-
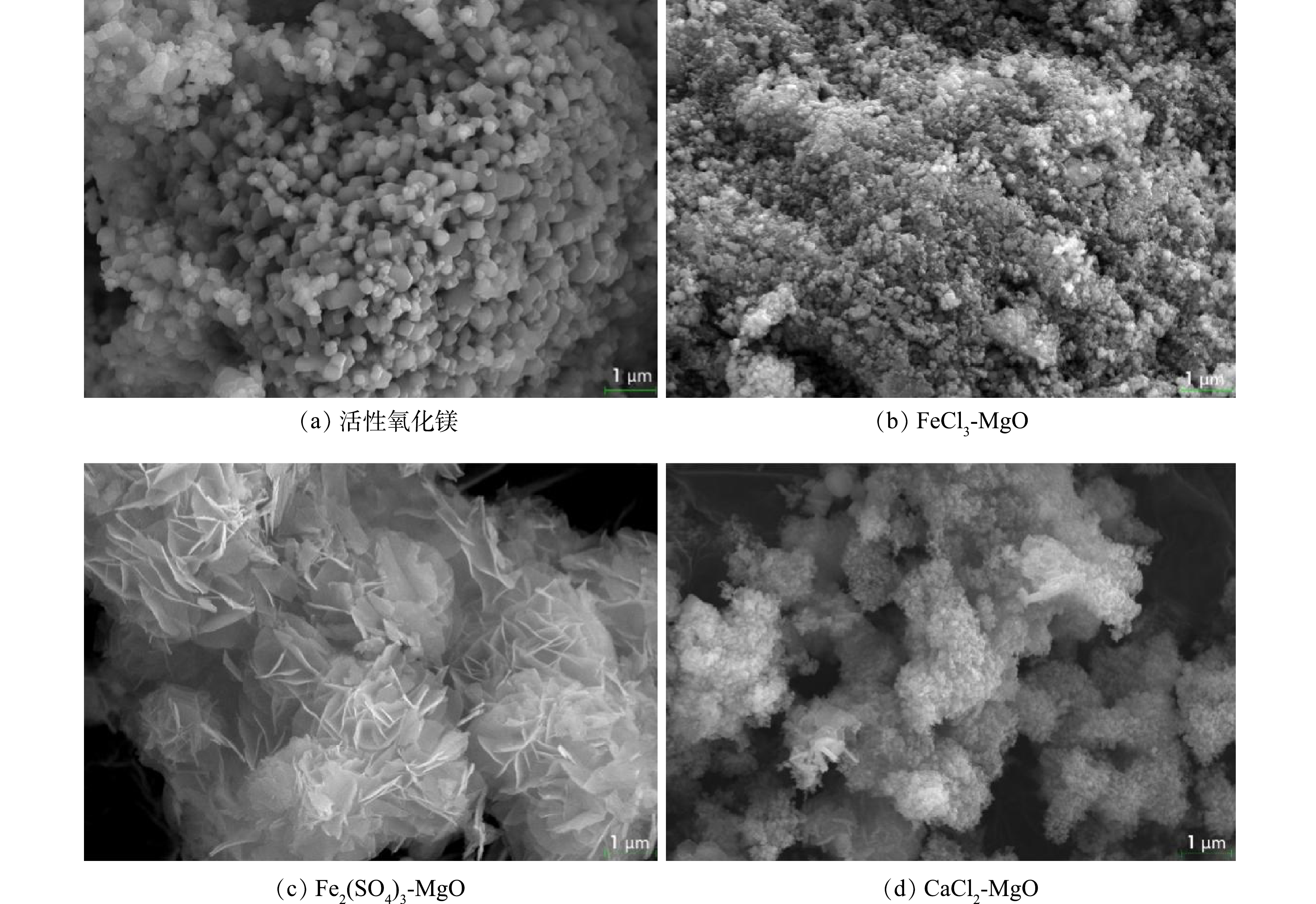
活性氧化镁、FeCl3-MgO、Fe2(SO4)3-MgO、CaCl2-MgO SEM表征结果如图1所示,活性氧化镁在电镜下结构松散,颗粒之间空隙较大,改性吸附剂表面变得粗糙,表面有凸起的颗粒,比表面积变得更大。BET比表面积测试结果表明,活性氧化镁、FeCl3-MgO、CaCl2-MgO的比表面积分别为17.724、47.423、56.642 m2·g−1,改性后的吸附剂比表面积均提升了2倍以上,特别是经过Fe2(SO4)3改性后的吸附剂,由于活性氧化镁在与水改性过程中生成了具有层状结构的氢氧化镁,而通过Fe2(SO4)3 改性过程中并没有破坏这一结构,而是在此结构上完成了负载,再经过高温焙烧后失去水时的层状结构被破坏转为交错的玫瑰状,使得比表面积进一步增大[15],比表面积由改性前的17.724 m2·g−1提高至62.492 m2·g−1。负载在氧化镁表面的铁盐和钙盐增加了吸附剂整体的正电性,F−本身带有负电,彼此之间的吸引有利于F−游离至改性吸附剂附近,改性吸附剂更大、更粗糙的比表面积有利于更多的与F−接触,使得吸附初期F−与吸附剂表面接触更加频繁,同时Fe与 F−直接的络合反应加快了反应速率。
-
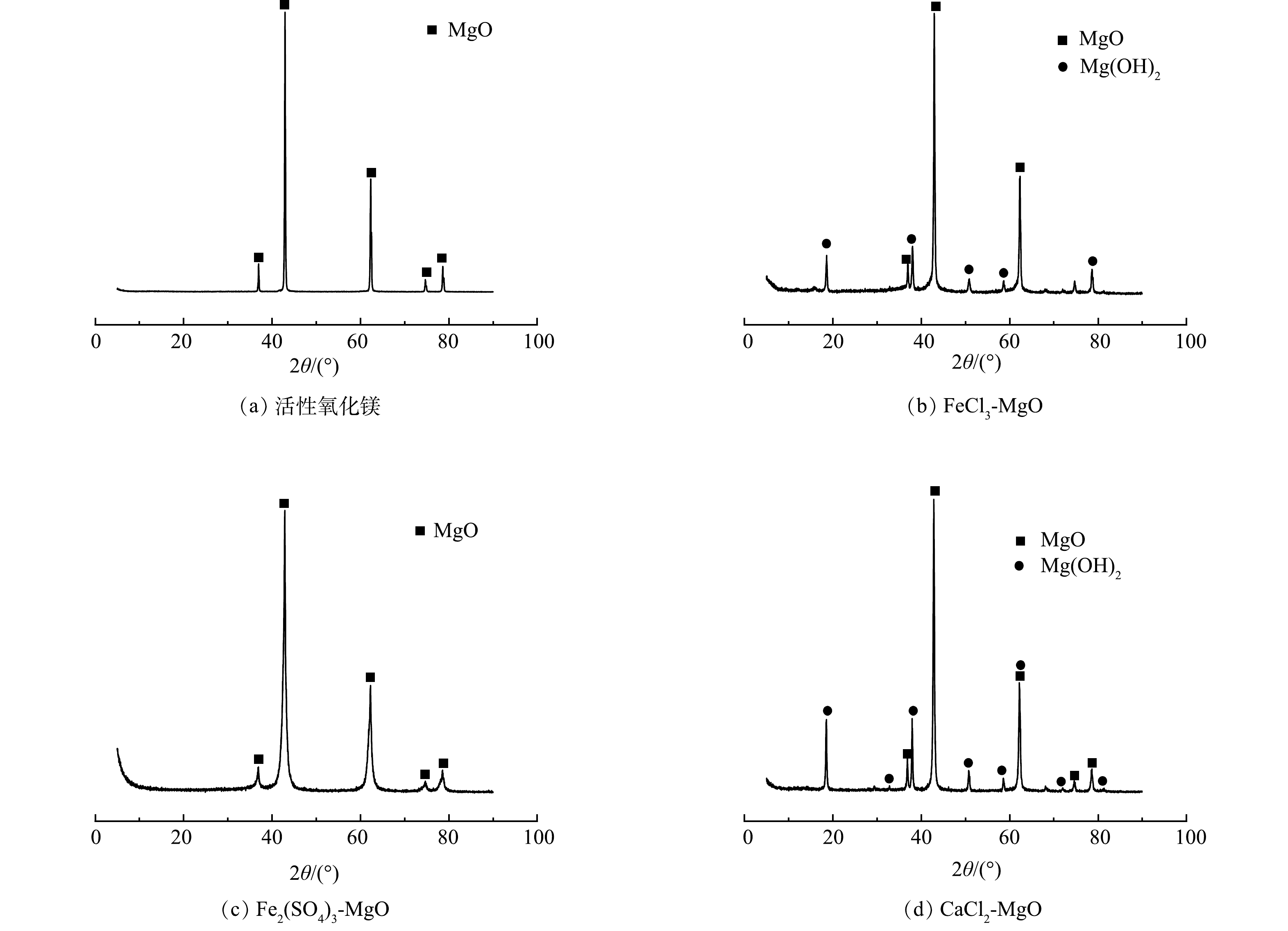
活性氧化镁、FeCl3-MgO、Fe2(SO4)3-MgO、CaCl2-MgO的XRD表征结果如图2所示。由图2(b)可以看出,在衍射角为37.00、42.95、62.35、74.75、78.65°处出现MgO衍射峰,说明MgO仍然是改性吸附剂的主体成分。在FeCl3-MgO的图谱中出现了少量的Mg(OH)2 (在18.60、38.05、50.83、58.50、68.50°附近的衍射峰),但改性前后各组分峰形和峰位置并未发生明显的变化。说明改性过程并没有改变活性氧化镁的晶格结构。在图2(b)中未出现铁的吸收峰,说明铁可能以无定型的物质形式附着在氧化镁表面[11]。
Fe2(SO4)3-MgO的XRD图谱中只有氧化镁出现并没有出现Mg(OH)2,原因是在最佳焙烧温度(500 ℃)下Mg(OH)2重新分解为氧化镁,可见铁盐的改性并没有改变活性氧化镁的物质结构,铁盐并没有出现稳定的物质结构,可能是以无定型金属化合物的形式附着在活性氧化镁表面。
CaCl2-MgO的XRD图谱和FeCl3-MgO的 XRD图谱类似,图中的衍射峰依旧是以MgO为主体,强度较低的峰位置出现少量的Mg(OH)2。这是因为在400 ℃的焙烧温度下,钙盐的改性对于氧化镁晶体结构影响较小,钙类物质并没有出现稳定的物质结晶,可能是其以无定型的形式附着在氧化镁表面。
-
活性氧化镁、FeCl3-MgO、Fe2(SO4)3-MgO、CaCl2-MgO FTIR红外光谱如图3所示。改性对于活性氧化镁表面官能团或者化学键有一定影响。可见,吸附前的活性氧化镁在1 439 、1 633 、3 434 cm−1出现的吸收峰归属为CO32−非对称伸缩振动、吸附水O—H的弯曲振动和伸缩振动。FeCl3-MgO在1 409 、1 437 、3 404 cm−1处出现的吸收峰指示的官能团或共价键与活性氧化镁一致,在3 628 cm−1和3 697 cm−1处出现的吸收峰归属为O—H的伸缩振动。Fe2(SO4)3-MgO在1 477 、1 634、3 436 cm−1处出现的吸收峰对应的官能团与活性氧化镁一致,1 089 cm−1处出现SO42−中的S=O振动的吸收峰,其形成可能来自于高价位S与Fe,Mg氧化物的双配位吸附[16-17]。CaCl2-MgO在1 448、1 638、3 427、3 696 cm−1处出现的吸收峰指示与活性氧化镁一致,在2 939 cm−1处出现的吸收峰与钙,水汽和二氧化碳生成的化合物有关,可见CaCl2-MgO对二氧化碳等物质的吸收高于活性氧化镁。
由图3可以看出,吸附后的FeCl3-MgO在426 cm−1处出现新的吸收峰,其归属为Mg—F键和Fe—F键的出现,CO32−的非对称伸缩振动吸收峰和吸附水O—H的伸缩振动吸收峰均出现蓝移,说明FeCl3-MgO表面吸收水和CO32−结合更稳定。Fe2(SO4)3-MgO吸附后在439 cm−1和3 700 cm−1处出现新的吸收峰,分别归属为Mg—F键,Fe—F键和结构水O-H的伸缩振动,指示为SO42−中S=O振动的吸收峰和CO32−非对称伸缩振动的吸收峰均出现红移,说明Fe2(SO4)3-MgO表面的SO42−和CO32−结合更不稳定。CaCl2-MgO在443 cm−1处出现吸收峰,归属为Mg—F键和Ca2+的移动。吸收水O—H的弯曲振动峰和CO32−非对称伸缩振动峰均出现蓝移,说明CaCl2-MgO表面吸收水和CO32−的结合稳定性增强。活性氧化镁、FeCl3-MgO、Fe2(SO4)3-MgO、CaCl2-MgO在含氟废水中水解后,吸附剂表面附着大量O—H,因此活性氧化镁基吸附剂F−与吸附剂表面的羟基的离子互换为除氟的主要过程,Mg-F键的出现是因为水中F−离子与O—H发生离子交换吸附于吸附剂表面,其过程如式(1)所示。
同时MgO也可与F−发生选择性吸附,其可由共价键和络合反应实现,而水中的MgOH+和MgF+又可通过静电吸引进一步吸附F−[18],Fe—F键的生成说明改性活性氧化镁表面的无定型铁水解后生成Fe(OH)3,之后主要通过络合反应 (配位反应) 实现Fe与F的结合,同时释放OH−,络合反应吸附能量比离子反应更强,对F−的选择性更高。钙盐改性后的氧化镁虽然没有Ca—F键的出现,但钙盐是以无定型的形式附着在MgO表面,Ca2+本身可以与F−发生反应生成CaF2沉淀,CaF2比CaCO3,Ca(HCO3)2等其他阴离子结合的盐类更稳定,因此,F−会优先与Ca2+结合,使得钙盐改性后的吸附剂对氟离子选择性吸附增强,抵抗其他阴离子干扰的能力增强,并且铁盐和钙盐对氟离子的亲和力很高,氟离子被吸附后不易被替换。
-
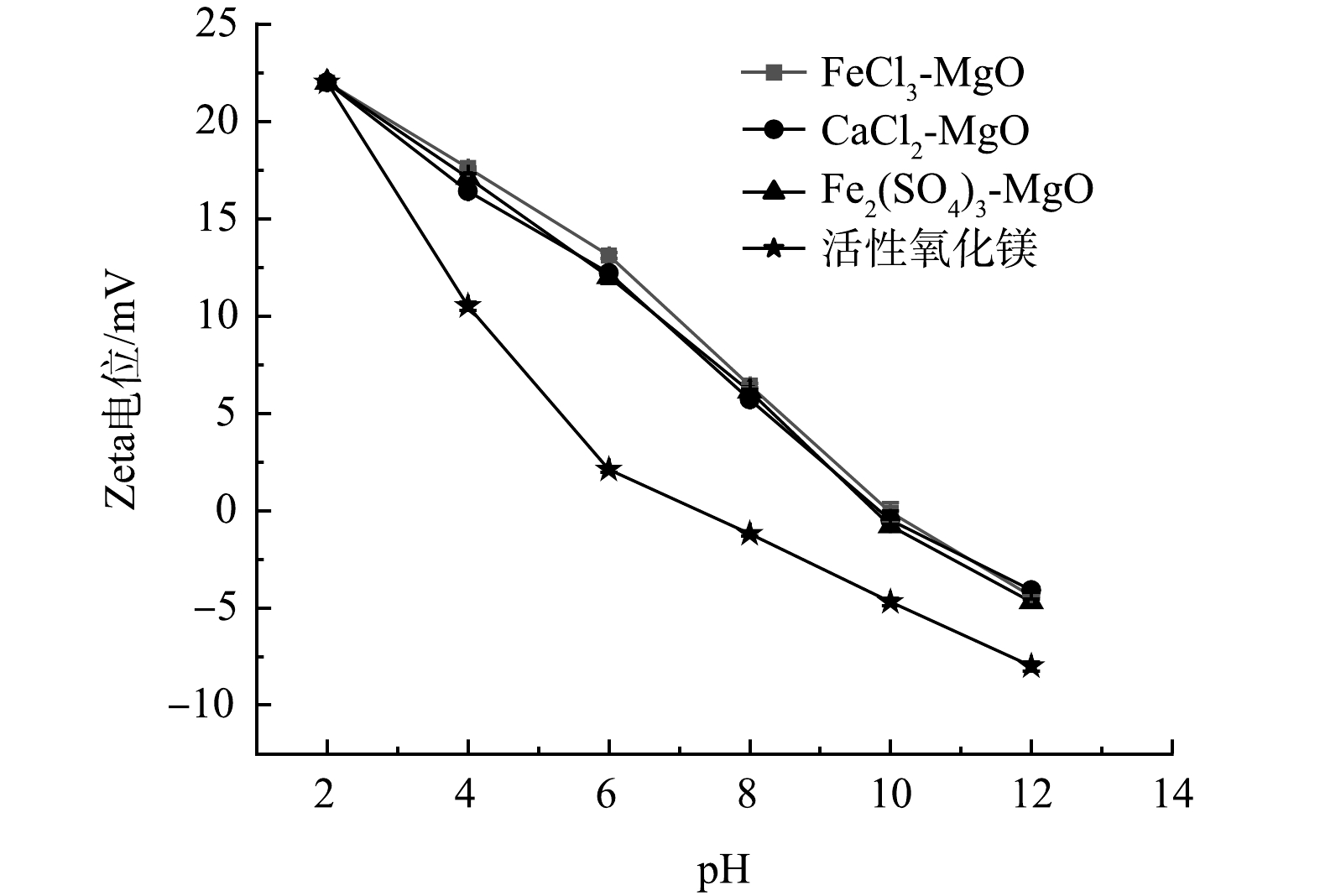
在pH分别为2、4、6、8、10、12的条件下,活性氧化镁、FeCl3-MgO、Fe2(SO4)3-MgO、CaCl2-MgO的Zeta电位测试结果如图4所示。可见,随着pH的升高,3种改性吸附剂的Zeta电位在不断下降,由最初的22 mV降至−4.2 mV左右。这是因为在pH较低时溶液中含有较多的H+,H+与改性吸附剂结合,使得吸附剂表面电负性降低;当pH升高时,溶液中含有较多的OH−,OH−与改性吸附剂结合,使得表面电负性增加。3种改性吸附剂的零点电位在9.97左右,当pH<9.97时,改性吸附剂表面带有正电,有利于吸附带有负电的氟离子;当pH>9.97时,吸附剂表面带有负电会排斥带有负电的氟离子。改性后的吸附剂零电位点所对应的pH较高,在较宽的pH范围内都有利于吸附氟离子,活性氧化镁由于零点电位较低,吸附氟离子的有利pH范围较窄。
-
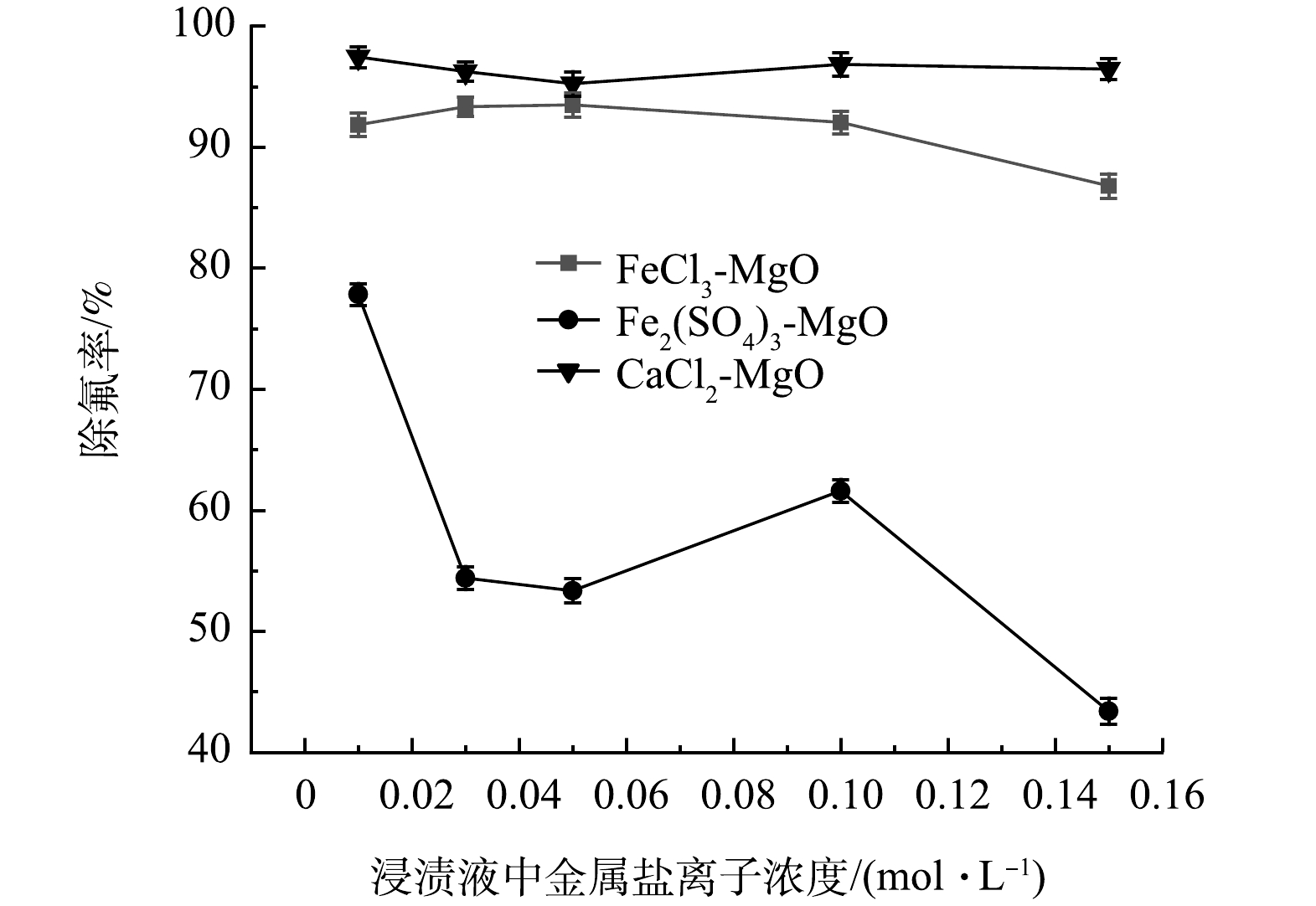
在温度为25 ℃,浸渍液中金属盐离子浓度分别为0.01、0.03、0.05、0.1 、0.15 mol·L−1,初始氟离子溶液质量浓度为20 mg·L−1,吸附时间为12 h,浸渍液中金属盐离子浓度对除氟率的影响如图5所示。可见,FeCl3-MgO会随着FeCl3浸渍液浓度增加除氟率先升高后降低。其原因是当FeCl3浓度较低时,浸渍改性会生成更多的氟离子吸附位点,当FeCl3浓度逐渐升高时,FeCl3会堵塞活性氧化镁表面的空隙,使得吸附位点与氟离子接触变少,导致除氟率下降,因此,FeCl3最佳浸渍浓度为0.05 mol·L−1。
随着CaCl2浸渍液离子浓度增加,CaCl2-MgO的除氟效果略微提升。这是因为少量CaCl2会提供更多的氟离子吸附位点,随着浓度的提高,过多的CaCl2会导致氧化镁表面吸附孔隙被堵塞,氟离子与活性氧化镁表面的吸附位点接触概率变低,导致除氟率下降。因此, CaCl2-MgO的最佳CaCl2浸渍液离子浓度为0.01 mol·L−1。
Fe2(SO4)3-MgO受Fe2(SO4)3浸渍液离子浓度变化影响较大。当Fe2(SO4)3浸渍液离子浓度浓度由0.01 mol·L−1升到0.03 mol·L−1时,Fe2(SO4)3-MgO的除氟率由80%迅速降至50%左右;当Fe2(SO4)3由0.05 mol·L−1增加到0.10 mol·L−1时,除氟率有一定上升,但除氟率仍低于Fe2(SO4)3为0.01 mol·L−1时制得的Fe2(SO4)3-MgO。这是因为Fe2(SO4)3有着较大的分子质量,当Fe2(SO4)3以无定型结构附着在氧化镁表面时,更容易堵塞氧化镁表面的吸附位点,使得氟离子难以被吸附,导致除氟率显著下降。因此,确定0.01 mol·L−1为Fe2(SO4)3-MgO的最佳浸渍液离子浓度。
-
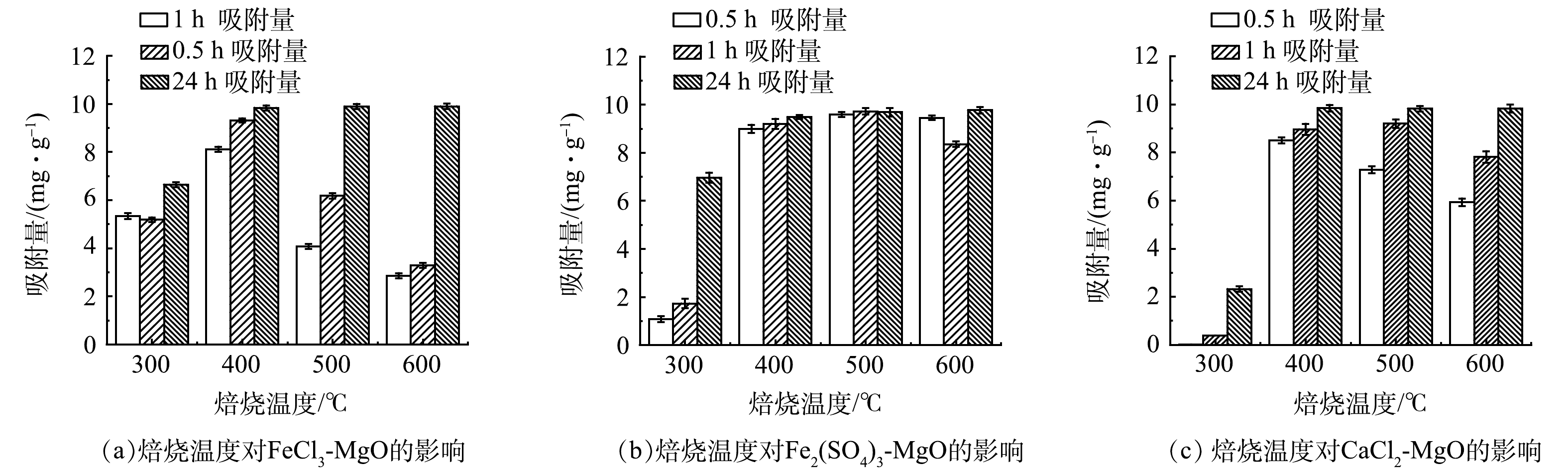
在温度为 25 ℃,初始氟离子质量浓度为20 mg·L−1,调节溶液pH至7,焙烧温度对吸附剂除氟效果的影响如图6所示。可见,随着焙烧温度增加,吸附容量随之增加,当达到一定温度后,吸附剂的吸附量基本维持不变, 400 ℃为FeCl3-MgO和CaCl2-MgO的最适焙烧温度,500 ℃为Fe2(SO4)3-MgO的最适焙烧温度。当焙烧温度过低时,吸附量较低,原因是吸附剂内结构水散失过少,不利于吸附位点的生成;焙烧温度过高,也会略微影响吸附量,原因是温度过高颗粒的过度结晶和团聚使吸附剂表面作用降低,吸附性能下降。焙烧使得铁盐和钙盐以无定型形态负载在活性氧化镁表面,当达到一定温度时,活性氧化镁表面负载量达到饱和,吸附剂的吸附量便基本维持不变。
-
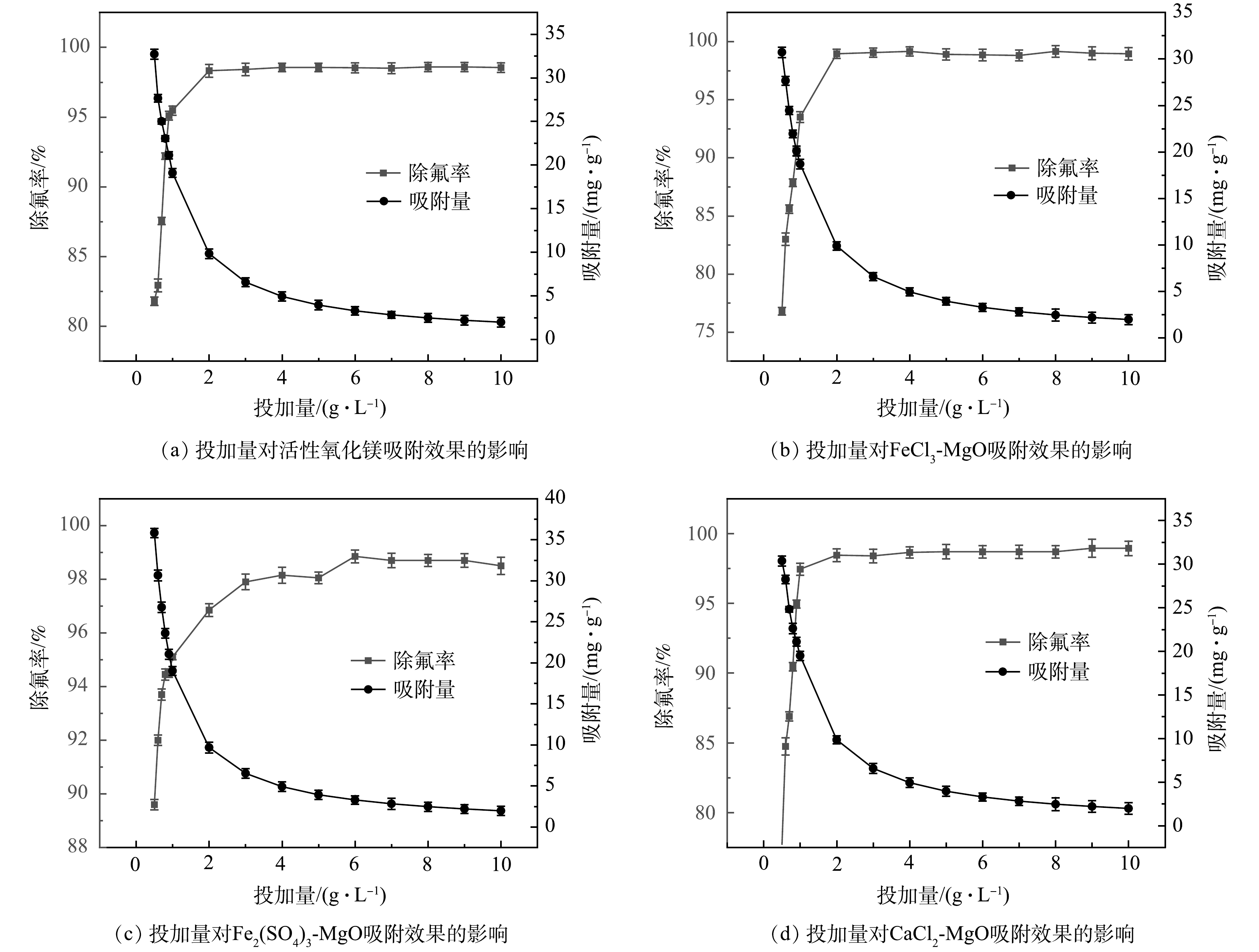
在温度为25 ℃时,分别向实验水样中投加不同剂量的活性氧化镁、FeCl3-MgO、Fe2(SO4)3-MgO、CaCl2-MgO,初始氟离子溶液质量浓度为20 mg·L−1,吸附时间12 h, 吸附剂投加量对于吸附效果的影响如图7所示。4种吸附剂的除氟率均随其投加量的增加先快速升高然后趋于稳定,最终达到吸附平衡。由图7(a)、图7(b)、图7(d)可见,活性氧化镁、FeCl3-MgO、CaCl2-MgO3种吸附剂在投加量为2 g·L−1时除氟率均达到最大,吸附剂投加量继续增加,除氟率基本维持不变。由图7(c)可见,Fe2(SO4)3-MgO除氟率随着投加量的增加快速升高,但除氟率始终略低于活性氧化镁、FeCl3-MgO、CaCl2-MgO,当吸附剂用量达到4.5 g·L−1左右,除氟率趋于稳定并与活性氧化镁,FeCl3-MgO和CaCl2-MgO的除氟率相当,继续增加吸附剂的投加量,除氟率基本保持不变,达到吸附平衡。
由图7可见,在吸附剂用量未达到3 g·L−1时,随着吸附剂用量的增加,4种吸附剂的吸附容量均会快速降低,当吸附剂投加量大于3 g·L−1,吸附容量会缓慢下降。这是因为刚开始投加吸附剂的时候,吸附氟离子的活性位点较多,随着投加量的增加,吸附位点逐渐被占据,利用率下降,当吸附剂用量达到3 g·L−1左右时,基本达到吸附平衡,这时吸附剂会缓慢吸收溶液中的氟离子,吸附位点基本被占据,吸附容量会缓慢下降。
-
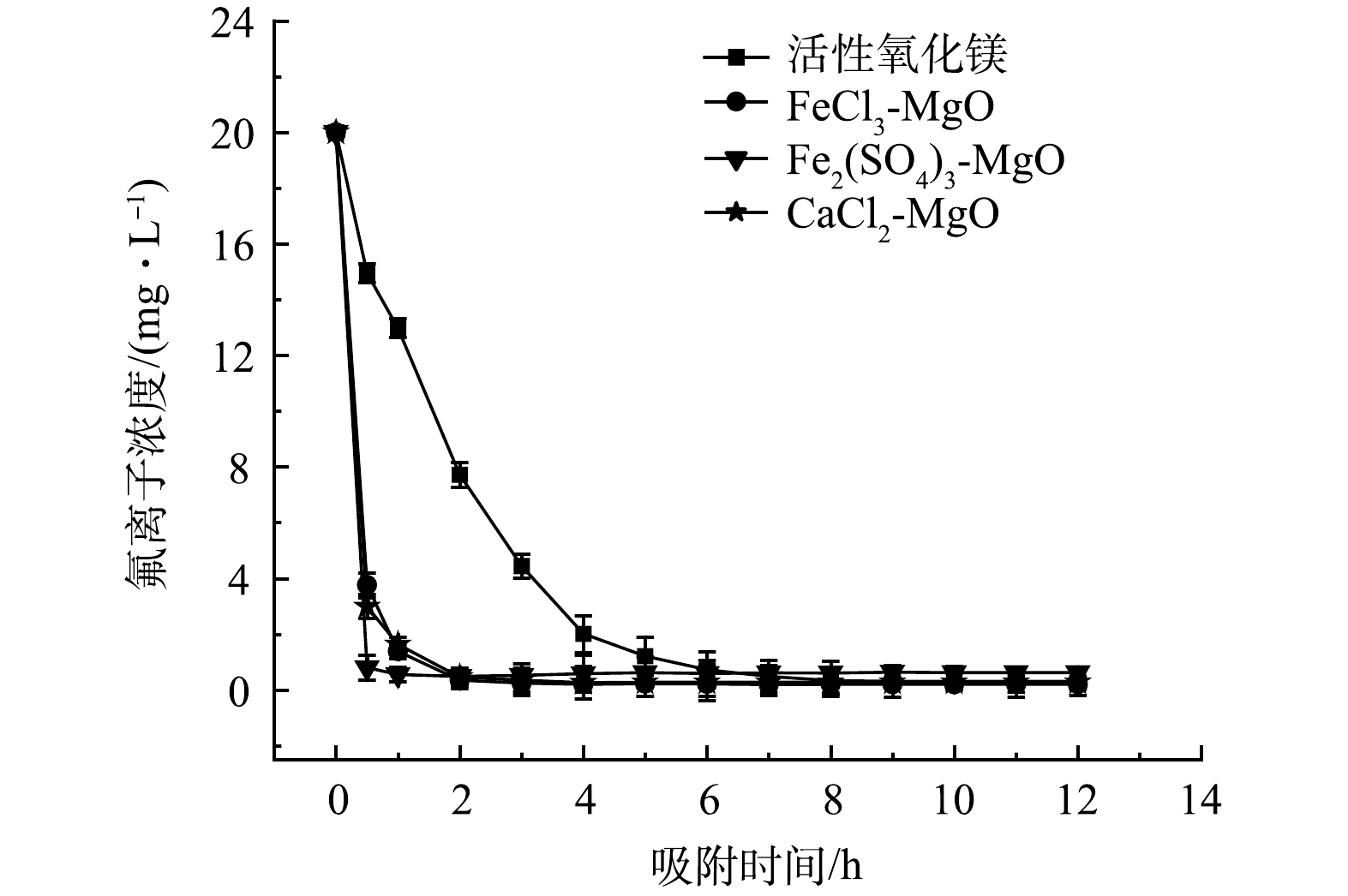
在温度为25 ℃,改性吸附剂投加量为2 g·L−1,初始氟离子溶液质量浓度为20 mg·L−1,吸附时间对于吸附效果的影响如图8所示。在吸附初期,4种吸附剂吸附速率均较快,随后,吸附速率减慢,直至达到吸附平衡,活性氧化镁吸附氟离子速率较慢,7 h达到吸附平衡,除氟效率低,CaCl2-MgO、FeCl3-MgO、Fe2(SO4)3-MgO除氟速率快,在2 h左右达到吸附平衡,除氟率均可达95%以上。
-
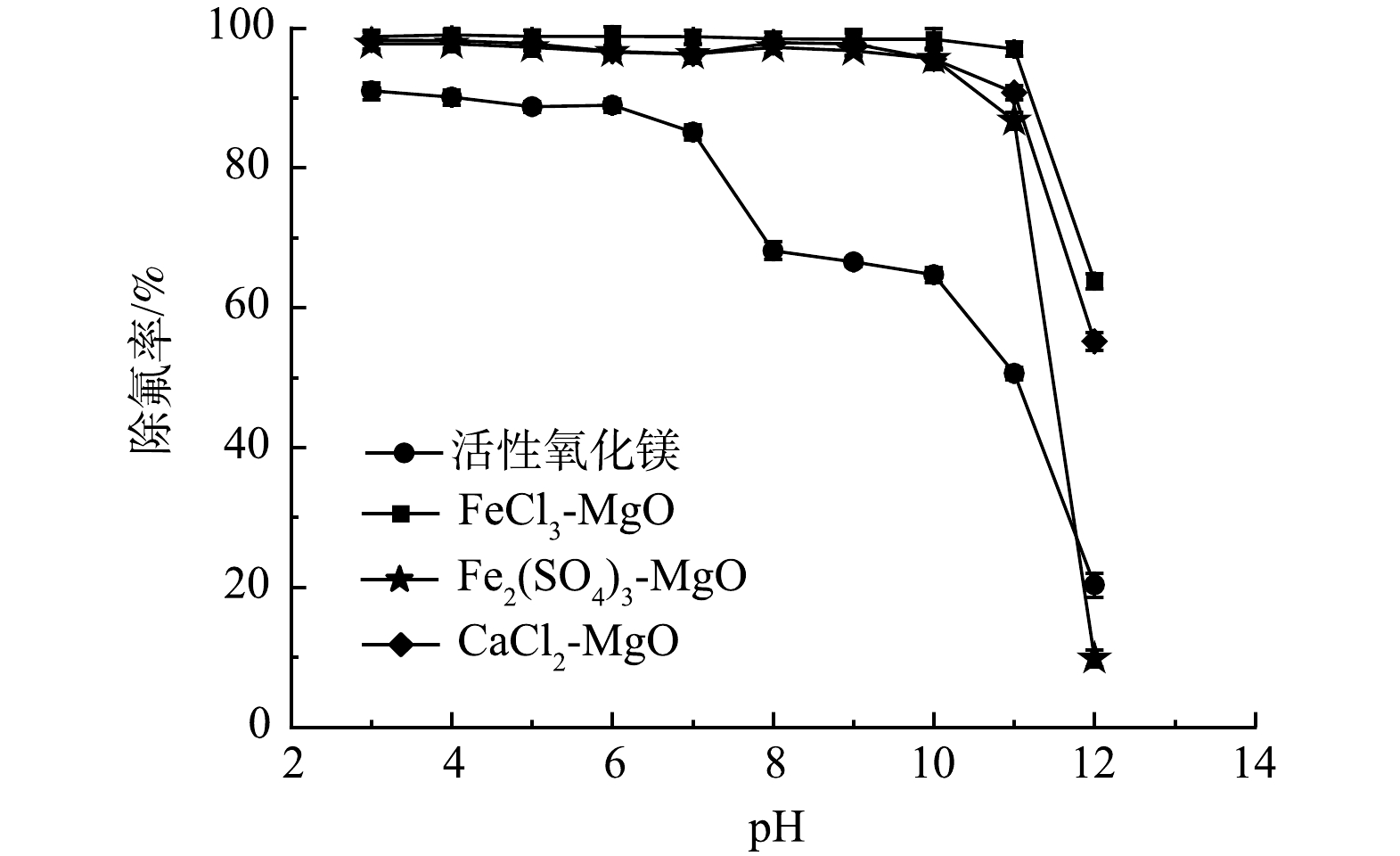
含氟溶液的pH会引起吸附剂表面电位的变化,影响吸附剂的吸附性能。在温度为25 ℃,改性吸附剂投加量为2 g·L−1,初始氟离子溶液质量浓度为20 mg·L−1,吸附时间为12 h,探究pH对去除率的影响。如图9所示, pH<7时活性氧化镁可以达到90%左右的去除率,当pH>7时活性氧化镁除氟率有明显的下降, pH>10时,活性氧化镁除氟率进一步下降。由此得出pH对于活性氧化镁除氟性能有很大的影响,活性氧化镁在酸性或者中性的环境下能有较好的除氟效果。随着pH升高,溶液中存在较多的OH−,OH−易于和金属吸附剂表面结合,使得活性氧化镁表面带负电,不利于吸附同为阴离子的氟离子。
改性后的3种吸附剂在pH>11后除氟率均有显著下降。这是因为改性后的吸附剂表面酸性位点增加,当pH升高时,溶液中的OH−会与吸附剂表面的酸性位点结合,从而提高吸附剂pH的缓冲能力,但吸附位点数量有限, OH−与吸附位点结合后使得F−不能与吸附位点结合,导致除氟率降低。由Zeta电位变化可以得出,改性后的吸附剂由于零电位点所对应pH较高,因此,在较宽的pH范围内均有良好的除氟效果。此外,有研究表明,SO42−与F−的交换可以在碱性条件下顺利进行,这也是经过Fe2(SO4)3改性后的活性氧化镁适宜pH扩大的原因之一。在pH<11的环境下,CaCl2-MgO、FeCl3-MgO、Fe2(SO4)3-MgO均能保持90%以上的去除率,改性后的吸附剂适应水溶液酸碱环境的能力显著增强。
-
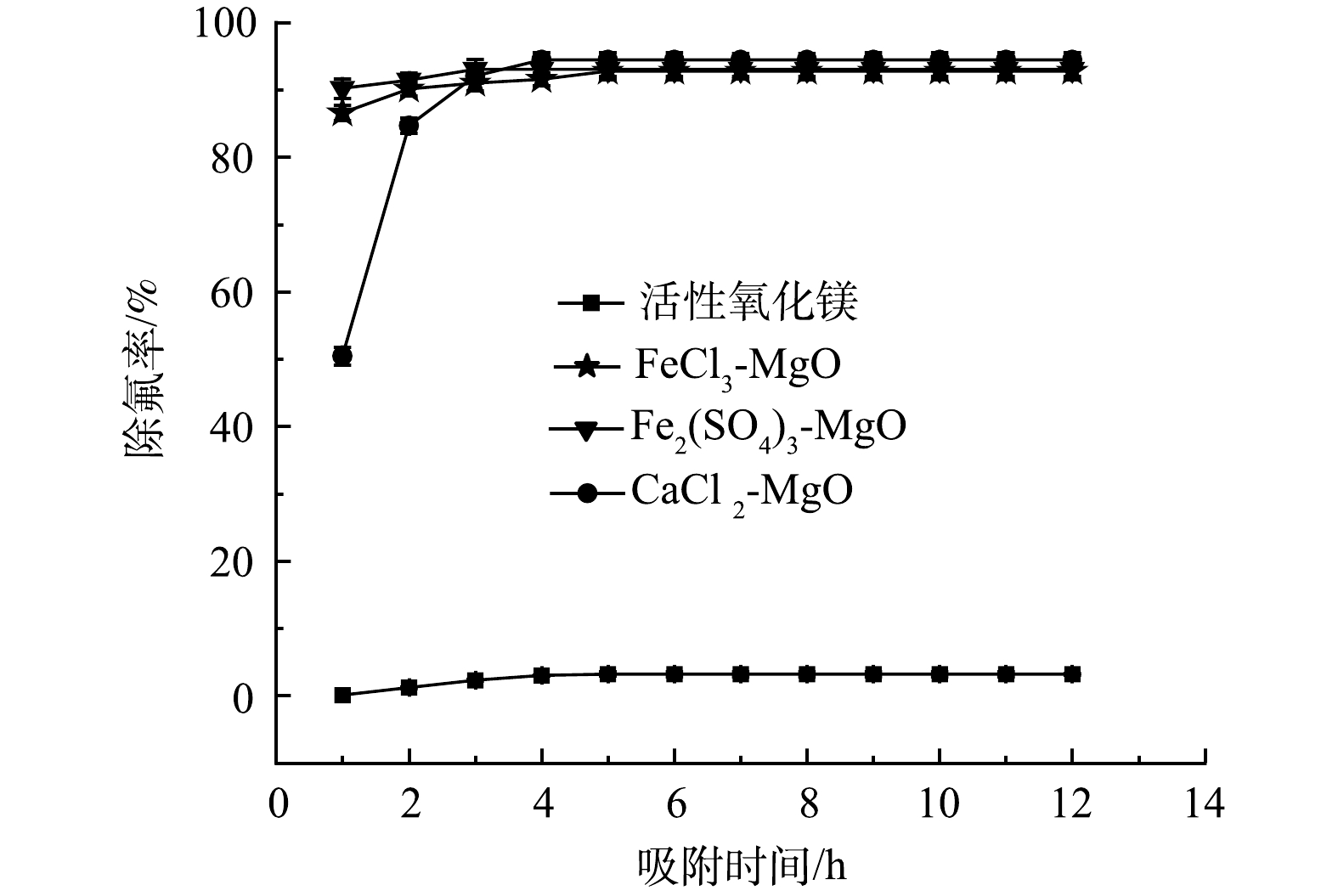
天然的含氟水是一个复杂的水体环境,在进行除氟处理时,要考虑水体中其他离子对除氟的影响。为此,在25 ℃,吸附剂投加量为2 g·L−1,初始氟离子质量浓度为5mg·L−1,吸附时间12 h,共存离子(Cl−、SO42−、CO32−、NO3−、HPO42−、Na+)的质量浓度为5 mg·L−1的条件下,考察吸附剂在复杂水体环境中的除氟效果,结果如图10所示。
活性氧化镁在实际含氟饮用水环境中达到吸附平衡后对氟离子的去除率不足10%,除氟效果差, CaCl2-MgO、FeCl3-MgO和Fe2(SO4)3-MgO在实际含氟饮用水溶液中达到吸附平衡后依然保持了90%以上的氟离子去除率,剩余F−质量浓度均小于1 mg·L−1,满足国家饮用水含氟标准,可见改性后的吸附剂对氟离子的选择性显著增强,在实际饮用水除氟中有良好的效果。
-
取0.1 g活性氧化镁、FeCl3-MgO、Fe2(SO4)3-MgO、CaCl2-MgO分别置于100 mL初始氟离子溶液质量浓度为5、10、20、25、100、200、300 mg·L−1的模拟含氟水中,在室温25 ℃下测量吸附12 h后的氟离子质量浓度,分别对其进行Langmuir(式(2))和Freundlich(式(3))等温吸附模型拟合,拟合结果如表1所示。
式中:Ce为吸附后溶液的平衡质量浓度,mg∙L−1;Qe为平衡吸附量,mg∙g−1;Qmax为最大吸附量,mg∙g−1;KL为 Langmuir 平衡常数,L∙mg−1;KF为Freundlich 平衡常数,L∙mg−1
4种吸附剂的Langmuir吸附等温模型拟合相关性系数R2均大于Freundlich吸附等温模型拟合相关性系数,说明活性氧化镁、FeCl3-MgO 、Fe2(SO4)3-MgO、CaCl2-MgO,对F−的吸附更倾向于单分子层吸附[19],FeCl3-MgO、Fe2(SO4)3-MgO、 CaCl2-MgO的最大吸附容量均有显著增加,经过氯化铁改性后的吸附剂吸附容量大于经过硫酸铁改性的吸附容量,原因是经过硫酸铁改性后的吸附剂吸附达到短暂平衡后又有F−溶出。
在Freundlich吸附等温模型中,1/n可反映吸附的有利程度,0<1/n<1有利于吸附的发生。由表1可以看出,4种改性吸附剂1/n均为0<1/n<1,说明4种吸附剂均有较好的吸附强度,3种改性吸附剂的1/n均小于活性氧化镁1/n,说明FeCl3、Fe2(SO4)3和CaCl2的改性有利于活性氧化镁的吸附 [20]。
-
取0.1 g CaCl2-MgO于烧杯中,加入超纯水100 mL,于25 ℃下以150 r·min−1振荡120 min,利用离心机将振荡后的固液分离,采用镁试剂测试水样中的镁离子含量,结果未检出镁离子,另外对CaCl2-MgO除氟后的水样进行检测,同样未检测出镁离子;取0.1 g 铁盐改性吸附剂于烧杯中,在上述相同的条件下重复实验未见有沉淀析出。表明Mg2+不会随着反应渗入溶液中,不会造成二次污染。
取0.1 g铁盐改性吸附剂于烧杯中,在上述相同的条件下取上清液加入少量氢氧化钠溶液,未见有沉淀出现;取0.1 g CaCl2-MgO于烧杯中,在上述相同的条件下取上清液加入少量碳酸钠溶液,未见出现沉淀,表明浸渍盐的离子并不会随着反应渗到溶液中。这说明改性吸附剂稳定性良好,不会造成新的污染。
取0.1 g改性吸附剂与烧杯中,加入超纯水100 mL,于25 ℃下以150 r·min−1振荡120 min,采用镁试剂测试水样中的镁离子含量,结果未检出镁离子。另外对改性吸附剂除氟后的水样进行检测,同样未检测出镁离子,表明改性吸附剂稳定性好,除氟过程未有其他离子溶出,不会造成二次污染。
取0.1 g铁盐改性吸附剂与烧杯中,在上述相同的操作条件下,未见有沉淀出现;取0.1 g CaCl2-MgO于烧杯中,在同样的操作条件下,未出现沉淀,表明浸渍盐的离子并不会随着反应渗到溶液中。这说明改性吸附剂稳定性良好,不会造成新的污染。
-
1)改性后的氧化镁内部结构更加紧密,比表面积显著增加,与氟离子结合的活性位点增多,且活性位点更加稳定,从而提高了对氟离子的吸附效率,由铁盐和钙盐改性后的吸附剂仍以氧化镁为主体,钙盐和铁盐是以无定型的形态附着在活性氧化镁表面。
2)活性氧化镁通过F−与OH−的离子交换去除废水中的氟离子,铁盐改性的吸附剂由于Fe-F键的出现,吸附过程不仅有离子交换还有络合反应,络合反应的反应能量大于离子交换,络合反应可加强吸附剂对氟离子的选择性吸附;钙盐改性的吸附剂尽管没有形成Ca-F键,但Ca2+可与F−反应直接生成CaF2沉淀,CaF2比其他阴离子与F−结合的稳定性高,经过钙盐,铁盐改性后的吸附剂抵抗共存阴离子干扰的能力大大增强
3)改性吸附剂吸附容量显著增加,吸附速率显著提高。适宜的浸渍浓度和适宜的焙烧温度会增强改性吸附剂的除氟效果,FeCl3-MgO和CaCl2-MgO的最佳焙烧温度是400 ℃,Fe2(SO4)3-MgO的最佳焙烧温度是500 ℃。
4)改性后的吸附剂有较高的零电位点,适宜pH范围变宽,pH在3~10内均可维持较高的除氟率。改性吸附剂符合Langmuir等温模型,改性吸附剂除氟后水样未有镁离子和浸渍盐离子溶出,不会造成2次污染。
金属盐改性活性氧化镁对饮用水中氟离子的去除性能
Removal performance of metal salt modified active magnesium oxide on fluoride ions in drinking water
-
摘要: 饮用水中氟含量超标会损害人体的健康,本研究研制了一种改性吸附剂用于去除饮用水中过量的氟离子。本研究采用浸渍,焙烧的方法将金属盐负载到活性氧化镁上,制备改性活性氧化镁,考察了其除氟效果和吸附除氟的主要影响因素。结果表明,经过钙盐,铁盐改性后的活性氧化镁最大吸附容量显著增加,在复杂水体环境中仍有突出的除氟效果;改性吸附剂适宜pH为3~10;金属盐改性活性氧化镁处理水样不会有Mg2+和浸渍盐离子溶出,是一种安全可靠的吸附除氟材料。Abstract: Excessive fluoride content in drinking water can harm human health. In this study, a modified adsorbent was developed to remove excessive fluoride ions from drinking water. The impregnation and calcination methods were used to load metal salts onto activated magnesium oxide for the preparation of modified activated magnesium oxide. The fluoride removal effect and the main influencing factors were studied. The results showed that the maximum adsorption capacity of activated magnesium oxide modified with calcium and iron salts significantly increased, and it still had outstanding fluoride removal effects in complex water environments; The pH values of 3~10 were suitable for the modified adsorbent; Metal salt modified active magnesium oxide treatment of water samples did not release Mg2+ and immersion salt ions, it is a type of safe and reliable material for adsorption and fluoride removal.
-

-
表 1 吸附剂的Langmuir和Freundlich等温吸附模型拟合参数
Table 1. Fitting parameters of Langmuir and Freundlich isothermal adsorption models for adsorbent
吸附剂 Langmuir Freundlich R2 Qmax/(mg∙g−1) KL/min R2 1/n KF/min−1 活性氧化镁 0.998 6 59.59 0.66 0.848 7 0.442 3 15.44 FeCl3-MgO 0.997 6 107.64 0.17 0.915 8 0.427 1 15.96 Fe2(SO4)3-MgO 0.997 2 89.29 0.15 0.886 4 0.414 9 13.12 CaCl2-MgO 0.994 6 108.34 0.22 0.875 4 0.398 4 18.71 -
[1] WANG H, YANG L, GAO P, et al. Fluoride exposure induces lysosomal dysfunction unveiled by an integrated transcriptomic and metabolomic study in bone marrow mesenchymal stem cells[J]. Ecotoxicology and Environmental Safety, 2022, 239: 113672. doi: 10.1016/j.ecoenv.2022.113672 [2] TANEJA L, KOCHAR C, KUMAR Y P, et al. Adsorption: A preferred technique for fluoride removal from water[J]. Materials Today:Proceedings, 2022, 71(2): 215-219. [3] LU N C, LIU J C. Removal of phosphate and fluoride from wastewater by a hybrid precipitation–microfiltration process[J]. Separation and Purification Technology, 2010, 74(3): 329-335. doi: 10.1016/j.seppur.2010.06.023 [4] 桑硕, 帖靖玺, 张南. 地下水除氟研究进展[J]. 科技创新与应用, 2022, 12(2): 78-82. [5] ANIS S F, HASHAIKEH R, HILAL N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review[J]. Desalination, 2019, 452: 159-195. doi: 10.1016/j.desal.2018.11.006 [6] SANINI B, LEICHTWEIS J, SILVESTRI S, et al. Impregnation of activated alumina with CeO2 for water defluoridation[J]. Materials Chemistry and Physics, 2022, 291: 126648. doi: 10.1016/j.matchemphys.2022.126648 [7] AYALEW A A. Comparative adsorptive performance of adsorbents developed from kaolin clay and limestone for de-fluoridation of groundwater[J]. South African Journal of Chemical Engineering, 2023, 44: 1-13. doi: 10.1016/j.sajce.2022.11.002 [8] LAONAPAKUL T, SUTHI T, OTSUKA Y, et al. Fluoride adsorption enhancement of Calcined-Kaolin/Hydroxyapatite composite[J]. Arabian Journal of Chemistry, 2022, 15(11): 104220. doi: 10.1016/j.arabjc.2022.104220 [9] THIRUNAVUKKARASU A, NITHYA R, SIVASHANKAR R. Continuous fixed-bed biosorption process: A review[J]. Chemical Engineering Journal Advances, 2021, 8: 100188. doi: 10.1016/j.ceja.2021.100188 [10] AMIN F, TALPUR F N, BALOUCH A, et al. Biosorption of fluoride from aqueous solution by white—rot fungus Pleurotus eryngii ATCC 90888[J]. Environmental Nanotechnology, Monitoring & Management, 2015, 3: 30-37. [11] 张小磊, 李尚明, 李红艳, 等. 负载镧镁改性活性氧化铝的除氟性能[J]. 环境工程学报, 2016, 10(8): 4189-4195. [12] BAKHTA S, SADAOUI Z, LASSI U, et al. Performances of metals modified activated carbons for fluoride removal from aqueous solutions[J]. Chemical Physics Letters, 2020, 754: 137705. doi: 10.1016/j.cplett.2020.137705 [13] 刘理华, 车王燕, 刘书群. 棒状氧化镁的改性及其吸附性能研究[J]. 淮北师范大学学报(自然科学版), 2023, 44(1): 52-57. [14] 王慧玲, 张彦燕, 徐薇, 等. 改性活性氧化镁的除氟效能及机制[J]. 环境工程学报, 2015, 9(5): 2125-2130. [15] WANG X Y, WEI J J, PENG W C, et al. Evaluation and DFT analysis of 3D porous rhombohedral Fe-modified MgO for removing fluoride efficiently[J]. Applied Surface Science, 2021, 552: 149423. doi: 10.1016/j.apsusc.2021.149423 [16] 潘柳依, 孙晓红, 王军峰, 等. 固体酸催化剂SO42-/TiO2-SnO2的合成及其催化合成丙烯酸异冰片酯[J]. 精细化工, 2018, 35(5): 791-795. [17] 胡美秋, 袁红, 景艳红. SO42-/Al2O3催化剂的表征及其在环氧化反应中的催化活性[J]. 中国油脂, 2019, 44(12): 55-58. [18] LI L X, XU D, LI X Q, et al. Excellent fluoride removal properties of porous hollow MgO microspheres[J]. New Journal of Chemistry, 2014, 38(11): 5445-5452. doi: 10.1039/C4NJ01361A [19] LU M, GUAN X H, XU X H, et al. Characteristic and mechanism of Cr(VI) adsorption by ammonium sulfamate-bacterial cellulose in aqueous solutions[J]. Chinese Chemical Letters, 2013, 24: 253-256. doi: 10.1016/j.cclet.2013.01.034 [20] 付娆, 张文龙, 冯江涛, 等. 锐钛矿型二氧化钛的低温合成及其吸附除氟性能的研究[J]. 环境工程, 2020, 38(2): 70-76. -



 下载:
下载: