-
随着全球医疗技术的发展和抗生素的广泛使用,医疗废水尤其是抗生素废水对人类和生态环境造成了严重的危害[1]。四环素作为世界上应用比较广泛的一类抗生素,主要用来治疗各种人类和动物疾病,然而研究表明,动物不能完全吸收和代谢摄入的抗生素,大部分抗生素则是以粪便的形式排放到环境中,四环素在环境中的积累会对土壤和自然水体造成污染,从而对人类的健康构成潜在威胁[2-4]。
目前有很多方法被应用在四环素的去除方面,包括吸附法、生物降解法、光降解法和氧化降解法等[5-8]。其中吸附法由于其操作简单、成本低、效率高和无副产物残留风险等优点得到了研究者的广泛关注[9-10]。在众多吸附材料中,碳材料凭借制备简单、吸附效果好和绿色环保等优点在吸附材料方面更占优势,常见的碳材料分为活性炭、石墨烯、生物炭和一些有机复合材料[11-12]。
海藻酸钠是一种能直接从褐藻中提取的天然生物大分子,无毒无害而且具有很好的生物相容性[13-14]。研究表明,海藻酸钠可以与多种金属离子发生凝胶反应,包括Mg2+、Ca2+、Ba2+、Co2+、Cu2+、Ni2+和Fe2+等[15-17]。燃煤电厂脱硫废水是工业水处理中比较难处理的一类水体,具有成分复杂、含盐量高、重金属污染物浓度高和腐蚀性强等特点,海藻酸钠可以有效利用脱硫废水中的重金属污染物作为交联剂原位实现海藻酸钠的凝胶化[18-20]。本研究采用与脱硫废水中重金属污染物络合形成的海藻酸钠水凝胶,通过碳化的方式来制备海藻酸钠基多孔碳气凝胶,在实现脱硫废水深度处理的同时有效利用处理完脱硫废水的凝胶废弃物。利用SEM、XRD、FTIR和BET等表征手段研究了海藻酸钠基多孔碳气凝胶的结构特征,通过一系列实验考察了在不同pH和反应时间的条件下海藻酸钠基多孔碳气凝胶对溶液中四环素的吸附效果,并对吸附动力学和吸附等温线进行了数据分析,最后通过结构特征和吸附效果来探究海藻酸钠基多孔碳气凝胶对水中抗生素的去除机理。
-
本研究中使用的化学试剂纯度均为分析纯。其中四环素(TC,C22H24N2O8,相对分子质量为444.44,CAS号为60-54-8)购自Macklin生化有限公司(中国上海),海藻酸钠、盐酸和氢氧化钠均购自国药集团化学试剂有限公司,研究用水均为超纯水。脱硫废水取自安徽某发电厂进入三联箱处理工艺之前的脱硫废水蓄水箱,水质情况:COD值为300~400 mg/L,硬度(以CaCO3计)为7 600~8 000 mg/L,pH为7.6~7.8,Ca2+为1 200~1 500 mg/L,Mg2+为1 000~1 200 mg/L,Ni2+的含量为0.06~0.08 mg/L,Zn2+的含量为0.3~0.4 mg/L,As3+的含量为0.02~0.03 mg/L,Cd2+的含量为0.16~0.18 mg/L,Pb2+的含量为0.18~0.2 mg/L,Cr3+的含量为0.005~0.006 mg/L,SO42−的浓度为2 200~2 300 mg/L。
-
材料的制备过程主要分为两部分。首先用电子天平(ME104E/02,Mettler-Toledo)称取60 g的海藻酸钠投加到1 000 mL的超纯水中,利用机械搅拌器(OS20-S ,DRAGONLAB)使其完全溶解形成均一溶液,得到60 g/L的海藻酸钠溶液。将海藻酸钠溶液通过蠕动泵(BT100-2J,Longer Pump)抽成均匀丝状投加到脱硫废水中,并用磁力搅拌器(90-4,上海振荣)进行搅拌,保证材料与脱硫废水充分接触,形成海藻酸钠水凝胶;其次,将上述丝状水凝胶进行冷冻干燥,先将丝状水凝胶放入超低温冰箱-40 ℃下冷冻12 h,然后放入冷冻干燥机(LGJ-10,北京松源)中干燥24 h。将冷干之后的水凝胶用泡沫镍进行包裹保护,放入管式炉(YMG10/6,合肥费舍罗),在30 mL/min恒定流量的氮气保护下进行碳化。管式炉的升温程序:先将样品以10 °C/min的升温速率加热至400 °C,并保持1 h。然后,以相同的升温速率将温度升至800 ℃,并在800 ℃下保持4 h。反应结束且待管式炉温度冷却至室温后,将样品取出备用。
用场发射扫描电子显微镜(FE-SEM,JEOL, JSM-7001F)对所制备的海藻酸钠基多孔碳气凝胶进行表面形貌的分析;场发射扫描电镜连接X射线光电子能谱(SEM-EDS)用来测定凝胶表面的元素种类与含量分析;用全自动比表面积与孔隙度仪(ASAP-2460,美国麦克)对凝胶的比表面积和孔径分布进行分析测定;用X 射线衍射仪(D8 advance,布鲁克)对材料的物相组成和晶化程度进行分析;用红外光谱仪(Vertex 70,布鲁克)来检测凝胶的傅里叶变换红外(FTIR)光谱图。
-
通过设计一系列批量实验来考察海藻酸钠基多孔碳气凝胶在不同的条件下对四环素的去除效果。
-
取100 mg的海藻酸钠基多孔碳气凝胶投加到100 mL的1 mg/L的四环素溶液中,于恒温振荡箱(DLHR-Q200,HDL Appatatus)中在25 ℃的条件下以160 r/min的振荡频率避光振荡。在振荡第10、30、60、120、240、360和480 min的时候进行取样分析。每组实验分别设置空白对照和3个平行实验,浓度取平行实验的平均值进行分析。用准一级动力学模型和准二级动力学模型对实验数据进行拟合,探究海藻酸钠基多孔碳气凝胶吸附溶液中四环素的动力学行为。
-
分别配制1、5、10、50、100和200 mg/L浓度的四环素溶液。取100 mg的海藻酸钠基多孔碳气凝胶投加到100 mL的上述四环素溶液中,于恒温振荡箱中在25 ℃的条件下以160 r/min的振荡频率避光振荡,反应8 h的时候进行取样。每组实验分别设置空白对照和3个平行实验,浓度取平行实验的平均值进行分析,将得到的实验数据用Langmuir和Freundlich模型进行拟合。
-
用0.05 mol/L的NaOH溶液和0.1 mol/L的HCl溶液来调节1 mg/L四环素溶液的pH值。利用台式pH计(LE438,Mettler-Toledo)测定溶液的pH值,将溶液的初始pH值分别调至2、4、6、8和10,然后称取100 mg海藻酸钠基多孔碳气凝胶投加到100 mL的上述四环素溶液中,于恒温振荡箱中在25 ℃的条件下以160 r/min的振荡频率避光振荡,反应6 h的时候进行取样分析。每组实验分别设置空白对照和3个平行实验,浓度取平行实验的平均值进行分析。
-
将所取水样用0.45 µm的水相滤头进行海藻酸钠基多孔碳气凝胶和四环素溶液的固液分离,然后用紫外分光光度计(UV-6100,上海美普达)在358 nm的波长下分析滤液中剩余四环素的浓度。
-
海藻酸钠基多孔碳气凝胶对溶液中的四环素的去除率和吸附容量(q)的计算方法,见式(1~2):
式中:q为吸附容量,mg/g;C0和Ct分别为初始时的溶液中四环素的浓度和平衡时的四环素浓度,mg/L;V为四环素溶液的体积,L;m为凝胶的投加量,g。
为了确定达到吸附平衡的时间,了解吸附过程的特点,将四环素的吸附动力学过程利用准一级动力学模型和准二级动力学模型进行模拟,具体模型见式(3~4):
式中:Qe为四环素的平衡吸附量,mg/g;Qt为t时刻四环素的吸附量,mg/g;t为吸附时间,min;k1为准一级动力学速率常数,min−1;k2为准二级动力学速率常数,g/(mg·min);
利用Langmuir 模型和Freundich 模型拟合海藻酸钠基多孔碳气凝胶对四环素溶液的等温吸附过程。
Langmuir具体模型,见式(5):
转换为线性模型,见式(6):
式中:qe为平衡吸附容量,mg/g;qmax为最大吸附容量,mg/g;KL为Langmuir吸附平衡常数;Ce为平衡时溶液中四环素的质量浓度,mg/L。
使用平衡参数RL来评价吸附的可行性,见式(7):
式中:RL的值表示吸附等温线的类型。RL = 0表示吸附不可逆;0 < RL < 1表示对吸附起促进作用;RL = 1表示吸附呈线性相关;RL > 1表示对吸附起抑制作用。
基于在非均匀表面上的吸附,Freundlich等温线以线性形式表示,见式(8):
式中,KF和n均为Freundlich常数,mg(1–1/n) L1/n /g。
-
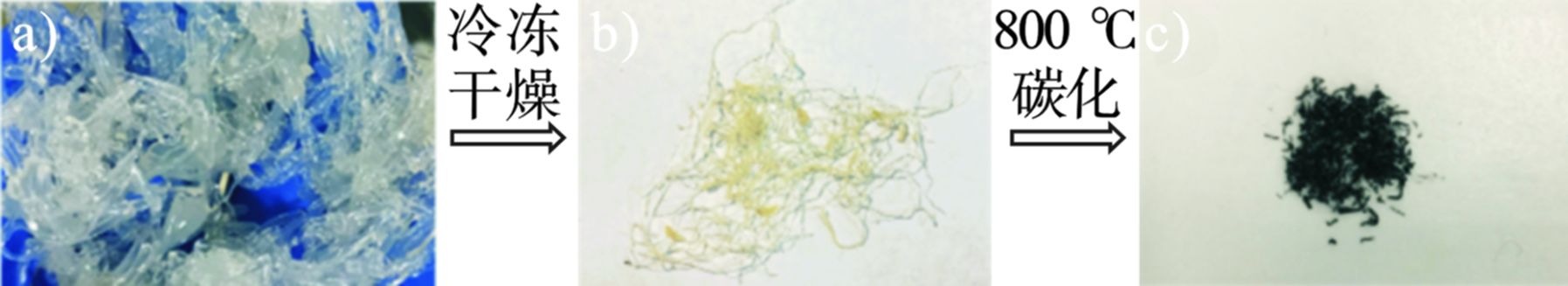
材料的制备过程见图1。首先将海藻酸钠投加到脱硫废水中,与脱硫废水中的重金属污染物迅速交联形成图1(a) 中的水凝胶,之后将处理过脱硫废水的废弃水凝胶进行冷冻干燥,再用管式炉800 ℃进行碳化,最终得到海藻酸钠基多孔碳气凝胶。
-
海藻酸钠基多孔碳气凝胶的SEM,见图2。通过观察凝胶的微观形貌结构,可以看出凝胶具有比较丰富的孔隙结构,虽然无序,但是孔径比较大,这样的结构有利于提高凝胶的吸附能力。对比图2(a)和(b)发现,吸附后的海藻酸钠基多孔碳气凝胶的孔隙率明显减少。海藻酸钠基多孔碳气凝胶的元素组成,见表1。表1可知,吸附前的凝胶元素组成主要是C和O,同时附带着Ca、Mg等金属元素,说明海藻酸钠凝胶成功络合了脱硫废水中的重金属污染物。对比吸附四环素后的海藻酸钠基多孔碳气凝胶发现,金属元素的含量明显降低,C和O的含量显著升高,推测是因为四环素成功占据了碳气凝胶上的活性位点。
-
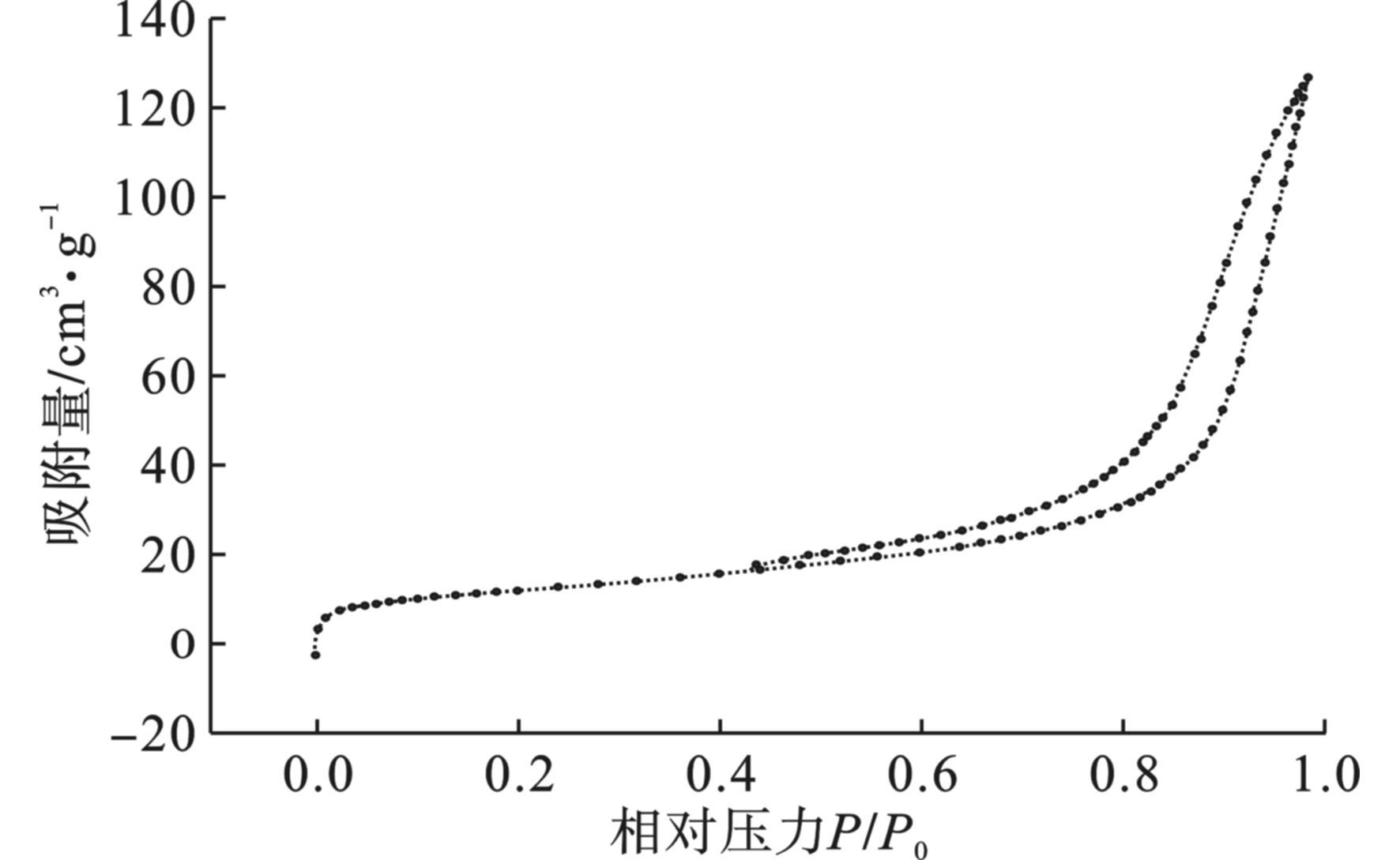
以氮气为吸附介质,在77 K和相对压力为10−6~1.0的范围内进行氮气吸附脱附测定凝胶的比表面积和孔结构。经过测定,海藻酸钠基多孔碳气凝胶的比表面积是52.37 m2/g。碳气凝胶对氮气的吸附脱附等温线见图3,等温线存在一个明显的滞后回环,这是Ⅳ类等温线的一个特征,表明海藻酸钠基多孔碳气凝胶的孔结构为中孔结构。
-
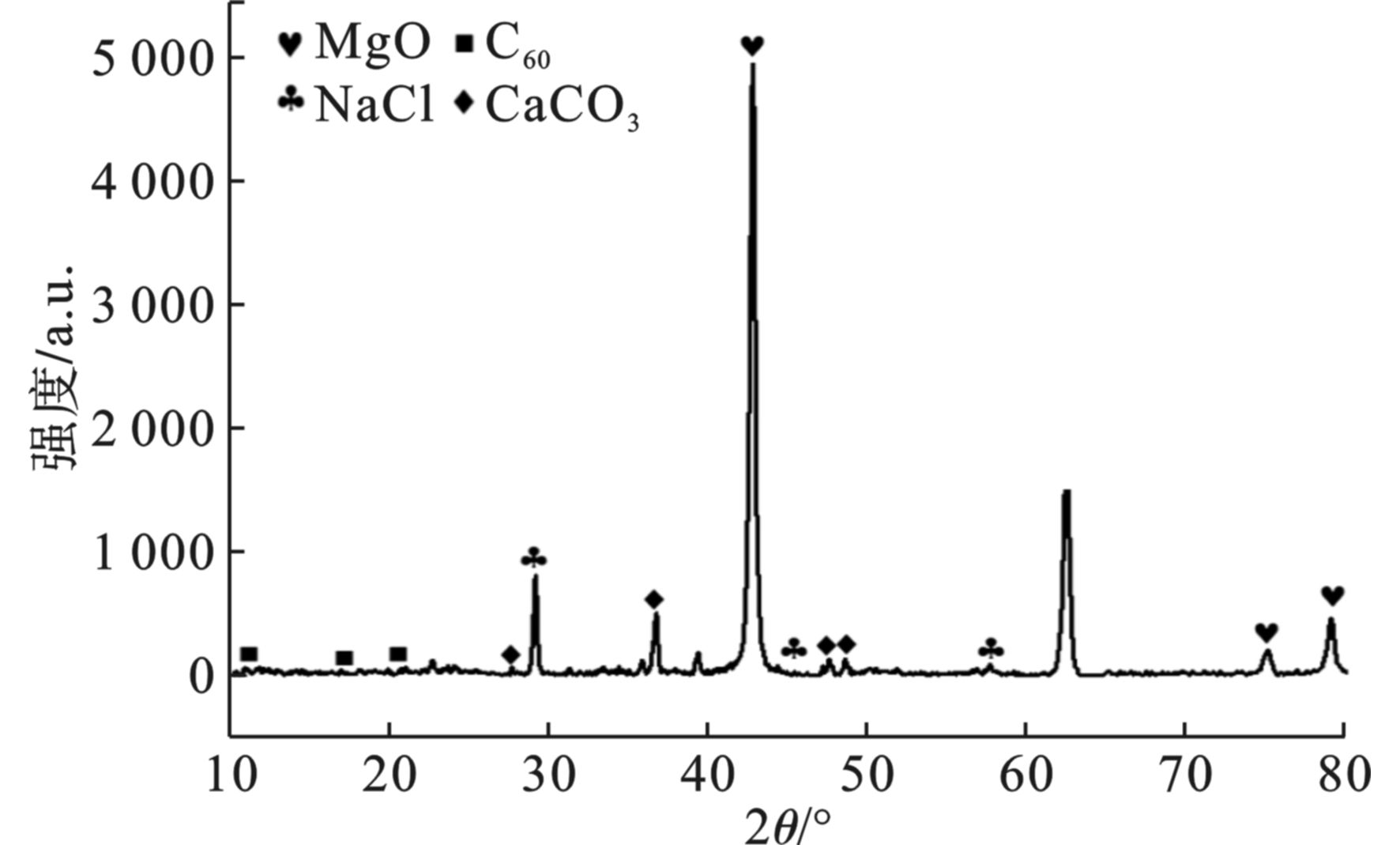
对海藻酸钠基多孔碳气凝胶进行X射线粉末衍射分析见图4。碳气凝胶在2θ为32°、42.5°和62.5°附近均出现衍射峰。通过与标准PDF卡片对比可知,海藻酸钠基多孔碳气凝胶中存在MgO、NaCl和CaCO3晶体,同时在碳化的过程中还生成了C60。
-
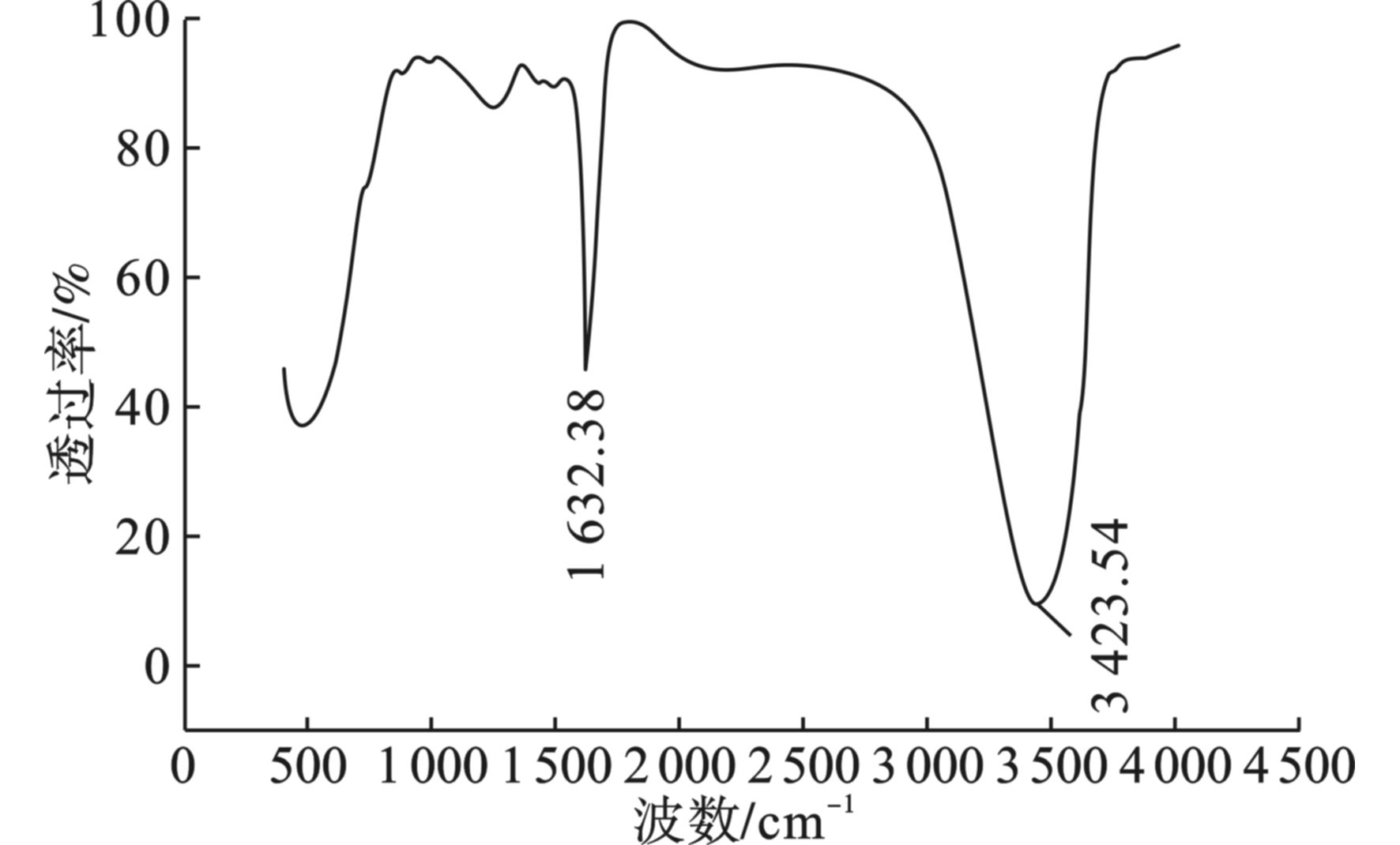
为了进一步研究海藻酸钠基多孔碳气凝胶中各组分的相互作用,对其进行FTIR分析,见图5。碳气凝胶在3 423 cm−1处存在羟基(—OH)的伸缩振动峰,而在1 632 cm−1处出现的微弱吸收峰,则与环状结构上的C=C和羰基(C=O)的伸缩振动峰有关[21-22]。
-
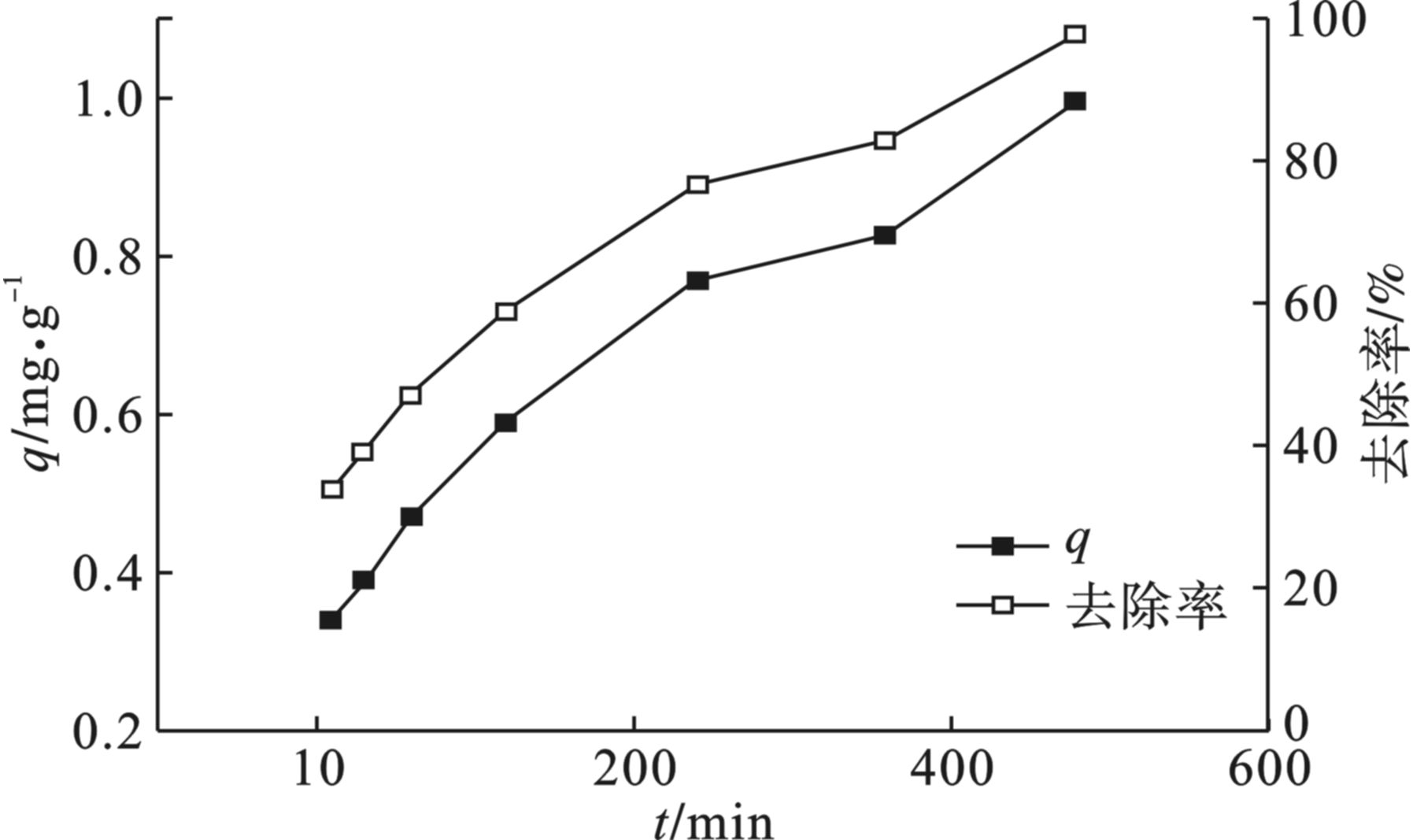
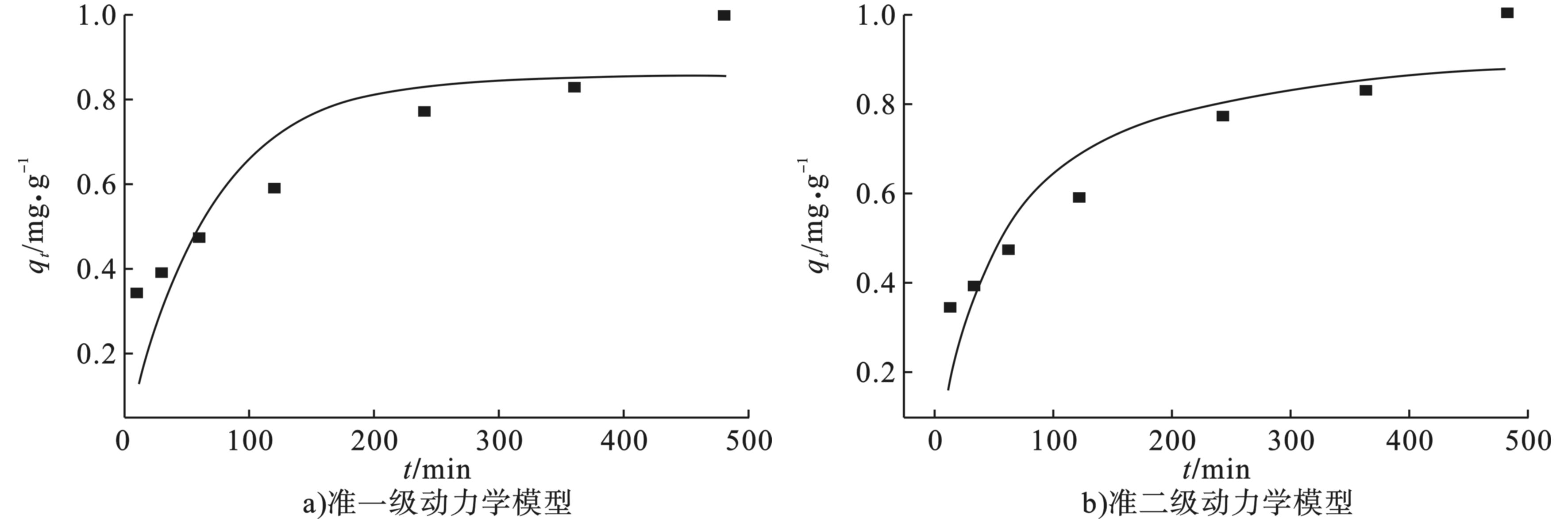
海藻酸钠基多孔碳气凝胶对四环素的吸附动力学研究见图6,碳气凝胶对水中四环素的吸附容量在短时间内随着反应时间的增加而迅速增大,去除率也随之增大,在反应时间到达240 min之后反应逐渐趋于平缓。这是由于在反应初期,凝胶上有相对较多的活性位点,四环素可以迅速与凝胶上的活性位点结合,达到去除的效果。从图中可以看出海藻酸钠基多孔碳气凝胶对溶液中的四环素去除率可以达到95%以上。
将海藻酸钠基多孔碳气凝胶对溶液中的四环素的吸附过程利用准一级动力学模型和准二级动力学模型进行拟合,拟合曲线见图7,具体数据见表2。比较两个动力学模型的可决系数(R2)可知,海藻酸钠基多孔碳气凝胶对溶液中的四环素的吸附行为更符合准二级动力学方程,而且拟合所得的平衡吸附量与实验所得的最大吸附量非常接近,且吸附过程以较快的速率进行,这与四环素本身含有较多的芳环结构有关,有研究表明抗生素的芳香环越多,碳基材料对它们的吸附速率越快,本研究中的海藻酸钠基多孔碳气凝胶和四环素上的芳环结构可以通过π-π作用结合进而去除溶液中的四环素[23]。
-
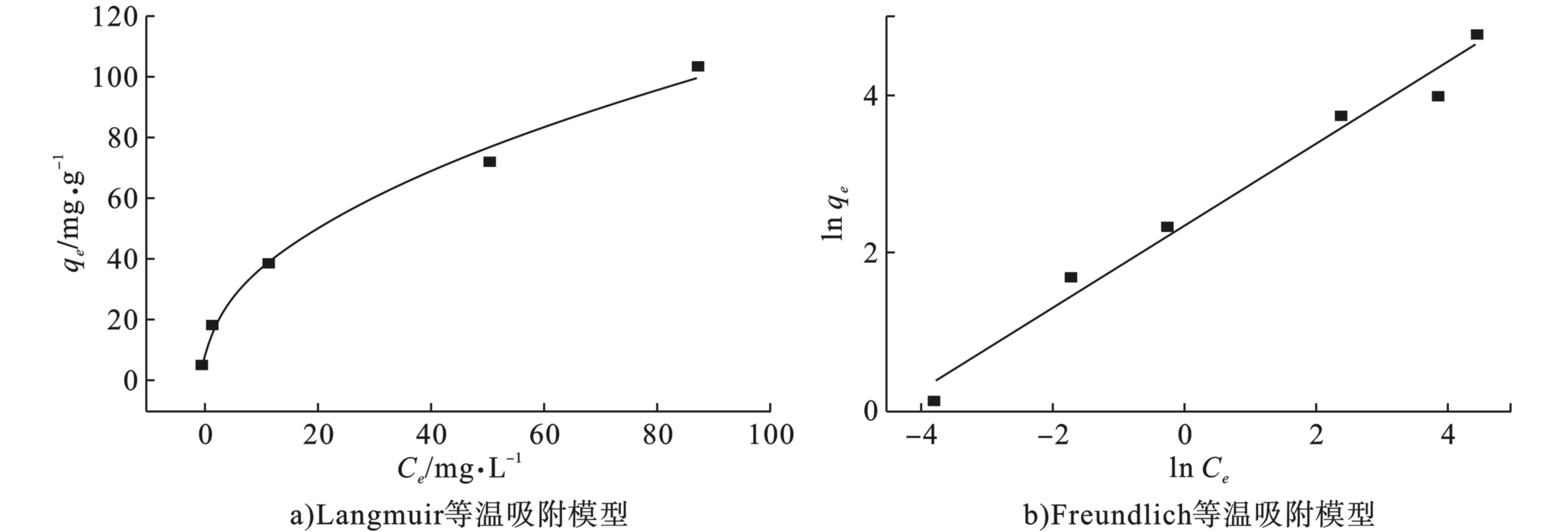
用Langmuir 和Freundlich 等温吸附模型对海藻酸钠基多孔碳气凝胶吸附四环素的过程进行拟合,见图8。2种模型的具体参数见表3,对比R2可以看出,Freundlich模型的R2为0.979 8,而Langmuir模型的R2为0.913 0,从拟合可以看出,用Freundlich等温吸附模型的拟合比Langmuir模型更高。因此海藻酸钠基多孔碳气凝胶对四环素的吸附过程更符合Freundlich模型,说明吸附过程是发生在非均匀表面的多分子层吸附[24]。
-
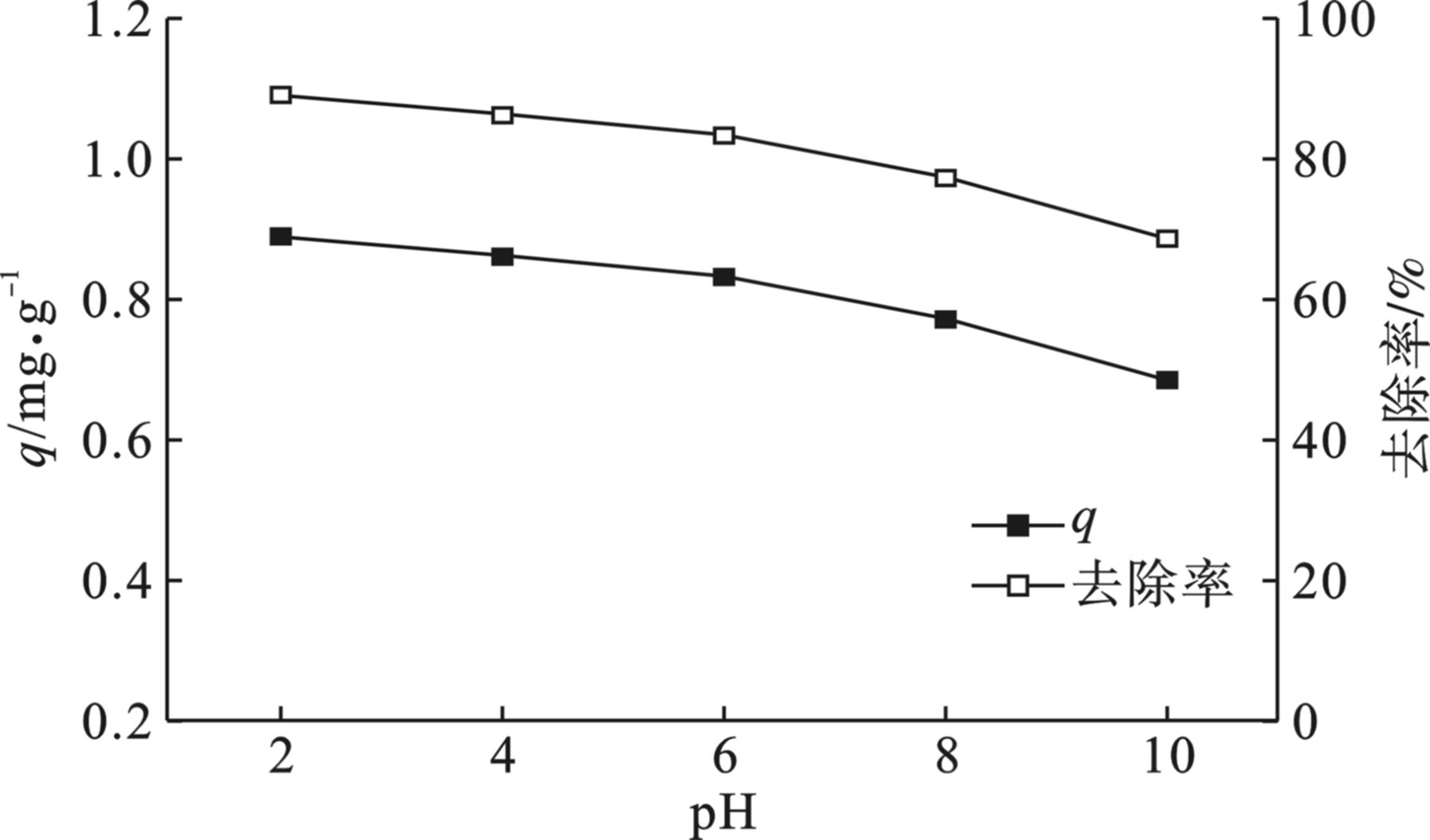
由于在不同的pH条件下四环素的存在形态以及生物炭的表面电荷不同,所以溶液的初始pH会影响海藻酸钠基多孔碳气凝胶的吸附效果。溶液初始pH在2~10范围内,碳气凝胶对四环素的吸附情况见图9。碳气凝胶对四环素的吸附量随着溶液pH的升高而逐渐降低。当溶液的pH在2~6的范围内时,凝胶对四环素有着较高的吸附量,且随pH 的升高,吸附量下降缓慢;当溶液的pH继续升高到10附近时,凝胶对四环素吸附量的下降趋势更加明显。这种趋势与四环素在不同的pH 下的存在形态有关,当pH < 4时,存在TCH3 +形态,阳离子与碳气凝胶发生强烈的静电吸附作用,因此在低pH 的环境下,海藻酸钠基多孔碳气凝胶对四环素的吸附效果更好;当pH>8时,主要存在TCH2−形态,阴离子与凝胶之间存在静电排斥作用,而且碱性过强时凝胶表面的活性位点会发生钝化,从而导致海藻酸钠基多孔碳气凝胶对四环素的吸附能力下降[25-26]。
-
(1) 利用与脱硫废水中重金属污染物络合形成的海藻酸钠水凝胶制备的海藻酸钠基多孔碳气凝胶的比表面积为52.37 m2/g,具有丰富的多孔结构。同时碳气凝胶中存在MgO、NaCl和CaCO3晶体以及 C60。通过FTIR分析,碳气凝胶中含有羟基(—OH)、C=C和羰基(C=O)结构。
(2)海藻酸钠基多孔碳气凝胶在较宽的pH范围内(2~6)均对溶液中的四环素具有良好的吸附能力,随着溶液pH值的增大,碳气凝胶对四环素的吸附量逐渐降低。海藻酸钠基多孔碳气凝胶对四环素的吸附过程更符合准二级动力学方程(R2=0.825 7),等温吸附过程更符合Freundlich 等温吸附模型(R2=0.979 8)。
(3)本研究提出的海藻酸钠基多孔碳气凝胶可以有效利用处理完脱硫废水的凝胶废弃物,不但可以实现脱硫废水处理的资源化利用,也可以为污水中抗生素污染物的处理提供新思路。
海藻酸钠基多孔碳气凝胶的制备及其对水中四环素去除
Preparation of sodium alginate-based porous carbon aerogel and its removal of tetracycline from aqueous solution
-
摘要: 采用脱硫废水中的重金属污染物原位交联使海藻酸钠形成水凝胶,再将该水凝胶在800 ℃的条件下制备为海藻酸钠基多孔碳气凝胶,利用场发射扫描电子显微镜(SEM)、比表面积分析仪、X射线衍射(XRD)和傅里叶红外光谱(FTIR)对该气凝胶进行了表征分析,并考察了该气凝胶对溶液中四环素的吸附行为。结果表明,该碳气凝胶具有丰富的孔隙结构,比表面积可以达到52.37 m2/g。 随着溶液初始pH值的增大,碳气凝胶对四环素的吸附量逐渐降低。碳气凝胶对四环素的吸附行为更符合准二级动力学方程(R2=0.825 7),最大吸附量可以达到112 mg/g,等温吸附过程对Freundlich 等温吸附模型比对Langmuir等温吸附模型拟合度更高,说明利用处理脱硫废水的凝胶废弃物制备的海藻酸钠基多孔碳气凝胶在去除水环境中的抗生素方面有较好的应用前景,实现了脱硫废水凝胶废弃物的资源化利用。Abstract: Sodium alginate hydrogel was formed by in situ cross-linking of heavy metal pollutants in desulfurization wastewater, and sodium alginate-based porous carbon aerogel was prepared at 800 ℃. Then the removal effect of sodium alginate hydrogel on tetracycline in solution were studied. The carbon aerogel was characterized by scanning electron microscopy (SEM), accelerated surface area and porosimetry system, X-ray diffraction (XRD) and Fourier transform infrared (FT-IR) spectroscopy. The results showed that the sodium alginate-based carbon aerogel had a rich pore structure, and the specific surface area could reach 52.37 m2/g. With the increase of the initial pH of the solution, the adsorption capacity of tetracycline by the carbon aerogel gradually decreased. The adsorption kinetic of tetracycline onto sodium alginate-based porous carbon aerogel was fitted to pseudo-second-order models (R2=0.825 7). The maximum adsorption capacity could reach 112 mg/g. The adsorption isotherm process was more in line with the Freundlich isotherm adsorption model than the Langmuir model, thus indicating that the sodium alginate-based porous carbon aerogel prepared by the gel waste of desulfurization wastewater had a good application prospect in removing antibiotics in the water environment. The desulfurization wastewater gel waste could be recycled as the resource.
-
Key words:
- desulfurization wastewater /
- sodium alginate /
- carbon aerogel /
- tetracycline /
- adsorption /
- resource utilization
-

-
表 1 吸附前后海藻酸钠基多孔碳气凝胶元素组成
% 元素种类 吸附前凝胶 吸附后凝胶 C 11.60 22.70 Mg 16.67 9.30 Cl 13.86 0.57 Ca 11.43 0.88 O 46.44 66.55 表 2 海藻酸钠基多孔碳气凝胶的吸附动力学参数
项目 准一级动力学模型 准二级动力学模型 qe/mg·g−1 0.8590 0.9690 k/min−1 0.0150 0.0210 R2 0.7218 0.8257 表 3 海藻酸钠基多孔碳气凝胶对四环素的吸附等温线参数
项目 Langmuir模型 Freundlich模型 KL/mg·L−1 0.0886 − KF/(mg1·1/n·L1/n)·g−1 − 9.5860 qm/mg·g−1 112 − 1/n − 0.5229 R2 0.9130 0.9798 -
[1] WANG X, LIU B, SHI B, et al. Transformation of leaf waste into 3D graphene for water treatment[J]. Desalination and Water Treatment, 2019, 168: 348 − 356. doi: 10.5004/dwt.2019.24654 [2] LIANG Y, PEI M, WANG D, et al. Improvement of soil ecosystem multifunctionality by dissipating manure-induced antibiotics and resistance genes[J]. Environmental Science & Technology, 2017, 51(9): 4988 − 4998. [3] 程扬, 沈启斌, 刘子丹, 等. 两种生物炭的制备及其对水溶液中四环素去除的影响因素[J]. 环境科学, 2019, 40(3): 1328 − 1336. doi: 10.13227/j.hjkx.201807076 [4] 周启星, 罗义, 王美娥. 抗生素的环境残留、生态毒性及抗性基因污染[J]. 生态毒理学报, 2007, 2(3): 243 − 251. [5] KHAN M, BAE H, JUNG J. Tetracycline degradation by ozonation in the aqueous phase: Proposed degradation intermediates and pathway[J]. Journal of Hazardous Materials, 2010, 181(1-3): 659 − 665. doi: 10.1016/j.jhazmat.2010.05.063 [6] 张树清, 张夫道, 刘秀梅, 等. 高温堆肥对畜禽粪中抗生素降解和重金属钝化的作用[J]. 中国农业科学, 2006, 39(2): 337 − 343. doi: 10.3321/j.issn:0578-1752.2006.02.018 [7] ZHUANG Y, YU F, MA J, et al. Facile synthesis of three-dimensional graphene–soy protein aerogel composites for tetracycline adsorption[J]. Desalination and Water Treatment, 2016, 57: 9510 − 9519. doi: 10.1080/19443994.2015.1029530 [8] 张玮玮, 弓爱君, 邱丽娜, 等. 废水中抗生素降解和去除方法的研究进展[J]. 中国抗生素杂志, 2013, 38(6): 401 − 410. doi: 10.3969/j.issn.1001-8689.2013.06.001 [9] PUTRA E, PRANOWO R, SUNARSO J, et al. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics[J]. Water Research, 2009, 43(9): 2419 − 2430. doi: 10.1016/j.watres.2009.02.039 [10] AKSU Z, TUNC Ö. Application of biosorption for penicillin G removal: comparison with activated carbon[J]. Process Biochemistry, 2005, 40(2): 831 − 847. doi: 10.1016/j.procbio.2004.02.014 [11] ÁLVAREZ-TORRELLAS S, RIBEIRO R S, GOMES H, et al. Removal of antibiotic compounds by adsorption using glycerol-based carbon materials[J]. Chemical Engineering Journal, 2016, 296: 277 − 288. doi: 10.1016/j.cej.2016.03.112 [12] YU F, LI Y, HAN S, et al. Adsorptive removal of antibiotics from aqueous solution using carbon materials[J]. Chemosphere, 2016, 153: 365 − 385. doi: 10.1016/j.chemosphere.2016.03.083 [13] ZHUANG Y, YU F, CHEN H, et al. Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity[J]. Journal of Materials Chemistry A, 2016, 4(28): 10885 − 10892. doi: 10.1039/C6TA02738E [14] NAKAUMA M, FUNAMI T, FANG Y, et al. Calcium binding and calcium-induced gelation of sodium alginate modified by low molecular-weight polyuronate[J]. Food Hydrocolloids, 2016, 55: 65 − 76. doi: 10.1016/j.foodhyd.2015.10.021 [15] LI X, QI Y, LI Y, et al. Novel magnetic beads based on sodium alginate gel crosslinked by zirconium(IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems[J]. Bioresource Technology, 2013, 142: 611 − 619. doi: 10.1016/j.biortech.2013.05.081 [16] KONG Y, ZHUANG Y, SHI B. Tetracycline removal by double-metal-crosslinked alginate/graphene hydrogels through an enhanced Fenton reaction[J]. Journal of Hazardous Materials, 2020, 382: 121060. doi: 10.1016/j.jhazmat.2019.121060 [17] CRUZ A, COUTO L, ESPLUGAS S, et al. Study of the contribution of homogeneous catalysis on heterogeneous Fe(III)/alginate mediated photo-Fenton process[J]. Chemical Engineering Journal, 2017, 318: 272 − 280. doi: 10.1016/j.cej.2016.09.014 [18] 叶春松, 黄建伟, 刘通, 等. 燃煤电厂烟气脱硫废水处理方法与技术进展[J]. 环境工程, 2017, 35(11): 10 − 13+51. doi: 10.13205/j.hjgc.201711003 [19] 杨跃伞, 苑志华, 张净瑞, 等. 燃煤电厂脱硫废水零排放技术研究进展[J]. 水处理技术, 2017, 43(6): 29 − 33. doi: 10.16796/j.cnki.1000-3770.2017.06.006 [20] 李兵, 张其龙, 王学同, 等. 燃煤电厂废水零排放处理技术[J]. 水处理技术, 2017, 43(6): 24 − 28+33. doi: 10.16796/j.cnki.1000-3770.2017.06.005 [21] LIU H, YANG F, ZHENG Y, et al. Improvement of metal adsorption onto chitosan/Sargassum sp. composite sorbent by an innovative ion-imprint technology[J]. Water Research, 2011, 45(1): 145 − 154. doi: 10.1016/j.watres.2010.08.017 [22] 丁文川, 田秀美, 王定勇, 等. 腐殖酸对生物炭去除水中Cr(Ⅵ)的影响机制研究[J]. 环境科学, 2012, 33(11): 3847 − 3853. [23] PENG B, CHEN L, QUE C, et al. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by π-π interactions[J]. Scientific Reports, 2016, 6(1): 1 − 10. doi: 10.1038/s41598-016-0001-8 [24] WANG H, FANG C, WANG Q, et al. Sorption of tetracycline on biochar derived from rice straw and swine manure[J]. RSC Advances, 2018, 8(29): 16260 − 16268. doi: 10.1039/C8RA01454J [25] OCAMPO-PÉREZ R, LEYVA-RAMOS R, RIVERA-UTRILLA J, et al. Modeling adsorption rate of tetracyclines on activated carbons from aqueous phase[J]. Chemical Engineering Research and Design, 2015, 104: 579 − 588. doi: 10.1016/j.cherd.2015.09.011 [26] LI H, HU J, MENG Y, et al. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather[J]. Science of the Total Environment, 2017, 603-604: 39 − 48. doi: 10.1016/j.scitotenv.2017.06.006 -



 下载:
下载: