-
随着工业技术的发展,人类文明的进步,环境问题日益成为人们关注的焦点[1-2]。大量的染料废水排入自然界,使得生态环境受到破坏,淡水资源逐渐减少,甚至威胁到人体生命健康[3]。半个多世纪前,研究人员发现二氧化钛(TiO2)可以在光激发条件下电解水产生氢气,此后,光催化材料被应用于多个领域,如二氧化碳还原、污染物降解等[4-6]。光催化是一种绿色、可靠并颇具研究价值的方法,可以利用太阳能净化环境[7],有望成为净化环境的有效途径之一[8-9]。然而,光催化材料存在光生电子与空穴易复合、导电性差等问题,通常通过掺杂、构造异质结或负载共催化剂等方式,提高半导体材料的光催化性能[5]。
过渡金属二硫族化合物(TMDs)由于其独特的光学、电学和机械性能而受到广泛关注[9]。其中,二硫化钼(MoS2)因其独特的层状结构和特性,而受到研究人员在催化、纳米摩擦学、电化学等领域的广泛研究[10-11]。近年来,三维二硫化钼纳米结构因其独特的性能和潜在的应用前景又一次引起了人们的广泛关注[12-13]。通过构建具有匹配能带结构的异质结,促进光生载流子的分离而提高异质结构半导体光催化材料活性[14]。迄今为止,为了提高MoS2的性能,人们已经合成了不同的MoS2基异质结材料,诸如MoS2/GaN,Fe3O4/MoS2,石墨烯/MoS2,CdS/MoS2,并表现出优异的光催化能力[10, 13, 15-16]。然而,MoS2的导电性较差,限制了其光生电子的传递。
二维(2D)过渡金属碳化物或碳氮化物(MXenes),由于其独特的性质被大量的合成和研究。Ti3C2具有优良的电子导电性和离子扩散能力,能够改善MoS2的导电性,促进电子的快速传输扩散,其丰富的表面氧化还原反应位点,还可以进一步提高污染物的降解性能[3]。研究发现,Ti3C2在水热反应条件下会被部分氧化生成TiO2。为方便表达,将反应后材料命名为p-Ti3C2,即复合产物为p-Ti3C2/MoS2纳米花[17]。
本研究通过添加Ti3C2提高半导体光催化材料的电子传递速率,构建p-Ti3C2/MoS2异质结复合催化材料促进电子与空穴对分离效率,首先,利用蚀刻法制备Ti3C2纳米片,其次,采用水热合成法将MoS2生长于Ti3C2纳米片上,形成被部分氧化的p-Ti3C2/MoS2异质结复合催化材料。并探究二者复合比例、光照条件以及废水pH等因素对污染物降解性能的影响。
全文HTML
-
实验所用试剂除特殊标注外均为分析纯,除Ti3AlC2外,均从上海阿拉丁生化科技股份有限公司处采购。
-
(1) Ti3C2制备
将1 g Ti3AlC2 粉末置于20 mL氢氟酸(HF)溶液(40%)中,磁力搅拌24 h以剥离Al层。然后将混合物以3500 r·min−1离心分离,用去离子水漂洗多次直至上清液的pH值达到5—6。最后将获得的湿沉淀物在80 ℃下干燥12 h后,得到部分氧化的固体p-Ti3C2。
(2) p-Ti3C2/MoS2复合材料制备
通过水热合成法将Ti3C2负载到MoS2上[18],取0.72 g 钼酸钠(Na2MoO4·2H2O)和0.69 g 硫脲(CS(NH2)2)溶于35 mL去离子水中超声处理1 h后,加入不同质量的p-Ti3C2粉末。将悬浮液转移到100 mL聚四氟乙烯内胆的高压釜中,在220 ℃条件下反应24 h。然后,对固液混合物进行离心分离,分别用蒸馏水和乙醇洗涤3次,直至上清液澄清,将产物在80 ℃下干燥12 h。最后,将得到的粉末研磨,得到不同p-Ti3C2质量分数(5%、10%、20%)的p-Ti3C2/MoS2复合材料,分别标记为TM-5、TM-10、TM-20,并通过相同的方法制得不添加p-Ti3C2的MoS2粉末。
-
采用D8 X射线粉末衍射仪对样品的晶体结构进行表征,工作电压和工作电流分别为40 kV和40 mA,扫描范围 5°—90°。采用Inspect F50扫描电子显微镜观察样品的表面形貌,CHI660E电化学工作站进行电化学性能测试,CV曲线的电位范围为−1.0—1.0 V,扫描速率为50 mV·s−1,I-t曲线的测试时间为400 s,电解质均为0.5 mol·L−1的Na2SO4溶液,在可见光照射下进行。采用Evolution 220紫外可见漫反射光谱仪测定光催化剂的紫外可见漫反射光谱(UV-Vis DRS),扫描范围为300—800 nm,BaSO4作为参比。采用JEOL 2100plus透射电子显微镜对样品组成进行表征,通过测量样品表面的晶格条纹间距,确定样品复合情况;并采用Bruker D8 ADVANCE X射线光电子分析仪确定样品中元素存在的方式。
-
通过改变光照条件探究光照对复合催化剂降解RhB的影响,选用30 W的卤素灯作为可见光源。20 mg的p-Ti3C2/MoS2复合材料加入50 mL初始浓度为10 mg·L−1的罗丹明B溶液中,黑暗条件下静置吸附30 min后,在可见光下进行降解测试。并将同等条件下吸附后的RhB溶液分别于自然光和黑暗条件下进行染料降解实验,采用UV-1901双光束紫外分光光度计测定不同降解时间的RhB溶液吸光度,分析其降解率。
选出合适的光照条件后,在同等条件下,将上述合成的4种材料分别加入罗丹明B溶液中,静置吸附30 min后,进行光催化降解,分析不同材料的降解率,并选出最优材料进行后续实验。将材料加入不同pH的罗丹明B溶液中,通过相同的实验条件探究pH对降解过程的影响。
1.1. 实验材料
1.2. 催化剂制备
1.3. 实验表征
1.4. 光催化降解实验
-
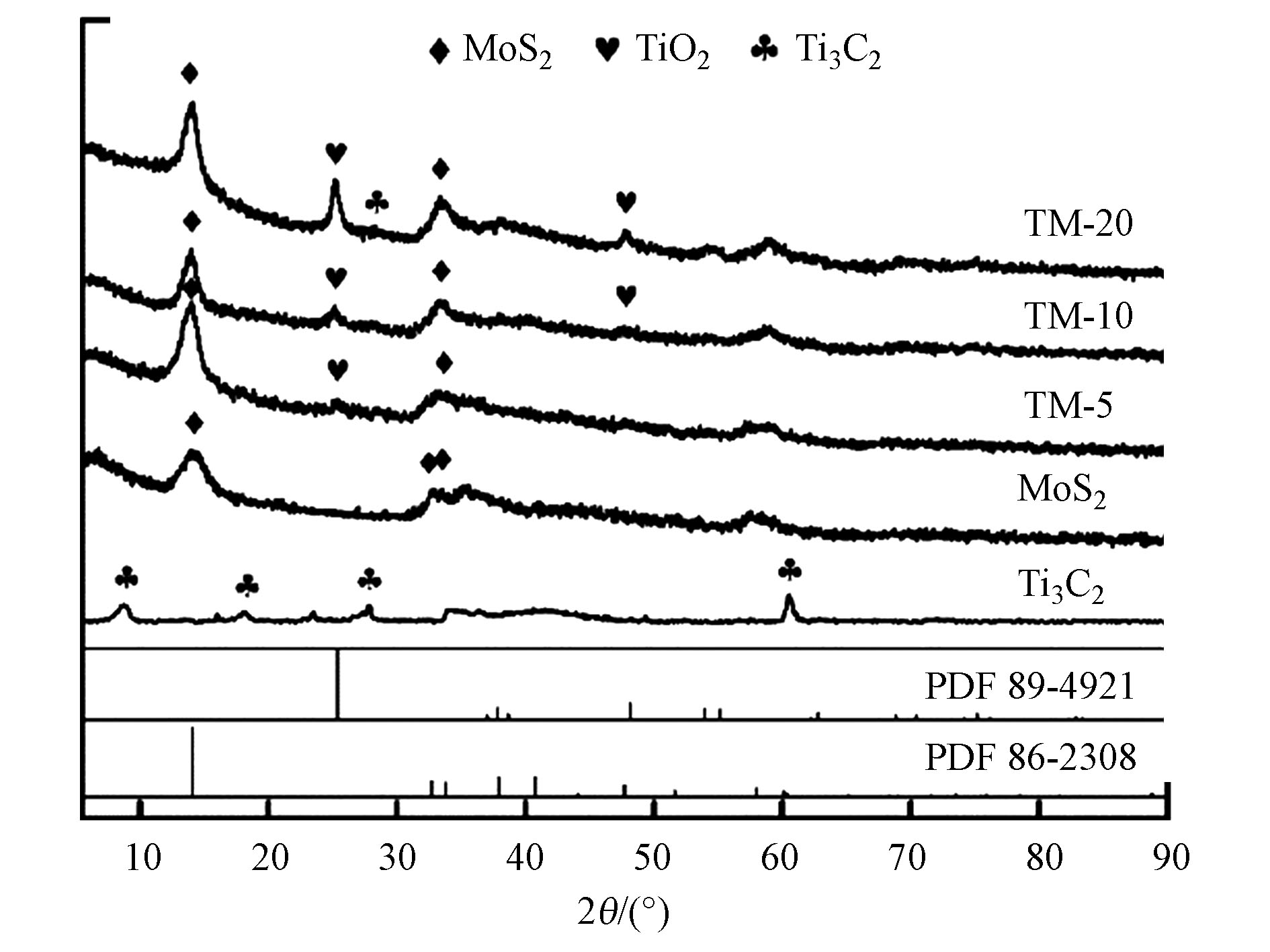
图1为MoS2、Ti3C2及其3种不同比例复合材料的XRD表征图。如图1所示,MoS2所测得的衍射峰同PDF#86-2308卡片的匹配度很高,说明本实验合成的MoS2为3R-MoS2,从TM-5和TM-10所测得的图像上也可明显找到属于MoS2的特征峰,这说明在加入适量Ti3C2的情况下,仍成功合成了3R-MoS2。此外,Ti3C2所表现出的衍射峰2θ = 8.7°、18.1°、28.0°和60.6°,与Ti3C2的特征峰有较高的匹配度[3]。再者,通过同PDF#89-4921的比对发现TiO2的衍射峰也出现在TM复合材料的曲线上,这与文献记载相符[18],说明Ti3C2在酸刻蚀与水热合成反应过程中被氧化。
由图1可知,在低比例复合材料中均未发现Ti3C2衍射峰,这可能是由于Ti3C2所呈现出的信号强度远不及MoS2及其复合材料所显示出的信号。但值得注意的是,在TM-20上发现2θ = 28.1°的有新衍射峰生成,与Ti3C2的特征峰有较好的匹配度。综上所述,p-Ti3C2/MoS2复合材料中,绝大多数的Ti3C2被部分氧化为TiO2,即形成p-Ti3C2,说明复合材料中同时存在有MoS2、TiO2和Ti3C2,且MoS2与TiO2占多数,Ti3C2占少数。
-
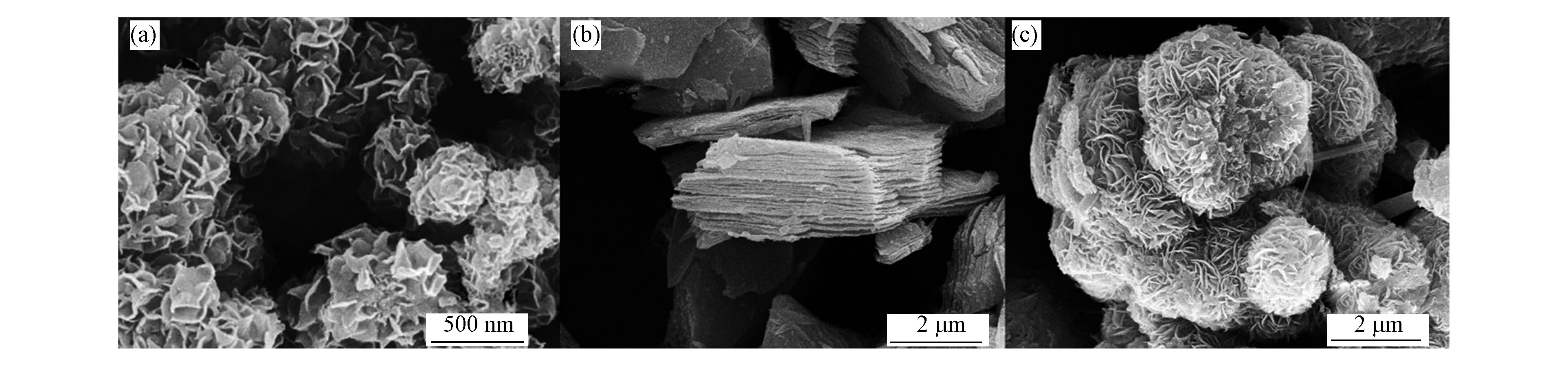
为探究材料的复合方式,对材料的微观形貌进行了SEM表征。如图2所示。图2(a)为MoS2的SEM图,可以看出MoS2 为纳米花状微球,团聚在一起,大小不一。图2(b)为Ti3C2纳米片的SEM图,显示为层片状结构,层层堆叠在一起。由图2(c)为TM-10复合物,可以看出其为花球结构,且没有层片结构,故可以推测MoS2 和p-Ti3C2 都参与了花球的形成,形成较大的团簇花球状,使其形貌较MoS2发生改变,这也与XRD的分析结论相符。
-
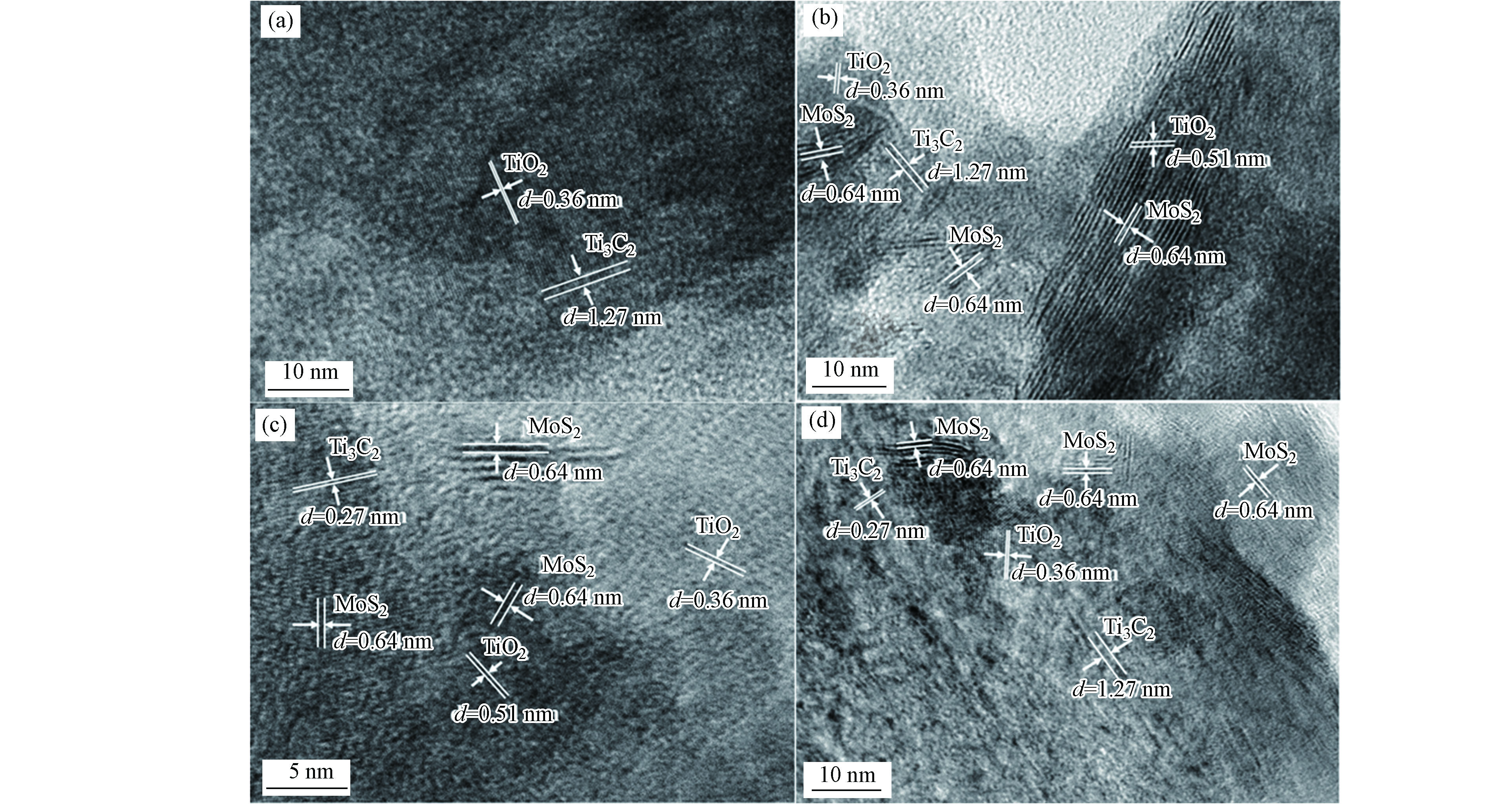
为进一步验证复合材料的组分以及异质结的复合情况,对材料进行TEM表征,测量其晶格条纹间距,图3为Ti3C2、TM-5、TM-10以及TM-20的TEM图。如图3(a)所示,可见间距为1.27 nm和0.36 nm的晶格条纹,说明材料中存在Ti3C2和TiO2,表明材料在蚀刻过程中已发生氧化反应,即Ti3C2中有一部分被氧化为TiO2[8, 18]。
此外,图3(b)(c)(d)分布着大量晶格间距为0.64 nm的条纹带,对应于二硫化钼的(003)晶面,说明MoS2已成功合成在材料上。图3(b)(c)中还存在0.27 nm的晶格条纹,对应于Ti3C2的(0110)晶面,说明材料中有Ti3C2存在[19]。图3(c)(d)中可观察到的0.51 nm的晶格条纹,对应于TiO2的特征晶格[20]。对比图3可以发现,Ti3C2所对应的晶格条纹(1.27 nm和0.27 nm)数量远少于TiO2(0.36 nm和0.27 nm),说明Ti3C2在水热反应过程中大部分被氧化为TiO2。
-
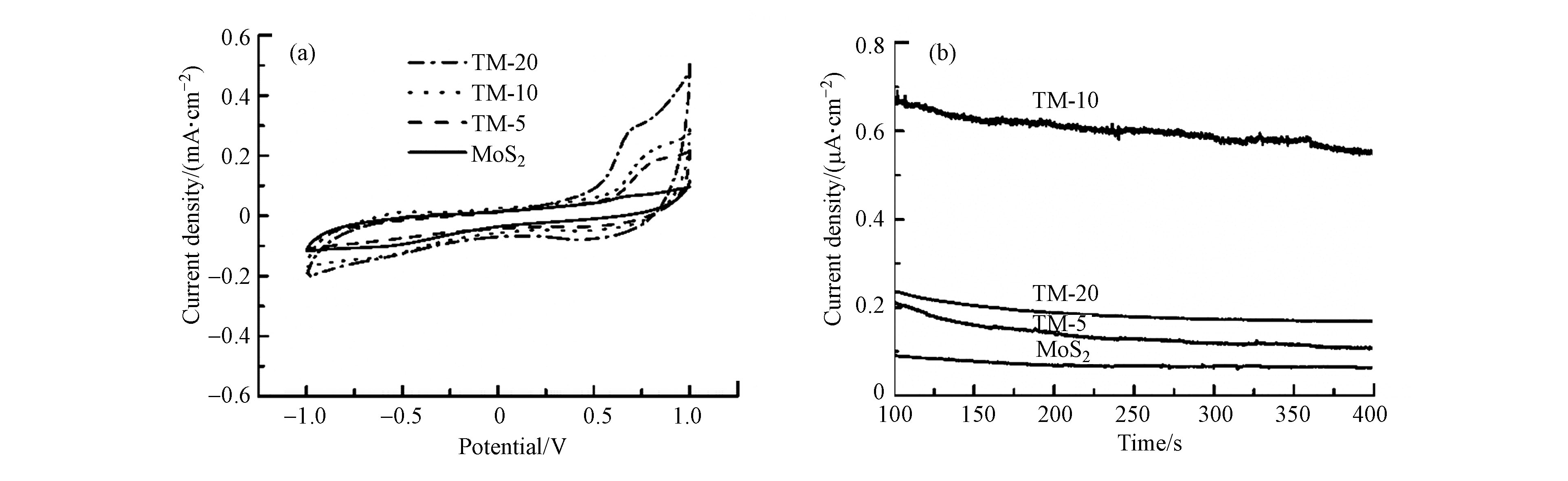
为进一步表征材料的电子传输能力,对MoS2、TM-5 、TM-10和TM-20进行了电化学测试。图4为4种材料的电化学分析图。其中,图4(a)为CV曲线图,图4(b)为I-t曲线图。由图4(a)可看出,原本单一的MoS2的电流及电压的峰并不明显,而p-Ti3C2的加入提高了材料的导电与催化性能。除此之外,由图4(b)I-t曲线可知,MoS2、TM-5、TM-10与TM-20与的电流密度分别为0.07 μA·cm−2、0.13 μA·cm−2、0.60 μA·cm−2和0.17 μA·cm−2,3种p-Ti3C2/MoS2电极的电流密度均高于MoS2,这表明p-Ti3C2/MoS2相较于MoS2有更强的传递电子能力,说明p-Ti3C2掺入有利于材料电子传递,能加快电子的转移。复合材料的电流均优于单一的MoS2,说明Ti3C2提高了MoS2的导电性能,其中TM-10具有较好的导电与电子传递性能。
-
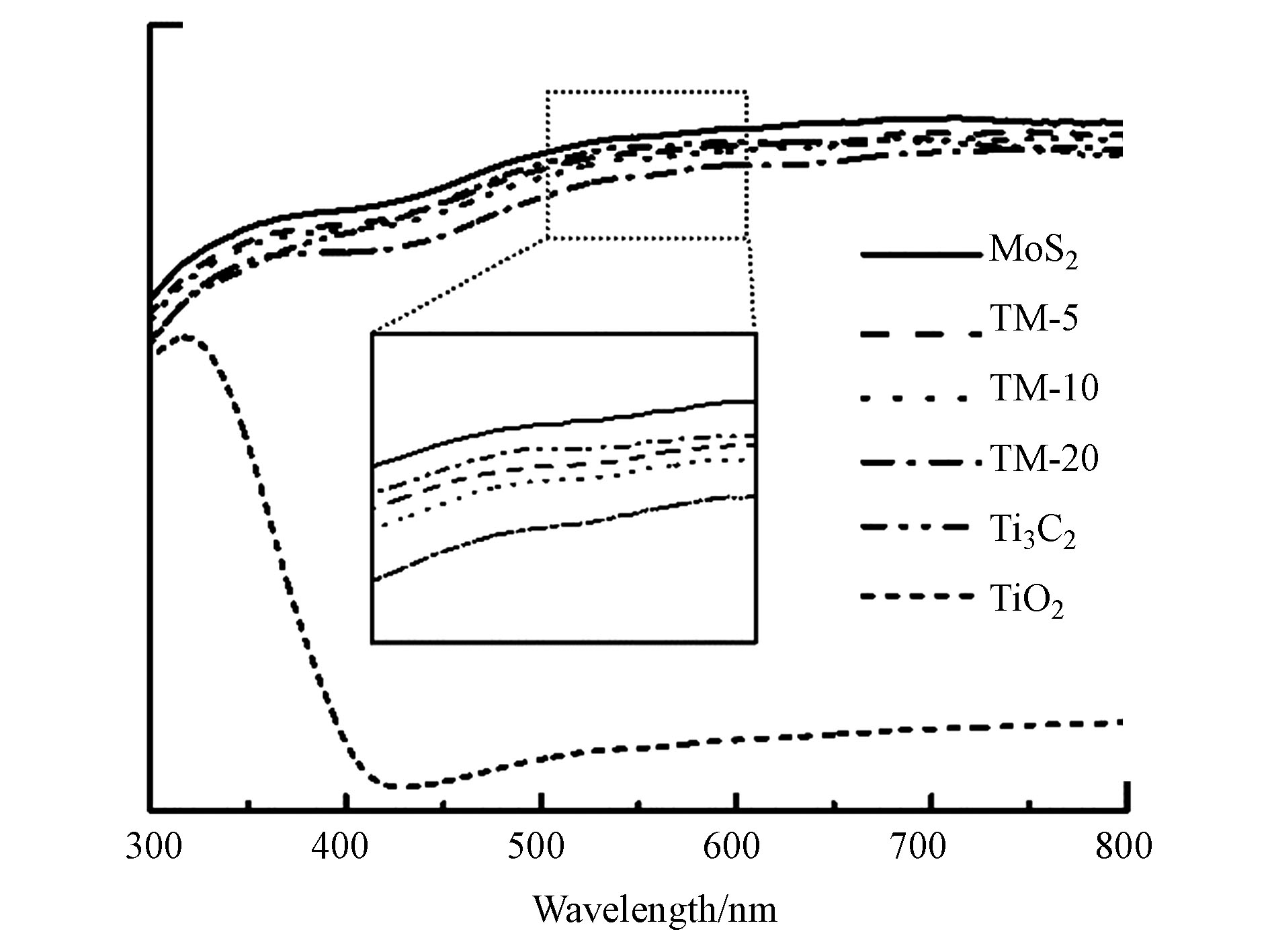
为了研究材料的光吸收性能,分别对TiO2、MoS2、Ti3C2、TM-5、TM-10以及TM-20进行紫外-可见漫反射光谱测试,如图5所示。由图5可以看出,MoS2、Ti3C2以及复合材料均对可见光(400—760 nm)有较好的吸收性能,而TiO2仅对波长小于420 nm的光(紫外光)表现出吸收能力,由此可推断出复合材料对于可见光的吸收能力会弱于MoS2,且其原因可能是复合材料中掺杂TiO2造成的。
此外,由图5可看出,3种复合材料对可见光的吸收波形与MoS2相近且接近,说明对于可见光的吸收能力可能主要取决于MoS2,由此可知,Ti3C2虽可提高材料的导电性能,但Ti3C2含量的增加,会影响材料对光的吸收性能,Ti3C2含量并非越多越好。
-
为进一步验证复合材料中钛元素的存在形式,对复合材料进行了XPS表征,如图6所示。图6(a)为TM-5、TM-10以及TM-20的Ti 2p XPS分析图,复合材料中XPS数据可被分为3组特征峰(Ti 2p3/2-Ti 2p1/2),其中,以459.3 eV(TM-5)、459.0 eV(TM-10)以及459.4 eV(TM-20)为中心的Ti3/2说明,材料中的钛元素主要以TiO2的形式存在[8]。而峰中心为456.1 eV(TM-5)、455.5 eV(TM-10)、456.1 eV(TM-20)处的特征峰说明以碳氧化合物TiCx(x<1)形式存在的钛元素极少,这与XRD以及TEM所得结论相同[8]。图6(b)为3种材料C 1s的XPS分析图, TM-5、TM-10和TM-203种材料分别在284.5、284.2、283.7 eV处显示出C-Ti-O的峰,说明其中存在Ti3C2/TiO2异质结[8]。
-
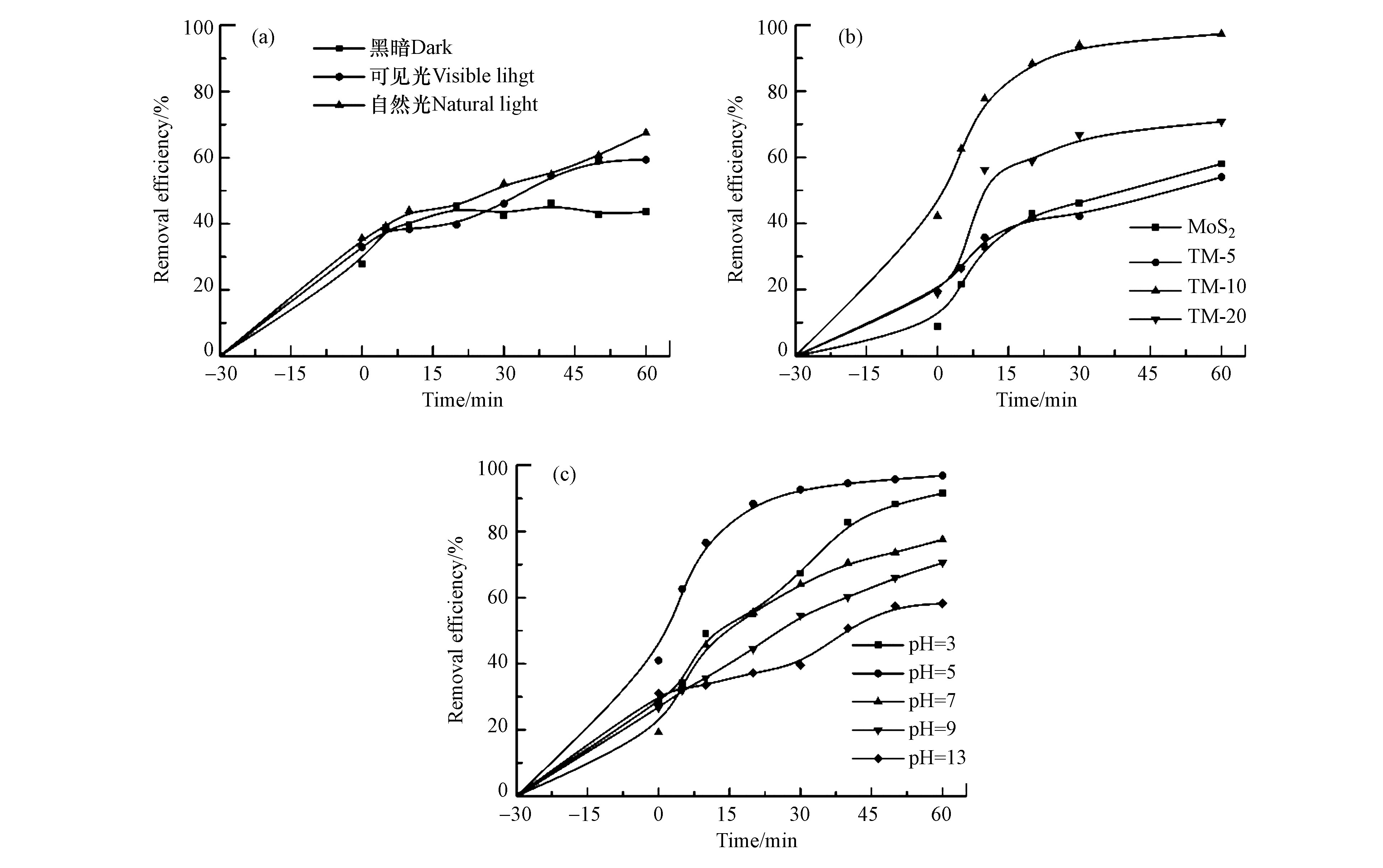
为探究光照条件,复合比例及降解环境的pH等条件对p-Ti3C2/MoS2复合材料催化降解罗丹明B性能的影响,本文中通过改变上述条件,在不同条件下进行污染物降解,实验结果如图7所示。采用TM-5进行测试,对比不同光照条件对RhB降解性能的影响,如图7(a)所示,在不同的光照条件下,去除曲线的发展趋势大致相同,均随时间增加而提高。黑暗条件下对罗丹明B进行吸附,但其去除率为43.6%。而在有光条件下,其去除率可相对提高。在可见光条件下(卤素灯),去除效率可提升至59.4%,自然光下更为可观,罗丹明B去除率提升至67.5%,是黑暗条件下的1.55倍,可见光下的1.14倍,但也可以看出,由于自然光的多变性和不稳定性,罗丹明B的降解速率时高时低。对照可知在相同时间内,自然光条件下 p-Ti3C2/MoS2催化材料对罗丹明B可以有更好的降解效果,但值得注意的是,自然光会随着时间的推移的改变,且其降解效率仅为卤素灯的1.14倍,故选用卤素灯为光源的可见光为外加条件进行后续实验。
如图7(b)所示,经过30 min的黑暗静置吸附后,不同p-Ti3C2含量的MoS2材料对污染物的吸附达到平衡。从图中可以看出,不同材料对罗丹明B的吸附效果不同,复合材料的吸附能力均高于MoS2。此外,通过四种材料的对比可以看出,p-Ti3C2的投加量非越高越好,TM-10在4种材料中展现出最佳的光催化能力,降解率达96.9%,是MoS2的1.67倍。如图7(c)所示,不同的pH条件会对TM-10降解罗丹明B的效果产生影响。在酸性条件下,TM-10显示出较强的催化降解能力,其降解效果均高于中性条件。而碱性条件下,降解效率的上升趋势就相对平稳,降解效果均低于中性条件。由图7可知,反应时间相同的情况下,pH 为5时,催化材料对罗丹明B降解效果更好。
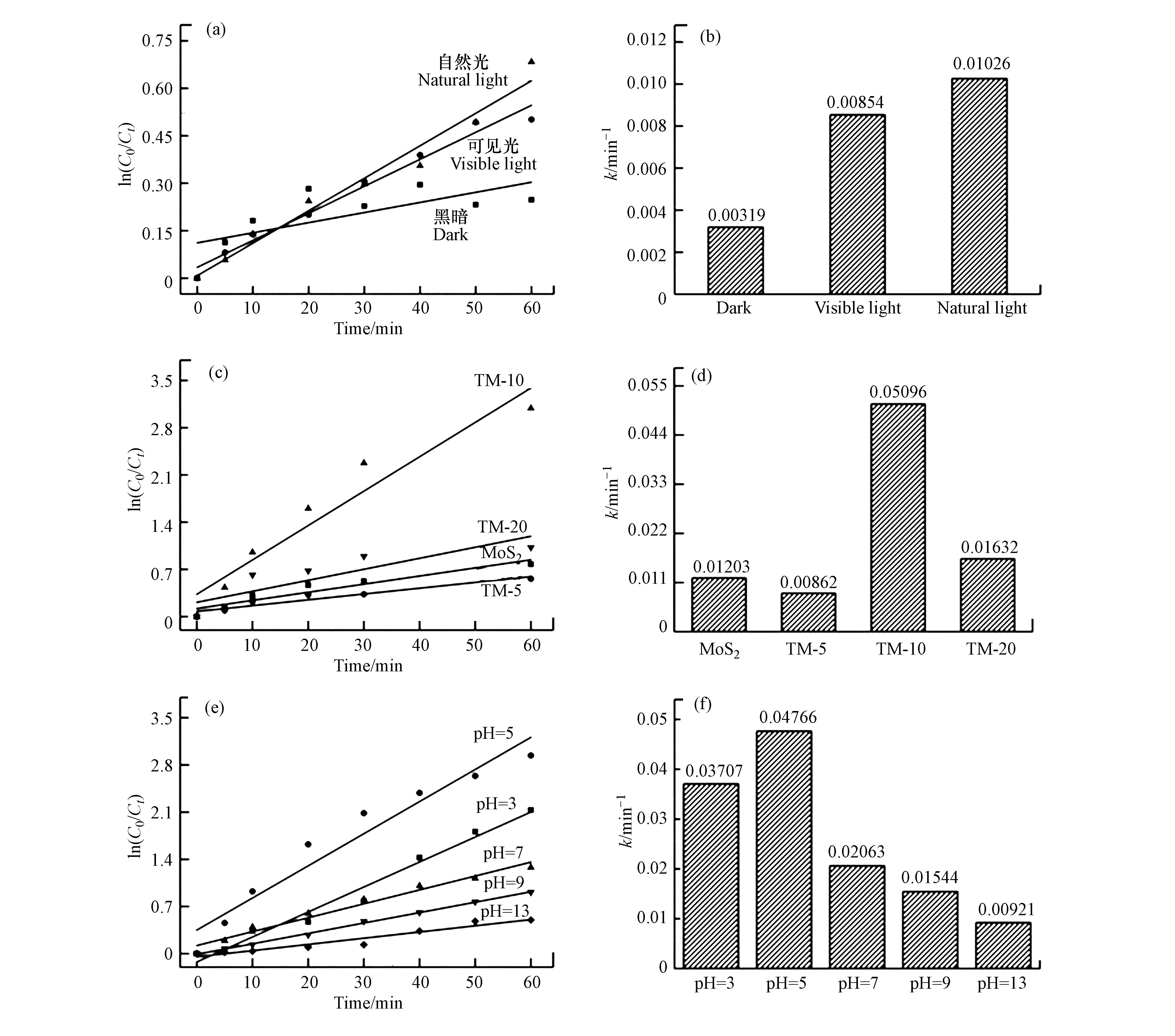
为进一步探究不同因素对RhB降解性能的影响,将降解率曲线进行拟合,根据准一级动力学方程:
式中,C0为初始污染物浓度,mg·L−1;Ct为取样时间为t时的污染物浓度,mg·L−1;k为动力学速率常数,min−1[21]。由拟合图8(a)可以看出,p-Ti3C2/MoS2在黑暗下对于RhB的去除数据不能与准一级动力学有较高的匹配度,起R2仅为0.42,远低于0.98(可见光)和0.97(自然光)。这进一步证明这一过程材料对RhB仅起到吸附作用,而少有降解反应发生。而图8(b)可知,自然光条件下材料对RhB的降解数据与可见光条件下对比,前者略高与后者。
此外,通过观察图8(c)和8(d)可以发现,TM-10,TM-20,MoS2以及TM-5对于RhB的降解速率依次减小,分别为0.0510,0.0163,0.0120、0.00862 min−1。其中,TM-10的降解速率常数是TM-5的5.91倍,TM-20的3.12倍,以及MoS2的4.24倍,展现出优异的光催化降解能力。
最后,由图8(e)和8(f)可以看出,反应速率随pH条件的改变而变化,反应速率常数k的最大值为0.0477 min−1,对应pH为5的酸性条件,这可以推测氢离子(H+)浓度在催化反应中起到一定的作用。
-
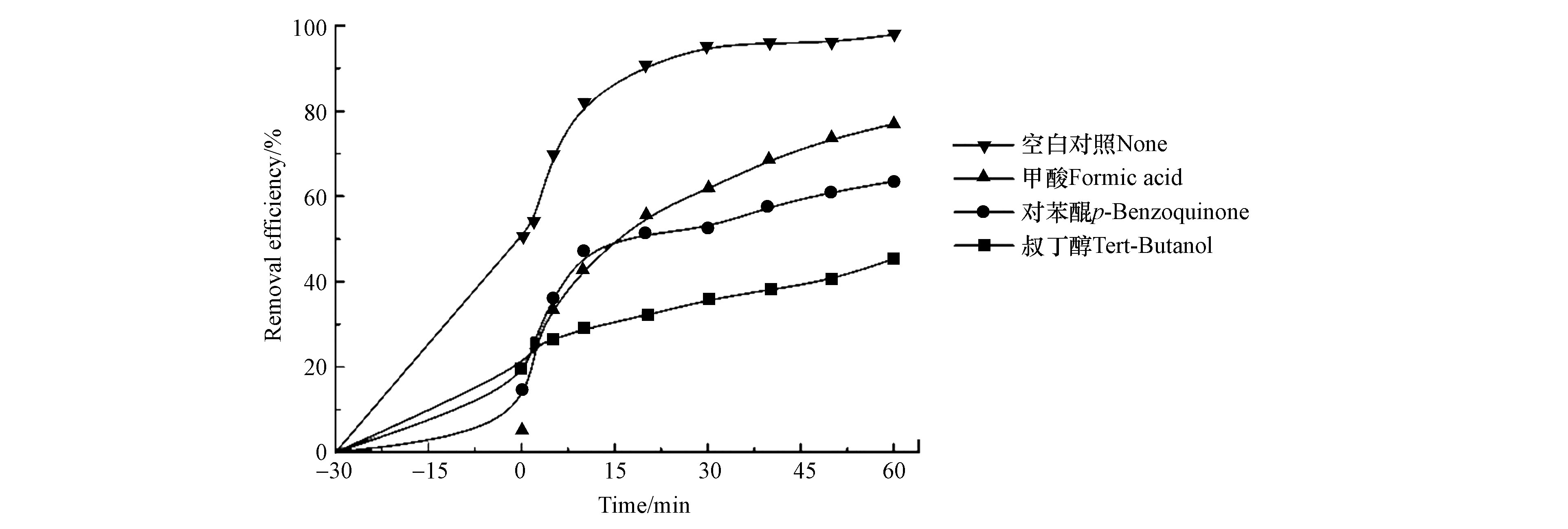
为进一步对光催化材料与污染物的降解性能进行探索,本文选用不同的自由基捕获剂来考察降解过程中起主要作用的活性物种,据此探究降解过程的反应机理。分别添加叔丁醇(TBA,1 mmol·L−1)、对苯醌(BQ, 1 mmol·L−1)以及甲酸(FA, 1 mmol·L−1)等3种捕获剂用以捕捉羟基自由基(·OH)、超氧自由基(·O2-)以及空穴(h+)。如图9所示,加入捕获剂后,TM-10对RhB的降解效率均有所降低,通过不同捕获剂的对比可知,在罗丹明B的降解过程中,加入TBA对降解效率的影响最大,可以判断出羟基自由基在降解中起主要作用,且仍有超氧自由基和空穴产生,都对污染物降解起促进作用。
基于上述结果,可推断出p-Ti3C2/MoS2的理论结构以及光催化降解反应机理,如图10(a)所示,MoS2纳米球生长在Ti3C2纳米片表面,同时,在水热反应过程中,Ti3C2纳米片的表面被部分氧化形成TiO2,TiO2生长于Ti3C2表面,且与MoS2接触形成异质结。此外,Ti3C2和TiO2对可见光的响应值均弱于MoS2,所以会降低复合材料对可见光的吸收[22]。
降解过程包括吸附与降解,催化材料先将污染物吸附在其表面后,再进行光催化降解,对降解机理的进一步分析如图10(b)所示,TiO2和MoS2上的电子-空穴对受到可见光能量的激发而发生分离,MoS2的价带(VB)电势和导带(CB)电势均高于TiO2,两者可形成Ⅱ型异质结[22]。因此,在TiO2/MoS2异质结结构中,TiO2上的h+会向MoS2迁移,而MoS2上光催化产生的e-则向TiO2迁移。此外,Ti3C2由于其特殊的氧化性质,可作为良好的空穴受体[8]。因此,MoS2以及TiO2上的h+也会被Ti3C2吸收,使得复合材料上产生的e-和h+进一步分离,从而降低e-和h+的复合率,延长了e-和h+的寿命。
复合物表面的e-活化氧气分子产生·O2−,·O2−又进一步与H+产生·OH,同时材料上的h+与H2O和OH−反应成·OH。由于·O2−分散在溶液中,而h+富集于Ti3C2上,所以,可以推出前一个反应不受发生地限制,更容易发生,故得出H+在催化作用中起一定积极作用。由于水电离平衡的存在,过多的H+会抑制OH−的生成,从而阻碍后一个反应的进行,对降解效果起一定的消极作用,二者之间存在一种平衡,这也与前文结论相符。由于h+、·OH和·O2−又会使RhB大分子分解成小分子物质,因此,在光催化过程中h+、·O2−,·OH都参与污染物的降解过程,其中,·OH在降解过程中起主导作用。
2.1. XRD分析
2.2. 形貌分析
2.3. TEM分析
2.4. 电化学分析
2.5. 紫外-可见漫反射光谱(DRS)分析
2.6. XPS分析
2.7. 光催化性能测试
2.7.1. 光照条件、p-Ti3C2含量与pH值对罗丹明B降解性能的影响
2.7.2. p-Ti3C2/MoS2降解罗丹明B的机理探究
-
本研究通过选择性刻蚀和水热合成法成功制备了掺杂不同比例Ti3C2的MoS2基复合材料,并以RhB为目标污染物,在不同条件下测试材料的光催化性能。研究发现,这种独特的结构虽然会降低原材料对光的吸收能力,但p-Ti3C2与MoS2复合可提高材料的电化学性质,从而提高材料的导电性。同时,p-Ti3C2 和MoS2 间形成Ⅱ型异质结,适量的掺杂有利于提高复合材料电子产生与分离速率。实验结果表明,在诸多比例的复合材料中,TM-10对RhB具有最佳的降解性能。在自然光或可见光条件下,均具有良好的光催化性能。在可见光下,其1 h去除率为MoS2的1.67倍,一级动力学速率常数为MoS2的5.91倍。研究发现,当溶液pH为5时,在可见光情况下TM-10对RhB废水的降解速率可达96.9%。由此可知,所制备的p-Ti3C2/MoS2复合材料在治理有机染料废水方面有着较大的应用前景。



 下载:
下载: