-
随着我国经济的高速发展,空气污染现象愈发严重。挥发性有机污染物是空气中的主要污染物,来自于室外的工业废气、机动车尾气、光化学烟雾等,以及室内装修、装饰材料等常温下释放出的甲苯、甲醛等[1-2]。挥发性有机污染物具有毒性和刺激性,甚至具有致癌作用,能损害血液成分和心血管系统,引起胃肠道紊乱,诱发免疫系统、内分泌系统及造血系统疾病,造成代谢缺陷,对生态环境和人类健康造成严重的危害。挥发性有机污染物的治理方法主要采用物理和化学吸附法,但存在不彻底、成本高等缺点。光催化技术因具有可直接利用太阳能、反应条件温和、无二次污染、能降解几乎所有的有机污染物等特点,被认为是一项极具应用前景的空气净化技术[3-5]。
在众多的半导体光催化剂中,石墨相氮化碳(g-C3N4)具有二维层状结构、较窄的禁带宽度(能吸收部分可见光)、合适的价带/导带位置、良好的稳定性、制备方法简单等优点,在环境和能源领域受到广泛的关注[6-8]。但g-C3N4存在光生载流子复合概率高和表面缺少活性位点等问题,使得其光催化活性受限。为了解决这一问题,在g-C3N4表面负载一些助催化剂,是改善g-C3N4光催化性能的有效方法[9-12]。其中,基于贵金属纳米颗粒局域表面等离共振效应的新型光催化剂,因其优异的可见光吸收特性和光催化活性,被认为是一种理想的可见光光催化材料[13-15]。一方面,等离激元纳米结构可以在较宽的光谱范围内扩展光催化剂的光捕获,为光催化反应提供更多的热电子参与反应。另一方面,等离激元引起的电磁场增强能加速光生载流子的分离,降低光生载流子的复合概率。
通常单一组分的贵金属等离激元纳米颗粒在光催化反应中依然表现出一定的局限性。为此,发展了一些双金属组分等离激元光催化剂体系,将等离激元(如Au、Ag等)作为光能收集器,与具有催化活性的金属组分(如Pt、Cu、Pd等)耦合形成合金、核壳或异质结构,可以实现较好的光催化性能。如LIU等在g-C3N4表面负载Pt-Au双金属纳米颗粒,Au与Pt的协同作用可以增强相邻Pt-Au位点上的局域分子偶极子,促进光生电荷的传输[16]。与Pt/g-C3N4和Au/g-C3N4相比,Pt-Au/g-C3N4光催化制氢的性能分别提高了4.9倍和2.3倍。如BABU等[17]在具有p-n异质结构的TiO2/B-g-C3N4表面沉积Au-Cu双金属纳米颗粒,Au-Cu之间的相互作用不仅增强了宽范围内的光吸收、电荷分离和迁移,还表现出优异的光催化性能。如SU等在g-C3N4表面锚定原子分散性质的Pt1Nx-Au1Nx氧化还原位点作为双功能电子供体-受体中心,可以将400~450 nm可见光的分离效率提高60%,光催化分解纯水的性能提高近10倍[18]。
由于g-C3N4存在电导率低、表面活性位点少等缺点,很难将双金属助催化剂直接沉积在其表面,并实现对有机污染物的高效降解。本研究在合成g-C3N4纳米片的基础上,首先在其表面负载还原氧化石墨烯纳米片制备rGO/g-C3N4复合材料,然后再沉积AuPt纳米颗粒得到AuPt/rGO/g-C3N4复合光催化剂。还原氧化石墨烯优异的电荷传输性能能够提高复合材料的电导率,促进光生电荷从g-C3N4到金属纳米颗粒的传输。还原氧化石墨烯和双金属Au-Pt之间的协同作用可以大幅增强g-C3N4光催化降解挥发性有机污染物的性能。
-
三聚氰胺(C3H6N6)、氯金酸(HAuCl4·4H2O)、硝酸铂(Pt(NO3)2)、乙二醇(C2H6O2)、甲醛(CH2O,37 wt%)、无水乙醇(C2H6O)等为国药集团化学试剂有限公司提供,所有试剂均为分析纯或以上规格。高纯氩气(99.999%)、氢气(99.999%)、空气(20vol.% O2/N2, 99.99%)等为南京上元气体厂提供。氧化石墨烯(GO)粉末通过改进的Hummers法合成[19]。
-
g-C3N4纳米片通过热分解三聚氰胺的方法制备,具有步骤如下:称取20 g的三聚氰胺粉末放入到100 mL带盖子的陶瓷坩埚中,以5 °C·min−1的升温速率在马弗炉中加热到550 °C,保温2 h后,将得到的产物充分研磨,再次在550 °C下退火3 h,最后得到g-C3N4纳米片,产率约为10%。
rGO/g-C3N4的合成过程如下:将300 mg的g-C3N4纳米片分散到80 mL的乙二醇中,并加入1 wt%的GO粉末,充分搅拌4 h。然后将悬浮液转移到含100 mL聚四氟乙烯内胆的反应釜中,加热至180 °C并保温24 h。将产物用无水乙醇和去离子水分别洗涤5次后,在60 °C的真空干燥箱中干燥12 h。
金属纳米颗粒在rGO/g-C3N4表面的沉积过程如下:将200 mg的rGO/g-C3N4粉末分散到100 mL的去离子水中,然后滴加一定量的HAuCl4和Pt(NO3)2溶液,搅拌均匀。将混合溶液加热至70 °C,并保温6 h。将产物用无水乙醇和去离子水分别洗涤3次后,在60 °C下真空干燥。最后,将干燥后的粉末用高纯氢气在250 °C下退火2 h。根据AuPt负载量(质量百分比分别为0.5%、1.0%、2.0%和3.0%,Au和Pt的摩尔比为1∶1)的不同,我们将复合光催化剂命名为AuPt/rGO/g-C3N4-0.5、AuPt/rGO/g-C3N4-1、AuPt/rGO/g-C3N4-2和AuPt/rGO/g-C3N4-3。为方便起见,AuPt/rGO/g-C3N4特指AuPt负载量为1%的AuPt/rGO/g-C3N4-1样品。作为对比,我们还采用相似的步骤合成了Au/rGO/g-C3N4和Pt/rGO/g-C3N4,Au和Pt的负载量也分别为1%。
-
采用Philips X’Pert MPD Pro型转靶X射线粉末衍射仪来分析样品的晶体结构;采用Renishawin Via Reflex型激光显微拉曼光谱仪来测试Raman光谱,激发波长为532 nm;采用日本株式会社JEM-
2100 型透射电子显微镜来观察样品的微观形貌;采用Thermo Scientific K-Alpha型X射线光电子能谱仪对样品的表面元素和结构方式进行分析;采用岛津UV-3600 型紫外-可见-红外分光光度计、FluoroLog-3-Tau and deltaflex型稳态/瞬态荧光光谱仪来分析样品的光谱性能;采用上海辰华CHI660E型电化学工作站和3mm玻碳电极来分析样品的电化学性能。 -
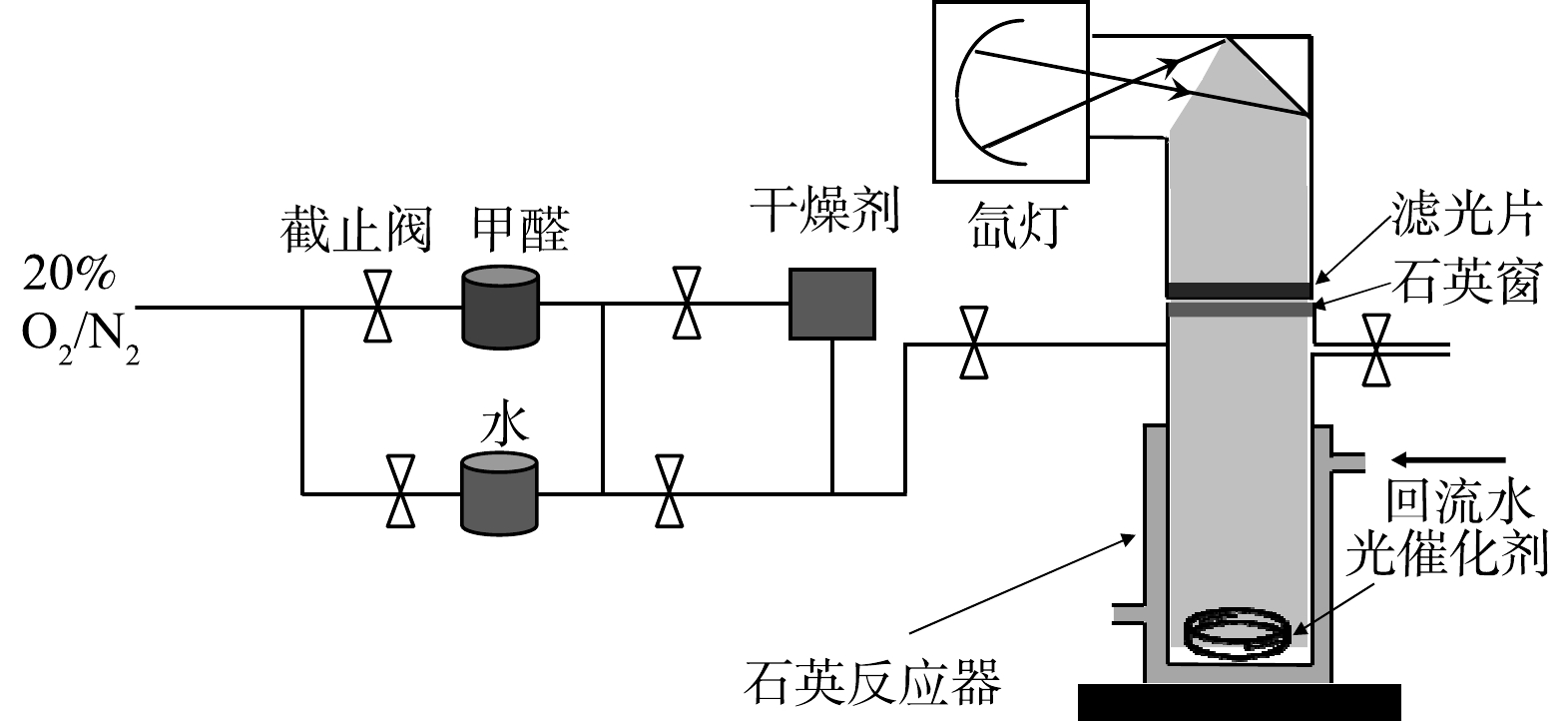
光催化降解挥发性有机污染物的实验在自行研制的石英反应器(400 mL)中进行,如图1所示。以甲醛作为模型化合物来评估光催化剂降解挥发性有机污染物的性能。采用北京泊菲莱的300 W氙灯(PLS-SXE300D)作为激发光源,分别在全谱激发(340 nm≤λ≤780 nm)和可见光激发(配UV420滤光片,420 nm≤λ≤780 nm)下进行光催化反应。反应物甲醛和产物CO2通过杭州科晓GC1690型气相色谱仪(配TCD和FID检测器)进行检测。石英窗与光催化剂的距离为8 cm。
光催化降解甲醛的实验过程如下:将50 mg 的光催化剂粉末分散到1 mL的无水乙醇中,超声30 min,然后均匀涂覆到直径5 cm 的培养皿表面,将培养皿至于石英反应器底部。反应前,通过高纯氩气吹扫石英反应器30 min,以排除反应器内残留的气体。反应器中甲醛的浓度、湿度通过高纯空气鼓泡甲醛溶液和水溶液的方式进行。甲醛的初始浓度通过气相色谱仪(岛津GC14C,毛细管填充柱,甲烷转化炉,FID检测器)检测,湿度通过电子湿度计测量。甲醛的初始浓度固定为 150 mg·m−3,相对湿度固定为70%。反应过程中,每隔一段时间采用气密性注射器(SGE,50 uL)采集气体并通过气相色谱仪分析甲醛和CO2的含量。
-
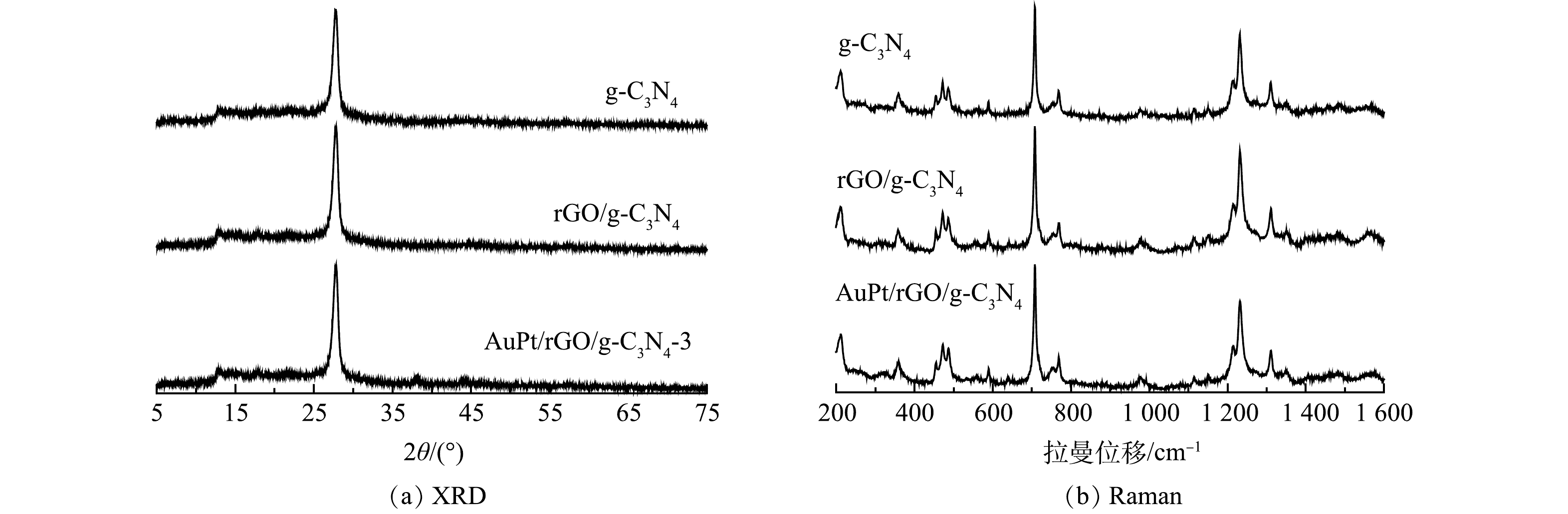
g-C3N4、rGO/g-C3N4和AuPt/rGO/g-C3N4-3的XRD谱图如图2(a)所示。对于g-C3N4,12.8°和27.9°处的衍射峰分别对应于石墨相氮化碳的(100)和(002)晶面。(100)晶面反映的是氮化碳面内3-s-三嗪单元的有序排列,而(002)晶面是由氮化碳的共轭芳香环的层间堆叠引起的[20-21]。还原氧化石墨烯和AuPt纳米颗粒的负载并没有改变g-C3N4中(100)和(002)晶面衍射峰的强度和位置。在AuPt/rGO/g-C3N4-3的XRD谱图中,38.1°和44.2°处的弱衍射峰对应于立方相Au的(001)和(200)晶面,未观察到石墨烯和Pt的特征衍射峰。当金属负载量低于3%时,也观察不到Au的衍射峰,这可能与他们的含量、分散等有关[22-22]。
g-C3N4、rGO/g-C3N4和AuPt/rGO/g-C3N4的Raman谱图如图2(b)所示。在700~1 600 cm−1内的特征峰对应于石墨相氮化碳[23]。753、976、1 115、1 151、1 233和1 311 cm−1处的振动归属于氮化碳中具有melon特征的芳香族C—N杂环的拉伸振动。对于rGO/g-C3N4和AuPt/rGO/g-C3N4,其Raman光谱与纯相g-C3N4几乎一致。XRD和Raman表征结果表明还原氧化石墨烯和AuPt纳米颗粒的负载并没有改变g-C3N4的结构。
-
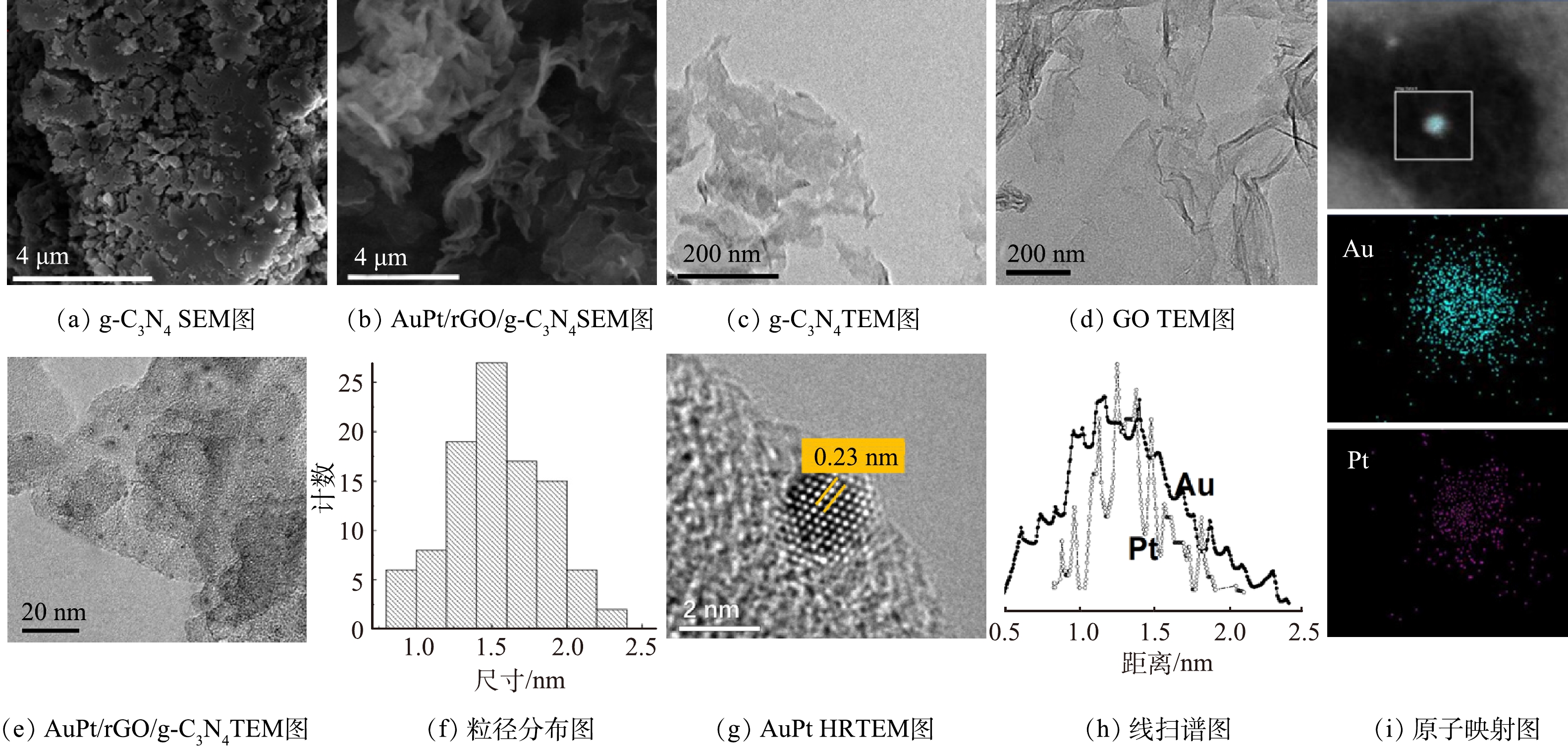
图3为g-C3N4、GO和AuPt/rGO/g-C3N4的SEM和TEM图片。经过2次热处理退火后,g-C3N4由团聚结构(图3(a))变为二维薄层纳米片结构(图3(b)、图3(c)),团聚现象大幅降低,这有利于金属纳米颗粒助催化剂在其表面的均匀沉积。采用改进Hummers法合成的GO,具有典型的二维层状材料卷曲结构特征(图3(d)),其平均直径在几微米到几十微米之间,表现为单层或寡层结构特征。对于AuPt/rGO/g-C3N4,还原氧化石墨烯和g-C3N4之间可以形成良好的2D-2D复合结构。还原氧化石墨烯具有优异的电子传输性能,可以促进光生电子在rGO/g-C3N4层状结构之间的传输。由图3(e)可以看出,金属纳米颗粒均匀地沉积在rGO/g-C3N4表面,其平均粒径约为1.5 nm(图3(f))。小粒径的金属纳米颗粒可以提高金属原子在光催化反应中的利用率,有利于光催化性能的增强。根据图3(g)的HRTEM图片,0.23 nm的晶格条纹间距介于Au(0.235 nm)和Pt(0.225 nm)之间,与AuPt合金结构(Au与Pt的摩尔比为1∶1)一致。图3(h)的EDS线扫谱图和图3(i)的原子映射图进一步证明了Au和Pt双金属之间以合金结构形式负载于rGO/g-C3N4表面。
-
图4为g-C3N4、Au/rGO/g-C3N4、Pt/rGO/g-C3N4和AuPt/rGO/g-C3N4的XPS谱图。对于g-C3N4,288.1 eV和284.8 eV处的特征峰(图4(a))对应于氮化碳结构中sp2杂化的N—C=N键和C=C键[24],在398.6、400.1和401.2 eV处的特征峰(图4(b))分别对应于氮化碳结构中sp2杂化的C=N、N—C3和C—N—H键[25]。对于Au4f和Pt4f的XPS谱图,其特征峰分别对应于金属态Au或Pt的4f7/2和4f5/2能级(图4(c)~(f)),表明Au和Pt是以金属态的形式负载于rGO/g-C3N4表面。对于Au4f,其4f7/2和4f5/2能级由Au/rGO/g-C3N4的83.6 eV和87.3 eV(图4(c))红移至AuPt/rGO/g-C3N4的83.8 eV和87.5 eV(图4(d));对于Pt4f,其4f7/2和4f5/2能级由Pt/rGO/g-C3N4的71.4 eV和74.3 eV(图4(e))蓝移至AuPt/rGO/g-C3N4的70.9 eV和74.1 eV(图4(f))。Au结合能的红移和Pt结合能的蓝移,表明在AuPt/rGO/g-C3N4中存在电荷的再平衡[26]。由于Au的功函数低于Pt,在AuPt/rGO/g-C3N4中存在光生电子从Au向Pt的迁移。第二金属Pt的引入有利于光生电荷的分离,提高光生载流子的寿命,并降低光生载流子的复合概率。
-
图5为g-C3N4及其复合物的紫外可见吸收光谱、光致激发发光光谱和荧光衰减曲线。由图5(a)的紫外可见吸收光谱中可以看出,g-C3N4的吸收带位于450 nm左右,归因于g-C3N4的特征吸收[27],与图5(b)中g-C3N4荧光发射峰的位置基本一致。根据Kubelka—Munk方程计算,g-C3N4的禁带宽度约为2.52 eV。还原氧化石墨烯和金属纳米颗粒的负载,并没有改变g-C3N4的吸收带,即g-C3N4的能带结构没有发生变化。对于AuPt/rGO/g-C3N4,在550 nm处出现新的特征峰,对应于纳米Au的表面等离共振吸收[28-29],与图5(a)中Au/G/g-C3N4的光谱一致。纳米Au的表面等离共振吸收可以为复合光催化剂吸收更多的可见光,为光催化反应提供更多的光电子参与反应,有利于增强光催化活性。如图5(b)所示,还原氧化石墨烯和金属纳米颗粒的负载后,复合光催化剂的荧光强度逐渐降低,说明助催化剂的引入抑制了e−-h+的复合[30]。AuPt双金属纳米颗粒和还原氧化石墨烯的协同作用,使得AuPt/rGO/g-C3N4表现出最弱的荧光强度,出现明显的荧光淬灭现象,说明双金属纳米颗粒AuPt和还原氧化石墨烯有效促进了光生载流子的分离。由图5(c)可以看出,还原氧化石墨烯的负载小幅增加了光生载流子的寿命,而AuPt双金属纳米颗粒和还原氧化石墨烯的负载大幅提高了光生载流子的寿命。这一结果表明,还原氧化石墨烯和AuPt双金属纳米颗粒的负载,不仅仅有助于光生载流子的分离,更重要的是延长了光生载流子的寿命。有效的光生电荷分离能够延长光生载流子的寿命,对于增强光催化性能至关重要[31]。
-
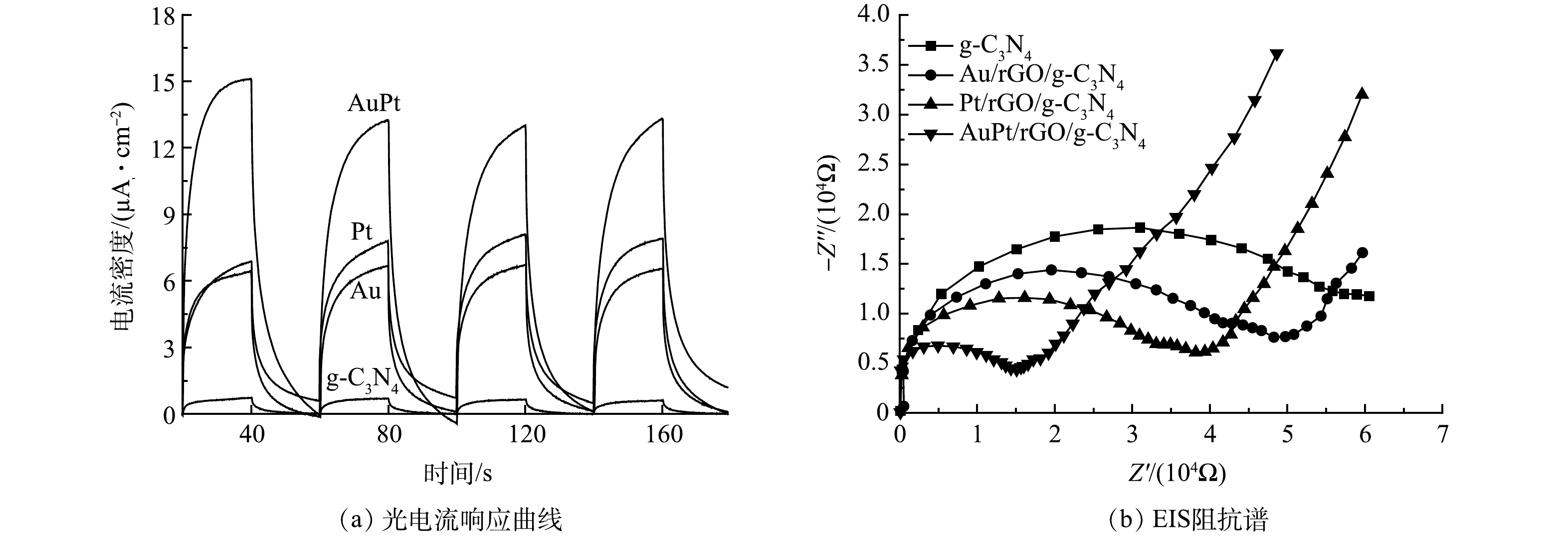
图6为g-C3N4、Au/rGO/g-C3N4、Pt/rGO/g-C3N4和AuPt/rGO/g-C3N4的电化学性能,包括光电流响应曲线和EIS阻抗谱。由图6(a)可以看出,具有双金属纳米颗粒的AuPt/rGO/g-C3N4的瞬态光电流密度高于具有单金属纳米颗粒的Au/rGO/g-C3N4和Pt/rGO/g-C3N4,而复合光催化剂的瞬态光电流密度远大于纯相的g-C3N4。如图6(b)所示,EIS阻抗谱可以进一步研究界面电子的迁移能力[32]。与光电流响应曲线类似,还原氧化石墨烯和AuPt双金属纳米颗粒的负载使得AuPt/rGO/g-C3N4表现出最小的半径,有效降低了界面阻抗,促进了光生载流子的迁移和传输。
-
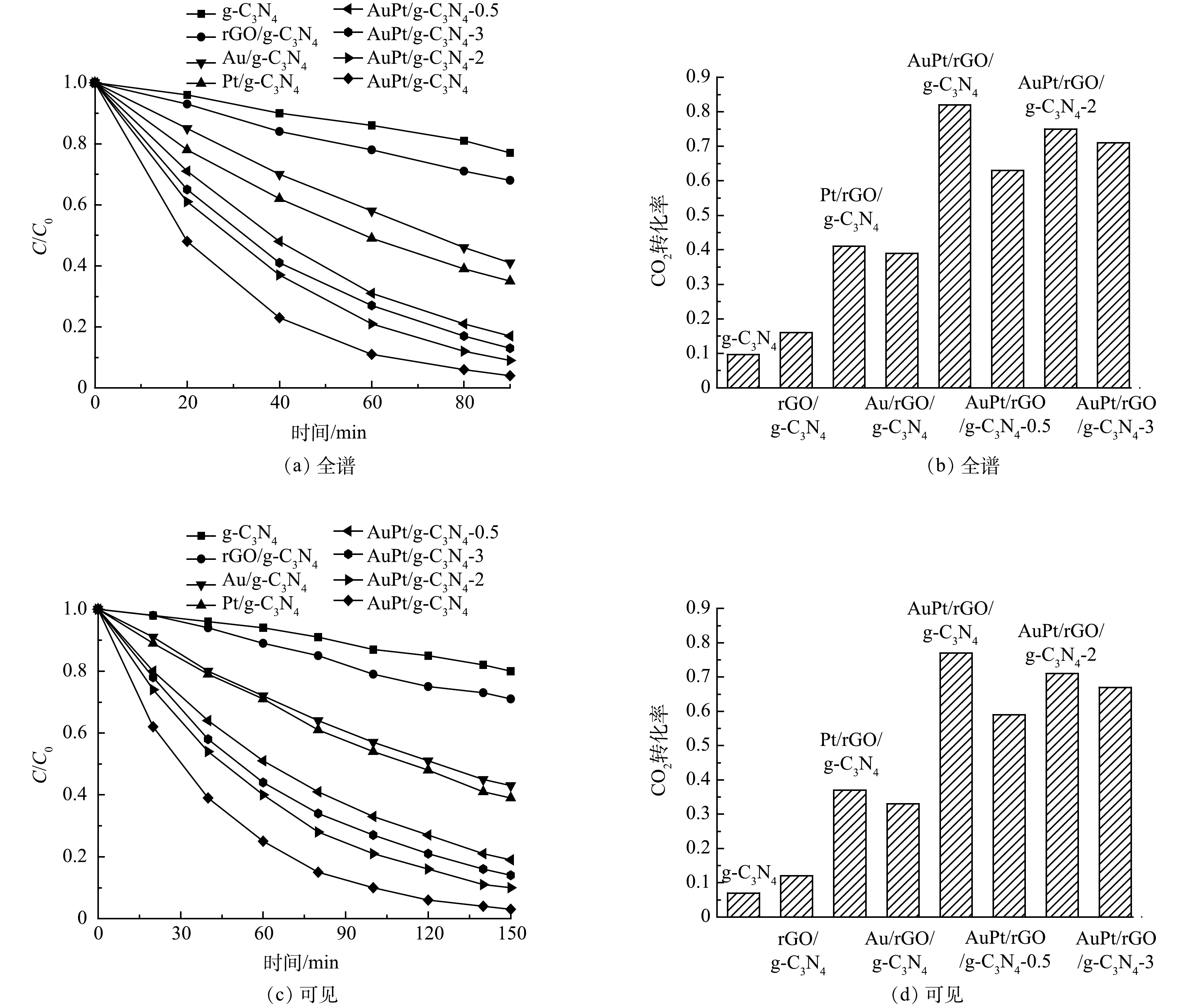
光催化降解挥发性有机污染物被认为是一种有效净化空气的方法,以甲醛为模型挥发性有机污染物,测试了不同的光催化剂在全谱激发(340 nm ≤ λ ≤ 780 nm)和可见光激发(420 nm ≤ λ ≤ 780 nm)下的光催化性能,结果如图7所示。由图7(a)和图7(b)可见,在全谱激发下,g-C3N4经过90 min的光催化反应,仅有23%的甲醛被降解,CO2的转化率仅为9.7%。在光催化降解甲醛反应过程中,甲醛首先被氧化为甲酸盐,甲酸盐进一步矿化成无机小分子CO2和H2O[33]。因此,CO2的转化率普遍低于甲醛的降解率。还原氧化石墨烯负载后,rGO/g-C3N4对甲醛的降解率提高至32%。Au或Pt在rGO/g-C3N4表面的沉积,能进一步将甲醛的降解率提高至59%或65%。当AuPt双金属纳米颗粒沉积到rGO/g-C3N4表面时,复合光催化剂的性能随着金属负载量的增加而增强。当AuPt的负载量为1 wt%时,复合光催化剂表现出最佳的光催化性能,甲醛降解率和CO2转化率分别达到96%和82%。当AuPt的负载量超过1%时,复合光催化剂的性能逐渐降低,但仍优于Au/rGO/g-C3N4、Pt/rGO/g-C3N4和g-C3N4。这表明:1)第二金属Pt的引入,可以促进光生电荷的进一步分离,有利于复合光催化剂性能的增强;2)金属的负载量会影响到金属颗粒的尺寸、分布以及其对光吸收和电荷分离的影响。在可见光激发下(图7(c)和图7(d)),所有光催化剂对甲醛的降解性能均有所降低。对于AuPt/rGO/g-C3N4,经过150 min的可见光反应,甲醛的降解率和CO2转化率分别为97%和77%。将AuPt/rGO/g-C3N4的可见光活性与g-C3N4复合光催化剂的性能进行了比较,如表1所示。结果表明,rGO和AuPt协同增强的AuPt/rGO/g-C3N4光催化剂在降解甲醛方面具有显著优势。
光催化降解有机污染物的反应可以用Langmuir-Hinshelwood动力学模型来解释。对图7(a)和图7(c)进行ln(C0/C)与t的拟合,得到表观一级反应速率常数,如表2所示。对于AuPt/rGO/g-C3N4,在全谱和可见光下的表观一级反应速率常数分别为0.035 5和0.023 2 min−1,分别为g-C3N4的12.5倍和15.5倍,表明AuPt双金属纳米颗粒和还原氧化石墨烯的协同负载显著提高了g-C3N4光催化降解甲醛等挥发性有机污染物的性能。
图8为AuPt/rGO/g-C3N4在可见光下光催化降解甲醛的稳定性,以及反应前后的XRD谱图和TEM图片。经过5轮的光催化反应,甲醛的降解率分别为97%、96%、95%、93%和92%,表现出良好的光催化稳定性。反应前后的XRD谱图没有明显变化,表明AuPt/rGO/g-C3N4的晶体结构没有发生改变。由图8(b)中的插图中可以看出,AuPt双金属纳米颗粒还是均匀地负载在rGO/g-C3N4表面,颗粒的平均粒径也没有变化。
-
通过上述对光催化剂的结构表征分析和性能测试,对AuPt/rGO/g-C3N4的光催化增强机制进行推测,结果如图9所示。在光照下(无论是全谱激发,还是可见光激发),在g-C3N4的价带和导带分别产生光生空穴和光生电子,在纳米Au的表面也会产生光生电子(或热电子)。首先,由于金属的费米能级低于半导体的费米能级(g-C3N4的费米能级接近于其导带电势),g-C3N4导带上的光生电子会倾向于迁移到金属颗粒表面。因为rGO具有高的电子迁移率(理论上,石墨烯在室温下的电子迁移率达到15 000 cm²·(V·s)−1), rGO的负载可以促进光生电荷从g-C3N4迁移至AuPt双金属纳米颗粒 [43-44]。其次,由于Pt的功函数(5.65)高于Au(5.1),在AuPt中Pt具有接受电子的倾向。第二金属Pt的引入,进一步促使光生电子从Au的表面迁移到Pt的表面,从而优先在Pt的表面发生还原反应[45]。rGO和AuPt双金属纳米颗粒的负载,有利于光生载流子的分离,有利于光催化氧化反应位和还原反应位在空间上的分离。最后,纳米Au 的表面等离共振效应还可以拓宽复合光催化剂的光谱吸收范围,为光催化反应提供更多的光生电子[46]。因此,与rGO/g-C3N4、Au/rGO/g-C3N4和Pt/rGO/g-C3N4相比,rGO和AuPt双金属纳米颗粒的协同作用,大幅增强了g-C3N4光催化降解甲醛的性能。
-
1)在合成g-C3N4纳米片的基础上,分别将还原氧化石墨烯和AuPt双金属纳米颗粒与g-C3N4复合,成功合成了一种高效的AuPt/rGO/g-C3N4复合光催化剂。
2)还原氧化石墨烯和AuPt双金属纳米颗粒的负载,不仅可以拓宽复合光催化剂的光谱吸收范围,还能有效促进光生载流子的分离、迁移和传输,延长光生载流子的寿命。
3) AuPt/rGO/g-C3N4表现出优异的光催化降解甲醛性能,在全谱和可见光激发下对甲醛的降解率分别为96% (90 min)和97% (150 min),CO2的转化率分别为82%和77%,相应的甲醛降解表观一级反应速率常数分别为0.035 5和0.023 2 min−1,分别是g-C3N4的12.5和15.5倍。
AuPt/还原氧化石墨烯/氮化碳的制备及其在室内挥发性有机物处理中的应用
Preparation of nano-Au alloy/reduced graphene oxide/g-C3N4 composites and their application in indoor volatile organic compound treatment
-
摘要: 光催化技术因可以直接利用太阳能、反应条件温和、无二次污染、能降解几乎所有的有机污染物等特点,有望成为解决空气污染的理想污染治理技术。在合成g-C3N4纳米片的基础上,分别将还原氧化石墨烯(rGO)和AuPt双金属纳米颗粒与g-C3N4复合,制备出AuPt/rGO/g-C3N4复合光催化剂。采用XRD、Raman、TEM、XPS、DRS、PL、电化学测试等多种表征手段对光催化剂的晶体结构、形貌、电子结构、光学性质和界面性质等进行了分析,并评估了其在全谱和可见光下降解甲醛的性能。研究结果表明,AuPt/rGO/g-C3N4表现出优异的光催化性能和光催化稳定性,这主要归功于拓宽的可见光吸收能力、有效的光生载流子分离以及延长的光生载流子寿命。在全谱和可见光激发下,AuPt/rGO/g-C3N4对甲醛的降解率分别为96%(90 min)和97%(150 min),CO2的转化率分别为82%和77%,相应的甲醛降解表观一级反应速率常数分别为0.035 5和0.023 2 min−1,分别是g-C3N4的12.5和15.5倍。Abstract: Photocatalytic technology is expected to become an ideal pollution control technology for solving air pollution due to its direct utilization of solar energy, mild reaction conditions, no secondary pollution, and ability to degrade almost all organic pollutants. On the basis of synthesizing g-C3N4 nanosheets, reduced graphene oxide (rGO) and AuPt bimetallic nanoparticles were respectively combined with g-C3N4 to fabricate AuPt/rGO/g-C3N4 photocatalytic composite. The crystal structure, morphology, electronic structure, optical and interface properties of AuPt/G/g-C3N4 were analyzed by techniques such as XRD, Raman, TEM, XPS, DRS, PL and electrochemical testing. The formaldehyde degradation performance by AuPt/rGO/g-C3N4 was assessed under full spectra or visible light conditions. The results indicated that AuPt/rGO/g-C3N4 exhibited excellent photocatalytic performance and stability, mainly due to its expanded visible light absorption capacity, effective separation of photogenerated carriers, and extended lifetime of photogenerated carriers. Under full spectra and visible light irradiation, the degradation rates of formaldehyde over AuPt/rGO/g-C3N4 were 96% (90 min) and 97% (150 min), respectively. The conversion rates of CO2 were 82% and 77%, respectively. The corresponding apparent first-order reaction rate constants for formaldehyde degradation were
0.0355 and0.0232 min−1, which were 12.5 and 15.5 times that of g-C3N4, respectively.-
Key words:
- photocatalysis /
- formaldehyde /
- carbon nitrate /
- rGO /
- bimetallic nanoparticles
-

-
表 1 AuPt/rGO/g-C3N4光催化剂与文献报道的g-C3N4及其复合光催化剂的比较
Table 1. Comparison of the AuPt/rGO/g-C3N4 photocatalyst with the previously reported ones
光催化剂 光源 光催化剂量/mg 甲醛质量浓度/(mg·m−3) 甲醛降解率/% 文献 Ag3PO4/g-C3N4 420 nm LED 50 0.5 22.4 [34] g-C3N4@HN 35 W 氙灯 2450 3 86.9 [35] 多孔g-C3N4 585 nm LED 500 0.45 56.32 [36] g-C3N4-TiO2/zeolites 300 W氙灯 150 0.9 90 [37] TiO2@g-C3N4/SiO2 300 W氙灯 15 83.5 [38] g-C3N4-Ag/SiO2 8W白光LED 500 0.5 65.6 [39] Ni/Ag/g-C3N4 300 W氙灯 0.5 90.19 [40] g-C3N4–TiO2 365 紫外灯 300 128 94 [41] g-C3N4/Bi/BiOBr 氙灯 100 160 80 [42] AuPt/rGO/g-C3N4 300 W氙灯 50 150 96 本研究 表 2 g-C3N4及其复合物在全谱和可见光下降解甲醛的的表观一级反应速率常数
Table 2. Apparent first-order reaction rate constant over g-C3N4 and g-C3N4-based composites
光催化剂 表观一级反应速率常数/min−1 全谱 可见光 g-C3N4 0.002 84 0.001 51 rGO/g-C3N4 0.004 32 0.002 44 Au/rGO/g-C3N4 0.011 7 0.006 34 Pt/rGO/g-C3N4 0.009 95 0.005 71 AuPt/rGO/g-C3N4 0.035 5 0.023 2 AuPt/rGO/g-C3N4-0.5 0.019 9 0.011 2 AuPt/rGO/g-C3N4-2 0.026 9 0.015 5 AuPt/rGO/g-C3N4-3 0.022 5 0.013 4 -
[1] ATKINSON R, AREY J. Atmospheric degradation of volatile organic compounds[J]. Chemical Reviews, 2003, 103(12): 4605-4638. doi: 10.1021/cr0206420 [2] KAMPA M, CASTANAS E. Human health effects of air pollution[J]. Environmental Pollution, 2008, 151(2): 362-367. doi: 10.1016/j.envpol.2007.06.012 [3] PELAEZ M, NOLAN N T, PILLAI S C, et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications[J]. Applied Catalysis B, 2012, 125: 331-349. doi: 10.1016/j.apcatb.2012.05.036 [4] GUO Y L, WEN M C, LI G Y, et al. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: a critical review[J]. Applied Catalysis B, 2021, 281: 119447. doi: 10.1016/j.apcatb.2020.119447 [5] REN H J, KOSHY P, CHEN W F, et al. Photocatalytic materials and technologies for air purification[J]. Journal of Hazardous Materials, 2017, 325: 340-366. doi: 10.1016/j.jhazmat.2016.08.072 [6] WANG X C, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials, 2009, 8(1): 76-80. doi: 10.1038/nmat2317 [7] TANG C S, CHENG M, LAI C, et al. Recent progress in the applications of non-metal modified graphitic carbon nitride in photocatalysis[J]. Coordination Chemistry Reviews, 2023, 474: 214846. doi: 10.1016/j.ccr.2022.214846 [8] LIN J, TIAN W, GUAN Z, et al. Functional carbon nitride materials in photo-Fenton-like catalysis for environmental remediation[J]. Advanced Functional Materials, 2022, 32(24): 2201743. doi: 10.1002/adfm.202201743 [9] XIA P, CAO S, ZHU B, et al. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria[J]. Angewandte Chemie-International Edition, 2020, 59(13): 5218-5225. doi: 10.1002/anie.201916012 [10] LIAO G, GONG Y, ZHANG L, et al. Semiconductor polymeric graphitic carbon nitride photocatalysts: the “holy grail” for the photocatalytic hydrogen evolution reaction under visible light[J]. Energy & Environmental Science, 2019, 12(7): 2080-2147. [11] XIAO Y, TIAN G, LI W, et al. Molecule self-assembly synthesis of porous few-layer carbon nitride for highly efficient photoredox catalysis[J]. Journal of the American Chemical Society, 2019, 141(6): 2508-2515. doi: 10.1021/jacs.8b12428 [12] ZHAO D, DONG C, BIN W, et al. Synergy of dopants and defects in graphitic carbon nitride with exceptionally modulated band structures for efficient photocatalytic oxygen evolution[J]. Advanced Materials, 2019, 31(43): 1903545. doi: 10.1002/adma.201903545 [13] LINIC S, CHRISTOPHER P, INGRAM D. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy[J]. Nature Materials, 2011, 10(12): 911-921. doi: 10.1038/nmat3151 [14] LI S, MIAO P, ZHANG Y, et al. Recent advances in plasmonic nanostructures for enhanced photocatalysis and electrocatalysis[J]. Advanced Materials, 2021, 33(6): 2000086. doi: 10.1002/adma.202000086 [15] KUMAR A, CHOUDHARY P, KUMAR A, et al. Recent advances in plasmonic photocatalysis based on TiO2 and noble metal nanoparticles for energy conversion, environmental remediation, and organic synthesis[J]. Small, 2022, 18(1): 2101638. doi: 10.1002/smll.202101638 [16] LIU X, HAO Z, WANG H, et al. Enhanced localized dipole of Pt-Au single-site catalyst for solar water splitting[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(8): e2119723119. [17] BABU P, DASH S, PARIDA K. Mechanistic insight the visible light driven hydrogen generation by plasmonic Au-Cu alloy mounted on TiO2@B-doped g-C3N4 heterojunction photocatalyst[J]. Journal of Alloys and Compounds, 2022, 909: 164754. doi: 10.1016/j.jallcom.2022.164754 [18] SU H, LIU M, CHENG W, et al. Heterogeneous single-site synergetic catalysis for spontaneous photocatalytic overall water splitting[J]. Journal of Materials Chemistry A, 2019, 7(18): 11170-11176. doi: 10.1039/C9TA01925A [19] CHEN J, LI Y, HUANG L, et al. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process[J]. Carbon, 2015, 81: 826-834. doi: 10.1016/j.carbon.2014.10.033 [20] 赵美花, 陈春连, 蒋芃, 等. Z型g-C3N4/WO3复合材料光催化降解土霉素[J]. 环境工程学报, 2023, 17(9): 2921-2927. doi: 10.12030/j.cjee.202304016 [21] NIU P, ZHANG L, LIU G, et al. Graphene-like carbon nitride nanosheets for improved photocatalytic activities[J]. Advanced Materials, 2012, 22(22): 4763-4770. [22] LIU X, HAO Z, WANG H, et al. Enhanced localized dipole of Pt-Au single-site catalyst for solar water splitting[J]. PNAS, 2022, 119(8): e2119723119. doi: 10.1073/pnas.2119723119 [23] MASLANA K, KALENCZUK R, ZIELINSKA B, et al. Synthesis and characterization of nitrogen-doped carbon nanotubes derived from g-C3N4[J]. Materials, 2023, 13: 1349. [24] WU X, ZHONG W, MA H, et al. Ultra-small molybdenum sulfide nanodot-coupled graphitic carbon nitride nanosheets: trifunctional ammonium tetrathiomolybdate-assisted synthesis and high photocatalytic hydrogen evolution[J]. Journal of Colloid and Interface Science, 2021, 586: 719-729. doi: 10.1016/j.jcis.2020.10.141 [25] XIAO X, GAO Y, ZHANG L, et al. A promoted charge separation/transfer system from Cu single atoms and C3N4 layers for efficient photocatalysis[J]. Advanced Materials, 2020, 32(33): 2003082. doi: 10.1002/adma.202003082 [26] AKSOY M, KORKUT S, METIN O. AuPt alloy nanoparticles supported on graphitic carbon nitride: in situ synthesis and superb catalytic performance in the light-assisted hydrolytic dehydrogenation of ammonia borane[J]. Applied Surface Science, 2022, 602: 154286. doi: 10.1016/j.apsusc.2022.154286 [27] ZHANG X, XIE X, WANG H, et al. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging[J]. Journal of the American Chemical Society, 2013, 135(1): 18-21. doi: 10.1021/ja308249k [28] LIU Z, HOU W, PAVASKAR P, et al. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination[J]. Nano Letters, 2011, 11(3): 1111-1116. doi: 10.1021/nl104005n [29] TIAN Y, TATSUMA T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles[J]. Journal of the American Chemical Society, 2005, 127(20): 7632-7637. doi: 10.1021/ja042192u [30] RAZIQ F, HE J, GAN J, et al. Promoting visible-light photocatalytic activities for carbon nitride based 0D/2D/2D hybrid system: beyond the conventional 4-elctron mechanism[J]. Applied Catalysis B, 2020, 270: 118870. doi: 10.1016/j.apcatb.2020.118870 [31] TIAN X, SUN Y, HE J, et al. Surface P atom grafting of g-C3N4 for improved local spatial charge separation and enhanced photocatalytic H2 production[J]. Journal of Materials Chemistry A, 2019, 7(13): 7628-7635. doi: 10.1039/C9TA00129H [32] LI S, CAI M, WANG C, et al. Ta3N5/CdS core-shell S-scheme heterojunction nanofibers for efficient photocatalytic removal of antibiotic tetracycline and Cr(VI): performance and mechanism insights[J]. Advanced Fiber Materials, 2023, 5: 994-1007. doi: 10.1007/s42765-022-00253-5 [33] ZHANG C, HE H, TANAKA K. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature[J]. Applied Catalysis B, 2006, 65(1-2): 37-43. doi: 10.1016/j.apcatb.2005.12.010 [34] RAO X, DOU H, LONG D, et al. Ag3PO4/g-C3N4 nanocomposites for photocatalytic degradation gas phase formaldehyde at continuous flow under 420 nm LED irradiation[J]. Chemosphere, 2020, 244: 125462. doi: 10.1016/j.chemosphere.2019.125462 [35] CHEN Z, ZHANG X, XU K, et al. Facile fabrication of nanocellulose-supported membrane composited with modified carbon nitride and HKUST-1 for efficient photocatalytic degradation of formaldehyde[J]. International Journal of Biological Macromolecules, 2024, 268: 131937. doi: 10.1016/j.ijbiomac.2024.131937 [36] KONG L, LI X, SONG P, et al. Porous graphitic carbon nitride nanosheets for photocatalytic degradation of formaldehyde gas[J]. Chemical Physics Letters, 2021, 762: 138132. doi: 10.1016/j.cplett.2020.138132 [37] LIU S, LIN W. A simple method to prepare g-C3N4-TiO2/waste zeolites as visible-light-responsive photocatalytic coatings for degradation of indoor formaldehyde[J]. Journal of Hazardous Materials, 2019, 368: 468-476. doi: 10.1016/j.jhazmat.2019.01.082 [38] ZHENG M, LIN Y, LIU S. TiO2@g-C3N4@SiO2 with superior visible-light degradation of formaldehyde for indoor humidity control coatings[J]. Materials Today Sustainability, 2023, 24: 100496. doi: 10.1016/j.mtsust.2023.100496 [39] 刘权锋, 彭炜东, 钟乘韡, 等. g-C3N4-Ag/SiO2复合材料光催化降解甲醛的应用[J]. 复合材料学报, 2022, 39(2): 628-636. [40] WANG Z, HUANG Z, YU J, et al. Growth of Ag/g-C3N4 nanocomposites on nickel foam to enhance photocatalytic degradation of formaldehyde under visible light[J]. Journal of Environmental Sciences, 2024, 137: 432-442. doi: 10.1016/j.jes.2023.02.003 [41] YU J, WANG S, LOW J, et al. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air[J]. Physical Chemistry Chemical Physics, 2013, 15(39): 16883-16890. doi: 10.1039/c3cp53131g [42] 彭江伟, 江卓婷, 姜奎兵, 等. Z-机制g-C3N4/Bi/BiOBr异质结光催化剂制备及其可见光降解甲醛气体研究[J]. 分子催化, 2023, 37(1): 53-62. [43] LIU M, XUE X, YU S, et al. Improving Photocatalytic Performance from Bi2WO6@MoS2/graphene Hybrids via Gradual Charge Transferred Pathway[J]. Scientific Reports, 2017, 7: 3637. doi: 10.1038/s41598-017-03911-6 [44] HOU F, LU K, LIU F, et al. Manipulating a TiO2-graphene-Ta3N5 heterojunction for efficient Z-scheme photocatalytic pure water splitting[J]. Materials Research Bulletin, 2022, 150: 111782. doi: 10.1016/j.materresbull.2022.111782 [45] LI L, WANG X, GU H, et al. Which is more efficient in promoting the photocatalytic H2 evolution performance of g-C3N4: monometallic nanocrystal, heterostructural nanocrystal, or bimetallic nanocrystal?[J]. Inorganic Chemistry, 2022, 61(11): 4760-4768. doi: 10.1021/acs.inorgchem.2c00171 [46] TSAO C, NARRA S, KAO J, et al. Dual-plasmonic Au@Cu7S4 yolk@shell nanocrystals for photocatalytic hydrogen production across visible to near infrared spectral region[J]. Nature Communications, 2024, 15(1): 413. doi: 10.1038/s41467-023-44664-3 -



 下载:
下载: