-
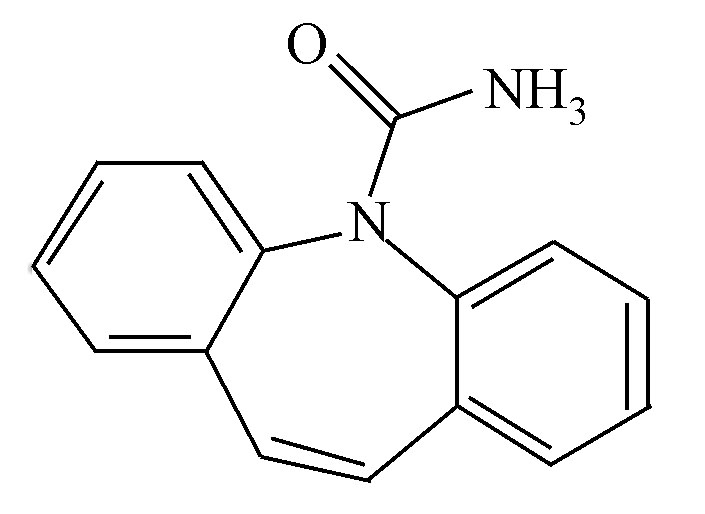
目前,药物和个人护理用品(pharmaceuticals and personal care products, PPCPs)广泛应用于动物疾病的防治、畜牧以及人类医疗等领域,对社会的发展和人类的健康做出了巨大的贡献[1]。但PPCPs进入动物或人体内均不能被全部吸收,大部分以原药或者其代谢产物通过尿液和粪便的形式排泄从而进入到环境中,因此有研究者在废水、地表水、海水、土壤等其他环境介质中都检测出了不同含量药物的存在[2],而且PPCPs还可以通过直接饮水和食物链富集等方式进入到人体当中,对人体的健康产生危害[3]。因此迫切地需要对PPCPs的环境风险和环境行为进行系统的研究。磺胺甲恶唑(SMX)和卡马西平(CBZ)作为代表性药物,两者环境暴露浓度高、迁移性高、难以降解且生物毒性大[4-7]。
碳基材料结构简单、具有丰富的孔隙结构、较大的比表面积和含氧官能团,对有机污染物具有较大的吸附能力,被广泛的用于去除环境中的有机污染物[8-10]。目前多数研究者仅仅关注其吸附能力的大小,如竞争吸附导致吸附量降低[11],或者协同作用导致吸附量增加[12],忽视了动力学吸附特性的研究,特别是共吸附体系中PPCPs所发生的竞争吸附动力学以及竞争吸持动力学,而且基本上是关于共吸附体系中离子[13]之间的动力学研究,但是几乎没有关于共吸附体系中竞争吸持动力学的研究。
本研究选取活性炭(AC)、单壁碳纳米管(SC)、氧化石墨烯(GO)为模型碳基吸附剂,SMX和CBZ作为模型药物,对单一体系以及共吸附体系中吸附动力学进行系统研究,为科学的预测PPCPs的环境行为和碳基材料在污染控制方面的应用提供理论基础。
全文HTML
-
磺胺甲恶唑(SMX)和卡马西平(CBZ)均购于Bio Basic Inc生物工程公司,具体的理化性质见表1;甲醇和乙腈为色谱纯试剂,均购于Sigma公司,其他试剂均为分析纯。活性炭(AC)购自于阿拉丁试剂有限公司;单壁碳纳米管(SC)购自中国科学院成都有机化学有限公司;氧化石墨烯(GO)购自南京吉仓纳米科技有限公司,3种碳基吸附材料的纯度均高于95%。然后用仪器检测碳基材料的形貌和理化性质。分别用元素分析仪(MicroCube,Elementar, Germany)检测材料的C、H、O、N、S等5种元素的含量;比表面积分析仪(Autosorb-1C,Quantachrome)测定材料的比表面积和孔径分布;透射电子显微镜(FEI/Philips XL30)扫描碳基材料的微观形貌。
-
将SMX(分析纯)和CBZ(分析纯)在背景液中分别配置成100 mg·L−1的储备液。背景液包含0.01 mol·L−1氯化钙(CaCl2,提供背景离子强度)和200 mg·L−1的叠氮钠(NaN3,抑制微生物生长)。吸附动力学实验是在40 mL带有四氟乙烯螺旋口的玻璃瓶中进行。AC、SC和GO的固液比(W/W)分别为:1∶40000、1∶20000和1∶100。在前期工作中,从共吸附体系的吸附等温线的数据中看出,在主要物质浓度为2 mg·L−1,竞争物质浓度为10 mg·L−1时两者的竞争作用最为显著。因此在动力学讨论中选择了此浓度。而且在这种浓度下,吸附SMX和CBZ达到平衡时的吸附率是在一定的范围内,这样就保证了数据的可靠性。所有样品瓶置于恒温摇床中,然后分别在1、2、3、5、8、12、24、36、72、120、168 h的时间点取出,经离心后取出0.2 mL的上清液,经0.45 μm的过滤头过滤并移到液相瓶中。然后用高效液相色谱仪(Agilent 1200)测定吸附质的剩余浓度。
SMX和CBZ的液相色谱分析条件是:紫外检测器、C18反相柱(5 μm,4.6×150 mm)、流动相配比(V∶V)乙腈∶水=40∶60、检测波长为265 nm,流速为1 mL·min−1,进样量为10 μL。SMX和CBZ的保留时间分别为1.9 min和3.8 min。
-
拟一级动力学模型(PFOM)和拟二级动力学模型(PSOM)被广泛的应用于有机污染物在碳材料上的吸附动力学的研究领域。Pan等[14]对大量文献的数据以及污染物在碳纳米管上的吸附动力学数据进行了比较和分析,对PSOM进行了修正,将PSOM模型的速率常数(k2a,h−1·mg−1·kg)改为了新的速率常数(k2a*=k2aQa),修正后的速率常数k2a*更适合分析动力学的过程。
通过Origin软件对SMX和CBZ在3种碳基材料上的吸附动力学曲线进行PFOM和改进后的PSOM拟合,动力学方程如下[15]:
拟一级动力学方程(PFOM):
拟二级动力学方程(PSOM):
式中,Qt是时间为t时的固相浓度(mg·kg−1),Qa为吸附平衡时的固相浓度(mg·kg −1),k1a和k2a分别为PFOM和PSOM吸附速率常数(h−1),k2a*为修正后的PSOM速率常数(h−1)。
1.1. 实验材料和表征
1.2. 吸附动力学实验
1.3. 数据处理
-
碳基材料一般具有较强的疏水性、较大的比表面积、发达的孔隙结构和丰富的表面官能团等特点[8]。本研究选用的3种碳质材料AC、SC和GO的性质差别较大,可以为SMX和CBZ的吸附提供不同的吸附位点。在前期工作中对3种吸附剂做了多种表征,用元素分析仪对吸附剂的C、H、N、S、O元素含量进行测定;用BET的方法(N2模式)测定吸附剂的比表面积(SA)、孔体积(TPV)和孔径,测定结果[16-17]见表2。
如表2所示,通过分析吸附剂的元素组成可知,3种吸附剂中SC的C含量是最高的,为96.30%,而AC和GO的C含量分别为71.50%和67.10%。但是AC和GO的O含量高于SC(2.15%),说明AC和GO的表面含有大量的含氧官能团。从BET的表征结果可知,3种吸附剂的比表面积的大小为AC>SC>GO的,且AC的比表面积是最大的,约是SC和GO的3倍。且AC含有丰富的孔隙,其孔体积为0.194 cm3·g−1,而SC和GO的孔体积是相近的,分别为0.061 cm3·g−1和0.058 cm3·g−1。
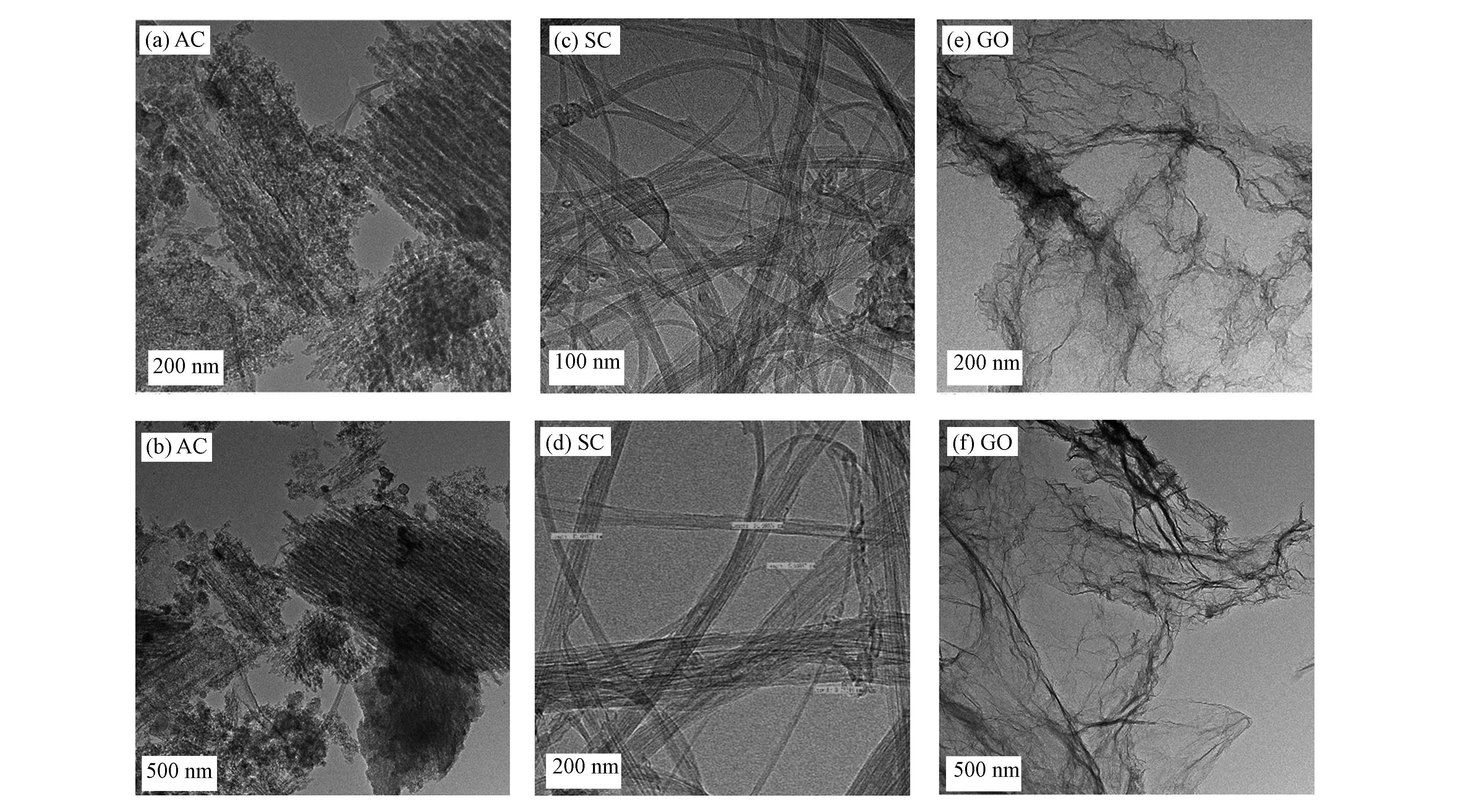
图1是3种吸附剂的TEM图.从AC的TEM图中可以看出,AC具有相对整齐的结构,孔隙结构发达,较大的比表面积可以为吸附提供了较多的吸附位点。从图1(d)中可以看出,SC的直径在几到十几纳米之间,说明是单壁碳纳米管,且单SC是连续光滑的,而且大多数SC是管与管之间紧靠成束装的。由图1(e、f)可知,GO呈透明薄纱状片层结构,且有明显的褶皱,GO表面或者边缘的褶皱提供了很好的吸附条件。
-
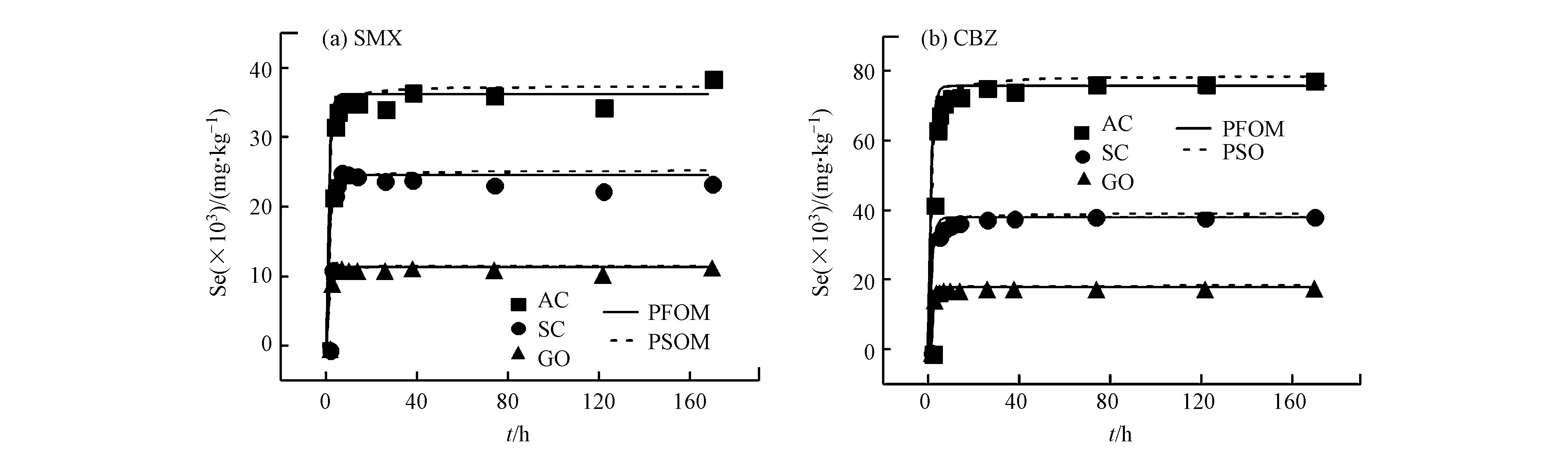
如图2所示是以吸附时间(t,单位为h)为横坐标,固相浓度(Se,单位是mg·kg−1)为纵坐标的3种碳基材料吸附单一SMX和CBZ的吸附动力学曲线。经过7 d的恒温震荡,所有碳基吸附剂对PPCPs的吸附均达到平衡。所有的碳基材料对SMX和CBZ的吸附过程呈现的都是先快后慢的趋势。SMX和CBZ的吸附量在12 h内快速的增加,随着时间的延长,吸附速率逐渐减慢,在24 h后基本达到平衡。这是因为随着吸附时间的增加,碳质吸附材料上的吸附位点在不断减少,从而导致吸附速率下降直至平衡。但是对于SMX和CBZ在不同的吸附剂上其吸附速率也会有所差别。
为了进一步研究吸附剂对SMX和CBZ的吸附机理和速率控制过程,采用PFOM和改进后的PSOM拟合SMX和CBZ的动力学数据并用吸附动力学速率常数来进行比较,拟合结果如表3所示。由表3拟合的结果可以看出,对于SMX和CBZ来说,PFOM和PSOM都可以较好的拟合吸附剂对吸附质的吸附,但是从R2adj的值来看,PFOM拟合的结果略优于改进后的PSOM。
从表3中可以看出,在3种吸附剂上CBZ的平衡吸附量Qa均高于SMX,CBZ与SMX相比,CBZ具有较高的Kow和较低的溶解度,它有着很强的疏水作用[18],疏水作用可能是导致CBZ的平衡吸附量高于SMX的原因之一。而且由于SMX和CBZ均含有苯环,可能会与碳质材料的表面形成较强的π-π作用,但是CBZ有2个苯环,SMX含有1个苯环,说明CBZ与吸附剂之间存在着较强的π-π作用。所以说CBZ在吸附剂上的平衡吸附量Qa高于SMX可能是由于CBZ的强疏水作用和较强的π-π 作用引起的。从PFOM的拟合结果来看,SMX和CBZ在3种吸附剂上的吸附速率常数顺序均为GO>AC>SC,而平衡吸附量则是AC>SC>GO。吸附速率与平衡吸附量之间并没有明显的相关性。在动力学研究中吸附速率可以代表吸附位点的可及性,表现出特定分子接触吸附位点的难易程度,而平衡吸附量又代表了吸附位点的有效性。比表面积被认为是影响吸附质在碳材料上的吸附速率主要原因[14]。即吸附速率与碳吸附材料的比表面积成正比,比表面积越大,吸附速率越快。但在本研究中,并没有发现有这一现象。AC的比表面积是SC和GO的比表面积的3倍,但是SMX和CBZ在AC上的吸附速率K1a分别为0.983 h−1和0.852 h−1,远低于GO。因此,比表面积不是影响这3种碳基材料吸附速率的主要因素。
刘伟等[19]认为,孔是吸附质分子在吸附剂中吸附驻留的地方,而且吸附质分子能否进入到吸附剂的孔中,与吸附剂孔的大小、吸附质分子的大小等有关。SMX和CBZ属于小分子的物质,而且王驰[20]根据高斯软件计算了SMX和CBZ的分子体积,SMX和CBZ的分子体积分别为0.246 nm3和0.166 nm3;也对SMX和CBZ进行了Polanyi-Manes模型拟合得到其饱和吸附量,发现SMX和CBZ的吸附量是大于理论的吸附量,在单一吸附体系中,AC、SC和GO的饱和吸附体积分别是0.414、0.198、0.378 cm3·g−1,但是3种吸附剂的孔体积为0.194 cm3·g−1,SC和GO的孔体积分别是0.058 cm3·g−1和0.061 cm3·g−1。所以孔隙填充机理(属于物理吸附)不是影响速率的主要原因。而且从表3的拟合结果中可以看出,SMX和CBZ在碳吸附材料上的吸附速率是GO>AC>SC,而三者吸附容量的大小顺序为AC>SC>GO。这就说明AC丰富的孔隙结构给SMX和CBZ提供了大量的有效吸附位点,但同时这些孔隙位点不容易进入,SMX和CBZ进入孔隙需要一定的时间,造成了吸附速率的下降。从表2的元素分析结果可知,AC和GO含氧量较多,SC的含氧量最少,说明GO和AC表面含有大量的含氧官能团,表面的含氧官能团又会对碳基材料的疏水性有巨大的影响。孟冠华等[21]认为含氧官能团能使AC的亲水性增加,水分子通过与含氧官能团之间的氢键作用吸附在AC上,阻止了有机物向AC上微孔的扩散,从而影响有机物在AC上的吸附速率。
对于GO来说,其表面也含有丰富的含氧官能团,而且对比其他两种吸附材料,SMX和CBZ在GO上的吸附速率是最快的。Wang等[22]认为,GO是具有3种主要的吸附位点:平整的表面位点、含氧官能团的边缘位点以及折叠形成的褶皱,褶皱的夹角通常比较小很难被吸附质分子吸收。由于CBZ含有苯环,CBZ则是主要是通过π-π作用吸附在GO表面;由于本实验是在中性的环境中进行的,从表1的SMX和CBZ的pKa中可以看出,SMX在中性的环境中会有微弱的电离,因此SMX与吸附剂之间还有微弱的静电作用,也能够与GO上的含氧官能团形成较强的氢键作用。所以SMX可能是通过静电辅助氢键作用(CAHB)吸附在GO表面[17],因此GO的平面结构可能是两种物质吸附速率较快的主要原因。
在3种碳质材料中,SMX和CBZ在SC上的吸附速率最低。SC形貌上可以看到管与管之间紧靠成束状存在,管壁与管壁之间形成了夹角和缝隙,SMX和CBZ分子到达这些吸附位点需要一定的时间,这可能是造成SMX和CBZ在SC上的吸附可及性较低的原因。
-
在自然的环境中,一般都是多种污染物共存的,相同或不同种类的抗生素也会发生相互影响,因此研究多种污染物共存所发生的作用具有重要的意义。本实验进一步讨论了两种污染物同时存在时,碳质吸附材料对SMX和CBZ的吸附速率的变化。
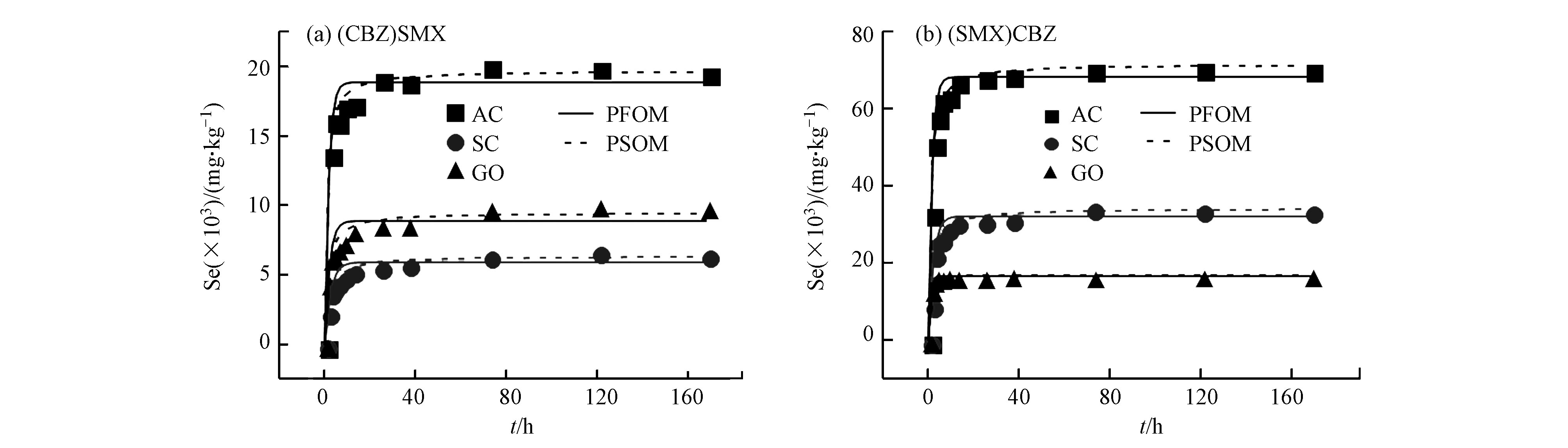
在共吸附体系中,SMX和CBZ的共吸附体系的动力学曲线均低于单一吸附体系(图3),表明SMX和CBZ的吸附都受到竞争物质的抑制。CBZ对SMX吸附的抑制更为显著,进一步表明3种碳质材料对CBZ有更高的吸附能力。为了进一步了解控制吸附速率的过程,单一吸附体系和共吸附体系中的吸附曲线通过PFOM进行了拟合,拟合结果见表4。存在CBZ竞争的情况下,SMX在3种吸附剂上的吸附动力学曲线出现了快速吸附而后逐渐下降的趋势,这一现象导致PFOM模型无法很好的描述该动力学过程。这个现象可能是由于SMX和CBZ主要竞争位点为吸附剂表面位点[23-25],SMX先快速的吸附在吸附剂表面上,对CBZ形成空间位阻,导致CBZ的竞争效应推迟,从而SMX的吸附出现了先快速增加而后下降的趋势。在单一吸附体系中,SMX就表现出了更快的吸附速率也可以说明这一问题。从CBZ在共吸附体系中的吸附动力学拟合结果中可以看出,SMX的加入导致CBZ的吸附速率降低,这也进一步证实了我们的推测。SMX为竞争物质时,CBZ在3种吸附剂上的吸附速率顺序为GO>AC>SC,与CBZ单一吸附体系的顺序一致。
-
竞争吸持实验就是先加入竞争物质,在摇床振荡1周后,竞争物质达到了吸附饱和,然后离心10 min,取出90%的上清液,然后再加入相同量的主要吸附物质和背景液,当主要吸附物质达到吸附饱和后,同样需要离心,离心以后取出上清液,用0.45 μm过滤头过滤后检测其含量。竞争吸持实验主要考察的是竞争物质在吸附剂上的吸持对主要物质吸附动力学的影响。用PFOM模型和PSOM模型拟合竞争吸持的动力学数据,拟合结果见表5。从表5和图4的竞争吸持动力学拟合结果来看,PFOM的可调可决系数R2adj在0.897—0.998之间,PSOM的可调可决系数R2adj在0.964—0.994之间,说明PSOM更符合SMX和CBZ在碳质吸附材料上的竞争吸持动力学。单一体系和共吸附体系中,SMX和CBZ符合PFOM模型,而其在竞争吸持体系中符合PSOM,这表明不同的竞争过程导致SMX和CBZ在不同的体系中符合不同的吸附动力学模型。
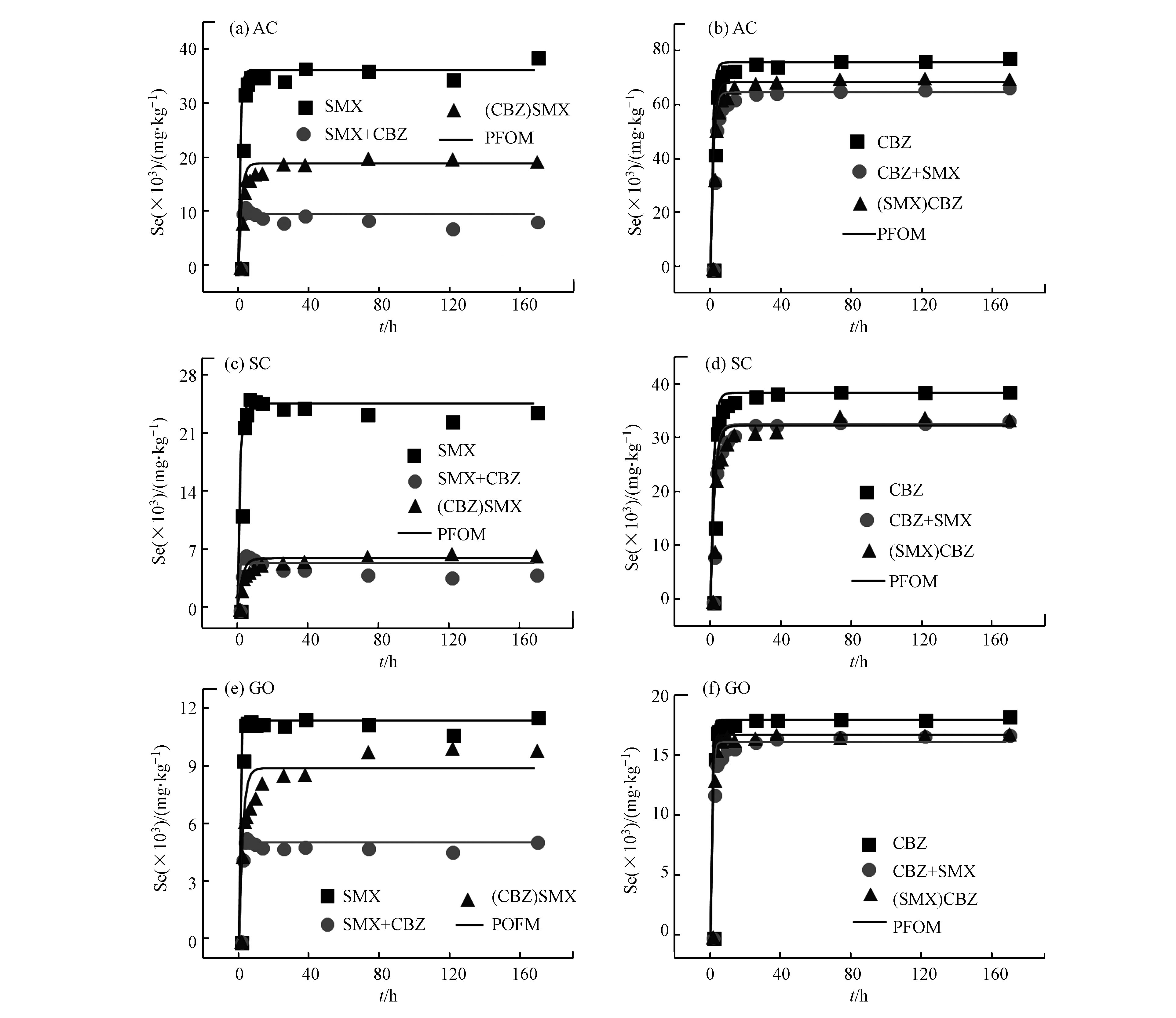
为了进一步地研究竞争吸持对碳质材料吸附SMX和CBZ的速率影响,图5比较了SMX和CBZ在单一吸附、共吸附和竞争吸持体系吸附动力学曲线。为了便于数据的比较,均用PFOM模型的数据进行比较的。通过比较3个体系的拟合结果可以看出,对于SMX和CBZ来说,无论是对比单一吸附还是共吸附体系,在同种吸附剂上竞争吸持体系的吸附速率都是最低的,但吸附容量的顺序为单一吸附>竞争吸持>共吸附体系(SC吸附CBZ体系除外)。这表明固体表面吸持的竞争物质形成的空间位阻会降低主要物质的可及性,同时污染物占据了吸附剂的共用位点也降低了主要物质的吸附容量。而共吸附体系中的液相中的竞争物质可能通过溶剂效应与主要物质形成静电作用,抑制了主要物质向吸附剂表面的迁移,导致共吸附体系中吸附量的降低。在竞争吸持体系中,CBZ作为竞争物质时对SMX吸附速率的影响高于SMX作为竞争物质,这可能是由于与SMX分子相比CBZ的分子结构更接近平面结构,对SMX分子造成的空间位阻更大。
2.1. 碳基吸附剂的性质表征
2.2. 单一吸附体系的吸附动力学曲线
2.3. 共吸附体系的吸附动力学曲线
2.4. 竞争吸持的吸附动力学曲线
-
本文研究了SMX和CBZ在3种碳质材料上的竞争吸附动力学,重点考察了吸附剂的性质和竞争物质加入顺序对吸附动力学的影响。主要结论如下:
(1)单一吸附体系中,SMX和CBZ在3种吸附剂上的吸附动力学符合PFOM动力学模型。SMX和CBZ在吸附剂上的吸附速率常数大小顺序均为GO>AC>SC。GO的平面结构有利于两种药物的快速吸附。且SMX和CBZ在碳质材料上的吸附是物理吸附和化学吸附共同作用的。
(2)在共吸附体系中由于竞争物质在吸附剂表面形成空间位阻,两种药物的吸附速率均低于单一吸附体系,且SMX的吸附会出现先升高后降低的现象。由于具有强的疏水作用和π-π作用,CBZ有更高的吸附能力和竞争能力。
(3)竞争吸持体系中两种药物的吸附速率远低于单一吸附和共吸附体系,且动力学曲线更符合PSOM模型。通过比较竞争吸持体系与共吸附体系的动力学过程,我们认为先加入的竞争物质吸附在固体会形成更强的空间位阻,降低吸附速率,但共吸附体系中液相中的竞争物质对吸附容量的抑制贡献更大。
(4)共吸附和竞争吸持两种竞争过程均会导致吸附剂对主要物质的吸附量减少且吸附速率降低,也就是说增加了主要物质的流动性和迁移性,不利于控制其环境风险。



 下载:
下载: