-
二噁英(polychlorinated dibenzo-p-dioxins and dibenzofurans,PCDD/Fs)是多氯代二苯并-对-二噁英(polychlorinated dibenzo-p-dioxins,PCDDs)和多氯代二苯并呋喃(polychlorinated dibenzofurans,PCDFs)的总称. PCDD/Fs是《关于持久性有机污染物的斯德哥尔摩公约》中首批持久性污染物之一,具有高毒性、环境持久性、长距离迁移性和生物累积性[1]. 在造纸过程中,PCDD/Fs主要产生于纸浆的含氯漂白阶段[2-6],其主要来源于未漂浆中二苯并-对-二噁英或二苯并呋喃(dibenzo-p-dioxin and dibenzofuran,DBD/F)的直接氯化和不可萃取态前体的转化[7-9]. 实际上,DBD/F广泛存在于环境体系中,在木材、水和空气中也检测到DBD/F[8, 10]. 工业过程中DBD/F的含量常常几个数量级地高于PCDD/Fs,且DBD/F的氯化也是PCDD/Fs生成的重要途径[11]. 而未漂浆中含量较高的酚类,如苯酚、愈创木酚和儿茶酚[12],它们在氯漂白中生成氯化的酚类[13],成为一种可吸附性有机卤化物而进入漂白浆和废液. 其中苯酚是氯化反应中最常见的酚类[14-16],愈创木酚是木素降解的主要结构单元[17],儿茶酚作为一种二羟基苯类物质,其氯化和降解也得到了研究[16, 18]. 我们最近对水溶液和纸浆体系中DBD/F氯化生成PCDD/Fs的动力学和途径做了探索[9]. 但是,目前鲜有对DBD/F和酚类共存体系的氯化研究. 由于DBD/F和酚类在环境中的普遍存在性,对酚类存在时DBD/F氯化生成PCDD/Fs的研究是有必要的.
本研究首先对实验环境中的DBD/F值进行表征检测,随后在纸浆氯漂白条件下进行氯化实验,对不同种类酚类和不同剂量酚类对DBD/F氯化生成PCDD/Fs的影响进行了研究.
-
试剂:二苯并-对-二噁英(DBD,>99.0%,TCI,Japan)、二苯并呋喃(DBF,>99%,Aldrich,USA)、苯酚(99%,北京百灵威科技有限公司)、愈创木酚(99%,北京百灵威科技有限公司)、邻苯二酚(99%,上海阿拉丁生化科技股份有限公司)、超纯水(18.2 MΩ,Millipore)、次氯酸钠溶液(分析纯,有效氯含量≥6.0%,游离碱含量(以NaOH计)≤4.0%,天津市科密欧化学试剂有限公司)、浓盐酸(优级纯,天津市大茂化学试剂厂)、硫代硫酸钠(5H2O,分析纯,≥99.0%,天津市大茂化学试剂厂)、无水硫酸钠(分析纯,≥99.0%,天津市大茂化学试剂厂)、63–100 µm硅胶(Sunchrom,Germany)、碱性氧化铝(ICN Biomedical Alumina B-Super I,Germany)、正己烷和二氯甲烷(农残级,Honeywell,USA)、壬烷(农残级,Fluka,Switzerland).
低氯代PCDD/Fs提取内标(MDDF-MDT,Wellington Laboratories,Guelph,Ontario,Canada)含8种13C12标记的内标(13C12-DBD,13C12-2-Cl1DD,13C12-2,3-Cl2DD,13C12-2,3,7-Cl3DD,13C12-DBF,13C12-2-Cl1DF,13C12-2,3-Cl2DF,13C12-2,3,8-Cl3DF);高氯代PCDD/Fs提取内标(EDF-8999,Cambridge Isotope Laboratories,Andover,MA,USA)含15种13C12标记的内标(13C12-2,3,7,8-Cl4DF,13C12-1,2,3,7,8-Cl5DF,13C12-2,3,4,7,8-Cl5DF,13C12-1,2,3,4,7,8-Cl6DF,13C12-1,2,3,6,7,8-Cl6DF,13C12-1,2,3,7,8,9-Cl6DF,13C12-2,3,4,6,7,8-Cl6DF,13C12-1,2,3,4,6,7,8-Cl7DF,13C12-1,2,3,4,7,8,9-Cl7DF,13C12-2,3,7,8-Cl4DD,13C12-1,2,3,7,8-Cl5DD,13C12-1,2,3,4,7,8-Cl6DD,13C12-1,2,3,6,7,8-Cl6DD,13C12-1,2,3,4,6,7,8-Cl7DD,13C12-Cl8DD). PCDD/Fs回收率内标(EDF-5999;Cambridge Isotope Laboratories,Andover,MA,USA)含2种13C12标记的内标13C12-1,2,3,4-Cl4DD和13C12-1,2,3,7,8,9-Cl6DD.
仪器:电子天平(JA-2003型,上海)、旋转蒸发仪(Buchi,Rotavapor R-205,Switzerland)、样品浓缩氮吹仪(Zymark,USA)、DFS高分辨气相色谱-高分辨质谱联用仪(Thermo Fisher Scientific,USA).
-
DBD/F空白实验:(1)溶剂:将过净化柱所用溶剂正己烷和二氯甲烷混合后加提取内标,直接浓缩定容测定;(2)净化柱(按照标准方法HJ 77.1−2008[19]装填的多层硅胶柱和氧化铝柱):对净化柱添加提取内标,按照净化样品所用的冲洗程序得到样品液,浓缩定容进行测定;(3)Milli-Q水:为尽可能减少水中可能存在的其他杂质的影响,实验中所有的用水均为Milli-Q超纯水. 水中可能存在的DBD/F污染,结合具体实验条件,加提取内标后用二氯甲烷萃取3次,合并萃取液,无水硫酸钠除水后浓缩定容分析;(4)样品前处理全程:以水进行前处理分析步骤,包括萃取、干燥、过净化柱、浓缩定容和仪器测定.
根据实验室模拟纸浆的氯化漂白条件[9],即未漂浆10 g,加水325 mL使纸浆浆浓达到3%,用浓HCl调节体系pH值约1.0,加入NaClO溶液使有效氯用量0.5 g(有效氯用量5%),25 ℃搅拌反应60 min. 在此条件下进行酚类存在时DBD/F氯化生成PCDD/Fs的研究,条件具体如下:
不同酚类体系中DBD/F的氯化:在325 mL Milli-Q水中加入0.5 g酚类(苯酚或愈创木酚或儿茶酚),再加入DBF和DBD,其中DBF/DBD的投入量分别为:0/0、1000/100、2000/200、4000/400 ng,浓HCl调节体系pH值为1,25 ℃搅拌,加入NaClO溶液(有效氯用量为0.5 g)开始氯化反应,反应时间为60 min,反应结束后加入0.2 mol·L−1 Na2S2O3溶液15 mL.
不同酚类用量时DBD/F的氯化:将2000 ng DBF和200 ng DBD加入到325 mL Milli-Q水中,再加入酚类(苯酚或愈创木酚或儿茶酚)溶解,酚类用量分别为0、0.25、0.5、0.75、1.0 g,用浓HCl调节体系pH值为1,25 ℃搅拌,加入NaClO溶液(有效氯用量为0.5 g)开始氯化反应,反应时间为60 min,反应结束后加入0.2 mol·L−1 Na2S2O3溶液15 mL.
-
对氯化反应后的样品,在其中加入10 µL低氯代13C12-PCDD/Fs提取内标和10 µL高氯代13C12-PCDD/Fs提取内标,加入35 mL二氯甲烷液液萃取3次,合并萃取液,用无水硫酸钠除水. 将样品的提取液旋转蒸发浓缩至1−2 mL,按照HJ 77.1−2008[19]标准方法中的活化和洗脱程序,进行多层硅胶柱和氧化铝柱净化处理,最终的净化液浓缩至1 mL,用气流平缓的N2吹至近干,加入10 µL 13C12-PCDD/Fs回收率内标,涡旋混匀,进行HRGC-HRMS分析.
-
HRGC-HRMS分析DBD/F和PCDD/Fs的色谱柱为Rtx-5 MS柱(60 m×0.25 mm i.d.×0.25 µm df). 载气为高纯氦气,恒流模式. 进样口温度270 ℃,进样量1 µL,不分流进样. 升温程序为初始温度140 ℃,保持1 min;以20 ℃·min−1上升到200 ℃,保持1 min;然后以5 ℃·min−1上升到220 ℃,保持16 min;以5 ℃·min−1上升到235 ℃,保持7 min;最后以5 ℃·min−1上升到310 ℃,保持15 min. 传输线温度280 ℃,高分辨质谱为EI电离源,离子源温度280 ℃,电离能45 eV,质谱分辨率10000,以选择离子监测(SIM)模式进行监测. 采用同位素稀释内标法对样品中的PCDD/Fs同类物进行定性定量测定. 用保留时间(或相对保留时间)和特征离子比例定性,同位素稀释内标法定量[9].
-
实验中所有玻璃器皿采用洗涤液进行清洗后用Milli-Q水冲洗烘干,在使用前用正己烷涮洗3−4次,并定期对试剂空白和操作空白进行确认. 仪器测试前进行调谐校准,定期校正保留时间和响应因子. 按照标准方法HJ 77.1−2008[19]中的要求进行检出限确认,实际样品中DBD、DBF和PCDD/Fs的检出限分别为0.79、55.70 、0.28−1.96 pg·L−1. 添加的23种13C12-PCDD/Fs提取内标平均回收率25%−110%.
-
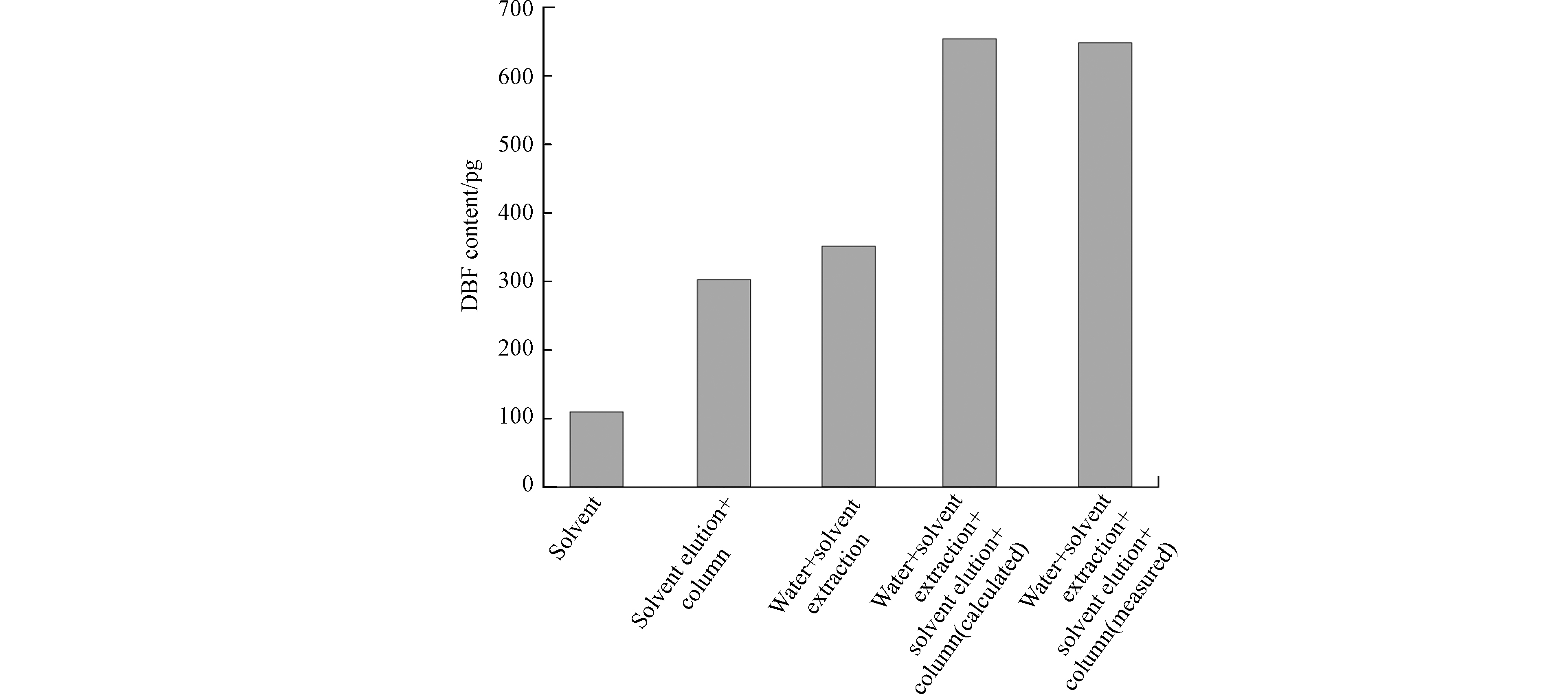
在实验分析环境下,除DBF外,未检测到DBD和其他PCDD/Fs异构体的存在. 实验分析条件中DBF的背景含量如图1所示. 分别对萃取和净化时所用的溶剂、净化柱、水溶液萃取分步骤检测确认其DBF空白值,得到正己烷和二氯甲烷溶剂DBF含量为109.68 pg,即溶剂中DBF浓度为0.73 pg·mL−1;净化柱带来约200 pg的DBF污染;Milli-Q水中DBF浓度为0.86 pg·mL−1. 所测得的DBF背景值与文献[8, 10]报道的相当. 由此计算样品前处理全程溶剂提取和净化柱净化中DBF空白值应为654.11 pg,此值与实测值648.17 pg基本相等,证明了实验的准确性和稳定性.
在最近的研究[9]中曾对所用的苯酚、愈创木酚和儿茶酚试剂中的DBD/F空白值进行了检测. 其中DBD/F含量较高的儿茶酚在本实验中进行了纯化处理. 结合对体系DBD/F空白值的检测,计算出在本实验条件下整个体系中总的DBD/F背景含量<1 ng,由此判定实验体系中的空白DBD/F值对后续实验的影响可以忽略不计.
-
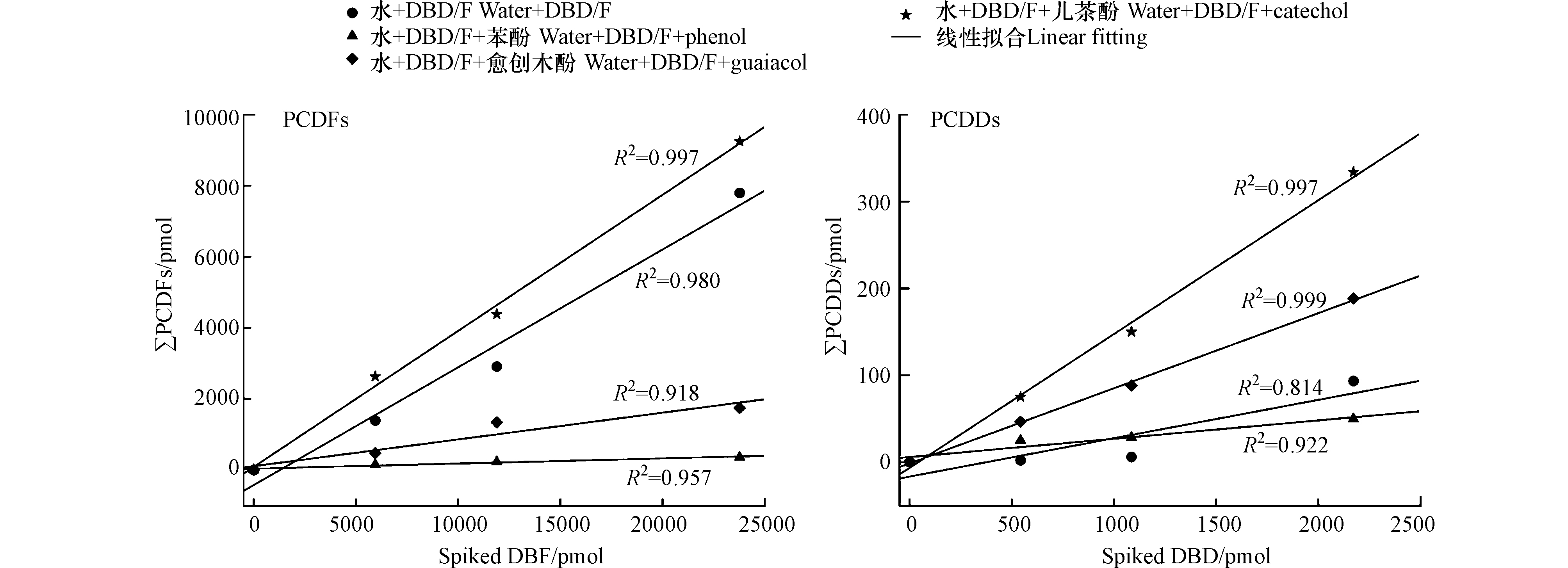
当体系存在一些纸浆中典型的酚类时,水溶液中DBD/F氯化生成PCDD/Fs的氯化行为发生改变. 结合前面对水、苯酚、愈创木酚、儿茶酚和分析过程中空白DBD/F的检测值,为排除实验全过程中体系固有的DBD/F对实验的干扰,DBF添加量为0、1000、2000、4000 ng,DBD添加量为0、100、200、400 ng,使其远远超过空白值,以此更准确反映DBD/F氯化生成PCDD/Fs的情况. 苯酚、愈创木酚和儿茶酚的添加量均为0.5 g,在不同氯化体系中梯度添加DBD/F生成PCDD/Fs的含量变化如图2所示.
由图2可知,在纯水溶液与苯酚、愈创木酚和儿茶酚水溶液中,DBD/F氯化生成PCDD/Fs的含量与DBD/F的添加量均呈现良好的线性关系(R2=0.814−0.999). 线性拟合直线的斜率代表了每增加1 pmol DBF和DBD而引起的∑PCDFs和∑PCDDs增加量(pmol),即DBF和DBD转化为相应∑PCDFs和∑PCDDs的转化率,也可以反映在不同酚类体系中DBF和DBD转化为PCDFs和PCDDs的难易程度. 由线性拟合直线的斜率,计算出在纯水溶液与苯酚、愈创木酚和儿茶酚水溶液中,DBF转化为∑PCDFs的转化率分别为33.1%、1.4%、7.5%、38.3%,DBD转化为∑PCDDs的转化率分别为4.4%、2.1%、8.7%、15.4%. 3种酚类体系中DBF和DBD的转化率呈现出相同的趋势:苯酚<愈创木酚<儿茶酚. 纯水体系中DBD/F氯化,DBF转化率大于DBD转化率. 苯酚和愈创木酚的加入,使得DBF的转化率分别下降了95.7%和77.3%,而同等条件下,儿茶酚的加入对DBF的转化无抑制效果. 如图2所示,愈创木酚和儿茶酚的投入对DBD氯化为∑PCDDs的转化并无抑制作用,不同酚类的作用强度不同.
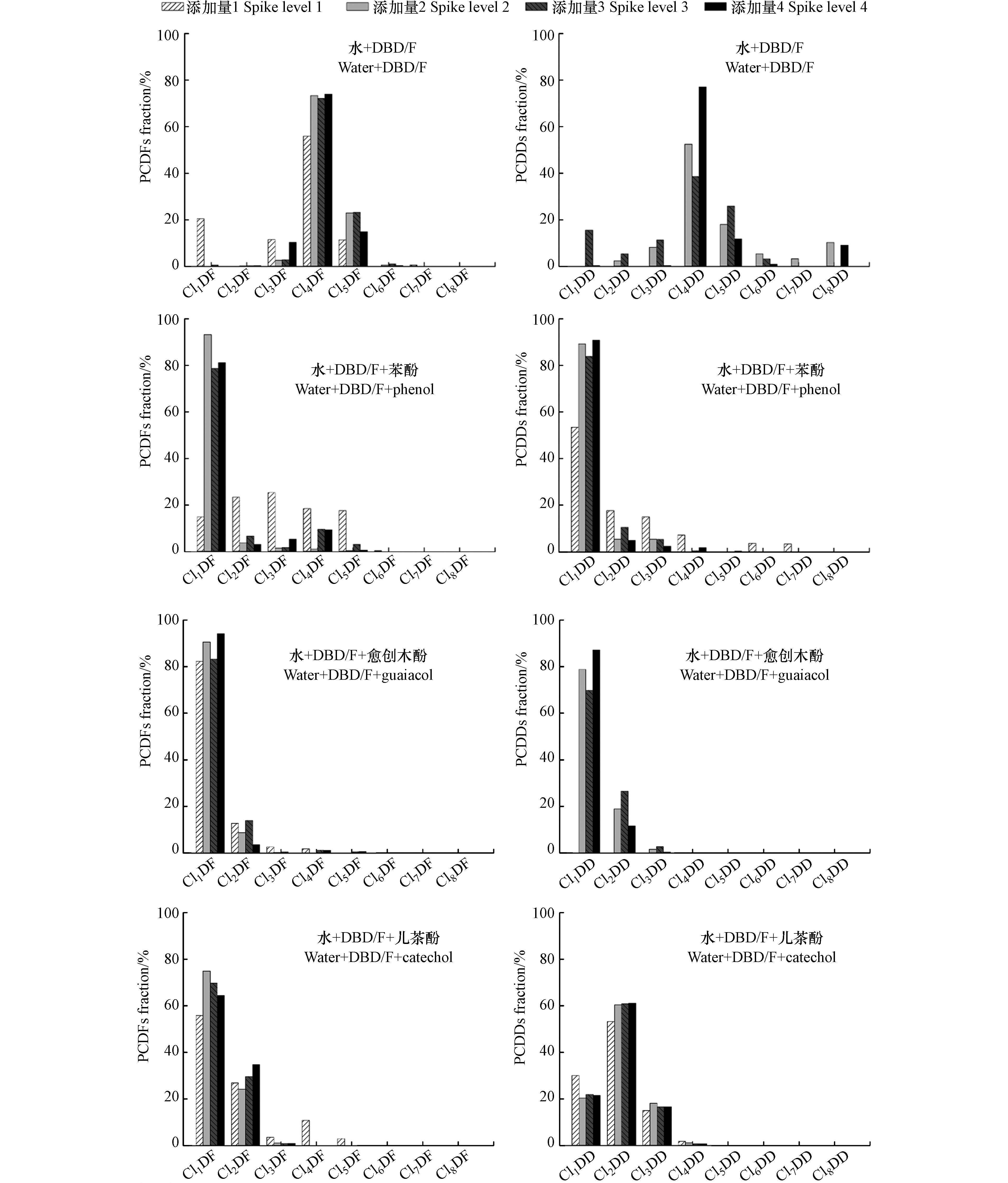
在水、苯酚、愈创木酚和儿茶酚体系中DBD/F氯化生成PCDD/Fs的同系物分布模式均不随DBD/F的添加量发生变化(如图3).
但酚类的加入使PCDD/Fs的同系物分布模式发生显著变化,PCDD/Fs的主导同系物由Cl4DD/F转化为Cl1DD/F,平均氯化度由4.0下降到1.5. 苯酚和愈创木酚体系中,Cl1DD/F占所有同系物的80%以上,而儿茶酚体系中Cl2DD/F的比例有所增加.
-
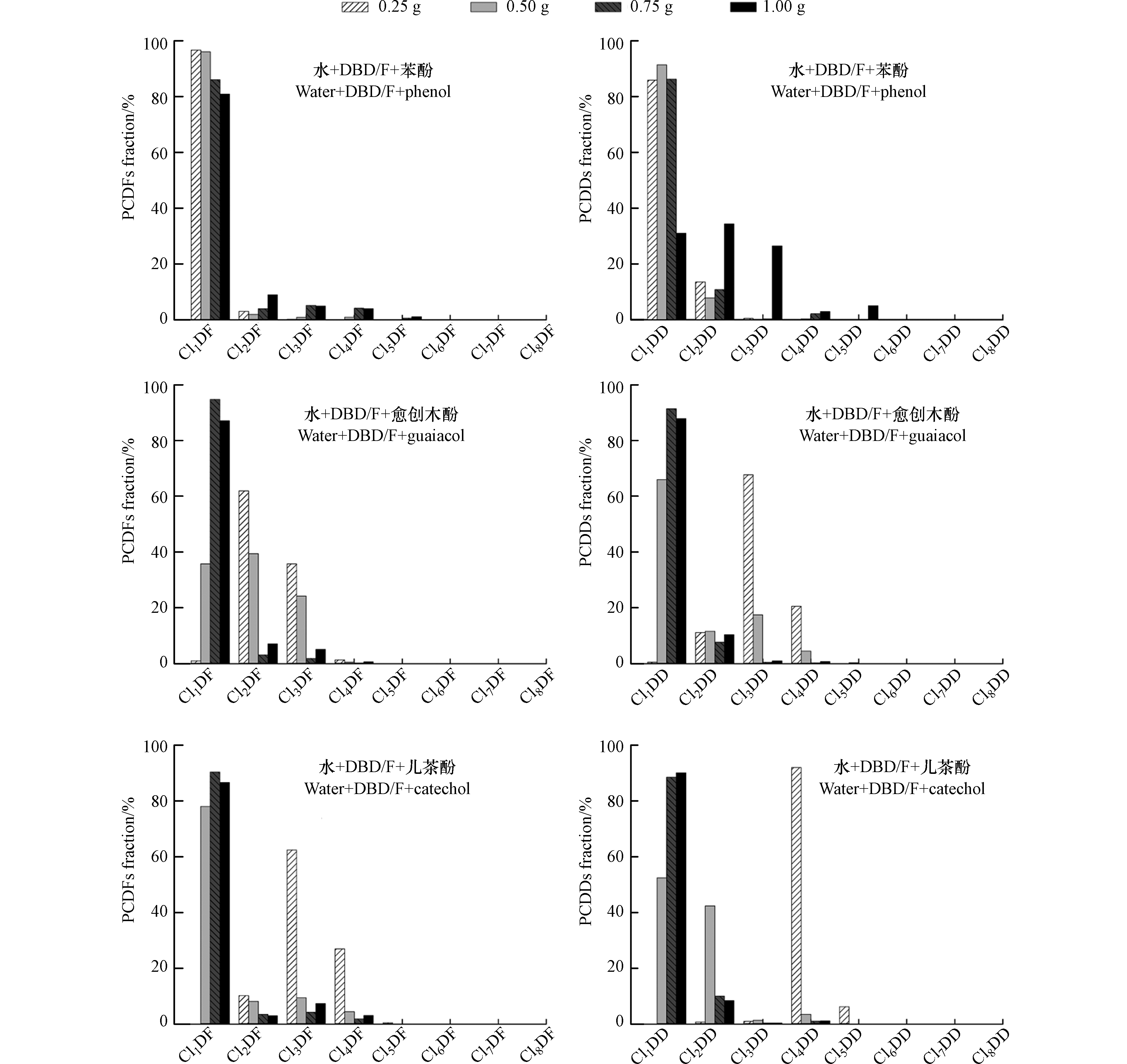
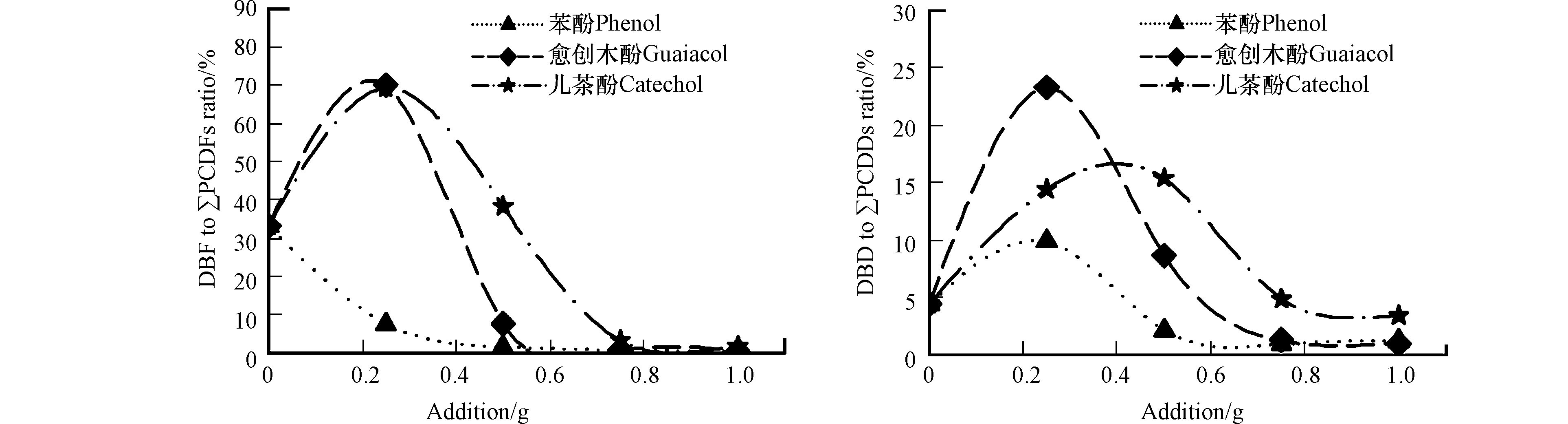
不同类型的酚类导致DBD/F氯化生成PCDD/Fs的含量、转化率和同系物分布模式发生变化,原因可能不仅与酚的种类有关,还与酚的投入量相关. 为考察酚类用量对DBD/F氯化生成PCDD/Fs的影响,实验中固定DBF和DBD的添加量分别为2000 ng和200 ng,3种酚类的投入量分别为0、0.25、0.5、0.75、1.0 g,按同样的实验条件进行氯化,其生成PCDD/Fs的同系物分布模式和含量分别如图4和图5所示. 酚类不同投入量时,体系中生成PCDD/Fs的分布模式未发生明显的变化,尤其对于添加苯酚的氯化体系(图4). 在愈创木酚和儿茶酚氯化体系中,在添加量为0.25 g时,其Cl3DD/F和Cl4DD/F的比例增加,比较接近于纯水溶液中DBD/F氯化生成PCDD/Fs的分布模式(图3).
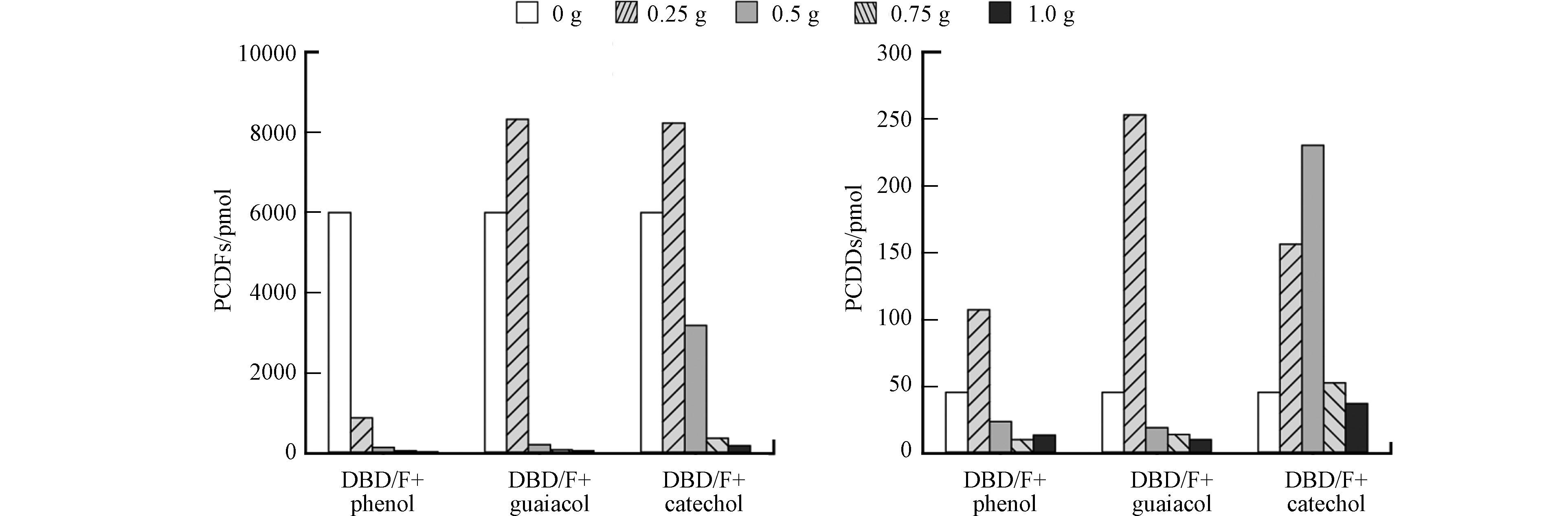
在愈创木酚和儿茶酚体系中,PCDFs的生成量在0.25 g时达到峰值;在苯酚和愈创木酚体系中,PCDDs的生成量也在0.25 g时达到峰值(图5). 特别地,苯酚的加入严重抑制了体系中DBF的氯化,而儿茶酚在加入0.5 g时,DBD氯化生成PCDDs的量达到峰值.
苯酚、愈创木酚和儿茶酚不同用量时DBD/F氯化生成PCDD/Fs的摩尔转化率如图6. 一定剂量酚类的加入会使体系中DBD/F氯化生成PCDD/Fs的转化率增大,当超出剂量范围后,DBD/F氯化生成PCDD/Fs得到抑制. 不同酚类的阈值不同,由图6呈现的趋势(由于只观测了有限个数量点,所以以虚线表示),3种酚类阈值顺序为:苯酚<愈创木酚<儿茶酚. 并且在此阈值范围内,存在一个使DBD/F氯化生成PCDD/Fs转化率最大的酚类用量值,实验观测的几个条件下,当酚类用量0.25 g时,愈创木酚和儿茶酚体系均表现出最大的DBD/F转化率,而苯酚体系的DBF氯化只观测到了抑制效应. 当超出酚类促进的阈值后,酚类的投入对DBD/F氯化生成PCDD/Fs表现为抑制效应. 同样剂量酚类投入下,其抑制效应大小表现为:苯酚>愈创木酚>儿茶酚.
在氯化的水溶液中,除了基本的氯化试剂氯气(Cl2)外,还存在多种氯氧化合物(ClOx),它们的浓度和存在形式随着体系条件的变化而变化[15, 20-21]. 氯化反应体系中,酚类和DBD/F均与多种形态的氯反应而生成相应的氯化产物或解离产物. 体系中对于DBD/F氯化生成PCDD/Fs的有效氯形态为Cl2,其他ClOx对生成的PCDD/Fs具有降解作用[22]. 由于体系中氯是大大过量的,当一部分酚类存在时,其消耗的Cl2对DBD/F的氯化并无显著影响,而酚类会消耗一部分ClOx,从而降低了ClOx对PCDD/Fs的破坏,使PCDD/Fs的降解得到降低,由此观测到DBD/F氯化生成PCDD/Fs的转化率增大. 但是随着酚类用量的增加,其大量消耗Cl2,导致体系中Cl2浓度极大下降,严重影响DBD/F氯化生成PCDD/Fs,造成最终观测到的PCDD/Fs转化率急剧下降. 不同的酚类消耗Cl2和ClOx的能力和速率不同,由此造成对DBD/F氯化生成PCDD/Fs促进和抑制效应的不同.
-
本研究在模拟纸浆氯漂白的实验条件下,对实验环境中的DBD/F进行检测,排除了背景DBD/F值对实验的干扰;随后考察了纸浆中含量较高的苯酚、愈创木酚和儿茶酚对DBD/F氯化生成PCDD/Fs的影响. 实验发现酚类的加入使DBD/F氯化生成PCDD/Fs的主导同系物由Cl4DD/F转化为Cl1DD/F,平均氯化度下降,并且不同酚类对DBD和DBF的氯化转化率的影响各不相同. 进一步研究了不同剂量酚类对DBD/F氯化生成PCDD/Fs的影响,发现一定剂量酚类的加入会使体系中DBD/F氯化生成PCDD/Fs的转化率增大,当超出剂量范围后,DBD/F氯化生成PCDD/Fs得到抑制. 不同酚类的阈值不同,3种酚类阈值顺序为:苯酚<愈创木酚<儿茶酚. 当超出酚类促进的阈值后,酚类的投入对DBD/F氯化生成PCDD/Fs表现为抑制效应. 同样剂量酚类投入下,其抑制效应大小表现为:苯酚>愈创木酚>儿茶酚. 可能是酚类结构的差异导致其消耗氯的种类和能力不同,从而对DBD/F氯化生成PCDD/Fs产生影响.
纸浆中代表性酚类对二苯并-对-二噁英/二苯并呋喃氯化生成多氯代二噁英的影响
Effect of representative phenols in pulp on the chlorination of dibenzo-p-dioxin/dibenzofuran into polychlorinated dibenzo-p-dioxins and dibenzofurans
-
摘要: 二苯并-对-二噁英/二苯并呋喃(DBD/F)的直接氯化是纸浆氯漂白过程中形成多氯代二噁英(PCDD/Fs)的重要途径. 纸浆中的酚类在氯化漂白过程中也将消耗氯而生成氯化产物. 本文在模拟纸浆氯漂白的实验条件下,对实验环境中的DBD/F进行检测,排除了背景DBD/F值对实验的干扰后,研究了纸浆中含量较高的酚类(苯酚、愈创木酚和儿茶酚)对DBD/F氯化生成PCDD/Fs的转化率和同系物模式的影响. 发现酚类的加入使DBD/F氯化生成PCDD/Fs的氯化度下降;一定剂量酚类的加入会使体系中DBD/F氯化生成PCDD/Fs的转化率增大,当超出剂量范围后,DBD/F氯化生成PCDD/Fs得到抑制,不同酚类的效应大小各不相同.
-
关键词:
- 酚类 /
- 二苯并-对-二噁英/二苯并呋喃 /
- 二噁英 /
- 氯化
Abstract: Direct chlorination of dibenzo-p-dioxin/dibenzofuran (DBD/F) is an important way to form polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) during chlorine bleaching of pulp. The phenols in the pulp also consume chlorine during the chlorine bleaching process to produce corresponding chlorinated products. In this study, under the condition of simulating the chlorine bleaching of pulp, the DBD/F in the experimental environment was detected, and the background DBD/F value was excluded from the experiment. The effect of phenols (phenol, guaiacol and catechol) with high content in pulp on the conversion efficiency and homolog distribution of PCDD/Fs generated from DBD/F chlorination was studied. It was found that the degree of chlorination of PCDD/Fs decreased with the addition of phenols. The addition of a certain amount of phenols increased the conversion rate of DBD/F to PCDD/Fs in the system. When the phenolic dosage was exceeded, the chlorination of DBD/F to PCDD/Fs was inhibited, and the effects of different phenols were different.-
Key words:
- phenols /
- DBD/F /
- PCDD/Fs /
- chlorination
-

-
图 3 水、苯酚、愈创木酚和儿茶酚体系中DBD/F氯化生成PCDD/Fs的同系物分布模式.Spike level 1−4分别表示酚类的投入量为0.5 g,而DBF/DBD的投入量为:0/0、1000/100、2000/200、4000/400 ng.
Figure 3. Homologous distribution patterns of PCDD/Fs generated by DBD/F chlorination in pure water and water spiked with phenol, guaiacol and catechol. Spike level 1−4 indicates that the input of phenols was 0.5 g, and the input of DBF/DBD was: 0/0, 1000/100, 2000/200, 4000/400 ng, respectively.
图 4 苯酚、愈创木酚和儿茶酚不同用量时DBD/F氯化生成PCDD/Fs的同系物分布模式.DBF和DBD的投入量分别为2000 ng和200 ng,酚类的投入量分别为0.25、0.5、0.75、1.0 g.
Figure 4. Homologues distribution patterns of PCDD/Fs generated by chlorination of DBD/F at different dosage of phenol, guaiacol and catechol wherein the input amounts of DBF and DBD were 2000 ng and 200 ng respectively, and the input amounts of phenols were 0.25, 0.5, 0.75 and 1.0 g, respectively.
图 5 苯酚、愈创木酚和儿茶酚不同用量时DBD/F氯化生成PCDD/Fs的含量DBF和DBD的投入量分别为2000 ng和200 ng,酚类的投入量分别为0、0.25、0.5、0.75、1.0 g.
Figure 5. Content of PCDD/Fs generated by chlorination of DBD/F at different dosage of phenol, guaiacol and catechol, wherein the input amounts of DBF and DBD were 2000 ng and 200 ng respectively, and the input amounts of phenols were 0, 0.25, 0.5, 0.75 and 1.0 g, respectively.
-
[1] United Nations Environment Programme (UNEP). Stockholm convention on persistent organic pollutants[EB/OL]. [2020-1-16], 2001. http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx. [2] ZHENG M H, BAO Z C, ZHANG B, et al. Polychlorinated dibenzo-p-dioxins and dibenzofurans in paper making from a pulp mill in China [J]. Chemosphere, 2001, 44(6): 1335-1337. doi: 10.1016/S0045-6535(00)00488-4 [3] BLANCO A, NEGRO C, MONTE C, et al. Peer reviewed: The challenges of sustainable papermaking [J]. Environmental Science & Technology, 2004, 38(21): 414A-420A. [4] WANG X L, NI Y W, ZHANG H J, et al. Formation and emission of PCDD/Fs in Chinese non-wood pulp and paper mills [J]. Environmental Science & Technology, 2012, 46(21): 12234-12240. [5] 夏科学, 倪余文, 张海军, 等. 纸浆氯漂白过程中二噁英的来源及生成机制研究进展 [J]. 环境化学, 2016, 35(9): 1823-1832. doi: 10.7524/j.issn.0254-6108.2016.09.2016012704 XIA K X, NI Y W, ZHANG H J, et al. Sources and formation mechanisms of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans in the process of pulp bleaching with chlorine [J]. Environmental Chemistry, 2016, 35(9): 1823-1832(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.09.2016012704
[6] AXEGARD P. The effect of the transition from elemental chlorine bleaching to chlorine dioxide bleaching in the pulp industry on the formation of PCDD/Fs [J]. Chemosphere, 2019, 236: 124386. doi: 10.1016/j.chemosphere.2019.124386 [7] LAFLEUR L, BRUNCK B, MCDONOUGH T, et al. Studies on the mechanism of PCDD/PCDF formation during the bleaching of pulp [J]. Chemosphere, 1990, 20(10): 1731-1738. [8] DIMMEL D R, RIGGS K B, PITTS G, et al. Formation mechanisms of polychlorinated dibenzo-p-dioxins and dibenzofurans during pulp chlorination [J]. Environmental Science & Technology, 1993, 27(12): 2553-2558. [9] XIA K X, NI Y W, ZHAN F Q, et al. Mechanistic aspects of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) formation from chlorine bleaching of non-wood pulp [J]. Journal of Hazardous materials, 2020, 386: 121652. doi: 10.1016/j.jhazmat.2019.121652 [10] VOSS R H, LUTHE C E, FLEMING B I, et al. Some new insights into the origins of dioxins formed during chemical pulp bleaching [J]. Pulp & Paper Canada, 1988, 89(12): 151-162. [11] ALTARAWNEH M, DLUGOGORSKI B Z, KENNEDY E M, et al. Mechanisms for formation, chlorination, dechlorination and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) [J]. Progress in Energy and Combustion Science, 2009, 35(3): 245-274. doi: 10.1016/j.pecs.2008.12.001 [12] WANG X L, CHEN J P, NI Y W. Polychlorinated dibenzo-p-dioxin and dibenzofuran precursors and formation mechanisms during non-woodpulp chlorine bleaching process [J]. Chemosphere, 2018, 211: 1-9. doi: 10.1016/j.chemosphere.2018.07.126 [13] MALHOTRA R, PRAKASH D, SHUKLA S K, et al. Comparative study of toxic chlorophenolic compounds generated in various bleaching sequences of wheat straw pulp [J]. Clean Technologies and Environmental Policy, 2013, 15(6): 999-1011. doi: 10.1007/s10098-013-0578-6 [14] GALLARD H, VON GUNTEN U. Chlorination of phenols: Kinetics and formation of chloroform [J]. Environmental Science & Technology, 2002, 36(5): 884-890. [15] LAU S S, ABRAHAM S M, ROBERTS A L. Chlorination revisited: Does Cl– serve as a catalyst in the chlorination of phenols? [J]. Environmental Science & Technology, 2016, 50(24): 13291-13298. [16] PRASSE C, VON GUNTEN U, SEDLAK D L. Chlorination of phenols revisited: Unexpected formation of α,β-unsaturated C4-dicarbonyl ring cleavage products [J]. Environmental Science & Technology, 2020, 54(2): 826-834. [17] DIER T K F, EGELE K, FOSSOG V, et al. Enhanced mass defect filtering to simplify and classify complex mixtures of lignin degradation products [J]. Analytical Chemistry, 2016, 88(2): 1328-1335. doi: 10.1021/acs.analchem.5b03790 [18] REBENNE L M, GONZALEZ A C, OLSON T M. Aqueous chlorination kinetics and mechanism of substituted dihydroxybenzenes [J]. Environmental Science & Technology, 1996, 30(7): 2235-2242. [19] 中华人民共和国环境保护部. 水质 二噁英类的测定 同位素稀释高分辨气相色谱-高分辨质谱法 [S]; HJ 77.1–2008; 2008.Ministry of Environmental Protection of the People’s Republic of China. Water-determination of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans-isotope dilution HRGC-HRMS [S]; HJ77.1-2008; 2008(in Chinese). [20] SIVEY J D, MCCULLOUGH C E, ROBERTS A L. Chlorine monoxide (Cl2O) and molecular chlorine (Cl2) as active chlorinating agents in reaction of dimethenamid with aqueous free chlorine [J]. Environmental Science & Technology, 2010, 44(9): 3357-3362. [21] LAU S S, REBER K P, ROBERTS A L. Aqueous chlorination kinetics of cyclic alkenes—Is HOCl the only chlorinating agent that matters? [J]. Environmental Science & Technology, 2019, 53(19): 11133-11141. [22] LI Q, YU J, CHEN W, et al. Degradation of triclosan by chlorine dioxide: Reaction mechanism,2,4-dichlorophenol accumulation and toxicity evaluation [J]. Chemosphere, 2018, 207: 449-456. doi: 10.1016/j.chemosphere.2018.05.065 -



 下载:
下载: