-
左氧氟沙星(levofloxacin,LEV)是一类人工合成的喹诺酮类广谱抗生素,在细菌感染方面具有良好的治疗效果,被广泛用于医疗、畜禽和水产养殖业. 由于使用量大、生物机体代谢率低,LEV大多以原药形式被排出,进入土壤、水体等环境介质[1]. 环境中残留的LEV会诱发细菌产生耐药性,也会与有机物或重金属反应,形成复杂化合物或螯合物,对生态环境和人类健康构成严重威胁[2]. 目前,传统生物处理技术对LEV去除率不到10%[3]。现有技术如膜分离和吸附法虽已用于抗生素废水处理,但存在耗时长、花费高且污染物去除效率低等缺点[4-5]. 因此,急需开发高效、节能、环保的LEV废水处理新技术.
国内外应用较多的有机废水处理技术中,高级氧化技术因其快速、高效等特点受到研究者广泛关注. Fenton反应是一种有效去除污染物的高级氧化技术,但均相Fenton过程存在反应条件苛刻(pH=2–3)、产生大量含铁泥浆及催化剂无法回收等问题[6]. 异相Fenton体系利用铁的氧化物(如针铁矿、磁铁矿等)或金属负载型催化剂,把自由的Fe离子固定,从而拓宽了体系的pH应用范围的同时可将催化剂回收利用[7]. 但Fe3+还原为Fe2+速率常数仅为0.001—0.02 mol−1·s−1,限制了整个体系的反应速率[8]. 而Cu具有与Fe类似的氧化还原特性,H2O2将Cu2+还原为Cu+的速率常数是Fe的23000倍[9]. Cu2O是一种常见的铜氧化物,具有低毒性、低成本和利于回收等优点. Kuo等[10]研究了Cu2O/H2O2体系对双酚A的降解,当Cu2O投加量0.112 g·L−1、H2O2浓度30 mmol·L−1、初始双酚A浓度10 mg·L−1,反应60 min,双酚A的去除率为78%. 此外,Cu2O也是半导体催化剂,在紫外光照下 Cu2O可高效降解甲基蓝、苯酚等新型污染物[11-13]. UV、Cu2O及H2O2组合体系可多种途径产生·OH,具有高效降解废水中有机污染物、降低治理成本的潜在优势. 但UV/Cu2O/H2O2组合体系耦合强化降解LEV的研究还鲜见报道.
因此,本研究考察UV/Cu2O/H2O2组合体系降解LEV的效果及影响因素,采用自由基淬灭剂确定该体系中的活性物种,检测降解中间产物,采用大肠杆菌为指示菌,评估降解过程中LEV抗菌性变化,此外研究无机阴离子对该降解过程的影响,旨在为LEV废水处理探索新方法.
全文HTML
-
氧化亚铜(Cu2O,分析纯),武汉曙欧科技有限公司生产;过氧化氢(H2O2,质量分数30%),营口润晟化工有限公司生产;左氧氟沙星(LEV,分析纯),上海曙灿有限公司生产;LB肉汤培养基购于北京奥博星生物技术有限责任公司;实验所用氢氧化钠、次氯酸、二乙基二硫代氨基甲酸钠、甲酚红、氨水、EDTA、柠檬酸铵、四氯化碳、氯化铵、氯化钠、硫酸钠、二水合磷酸二氢钠、硝酸钠、甲醇、一水合草酸铵(ammonium oxalate, AO)、硝酸银均为分析纯,购于国药集团化学试剂有限公司.
-
为探究UV/Cu2O/H2O2降解LEV的特性和规律,设计批次试验. 在多通道光催化反应仪中,反应溶液通过冷却水循环控制在(25±2 )℃,UV功率为3 W,反应溶液的初始pH值用0.1 mol·L−1 HClO和NaOH溶液调节,初始LEV浓度为20 mg·L−1,分别加入一定量的Cu2O 和H2O2. 每间隔一定时间取出5 mL反应液,快速加入过量甲醇,过0.45 μm滤膜后测定,设置3组平行.
自由基淬灭剂和无机阴离子实验,选取5 mmol·L−1的CH3OH、AO、AgNO3,200 mg·L−1的Cl−、
${\rm{SO}}_4^{2-} $ 、${\rm{H}}_2{\rm{PO}}_4^{-} $ 、${\rm{NO}}_3^{-} $ ,初始LEV浓度为20 mg·L−1,Cu2O投加量为0.1 g·L−1,H2O2浓度为2 mmol·L−1,pH=3.2,反应温度(25±2 )℃. -
LEV及其降解产物的抗菌性实验,按照不同时间取出一定量样品,加入过量Na2S2O3,过滤后,注入到40 mL灭菌的LB培养基中,加入0.05 mL的OD600为0.1单位的大肠杆菌(E.coli)悬浊液,以去离子水替代样品进行对照实验,同时配置单独Cu2O、Na2S2O3、H2O2溶液,探究其对E.coli生长的影响. 将培养基于36.5 ℃、180 r·min−1摇床中连续振荡8 h,设置3组平行.
-
LEV的测定采用紫外分光光度法:取一定量的LEV标准溶液和反应溶液过滤后,稀释至所需浓度,调节溶液pH=3.2,在波长293 nm测量其吸光值[14],根据下式计算LEV去除率:
式中,ηLEV为LEV去除率(%);ρ0为LEV初始质量浓度(mg·L−1);ρt为反应t(h)后LEV质量浓度(mg·L−1).
采用二乙基二硫代氨基甲酸钠萃取光度法(HJ 485—2009)测定铜含量. 采用液相色谱–质谱(Agilent technologies 6420 Triple Quad LC/MS)确定LEV降解的中间产物,LC分析条件为:色谱柱Agilent Poroshell 120 EC-C18(50 mm×3 mm,2.7 μm);流动相为水−0.1%(体积分数)甲酸水溶液(体积比10∶90);流速0.3 mL·min−1;检测波长290 nm;进样量3 μL. MS条件:电喷雾ESI源,正离子电离模式;扫描方式:全扫描(scan). 扫描范围(m/z):50—500.
采用紫外分光光度法测定E.coli光密度:取一定量的培养基溶液,在波长600 nm测量其吸光值[15],根据下式计算E.coli生长抑制率:
式中,OD600(样品)为E.coli在样品中的光密度;OD600(空白)为E.coli在去离子水中的光密度.
1.1. 主要试剂
1.2. 批次试验
1.3. 转化产物的抗菌性分析
1.4. 测定方法
-
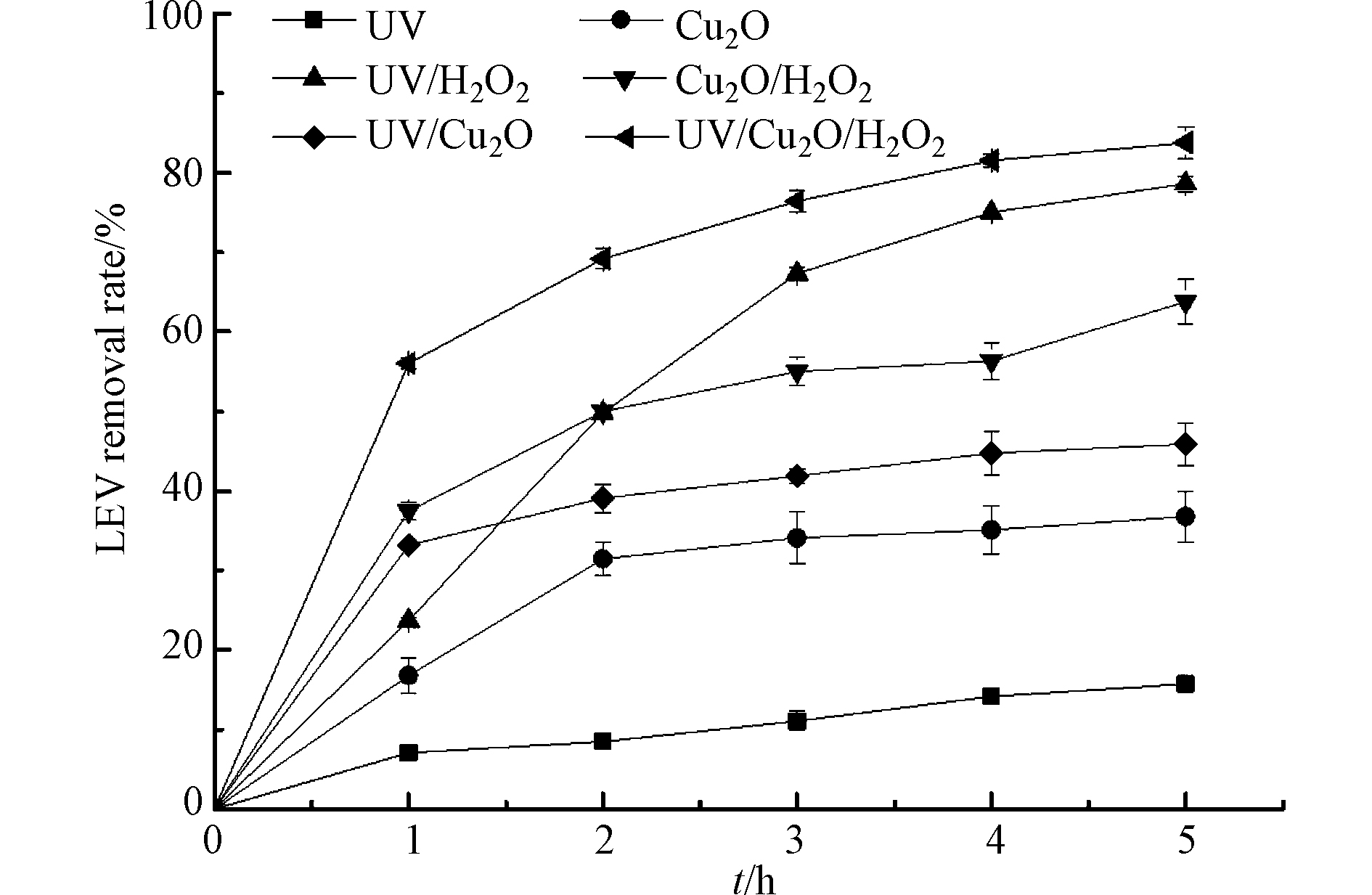
实验首先比较了单一UV、单一Cu2O、UV/H2O2、Cu2O/H2O2、UV/Cu2O 和UV/Cu2O/H2O2 等6种体系对LEV的去除效果,结果如图1所示.
UV照射2 h时,溶液中LEV的去除率仅有8.5%. 孟婷等[14]的研究发现,LEV初始浓度20 mg·L−1,pH=7.0,14 W UV照射2 h时,LEV去除率约为70.0%,高于本研究结果. 这可能是因为实验采用的UV功率不同,本实验采用3 W UV,功率较小,因此对LEV的直接光降解作用较小. 在无光照仅有Cu2O的处理中反应2 h时,LEV的去除率是31.5%,并且随着时间延长,不再发生明显变化,这是由于Cu2O对LEV的吸附而导致其浓度下降. 在UV/H2O2、Cu2O/H2O2和UV/Cu2O体系中,反应5 h时,LEV去除率分别为78.6%、63.8%和45.9%;而在UV/Cu2O/H2O2体系中,反应5 h时,LEV去除率可达83.8%,表明UV/Cu2O/H2O2组合体系比UV/H2O2体系、类Fenton体系和光催化体系更利于LEV的降解. 分析认为,UV/Cu2O/H2O2体系可通过以下途径产生·OH:一是H2O2促进Cu2O表面的≡Cu+与≡Cu2+直接转化,≡Cu+与H2O2反应生成·OH[10],反应式(1)—(3);另外半导体Cu2O在UV照射下,产生h+和e−,h+氧化OH−生成·OH,e−还原H2O2生成·OH[16],反应式(4)—(6);此外H2O2在UV下分解为·OH[17],反应式(7). ·OH与吸附在Cu2O表面的LEV反应,将其降解为简单物质,最终氧化为CO2和H2O,反应式(8)—(9).
式中,“≡”表示Cu2O表面.
-
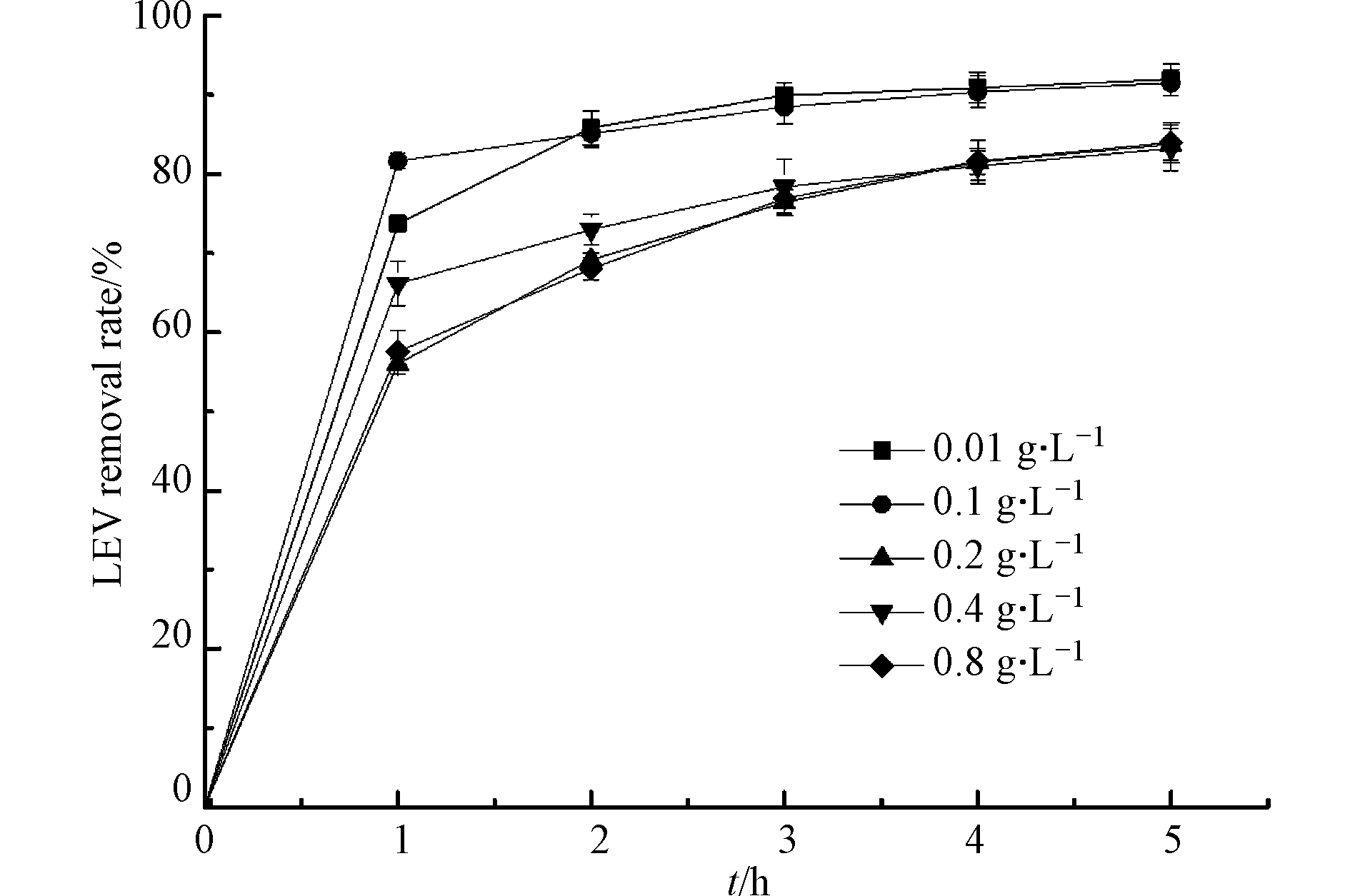
Cu2O投加量在0.01—0.8 g·L−1范围考察UV/Cu2O/H2O2体系对LEV降解的影响,结果如图2所示. 可知,Cu2O投加量依次为0.01、0.1、0.2、0.4、0.8 g·L−1时,反应1 h,LEV的去除率分别为73.7%、81.6%、56.0%、66.2%和57.5%. Cu2O投加量为0.1 g·L−1时,效果最好. 这是因为随着Cu2O质量浓度增加,体系内产生了更多的活性位点,进而产生更多的·OH,从而促进LEV的降解;但继续增加Cu2O含量,LEV降解效果变差,原因是Cu2O添加量过多,引起光散射,使得量子产率下降,影响反应液的透光率,减慢反应速率[18-19].
-
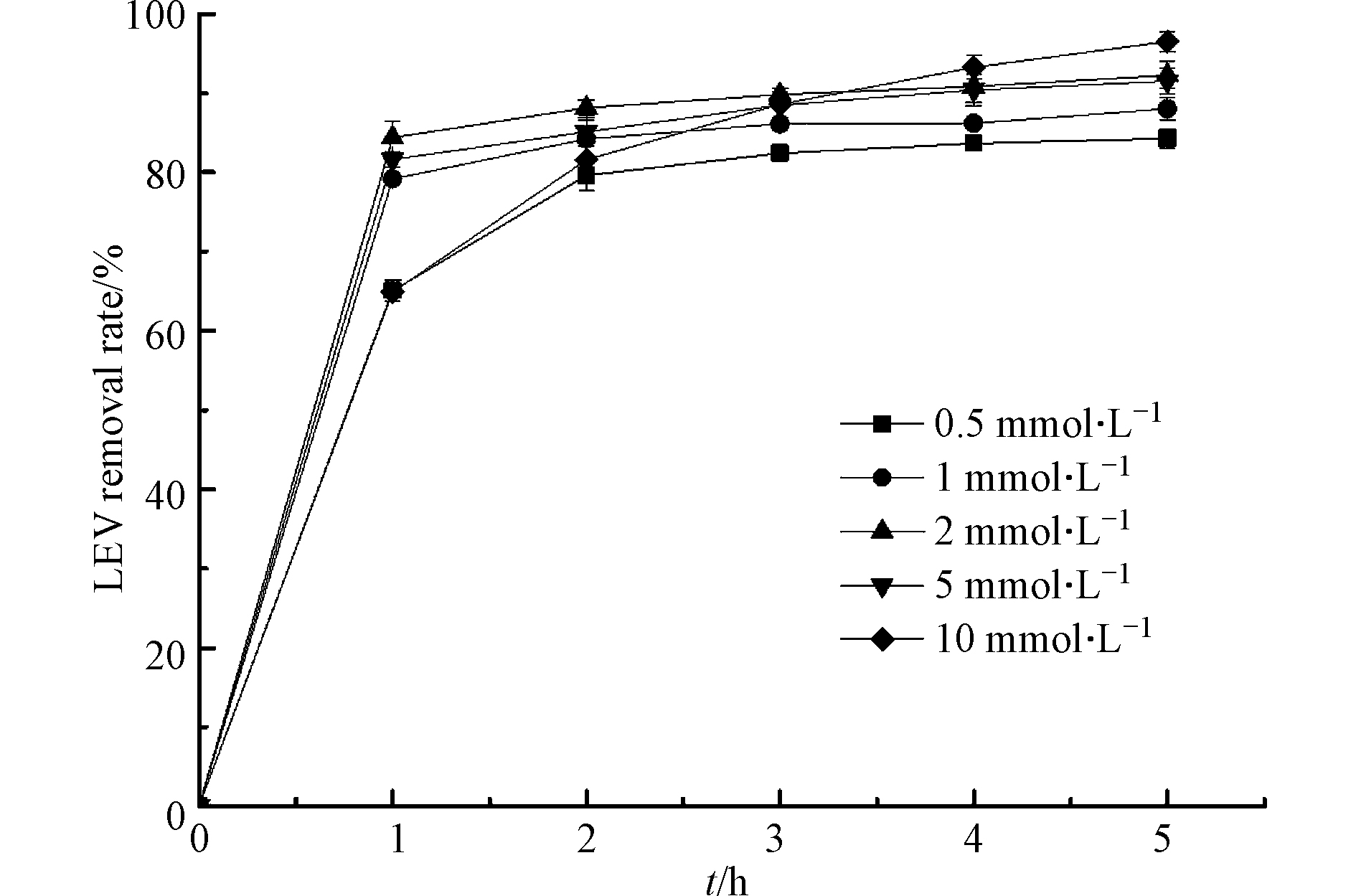
H2O2是产生·OH的重要来源,它的用量将直接影响·OH的生成,进而影响LEV的降解效率. 考察不同H2O2浓度对LEV降解的影响,结果见图3. 由图3可知,反应1 h时,随着H2O2浓度升高,LEV的去除率先升高后下降. 这是因为H2O2对LEV的降解有促进作用和抑制作用的双重性,当H2O2浓度较低时,体系中·OH生成速率随着H2O2浓度的增加而增加,单位时间内·OH与LEV的碰撞机率变大,从而增加了LEV的降解率;但过量的H2O2不仅会捕获·OH生成氧化性弱的·OOH(1.7 V),而且还会导致自身的无效分解[20],反应式如(10)—(11). 以上表明,最佳H2O2浓度为2 mmol·L−1.
-
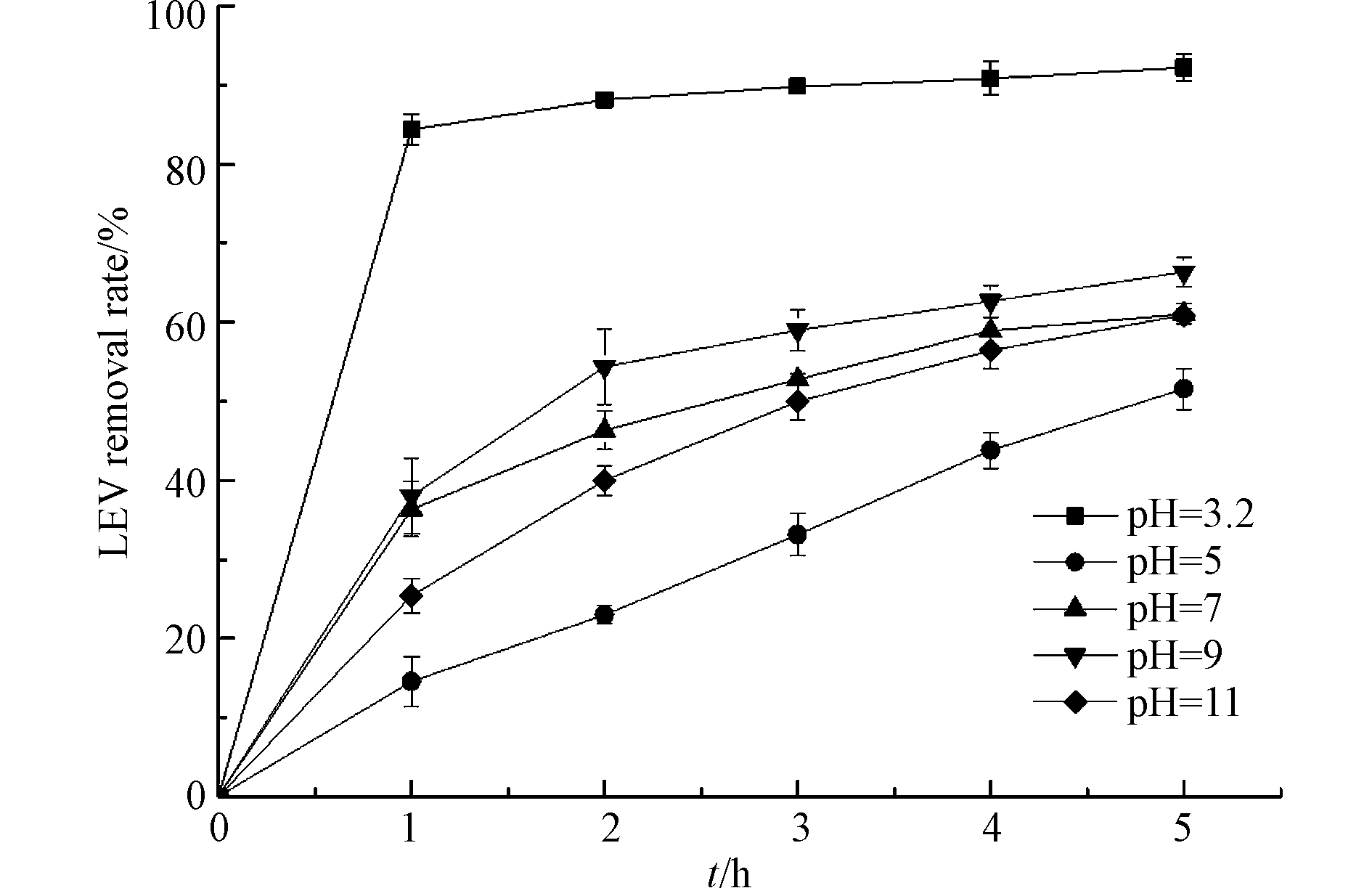
pH是类Fenton反应的重要影响因素,考察不同pH对LEV降解的影响,结果见图4. 由图4可知,溶液初始pH值为5、7、9和11,反应5 h时,LEV的去除率分别为51.6%、61.0%、66.4%和60.9%,这表明弱碱性条件比中性、碱性和弱酸性条件更利于UV/Cu2O/H2O2体系降解LEV. 这是因为偏碱性的环境中OH−与h+大量反应生成·OH,增加目标物的氧化效率;但过量的OH−与LEV在Cu2O表面产生竞争吸附,降低降解效率. 相比于初始pH值为9的体系,溶液初始pH值为3.2,反应5 h时,LEV的去除率升高了25.9%. 这可能是因为有少量Cu2O溶解形成离子态的铜,与H2O2组成均相类Fenton体系,加速了·OH生成,继而加快LEV降解[21-22],反应式如(12)—(13).
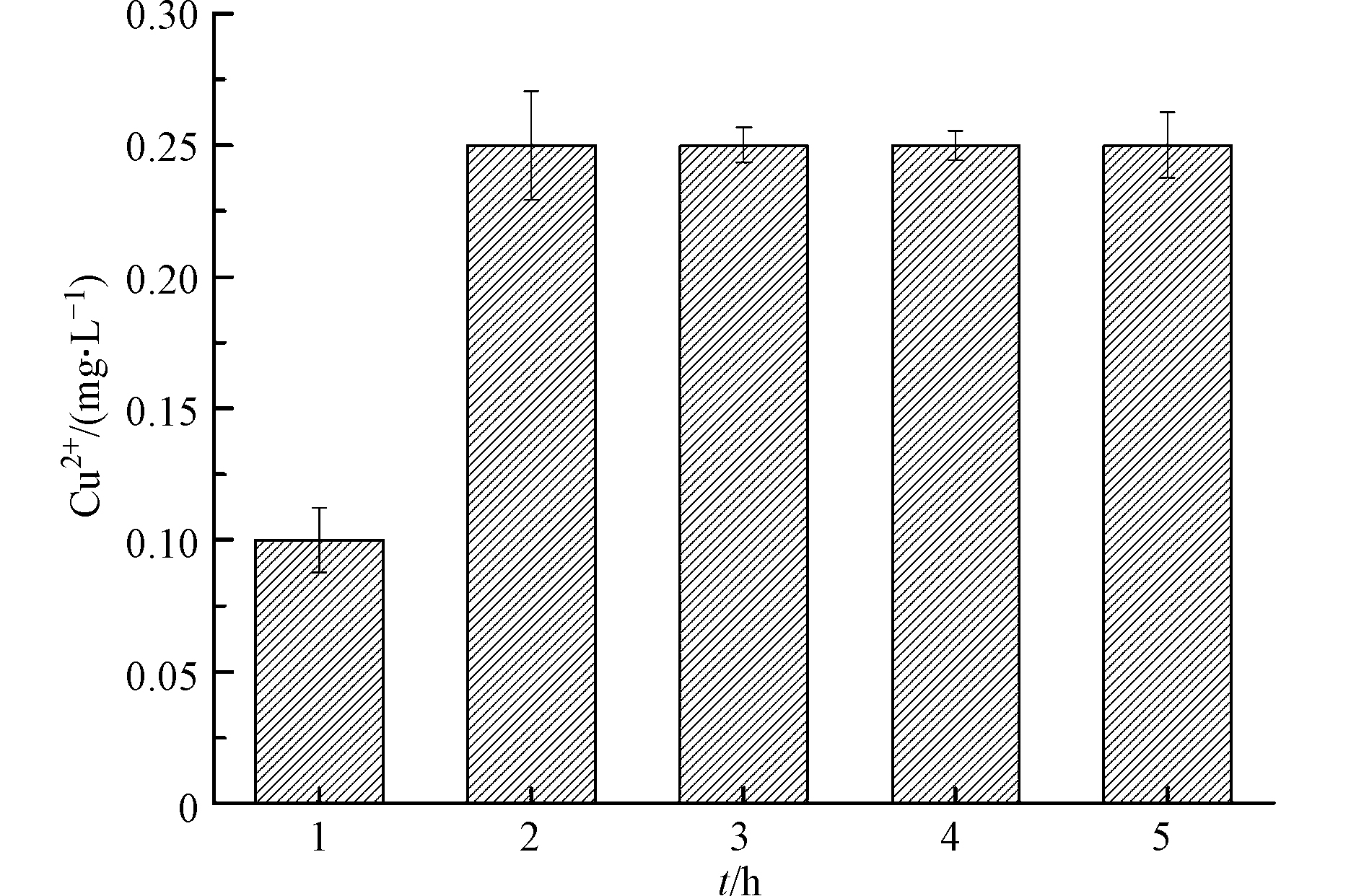
异相Fenton体系中,铜价态的反复变化会促使铜从催化剂表面以离子态溶出[21]。环境中高浓度铜离子可通过食物链进入人体,引发铜超标,使人体出现肝损伤、肝硬化等毒性效应[23]。因此,为了评估UV/Cu2O/H2O2工艺在应用时的安全性,考察溶液初始pH值为3.2 时Cu2+离子的溶出量,结果如图5所示. 由图5可知,反应5 h时,Cu2+溶出量仅为0.25 mg·L−1,低于生活饮用水卫生标准GB5749—2006(1 mg·L−1),这说明UV/Cu2O/H2O2在降解LEV过程中,不会产生二次污染. 综合考虑污染物去除率和铜离子溶出量,UV/Cu2O/H2O2体系降解LEV最适pH值为3.2.
-
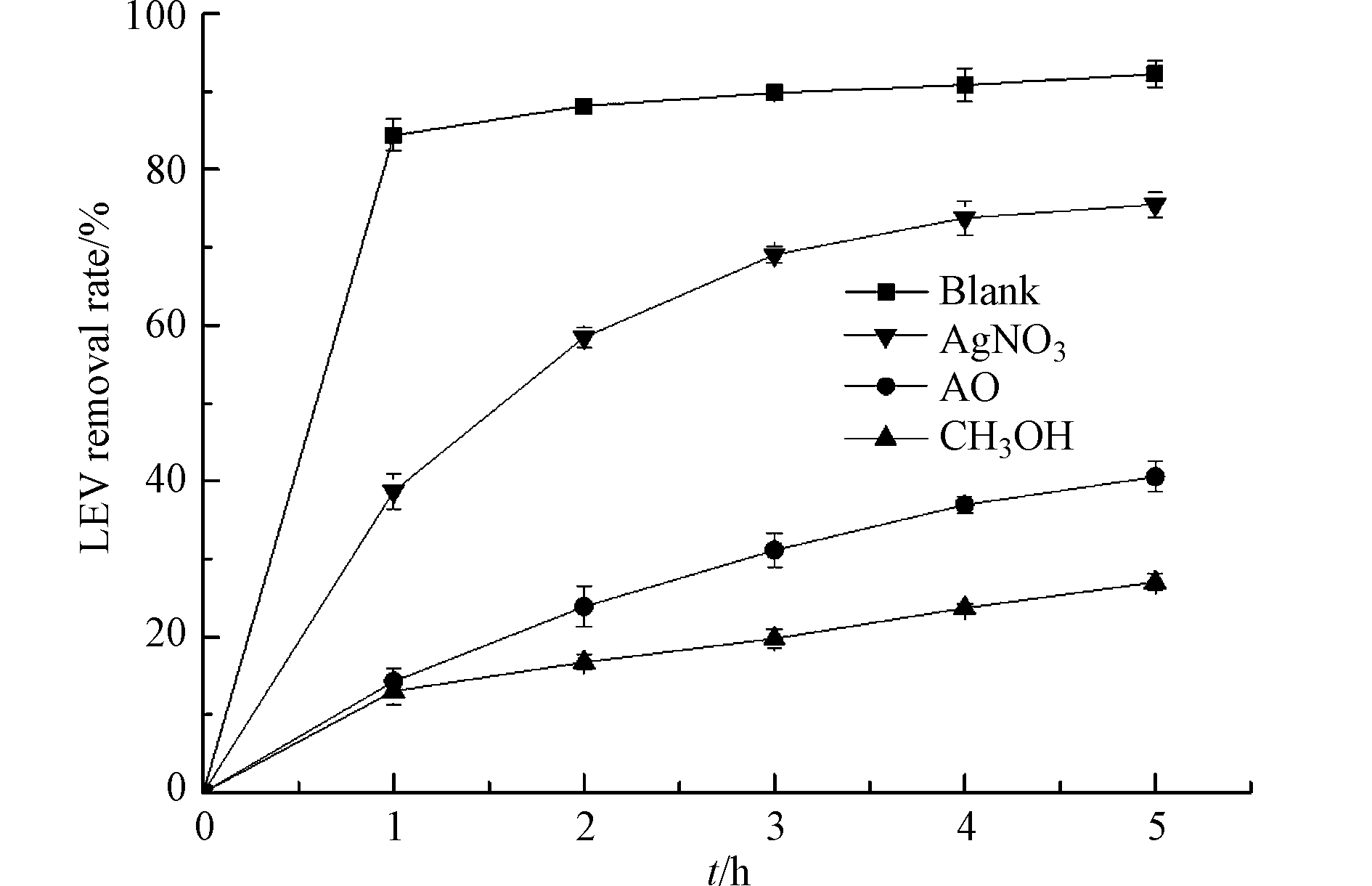
甲醇可捕获·OH,AgNO3可捕获e−,AO可捕获h+ [24]. 为了进一步确认反应机理,向UV/Cu2O/H2O2体系中分别加入5 mmol·L−1的甲醇、AgNO3和AO,结果如图6所示. 结果表明,添加甲醇和AO,LEV的降解明显受到抑制,反应5 h时,LEV的去除率为27%和40.5%. 当体系中添加AgNO3,LEV去除率受到的抑制程度较小,反应5 h时,LEV的去除率为75.5%. 以上结果表明,UV/Cu2O/H2O2体系降解LEV的主要活性物种是·OH和h+.
-
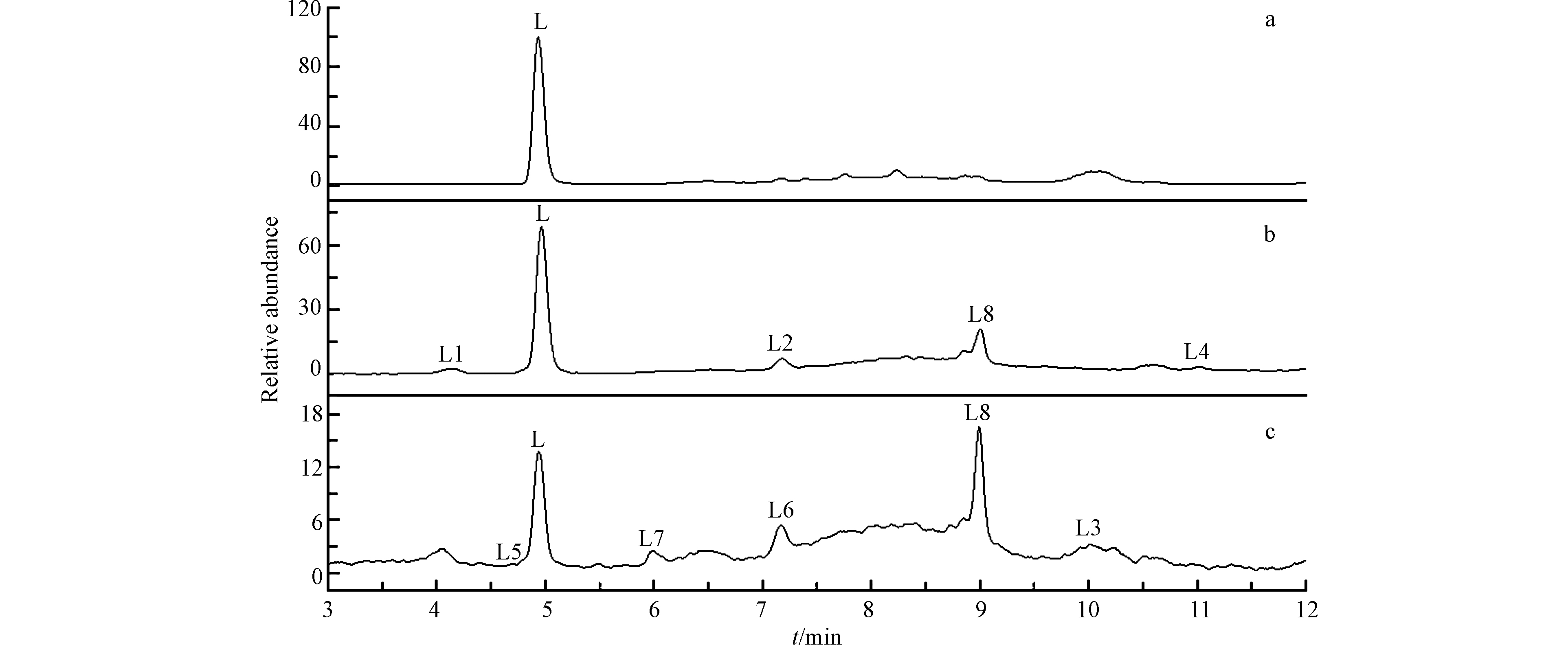
利用HPLC-MS技术,测定UV/Cu2O/H2O2降解LEV过程中出现的中间产物,反应0 h、1 h和5 h时的离子流色谱图如图7所示.
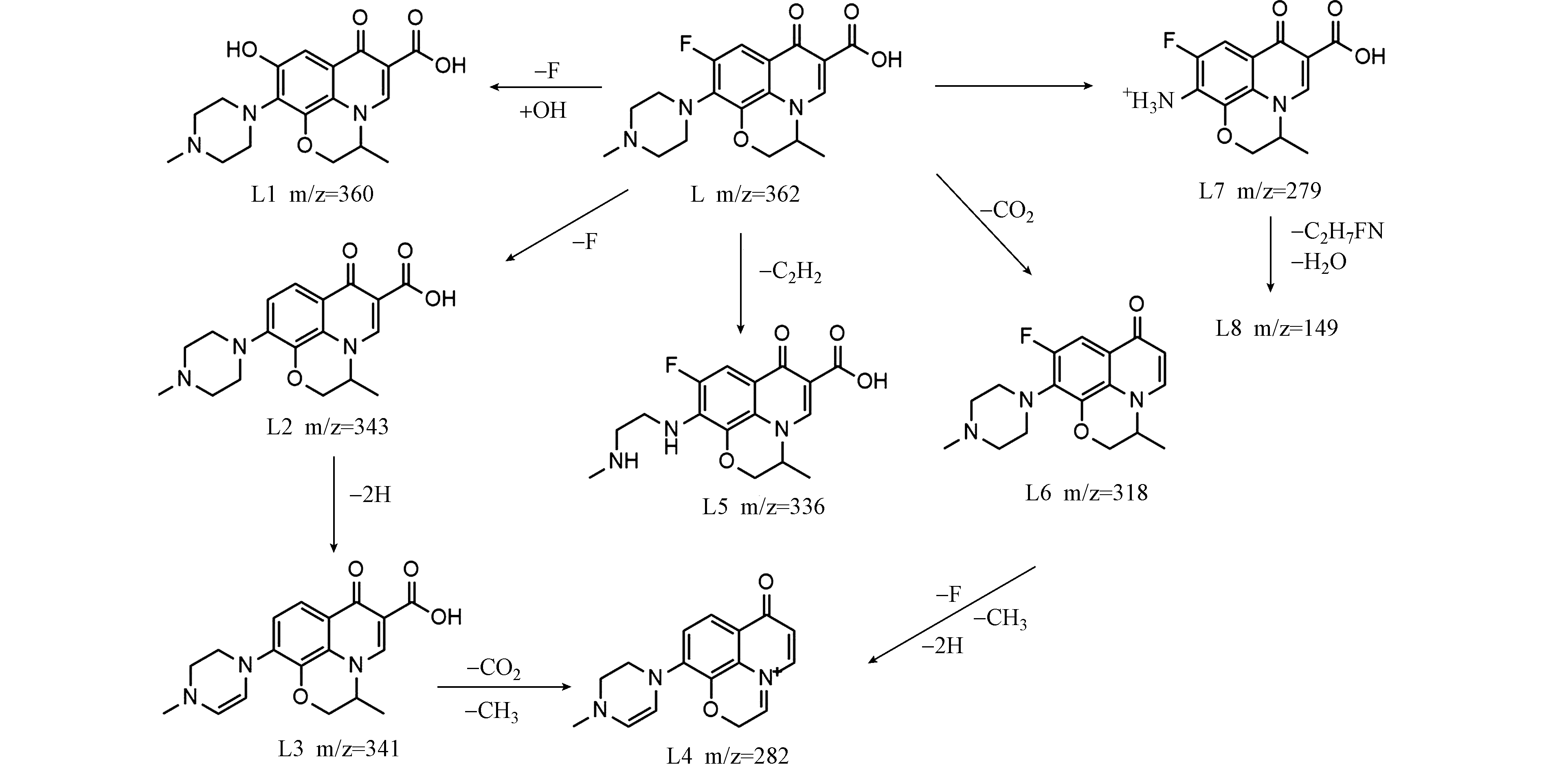
由图7(a)可见,在t=0 h时,只检测到LEV的存在,质荷比(m/z)362,保留时间为tR=4.95 min(L);当t=1 h和t=5 h时,共检测到8种中间产物,质荷比(m/z)分别为:360(L1)、343(L2)、341(L3)、282(L4)、336(L5)、318(L6)、279(L7)和149(L8). 根据分子量、理论分子量和前人相关报道,推测UV/Cu2O/H2O2降解LEV中间产物的结构和反应路径如图8所示.
LEV的降解路径之一是羟基取代氟生成L1[25]. LEV的另一条路径为先还原脱氟生成L2,再脱氢生成L3,进而去甲基、去二氧化碳生成L4[26]. LEV的第三条降解路径为胺侧链被氧化生成L5[27]. LEV的第四条降解途径为脱二氧化碳生成L6,进而还原脱氟、去甲基和脱氢生成L4[28]. LEV的第五条降解路径为去哌嗪环生成L7,进一步被氧化后脱水生成L8[29]. 以上分析表明UV/Cu2O/H2O2通过逐步去除LEV官能团,将其降解为小分子物质.
-
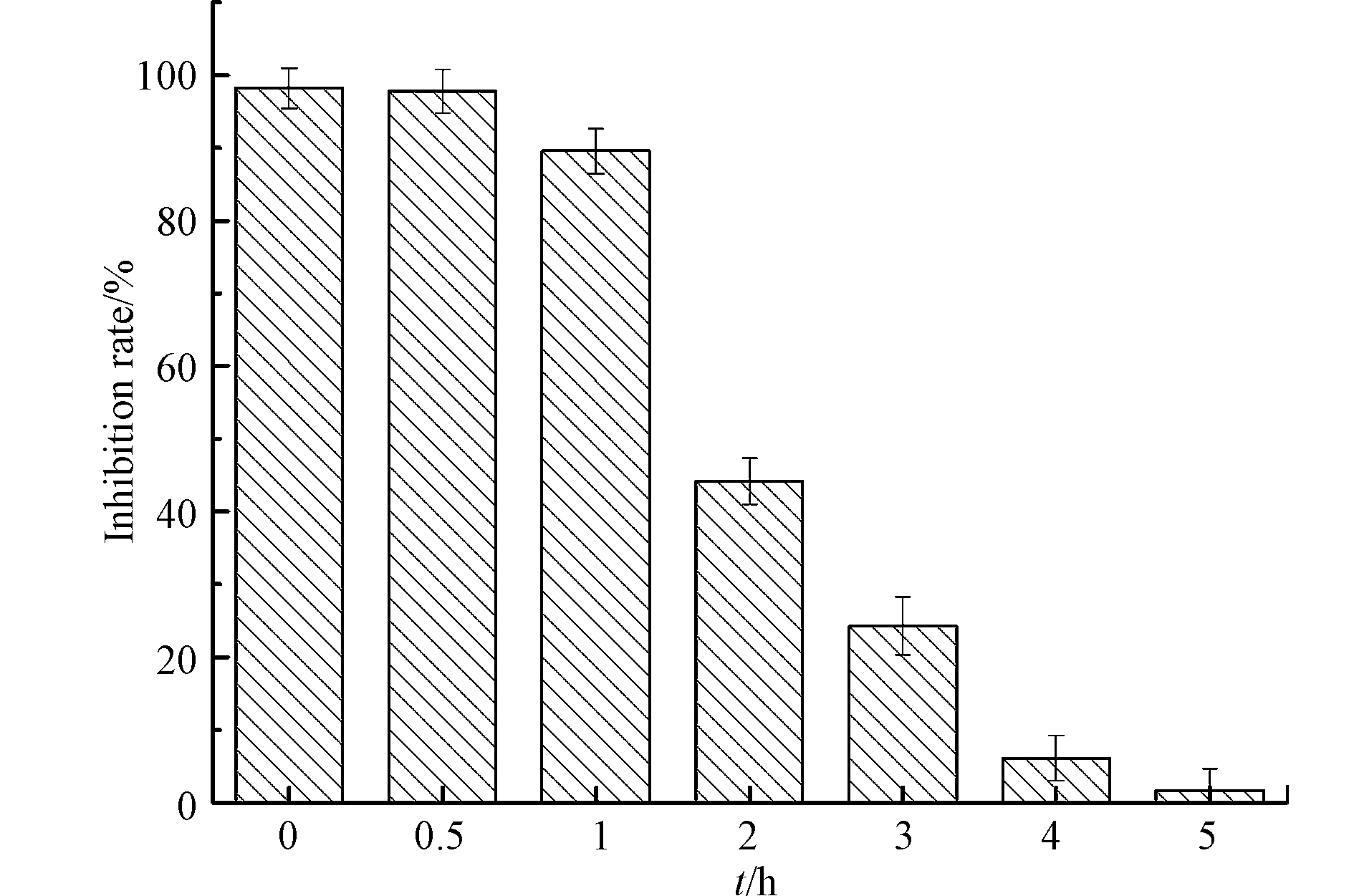
利用E.coli生长抑制率评估LEV降解产物的抗菌性变化,结果见图9. Cu2O、Na2S2O3、H2O2对大肠杆菌无明显抑菌作用. LEV对E.coli具有抗菌性,随着反应进行,LEV的降解样品对E.coli的生长抑制率逐渐降低,至5 h时仅为1.7%,表明UV/Cu2O/H2O2体系不仅能够降解LEV,而且能有效去除其抗菌性. 这与龚月湘采用电化学技术降解左氧氟沙星的研究结果类似[30].
-
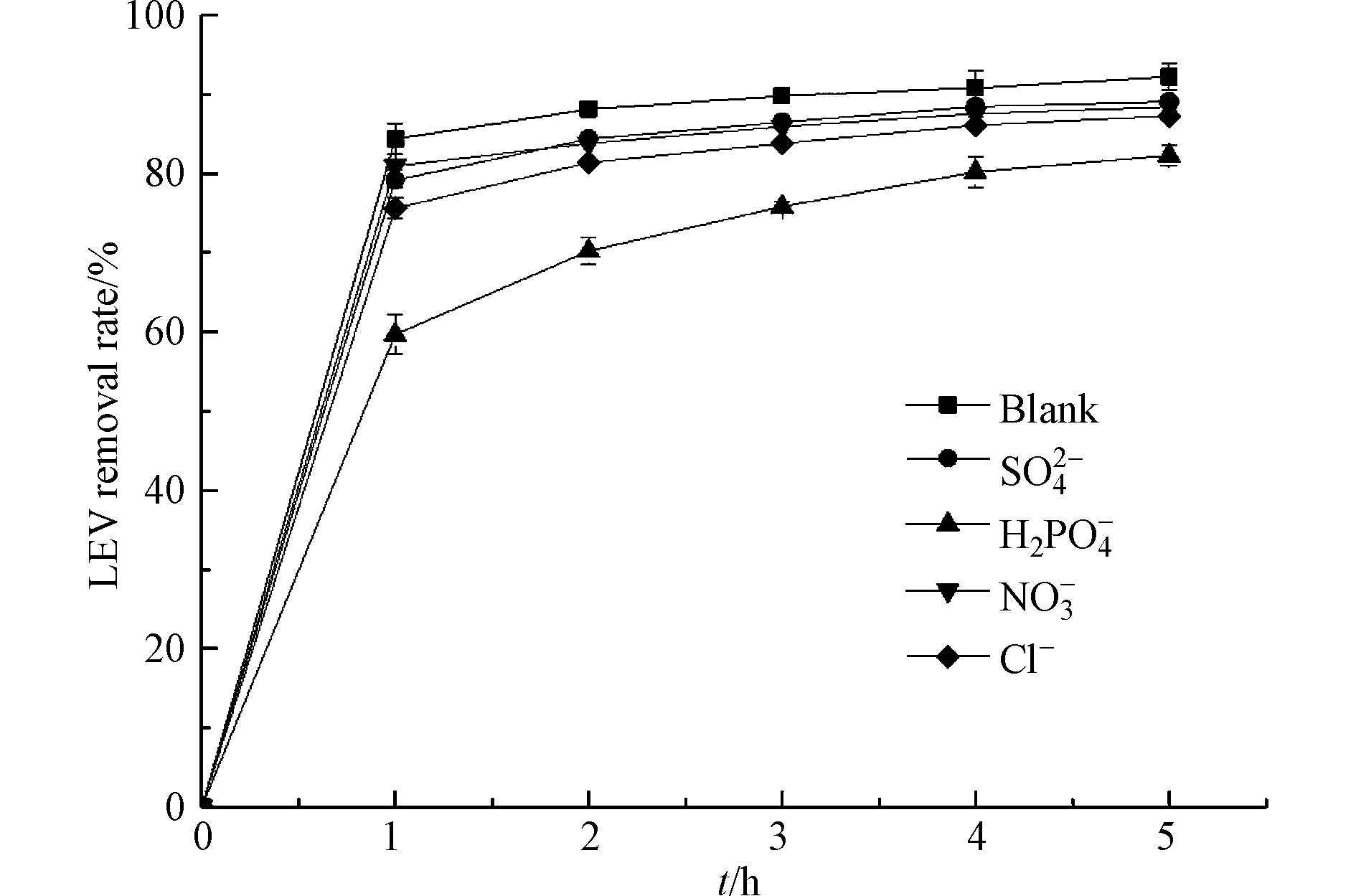
抗生素废水中常含有多种无机阴离子,这些离子对高级氧化技术的反应速率有着不可忽视的影响. 考察4种不同阴离子(
${\rm{H}}_2{\rm{PO}}_4^{-} $ 、Cl−、${\rm{SO}}_4^{2-} $ 、${\rm{NO}}_3^{-} $ )对LEV降解的影响(图10). 由图10可知,Cl−、${\rm{SO}}_4^{2-} $ 和${\rm{NO}}_3^{-} $ 对UV/Cu2O/H2O2体系降解LEV的抑制作用较小,而${\rm{H}}_2{\rm{PO}}_4^{-} $ 的抑制作用较大. 当UV/Cu2O/H2O2体系中加入5 mmol·L−1${\rm{H}}_2{\rm{PO}}_4^{-} $ ,反应1 h时LEV的去除率为59.7%,与空白相比降低了24.7%;反应5 h时LEV的去除率为82.3%,与空白相比降低了10%. 研究表明,${\rm{H}}_2{\rm{PO}}_4^{-} $ 可捕获·OH和h+,生成弱氧化性的$ {{\rm{H}}_2}{\rm{P}}{{\rm{O}}_4}^\cdot $ [31],从而抑制LEV降解,反应式如(14)—(15). 虽然${\rm{H}}_2{\rm{PO}}_4^{-} $ 可抑制UV/Cu2O/H2O2体系降解LEV,但随着反应时间延长,该抑制作用可逐渐降低.在UV/Cu2O/H2O2体系中分别加入Cl−和
${\rm{SO}}_4^{2-} $ ,反应1 h时,LEV去除率分别为75.6%和79.2%. 尽管Cl−和${\rm{SO}}_4^{2-} $ 可捕获·OH,生成Cl2·-和SO4·-,反应式如(16)—(17),但Cl2·-(2.09 V)和SO4·-(2.43 V)也是强氧化性的自由基[32],因此对催化氧化的抑制作用较弱. NO3−对UV/Cu2O/H2O2体系降解LEV的抑制作用最小,反应1 h时,LEV去除率为80.9%,与空白相比仅降低3.5%. 这是因为在UV下,${\rm{NO}}_3^{-} $ 对光催化降解有机物具有促进和抑制的双重性,一方面${\rm{NO}}_3^{-} $ 可与H2O反应生成·OH, 如反应式(18);另一方面NO3−对UV吸收能力强,降低了Cu2O和H2O2对UV的利用率[33],这两方面的综合作用使体系内·OH生成量减少,从而抑制LEV的降解.
2.1. 反应条件对LEV降解的影响
2.2. Cu2O投加量对LEV降解的影响
2.3. H2O2浓度对LEV降解的影响
2.4. 反应溶液初始pH值对LEV降解的影响
2.5. 自由基淬灭剂对LEV降解的影响
2.6. UV/Cu2O/H2O2体系降解LEV的产物分析
2.7. UV/Cu2O/H2O2体系降解LEV的抗菌性变化
2.8. 无机阴离子对LEV降解的影响
-
(1)与单一UV、单一Cu2O、UV/H2O2、Cu2O/H2O2和UV/Cu2O相比,UV/Cu2O/H2O2体系显著提高了LEV的去除率. UV/Cu2O/H2O2体系降解LEV的最佳反应条件是:当Cu2O投加量为0.1 g·L−1,H2O2浓度为2 mmol·L−1,pH=3.2,LEV初始浓度20 mg·L−1,反应5 h,LEV去除率达92.3%.
(2)UV/Cu2O/H2O2体系降解LEV的主要活性物种是·OH和h+.
(3)采用LC-MS方法检测到8种中间产物. UV/Cu2O/H2O2体系通过逐步去除LEV官能团,将其降解为小分子物质,最终产物趋于无抗菌性.
(4)Cl−、
${\rm{SO}}_4^{2-} $ 和${\rm{NO}}_3^{-} $ 对UV/Cu2O/H2O2体系降解LEV的抑制作用较小,而${\rm{H}}_2{\rm{PO}}_4^{-} $ 的抑制作用较大. 随着反应时间延长,${\rm{H}}_2{\rm{PO}}_4^{-} $ 对UV/Cu2O/H2O2体系降解LEV的抑制作用可逐渐降低.









 下载:
下载: