-
气候问题已成为全球性问题,根据CAIT(世界资源研究所)相关数据分析,在过去十年中,世界CO2排放量以每年2.4%的增长率增加[1]。中国碳排放量位居全球首位,实施低碳发展战略,对缓减全球变暖具有重要现实意义[2]。
碱金属基固体吸附剂低温脱除烟气中CO2技术反应温度低、吸附剂与CO2反应速率快、转化率高、无二次污染[3]。以胺基为活性组分加多孔材料的新型二氧化碳吸附剂研究相对成熟。其中胺基改性介孔二氧化硅气凝胶吸附剂(AMSA)的吸附容量在25℃时可达6.97 mmol·g-1 [4]。钠基吸附剂资源丰富、价格低廉,但吸附剂反应活性低[5]。钙基吸附剂在碳酸化之后因内部结构烧结而导致吸附率急剧下降,且废气中的SO2与CaO反应生成CaSO4无法再生,使CaO有效含量下降,吸附性能降低[6]。关于以钾基为活性组分的CO2吸附剂是近年研究热点,根据载体材料不同,国内外学者进行了一系列脱碳特性研究,见表1。研究表明,钾基碳酸盐与微观结构发达、机械强度高的载体材料结合,碳酸钾利用率得以提升,碳酸化性能得到极大改善[7]。
由于载体本身微观特性影响,K2CO3负载能力有限,使吸附剂微观结构和吸附容量因饱和负载而被限制。因此,需要制备一种具有良好微观特性的载体材料,负载活性组分K2CO3制成高CO2吸附量、低成本的改性钾基吸附剂。气凝胶是一种高比表面积、高孔隙率、低密度的非晶纳米多孔材料,在吸附、催化、催化剂载体领域有广泛应用。余煜玺等以仲丁醇铝为前驱体制备氧化铝气凝胶热稳定性高,比表面积达744.50 m2·g-1 [16]。Guo等用TEOS为硅源制备硅凝胶负载K2CO3做吸附剂, CO2吸附量可达1.32 mmol·g−1,碳酸化性能优良[14]。以TEOS和有机醇盐作为溶胶的硅源铝源不仅成本高而且毒性较大[16]且上述研究仅限于超低CO2浓度,无法满足燃煤电厂烟气气氛。
燃煤电厂除尘器排放的飞灰是目前世界上排放量最大的工业废料之一,主要化学成分为SiO2和Al2O3。刘博等[18]利用煤矸石制备二元复合气凝胶比表面积达483.23 m2·g−1,比孔容积1.87 cm3·g−1;陈娜等[19]分别以六甲基二硅胺烷和正丁醇为表面改性剂制备SiO2-Al2O3复合气凝胶比表面积分别为114 m2·g−1、183 m2·g−1;蒲鲲等[20]利用飞灰制备SiO2-Al2O3复合气凝胶比表面积较小,仅为44.47 m2·g−1。
钾基吸附剂对CO2的脱除与活性组分相关,上述研究侧重载体制备、凝胶结构差距较大、以分解率为指标误差较大,且由SiO2-Al2O3复合气凝胶负载K2CO3的碳酸化反应研究相对较少,机理解释不充分。基于此,本文旨在利用飞灰制备SiO2-Al2O3复合气凝胶、K2CO3负载改性,集制备和负载于一体,用作 CO2吸附剂。研究不同负载率下CO2吸附性能,探究吸附剂脱碳特性及循环机理,为今后脱碳提供理论依据。
-
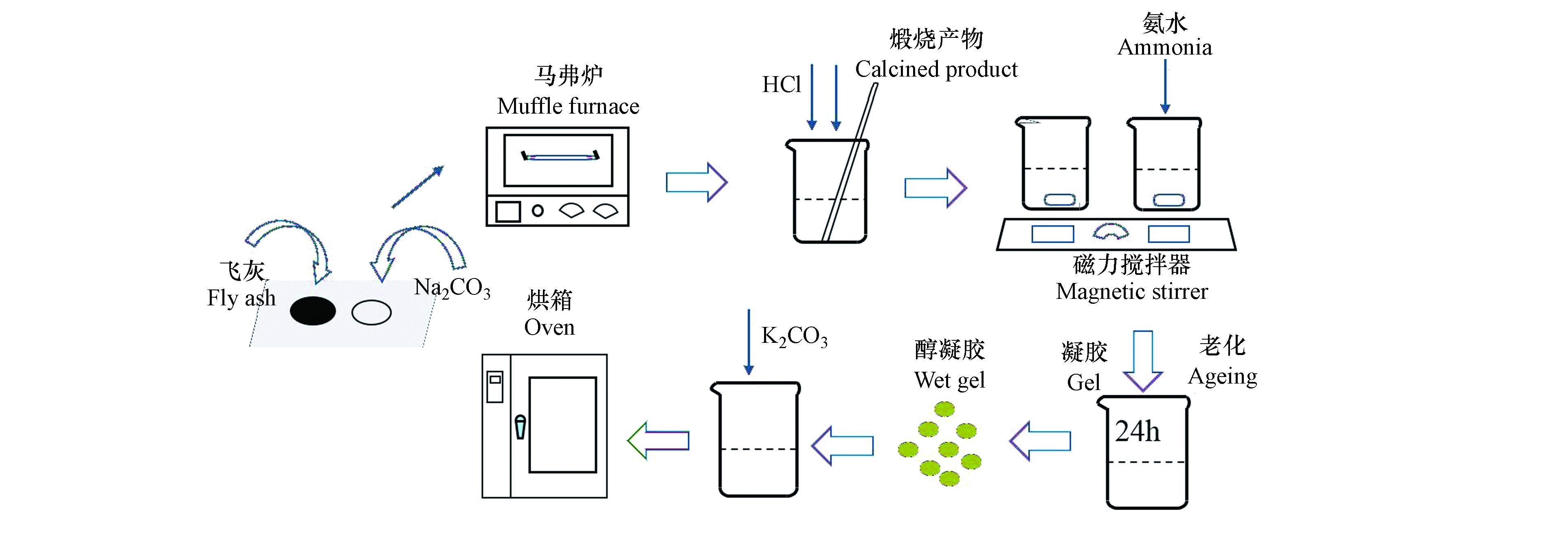
吸附剂制备流程图如图1所示。具体制备流程如下:(1)飞灰与Na2CO3按比例充分混合置入马弗炉,程序升温并煅烧;(2)将一定浓度盐酸倒入煅烧产物,磁力搅拌器40℃反应50 min,离心分离上清液;(3)滴加氨水玻璃棒搅拌5 min,室温密封静置,待凝胶后加乙醇层密封50℃水浴老化24 h形成醇凝胶,每隔8 h更换乙醇层;(4)将不同负载量K2CO3(0%、10%、20%、25%、30%、40%)与醇凝胶混合溶解在适量去离子水中,室温下磁力搅拌器搅拌12 h充分浸渍;(5)混合物放入100℃烘箱12 h脱除样品中的游离水,之后300℃马弗炉焙烧2 h即制备完成。
-
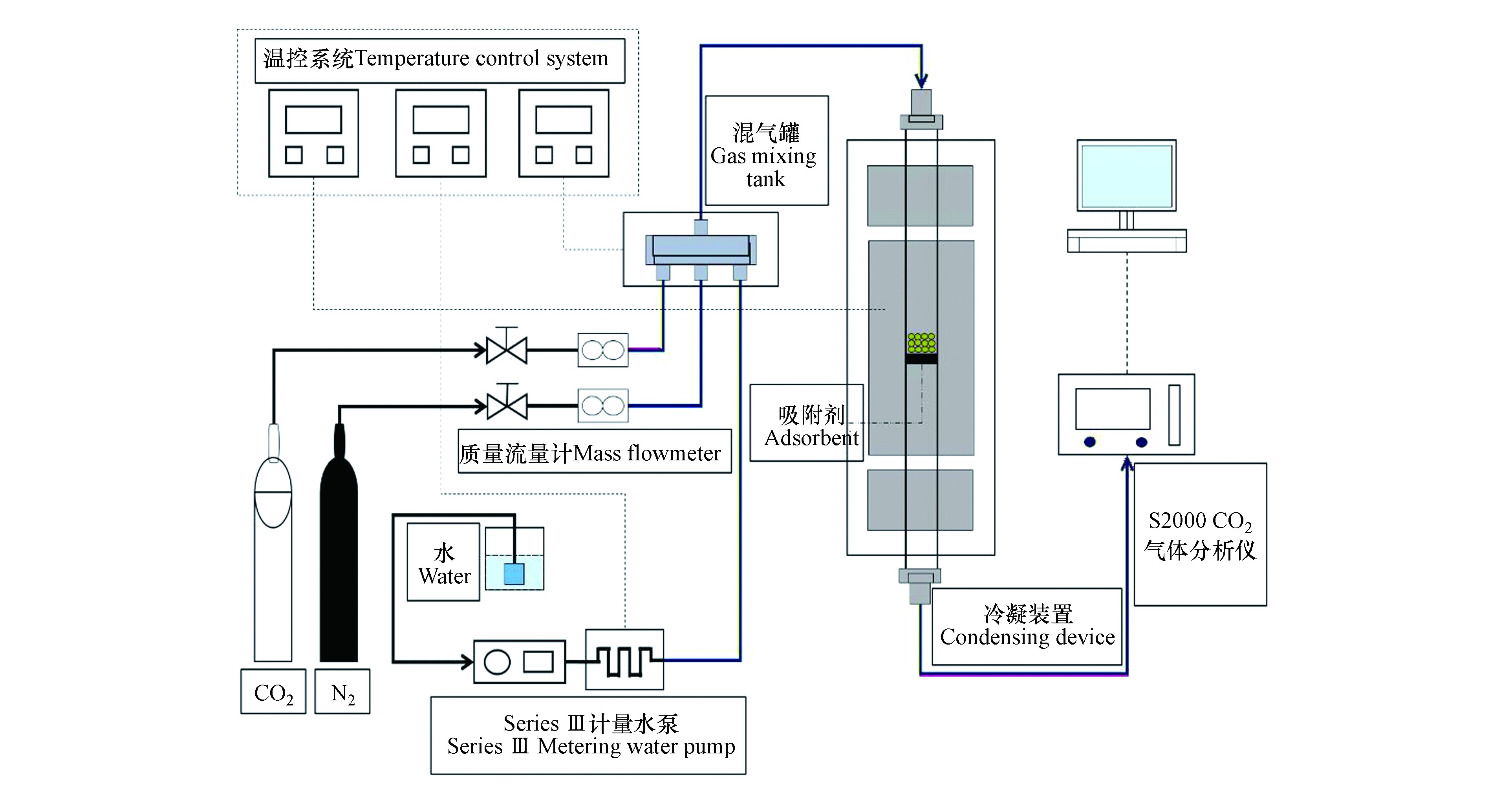
一般燃煤电站锅炉烟气由10%—15% CO2、8%—17%水蒸气、少量O2、微量SO2和NOХ以及大部分N2组成[21]。为模拟实际烟气环境,入口CO2和水蒸气体积浓度均设为10%,N2浓度80%,总气量500 mL·min−1,试验系统如图2所示。CO2和N2由钢瓶提供,经质量流量计控制开度,水蒸气由Series Ш计量水泵经电加热汽化产生,与CO2和N2混合进入混气罐。混气干燥脱水后通过S2000 CO2气体分析仪,待出口与入口CO2浓度一致,反应结束,记录试验数据并进行有效计算。
采用单位质量吸附剂累计吸附量q(mmol·g−1)、CO2穿透率η(%)研究吸附剂脱碳特性,分别通过式(1)和(2)进行计算(按照标准工况):
式中,C1、C2为入口、出口CO2浓度,%;Q为模拟烟气流量,mL·min−1;t为反应时间,min;m为固定床钾基吸附剂质量,g;T为反应温度,K;T0 为绝对零度273 K;Vm 为标况下的气体摩尔体积22.4 mol·L−1。
-
本文采用Tescan Mira3场发射扫描电镜进行SEM测试,获得吸附剂的表面微观形貌;采用N2吸附-脱附仪对钾基吸附剂进行N2吸附-脱附试验,获得吸脱附等温线,探究吸附剂孔隙结构特征。通过BET方程及BJH算法对各样品比表面积、比孔容积等相关孔隙结构参数进行分析;采用荷兰E3型XRF测试仪,进行全元素扫描通过转换计算获得其化学组成;采用DX2700B型XRD测试仪对晶体结构进行分析。
-
经XRF测试仪得煤粉炉飞灰化学组成如表2所示,主要成分为SiO2和Al2O3,质量分数合约为86%,还含有少量的铁、钙、钛、钾、硫等杂质,硅铝含量巨大,能够作为很好的硅铝气凝胶原材料。
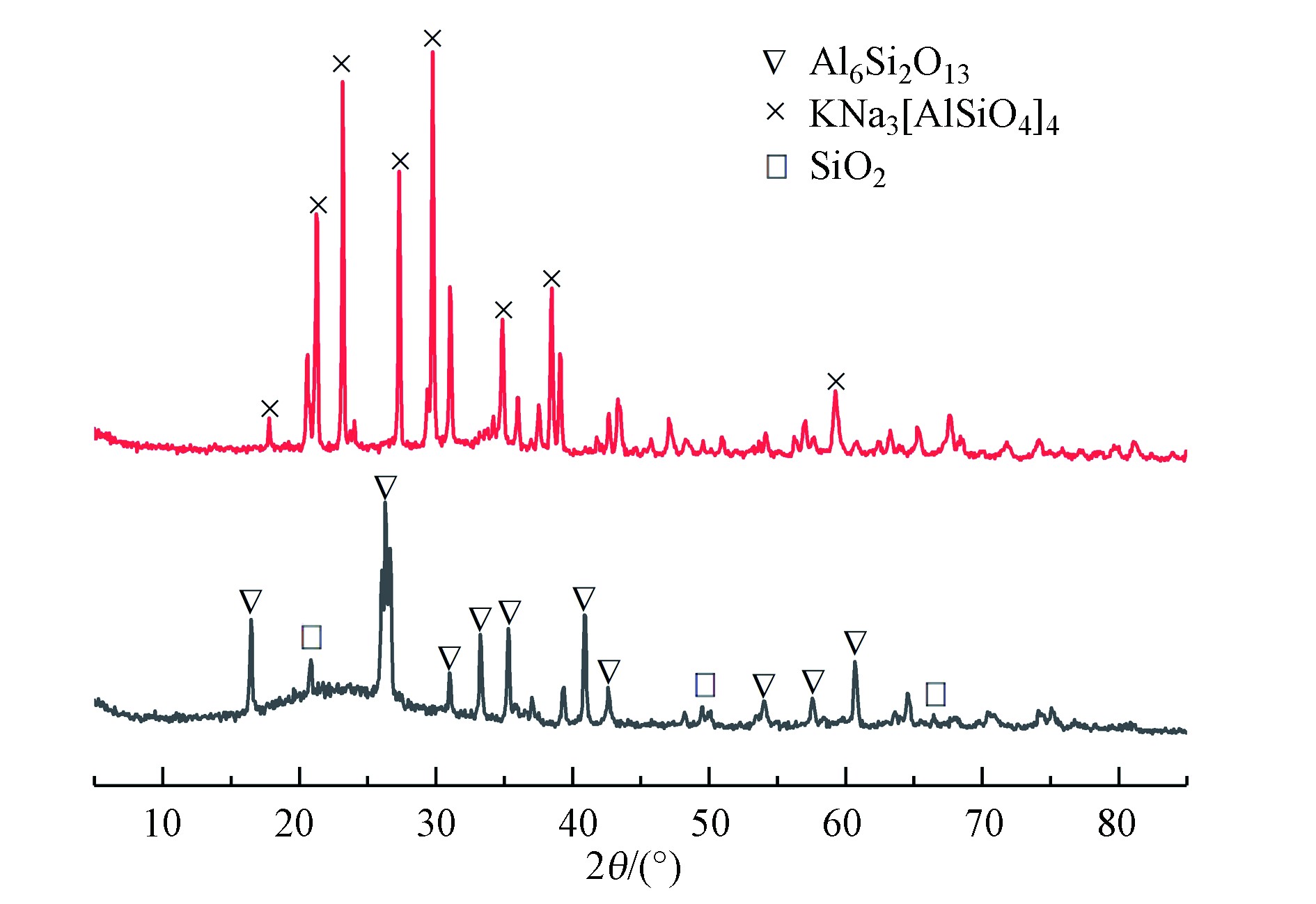
硅铝在飞灰中主要以矿物组分莫来石(Al6Si2O13)和石英(SiO2)存在。由于矿物晶体反应活性低,为充分提取硅铝组分,需要在高温条件下对飞灰进行碱熔烧结活化处理,将飞灰中不溶于酸的以莫来石为主的物相与碱(Na2CO3)经高温烧结反应转变为易溶于酸的以霞石(KNa3[AlSiO4]4)为主的物相[20],提高反应活性将硅铝组分分离。
碱熔烧结活化[19]是飞灰制备SiO2-Al2O3复合气凝胶的关键。通常影响碱熔烧结活化反应的主要因素有:反应温度、反应时间和碱添加比例。为确定最佳煅烧制度,制备孔隙结构丰富的SiO2-Al2O3复合气凝胶,本文选用SiO2-Al2O3复合气凝胶的比表面积作为衡量活化效果优劣的指标,设计三因素三水平的正交试验L9(33),如表3和表4所示。
表4中Ki为各因素水平指标之和平均值;Ki值越大,表示某水平对试验指标影响越大;R为极差,R最大,表示该参数在试验范围内对试验指标影响最大。极差分析结果显示,影响碱熔烧结活化的各因素主次作用为:反应时间>反应温度>碱添加比例,综合考虑各因素主次作用、能耗影响及极差结果,本试验反应条件的最优组合选为:900℃、反应60 min、Na2CO3添加比例0.5。
比较煅烧产物与飞灰晶相结构,物相分析结果如图3所示,飞灰中主要物相组分为莫来石(Al6Si2O13)和SiO2,碱熔烧结活化后Al6Si2O13和SiO2对应衍射峰位减弱甚至消失,霞石(KNa3[AlSiO4]4)对应衍射峰位解析明显。说明在高温下Na2CO3活化反应过程中,Si—O—Si、Si—O—Al内键断裂,晶相结构遭到破坏[19],莫来石的共价键转化为霞石的离子键,惰性的铝硅组分得到充分活化,形成在三维空间均匀分布的KNa3[AlSiO4]4架状结构[21],为SiO2-Al2O3复合气凝胶的制备提供理论依据。
-
本试验将K2CO3负载到最佳煅烧制度所制备SiO2-Al2O3复合气凝胶制成负载型吸附剂,提高钾CO2捕获率;同时研究不同负载条件下改性钾基吸附剂的碳酸化特性,结合表观形貌及微观特性,研究吸附剂吸附机理。
(1) 负载性能
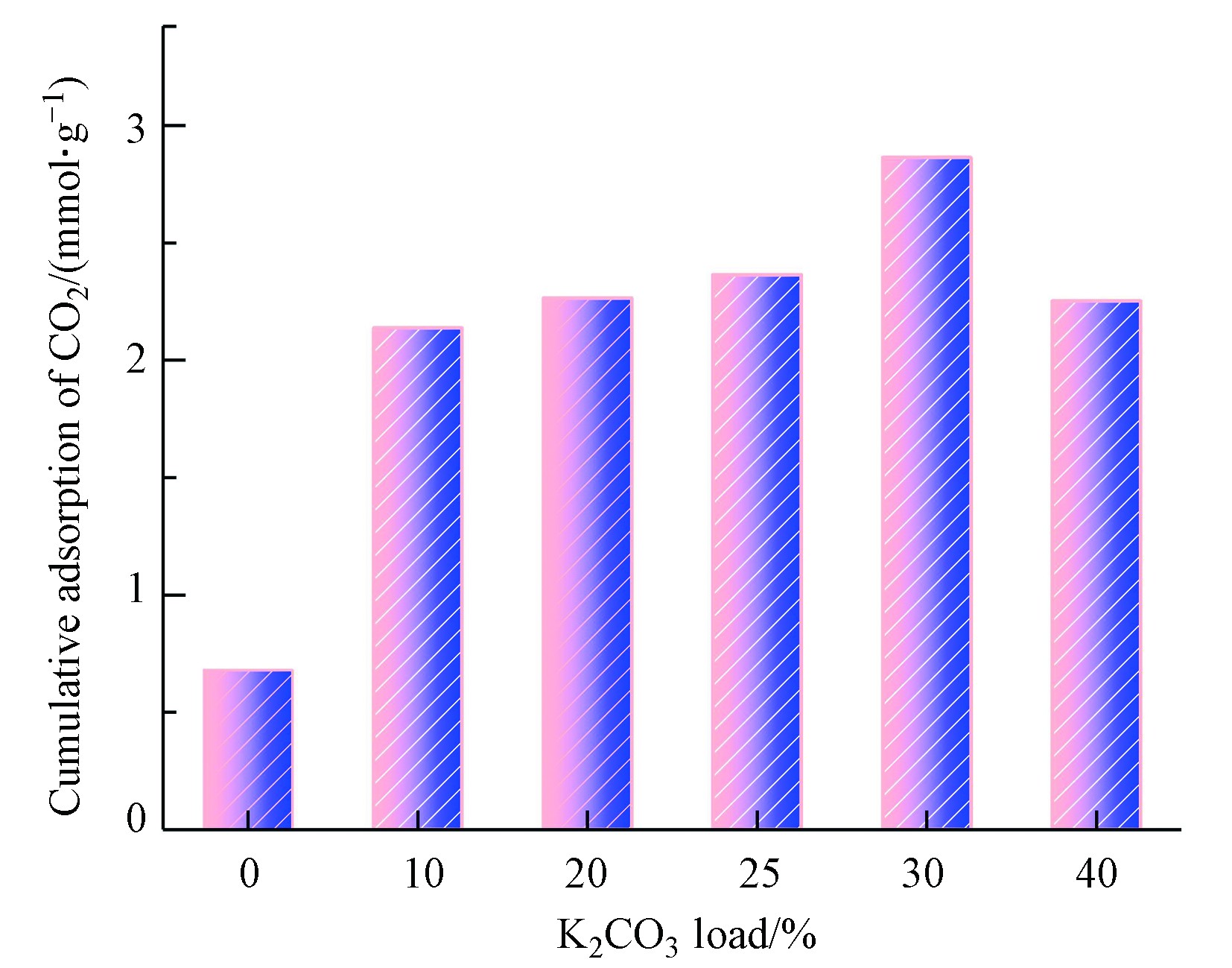
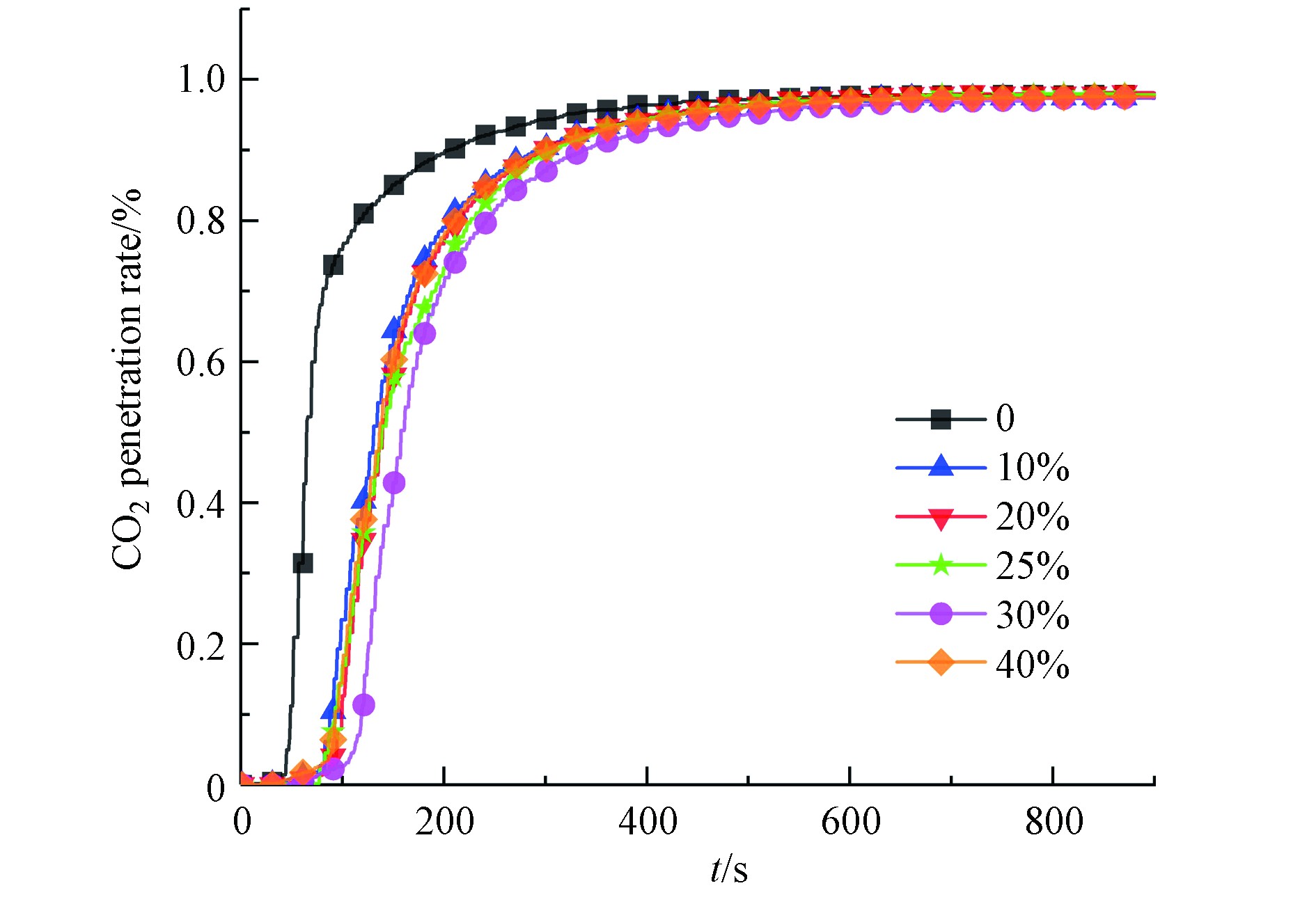
模拟燃煤电站锅炉烟气在60℃、10%CO2+10%H2O+80%N2气氛条件下进行碳酸化试验,研究10%、20%、25%、30%、40%负载量下钾基吸附剂的CO2捕获性能,结果如图4和5所示。
SiO2-Al2O3复合气凝胶载体对CO2的吸附量最低,仅为0.68 mmol·g−1;在10%—40%范围内,随负载组分占比增加负载型吸附剂的CO2吸附量呈先增大后减小的趋势,在负载量为30%时达到最大,为2.86 mmol·g−1,碳酸化性能优良,说明CO2的吸附主要是通过K2CO3的化学反应来实现的。
当负载量从10%增加到30%,活性组分K2CO3逐渐增多,促进反应向正反应方向进行。活性组分增加到一定程度,CO2吸附量反而减小,这是由于K2CO3在载体表面的分布已经达到饱和状态,K2CO3负载量越多,载体表面可附着K2CO3活性位点已被占据,活性组分与气体的接触达到饱和[22];同时过量K2CO3负载会堵塞孔结构,破坏CO2扩散过程,降低K2CO3的扩散和利用效率;其次,在碳酸化反应过程中,载体表面有KHCO3生成 [23],不利于反应的进行。
相同试验条件下,同一时刻η值越高,对应吸附剂样品脱碳性能越差。观察不同负载量吸附剂 CO2穿透曲线,同一时刻内,30%负载量下η值最低,吸附剂CO2穿透时间最长,吸附效果最佳,与累计吸附量结果保持一致。
(2) 微观特性
影响吸附剂脱碳特性的孔隙结构参数主要有比表面积、累积孔体积、相对比孔容积等[24]。当负载量从10%增加到40%,N2吸附量逐渐下降,比表面积和累积孔体积相对于纯载体均有所减少(如表5所示),同时K2CO3的负载使吸附剂的孔隙丰富度减小,这是因为负载过程中,K2CO3会大量附着在载体表面和孔隙内[25]。此时引入单位容积下的比表面积Z,用以表征其孔隙丰富程度,如式(3)所示:
式中,S0为吸附剂BET比表面积,m2·g−1;V0为吸附剂比孔容积总和,cm3·g−1。
对不同负载条件下吸附剂样品进行低温N2吸附-脱附试验,探究吸附剂孔隙结构特征。同时对各样品吸-脱附等温线和孔径分布曲线进行分析,结果如下。
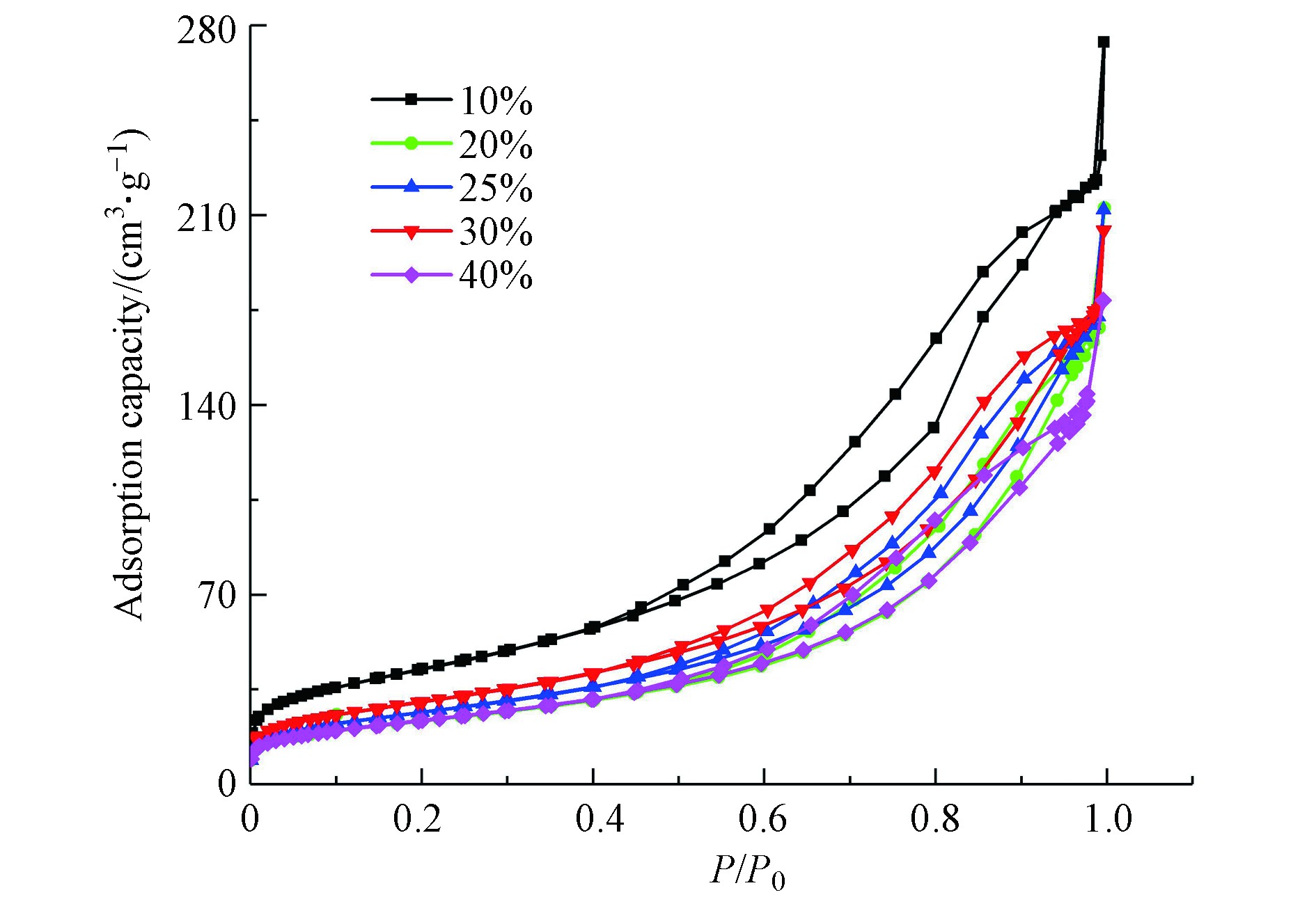
观察SiO2-Al2O3复合气凝胶和吸附剂吸脱附等温线(见图6),根据IUPAC(国际纯粹与应用化学联合会)的分类,属于Ⅳ型等温线[26],表明是孔径分布较为均匀的有序介孔材料,有利于气体CO2在孔隙内的扩散和吸附,且负载后吸附剂的吸脱附等温线并未发生明显变化,说明负载对气凝胶孔结构影响较小。
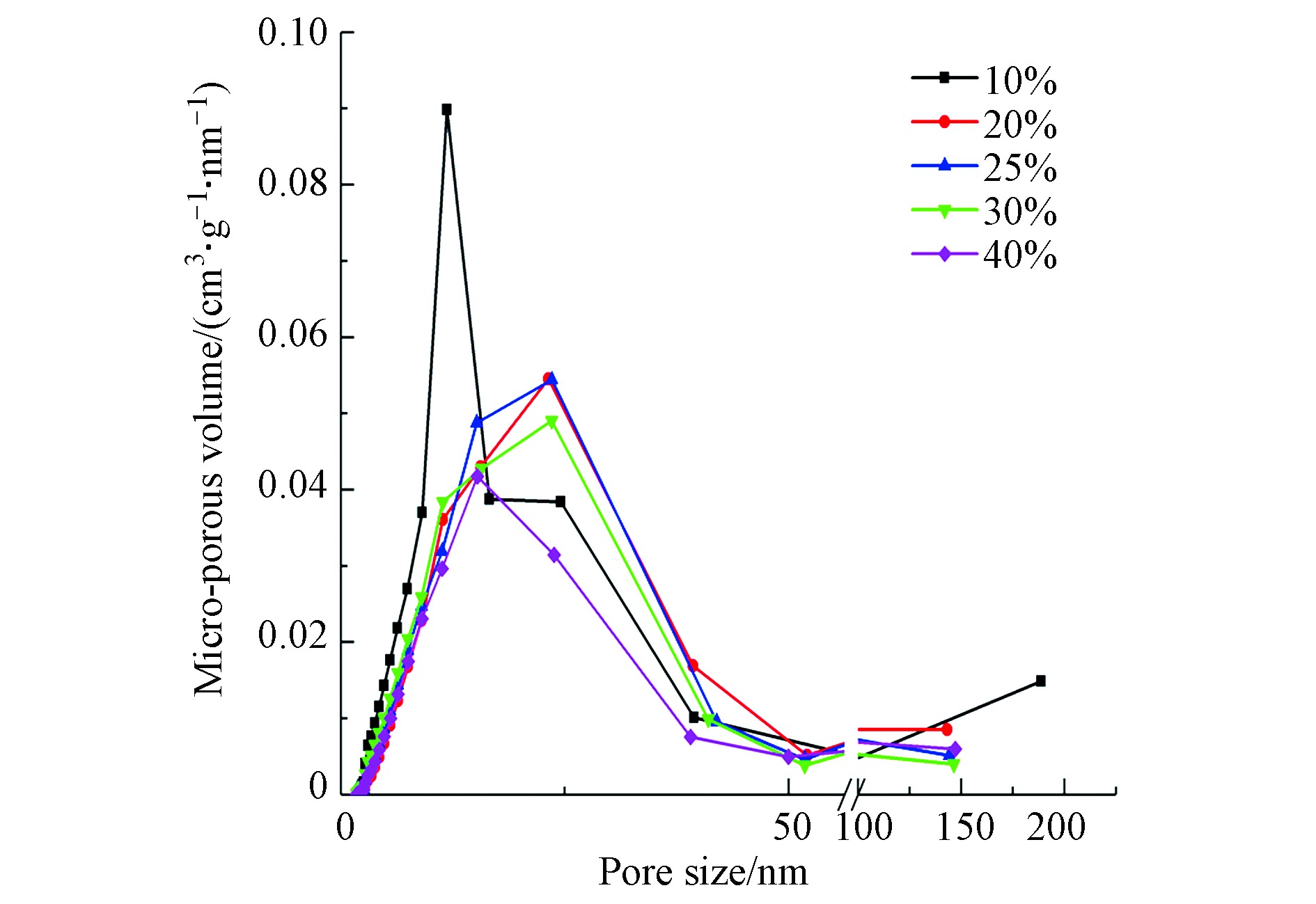
图7为不同负载量下吸附剂的孔径分布曲线,孔径均匀分布在3—50 nm之间,主要由介孔和少部分大孔组成。较大比表面积和比孔容积的吸附剂一方面可以提供更多活性位点;另一方面,使活性组分K2CO3的分布更为均匀,有利于CO2分子与K2CO3的接触,促进碳酸化反应[27]。孔径分布曲线中峰值代表孔径分布最广的孔,随负载量增加,峰值先逐渐向右下方偏移,再向左下方偏移,孔体积明显减小。当负载量从10%增加到40%时,介孔的孔体积百分比由94.21%降低到89.32%,大孔的孔体积百分比由5.45%增加到10.50%,说明活性组分K2CO3主要填充在介孔中;平均孔径为累计总孔内表面积与累计孔体积之比,随介孔相对比孔容积减小,大孔相对比孔容积增大,使平均孔径出现增大趋势。
(3) 表观形貌
图8为不同K2CO3负载量吸附剂SEM颗粒形态分布。分析发现,SiO2-Al2O3复合气凝胶载体(图8a)表面疏松,孔隙结构丰富;当K2CO3负载量从10%(图8b)增加到20%时(图8c),颗粒孔隙率开始降低,K2CO3主要填充颗粒间空隙及一部分介孔,同时K2CO3晶体以白色小颗粒分布在载体表面;K2CO3负载量从25%(图8d)继续增加至30%(图8e)时,吸附剂表面由小颗粒堆叠而成且开始变得更加致密,说明负载改变了载体的表观形貌,使K2CO3分布更为均匀,有利于气体向固体表面的扩散,与陈少卿[11]等的结论一致。此外,载体表面还存在白色晶粒状物质[28],可能是由于在制备过程中引入大量氯离子导致,晶粒状物质为氯离子和其它金属离子组成的盐类化合物(如FeCl3、KCl等)。
观察负载量为40%(图8f)的吸附剂表面,过量的K2CO3负载使孔结构堵塞,破坏CO2扩散过程。载体表面的绝大多数孔道被填充和覆盖后,K2CO3晶体开始出现大块团聚现象,多孔结构较少。负载量为30%时的钾基吸附剂颗粒分布较为均匀,形貌较好。
-
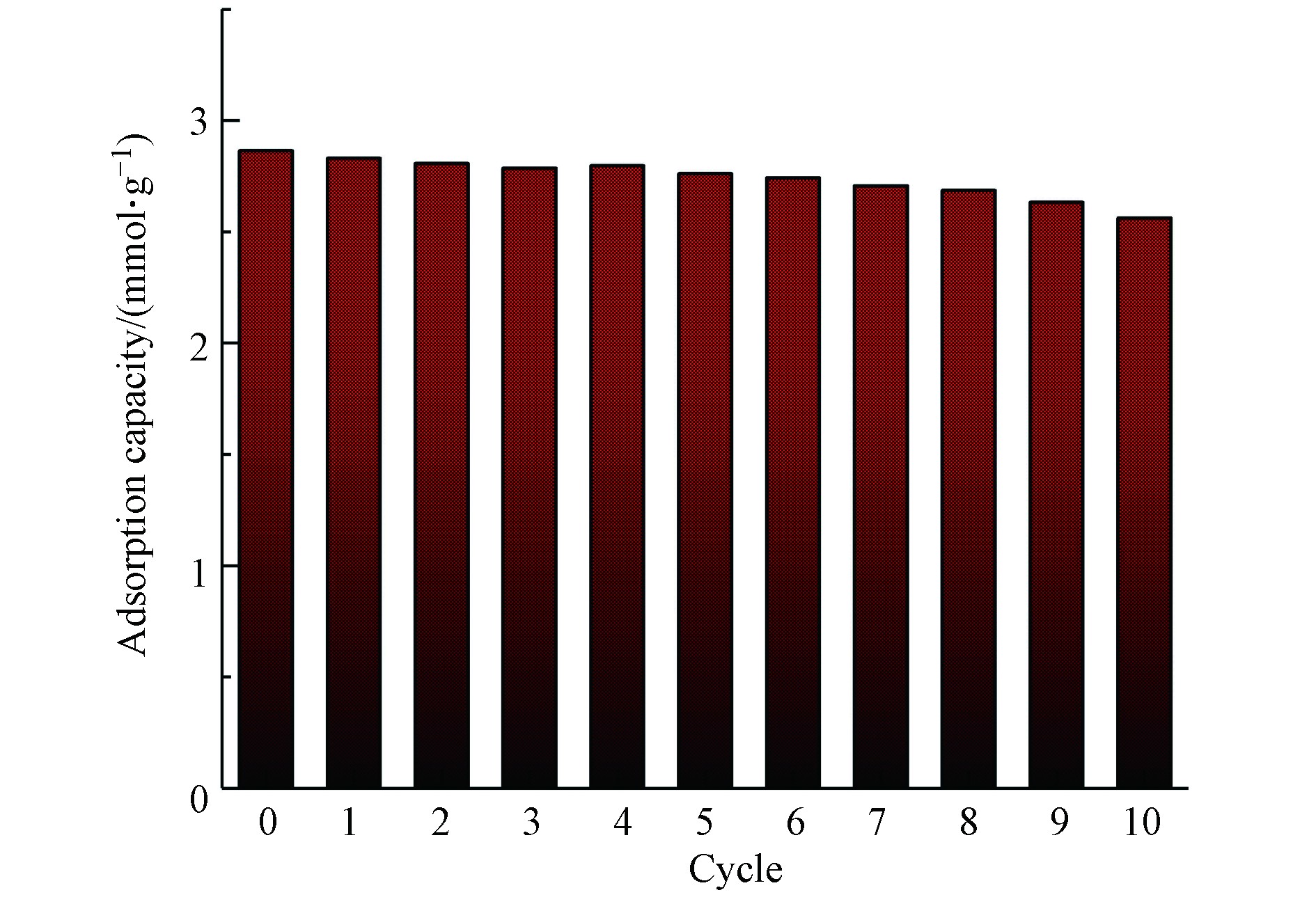
循环脱碳特性是衡量钾基吸附剂能否广泛用于实际的重要指标。本文对上述所制备吸附剂进行10次吸附-再生循环试验,设置总气量500 mL·min−1,在吸附温度60℃、吸附气氛80%N2+10%H2O+10%CO2、再生温度120℃、再生气氛纯氮气条件下,吸附剂的累计CO2吸附量随循环次数变化如图9所示。由图9可知,吸附剂累计吸附量随吸脱附循环次数增加,呈逐渐下降的趋势,经过10次吸附-再生循环后,CO2吸附量从2.86 mmol·g−1降低到2.56 mmol·g−1,仍有较好的吸附效果,吸附剂再生率可达89.51%。对10次循环再生后的样品进行孔隙结构分析见表6,发现比表面积和比孔容积均变化不大,说明所制备吸附剂结构稳定,循环脱碳性能良好。
-
(1)以飞灰为原料利用溶胶-凝胶法经常压干燥制备孔隙结构良好的SiO2-Al2O3复合气凝胶。以SiO2-Al2O3复合气凝胶比表面积为衡量指标,经正交试验确定碱熔烧结活化的最佳煅烧制度为:900℃、反应60 min、Na2CO3添加比例0.5。
(2)K2CO3负载量从10%增加到40%,比表面积和累积孔体积均有所减小,CO2累计吸附量呈先增大后减小的趋势,当负载量为30%时,CO2累计吸附量最大为2.86 mmol·g−1,与CO2穿透率结果保持一致。
(3)负载量越多,载体表面可附着K2CO3活性位点已被占据,活性组分与气体的接触达到饱和;过量K2CO3负载会堵塞孔结构,破坏CO2扩散,降低扩散和利用效率。在反应过程中,载体表面生成KHCO3,不利于反应进行。
(4)介孔的孔体积百分比由94.21%降低到89.32%,说明活性组分K2CO3主要填充在介孔中。较大比表面积和比孔容积的吸附剂一方面可以提供更多活性位点;另一方面,使活性组分K2CO3的分布更为均匀,有利于气体向固体表面扩散,促进碳酸化反应。
(5)经过10次吸附-再生循环试验,吸附剂的CO2吸附量下降幅度为10.49%,同时吸附剂微观结构参数并未发生很大变化,结构稳定、循环脱碳性能良好。
碳酸钾改性SiO2-Al2O3复合气凝胶制备及脱碳特性
Preparation and decarburization characteristics of SiO2-Al2O3 composite aerogel modified by potassium carbonate
-
摘要: 本文对碳酸钾改性SiO2-Al2O3复合气凝胶的制备、K2CO3碳酸化特性及再生循环脱碳特性进行了研究,采用固定床反应器研究负载率对CO2吸附的影响,结合SEM、BET对样品微观结构进行分析。结果表明,Na2CO3碱熔烧结过程中,Si—O—Si、Si—O—Al键断裂,晶相结构被破坏,莫来石的共价键转化为霞石的离子键;经正交试验以气凝胶比表面积为衡量指标确定最佳煅烧条件为:900℃、反应60 min、Na2CO3添加比例0.5;K2CO3负载量越多,载体表面相应活性位点越少,且负载量为30%时,CO2吸附量最大为2.86 mmol·g−1;过量K2CO3会堵塞孔结构,破坏CO2扩散,降低扩散和利用效率;介孔孔体积百分比由94.21%下降至89.32%,说明活性组分K2CO3主要填充在介孔;经过10次循环-再生试验,吸附剂的CO2吸附量下降幅度为10.49%,吸附剂孔隙结构稳定,脱碳性能优良。
-
关键词:
- SiO2-Al2O3复合气凝胶 /
- 吸附CO2 /
- 碳酸化特性 /
- 微观特性
Abstract: The preparation of potassium carbonate modified SiO2-Al2O3 composite aerogel, the carbonation characteristics of K2CO3 and the decarbonization characteristics of the regeneration cycle were studied. Using a fixed bed reactor to study the effect of loading rate on CO2 adsorption. Analyze the microstructure of samples combined with SEM and BET. The results show that during the Na2CO3 alkali fusion and sintering process, the Si—O—Si and Si—O—Al bonds are broken, the crystal structure is destroyed, and the covalent bond of mullite is transformed into ionic bond of nepheline. The orthogonal test uses the specific surface area of the aerogel to determine the best calcination system as follows: 900℃, 60 min reaction, and 0.5 Na2CO3 addition ratio. The more K2CO3 loading, the fewer K2CO3 active sites can be attached to the surface of the carrier. When the load is 30%, the maximum CO2 adsorption capacity is 2.86 mmol·g-1. Excessive K2CO3 will block the pore structure, destroy CO2 diffusion, and reduce diffusion and utilization efficiency. The volume percentage of mesopores decreased from 94.21% to 89.32%, indicating that the active component K2CO3 was mainly filled in the mesopores. After 10 cycles-regeneration tests, the CO2 adsorption capacity of the adsorbent decreased by 10.49%, the pore structure of the adsorbent was stable, and the decarburization performance was excellent. -

-
表 1 K2CO3基吸附剂CO2吸附量对比
Table 1. Comparison of CO2 adsorption capacity of K2CO3-based adsorbent
吸附剂
AdsorbentK2CO3负载量/%wt
K2CO3 load吸附条件
Adsorption conditions转化率/%
Conversion ratesCO2吸附量/(mmol·g−1)
CO2 adsorption capacity参考文献
ReferencesK2CO3/AC 30 60℃,1% CO2+9%H2O — 1.95 [8-9] 30 20℃,0.5% CO2+1.8%H2O — 0.87 [10] K2CO3/AC1 30 60℃,15% CO2+15%H2O 89.20 — [11] K2CO3/AC2 30 60℃,15% CO2+15%H2O 87.90 — [11] K2CO3/Al2O3 30 60℃,1% CO2+9%H2O — 1.93 [8-9] 24.5 60℃,18% CO2+18%H2O 95.20 -- [12] 30 20℃,0.5% CO2+1.8%H2O — 1.18 [10] K2CO3/MgO 30 60℃,1% CO2+9%H2O — 2.70 [8-9] K2CO3/5A 33 70℃,5%CO2+10%H2O 78.20 — [13] 30 20℃,0.5% CO2+1.8%H2O — 0.34 [10] K2CO3/SG 20 20℃,1% CO2+2%H2O 88.62 1.32 [14] 30 60℃,15% CO2+15%H2O 18.80 — [11] K2CO3/SiO2 30 60℃,1% CO2+9%H2O — 0.23 [8-9] K2CO3/ZrO2 30 60℃,1%CO2+9-11%H2O — 1.73-1.87 [6,15] K2CO3/DT 19.1 60℃,18% CO2+18%H2O 33.90 — [12] K2CO3/TiO2 30 60℃,1% CO2+9%H2O — 1.89-2.05 [8-9] 表 2 煤粉炉飞灰化学组成(来自太原古交电厂)
Table 2. Chemical composition of fly ash from pulverized coal furnace
主要成分
Main ingredients质量分数/%
Quality scoreSiO2 50.26 Al2O3 35.88 Fe2O3 6.00 CaO 2.43 TiO2 1.54 K2O 1.35 SO3 1.11 other 1.43 表 3 因素水平表
Table 3. Factor Level Table
水平
Level因素
Factor反应温度/℃
Temperature reflex反应时间/min
Reaction time碱添加比例/(m灰:m碳酸钠)
Alkali addition ratio1 800 60 0.5 2 850 90 0.6 3 900 120 0.7 表 4 正交试验L9(33)和极差分析
Table 4. Orthogonal test L9 (33) and range analysis
试验号
Test number因素
factor比表面积/(m2 ·g−1)
Specific surface area反应温度 /℃
Temperature reflex反应时间
Reaction time碱添加比例/(m灰:m碳酸钠)
Alkali addition ratio1 800 60 0.5 274 2 800 90 0.6 143 3 800 120 0.7 202 4 850 60 0.6 219 5 850 90 0.7 215 6 850 120 0.5 231 7 900 60 0.7 239 8 900 90 0.5 209 9 900 120 0.6 276 K1 206 244 238 — K2 222 189 213 — K3 241 236 219 — R 35 55 25 — 表 5 不同负载量吸附剂孔结构参数
Table 5. Structure parameters of adsorbent pores with different loadings
K2CO3负载量/%
K2CO3 loadBET比表面积/
(m2·g-1)
BET specific
surface area累积孔体积/
(cm3·g-1)
Cumulative pore
volume平均孔径/nm
Average pore
diameter孔隙丰富度
Pore
richness Z相对比孔容积/%
Relative pore volume微孔
Microporous介孔
Mesoporous大孔
Big hole10 153.9782 0.3639 8.4944 423.1600 0.2130 93.0500 6.7370 20 110.7819 0.2749 8.9543 402.9385 0.3338 94.2127 5.4536 25 96.6573 0.2695 9.9113 358.6302 0.0988 92.7080 7.1932 30 90.4104 0.2626 11.2873 344.2894 0.0282 90.8132 9.1586 40 85.2602 0.2254 9.5784 378.2618 0.1780 89.3174 10.5027 表 6 钾基吸附剂循环结构参数变化
Table 6. Changes of potassium-based adsorbent circulation structure parameters
BET比表面/(m2 ·g−1)
BET specific surface累积孔体积/(cm3 ·g−1)
Cumulative pore volume最可几孔径/nm
Most probable aperture孔隙丰富度
Pore richness Z30%负载量吸附剂 90.4104 0.2626 11.2873 321.3945 10次循环再生后 85.3012 0.2263 9.7932 368.5217 -
[1] 孙乔. 我国碳排放市场建设现状分析 [J]. 价值工程, 2019, 38(26): 120-121. SUN Q. Analysis of the current situation of China’s carbon emission market construction [J]. Value Engineering, 2019, 38(26): 120-121(in Chinese).
[2] 张婷婷, 马文林, 亓学奎, 等. 北京城区PM2.5有机碳和元素碳的污染特征及来源分析 [J]. 环境化学, 2018, 12: 2758-2766. doi: 10.7524/j.issn.0254-6108.2018051701 ZHANG T T, MA W L, QI X K, et al. Characteristics and sources of organic carbon and element carbon in PM2.5 in the urban areas of Beijing [J]. Environmental Chemistry, 2018, 12: 2758-2766(in Chinese). doi: 10.7524/j.issn.0254-6108.2018051701
[3] 赵传文, 陈晓平, 赵长遂. 碱金属基吸收剂干法脱除CO2技术的研究进展 [J]. 动力工程, 2008, 28(6): 827-833. ZHAO C W, CHEN X P, ZHAO C S. Research progress of CO2 capture technology using dry alkali-based sorbents [J]. Journal of Power Engineering, 2008, 28(6): 827-833(in Chinese).
[4] CUI S, CHENG W, SHEN X D. Mesoporous amine-modified SiO2 aerogel: A potential CO2 sorbent [J]. Energy & Environmental Science, 2011, 4(6): 2070-2074. [5] 董伟, 陈晓平, 余帆, 等. 钠基负载型固体CO2吸收剂碳酸化反应特性 [J]. 煤炭学报, 2015, 40(9): 2200-2206. DONG W, CHEN X P, YU F, et al. Carbonation characteristics of sodium-based solid sorbents for CO2 capture [J]. Journal of China Coal Society, 2015, 40(9): 2200-2206(in Chinese).
[6] 梁成. 生物质模板改性钙基吸收剂颗粒循环脱碳性能研究[D]. 南京: 南京师范大学, 2019. LIANG C. Investigations on CO2 capture characteristics of dry potassium-based sorbent[D]. Nanjing: Nanjing Normal University, 2019(in Chinese).
[7] 杨泛明, 符健斌, 邹创乾, 等. MgCl2改性碳材料的制备及其CO2吸附性能 [J]. 环境化学, 2019, 38(11): 2555-2562. YANG F M, FU J B, ZOU C Q, et al. Synthesis of MgCl2 modified carbon materials for CO2 adsorption [J]. Environmental Chemistry, 2019, 38(11): 2555-2562(in Chinese).
[8] LEE S C, KIM J C. Dry potassium-based sorbents for CO2 capture [J]. Catalysis Surveys from Asia, 2007, 11(4): 171-185. doi: 10.1007/s10563-007-9035-z [9] LEE S C, CHOI B Y, LEE T J, et al. CO2 absorption and regeneration of alkali metal-based solid sorbents [J]. Catal Today, 2006, 111(3): 385-390. [10] ZHAO C W, GUO Y F, LI C H, et al. Removal of low concentration CO2 at ambient temperature using several potassium-based sorbents [J]. Applied Energy, 2014, 124(7): 241-247. [11] 赵传文. 钾基固体吸收剂脱除CO2特性及机理研究[D]. 南京: 东南大学, 2011. ZHAO C W. Investigations on CO2 capture characteristics of dry potassium-based sorbent[D]. Nanjing: Southeast University, 2011(in Chinese).
[12] LEE S C, CHAE H J, LEE S J, et al. Novel regenerable potassium-based dry sorbents for CO2 capture at low temperatures [J]. Journal of Molecular Catalysis. B:Enzymatic, 2009, 56(2): 179-184. [13] 刘燕燕, 徐樑, 宋凯, 等. 负载型K2CO3/5A吸附剂的碳酸化反应特性研究 [J]. 化学工程, 2018, 46(7): 12-16. doi: 10.3969/j.issn.1005-9954.2018.07.003 LIU Y Y, XU L, SONG K, et al. Carbonation characteristics of supported K2CO3/5A adsorbent [J]. Chemical Engineering, 2018, 46(7): 12-16(in Chinese). doi: 10.3969/j.issn.1005-9954.2018.07.003
[14] GUO Y F, ZHAO C W, SUN J. Facile synthesis of silica aerogel supported K2CO3 sorbents with enhanced CO2 capture capacity for ultra-dilute flue gas treatment [J]. Fuel, 2018, 215(3): 735-743. [15] 陈少卿, 赵长遂, 赵传文. 钾基固体吸收剂脱除烟气中CO2技术的研究进展 [J]. 动力工程学报, 2010, 30(7): 542-549. CHEN S Q, ZHAO C S, ZHAO C W. Development of CO2 capture technology using solid potassium-based sorbents [J]. Journal of Power Engineering, 2010, 30(7): 542-549(in Chinese).
[16] 冯坚, 高庆福, 武纬, 等. 硅含量对Al2O3-SiO2气凝胶结构和性能的影响 [J]. 无机化学学报, 2009, 25(10): 1758-1763. doi: 10.3321/j.issn:1001-4861.2009.10.010 FENG J, GAO Q F, WU W, et al. Effect of silica content on structure and properties of Al2O3-SiO2 aerogeis [J]. Chinese Journal of Inorganic Chemistry, 2009, 25(10): 1758-1763(in Chinese). doi: 10.3321/j.issn:1001-4861.2009.10.010
[17] 余煜玺, 马锐, 王贯春, 等. 高比表面积、低密度块状Al2O3气凝胶的制备及表征 [J]. 材料工程, 2019, 47(12): 136-142. doi: 10.11868/j.issn.1001-4381.2017.000852 YU Y X, MA R, WANG G C, et al. Preparation and characterization of Al2O3 bulk aerogel with high specific surface area and low density [J]. Journal of Materials Engineering, 2019, 47(12): 136-142(in Chinese). doi: 10.11868/j.issn.1001-4381.2017.000852
[18] 刘博, 刘墨祥, 陈晓平. 用废弃煤矸石制备高比表面积的SiO2-Al2O3二元复合气凝胶 [J]. 化工学报, 2017, 68(5): 2096-2104. LIU B, LIU M X, CHEN X P. Preparation of SiO2-Al2O3 composite aerogel with high specific surface area by sol-gel method from coal gangue [J]. CIESC Journal, 2017, 68(5): 2096-2104(in Chinese).
[19] 陈娜, 严云, 胡志华, 等. 用粉煤灰制备SiO2-Al2O3气凝胶的研究 [J]. 武汉理工大学学报, 2011, 33(2): 37-41. doi: 10.3963/j.issn.1671-4431.2011.02.009 CHEN N, YAN Y, HU Z H, et al. Research of SiO2-Al2O3 aerogel with fly ash [J]. Journal of Wuhan University of Technology, 2011, 33(2): 37-41(in Chinese). doi: 10.3963/j.issn.1671-4431.2011.02.009
[20] 蒲鲲, 陈宁, 李斌. 粉煤灰制备硅—铝气凝胶的研究 [J]. 建材发展导向, 2017, 15(18): 1672-1675. PU K, CHEN N, LI B. Study on the preparation of silicon-aluminum aerogel from fly ash [J]. Development Opment Guide to Building Materials, 2017, 15(18): 1672-1675(in Chinese).
[21] 许志康. 钠基CO2固体吸附剂制备改性及成型研究[D]. 南京: 东南大学, 2019. XU Z K. Study on the preparation & modification and pelletization of sodium-based CO2 solid sorbents[D]. Nanjing: Southeast University, 2019(in Chinese).
[22] GUO B H, WANG Y L, GUO J N, et al. Experiment and kinetic model study on modified potassium-based CO2 adsorbent [J]. Chemical Engineering Journal, 2020, 399: 125849. doi: 10.1016/j.cej.2020.125849 [23] AMIRI M, SHAHHOSSEINI S. Optimization of CO2 capture from simulated flue gas using K2CO3/Al2O3 in a micro fluidized bed reactor [J]. Energy and Fuels, 2018, 32(7): 7978-7990. doi: 10.1021/acs.energyfuels.8b00789 [24] KONG Y, SHEN X D, CUI S, et al. Development of monolithic adsorbent via polymeric sol-gel process for low-concentration CO2 capture [J]. Applied Energy, 2015, 147: 308-317. doi: 10.1016/j.apenergy.2015.03.011 [25] LINNEEN N, PFEFFER R, LIN Y S. CO2 capture using particulate silica aerogel immobilized with tetraethylenepentamine [J]. Microporous and Mesoporous Materials, 2013, 176: 123-131. doi: 10.1016/j.micromeso.2013.02.052 [26] 何余生, 李忠, 奚红霞. 气固吸附等温线的研究进展 [J]. 离子交换与吸附, 2014, 20(4): 276-289. HE Y S, LI Z, XI H X. Research progress of gas-solid adsorption isotherms [J]. Ion Exchange and Adsorption, 2014, 20(4): 276-289(in Chinese).
[27] KONG Y, JIANG G D, FAN M H, et al. A new aerogel based CO2 adsorbent developed using a simple sol-gel method along with supercritical drying [J]. Chemical Communication, 2014, 50(81): 12158-12161. doi: 10.1039/C4CC06424K [28] HUANG Y Q, LIU H C, YUAN H Y, et al. Migration and speciation transformation of K and Cl caused by interaction of KCl with organics during devolatilization of KCl-loaded model biomass compounds [J]. Fuel, 2020, 277: 118205-118215. doi: 10.1016/j.fuel.2020.118205 -



 下载:
下载: