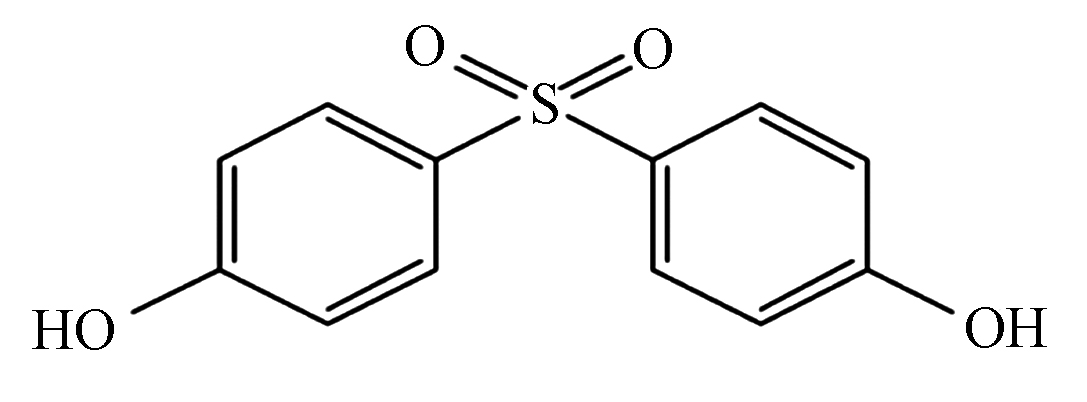
-
双酚A(bisphenol A, BPA)作为一种有机化工原料,广泛应用于聚碳酸酯和环氧树脂等材料的合成[1]。然而由于BPA具有内分泌干扰效应,许多国家已限制或禁止其用于食品包装,婴儿用品和热敏纸等产品的生产[2]。双酚S(bisphenol S, BPS)因其结构和性质与BPA相似,且含有的刚性O=S=O官能团使其表现出比BPA更高的热稳定性[3],被视为BPA的“安全替代物” [4]。随着BPS的广泛应用使其不可避免的进入环境中,目前BPS已在食物、个人护理产品、沉积物、甚至人体尿液中被检测到[5-6]。有研究报道BPS具有与BPA类似的内分泌干扰效应,如较低浓度(<100 μg·L−1)的BPS会引起斑马鱼的发育毒性,损坏其正常繁殖,并干扰其体内的类固醇平衡[7];高浓度的BPS(3.0 mg·L−1)还会对斑马鱼的胚胎神经系统产生严重影响[8]。因此,亟需开展工作去研究BPS在环境中的归趋及环境风险等问题。
土壤是有机污染物在环境中主要的汇,有机污染物在土壤中的吸附行为将影响其在土壤中的迁移转化和生物有效性。当前,已有大量文献研究了BPA在土壤上的环境行为和吸附机制[9-11],而对BPS的相关研究主要集中在毒性、内分泌干扰效应[11-12]方面。虽然近期有少量研究关注BPS在土壤中的吸附解吸等行为[13],但BPS在土壤中吸附机制尚未清晰。另外,人为耕作中的施肥、翻耕等过程将显著改变土壤的理化性质[14],势必对BPS在土壤中的吸附产生影响。因此,本研究将对比分析人为耕作对BPS吸附的影响,深入探究BPS在土壤中的吸附机制,为理解BPS在土壤中的迁移转化及环境风险评价提供重要的视角。
本研究选取云南两个典型地区的耕作和未耕作的土壤,以及滇池周边的泥炭土作为高有机质土壤的参照,研究BPS以及BPA在不同土壤中的吸附行为,旨在揭示BPS在土壤中的吸附机制,为理解BPS环境行为和风险评估提供基础数据。
-
BPA和BPS均为分析纯(99.9%),其中BPA购自中国国药集团化学试剂有限公司,BPS购自阿拉丁中国试剂有限公司。两种化合物的理化性质及分子结构见表1。
土壤样品收集于云南典型地区,如元阳、蒙自。其中,元阳万亩梯田已耕作千年,蒙自是道地药材三七的主产地。采集滇池周边的泥炭土作为高有机质土壤的参照。采样点均远离工业区,以保证污染物含量相对较低。梯田未耕作(YN)和梯田水稻田(YP)以及梯田旱地(YD)均取自云南元阳,位于东经102.82062°—102.82661°, 北纬23.22097°—24.23479°。未种植三七土(MN)和三七种植土(MP)取自蒙自,位于东经103.78113°—103.78977°, 北纬23.40204°—23.41552°。泥炭土(DP)取自滇池周围,其经纬度为东经102.69186°—102.69202°, 北纬24.73001°—24.73101°。土样采集后,风干,剔除其中的枯枝落叶等植物残体和石块,并将土壤样品碾碎,但应尽量保持颗粒的完整性,随后过2 mm筛子,避光保存以备用。
-
将BPA和BPS溶于0.02 mol·L−1 NaCl(保持离子强度)和200 mg·L−1 NaN3(抑制微生物活性)的背景液中,配制成100 mg·L−1的储备液,并用背景液将储备液逐级稀释为1、2、4、6、10、20、34、64 mg·L−1,且每个浓度点设置两个平行样。根据预实验,BPA和BPS的固液比均为25∶2时,能确保吸附率在20%—80%之间。吸附实验在8 mL带聚四氟乙烯垫螺旋口塞玻璃瓶中进行,并将所有样品瓶置于恒温振荡器(25 °C)中,以80 r·min−1转速平衡7 d。根据预实验的结果,吸附在7 d后达到表观平衡,因此7 d后,以2000 r·min−1转速离心15 min,取上清液于高效液相色谱仪(HPLC, Agilent Technologies 1260)定量分析。液相色谱分析条件如下: C18反向色谱柱(5 mm, 4.6 mm×150 mm)。BPA和BPS流动相分别为乙腈:去离子水=40∶60 (V∶V)、乙腈:去离子水=35∶65 (V∶V),紫外检测波长分别为280 nm和257 nm。流动相的流速均为1 mL·min−1。
-
土壤样品的C、H、O、N和S等元素通过元素分析仪(MicroCube, Elemental, Germany)测定得到。通过比表面积分析仪(Autosorb-1C, Quantachromeo)应用BET方法分析土样的比表面积。采用X射线衍射分析仪(XRD, D/Max2200, Rigaku, Japan)对土样的矿物成分进行分析。应用傅里叶红外光谱仪(Varian 640-IR, USA)来分析土样的表面官能团的特征。
-
采用Freundlich (FM) 模型对实验数据进行拟合,以期探究不同耕作方式的土壤对BPA及其替代物BPS的吸附机制。FM方程式如下:
式中,Se (mg·kg−1)和Ce (mg·L−1)分别为固相平衡浓度和液相浓度;KF [(mg·kg−1)/(mg·L−1)n]为Freundlich吸附系数,n为Freundlich非线性系数。
由于不同方程中参数个数不同,可决系数(r2)不能直接用于比较拟合效果。因此,本研究采用可调可决系数(r2adj)来确定模型的拟合优度,调整方程如下:
式中,m为拟合方程中的参数个数;N为拟合的数据个数。
-
土壤样品的主要元素组成、理化性质见表2。由表2可知,人为耕作活动对土壤有机碳的影响随土壤所在区域的不同而表现出较大的差异。耕作后,元阳土壤有机碳的含量增加,但蒙自土壤有机碳含量减少。元阳背景土壤中有机含量较低,耕作过程中施加的肥料及秸秆还田会导致土壤有机质碳增加。另外,所种植的水稻收获后其根部残留在土壤中,通过微生物作用进一步转化成土壤有机质,也将导致土壤有机碳增加。蒙自背景土壤有机碳含量高于元阳土壤,但耕作后土壤的有机碳却减少。这可能是由于所种植三七成熟后主要收获其根部,同时在种植三七的过程中,其根部会分泌较多的小分子有机酸,有机酸会破坏土壤矿物结构[17-18],减弱矿物对有机碳的保护,从而促使土壤有机碳流失或发生转化[19-20]。泥炭土中由于残留了较多的由动植物残体形成的腐殖质,故其有机碳含量明显高于其他土壤。农耕后元阳和蒙自土样的比表面积明显减少,一般情况下土壤有机质(SOM)含量降低,土壤的孔隙结构减少,相应的比表面积会减少。而元阳土壤的SOM增加比表面积却减少可能是因为SOM会掩盖矿物颗粒上的孔并且促进土壤颗粒的团聚[21]。Pignatello等[22]认为尽管土壤富含大量的微孔,但由于SOM对孔的掩盖使得基于N2的BET方法无法测得微孔的数据。Drillia等[23]也观察到当土壤有机碳含量增加时比表面积却减少的现象。
H/C和(O+N)/C的比值能分别指示土壤有机质的芳香性和极性[24]。元阳土样农业耕作后,(O+N)/C和H/C比值均显著降低,这可能是耕作过程中施肥增加了土壤C含量,从而使土壤的憎水性和芳香性增高,即土壤从“软质碳”过渡到“硬质碳”,元阳土样的红外数据也表明耕作后脂肪族化合物(C—O—C)的含量有所降低(图1)。而在种植三七的蒙自土壤(MP)耕作后,H/C和(O+N)/C比值却呈现出上升的趋势,这主要由于耕作后土壤有机碳有所降低。三七根部分泌的有机酸破坏土壤矿物[16],促进土壤有机碳的转化[20]从而导致土壤C含量降低,而O含量的升高可能是分泌的酸残留在土壤中所导致。Drever等[18]和Keiluweit等[20]研究发现根系分泌物能够促进矿物的溶解,释放有机物,导致碳损失。蒙自土样的红外数据也表明耕作后脂肪族化合物(C—O—C)的含量有所升高(图1)。
阳离子交换量(CEC)是评价土壤土壤缓冲性能和肥力的重要指标[25]。农耕后土壤的CEC显著提升,是因为长期耕作过程中施加肥料所导致的。张继光等[26]研究发现,长期施肥可显著提升红壤旱地土壤的CEC。另外,尽管DP的C含量比其它土壤高出一个数量级,但CEC相当,Mader等[27]认为碳化程度高的土壤富含更紧致的腐殖质,从而导致土壤的CEC的降低。
-
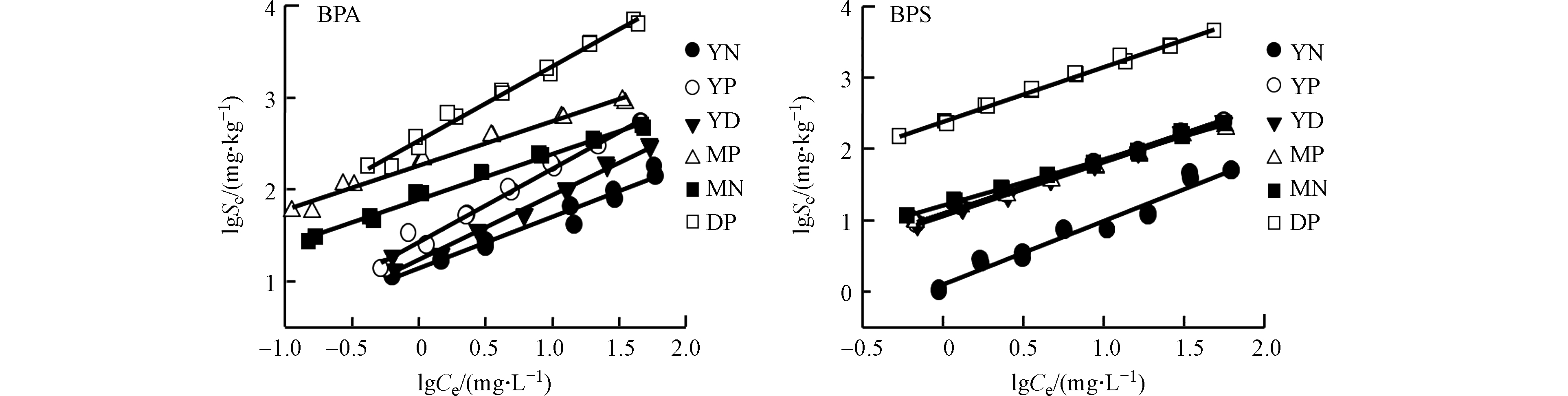
BPA和BPS在不同土壤上的吸附等温线如图2所示。通过Freundlich吸附模型拟合后,可调可决系数r2adj在0.953—0.997之间,表明Freundlich吸附模型能较好拟合BPS和BPA土壤样品的吸附等温线,因此,可采用Freundlich拟合结果对BPS和BPA在土壤中的吸附行为和吸附机制进行分析。参数n值可反映吸附的非线性程度,当n越接近0时,吸附等温线的非线性越强,表明土壤中有机质的浓缩程度越高,对有机物的结合能力也就越强[27]。BPA和BPS的n值的范围分别为0.483—0.801和0.648—0.889(表3),表现出较高的非线性吸附。
通过计算单点吸附系数Kd (Ce=1 mg·L−1和Ce=10 mg·L−1)来比较BPS和BPA在不同土壤上的吸附性能。由表3可知,BPS和BPA的Kd值随其初始浓度的增加而减小,这与其非线性吸附有关。同时,也表明BPS和BPA在土壤上的吸附首先是占据高能量点位的过程。Sun等[11]在研究菲在土壤上的吸附等温线时也得到了类似的结果。耕作后,除蒙自土壤(MP)上吸附BPS的Kd值有所降低外,其他土壤吸附BPS和BPA的Kd值均明显增加。MP上吸附BPS的Kd值有所降低是由于MP土壤的有机碳含量低于MN,憎水性作用减弱而导致吸附降低。但MP吸附BPA的Kd值却高于MN表明除憎水性作用外,BPA在MP上的吸附还受其他吸附机制的影响。
-
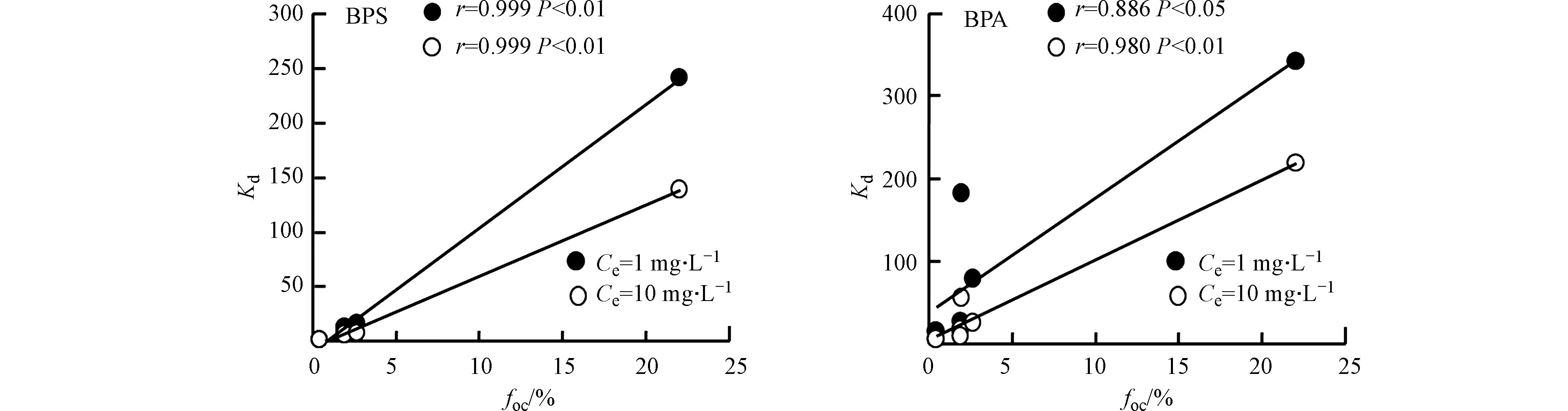
为了探究土壤有机碳对BPS和BPA吸附的影响,应用SPSS 19.0统计软件分析了单点吸附系数Kd (Ce=1 mg·L−1和Ce=10 mg·L−1)与土壤有机碳(foc)含量之间的相关性。如图3所示,BPA和BPS在低浓度(Ce=1 mg·L−1)和高浓度(Ce=10 mg·L−1)下的Kd与foc均显著正相关,表明foc是影响BPA和BPS在土壤上的吸附的主导因素,BPS和BPA在土壤上的吸附以憎水性作用为主。Sun等[11]研究发现憎水性是影响BPA等有机物在河流沉积物中吸附的重要因素。
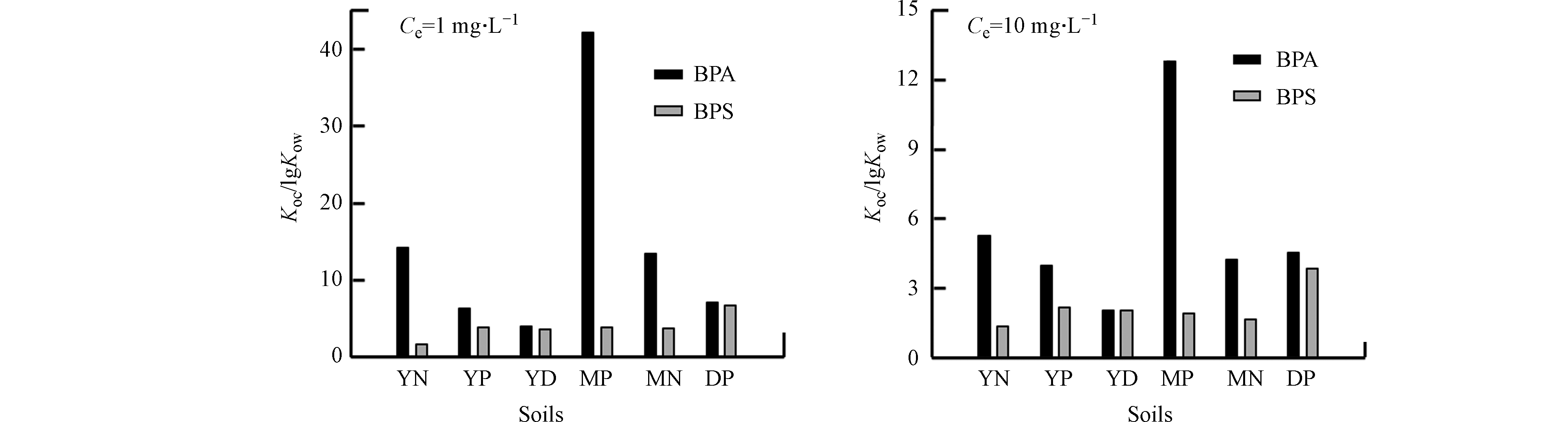
但用 foc对Kd进行标准化获得Koc(Koc=Kd/foc)后发现(表3),耕作前后BPS和BPA的Koc仍差异较大,表明除土壤有机碳外,还有其他机制影响BPS和BPA在土壤上的吸附过程。为进一步探究其它吸附机制,将Koc进行正辛醇-水分配系数标准化(Koc/lg Kow)以屏蔽憎水性作用作用(图4)。由图4可知,在Ce=1 mg·L−1和Ce=10 mg·L−1时,BPA的Koc/lg Kow比值均大于BPS,尤其在MP土壤上尤为明显.
之前研究表明有机污染在土壤上的吸附除憎水性作用外,静电作用、氢键作用以及电子供-受体作用也对吸附有贡献[28-29]。实验所用土壤样品的pHzpc为2.24—3.69(表2),吸附实验平衡前后的pH范围为5.4—6.3,表明在吸附过程中土壤颗粒始终带负电。BPS和BPA的pKa分别为8.2和10.1,即BPS和BPA在实验体系下以中性分子为主。因此,BPA和BPS在土壤上的吸附差异与静电作用无关。BPS和BPA分子结构上的羟基数量和位置是一致的,可排除氢键作用的影响。BPS和BPA的分子结构主要区别为连接两个苯环的官能团有所不同,BPS中连接两个苯环的为磺酸基团,而BPA为丙烷。磺酸基团为强吸电子基团[15],而丙烷为供电基团,另外BPS和BPA上的羟基为供电基团。因此BPA分子整体可作为电子供体,而BPS分子的供-受电子能力较弱。土壤有机质含有丰富的官能团,同时含有缺电子结构(如:醌)和富电子结构(如:酚)[30-31]。因此BPA与土壤有机质之间的电子共受体作用将强于BPS,导致BPA的Koc/lg Kow比值均大于BPS。
另外,通过Koc/lg Kow与(O+N)/C的相关性分析可以发现(图5),BPA体系中Koc/lg Kow与(O+N)/C成显著正相关,而BPS体系中Koc/lg Kow与(O+N)/C无明显相关性,这也进一步表明BPA的吸附与土壤表面官能团相关。
值得注意的是,BPA在MP土样上的Koc/lg Kow值远高于其它土样(图4)。李祖然等[32]观察到三七会分泌柠檬酸等低分子量的有机酸,而有机酸的—COOH可以作为电子受体。因此,MP土壤中三七根所分泌的柠檬酸会增强土壤的电子受体能力,从而增强BPA-MP之间的电子供-受体作用,使得BPA在MP土壤中的Koc/lg Kow比值最高。
-
(1)人为耕作会增加土壤有机碳的含量,但某些作物根系分泌的小分子有机酸会破坏土壤矿物结构而导致土壤有机碳降低。
(2)土壤有机碳是影响BPA和BPS在土壤中吸附的主导因素,BPA和BPS在土壤上的吸附以憎水性作用为主。
(3)由于官能团供-受电能力的差异,BPA与土壤有机质之间的电子供-受体作用强于BPS,综合憎水性作用和电子供受体作用,BPA在土壤上的吸附高于BPS。
双酚S及双酚A在云南典型耕作土壤上的吸附机制
Adsorption mechanism of bisphenol S and bisphenol A on typical cultivated soil in Yunnan Province
-
摘要: 近年来,双酚S(BPS)作为双酚A(BPA)的替代物被大量引入土壤环境,研究发现BPS 具有与BPA类似的内分泌干扰效应,亟需开展工作研究BPS在土壤中的环境行为。本研究选取云南元阳、蒙自两地区耕作前后的土壤样品,通过批量吸附实验研究BPS以及BPA在云南典型耕作土壤中的吸附机制。结果表明,Freundlich模型能较好拟合BPS和BPA在土壤上的吸附等温线(r2adj:0.953—0.997),且表现出较高的非线性吸附。吸附系数Kd与土壤有机碳(foc)呈显著正相关,表明BPS和BPA在土壤上的吸附以憎水性作用为主。排除憎水性作用后,BPA在土壤上的吸附高于BPS 是由于BPA与土壤有机质之间的电子供-受体作用强于BPS所导致的。蒙自耕作土壤中(MP)三七根所分泌的有机酸可能会破坏土壤矿物结构而导致土壤有机质有所降低,但有机酸分子中的羧基可增强土壤的电子受体能力,使得BPA与MP土壤之间形成强烈的电子供-受体作用而使其吸附增强。Abstract: Bisphenol S has been introduced into the soil environment as a substitute for Bisphenol A. However, studies have shown that BPS has similar estrogenic activity as BPA, and it is essential to investigate the environmental behavior of BPS in soil. The native and cultivated soil in Yuanyang and Mengzi of Yunnan were collected, and the adsorption mechanism of BPS and BPA on typical native and cultivated soil in Yunnan was studied through batch adsorption experiments. The results showed that the Freundlich model could well fit the adsorption isotherms of BPS and BPA on soils (r2adj: 0.953—0.997), and the adsorption isotherms exhibited significant nonlinear. The adsorption coefficient Kd is significantly positive correlated with soil organic carbon (foc), indicating that the adsorption of BPS and BPA on the soils is dominated by hydrophobicity effect. Higher adsorption of BPA on soils than that of BPS when hydrophobic effects were excluded is owing to the electron donor-acceptor interaction between BPA and soil organic matter is stronger than that of BPS. The organic acids secreted by the roots of Notoginseng in Mengzi tillage soil (MP) may destroy the soil mineral structure and then lead to a decrease in soil organic matter. However, the carboxyl groups in the organic acid molecules can enhance the electron-donating ability of the MP, then strengthen the electron donor-acceptor interaction between BPA and MP, resulting in higher adsorption of BPA than BPS on MP.
-
Key words:
- BPS /
- artificial farming /
- adsorption mechanism /
- root exudates
-

-
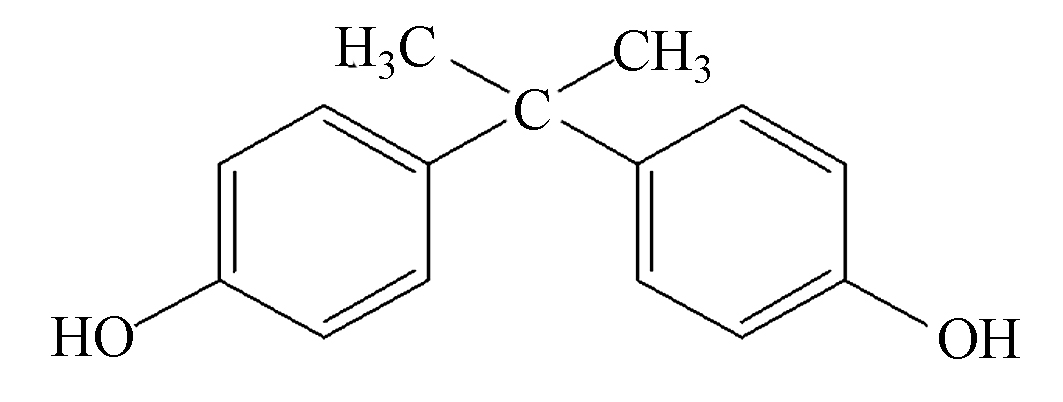
表 1 BPS和BPA的理化性质及分子结构
Table 1. The physicochemical properties and molecular structure of BPS and BPA
表 2 土壤样品的理化性质
Table 2. The physicochemical properties of soil samples
样品 元素含量Element content /% 原子比Atomic ratio pHzpc CEC/
(cmol·kg−1)SA/
(m2·g−1)C H O N S H/C (N+O)/C YN 0.45 0.97 7.90 0.06 0.08 25.68 13.24 2.24 2.12 33.94 YP 1.93 1.01 8.38 0.18 0.04 6.25 3.32 2.87 6.68 18.36 YD 1.94 1.02 8.59 0.19 0.05 6.30 3.40 2.78 4.83 18.36 MN 2.66 1.80 13.96 0.23 0.07 8.13 4.01 3.69 5.33 82.01 MP 1.96 1.71 15.51 0.18 0.06 10.47 6.02 3.63 8.18 35.02 DP 22.04 3.01 17.03 1.27 0.38 1.64 0.63 3.54 7.81 2.62 表 3 BPS和BPA在土壤上的吸附等温线拟合参数
Table 3. Isotherms fitting results of BPA and BPS on soils by Freundlich model
样品
Samples土样
Soillg KFa n r2adj SEEb Kd Koc Ce=1 mg·L−1 Ce=10 mg·L−1 Ce=1 mg·L−1 Ce=10 mg·L−1 BPS YN 0.093 0.889 0.953 0.121 1.24 0.96 2.75 2.13 YP 1.089 0.748 0.997 0.025 12.27 6.88 6.33 3.55 YD 1.051 0.758 0.997 0.027 11.26 6.45 5.80 3.32 MN 1.204 0.648 0.996 0.028 16.00 7.12 6.01 2.67 MP 1.091 0.690 0.995 0.033 12.32 6.03 6.29 3.08 DP 2.383 0.760 0.996 0.033 241.27 138.71 10.95 6.29 YN 1.146 0.572 0.985 0.053 14.00 5.22 30.99 11.56 BPA YP 1.424 0.801 0.985 0.067 26.52 16.77 13.68 8.65 YD 1.228 0.707 0.977 0.072 16.91 8.61 8.71 4.43 MN 1.892 0.497 0.987 0.050 78.02 24.51 29.30 9.20 MP 2.258 0.483 0.980 0.062 181.05 55.03 92.46 28.10 DP 2.533 0.806 0.986 0.064 341.27 218.17 15.48 9.90 a lg KF的单位为[(mg·kg−1)/(mg·L−1)n];b SEE为标准估计误差;r2adj为可调可决系数。 -
[1] LI J, ZHENG L, WANG S L, et al. Sorption mechanisms of lead on silicon-rich biochar in aqueous solution: spectroscopic investigation [J]. Science of the Total Environment, 2019, 672: 572-582. doi: 10.1016/j.scitotenv.2019.04.003 [2] LEE E H, LEE S K, KIM M J, et al. Simple and rapid detection of bisphenol A using a gold nanoparticle-based colorimetric aptasensor [J]. Food Chemistry, 2019, 287: 205-213. doi: 10.1016/j.foodchem.2019.02.079 [3] RWEI S P, KAO S C, LIOU G S, et al. Curing and pyrolysis of epoxy resins containing 2-(6-oxido-6 H -dibenz (c, e )(1, 2)oxaphosphorin-6-yl)-1, 4-naphthalenediol or bisphenol S [J]. Colloid and Polymer Science, 2003, 281(5): 407-415. doi: 10.1007/s00396-002-0787-8 [4] YANG T, WANG L, LIU Y, et al. Comparative study on ferrate oxidation of BPS and BPAF: Kinetics, reaction mechanism, and the improvement on their biodegradability [J]. Water Research, 2019, 148: 115-125. doi: 10.1016/j.watres.2018.10.018 [5] LIAO C, KANNAN K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure [J]. J Agric Food Chem, 2013, 61(19): 4655-4622. doi: 10.1021/jf400445n [6] LIAO C, KANNAN K. A Survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States [J]. Archives of Environmental Contamination and Toxicology, 2014, 67(1): 50-59. doi: 10.1007/s00244-014-0016-8 [7] NADERI M, WONG M Y L, GHOLAMI F. Developmental exposure of zebrafish (danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults [J]. Aquat Toxicol, 2014, 148: 195-203. doi: 10.1016/j.aquatox.2014.01.009 [8] GU J, ZHANG J, CHEN Y, et al. Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae (Danio rerio) [J]. Chemosphere, 2019, 217: 629-635. doi: 10.1016/j.chemosphere.2018.10.218 [9] 王子莹, 金洁, 张哲赟, 等. 土壤和沉积物中有机质对双酚A和17α-乙炔基雌二醇的吸附行为 [J]. 环境化学, 2012, 31(5): 625-630. WANG Z Y, JIN J, ZHANG Z, et al. Sorption of 17α-ethinyl estradiol and bisphenol A by different soil/sediment organic matter fractions [J]. Environmental Chemistry, 2012, 31(5): 625-630(in Chinese).
[10] CHOI Y J, LEE L S. Partitioning behavior of bisphenol alternatives BPS and BPAF compared to BPA [J]. Environmental Science & Technology, 2017, 51(7): 3725-3732. [11] SUN K, KANG M, ZHANG Z, et al. Impact of deashing treatment on biochar structural properties and potential sorption mechanisms of phenanthrene [J]. Environmental Science & Technology, 2013, 47(20): 11473-11481. [12] 沈杰, 刘建超, 陆光华, 等. 双酚S和双酚F在水环境中的分布、毒理效应及其生态风险研究进展 [J]. 生态毒理学报, 2018, 13(5): 37-48. doi: 10.7524/AJE.1673-5897.20171009001 SHEN J, LIU J C, LU G H, et al. A review of the occurrence, toxicology and ecological risk assessment of bisphenol S and F in aquatic environment [J]. Asian Journal of Ecotoxicology, 2018, 13(5): 37-48(in Chinese). doi: 10.7524/AJE.1673-5897.20171009001
[13] 黄晓妍, 裴志国, 罗磊, 等. 双酚S在两种典型地带性土壤中的吸附/解吸行为研究 [J]. 环境科学学报, 2020, 40(4): 1452-1459. HUANG X Y, PEI Z G, LUO L, et al. Sorption and desorption of bisphenol S in two typical zonal soils [J]. Acta Scientiae Circumstantiae, 2020, 40(4): 1452-1459(in Chinese).
[14] ABDOLLAHI L, SCHJONNING P, ELMHOLT S, et al. The effects of organic matter application and intensive tillage and traffic on soil structure formation and stability [J]. Soil & Tillage Research, 2014, 136: 28-37. [15] GUO H, LI H, LIANG N, et al. Structural benefits of bisphenol S and its analogs resulting in their high sorption on carbon nanotubes and graphite [J]. Environmental Science and Pollution Research, 2016, 23(9): 8976-8984. doi: 10.1007/s11356-016-6040-7 [16] HOU J, PAN B, NIU X, et al. Sulfamethoxazole sorption by sediment fractions in to comparison pyrene and bisphenol A [J]. Environ Pollut, 2010, 158: 2826-2832. doi: 10.1016/j.envpol.2010.06.023 [17] YU G. Root exudates and microbial communities drive mineral dissolution and the formation of nano-size minerals in soils: Implications for Soil Carbon Storage [M]. Berlin : Springer , 2018. [18] DREVER J I, STILLINGS L L. The role of organic acids in mineral weathering [J]. Colloids & Surfaces A Physicochemical & Engineering Aspects, 1997, 120(1/3): 167-181. [19] 李浩成, 左应梅, 杨绍兵, 等. 三七根系分泌物在连作障碍中的生态效应及缓解方法 [J]. 中国农业科技导报, 2020, 22(8): 159-167. LI H C, ZUO Y M, YANG S B, et al. Ecological effects and mitigation methods of panax notoginseng root exudates in continuous cropping obstacles [J]. Journal of Agricultural Science and Technology, 2020, 22(8): 159-167(in Chinese).
[20] KEILUWEIT M, BOUGOURE J J, NICO P S, et al. Mineral protection of soil carbon counteracted by root exudates [J]. Nature Climate Change, 2015, 5(6): 588-595. doi: 10.1038/nclimate2580 [21] ZHOU D, CHEN B, WU M, et al. Ofloxacin sorption in soils after long-term tillage:The contribution of organic and mineral compositions [J]. Science of the Total Environment, 2014, 497: 665-670. [22] PIGNATELLO J J, SEOKJOON KWON A, LU Y. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): attenuation of surface activity by humic and fulvic acids [J]. Environ Sci Technol, 2006, 40(24): 7757-7763. doi: 10.1021/es061307m [23] DRILLIA P, STAMATELATOU K, LYBERATOS G. Fate and mobility of pharmaceuticals in solid matrices [J]. Chemosphere, 2005, 60(8): 1034-1044. doi: 10.1016/j.chemosphere.2005.01.032 [24] GUNASEKARA A S, SIMPSON M J, XING B S. Identification and characterization of sorption domains in soil organic matter using structurally modified humic acids [J]. Environmental Science & Technology, 2003, 37(5): 852-858. [25] ZHAO X, ARSHAD M, LI N, et al. Determination of the optimal mathematical model, sample size, digital data and transect spacing to map CEC (Cation exchange capacity) in a sugarcane field [J]. Computers and Electronics in Agriculture, 2020, 173: 105436. doi: 10.1016/j.compag.2020.105436 [26] 张继光, 秦江涛, 要文倩, 等 长期施肥对红壤旱地土壤活性有机碳和酶活性的影响 [J]. 土壤, 2010, 42(3): 364-371. ZHANG J G, QIN J T, YAO W Q, et al. Effects of long-term fertilization on soil active organic carbon and soil enzyme. activities in upland red soils[J]. Soil, 2010, 42(3): 364-371 (in Chinese).
[27] MADER B T, UWEGOSS K, EISENREICH S J. Sorption of nonionic, hydrophobic organic chemicals to mineral surfaces [J]. Environmental Science & Technology, 1997, 31(4): 1079-1086. [28] VOICE T C, WEBER W J. Sorption of hydrophobic compounds by sediments, soils and suspended solids—I. Theory and background [J]. Water Research, 1983, 17(10): 1433-1441. doi: 10.1016/0043-1354(83)90275-0 [29] ZHU D, PIGNATRLLO J. Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model [J]. Environmental science & Technology, 2005, 39(7): 2033-2041. [30] ZHU D Q, HYUN S H, PIGNATELLO J J, et al. Evidence for pi-pi electron donor-acceptor interactions between pi-donor aromatic compounds and pi-acceptor sites in soil organic matter through pH effects on sorption [J]. Environmental Science & Technology, 2004, 38(16): 4361-4368. [31] SENESI N. Binding mechanisms of pesticides to soil humic substances [J]. The Science of the total environment, 1992, 123/124: 63-76. doi: 10.1016/0048-9697(92)90133-D [32] 李祖然, 闵强, 孙晶晶, 等. As胁迫对二年生三七生长、根部As含量和根系分泌物的影响 [J]. 北京农学院学报, 2015, 30(3): 86-91. LI Z R, MIN Q,SUN J J, et al. Effect of As stress on the growth, the root As contents and root exudates in 2-year-old Panax notoginseng [J]. Journal of Beijing University of Agriculture, 2015, 30(3): 86-91(in Chinese).
-




 下载:
下载: