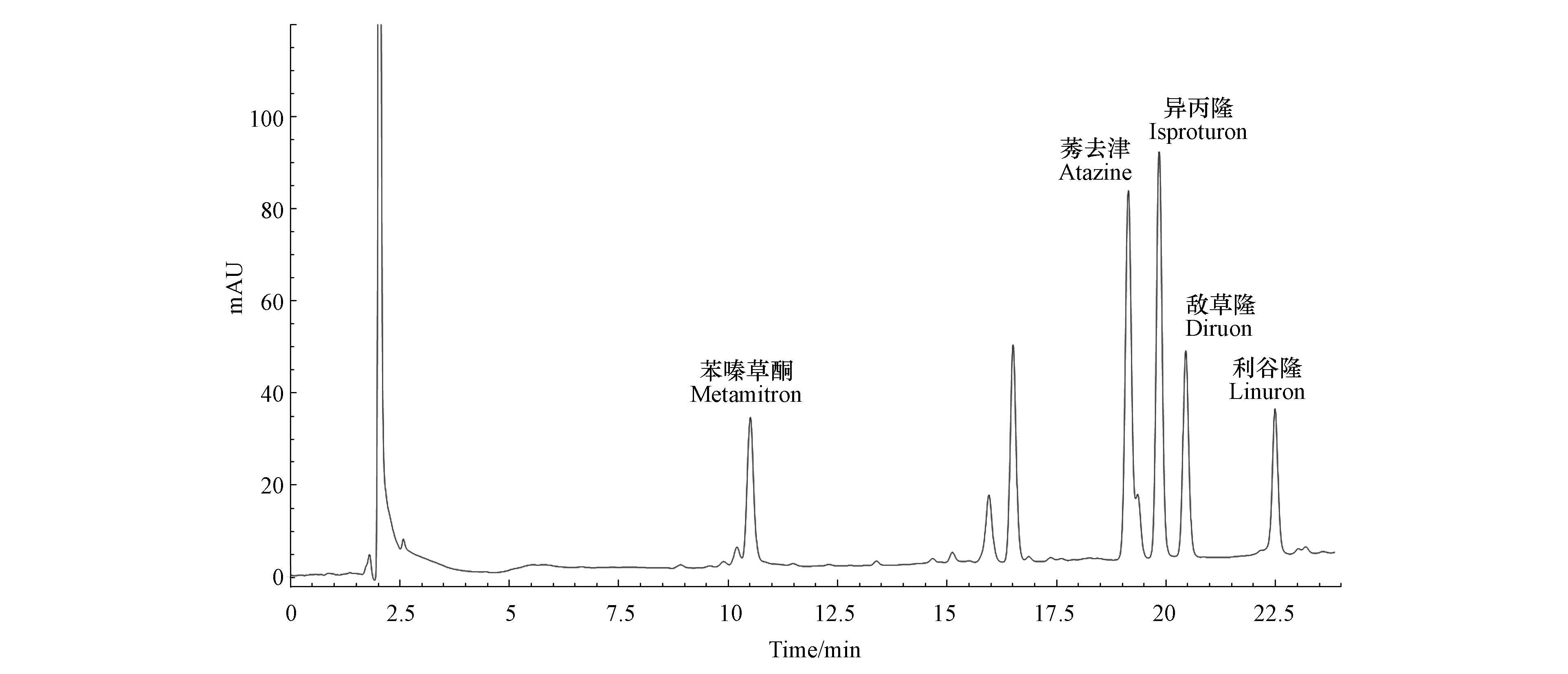
-
磁性固相萃取[1]作为一种新型的前处理技术,其对分析物的萃取是利用磁性或磁性修饰材料进行富集,利用外部磁场和基质样品进行分离,不需要过滤除杂,使分离更简单、更快速[2]。离子液体是近年来发展起来的一种对环境友好的绿色溶剂,由于其黏度较大,大部分溶于有机溶剂或者水,不易于与样品分离,因而将离子液体固着于金属或键合于硅胶、聚酯纤维表面作为吸附材料,用于一些有机污染物的去除和分析前处理方法中[3-4]。
石墨烯形成的磁性材料具有巨大的比表面积,强顺磁性,在分析领域受到广泛关注,在污染物分析中应用于水样中氨基甲酸酯类杀虫剂、酰胺类除草剂、烟碱类杀虫剂、三唑类杀菌剂和多环芳烃、邻苯二甲酸酯的富集[5-11]。离子液体可以通过选择不同的阴、阳离子改变其极性和性能以及对目标分析物的溶解性。因此,将离子液体和磁性石墨烯联合使用,大大地拓展了二者在分析领域的应用。Cao[12]和Zhang等[13]用离子液体和磁性石墨烯复合物作为吸附剂用于对水中硝基苯和植物油中PAH的富集,Chen等[14]用聚合离子液体磁性石墨烯QuEChERS方法萃取了蔬菜中的防腐剂,Wu和Cai等[15-16]用离子液体包被磁性氧化石墨烯萃取了生物样品中的头孢菌素和水中的氯酚类物质,Liu等[17]用化学共沉淀法合成离子液体磁性石墨烯富集水中的藻毒素,Zhang等[18]用离子液体修饰磁性石墨烯,增强了对水中As3+和As5+的去除性能。也有报道使用离子液体功能化磁性石墨烯/聚吡咯富集尿液中的甲氨蝶呤[19],用离子液体改性二硫化钼和还原石墨烯氧化物磁性纳米复合材料用于分离染料[20],离子液体和环糊精功能化的磁性石墨烯氧化物高效富集植物生长调节剂[21]。而离子液体和磁性石墨烯联合用于富集环境水中的除草剂尚鲜见报道。
综上所述,离子液体用于包被磁性氧化石墨烯大多采用极性较弱的烷基咪唑类离子液体,对于极性较强的物质富集能力较差。本文选择三嗪类、脲类极性较强常用除草剂作为研究对象,采用羟基化离子液体包被磁性氧化石墨烯作为富集材料,建立环境水中除草剂磁性固相萃取残留分析方法,为环境水中农药污染物提取和分离提供技术参考。
-
甲醇、乙腈、乙醇(色谱纯,美国Mreda)、磁性氧化石墨烯(南京先锋纳米科技有限公司)、无水氯化钠(北京化学试剂公司提供)、5种农药标准品(苯嗪草酮、莠去津、异丙隆、敌草隆、利谷隆)的纯度均大于98%(中国标准物质有限公司)、1-羟基己基-3甲基咪唑溴盐(HFIL)(上海成捷化学有限公司)。
-
安捷伦1260HPLC系统(配有DAD二极管阵列检测器)。TDL-50B低速台式离心机(上海安亭科学仪器厂),WD-9415E型超声波清洗器(北京市六一仪器厂),傅里叶红外光谱(Thermo Scientific)用于离子液体包被磁性氧化石墨烯材料的红外光谱扫描测定。
-
色谱柱:Agilent Zorbax Eclipse XDB-C18柱(4.6 mm×150 mm,5.4 µm)。甲醇和水作为流动相;检测波长:230 nm。采用梯度洗脱进行分离,0 min,80%水;10 min,50%水;25 min,20%水;28 min,10%水;30 min,80%水。流速1 mL·min−1。进样量20 μL。
-
称取6 mg的磁性石墨烯和2 mg的离子液体混合于玻璃试管中,加入2 mL添加除草剂的水溶液,超声10 min,离子液体会吸附到磁性石墨烯上。在玻璃管底部加一块强磁性磁铁,使固相吸附剂从混合溶液中分离,沉到试管底部。吸去上层水,加入300 µL的乙醇,超声解吸5 min,将磁性石墨烯吸附的除草剂洗脱下来,然后外加磁铁进行固液分离,将上清液经滤膜过滤到自动进样瓶中进行液相色谱检测。
-
为了验证离子液体是否能够成功地包被磁性氧化石墨烯表面,采用FTIR进行扫描测定。红外光谱如图1,光谱之间有许多吸收信号相互匹配,如典型峰位在1045 cm−1左右,对应于C—O的特征波段。包覆Fe3O4/GO 的光谱中出现2930、2857 cm−1的C—H波段伸缩振动和3330 cm−1左右O—H波段振动,表明1-羟基己基-3甲基咪唑溴盐成功地包覆在Fe3O4/GO表面。
-
为了优化萃取条件,在2 mL水中添加分析物混合标样,使添加浓度为50 μg·L−1。改变其中的条件之一,其它条件不变,对影响富集效率的主要因素进行优化,如HFIL和Fe3O4/GO的比例、萃取时间、解吸时间、解吸剂种类、盐浓度等。添加回收率(ER)用下列公式计算:
式中,CS、C0、V0、Vs分别表示测试浓度、水中初始添加浓度、解吸液体积、水样的体积。
-
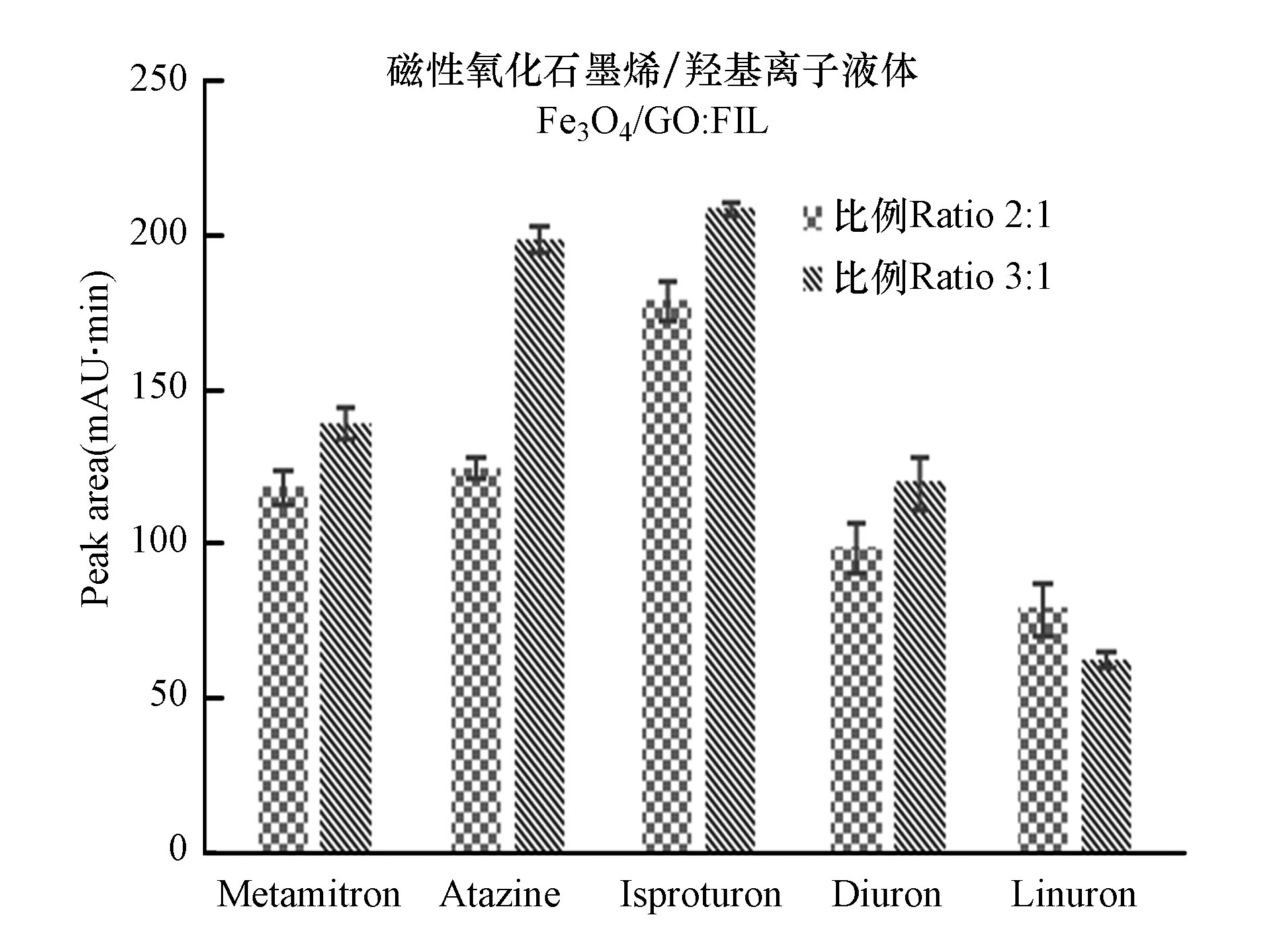
在萃取过程中,磁性氧化石墨烯与离子液体的配比是本方法中主要影响因素,因为半胶束和胶束均是在Fe3O4/GO表面形成的,两者之间是靠疏水作用、静电吸引和π-π相互作用。通过加入Fe3O4/GO 6 mg、离子液体分别为3 mg、2 mg,磁性氧化石墨烯和离子液体分别为2∶1、3∶1两种不同比例的混合物对5种除草剂萃取效率的影响进行比较。图2显示,磁性氧化石墨烯与离子液体的比例不同时,对萃取效率有着很显著的影响,当比例为3∶1时峰面积明显高于2∶1,离子液体含量增加萃取效率降低,可能是由于2 mg时胶束已经形成。随着离子液体含量的增加,分析物在溶液中重新分配。因此使用磁性氧化石墨烯与离子液体的比例3∶1进行以下实验。
-
有机溶剂很容易破坏半胶束结构,解吸溶剂种类和体积对于从吸附剂中有效解吸分析物至关重要。因此首先选用甲醇、乙醇、乙腈各200 µL进行解吸附。结果如图3(a)所示,用乙醇作为解吸剂时,5种农药的响应值较高,萃取效果较好。然后对解吸剂体积200—500 µL进行解吸效率对比,结果如图3(b)所示,300 µL解吸效果较好。同时对解吸时间3、5、8 min进行优化,5 min已经能够完全解吸。
-
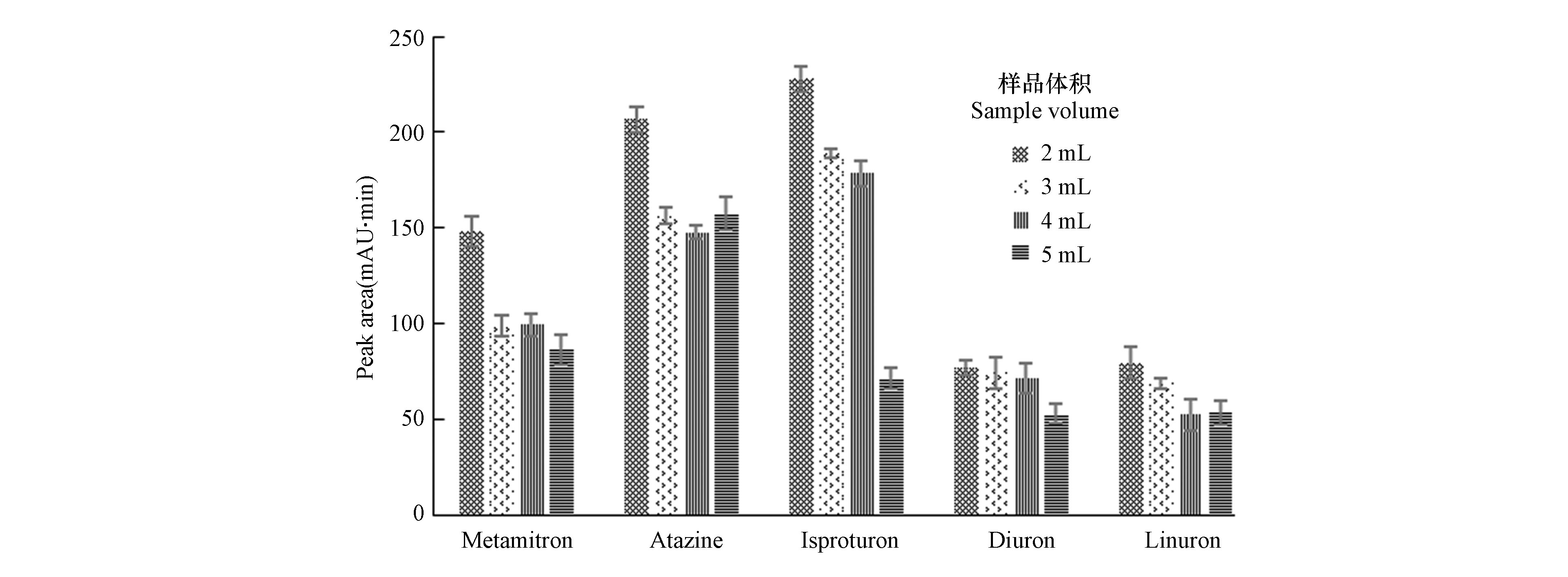
样品水体积和富集材料比例对于分析物的提取效率影响较大。本研究在上述优化条件吸附剂固定的情况下(即6 mg Fe3O4 /GO+2 mg HFIL),分别对2、3、4、5 mL水样添加相同浓度除草剂进行萃取,解吸后用乙醇稀释,使进样浓度相同。结果如图4所示,随着样品水的体积的不断增加,离子液体磁性氧化石墨烯不足以支持萃取导致峰面积逐渐减小。因此水样体积为2 mL时,萃取效果最佳。
-
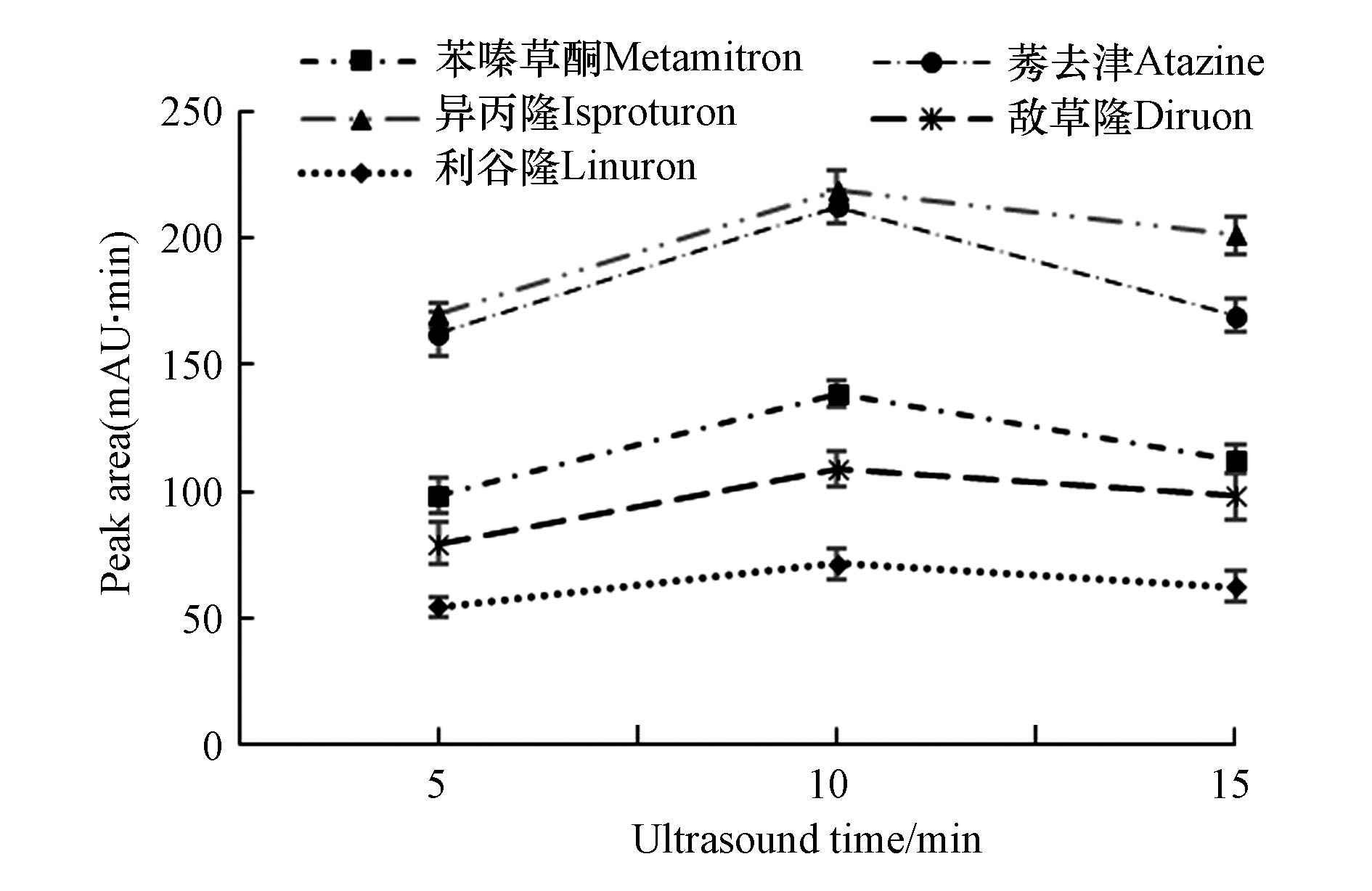
超声处理可以加快分析物的传质速度,在固液两相间达到平衡。选择在超声时间5—15 min内对萃取效果的影响进行比较,结果显示图5,在超声时间为5 min时,由于时间过短,分析物没有完全吸附,而达到平衡,5种农药的峰面积最低。当时间为10 min时,峰面积响应最高。当时间为15 min时有所下降,可能由于超声产生多余热量使离子液体脱落,分析物吸附率降低。故选择超声时间为10 min。
-
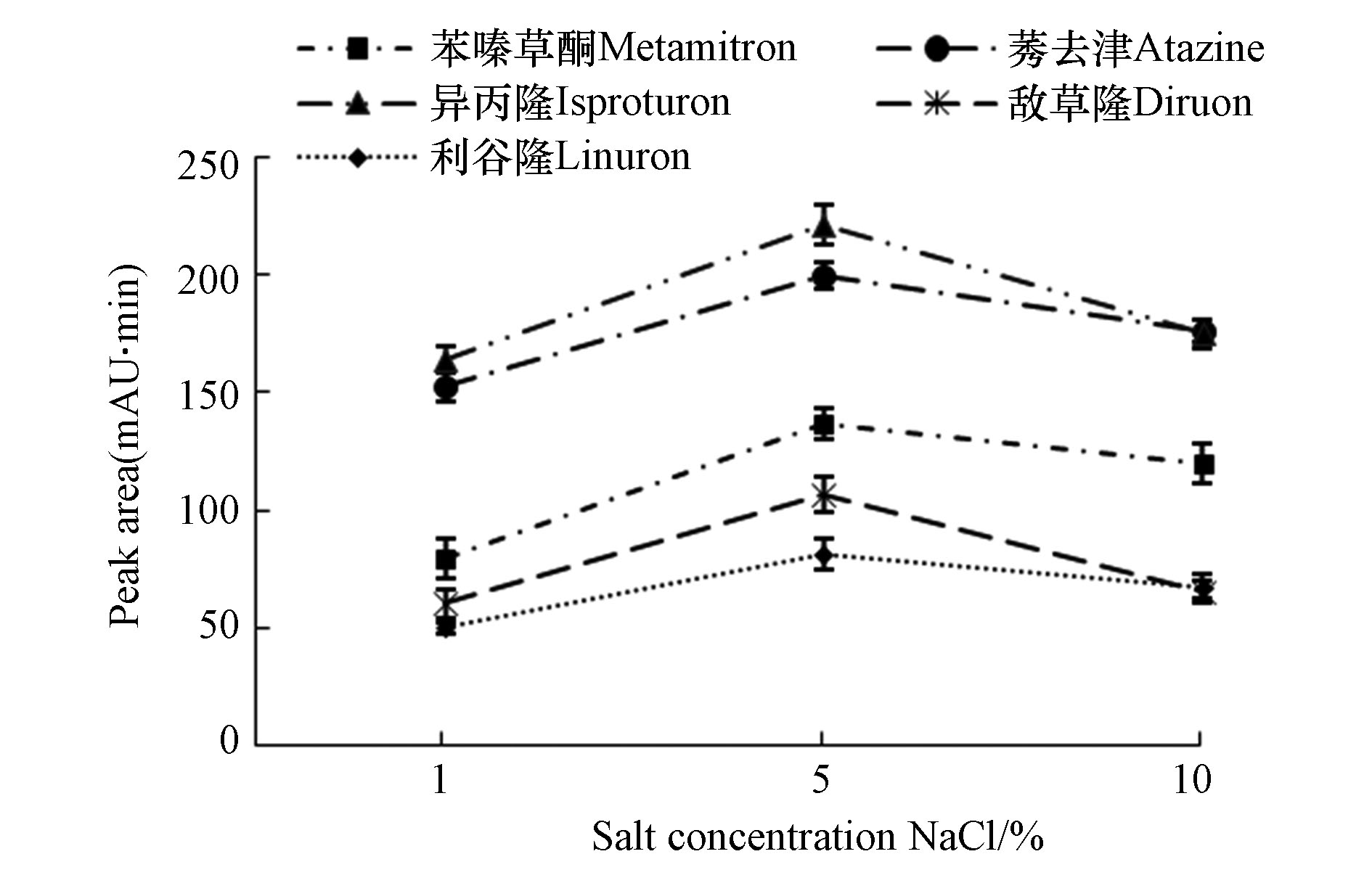
添加盐通常会降低有机化合物在水中的溶解度,有利于提高分析物的提取效率。在水中添加NaCl,形成1%、5%、10% NaCl水溶液,进行影响萃取效果比较研究。结果如图6所示,5% NaCl的水溶液,5种农药的峰面积最大,萃取效果较好。
-
为验证方法的可行性和可靠性,对该方法的线性、回收率、精密度、检出限、定量限进行评价,结果如表1所示,在10—500 µgL−1范围内线性良好,线性相关系数均在0.9955—0.9987之间。添加浓度为50 μg·L−1时,5个重复的平均回收率为81.2%—90.2%。相对标准偏差为1.9%—3.9%;最小检出限以最低线性浓度3倍信噪比计算为0.036—0.053 μg·L−1,定量限以10倍信噪比计算为0.12—0.18 µg·L−1.
-
为了验证该方法的适用性,将该方法应用于所采不同环境水样(田间水、河水和地表水)进行除草剂含量测定,均未检出目标分析物,为了确定基质效应对该方法的影响,分别在3种环境水样中添加50、100、500 µg·L−1的3种浓度进行回收率实验。结果见表2,回收率在78.9%—90.9%之间,相对标准偏差在3.0%—9.3%之间。结果表明,基质对萃取效果无显著影响,该方法对测定实际水样中除草剂方法具有有效性,可行性,田间水样品色谱图如图7,因此该方法能够适用于环境水样中5种除草剂富集和分析。
-
本研究采用羟基咪唑离子液体包被磁性氧化石墨烯胶束磁性固相萃取高效液相色谱测定环境水中的除草剂,该方法的检出限均低于SPME[23]和分散液液微萃取[24-25]与SPE[22]相近,但是比SPE需要样品量少,萃取时间短,洗脱溶剂少(表3)。这些结果表明,该方法快速、重复性好、操作简便,安全环保,可用于环境水样除草剂的富集和分析。
-
本文采用羟基功能化离子液体半胶束磁性氧化石墨烯固相萃取的方法,对水中的三嗪类和脲类5种除草剂进行萃取、富集一步完成,快速、简单、减少了有机溶剂的使用,与传统方法固相萃取相比,既避免了反复萃取又避免过SPE小柱费时,既环保又节约成本。试验对磁性固相萃取的条件如磁性氧化石墨烯与离子液体的质量比、萃取时间、解吸条件、离子强度等进行了优化。在最佳条件下,5种除草剂的平均回收率在81.2%—90.2%之间,相对标准偏差为1.9%—3.9%,最小检出限为0.036—0.053 μg·L−1,定量限为0.12—0.18 µg·L−1,并将该方法用于实际样品的检测,基质效应没有明显影响,适用于环境水中除草剂的萃取和检测。
羟基离子液体磁性氧化石墨烯混合半胶束磁性固相萃取高效液相色谱测定环境水中的除草剂
Mixed hemimicelles magnetic solid-phase extraction based on hydroxyl ionic liquid-coated magnetic graphene oxide for the determination of herbicides in enviormental water samples coupled with high-performance liquid chromatography
-
摘要: 本文建立了羟基离子液体磁性氧化石墨烯混合半胶束磁性固相萃取高效液相色谱测定环境水中除草剂的分析方法。利用具有大的表面积和较强的吸附能力的羟基功能化离子液体1-羟己基-3-甲基咪唑溴盐(HFIL)包被磁性氧化石墨烯作为磁性固相萃取吸附材料,富集环境水的除草剂。对萃取效率的一些影响因素进行优化,如磁性氧化石墨烯与离子液体的质量比、萃取时间、解吸条件、离子强度等,从而选择最佳萃取条件。在最佳条件下,5种除草剂的平均回收率在81.2%—90.2%之间,相对标准偏差为1.9%—3.9%。浓度在10—500 μg·L−1范围内线性良好,相关系数在0.9955—0.9987之间,最小检出限为0.036—0.053 μg·L−1,并且将建立的方法应用于实际环境水样5种除草剂的测定。Abstract: In this study, a novel mixed hemimicelles magnetic solid phase extraction method based on hydroxyl ionic liquid-coated magnetic graphene oxide (Fe3O4/GO) was developed for the simultaneous extraction and determination of herbicides in enviormental water samples coupled with high-performance liquid chromatography. Fe3O4/GO after modification with 1-hydroxyl hexyl-3-methylimidazoliumbis bromide (HFIL) has high surface area and excellent adsorption capacity, which were used as magnetic solid-phase extraction (MSPE) adsorbent to enrich herbicides in environmental water samples. The factors affecting the extraction efficiency such as the ratio of the amout of Fe3O4/GO and ionic liquid, extraction time, desorption conditions and ionic strength were investigated to select the best extraction conditions. Under optimal conditions, the recoveries of five target compounds were obtained ranging from 81.2% to 90.2% and the relative standard deviations(RSDs, n=5) were 1.9%—3.9%. Linearities were achieved in the range of 10—500 μg·L−1 with correlation coefficients ranging from 0.9955 to 0.9987. The limits of detection were in the range of 0.036—0.053 μg·L−1. The method was successfully applied to the determination of the target five herbicides in the environmental water samples.
-

-
表 1 线性、相关系数 (R2)、回收率、LOD和LOQ
Table 1. The linearity, correlation coefficients (R2), LOD and LOQ
分析物
Analyte回归方程
Regression equation相关系数
Coefficient of determination(R2)RSD/% 平均添加回收率
Recovery/%检出限
LOD/(µg·L−1)定量限
LOQ / (µg·L−1)苯嗪草酮
Metamitrony=92.643x+27.154 0.9970 3.9 86.3 0.053 0.18 莠去津
Ataziney=87.747x+18.523 0.9987 3.2 81.2 0.042 0.14 异丙隆
Isproturony=73.468x+21.17 0.9974 1.9 86.2 0.036 0.12 敌草隆
Diurony=75.585x+21.706 0.9975 2.6 89.2 0.048 0.16 利谷隆
Linurony=36.875x+12.829 0.9955 3.4 90.2 0.051 0.17 表 2 3种实际水样中3种浓度添加回收率和RSD
Table 2. Recovery and RSD of three concentrations spicked in three real enviormental water
除草剂Herbicide 添加水平Fortified/(μg·L−1) 河水(River water) 田间水(Feild water) 地表水(Suface water) 回收率Recovery/% RSD/% 回收率Recovery/% RSD/% 回收率Recovery/% RSD/% 苯嗪草酮Metamitron 50 80.3 8.7 76.9 5.8 82.7 4.3 100 83.8 6.5 80.9 9.3 80.8 7.9 500 79.2 3.9 81.8 6.7 78.9 5.9 莠去津Atazine 50 87.6 6.6 88.6 3.0 85.5 3.7 100 80.3 7.5 84.7 4.9 82.0 5.8 500 81.8 5.0 80.0 5.5 79.4 6.2 异丙隆Isproturon 50 86.2 3.8 82.3 6.2 83.9 8.4 100 85.3 9.3 83.0 8.0 81.2 9.6 500 79.2 6.7 80.4 6.4 82.7 4.1 敌草隆Diuron 50 80.3 8.8 83.1 3.1 80.1 6.0 100 84.6 6.4 88.8 6.9 87.3 6.7 500 90.9 4.2 86.4 5.6 88.7 8.9 利谷隆Linuron 50 90.6 8.0 91.7 7.1 88.1 6.3 100 87.5 4.9 88.3 6.4 84.3 5.8 500 86.2 6.4 82.5 2.8 85.1 7.5 表 3 本方法与其它方法比较
Table 3. Comparison of the proposed method with other methods for the determination of hebicides
方法
Method样品体积
Sample volume/mL萃取时间
Extraction time /min线性范围
Linear range/(µg·L−1)回收率
Recovery /%相对标准偏差
RSD/%检出限
LOD/(µg·L−1)本方法 2 10 10—500 81.2—90.2 1.9—3.9 0.036—0.053 固相萃取(SPE)[22] 500 >60 0.05—2 70—90 5—20 0.021—0.042 固相微萃取SPME[23] 3 40 5—1000 85—113 2.4—5.9 0.5—5.1 分配分散液液微萃取(PDLLME)[24] 5 Few seconds 0.5—100 91—104 0.4—5.9 0.10—0.28 分散液液微萃取(DLLME)[25] 25 10 1—6 81—102 5—10 0.19—1.12 -
[1] CHEN L, WANG T, TONG J. Application of derivatized magnetic materials to the separation and the preconcentration of pollutants in water samples [J]. Trends in Analytical Chemistry, 2011, 30(7): 1095-1108. doi: 10.1016/j.trac.2011.02.013 [2] SAJID M. Magnetic ionic liquids in analytical sample preparation: A literature review [J]. TrAC Trends in Analytical Chemistry, 2019, 113: 210-223. doi: 10.1016/j.trac.2019.02.007 [3] LAWAL I A, KLINK M, NDUNGU P, et al. Brief bibliometric analysis of“ionic liquid”applications and its review as a substitute for common adsorbent modifier for the adsorption of organic pollutants [J]. Environmental Research, 2019, 175: 34-51. doi: 10.1016/j.envres.2019.05.005 [4] SZALATYTJ, KLAPISZEWSKI L, JESIONOWSKI T. Recent developments in modification of lignin using ionic liquids for the fabrication of advanced materials: A review [J]. Journal of Molecular Liquids, 2020, 3011: 112417. [5] WU Q H, ZHAO G Y, FENG C, et al. Preparation of grapheme-based magnetic nanocomposite for the extraction of carbamate pesticides from enviormental water samples [J]. Journal of Chromatography A, 2011, 1218: 7936-7942. doi: 10.1016/j.chroma.2011.09.027 [6] ZHAO G Y, SONG S J, WANG C, et al. Determination of triazine herbicides in environmental water samples by high-performance liquid chromatography using graphene-coated magnetic nanoparticles as adsorbent [J]. Analytica Chimica Acta, 2011, 708: 155-159. doi: 10.1016/j.aca.2011.10.006 [7] WANG W N, LI Y P, WU Q H, et al. Extraction of neonicotinoid insecticides from environmental water samples with magnetic graphene nanoparticles as adsorbent followed by determination with HPLC [J]. Analytical Methods, 2012, 4: 766-772. doi: 10.1039/c2ay05734d [8] WANG W N, MA X X, WU Q H, et al. The use of graphene-based magnetic nanoparticles as adsorbent for extraction of triazole fungicides from environmental water [J]. Journal of Sepration Science, 2012, 35: 2266-2272. doi: 10.1002/jssc.201200285 [9] WU Q H, LIU M, MA X X, et al. Extraction of phthalate esters from water and beverages using a graphene-based magnetic nanocomposite prior to their determination by HPLC [J]. Microchim Acta, 2012, 177: 23-30. doi: 10.1007/s00604-011-0752-7 [10] WANG W N, MA R Y, WU Q H, et al. Magnetic microsphere-confined graphene for the extraction of polycyclic aromatic hydrocarbons from environmental water samples coupled with HPLC-fluorescence analysis [J]. Journal of Chromatography A, 2013, 1293: 20-27. doi: 10.1016/j.chroma.2013.03.071 [11] WANG W N, MA R Y, WU Q H, et al. Fabrication of magnetic microsphere-confined graphene for the preconcentration of some phthalate esters from environmental water and soybean milk samples followed by their determination by HPLC [J]. Talanta, 2013, 109: 133-140. doi: 10.1016/j.talanta.2013.02.008 [12] CAO X J, SHEN L X, YE X M, et al. Ultrasound-assisted magnetic solid-phase extraction based ionic liquid-coated Fe3O4@graphene for the determination of nitrobenzene compounds in environmental water samples [J]. Analyst, 2014, 139: 1938-1944. doi: 10.1039/c3an01937c [13] ZHANG Y, ZHOU H, ZHANG Z H, et al. Three-dimensional ionic liquid functionalized magnetic graphene oxide nanocomposite for the magnetic dispersive solid phase extraction of 16 polycyclic aromatic hydrocarbons in vegetable oils [J]. Journal of Chromatography A, 2017, 1489: 29-38. doi: 10.1016/j.chroma.2017.02.010 [14] CHEN Y L, CAO S R, ZHANG L, et al. Preparation of size-controlled magnetite nanoparticles with agraphene and polymeric ionic liquid coating for the quick, easy, cheap, effective, rugged and safe extraction of preservatives from vegetables [J]. Journal of Chromatography A, 2016, 1448: 9-19. doi: 10.1016/j.chroma.2016.04.045 [15] WU J R, ZHAO H Y, XIAO D L, et al. Mixed hemimicelles solid-phase extraction of cephalosporins in biological samples with ionic liquid-coated magnetic graphene oxide nanoparticles coupled with high-performance liquid chromatographic analysis [J]. Journal of Chromatography A, 2016, 1454: 1-8. doi: 10.1016/j.chroma.2016.05.071 [16] CAI M Q, SU J, HU J Q, et al. Planar graphene oxide-based magnetic ionic liquid nanomaterial for extraction of chlorophenols from environmental water samples coupled with liquid chromatography–tandem mass spectrometry [J]. Journal of Chromatography A, 2016, 1459: 38-46. doi: 10.1016/j.chroma.2016.06.086 [17] LIU X Y, GAO S Q, LI X Y, et al. Determination of microcystins in environmental water samples with ionic liquid magnetic graphene [J]. Ecotoxicology and Environmental Safety, 2019, 176: 20-26. doi: 10.1016/j.ecoenv.2019.03.063 [18] ZHANG M Y, MA X G, LI J, et al. Enhanced removal of As(Ⅲ) and As(Ⅴ) from aqueous solution using ionic liquid-modifified magnetic graphene oxide [J]. Chemosphere, 2019, 234: 196-203. doi: 10.1016/j.chemosphere.2019.06.057 [19] HAMIDI S, AZAMI A, AGHDAM E M. A novel mixed hemimicelles dispersive micro-solid phase extraction using ionic liquid functionalized magnetic graphene oxide/polypyrrole for extraction and pre-concentration of methotrexate from urine samples followed by the spectrophotometric method [J]. Chimica Acta, 2019, 48: 179-188. [20] NI R, WANG Y Z, WEI X X, et al. Ionic liquid modified molybdenum disulfide and reduced graphene oxide magnetic nanocomposite for the magnetic separation of dye from aqueous solution [J]. Analytica Chimica Acta, 2019, 1054: 47-58. doi: 10.1016/j.aca.2018.12.037 [21] CAO S R, CHEN J Y, LAI G Y, et al. A high efficient adsorbent for plant growth regulators based on ionic liquid and β-cyclodextrin functionalized magnetic graphene oxide [J]. Talanta, 2019, 1941: 14-25. [22] CARABIAS-MARTÍNEZ R, RODRÍGUEZ-GONZALO E, HERRERO-HERNÁNDEZ E, et al. Simultaneous determination of phenyl- and sulfonylurea herbicides in water by solid-phase extraction and liquid chromatography with UV diode array or mass spectrometric detection [J]. Analytica Chimica Acta, 2004, 517: 71-79. doi: 10.1016/j.aca.2004.05.007 [23] LIN H H, SUNG Y H, HUANG S D. Solid-phase microextraction coupled with high-performance liquid chromatography for the determination of phenylurea herbicides in aqueous samples [J]. Journal of Chromatography A, 2003, 1012: 57-66. doi: 10.1016/S0021-9673(03)01169-5 [24] CHOU T Y, LIN S L, FUH M R. Determination of phenylurea herbicides in aqueous samples using partitioned dispersive liquid–liquid microextraction [J]. Talanta, 2009, 80: 493-498. doi: 10.1016/j.talanta.2009.07.005 [25] RODRÍGUEZ-GONZÁLEZ E, BECEIRO-GONZÁLEZ E, GONZÁLEZ-CASTRO M J, et al. An environmentally friendly method for the determination of triazine herbicides in estuarine seawater samples by dispersive liquid–liquid microextraction [J]. Environmentao Science Pollution Research, 2015, 22: 618-626. doi: 10.1007/s11356-014-3383-9 -



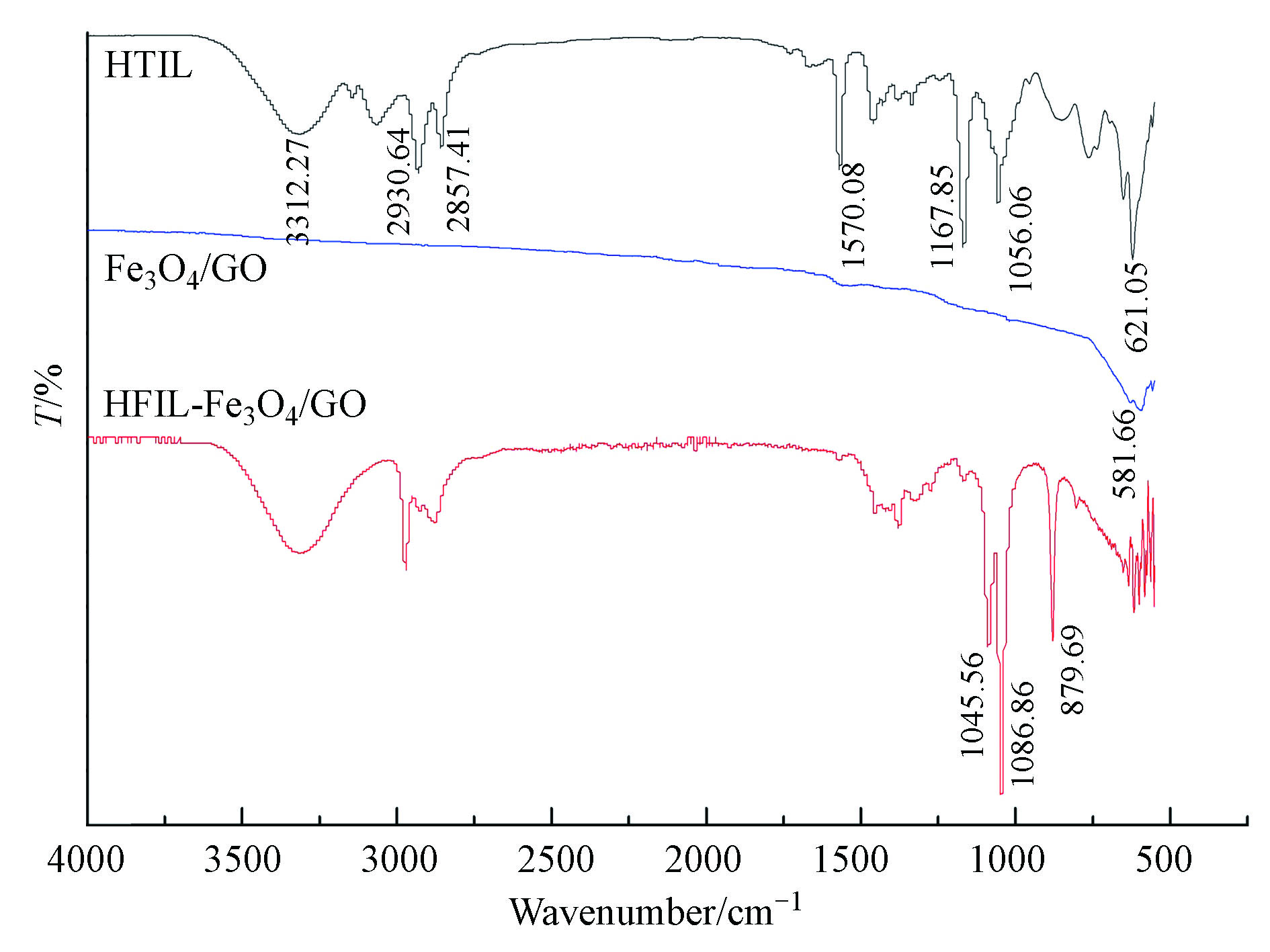
 下载:
下载: