-
磷作为细胞中脱氧核糖核酸、核糖核酸的构成元素之一,在生物的生长发育、遗传代谢方面起着至关重要的作用[1-2]。天然水体中的可溶性磷酸盐大部分由各种价态的正磷酸盐组成,主要以H3PO4、
${\rm{H}}_2{\rm{PO}}_4^{-} $ 、${\rm{HPO}}_4^{2-} $ 、${\rm{PO}}_4^{3-} $ 形式存在[3],可作为营养物质被藻类吸收。其他形式的可溶性磷酸盐也会逐渐被分解成正磷酸盐[4],其中包括农药和肥料中含有的有机磷酸盐以及洗衣粉中含有的聚合磷酸盐等。近年来随着工农业的不断发展,过量废水排放引发生态环境失衡,高浓度磷导致水体富营养化,有毒藻类大量生长,从而造成溶解氧含量枯竭,水质下降,正常水生生物死亡[5-,6]。在临床诊断中,血清中的磷酸盐水平被确立为常规血清检验的一部分。体液中的磷酸盐水平可以为诊断甲状旁腺功能亢进,维生素D缺乏症等疾病提供有价值的参考信息[7]。当血液中磷酸盐浓度超过正常水平时(即高磷血症),沉淀钙会减少维生素D的产生以及干扰甲状腺旁激素介导的骨吸收,进而引起低钙血症[8]。另一方面,当磷酸盐浓度低于正常水平时,细胞内ATP水平降低,可能导致依赖磷酸盐化合物的细胞出现功能性损伤[9].关于磷酸盐浓度的规定已有相关报道,欧盟立法规定将水中含有0.1 mg·L−1的磷酸盐作为判断蓝藻爆发的指标[10]。天然河水中磷酸盐被允许的最大含量在0.32×10−6 mol·L−1(9.8 μg·L−1),废水中磷酸盐被允许的浓度在0.0143—0.143×10−3 mol·L−1(0.4418 —4.418 mg·L−1)范围内[11]。作为临床诊断标准,人体唾液中磷酸盐正常检测浓度在5—14×10−3 mol·L−1(154.5 —432.6 mg·L−1)[12],人体血液中磷酸盐正常检测浓度在0.81—1.45×10−3 mol·L−1(25.029—43.26 mg·L−1)范围内[13]。因此不仅是从生态环境角度还是生物医学角度,磷酸盐测定是一个值得关注的问题,尽管关于磷酸盐的测定方法报道很多,但能够实现准确快速且实时监控磷酸盐还存在一些技术难点。相关发表的文章中针检测磷酸盐水平的方法主要有比色法、色谱法、光学荧光法以及电化学法[14-17],各篇文章研究的主要目的在于开发灵敏度更高、检测限更低、抗干扰能力更强,且能够提高经济效益,满足社会发展需要的方法。目前检测水溶液中磷酸盐的标准方法是Murphy和Riley提出的比色法[18]。该方法是以抗坏血酸为还原剂,将钼酸铵与抗坏血酸与锑(Ⅲ)添加到磷酸盐样品中,生成蓝色的磷钼酸盐络合物,最后用分光光度计光学测定磷酸盐的浓度,反应方程式如下:
然而磷钼蓝法易受到砷酸盐、硅酸盐、硫化物等化合物以及样品浊度的干扰[19],实验过程繁琐冗长,操作环境要求较高无法实现在线监测,并且操作中使用的化学物质对健康有害,如钼。电化学方法具有弥补上述部分缺陷的优势,它具有操作简单、携带方便、灵敏度高、检测限低,对分析物可以进行连续快速分析的优越性能[20]。
随着经济的快速发展,我国内陆湖泊河流等水生环境面临的压力与日俱增,城市建设对于环境监控的需求日趋增大,因此研究和开发适用于环境检测的实时在线监控系统是当前面临的挑战[21]。随着无线传感网络的出现,卫星遥感监测方法也变得很流行[22],其中电化学传感器可作为系统的核心部分,识别样本并将样品浓度转化为电信号后,输出可分析的数据。电化学传感器中离子选择电极是最早和最简单的传感器之一,用于直接进行电化学检测磷酸盐,其中离子选择性电极可选择掺杂或者不掺杂离子载体的金属电极或者聚合物膜修饰电极。其他电化学方法还包括安培分析、电导分析等。电化学传感器在原位实时、快速检测以及小型化、智能化等方面具有较突出的优势,不仅在环境领域受到广泛关注,在食品安全、医学检验等领域得到广泛应用。
本文简要综述了近年来实际应用于检测无机磷酸盐的电化学技术的研究现状,其中包括电位法、安培法、电导法等,以及针对各种传感器在检测限、选择性等性能方面进行比较探讨。
-
相关文献已经报道,金属电极和离子选择性电极已经成功应用在检测阳性离子(Zn2+、Cd2+、Pb2+、Cu2+等)领域[23-25],但在检测阴离子(F−、OAc−、
${\rm{HCO}}_3^{-} $ 、H2PO4−等)方面还存在选择性不足,适应性不强等问题[26]。其中磷酸盐阴离子属于四面体氧阴离子家族,较大尺寸的磷酸盐会降低有效核电荷密度,并使它们的理想点电荷降低,从而降低其静电结合效率的程度[27]。水性介质的pH会影响磷酸盐的存在形式,例如H3PO4$ \Leftrightarrow $ H2PO4−$ \Leftrightarrow $ HPO42−$ \Leftrightarrow $ PO43-分别在pKa1≈2.12,pKa2≈7.21和pKa3≈12.67的情况下互相转化[28]. 此外,由于中心的磷原子被氧原子包围,所以磷酸根具有相对较高的水合能。感胶离子序末端阴离子的位置反应出磷酸盐的亲水性,由于磷酸盐比大多数疏水性阴离子更容易保留在溶液中,所以很大程度上限制了磷酸盐离子选择性膜的选择性[4,29]。目前针对电位法检测无机磷酸盐的研究见表1,其中按IUPAC(国际纯粹与应用化学联合会)定义,根据敏感膜的不同性状,将离子选择性电极分为两大类。 -
基于聚合物膜的电极是通过使用离子交换机制加快离子通过中心离子载体化合物的速度,这可以改善感胶离子序末端离子的选择性电位响应。表1中简要概述了近几年关于离子选择性电极方面的研究。Satoh等[30]建立了一种基于双(二溴苯基锡烷基)甲烷掺杂聚氯乙烯(PVC)膜的微型传感器。这种微型传感器表现出优异的选择性,可以在不同无机磷种类中主要对HPO42−做出反应,且检测限可以达到0.5 μmol·L−1,其中不足之处在于容易受到OH−的干扰且使用寿命很短。Topcu等[31]将壳聚糖-蒙皂石生物复合材料作为PVC膜电位传感器结构中的电活性组分,用于直接和高度选择性地测定HPO42−。该电位型离子选择性电极(PISE)在浓度为1×10−6—1×10−2 mol·L−1 HPO42−水溶液中表现出良好的电位响应,并且检测限为6×10−7 mol·L−1。此外,有机锡离子载体也被整合到PVC膜中,锡(IV)通过从中心取出电子来促进磷酸盐中的氧原子与有机络合物结合。Liu等[32]将双核有机锡化物掺杂入液体聚合物膜中作为磷酸盐离子载体,在浓度为5×10−6—10−1 mol·L−1范围内表现出优异的性能,检测限为10−6 mol·L−1很好地满足环境水样的监控,但缺陷在于一个月后使用性能大幅度下降。
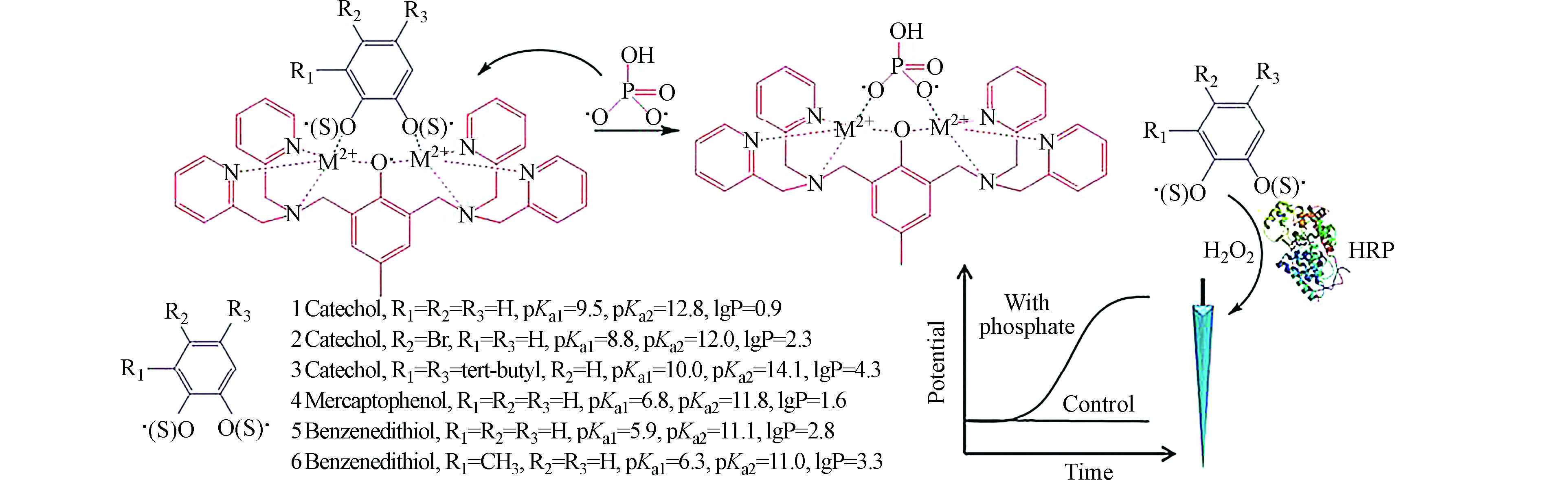
在大多数情况下,金属络合物通过与不同的磷酸盐阴离子形成有效的金属-PPi键从而能有效地进行选择性识别PPi。目前,许多金属离子已经被使用在离子选择性电极中,例如Zn2+-BPMP和Cu2+-BPMP(BPMB=双(2,6-二(2-吡啶基甲基)氨基甲基)-4-甲基苯酚)掺杂的聚合物膜被用作磷酸盐受体。李龙[33]以BPMP为与金属离子配合物作为分子识别体,以与儿茶酚具有类似分子结构的物质作为指示剂,通过磷酸根离子取代指示剂与金属离子络合产生的电信号,实现对磷酸根的检测,后续使用H2O2的氧化反应产生的电位信号变化对磷酸根进行定量检测,信号反应机制如图1。这种方法优势在于强亲脂性阴离子不干扰磷酸盐的检测,且证明Cu2+-BPMP比Zn2+配合物更灵敏,更具有选择性。
-
固态离子选择性检测具有许多优点,检测传感器易制备、易保存、易携带,易小型化,为建立长期在线监测提供更大可能性,但同时需要定期维护,增加经济成本[40-42]。Xiao等[43]在1995年提出了第一个钴基磷酸盐传感器。传感器对于磷酸盐的选择性响应是由于钴在缓慢氧化后,在电极表面氧和Co2+还原的作用下形成了磷酸钴沉淀。同时,钼电极也用于磷酸盐的测定[1,38],文章基于钼和HPO42−在碱性条件下的反应,开发了基于钼的磷酸根离子选择电极(ISE)。研究了pH对传感器性能的影响,在pH 8.5时得到最佳响应,且检测限为1.9×10−6 mol·L−1。传感器制备过程简单便宜,使用周期为3个月,并具有良好的重现性和选择性。相关报道也指出锆对于磷酸根具有极强的亲和力和非常长出色的选择性,并且它本身不会损害环境的稳定性以及人类的安全[44],锆金属配合物应用于检测磷酸盐领域是很好的研究方向,然而目前针对此思路的报道相对较少。
近年来关于固态离子检测的思路逐渐多样化。Ejhieh等[34]提出了一种基于表面活性剂改性沸石颗粒的磷酸盐选择性电极(SMZ-CPE),电极所达到的检测限为1.28×10−5 mol·L−1,且在pH4—12 以及1×10−4—1×10−3 mol·L−1 NaNO3的溶液中仍保持稳定的响应电位。这种电极不仅用于直接电位法,还可以用于电位滴定法。Tafesse等[35]构建出一种由铝粉(Al),磷酸铝(AlPO4)和铜粉(Cu)的可变混合物组成的磁盘,制造出高度灵敏的磷酸盐传感器用于环境水样监测。此传感器具有小于60 s的快速响应时间且检测限低于1×10−6 mol·L−1,选择性强,可抗多种水溶性离子的干扰。姜涛钦等[36]以玻碳电极为基底,聚合硫酸钴与硫酸钠混合溶液制备出固态磷酸盐离子选择性电极,检测下限为6.3×10−5 mol·L−1。
基于其他材料和理论来发展固态选择性检测也有相关报道[45],例如丝网印刷电极(SPE),分子印迹传感器(MIP),场效应晶体管等。Kabir等[39]开发了一种新型四水合钼酸铵合银纳米线(AMT/AgNMs)修饰的丝网印刷电极,此电极在5×10−6—1×10−3 mol·L−1浓度范围内对磷酸盐高度敏感,检测限是3×10−6 mol·L−1。研究开发的传感电极可以是一个潜在的低成本系统,因为它具有易于制造,体积微小的特点,所以需要较少的收集设备和现场部署。Kugimiya等[46]构建了一种以硫脲为功能单体,用于检测和定量水溶液中的腺苷-3',5'-环一磷酸(cAMP)的分子印迹传感器(MIP)。研究将印迹分子涂覆在离子敏感场效应晶体管表面并进行原位聚合,实验绘制了cAMP在3.1—31 mg·L−1浓度范围内的标准曲线,而没有印迹分子的聚合物则没有响应,其中对3',5'-单磷酸腺苷(AMP),5'-单磷酸腺苷(GMP)和鸟苷-3',5'-环一磷酸(cGMP)的溶液响应非常有限。
-
安培法是指在恒定电压条件下,测量目标分析物在电极上产生的电流来进行定量的方法,如表2。测定磷酸盐最常用的安培分析法是电化学还原钼酸盐技术,这种技术可以减少实验试剂的使用,提供了便携性和优异的灵敏度,在实地监测磷酸盐方面具有很大优势。而伏安法则指工作电极上的电势发生变化时测量电流,且分析物浓度与被测物质的氧化或者还原峰电流成比例关系。
-
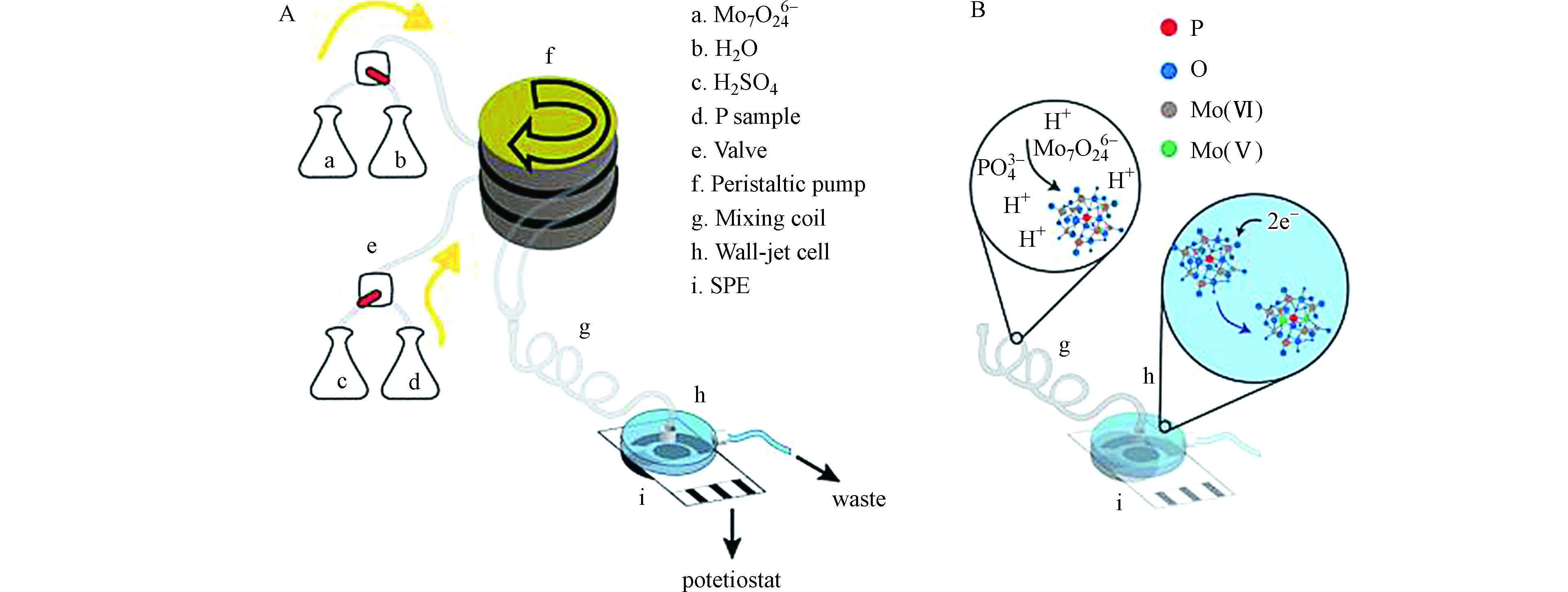
目前,在检测水中磷酸盐的安培传感器技术应用中,关于电化学还原钼酸铵的技术有很多。Cinti等[47]基于比色法原理构建了一种新颖的无试剂纸基丝网印刷电极。滤纸提供了一个有效的电分析平台,可以检测标准溶液和实际河流水样品中的磷酸根离子。这种新颖且高度可持续的配置具有优异的稳定性和灵敏性,研究显示30 d以后电流响应无变化,并且在10—300 μmol·L−1宽线性范围内实现4 μmol·L−1的检测限。低成本且可持续的诊断测试条不使用昂贵的实验室设施,可在资源匮乏的地方开发利用。很多相关文献也报道了安培检测在流动注射分析中的应用。Talarico等[48]基于微型传感器的安培检测,开发了一种连续和长期监测磷酸盐水平的自动流动分析系统。分析系统的装置图及电极表面的反应原理如图2所示,该系统结合了试剂和废物储存,并连接到便携式恒电位仪,用于快速检测和定量河水和湖水中的磷酸盐。炭黑纳米粒子(CBNPs)修饰的丝网印刷电极在125 mV电位下对磷酸盐络合物进行电化学还原,不会存在污垢问题,且检测限为6 μmol·L−1,满足监测环境的要求。Quintana等[49]以碳糊电极为指示电极,使用批量进样分析技术(BIA)技术对海水中的正磷酸盐进行安培检测。在电极上施加+0.3 V的电压时,酸性介质中磷酸盐与钼酸铵反应生成磷钼酸盐络合物发生还原反应。这种方法的主要特征在于设备简单、试剂消耗量有限、低检测限(0.3 μmol·L−1)、宽线性范围(1—20 μmol·L−1)以及能够完全消除硅酸盐的干扰。这种方法还应用于测定罗马地下墓穴手机的蓝细菌生物膜中的正磷酸盐。
-
安培型传感器具有操作简便、检测限低、响应时间短、以及成本低的优势,但同时存在选择性不佳的不足。而常用的分光光度法,离子色谱法则表现出优异的选择性,但受到反应时间长、操作复杂、试剂消耗大等缺点的限制。目前有相关报道将安培法与这两种方法联用,利用电化学还原技术代替试剂还原或者改用电化学检测器,既解决了安培法选择性差的问题,又解决了分光光度法时间和经济成本高,以及离子色谱法操作繁琐费时的问题。Mariaulle等[56]设计了用于非电活性离子的安培传感器,在流动注射分析中进行了评估,并将其用作离子色谱法中的新型检测器。检测器由碳糊电极组成,该电极经阳离子或阴离子聚合物改性,含有适合的电荷转移介体(Fe(CN)63-或Cu2+),并以间接安培法运行。检测机制包括电极表面的聚合物颗粒中非氧化还原离子分析物和电活性介体之间的离子交换,以及电极溶液界面处聚合物中浸出的介体物种的电化学转变。研究中建立的系统还成功用于分析阳离子Li+、Na+、K+、
${\rm{NH}}_4^{+} $ 、Ca2+、Mg2+)和阴离子(F−、Cl−、NO2−、NO−、${\rm{SO}}_4^{2-} $ 、${\rm{PO}}_4^{3-} $ )混合物的色谱分离。Torres等[57]提出了一种新颖的自动连续进样程序,用于分光光度法测定正磷酸盐,不需要使用不稳定的化学试剂去还原络合物。该进样程序基于使用不锈钢管状流通式工作电极,采用电化学安培法还原磷钼酸络合物,并在线生成可检测物质的方法。该检测程序具有极宽的动态范围(即0.3—800 mg·L−1),且对硅酸盐的干扰具有耐受性。该方法已经成功用于测定废水,饮料和含有高浓度目标分析物的生物样品中的正磷酸盐。 -
伏安法[35,58]是利用分析物浓度与被测物质的氧化或者还原峰电流之间的比例关系来进行检测,检测环境采用由工作电极、参比电极和辅助电极组成的三电极体系。三电极体系优势在于结果曲线稳定,精密度高,氧化还原峰明显,有利于清晰的读取研究所需要的峰值电流与电位。He等[58]构建了一种由泡沫镍(NF)和一步水热法而在NF表面生成纳米花状镍水合氢氧化物(NHH/NF)组成的工作电极,利用伏安法对磷酸盐进行检测。工作电极表现出210 μA·(mmol·L−1)−1·cm−2和87 μA·(mmol·L−1)−1·cm−2的高灵敏度,对应磷酸盐的检测范围分别是10—14000 μmol·L−1和14000—50000 μmol·L−1。NHH层的纳米结构不仅提供了较大的表面积和快速的电子传输,而且还保护了NF基板不受电解质和干扰物质的降解,从而实现了良好的稳定性和选择性,此方法成功应用于实际废水检测。
-
生物电分析法是利用电化学的基本原理和方法,在生物体和有机组织的整体,分子和细胞两个不同水平上研究或模拟研究电荷(包括电子、离子等)在生物体系和其相应模型体系中分布、传输和转移及转化规律的方法。生物电分析法发展迅速,相关技术包括生物传感器、微电极等,应用于环境保护、食品控制、燃料电池等多个领域。其中生物传感器是一种将生物化学反应能转换成电信号的装置,主要包括酶传感器,免疫传感器,DNA生物传感器等。通常用于测定磷酸盐的生物传感器[59]基于单酶或多酶反应,其中磷酸盐充当抑制剂或底物。酶传感器具有识别性强,灵敏度高等优点,但同时因为其特殊的生物材料也导致存在使用期限短,检测环境要求高等不足。目前发展的针对无机磷酸盐检测的主要点酶传感器见表2,根据酶的使用数量,将磷酸盐酶传感器主要分为以下三类。
-
单酶传感器使用材料较少,因此具有成本优势。此外,传感器还涉及到简单的酶固定化程序,从而可以减少来自样品和其他成分的干扰。单酶磷酸盐传感器系统中,丙酮酸氧化酶(PyOx)是使用最广泛的酶,其催化反应为:
因此基于POD的生物传感器主要是基于H2O2的电催化氧化,反应过程中PyOx需要焦磷酸硫胺素、黄素腺嘌呤二核苷酸以及Mg2+提供辅助催化作用。目前不少单酶传感器应用到自然水体磷酸盐检测中。He等[50]利用载有金纳米棒负载纳米氧化铜(AuNRs@Cu2O-NDs)的聚(二烯丙基二甲基氯化铵)(PDDA)对石墨烯(Gr)进行功能化修饰,并以此用来固定PyOx,基于以上材料构建了一种新型的灵敏的电化学生物传感器。具有良好导电能力的PDDA-Gr可以增加活性位点并作为生物传感器的支撑底物;AuNRs@Cu2O-NDs复合材料可以增强PyOx的固定能力并实现电子快速转移,以此放大电信号。此生物传感器在0.01—80 μmol·L−1宽线性范围中显示出良好的电流响应,且检测限为0.4 nmol·L−1,成功应用于食品中磷酸盐含量的检测。Ogabiela等[51]利用循环伏安法和安培法证实了通过与牛血清蛋白(BSA)和戊醛(GLA)交联,在金纳米线阵列(AuNMA)上成功整合了PyOx和辅助因子。此传感器在重复使用2周内表现出最佳性能,具有很好的稳定性,对主要阴离子(Cl−、NO3−、NO2−、F−)具有良好的选择性。传感器检测为0.1 μmol·L−1,成功应用于池塘水样中磷酸盐的检测。
-
双酶磷酸盐生物传感器最常使用的两种酶是核苷磷酸化酶(NP)和黄嘌呤氧化酶(XOD)[52],两种酶的催化反应如下:
因此,磷酸盐浓度的测定可以通过监测O2的消耗量或者酶反应过程中过氧化氢(H2O2)和尿酸的产生量来实现。Lawal等[52]通过恒电流聚合作用将两种酶包埋在聚吡咯膜中,设置不同聚合参数和辅助因子,制备出两种电化学(电位和安培)磷酸盐生物传感器。通过比较,电位型传感器最低检测浓度为20 mmol·L−1,线性范围为20—200 mmol·L−1,安培型传感器最低检测浓度为10 mmol·L−1,线性范围为0.1—1 mmol·L−1。最终,选择电位型传感器用于实际样品分析和环境监测。此研究团队又将这两种酶与戊二醛(GLA)和牛血清白蛋白(BSA)进行交联,制造出灵敏,可靠的双层电位磷酸盐生物传感器。内层BSA–GLA可改善外层BSA–GLA–PNP–XOD的附着力,并确保磷酸盐生物传感器的稳定性。该传感器稳定性强,使用一个多月后,初始电位值仅变化10%;灵敏度高,检测限低至20 μmol·L−1[53]。其他确定磷酸盐的双酶组合还包括碱性磷酸酶(ALP)和葡萄糖氧化酶(GOD)等[60]。
-
酶生物传感器主要是通过测量酶催化作用产生或者消耗化学活性物质来对磷酸盐进行定性或者定量,但只有少数酶具有产生这些氧化还原活性物质的能力,因此这限制了单酶传感器开发数量。目前针对这个问题,可以通过耦合反应循环途径中不同的酶,以此开发多酶传感器来解决。其中多是基于以下四种不同酶,包括麦芽糖磷酸化酶(MP),酸性磷酸酶(AP),葡萄糖氧化酶(GOD)以及突变酶(MR),其中相互之间催化反应关系如下[54]:
Conrath等[54]将这四种酶共同固定在再生的纤维素膜上,该膜安装在铂电极的顶端,用于检测酶促反应形成的过氧化氢。由此构造的多酶传感器在0.1—1 μmol·L−1浓度范围内呈现良好响应,检测限为10−8 mol·L−1。后续结合流动分析系统改善测量设备,减少由于批次分析造成的葡萄糖累积从而导致的漂移。实际监测废水,可以通过在FIA系统的采样部分安装固定了GOD和过氧化氢酶的滤芯来排除葡萄糖和过氧化氢的干扰。Mousty等[55]将MP、MR和GOD混合物包裹在无机皂石黏土中制造出用于磷酸盐的生物传感器。传感器检测范围1—50 μmol·L−1,检测限为1 μmol·L−1,最大响应电流稳定至少2周,成功应用于地表水中磷酸盐的检测。目前多酶传感器也存在急需解决的缺陷,数量过多的酶将会导致过多的非特异性反应,并且酶的不稳定性也会引起传感器性能的波动。因此在平面中固定三到四种酶,且各种酶在固定化条件下仍然保持活性是一项艰难的任务。
-
文献中还报道了通过某些非传统方法来检测磷酸根离子,如电导分析法[61,62]、电化学发光法[63]、以及基于微流控的离子通道感应系统[64]。非传统方法具有自身独特的优势,但由于发展时间较短,也存在一些不可忽略的弊端。比如电导分析法是测量电阻率的另一种方法,具有响应迅速,灵敏度高的优点,但仍然面临选择性偏差的缺点。电化学发光法较荧光法相比,不需要外来的光源,从而减少了光散射,降低了噪音信号的干扰,提高了检测的灵敏度;缺陷是选择性差,会对一系列化合物作出反应。微流控技术传质效率及精密度高,但存在价格昂贵的问题。Zhang等[62]构建的单酶传感器可以在20—50 ℃温度下正常工作,并且约10 s内达到稳态电导的90%。传感器具有两个线性浓度范围(1.0—20 μmol·L−1;20—400 μmol·L−1),检测限为1.0 μmol·L−1;并且其他阴离子(如
${\rm{SO}}_4^{2-} $ 、Cl−、${\rm{NO}}_3^{-} $ 、${\rm{NO}}_2^{-} $ 和${\rm{HCO}}_3^{-} $ )无明显干扰。然而,传感器对pH和稳定比较敏感,三周后性能有所下降。Xue等[63]利用磷钼酸于质子化的罗丹明B形成的疏水性复合物提取到碳糊电极上。在施加1.3 V电压后,罗丹明B被氧化产生电化学发光信号。其中,信号强度与磷酸盐浓度在2.0×10−10—1.0×10−8 g·mL−1之间呈现出良好的线性关系,并且传感器表现出灵敏度高选择性强的优点。 -
为了满足社会发展对磷酸盐含量监测的需求,需要一种经济高效的传感器,该传感器不仅灵敏性强,还具有抵抗外界环境干扰的能力以及能够适用于实地检测。离子选择性电极成本低,仪器要求低,商业生产中具有很大优势,且适用于实地分析,临床检测和遥感。当前新技术的发展也为样品的测量带来了新的进展,包括微型传感器,纳米复合材料,金属络合物等。然而,离子选择性电极仍然面临着选择性或耐久性差的问题。基于现场测量的另一种广泛使用的技术是电化学还原钼酸铵对磷酸盐进行检测。电位选择性和便携性与微型化相结合,使得利用电化学还原钼酸铵测定磷酸盐具有很高的通用性。纳米材料修饰电极为酶的固定提供了更大的表面积和更快的离子转移速度,例如金纳米阵列,聚吡咯膜等。与其他传感器相比,纳米生物传感器表现出更宽的磷酸盐浓度线性范围。此外,丝网印刷电极的应用很好的解决部分生物传感器不稳定的问题。然而,环境温度以及酶活性给传感器带来的影响是不可忽视的,这是限制生物传感器进行商业化的重要因素。总之,电化学方法适用于对环境水样中的磷酸盐浓度进行测量,但实现实地连续监测海水、河水以及废水中的磷酸盐仍需要不断改进研究方法。此外,生物医学领域所需的传感器与环境领域的有所不同,两者是在不同的复杂介质中工作,因此针对生物医学领域的研究也需不断加强。
电化学方法在无机磷酸盐检测中的应用
Applications of electrochemical methods in inorganic phosphate detection
-
摘要: 过多的磷将导致严重的生态环境和生理健康危害,包括水体富营养化,水生生物死亡以及人类的高磷血症等。因此,针对磷酸盐检测技术的研究变得至关重要。与传统比色法相比,电化学方法具有很多优势,包括选择性强,成本低,响应时间短且能满足在线监测的要求。本篇综述讨论电化学传感器在检测无机磷酸盐方面的研究进展,按照使用的电化学检测技术(电位法、安培法、伏安法、电导分析法等)对各种传感器进行分类。根据灵敏度、特异性、应用范围以及现场测量适用性等性能,对各类传感器的优劣势进行分析比较。Abstract: Excess phosphate in water could lead to serious ecological and physiological problems, including eutrophication, death of aquatic organisms and hyperphosphatemia of people. Hence, it is necessary and exigent to study analytical techniques for detection of phosphate. Electrochemical methods for phosphate sensing have many advantages over colourimetry, including excellent selectivity, cost-effectiveness, short response time and online operation. The review considers the progress in the development of phosphate sensors prepared by electrochemical methods. The various sensing strategies can be classified as potentiometry, amperometry, voltammetry, conductometric analysis and others on the basis of electrochemical detection technology. The advantages and disadvantages of various phosphate sensors are assessed detailedly in this review on basis of their performances, including sensitivity, selectivity, application and suitability for field measurements.
-
Key words:
- inorganic phosphate /
- electrochemical sensing /
- potentiometry /
- amperometry /
- biosensors
-

-
图 1 磷酸盐传感的信号反应机制[33]
Figure 1. Response mechanism of the phosphate sensing
图 2 (A)磷酸盐传感的连续流动装置图;(B)电极表面磷钼酸盐络合物的形成及还原反应[48]
Figure 2. (A)Schematic representation of the apparatus for continuous flow for phosphate analysis; (B)Reaction involved in the formation of the phosphomolybdate complex and its reduction at the working electrode surface
表 1 电位法
Table 1. Potentiometry
方法类型
Methods电极
Electrodes线性范围/ (mol·L−1)
Concertration range检测限 (μmol·L−1)
Detection limit应用领域
Application参考文献
Reference电位法——离子载体膜选择性电极 双(二溴苯基锡烷基)甲烷/聚氯乙烯膜 5×10−5—5×10−3 0.5 — [30] 壳聚糖-蒙皂石/聚氯乙烯膜 1×10−6—1×10−5 0.6 — [31] 有机锡/聚氯乙烯膜 5×10−6—1×10−1 1 — [32] Cu2+−BPMP/聚氯乙烯膜 3×10−6—5×10−5 0.5 矿泉水 [33] 电位法——刚性基质膜选择性电极 沸石/碳糊电极 1.58×10−5—1×10−2 12.8 肥料 [34] 铝-磷酸铝-铜粉/玻璃管 1×10−6—1×10−1 <1 河水、湖水 [35] 硫酸钴-硫酸钠膜/玻碳电极 1×10−4—1×10−1 63 — [36] 钴-氧化钴/镁铝复合金属氧化
物/聚氯乙烯膜1×10−4—1×10−1 76.5 — [37] 钼电极 1×10−5—1×10−1 1.9 河水 [38] 钼酸铵/银纳米线/丝网印刷电极 5×10−6—1×10−3 3 湖水 [39] 表 2 安培法及电化学生物传感器
Table 2. Amperometry and Electrochemical biosensors
方法类型
Methods电极
Electrodes线性范围/ (mol·L−1)
Concertration range检测限/ (μmol·L−1)
Detection limit应用领域
Application参考文献
Reference安培法——电化学还原钼酸铵 纸基/丝网印刷电极 1×10−5—3×10−4 4 河水 [47] 炭黑丝网印刷电极/
自动注射
分析系统1×10−6—8×10−5 6 水龙头,河水,湖水 [48] 碳糊电极 1×10−6—2×10−5 0.3 海水 [49] 生物电分析法——
单酶传感器金纳米阵列-氧化亚铜-聚(二烯丙基二甲基氯化铵)/PyOx 1×10−8—8×10−5 4×10−4 食品 [50] 金纳米阵列/PyOx 1.25×10−7—1×10−3 0.1 池塘水 [51] 生物电分析法——
双酶传感器聚吡咯膜/NP-XOD 电位型:2×10−2—2×10−1 2×104 河水 [52] 安培型:1×10−4—1×10−3 1×104 — 生物电分析法——
双酶传感器牛血清白蛋白-戊二醛/NP-XOD-
牛血清白蛋白-戊二醛1×10−6—5×10−5 20 河水 [53] 生物电分析法——
多酶传感器MP-AP-MR-GOD/铂电极 1×10−7—1×10−6 0.01 废水 [54] MP-MR-GOD/无机皂石黏土 1×10−6—5×10−5 1 地表水 [55] 注:PyOx:丙酮酸氧化酶、NP:核苷磷酸化酶、XOD:黄嘌呤氧化酶、MP:麦芽糖磷酸化酶、AP:酸性磷酸酶、MR:突变酶、GOD:葡萄糖氧化酶. -
[1] 骆芳萍. 基于金属钼离子选择电极的磷酸根检测方法的研究[D]. 无锡: 江南大学, 2019. LUO F P. Study on phosphate detection method based onmetal molybdenum ion selective electrode[D]. Wuxi: Jiangnan University, 2019(in Chinese).
[2] 崔丽娜. 水质磷酸盐检测技术研究[D]. 上海: 上海海洋大学, 2018. CUI L N. Study on the determination technology of phosphate in water[D]. Shanghai: Shanghai Ocean University, 2018(in Chinese).
[3] KATAYEV E, USTYNYUK Y A, SESSLER J L. Receptors for tetrahedral oxyanions [J]. Coordination Chemistry Reviews, 2006, 250(23): 3004-3037. [4] WARWICK C, GUERREIRO A, SOARES A. Sensing and analysis of soluble phosphates in environmental samples: A review [J]. Biosensors and Bioelectronics, 2012, 41(1): 1-11. [5] 喻航. 水体富营养化的危害及防治对策 [J]. 智能城市, 2019, 5(17): 147-148. YU H. Harm of water eutrophication and prevention measures [J]. Intelligent City, 2019, 5(17): 147-148(in Chinese).
[6] TIESSEN H. Phosphorus in the Global Environment[M]. Berlin: Springer Netherlands, 2008: 631-632. [7] KAWASAKI H, SATO K, OGAWA J, et al. Determination of inorganic phosphate by flow injection method with immobilized enzymes and chemiluminescence detection [J]. Analytical Biochemistry, 1989, 182(2): 366-370. doi: 10.1016/0003-2697(89)90609-X [8] 韦珍妮. 浅谈高磷血症致慢性肾脏病血管钙化的研究及治疗进展 [J]. 临床检验杂志(电子版), 2020, 9(1): 241-242. WEI Z N. Discussing the research and treatment progress of vascular calcification of chronic kidney disease caused by hyperphosphatemia [J]. Clinical Laboratory Journal (Electronic Edition), 2020, 9(1): 241-242(in Chinese).
[9] VORUM H, DITZEL J. Disturbance of Inorganic Phosphate Metabolism in Diabetes Mellitus: Its Relevance to the Pathogenesis of Diabetic Retinopathy [J]. Journal of Ophthalmology, 2014, 2014(8): 1-8. [10] HANRAHAN G, GLEDHILL M, FLETCHER P J, et al. High temporal resolution field monitoring of phosphate in the river frome using flow injection with diode array detection [J]. Analytica Chimica Acta, 2001, 440(1): 55-62. doi: 10.1016/S0003-2670(00)01395-7 [11] BERCHMANS S, ISSA T B, SINGH P. Determination of inorganic phosphate by electroanalytical methods: A review [J]. Analytica Chimica Acta, 2012, 729(11): 7-20. [12] CAREY C M, VOGEL G L. Measurement of calcium activity in oral fluids by ion selective electrode: Method evaluation and simplified calculation of ion activity products [J]. Journal of Research of the National Institute of Standards & Technology, 2000, 105(2): 267. [13] TOBEY S L, ANSLYN E V. Determination of inorganic phosphate in serum and saliva using a synthetic receptor [J]. Organic Letters, 2003, 5(12): 2029-2031. doi: 10.1021/ol034427x [14] LIU W Q, DU Z F, QIAN Y, et al. A specific colorimetric probe for phosphate detection based on anti-aggregation of gold nanoparticles [J]. Sensors & Actuators B Chemical, 2013, 176(1): 927-931. [15] WU H F, TONG C L. A specific turn-on fluorescent sensing for ultrasensitive and selective detection of phosphate in environmental samples based on antenna effect-improved FRET by surfactant [J]. Acs Sensors, 2018, 3(8): 1539-1545. doi: 10.1021/acssensors.8b00343 [16] CHEN C, ZHAO J H, LU Y Z, et al. A fluorescence immunoassay based on the phosphate-triggered fluorescence turn-on detection of alkaline phosphatase [J]. Analytical Chemistry, 2018, 90(5): 3505-3511. doi: 10.1021/acs.analchem.7b05325 [17] OGATA F, DAISUKE I, MEGUMU T. Adsorption of phosphate ion in aqueous solutions by calcined cobalt hydroxide at different temperatures [J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1570-1577. doi: 10.1016/j.jece.2015.05.028 [18] MURPHY J, RILEY J P. Modified single solution method for the determination of phosphate in natural water [J]. Analytica Chimica Acta, 1962, 27(1): 31-36. [19] UDNAN Y, MCKELVIE I D, GRACE M R, et al. Evaluation of on-line preconcentration and flow-injection amperometry for phosphate determination in fresh and marine waters [J]. Talanta, 2005, 66(2): 461-466. doi: 10.1016/j.talanta.2004.12.064 [20] 丁家旺, 秦伟. 电化学传感技术在海洋环境监测中的应用 [J]. 环境化学, 2013, 33(1): 53-61. DING J W, QING W. Applications of electrochemical sensing technologies in marine environmental monitoring [J]. Environmental Chemistry, 2013, 33(1): 53-61(in Chinese).
[21] MILLS G, FONES G. A review ofin situmethods and sensors for monitoring the marine environment [J]. Sensor Review, 2012, 32(1): 17-28. doi: 10.1108/02602281211197116 [22] 王维虎. 基于物联网的湖泊水域岸线及水质监控技术的研究[D]. 武汉: 华中师范大学, 2015. WANG W H. The research of monitoring technology shoreline and water quality of lake bassed on IOT[D]. Wuhan: Central China Normal University, 2015(in Chinese).
[23] 吴玲. 一种高灵敏检测尿酸的聚阳离子纳米复合膜电化学传感器//[C]. 北京: 中国化学会第29届学术年会2014: 300. WU L. A highly sensitive polycation nanocomposite membrane electrochemical sensor for detecting uric acid[C]. Beijing: the 29 th Academic Annual Conference of Chinese Chemical Society 2014: 300(in Chinese).
[24] 付海曦, 刘威, 张春辉, 等. 水体中重金属离子的检测方法研究进展 [J]. 理化检验(化学分册), 2012, 48(4): 496-503. FU H XI, LIU W, ZHANG C H, et al. Recent advance of detection method of heavy metal ions in water [J]. Physical and Chemical Inspection (Chemical Volume), 2012, 48(4): 496-503(in Chinese).
[25] HUSSAIN R, KHAN M Q, KHAN A A. Electrochemical sensing of Pb2+ ion in water by ion selective membrane electrode based on Polypyrrole titanium(IV)sulphosalicylo phosphate cation exchange nanocomposite [J]. Groundwater for Sustainable Development, 2019, 8(1): 216-225. [26] BAIG U, KHAN A A. Polyurethane-based cation exchange composite membranes: Preparation, characterization and its application in development of ion-selective electrode for detection of copper(II) [J]. Journal of Industrial and Engineering Chemistry, 2015, 29(1): 392-399. [27] PAL S, GHOSH T K, GHOSH R, et al. Recent advances in recognition, sensing and extraction of phosphates: 2015 onwards [J]. Coordination Chemistry Reviews, 2020: 405. [28] EVGENY A K, YURI A U, JONATHAN L S. Receptors for tetrahedral oxyanions [J]. Coordination Chemistry Reviews, 2006, 250(23-24): 3004-3037. doi: 10.1016/j.ccr.2006.04.013 [29] HYDE A M, ZULTANSKI S L, WALDMAN J H, et al. General principles and strategies for salting-out informed by the Hofmeister series [J]. Organic Process Research & Development, 2017, 21(9): 1355-1370. [30] SATOH H, MIYAZAKI Y, TANIUCHI S, et al. Improvement of a phosphate ion-selective microsensor using bis(dibromophenylstannyl)methane as a carrier. [J]. Analytical Chemistry, 2017, 33(1): 825-830. [31] TOPCU C, CAGLAR B, ONDER A, et al. Structural characterization of chitosan-smectite nanocomposite and its application in the development of a novel potentiometric monohydrogen phosphate-selective sensor [J]. Materials Research Bulletin, 2018, 98(1): 288-299. [32] LIU D, CHEN W C, YANG R H, et al. Polymeric membrane phosphate sensitive electrode based on binuclear organotin compound [J]. Analytica Chimica Acta, 1997, 338(3): 209-214. doi: 10.1016/S0003-2670(96)00382-0 [33] 李龙. 基于新型分子识别机制的电位型传感器技术研究[D]. 烟台: 中国科学院烟台海岸带研究所, 2016. LI L. Studies on Potentiometric Sensing Platforms Based on Novel Molecular Recognition Mechanisms[D] Yantai: Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, 2016(in Chinese).
[34] EJHIEH A N, MASOUDIPOUR N. Application of a new potentiometric method for determination of phosphate based on a surfactant-modified zeolite carbon-paste electrode (SMZ-CPE) [J]. Analytica Chimica Acta, 2010, 658(1): 68-74. doi: 10.1016/j.aca.2009.10.064 [35] TAFESSE F, ENEMCHUKWU M. Fabrication of new solid state phosphate selective electrodes for environmental monitoring [J]. Talanta, 2011, 83(5): 1495. [36] 姜涛钦, 杨慧中. 一种固态磷酸根离子选择电极 [J]. 环境工程学报, 2016, 10(10): 5631-5636. doi: 10.12030/j.cjee.201504250 JIANG T X, YANG H Z. A solid-state phosphate ion-selective electrode [J]. Journal of Environmental Engineering, 2016, 10(10): 5631-5636(in Chinese). doi: 10.12030/j.cjee.201504250
[37] 李琳娜. 基于离子选择电极的磷酸根检测的研究[D]. 无锡: 江南大学, 2015. LI L N. Study on phosphate detection based on ion selective electrode[D]. Wuxi: Jiangnan University, 2015(in Chinese).
[38] YANG H Z, JIANG T Q, YU X D, et al. Phosphate sensor using molybdenum [J]. Journal of the Electrochemical Society, 2016, 163(9): 479-484. doi: 10.1149/2.0161609jes [39] KABIR M F, RAHMAN M T, GURUNG A, et al. Electrochemical phosphate sensors using silver nanowires treated screen printed electrodes [J]. IEEE Sensors Journal, 2018, 18(9): 3480-3485. doi: 10.1109/JSEN.2018.2808163 [40] 宋彩侨. 固态离子选择性电极检测血清钾离子[D]. 大连: 大连理工大学, 2019. SONG C Q. Determination of serum potassium ion by solid-state ion-selective electrodes[D]. Dalian: Dalian University of Technology, 2019(in Chinese).
[41] 秦溧. 基于二维MOF材料的纳米酶传感器阵列对磷酸盐的检测及其水解过程的监控[D]. 南京: 南京大学, 2019. QIN S. 2D-MOF nanozyme sensor arrays for probing phosphates and enzymatic hydrolysis[D]. Nanjing: Nanjing University, 2019(in Chinese).
[42] 卢圆圆. 化学修饰电极对土壤重金属和磷酸盐离子的灵敏检测[D]. 杭州: 浙江大学, 2019. LU Y Y. The sensitive detection of heavy mental ions and phosphate anions in soil by chemically modified electrodes[D]. Hangzhou: Zhejiang University, 2019(in Chinese).
[43] XIAO D, YUAN H Y, LI J, et al. Surface modified cobalt-based sensor as a phosphate-sensitive electrode [J]. Analytical Chemistry, 1995, 67(2): 288-291. doi: 10.1021/ac00098a009 [44] 吴星. 改性UiO-66和氢氧化锆对磷酸根去除性能研究[D]. 南昌: 南昌航空大学, 2018. WU X. Study on the removal performance of phosphate by modified UiO-66 and zirconium hydroxide[D]. Nangchang: Engineering Nanchang Hangkong University, 2018(in Chinese).
[45] KHALED E, HASSAN H. N. A, GIRGIS A, METELKA R. Construction of novel simple phosphate screen-printed and carbon paste ion-selective electrodes [J]. Talanta, 2008, 77(2): 737-743. doi: 10.1016/j.talanta.2008.07.018 [46] KUGIMIYA A, KOHARA K. Biomimetic sensor for cAMP using an ion-sensitive field-effect transistor [J]. Materials Science & Engineering C, 2009, 29(3): 959-962. [47] CINTI S, TALARICO D, PALLESC G, et al. Novel reagentless paper-based screen-printed electrochemical sensor to detect phosphate [J]. Analytica Chimica Acta, 2016, 919(1): 78-84. [48] TALARICO D, CINTI S, ARDUINI F, et al. Phosphate detection through a cost-effective carbon black nanoparticle-modified screen-printed electrode embedded in a continuous flow system [J]. Environmental Science & Technology, 2015, 49(13): 7934-7939. [49] QUINTANA J C, IDRISSI L, PALLESCHI G, et al. Investigation of amperometric detection of phosphate: Application in seawater and cyanobacterial biofilm samples [J]. Talanta, 2004, 63(3): 574. [50] HE B S, LIU H. Electrochemical biosensor based on pyruvate oxidase immobilized AuNRs@Cu2O-NDs as electroactive probes loaded poly (diallyldimethylammonium chloride) functionalized graphene for the detection of phosphate[J]. Sensors and Actuators B: Chemical, 2020, 304: 127-303. [51] OGABIELA E, ADELOJU S B, CUI J W, et al. A novel ultrasensitive phosphate amperometric nanobiosensor based on the integration of pyruvate oxidase with highly ordered gold nanowires array [J]. Biosensors & Bioelectronics, 2015, 71(1): 278-285. [52] LAWAL A, ADELOJU S B. Polypyrrole based amperometric and potentiometric phosphate biosensors: A comparative study B [J]. Biosensors & Bioelectronics, 2013, 40(1): 377-384. [53] ADELOJU S B, LAWAL A T. Fabrication of a bilayer potentiometric phosphate biosensor by cross-link immobilization with bovine serum albumin and glutaraldehyde [J]. Analytica Chimica Acta, 2011, 691(1-2): 89-94. doi: 10.1016/j.aca.2011.02.020 [54] CONRATH N, GRUNDING B, HUWEL S, et al. A novel enzyme sensor for the determination of inorganic phosphate [J]. Analytica Chimica Acta, 1995, 309(1-3): 47-52. doi: 10.1016/0003-2670(95)00065-8 [55] MOUSTY C, COSNIER S, SHAN D, et al. Trienzymatic biosensor for the determination of inorganic phosphate [J]. Analytica Chimica Acta, 2001, 443(1): 1-8. doi: 10.1016/S0003-2670(01)01188-6 [56] MARIAULLE P, SINAPI F, LAMBERTS L, et al. Application of electrodes modified with ion-exchange polymers for the amperometric detection of non-redox cations and anions in combination to ion chromatography [J]. Electrochimica Acta, 2001, 46(23): 3543-3553. doi: 10.1016/S0013-4686(01)00636-3 [57] TORRES F M, ESTELA J M. Sequential injection spectrophotometric determination of orthophosphate in beverages, wastewaters and urine samples by electrogeneration of molybdenum blue using tubular flow-through electrodes [J]. Analytica Chimica Acta, 2004, 510(1): 61-68. doi: 10.1016/j.aca.2003.12.051 [58] HE J, SUN H, DAI J, et al. In situ growth of nanoflake and nanoflower-like Ni hydrated hydroxide on the surface of Ni foam as a free-standing electrode for high-performance phosphate detection[J]. Journal of Hazardous Materials, 2020, 392: 122-313. https://doi.org/10.1016/j.jhazmat.2020.122313. [59] UPADHYAY L S B, VERMA N. Recent advances in phosphate biosensors [J]. Biotechnology Letters, 2015, 37(7): 1335-1345. doi: 10.1007/s10529-015-1823-3 [60] COSNIER S, GONDRA C, WATELET J C, et al. A bienzyme electrode (alkaline phosphatase-polyphenol oxidase) for the amperometric determination of phosphate [J]. Analytical Chemistry, 1998, 70(18): 3952-3956. doi: 10.1021/ac980125a [61] HOUSSEMEDDINE E, CLAUDE D. A self-assembled monolayers based conductometric algal whole cell biosensor for water monitoring [J]. Microchimica Acta, 2008, 163(3-4): 79-184. [62] ZHANG Z Q, JAFFREZIC R N, BESSUEILLE F O, et al. Development of a conductometric phosphate biosensor based on tri-layer maltose phosphorylase composite films [J]. Analytica Chimica Acta, 2008, 615(1): 73-79. doi: 10.1016/j.aca.2008.03.044 [63] XUE Y, ZHENG X Y, LI G X. Determination of phosphate in water by means of a new electrochemiluminescence technique based on the combination of liquid–liquid extraction with benzene-modified carbon paste electrode [J]. Talanta, 2007, 72(2): 450-456. doi: 10.1016/j.talanta.2006.11.003 [64] XU J J, PENG Y, BAO N, et al. In-channel indirect amperometric detection of nonelectroactive anions for electrophoresis on a poly(dimethylsiloxane) microchip [J]. Electrophoresis, 2005, 26(19): 3615-3621. doi: 10.1002/elps.200410401 -



 下载:
下载: