-
抗生素由于良好的抑菌杀菌性能被广泛应用于医疗卫生、禽畜及水产养殖等领域[1]。然而,由于抗生素滥用导致大量耐药菌产生,引发了一系列难治性感染。因此,探索和研发抗生素的替代品变得迫在眉睫[2]。群体感应抑制剂(quorum sensing inhibitor,QSIs)是作用于细菌群体感应(quorum sensing,QS)系统的一类新型抗菌药物,它可以通过抑制信号分子合成、促进信号分子降解、阻断信号分子与受体结合以及抑制信号分子级联转导水平等方式抑制细菌的QS系统[3]。相比于传统抗生素,QSIs不会直接对细菌产生毒性作用,不易使细菌产生耐药性[4]。因此,QSIs被认为是未来最理想的抗生素替代品。根据化学结构的不同,QSIs可分为二酮呱嗪类、酰基高丝氨酸内酯类(N-Acyl homoserine lactone,AHL)、寡肽类以及激素类[5]等。其中,高丝氨酸内酯类QSIs(本文简称为AHL-QSIs)最为引人关注,其作用机制是与AHL信号分子竞争结合受体蛋白来干扰QS系统[6]。鉴于AHL-QSIs在医疗及养殖领域中广泛的应用前景,有必要探究其潜在的生态风险。
目前,已有研究者探索了AHL-QSIs对细菌的毒性作用,发现其可以对细菌产生低浓度刺激、高浓度抑制的毒物兴奋效应(即Hormesis效应)。例如,Wang等[7]等在测定6种AHL-QSIs(3-苯并呋喃酮、D-脯氨醇、2-甲基四氢呋喃-3-酮、(R)-羟基四氢吡咯、γ-戊内酯、1-(1-吡咯烷)环己烯)对大肠杆菌的生长毒性效应时发现,这些AHL-QSIs在第14 h会对大肠杆菌的生长产生Hormesis效应。Zhang[8]等的研究表明,呋喃酮乙酸酯(一种AHL-QSIs)对枯草芽孢杆菌的生长在第3 h也有明显的Hormesis效应。虽然AHL-QSIs对细菌的Hormesis效应已被人们发现,但关于这种效应的具体机制还有待进一步探索。已有研究表明,细菌Hormesis效应的发生与细菌自身的QS系统密切相关。例如Sun[9]等发现磺胺类抗生素引起大肠杆菌生长的Hormesis效应可能是由磺胺类抗生素作用于QS系统中的关键蛋白并刺激其表达所导致。Deng[10]等在研究磺胺类抗生素对明亮发光杆菌的毒性效应时也发现,Hormesis效应源于磺胺类抗生素作用于明亮发光杆菌的QS系统。鉴于以上研究进展,探索AHL-QSIs对细菌的QS系统的作用,可能是了解AHL-QSIs诱导Hormesis效应并阐明其作用机制的一个有效手段。
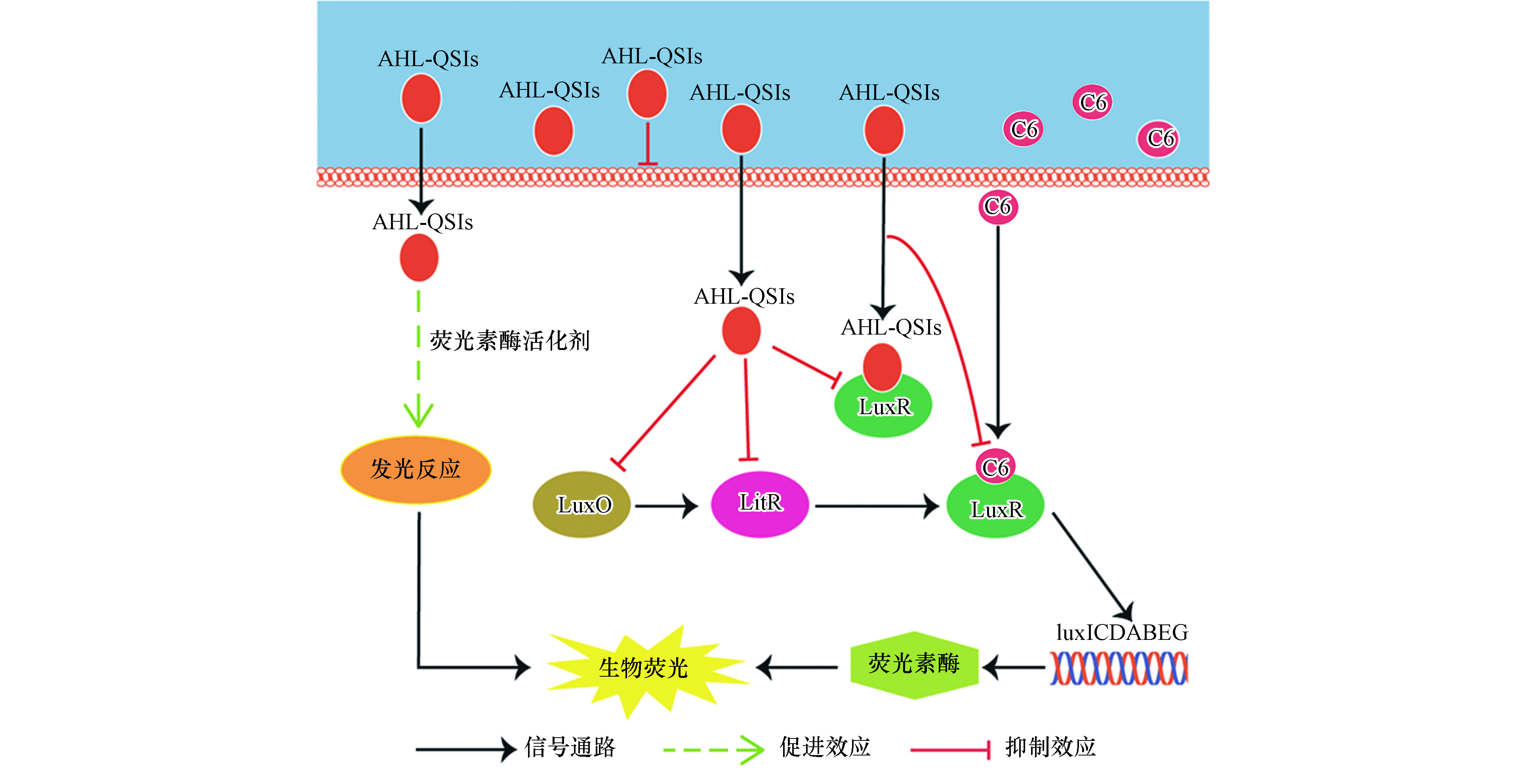
费氏弧菌(Aliivibrio fischeri,A. fischeri)是首次发现具有QS现象的革兰氏阴性菌[11],其QS系统较为清晰,如图1所示,主要调控A. fischeri的生物荧光[12-14]。在细菌缺乏C8信号分子和AI-2信号分子时,磷酸化的AinR蛋白和磷酸化的LuxP/Q蛋白会将磷酸分子传递给LuxU蛋白,再传递给LuxO蛋白,而磷酸化的LuxO蛋白是qrr1基因的转录激活剂,可以激活qrr1基因编码产生Qrr1蛋白;Qrr1蛋白在蛋白Hfq的协同作用下会抑制litR基因向LitR蛋白的编码,从而阻断后续的信号通路。当信号分子C8和AI-2含量增加时,磷酸转移路径会被抑制,导致LitR蛋白稳定表达,继而转录激活luxR基因,编码LuxR蛋白;LuxR蛋白能够和信号分子C6结合形成复合物,激活luxICDABEG基因;luxA和luxB两种基因能够分别编码荧光素酶的α亚基和β亚基[15],而荧光素酶能够催化A. fischeri体内的生物发光反应,进而产生生物荧光。
本课题组前期实验发现,AHL-QSIs对A. fischeri的发光在延滞期具有Hormesis效应,且这种Hormesis效应会随生长周期的变化而变化[9]。因此本文以A. fischeri为受试模式生物,3种AHL-QSIs(2(5H)-呋喃酮(2(5H)-Furanone,2,5-D-3(2H)-F)、D-脯氨醇(D-Prolinol,D-P)以及N-乙烯基吡咯烷酮(N-Vinyl-2-pyrrolidone,NV2P))为受试化合物,测定了它们对A. fischeri生物荧光、生长量、相关蛋白表达量以及模拟发光反应的影响。初步探究了AHL-QSIs对A. fischeri生物荧光随时间变化的Hormesis效应及其机制,以期为AHL-QSIs的后续应用以及使用及环境生态风险评价提供研究基础。
全文HTML
-
2,5-D-3(2H)-F、D-P以及NV2P均购自Sigma-Aldrich化学制品有限公司(圣路易斯,密苏里州,美国),纯度均为分析纯(98%)以上,具体信息见表1。
实验模式生物A. fischeri(ATCC7744)购自美国模式培养物保藏所(马纳萨斯,维吉尼亚州,美国)。A. fischeri在培养24 h后,其生长周期可以分为3个阶段:(1)延滞期(1—8 h),细菌生长缓慢并且生物荧光较低,此时QS较弱,C6和LuxR的浓度较低;(2)对数期(9—17 h),细菌快速生长并且生物荧光快速增加,此时QS较强,有大量的C6和LuxR产生;(3)平台期(18—24 h),细菌的生长逐渐减缓并且生物荧光减弱,此时QS开始逐渐变弱,C6和LuxR的产生也逐渐变少[16]。
-
在无菌操作台上,用灭好菌的接种环接取适量菌种放入5 mL的单倍液体培养基中,置于22 ℃培养箱中振荡培养至对数生长期(12 —14 h)成为摇瓶菌液。实验时取0.2 mL摇瓶菌液加入40 mL的2%氯化钠溶液中,持续搅拌40 min后用于毒性试验。
-
(1)慢性毒性实验
取待测化合物用适量DMSO配制成标准溶液,实验时用2%(质量分数)NaCl稀释成等对数梯度系列,取80 μL加入96孔板中。同时取80 μL的2% NaCl作为对照。在每个样品中加入80 μL 2.5倍培养基和40 μL的工作菌液(初始发光值需调至12000—15000)。每个浓度点至少做3个平行。振荡、混匀,放入Luminoskan Ascent发光分析仪(赛默飞世尔科技有限公司,美国)或Bioscreen C全自动生长分析仪(Bioscreen测试服务股份有限公司,美国)中培养24 h,每隔1 h测定RLU(relative light units,相对光度单位)或OD600(optical density,光密度)值。
(2)急性毒性实验
急性毒性实验的方法与慢性毒性方法类似,急性毒性实验是将A. fischeri直接暴露于不同浓度的污染物,不需要加入2.5倍培养基,15 min后测定每个浓度点的毒性;慢性毒性实验则是将 A. fischeri直接暴露于不同浓度的污染物,但是需要加入2.5倍培养基,24 h后测定每个浓度点的毒性。在急性毒性实验中,暴露时间较短,细菌数目变化很小,因此仅以生物荧光作为测试终点。首先配制待测化合物标准溶液,实验时用 2% NaCl 稀释成等对数梯度系列,取 80 μL加入96孔板相应位置(例如:2,5-D-3(2H)-F设置了16个浓度点,分别为lgC=−3.87,−3.74,−3.62,−3.48,−3.36,−3.23,−3.10,−2.97,−2.87,−2.74,−2.62,−2.48,−2.36,−2.23,−2.10,−1.97 mol·L−1),空白样加入80 μL的 2% NaCl溶液;每个孔中再各加入80 μL 2% NaCl溶液,并加入40 μL工作菌液(初始发光值需调至200000左右)。其中,每个浓度点至少做3个平行。振荡混匀后静置15 min,于酶标仪(Mithras LB940,德国伯托科技有限公司,德国)测定RLU值。
(3)毒性表征
化合物毒性用其对A. fischeri的发光抑制率和生长抑制率表征,如式(1)所示:
其中,X0代表对照组的RLU值或者OD600值,X代表实验组的RLU值或者OD600值,对照组和实验组均为3组平行。
-
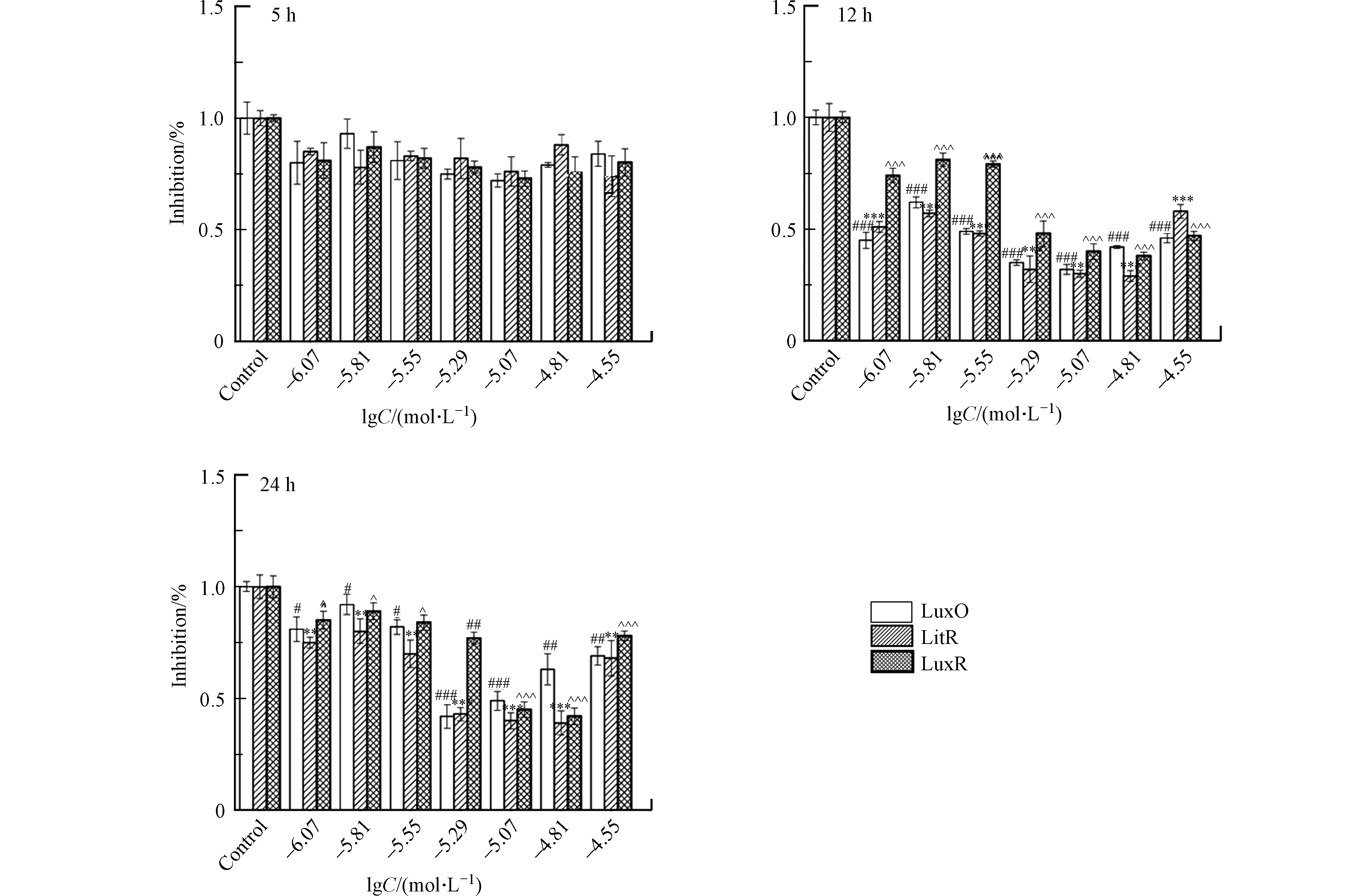
在暴露于一系列浓度(lgC=−6.07,−5.81,−5.55,−5.29,−5.07,−4.81,−4.55 mol·L−1)的受试化合物之后,通过实时荧光定量PCR(Quantitative real-time PCR, qPCR)测定A. fischeri中LuxO、LitR及LuxR蛋白mRNA的相对表达量。首先,使用总RNA提取试剂盒(Total RNA Kit,Omega 生物科技有限公司,美国)提取A. fischeri中的总RNA。然后,在Applied Biosystem Veriti热循环仪(赛默飞世尔科技有限公司,美国)上,使用PrimeScriptTM 1st stand cDNA合成试剂盒(Takara生物技术有限公司,日本)对1 μg 的RNA进行逆转录,生成cDNA。最后,在7500 实时荧光定量PCR仪(赛默飞世尔科技有限公司,美国)上利用SYBR@ Premix Ex TapTM II 试剂盒(Takara生物技术有限公司,日本)使cDNA进行多聚核苷酸链式反应,PCR参数为:(1)95 ℃,30 s;1个循环;(2)95 ℃,5 s;60 ℃,34 s;40个循环;(3)95 ℃,15 s;60 ℃,1 min;15个循环;(4)95 ℃,15 s;60 ℃,15 min。所测蛋白mRNA的引物序列是基于ncbi数据库序列,如表2所示。实验设置3组平行,结果以平均值±标准偏差的形式展示。在进行柯尔莫诺夫-斯米尔诺夫检验之后,使用GraphPad Prism 5软件(GraphPad软件公司,拉荷亚,加利福尼亚州,美国)对实验结果进行单因素方差分析及Tukey多重比较检验来判别实验组和对照组之间的差异。差异的显著性分为:P<0.05、P<0.01、P<0.001,分别用#,##和###表示蛋白LuxO mRNA相对表达量的下降;分别用*,**和***表示蛋白LitR mRNA相对表达量的下降;分别用^,^^和^^^表示蛋白LuxR mRNA相对表达量的下降。
-
A. fischeri的生物荧光来源于荧光素酶催化的发光反应[17-18](式2),
根据Sigma-Aldrich化学品有限公司(圣路易斯,密苏里州,美国)提供的荧光素酶活性检测方法对A. fischeri的发光反应进行了模拟,探究了受试化合物对体外模拟发光反应的影响。实验在96孔板中进行,每孔按顺序依次加入100 μL的Sorensen缓冲溶液(pH值为6.8,由KH2PO4和Na2HPO4组成)、10 μL的巯基乙醇、100 μL的化合物(作为实验组)或超纯水(作为对照组)、10 μL的荧光素酶、10 μL的还原型辅酶Ⅰ(Nicotinamide adenine dinucleotide,简称NADH)、10 μL的黄素单核苷酸(Flavin mononucleotide,简称FMN)和10 μL的癸醛。在25 ℃下反应15 min之后,在酶标仪(Mithras LB940,德国伯托科技有限公司,德国)上对每孔连续测定10 s以获得每孔反应的RLU值。毒性结果采用抑制率(式3)来表示:
其中,RLU0为对照值,RLU为实验值,对照组和实验组均为3组平行。
1.1. 试剂与生物
1.2. 工作菌液配制
1.3. 毒性实验
1.4. 蛋白质mRNA相对表达量测定实验
1.5. 发光反应体外模拟实验
-
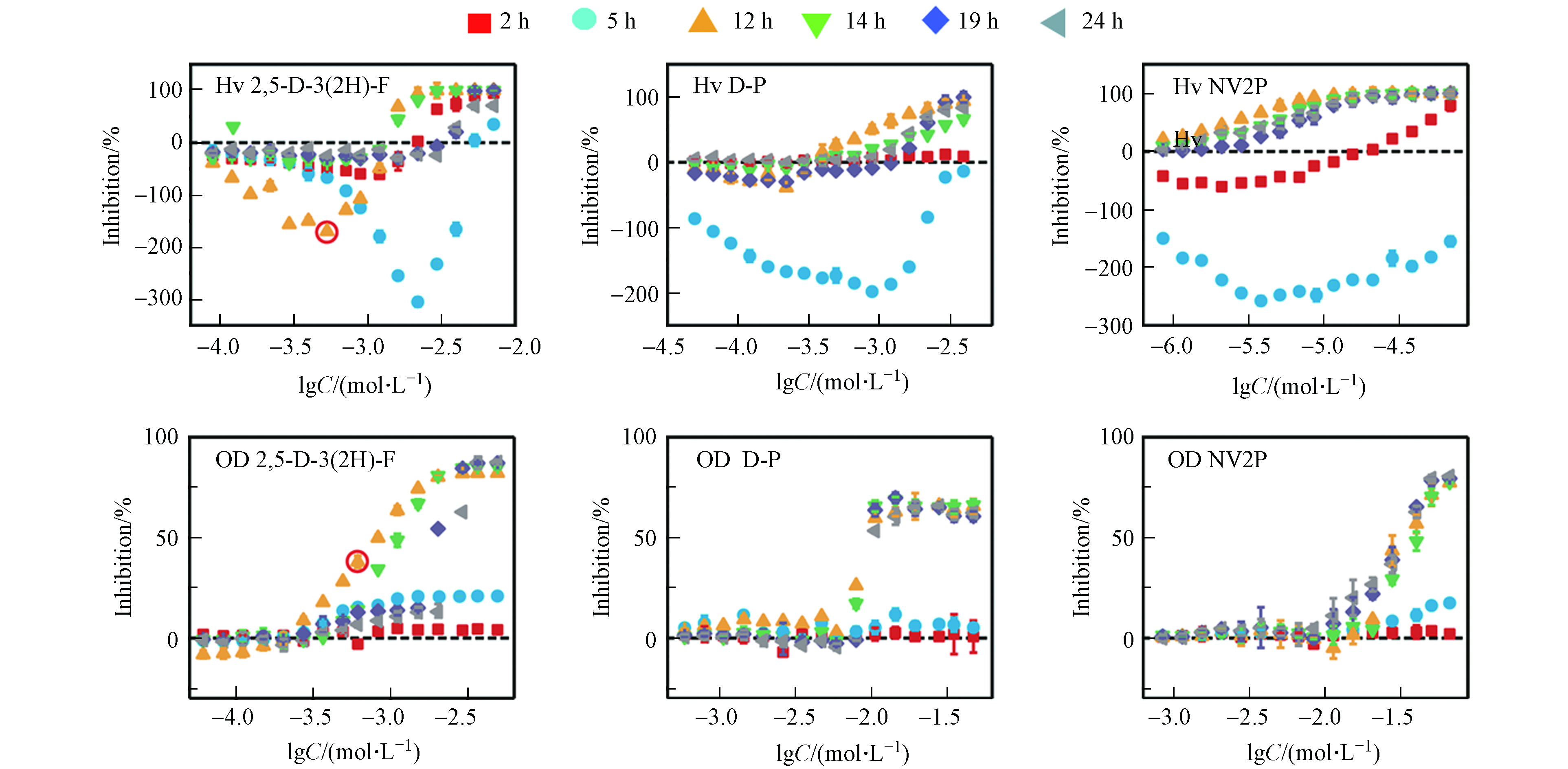
本文以2 h和5 h作为延滞期的代表、以12 h和14 h作为对数期的代表、以19 h和24 h作为平台期的代表,测定了2,5-D-3(2H)-F、D-P和NV2P对A. fischeri生物荧光在不同时间点下的毒性作用。如图2所示,这3种AHL-QSIs均引起了A. fischeri生物荧光随时间变化的Hormesis效应。在延滞期(2 h和5 h),3种AHL-QSIs对A. fischeri生物荧光产生了明显的促进效应,且最大促进率随着时间的增加而逐渐变大;在对数期(12 h和14 h),2,5-D-3(2H)-F和D-P对A. fischeri生物荧光表现为低浓度刺激、高浓度抑制的“J”型的Hormesis剂量-效应关系,而NV2P则对A. fischeri生物荧光只有抑制作用;在平台期(19 h和24 h),2,5-D-3(2H)-F依旧对A. fischeri生物荧光具有Hormesis效应,而D-P和NV2P对A. fischeri生物荧光只有抑制作用,并表现为一种“S”型的剂量-效应关系。相比于生物荧光,AHL-QSIs对A. fischeri的生长在3个时期内均没有表现出Hormesis效应,但具有一致的变化趋势:在延滞期,化合物对A. fischeri的生长没有明显的抑制作用;在对数生长期和平台期,化合物对A. fischeri的生长逐渐表现为抑制现象,并且最大抑制率随暴露时间的延长逐渐稳定,呈现出“S”型的剂量-效应曲线。有研究发现,AHL-QSIs可以作为非极性麻醉剂与细胞膜非共价键结合从而改变膜的功能,进而抑制细菌生长[19]。同时,对A. fischeri生长的抑制能够直接减弱A. fischeri的生物荧光[20]。例如,从第5 h到第12 h,2,5-D-3(2H)-F对A. fischeri生长的抑制率从21 %增加到82 %,而对A. fischeri的生物荧光的最大促进率从620 %减少到150 %。此外,在第12 h,2,5-D-3(2H)-F于lgC=−3 mol·L−1的浓度下对A. fischeri的生长有明显的抑制作用,但在同等浓度下2,5-D-3(2H)-F依然能促进A. fischeri的生物荧光(已在图2标出)。这说明受试化合物能够同时对A. fischeri的生物荧光产生刺激作用和抑制作用,且受试化合物对A. fischeri生物荧光表观的影响是刺激作用和抑制作用的叠加。有此前研究可知,AHL-QSIs对A. fischeri生物荧光的抑制作用源于对细菌生长的抑制以及与C6竞争结合LuxR蛋白减少荧光素酶的含量;那么,AHL-QSIs对A. fischeri生物荧光的刺激作用的来源是什么?刺激作用和抑制作用是如何相互作用导致随时间变化的Hormesis效应?
如公式(2)所示,A. fischeri的生物荧光来源于荧光素酶催化的发光反应。由此可见,AHL-QSIs对A. fischeri生物荧光的刺激作用可能源于对荧光素酶含量的增加或是对荧光素酶活性的刺激。由A. fischeri的生物荧光来源于荧光素酶催化的发光反应,而荧光素酶的含量则是受A. fischeri的QS系统调控(见图1)。除此之外,相关研究发现,荧光素酶活性可能是由于外界化合物作为荧光素酶的活化剂刺激了荧光素酶的活性[21]。因此,我们接下来想通过对相关蛋白表达量和模拟发光反应实验来进行验证.
-
A. fischeri的生物荧光是细菌生长到一定阶段后在其QS系统调控下产生的一种现象[20]。基于A. fischeri的QS系统(图1)可以看出,LuxO、LitR和LuxR蛋白均是调控A. fischeri生物荧光的关键蛋白。结合细菌的生长周期,本文测试了第5、12、24 h(分别代表延滞期,对数生长期和平台期)NV2P(作为AHL-QSIs代表)对LuxO,LitR和LuxR等3b个蛋白mRNA相对表达量的影响(如图3)。结果表明,在第5小时,NV2P对3个蛋白mRNA相对表达量没有明显的影响;而在第12和24 小时,NV2P会明显抑制3个蛋白mRNA相对表达量。结合A. fischeri的QS系统,不难看出LuxO和LitR是LuxR的上游蛋白。因此,AHL-QSIs能够在细菌进入对数期之后抑制细菌的生长从而抑制LuxO蛋白的合成,进而阻断LitR蛋白和LuxR蛋白的表达,最终导致荧光素酶的含量减少,表现为对A. fischeri生物荧光的抑制作用。该实验结果说明AHL-QSIs不能通过提高A. fischeri 中荧光素酶的含量来刺激其生物荧光。
-
由于急性毒性实验暴露时间较短(15 min)、细菌密度低,信号分子还未开始积累,化合物对细菌生长及群体感应的作用都很小,细胞个数基本保持不变,故急性毒性实验能够反映化合物对个体细菌的影响。本文测定了2,5-D-3(2H)-F、D-P和NV2P对A. fischeri生物荧光的急性毒性实验,结果如图4(a)所示,3种受试化合物对A. fischeri的急性发光均有明显的刺激效应。其中,2,5-D-3(2H)-F于lgC=−3.87 mol·L−1的浓度下最大刺激为49%;D-P于lgC=−3.84 mol·L−1的浓度下最大刺激为23% NV2P于lgC=−2.79 mol·L−1的浓度下最大刺激为28%。因此,推测AHL-QSIs可能通过刺激A. fischeri的发光反应最终刺激细菌的生物荧光。为了进一步验证这一推论,本文测定了NV2P(作为AHL-QSIs的代表)对体外模拟荧光素酶促进发光反应的影响,结果如图4(b)所示,NV2P在一定的浓度范围对体外模拟发光反应有明显的促进作用。因此,推测AHL-QSIs能够作为荧光素酶的活化剂暴露荧光素酶的活性位点从而刺激个体发光反应[21],最终促进A. fischeri的生物荧光。
-
综合上述结果及分析,基于受体/信号通路理论[22],AHL-QSIs对A. fischeri生物荧光随时间变化的Hormesis效应的机制推测如下(如图5).
(1)延滞期(2 h和5 h):细菌生长缓慢且生物荧光本底值较低,信号分子C6的含量很低。此外,缓慢的细菌生长使得AHL-QSIs对细菌生长的抑制作用无法显现出来。在延滞前期(第2小时),AHL-QSIs在低浓度时可以通过提高荧光素酶的活性进而刺激A. fischeri生物荧光;但当AHL-QSIs处于高浓度时,更多的AHL-QSIs会与QS系统中的LuxR蛋白结合,使得luxICDABEG基因无法被激活,荧光素酶受体被限制,最终表现为对生物荧光的抑制作用。在延滞后期(第5 小时),细菌要为接下来的对数期做准备,因此细菌体内分泌释放出少量的C6,LuxR蛋白的含量也会增加,少量的C6与LuxR蛋白结合形成复合物使得荧光素酶的含量有所增加,因而AHL-QSIs通过提高荧光素酶活性所表现出的刺激作用进一步增强;此外,由于此时高浓度的AHL-QSIs对细菌的生物荧光产生了抑制作用,因此在第5 h,AHL-QSIs对A. fischeri的生物荧光的促进作用并没有随AHL-QSIs浓度的增加而增加,而是先增大后减小。
(2)对数期(12 h和14 h):细菌快速生长,高浓度AHL-QSIs对细菌生长的抑制作用表现出来;同时,信号分子C6和LuxR迅速积累,细菌生物荧光的本底值增大,此时QS较强。在本时期,AHL-QSIs依然可以在低浓度时通过促进发光反应刺激生物荧光;而对生物荧光的抑制作用则来源于高浓度的AHL-QSIs一方面作为非极性麻醉剂与细胞膜非共价键结合从而改变膜的功能,对细菌的生长产生抑制作用(图2),另一方面还会与C6竞争结合LuxR减少荧光素酶含量。此外,随着暴露时间的延长,生物荧光本底值逐渐增强,这使得AHL-QSIs对A. fischeri生物荧光的刺激作用会随时间逐渐降低。而NV2P对生物荧光促进效应的缺失可能是因为其对荧光素酶活性的刺激作用相比于另外两种AHL-QSIs较小,而高本底值的生物荧光和对QS的抑制作用最终掩盖了其对生物荧光的促进作用,故只表现为抑制效应。
(3)平台期(19 h和24 h):细菌密度基本保持不变,虽然C6的产生量开始下降,但其含量依然较高。在此阶段,A. fischeri生物荧光的本底值依然处于高本底值状态,这使得AHL-QSIs对A. fischeri生物荧光的刺激效应会被掩盖,最终表现为对A. fischeri生物荧光的抑制作用。
2.1. AHL-QSIs对A. fischeri生物荧光和生长的毒性效应
2.2. AHL-QSIs对A. fischeri中蛋白mRNA的相对表达量的影响
2.3. AHL-QSIs对A. fischeri个体发光反应的影响
2.4. AHL-QSIs对A. fischeri生物荧光随时间变化Hormesis效应的机制探究
-
本研究表明,AHL-QSIs对A. fischeri的生物荧光均产生了随时间变化的低浓度促进、高浓度抑制的Hormesis现象。当AHL-QSIs在低浓度时可以通过增强荧光素酶的活性进而促进个体发光反应,最终刺激A. fischeri的生物荧光;随着AHL-QSIs浓度的增加,更多的AHL-QSIs不仅会改变细胞膜的通透性而抑制细菌的生长,还可以与C6竞争结合LuxR减少荧光素酶含量,最终抑制A. fischeri的生物荧光。另外,在细菌不同的生长阶段,AHL-QSIs对A. fischeri生物荧光的刺激作用和抑制作用会随细菌生长状态的不同而发生改变,随时间变化的刺激作用和抑制作用的相互作用最终导致了其Hormesis效应随时间的变化而变化。
本文将有助于为AHL-QSIs的环境生态风险评价提供研究基础,并为其对A. fischeri生物荧光随时间变化Hormesis效应的机制提供参考。




 下载:
下载: