-
双酚A作为一种内分泌干扰物,是环境中存在最广的一类双酚类污染物,会使动物产生雌性早熟、性发育紊乱,具有胚胎毒性和致畸性,并且能够引起内分泌紊乱等[1-3]. 随着社会的快速发展,电子产品、塑料袋以及化妆品的不当处理,导致BPA在环境中日益累积,危及人们的用水安全[4]. 因此,探究如何有效去除水中BPA具有重要的研究意义.
高级氧化技术(AOPs)是目前水处理中最常见的方法之一. 过一硫酸盐(peroxymonosulfate,PMS)因其具有化学稳定性好、方便运输、易溶于水等优点而常被用作氧化剂[5]. 活化过一硫酸盐产生的硫酸根自由基(
${\rm{SO}}_4^{ \cdot - } $ ),相比于传统芬顿产生羟基自由基(·OH)拥有更高的氧化还原电位,且${\rm{SO}}_4^{ \cdot - } $ 半衰期更长(30—40 μs)、pH适应范围更广[6-7].PMS的活化方法被研究人员广泛探究,如热活化[8]、超声活化[9]以及紫外线辐射活化[10]等,但都存在着能量消耗过大的缺点.过渡金属离子作为活化PMS最有效的方法,能够有效降解污染物,如Co2+、Fe2+、Mn2+等[11-13],取得了相当大的进展.同样的,金属析出对环境造成二次污染的问题不可避免. 因此,研究人员把目光转向了非金属催化剂.活性炭、碳纳米管、多孔碳以及石墨烯等[14-17]碳材料均展现出一定的活化PMS的能力,但和过渡金属的优异性能相比,效率仍相差甚远. 因此,研究人员对其进行改性,O、S、N等元素的掺杂可以极大提高其催化性能. Gao等[18]通过一步热聚合法合成氧掺杂的氮化碳活化PMS降解BPA,在60 min内可以将其完全去除. Zhang等[19]直接煅烧三硫氰尿酸得到硫掺杂的氮化碳,在外加光源的协同作用下有效的活化PMS.Wang等[20]以尿素为前驱体制备出氮掺杂的氧化石墨烯,大大提高了对磺胺甲恶唑的降解效率. 研究表明,氮相比于其他非金属元素,对催化剂性能的提高更优,主要因为氮比碳的电负性更高,可以改变邻碳的电荷分布,并提高对PMS的吸附性能[21-22].
基于已有的研究成果[23],本研究拟采用尿素和2-萘甲醛为前驱体,通过一步热聚合法制备氮掺杂的碳材料(NCN-x). 以NCN-x为催化剂活化PMS降解双酚A,并探究了催化剂的添加量、PMS的添加量、反应温度以及初始pH值等对降解效率的影响;进一步通过捕获实验来判断反应体系中主要的活性物种.
-
-
采用一步热聚合的方法制备氮掺杂的碳催化剂,其制备过程如下:以尿素和2-萘甲醛为前驱体. 称取20 g尿素和一定量的2-萘甲醛(分别为0.2、0.4、0.6、0.8、1.0 g)机械研磨均匀,在马弗炉中于550 ℃煅烧2 h,升温速率为5.0 ℃·min−1. 将所得到的催化剂用大量超纯水和乙醇洗涤,然后在60 ℃的烘箱中烘干,命名为NCN-x(x为2-萘甲醛对尿素的质量百分比). 为了对比,单独将20 g尿素在马弗炉采用同样的制备过程合成石墨相氮化碳(g-C3N4).
-
本实验采用扫描电子显微镜(日本电子JSM-IT500HR)对催化剂的表面形貌进行分析,日本电子JFC-1600型离子溅射仪(喷金仪)对材料进行喷金;采用傅里叶红外光谱仪(美国NICOLFT公司NEXUS870型)用于研究分子结构和物质化学组成,扫描速率为4 cm−1·min−1,扫描范围为500—4000 cm−1;采用X射线衍射仪(Bruker-AXS D8 Advance)用于研究催化剂的物相组成成分、晶型结构和晶胞参数,设置电压为40 kV,电流位40 mA,扫描范围为5°—80°;采用X射线光电子能谱仪(日本ULVAC-PHI公司,PHI5000 VersaProbe)对催化剂的元素组成和含量以及分子结构等进行分析.
-
本实验以双酚A(BPA)为目标污染物,具体实验过程为:量取50 mL浓度为30 mg·L−1的BPA加入到锥形瓶中,接着加入催化剂,达到吸附平衡后,加入一定量的PMS引发反应. 设置水浴温度为30 ℃,转速为800 ·min−1. 在设定的时间间隔取样,每次用注射器取0.5 mL样品,通过0.22 μm的膜过滤,然后加入到1 mL的甲醇中终止反应.不添加PMS,其余实验条件不变,来评价催化剂的吸附性,空白对照为不加催化剂.
为了探究反应体系中各种因素的影响,做了催化剂不同的添加量、PMS不同的添加量、温度以及初始pH值条件实验. 采用1 mol·L−1的NaOH和1 mol·L−1的HCl调控pH值. 所有的实验均做3次平行,并绘制误差线.
-
双酚A的浓度通过配备有Waters 2489 UV/Visible检测器的高效液相色谱(HPLC)来检测. 将20 μL样品注入iChrom C18色谱柱(4.6 mm×250 mm,2.5 μm)中以分离分析物,并将色谱柱温度保持在30 ℃. 流动相由甲醇和水(体积比 = 85∶15)组成,流速为1 mL·min−1,检测波长为230 nm,BPA洗脱时间为3.5 min.
总有机碳的浓度测定采用Elementar vairo TOC仪器.
-
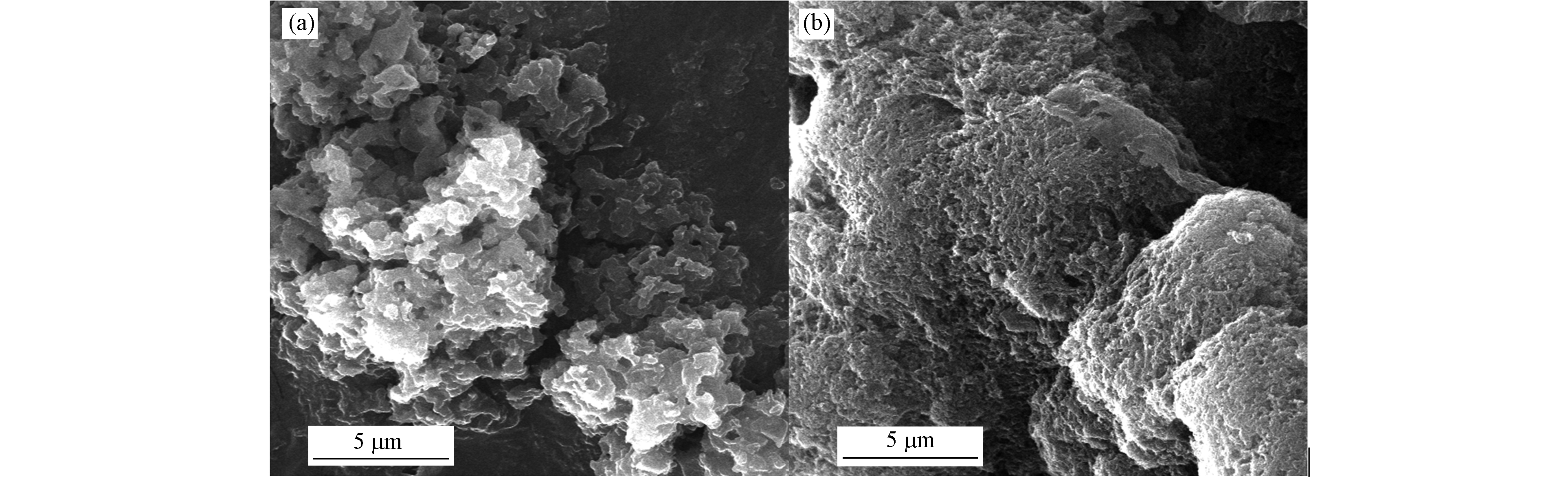
利用扫描电子显微镜(SEM)对催化剂的表面形貌进行分析. 图1a是g-C3N4的SEM图,其结构为层层堆叠的块状. 当2-萘甲醛加入尿素的热聚合过程中时,产物NCN-3的SEM图如图1b所示,NCN-3的表面更加粗糙. 这可能因为2-萘甲醛加入后与尿素热解的产物发生反应,在气体的冲击下影响其聚合过程而导致的. 在用于催化反应时,NCN-3中凹凸不平的表面也可为其提供更多的活性位点.
-
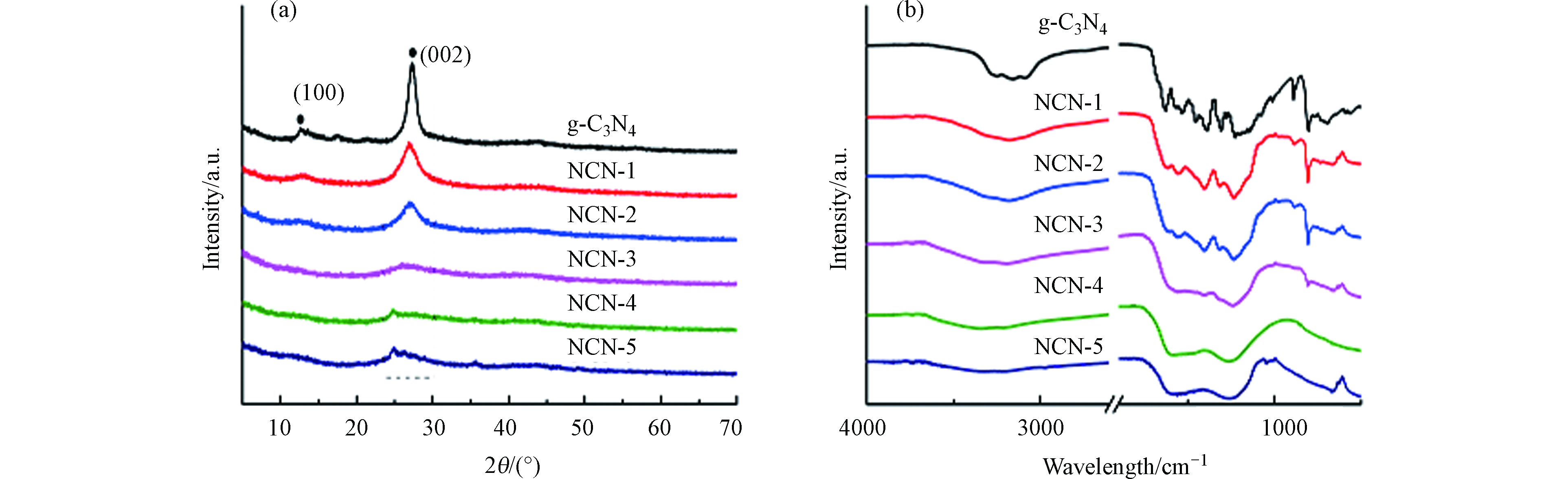
XRD谱图能够反应催化剂的晶面特征. 如图2a所示,2θ为13.1°和27.4°两处一强一弱的特征峰分别代表g-C3N4的(100)晶面和(002)晶面,即分别为其面内的七嗪单元结构和片层层间距[24]. 随着2-萘甲醛比例的增加,NCN-x中13.1°处的峰逐渐减弱,说明催化剂晶体中的七嗪结构被破坏,这是因为尿素在分解过程中,含胺类化合物与2-萘甲醛发生席夫碱反应,从而影响了g-C3N4的聚合过程[25-26]. 同样的,NCN-x中27.4°处的峰也明显减弱,且峰位置向小角度移动,说明NCN-x的层间距变大[27].
-
通过傅立叶变换红外光谱对g-C3N4与NCN-x的化学结构进行分析. 如图2b所示,g-C3N4在806 cm−1处有明显的尖峰,对应于七嗪结构的呼吸振动峰. 在1200—1600 cm−1之间出现多个峰,对应于七嗪单元的伸缩振动模式,而在3000—3500 cm−1处的宽峰则对应-NH和-OH的伸缩振动[28]. 随着NCN-x合成过程中2-萘甲醛的加入,NCN-x在806 cm−1处的峰强逐渐减小,说明七嗪单元结构被破坏,这与XRD得到的结果一致. NCN-x催化剂在3000—3500 cm−1处的峰减弱并变宽是由于-NH被C-N所取代. 可见,在尿素聚合时2-萘甲醛的加入阻碍了g-C3N4的形成过程,而促成了含氮碳材料NCN-x的形成.
-
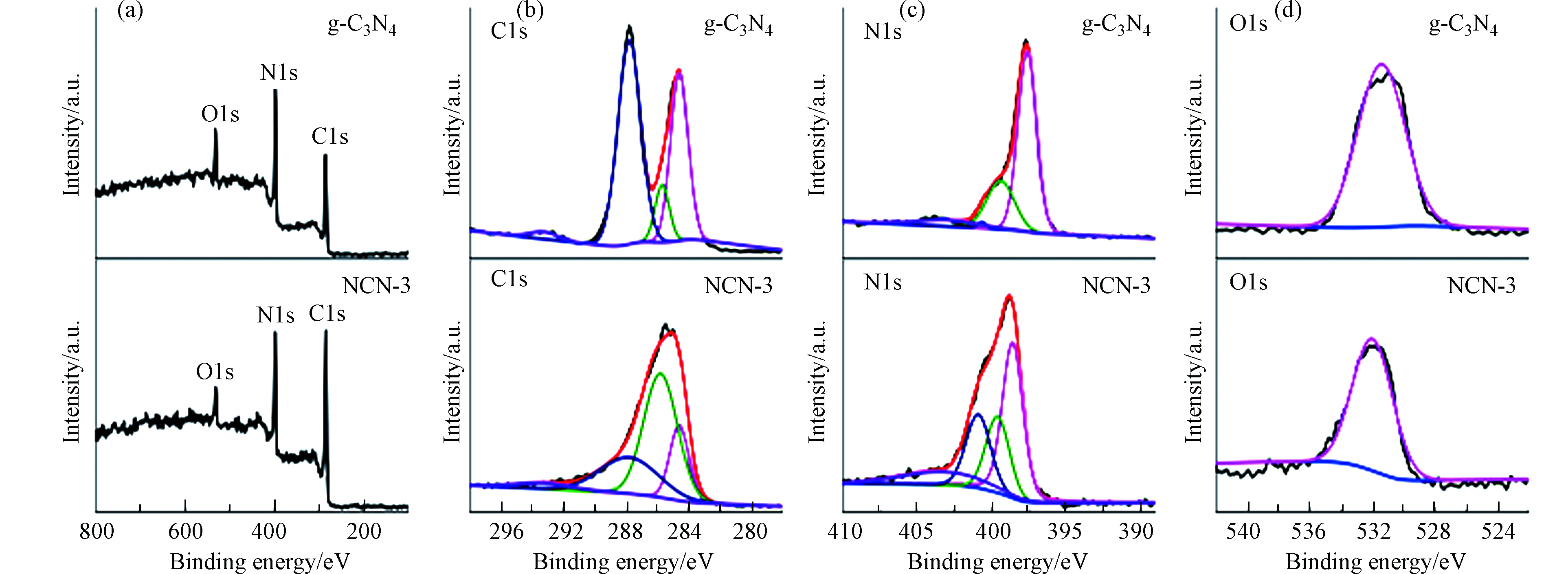
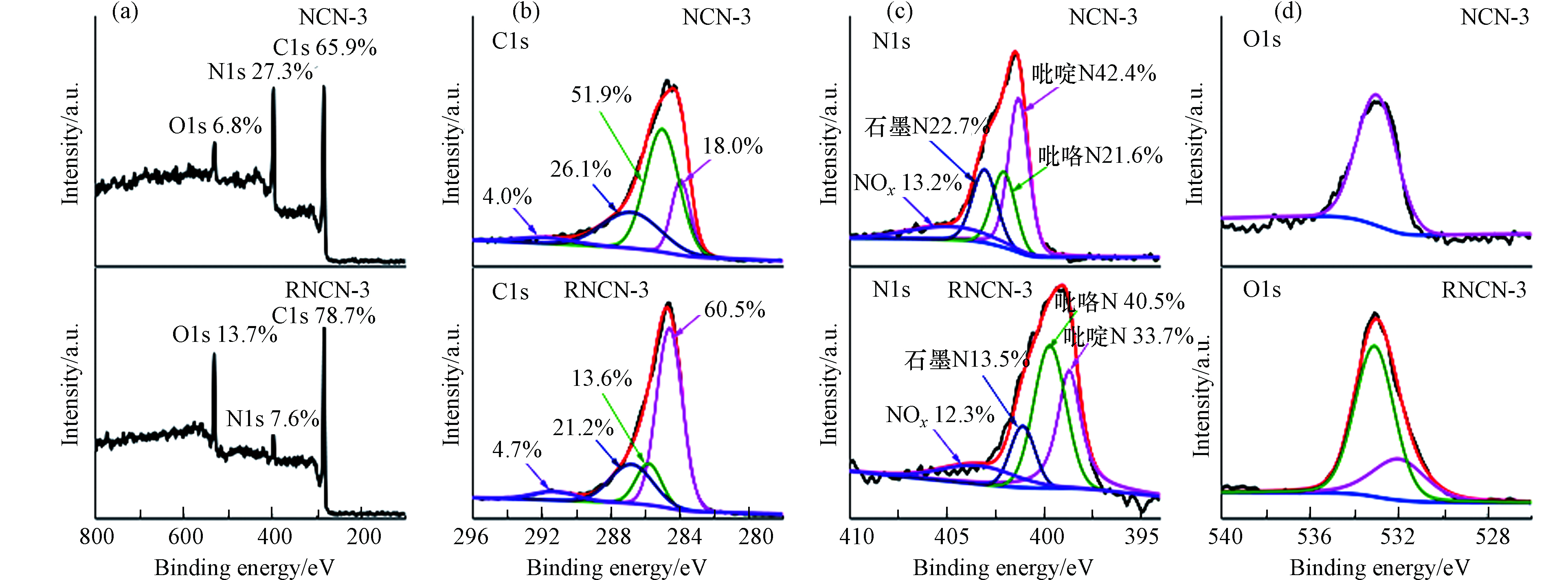
用X射线光电子能谱(XPS)来研究g-C3N4和NCN-3中化学组成以及各元素价态.结果如图3a所示,g-C3N4与NCN-3均由C、N、O组成. 与g-C3N4相比,NCN-3中C元素所占的比重大大增加,这可能是因为2-萘甲醛中苯环中的碳引入导致的. 由图3b可以看出,NCN-3的C1s谱图在结合能为284.6、285.8、287.8、293.3 eV处出现4个特征峰,分别对应于石墨碳基准峰、环氧基团中C-O键、七嗪单元中的sp2杂化碳和π电子激发[29]. 这与g-C3N4在284.6、285.7、287.8、293.3 eV的峰位置基本保持一致,这表明NCN-3仍保留一部分g-C3N4的结构.NCN-3的N1s谱图在结合能为398.6、399.6、400.9、403.3 eV的峰分别对应于吡啶N、吡咯N、石墨N以及N的氧化物(NOx). 如表3所示,NCN-3中石墨N所占的比例为g-C3N4中的22.7倍. 此外,NCN-3的O1s谱图在532.0 eV处的出峰归因于催化剂表面上吸附的二氧化碳(C=O)和水中的O原子[30-31].
分析了N元素在催化剂中的含量,如表4,NCN-1催化剂中N原子浓度大于g-C3N4中的N。因为2-萘甲醛的加入,促进了NCN-x的碳化,催化剂的产量降低,导致N原子浓度相对升高. 而随着2-萘甲醛的比例逐渐增大,N原子浓度逐渐降低,这是因为对苯二甲醛不含N元素,而有较多的C元素,C元素的掺入使得N原子浓度相对降低.
-
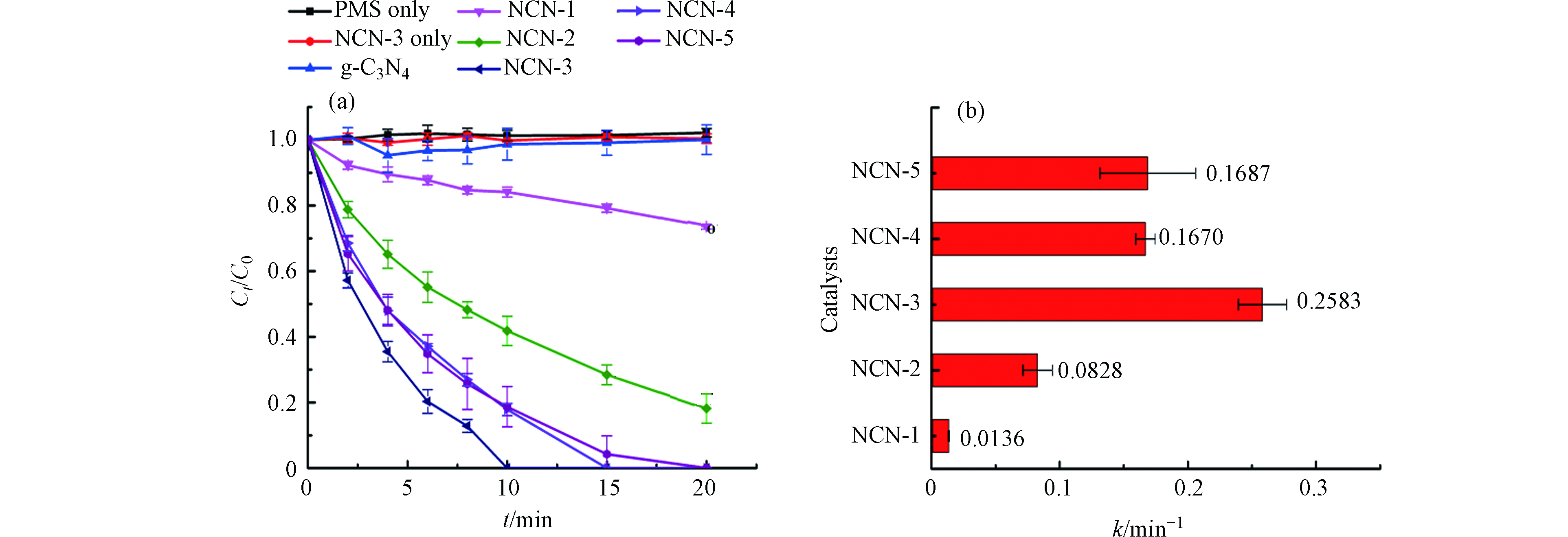
利用不同2-萘甲醛的添加量制备的NCN-x,在活化PMS降解双酚A时效果差异较大.如图4a所示,当NCN-3和PMS单独作用于BPA时,BPA的浓度几乎没有变化,说明NCN-3对BPA没有吸附作用;此外,PMS也不能单独降解BPA. 当2-萘甲醛添加量为0,用g-C3N4活化PMS降解BPA时,BPA的浓度没有变化,说明g-C3N4不能活化PMS. 而随着NCN-x中2-萘甲醛添加量的增大,其对BPA的降解效果逐渐增大,当2-萘甲醛的比例达到3%(即为NCN-3)时,效果最佳,10 min左右即可完全去除BPA. 当2-萘甲醛的含量由1%增大至3%时,对应催化剂的速率常数从0.0136 min−1增加到0.2583 min−1,扩大了近19倍,这是因为石墨N的引入会破坏sp2杂化碳层的化学惰性并产生新的活性位点,并使得邻C产生较高的正电荷密度,有利于吸附PMS. 随着2-萘甲醛的比例继续增大到4%和5%时,催化剂的活性又逐渐降低. 这可能是由于2-萘甲醛的量过多,催化剂的层间距过大,结构破碎,导致了电荷载流子的振动能量更容易散逸[32].
-
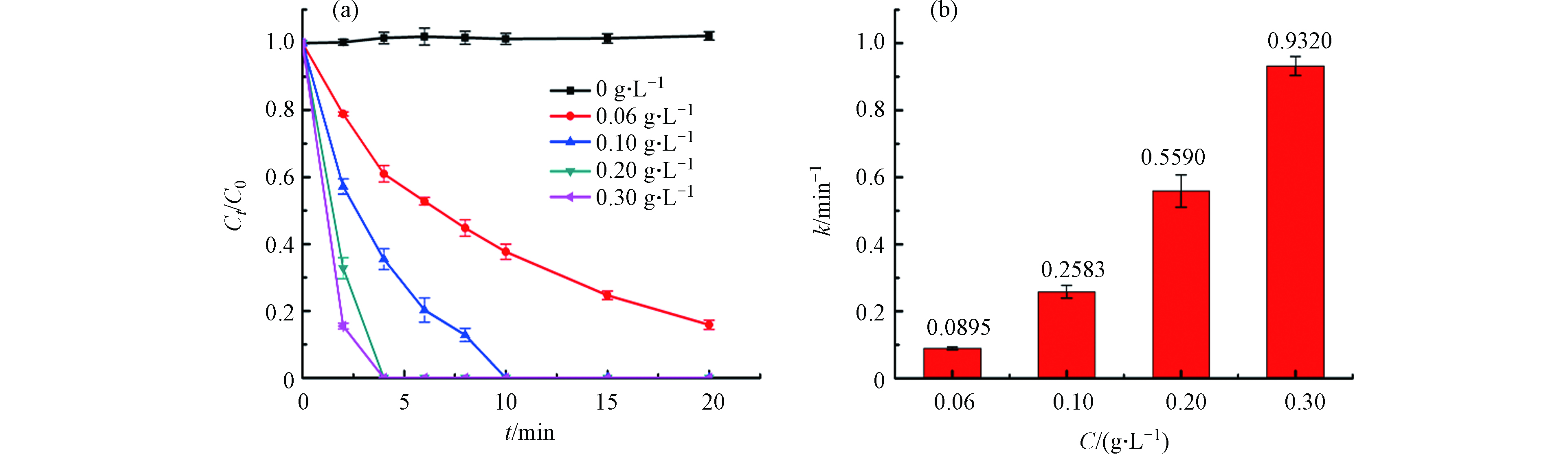
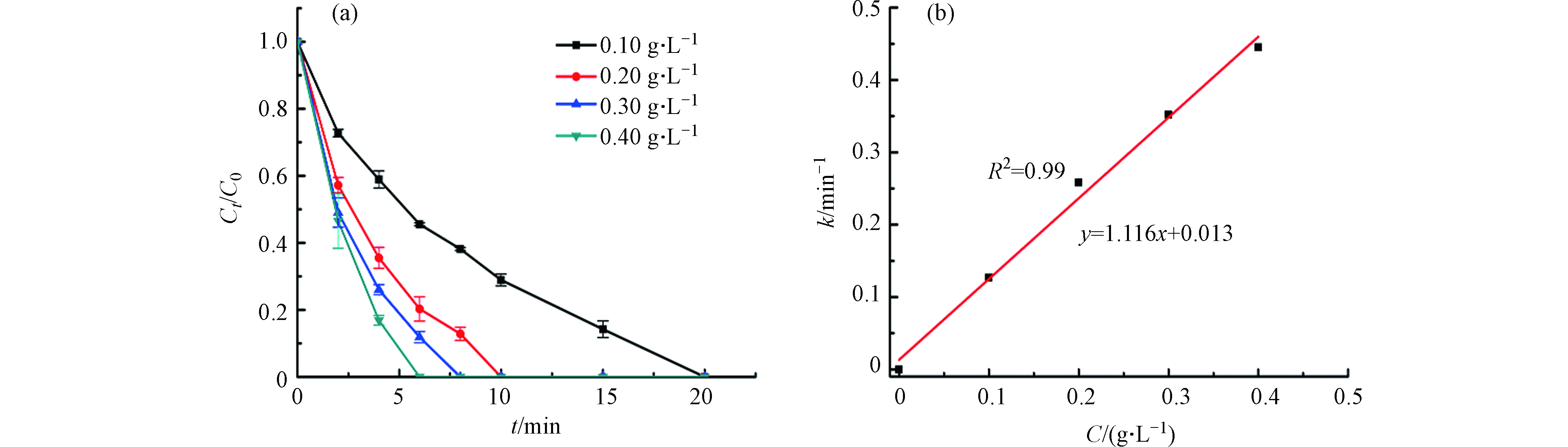
进一步探究催化剂的投加量对活化PMS降解BPA的影响,从图5a可以看出,随着催化剂投加量的提升,BPA的降解速率越来越快,当浓度达到0.20 g·L−1时,BPA在4 min左右就被完全去除. 图5b展示的是不同催化剂浓度的速率常数,NCN-3的浓度从0.06 g·L−1增加到0.30 g·L−1时,速率常数k从0.0895 min−1提升到0.9320> min−1,增大了10.4倍. 这是因为增加催化剂的量则意味着可以提供更多的活性位点,促进活性物种的生成,进一步促进催化反应[33].
-
图6a显示了PMS浓度对BPA降解效率的影响,当PMS的浓度为0.10 g·L−1时,BPA被完全降解需要20 min,而当0.40 g·L−1的PMS加入到反应体系中时,仅6 min左右即可将BPA完全去除,这是因为PMS浓度的提升利于催化剂与PMS更好的接触[34].
PMS浓度为0.10、0.20、0.30、0.40 g·L−1,对应的速率常数k分别为0.1267、0.2583、0.3521、0.4452 min−1. 从图6b可以看出速率常数k与PMS的浓度符合线性关系(k = 1.116x+0.013),表明在反应过程中的活性物种主要源自PMS的活化反应产物[35].
-
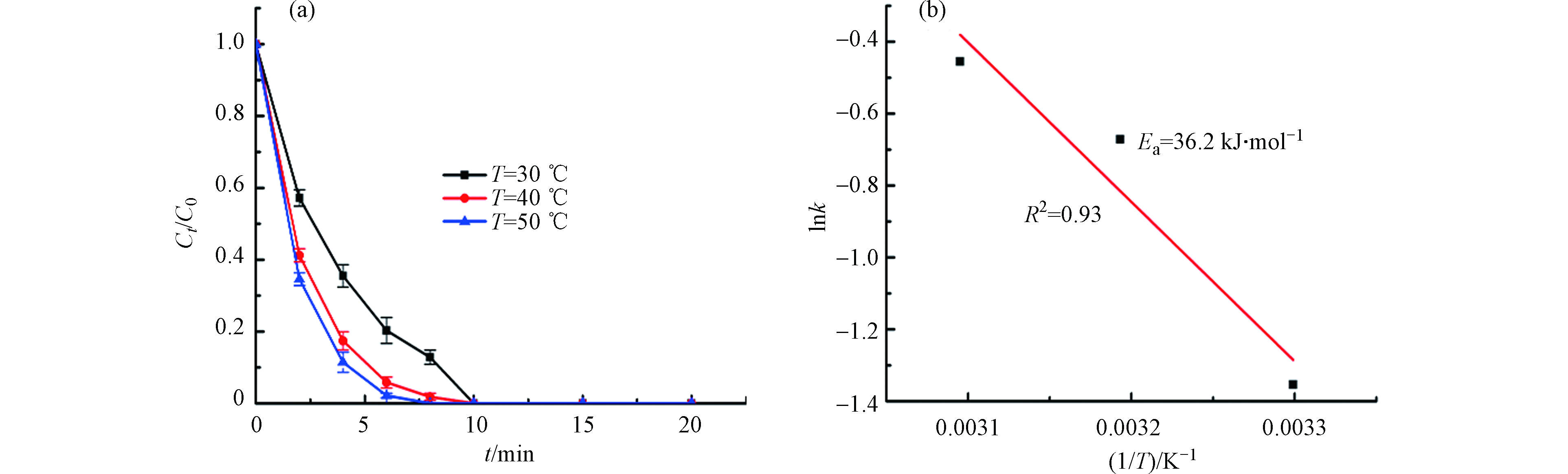
反应温度是活化PMS降解BPA的一个重要影响参数. 如图7a,随着温度的升高,BPA的降解速率有略微的提升,这得益于温度升高有助于PMS的分解[36]. 从图7b得知,反应速率常数与反应温度之间具有线性相关性.
根据Arrhenius方程(1),计算可得表观活化能为36.2 kJ·mol−1,这表明反应温度的变化对BPA的降解效率影响相对较小[37],且低于之前文献报导的催化剂用于活化PMS降解BPA的催化剂的活化能,如Co3O4-Bi2O3(50.5 kJ·mol−1)[38]和B-OMC(64.04 kJ·mol−1)[39].
其中,Ea是Arrhenius活化能(kJ·mol−1),A是指前因子(单位同k),R是通用气体常数(8.314 J·mol−1·K−1),T是系统反应温度(K).
-
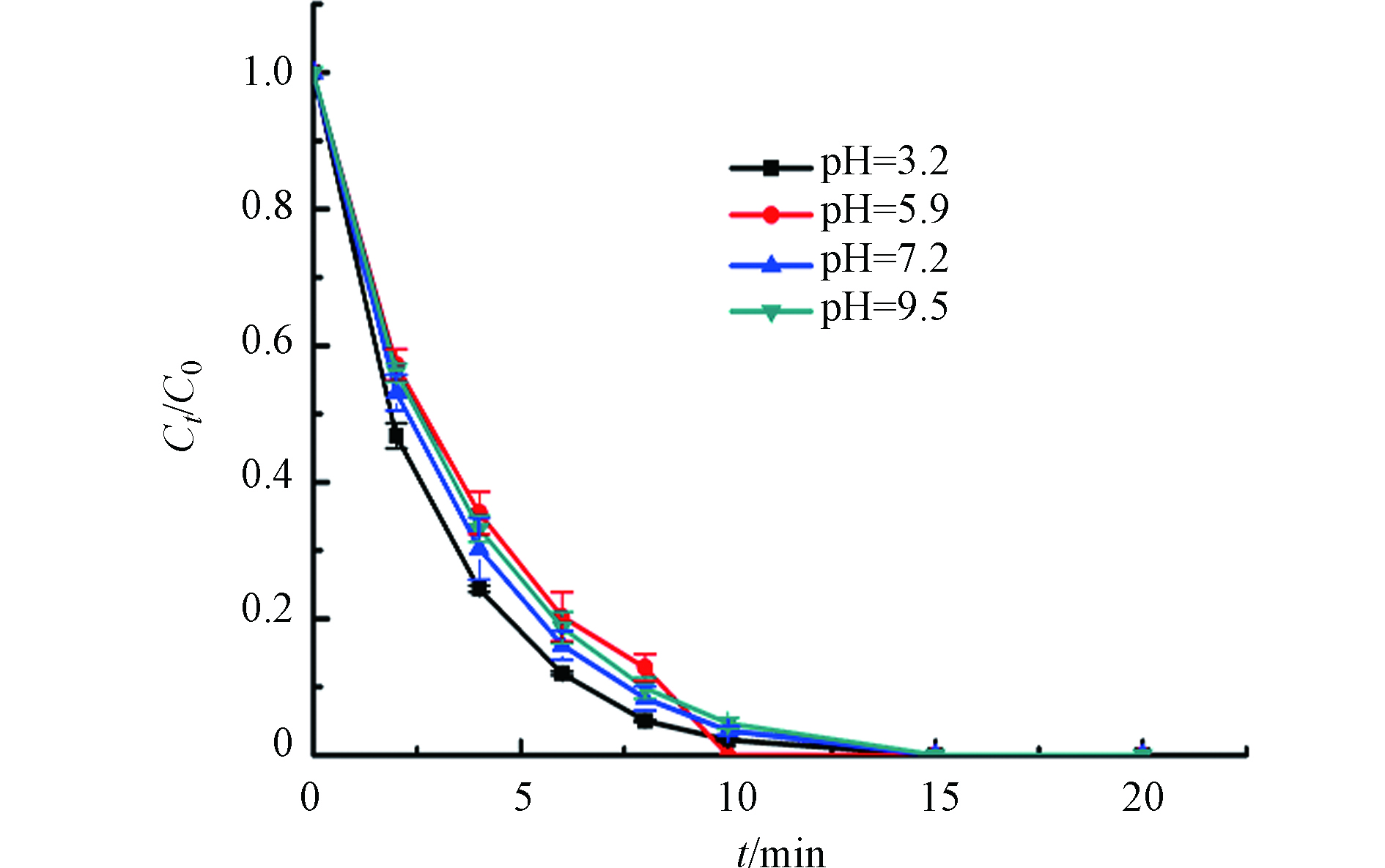
在实际处理废水中,pH值对污水处理要求非常高,如利用可溶性铁盐的经典Fenton芬顿氧化在酸性的条件下效果最佳,但会导致昂贵的费用[40]. 而从图8中可以看出,NCN-3/PMS反应体系的pH值从3.2提高至9.5时,对BPA的降解影响较小,20 min内都可以将其完全降解. 这说明催化剂具有很好的抗pH值干扰性,在酸性、中性以及碱性条件下都能够保持较高的活性[41]. 此外,原始BPA溶液的pH值为5.9,不添加酸或碱调节,这也节省了费用、减少了其他离子的干扰[42].
-
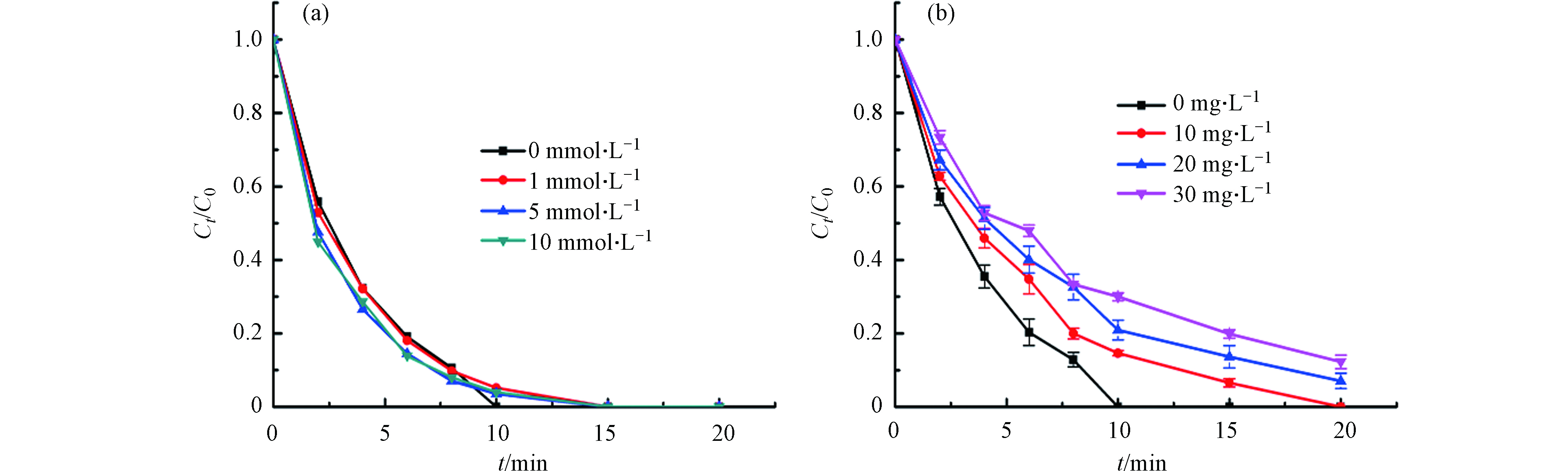
自然界的实际水体中,含有大量的干扰离子,可能会影响反应体系中的降解效率. 从图9a中可以发现,Cl-浓度为1 mmol·L−1时对BPA降解的影响并不明显,该结果与之前的报道类似[43]. 当其浓度为5 mmol·L−1和10 mmol·L−1时,降解效率仅有略微的提升. 这可能是因为在NCN-3/PMS/BPA体系中,Cl−与PMS反应生成了HOCl,促进了BPA的降解[37].
-
实际水体中,有许多溶解性有机物质.本实验选用腐殖酸(HA)作为模型有机物质,探究其对活化PMS降解BPA的影响. 如图9b所示,随着反应体系中HA浓度的增大,BPA的降解效果受到抑制,当浓度达到30 mg·L−1时,只能降解88%. 研究表明,HA与BPA在降解过程中存在着竞争关系,从而导致NCN-3的活化效果出现逐渐下降的趋势[44].
-
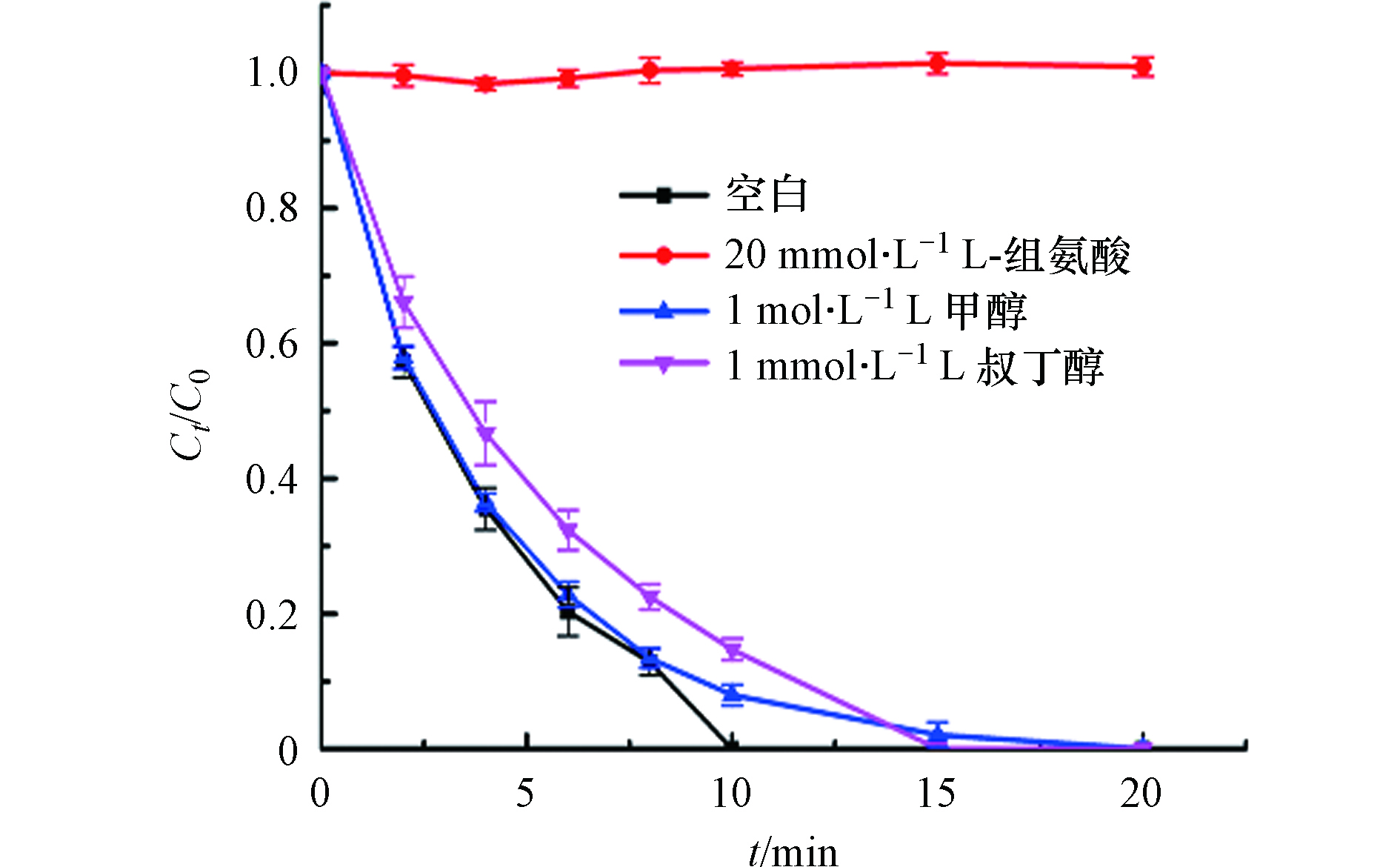
为了探究每个活性物种对BPA降解的贡献,采用自由基猝灭实验,甲醇、叔丁醇和L-组氨酸分别用来捕获
${\rm{SO}}_4^{ \cdot - } $ 、·OH和1O2,反应速率常数如表5. 从图10可以看出,当加入甲醇和叔丁醇时,BPA的降解效率没有明显的改变,而加入少量的(20 mmol·L−1)L-组氨酸时,BPA基本上没有降解. 这说明在BPA的降解过程中,自由基途径和非自由基途径协同作用,自由基途径的效果甚微,非自由基途径占主导地位[34]. 此外,图中显示叔丁醇产生的抑制作用略大于甲醇产生的,这可能是因为一部分叔丁醇吸附在了催化剂的表面覆盖了其活性位点,导致了催化性能变差[46]. -
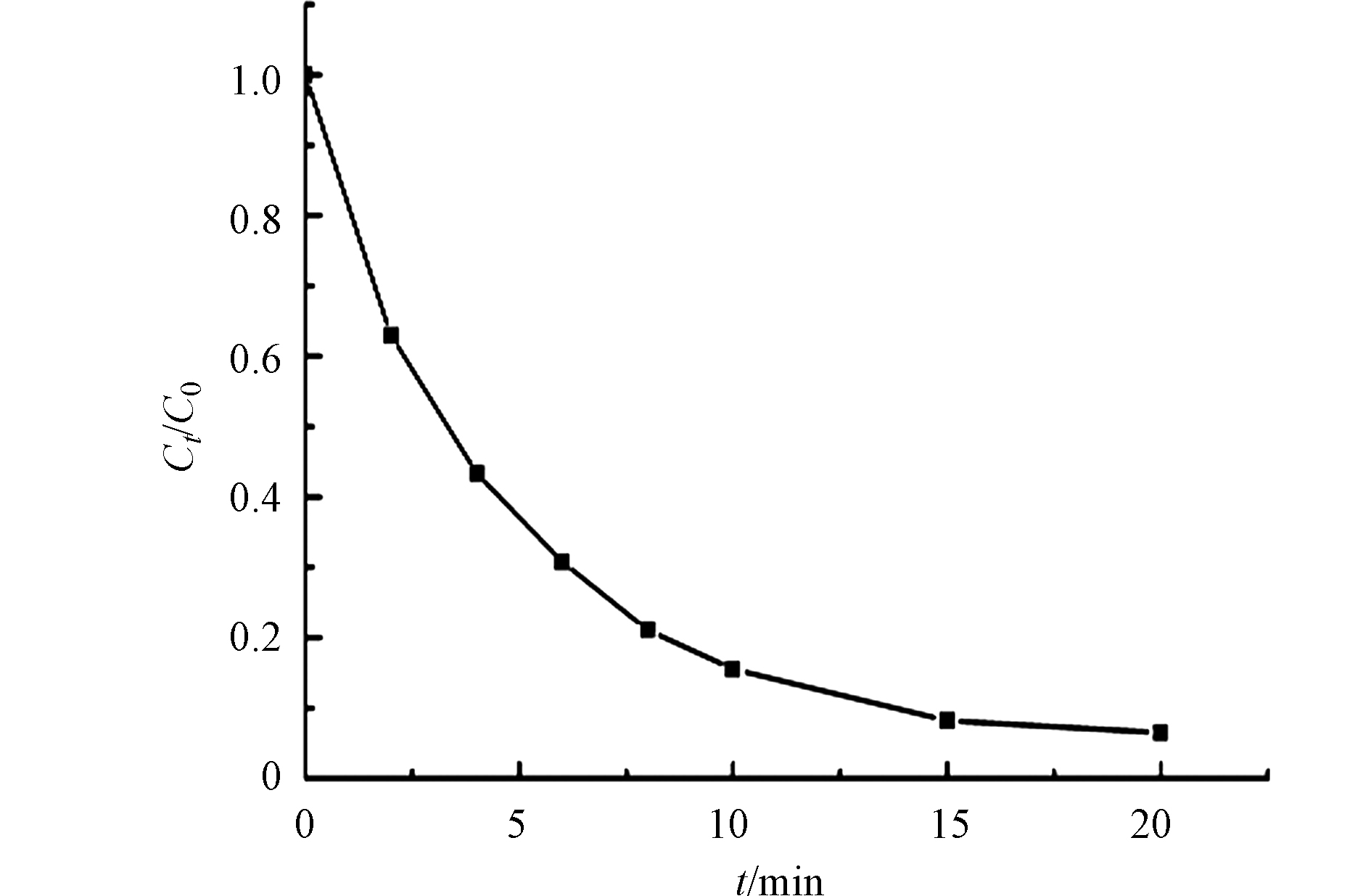
TOC的去除率可以反应污染物被矿化的程度. 由图11可知,在NCN-3/PMS系统中,TOC的浓度逐渐降低,在20 min后,TOC的去除率按到了90%以上,说明BPA基本上被转化成H2O和CO2,NCN-3可以作为一个有效的催化剂活化PMS.
-
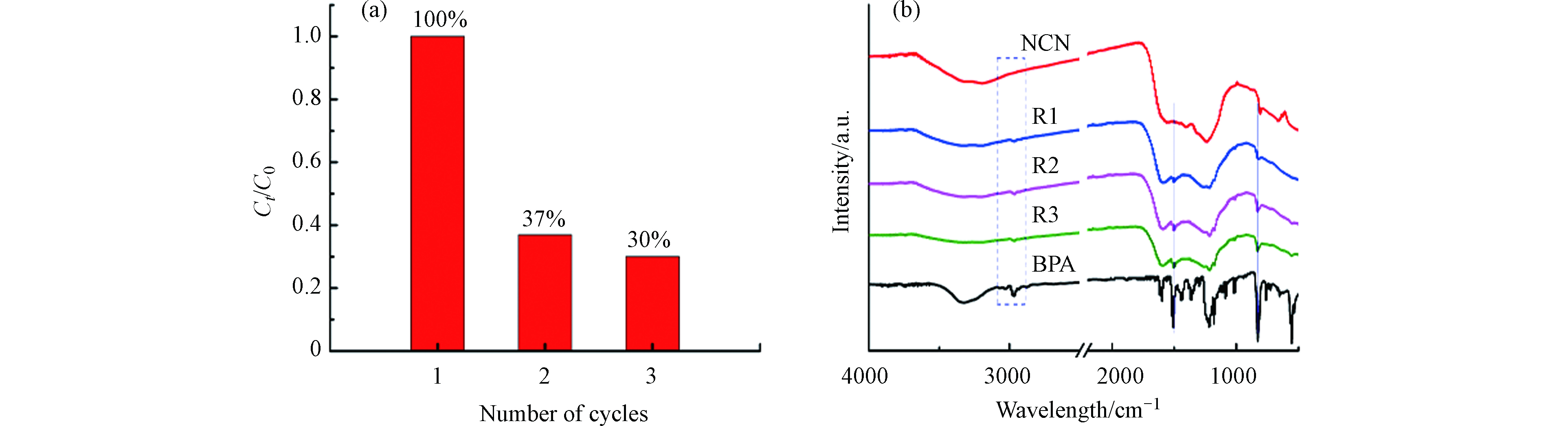
碳材料作为催化剂活化PMS降解污染物时,其稳定性一直是应用的难点. 如图12a,在NCN-3/PMS体系反应20 min后,NCN-3在第二次对BPA的降解效率仅有37%,催化剂的性能大大降低,第三次仅有30%,有略微下降,说明NCN-3的稳定性不好. 因此,对反应前后的催化剂进行红外表征,如图12b,R1、R2和R3分别为第一次、第二次和第三次回收后的催化剂,可以发现回收后的催化剂有BPA的特征峰出现,特别是在1500 cm−1处,有苯环的特征峰出现,这说明BPA或者中间产物吸附在NCN-3的表面,掩盖了活性位点,导致催化性能的降低;而PMS也具有一定的氧化能力,NCN-3表面官能团部分被氧化.
-
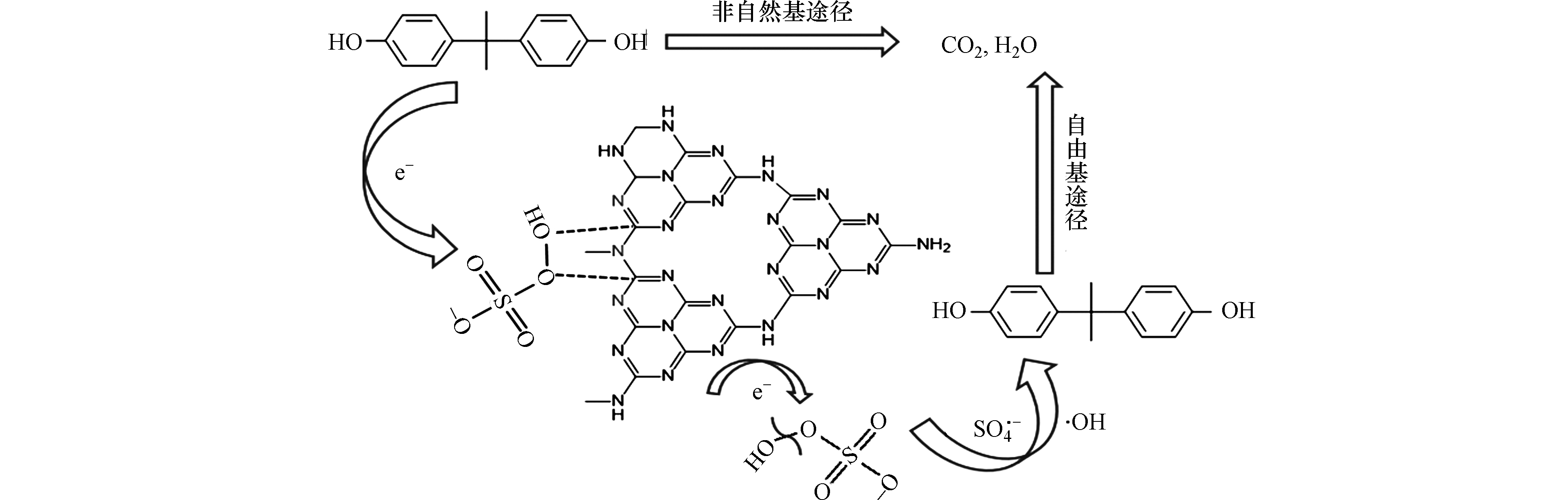
由自由基的猝灭实验结果可知,NCN-3活化PMS降解双酚A的过程中,如图13所示,非自由基途径占主要作用,自由基途径作用较小. NCN-3有着较高的石墨N比例,相比C原子,石墨N的存在会将相邻碳原子的电子吸引过来,使邻碳产生较高的正电荷密度,从而有效提高了催化剂对带负电的PMS的吸附能力,形成复合中间体[47]. 电子通过复合体从污染物(电子供体)转移到PMS(电子受体),PMS被消耗产生1O2,污染物则通过电子转移和1O2协同作用被矿化成H2O、CO2和小分子物质[48-49],这是双酚A降解的非自由基途径. 自由基途径则是NCN-3的边缘缺陷等为主要活性位点,PMS接受电子产生
${\rm{SO}}_4^{ \cdot - } $ 和·OH去攻击BPA,使其转化为H2O、CO2和小分子物质.为了探究催化剂的活性位点,分析了3次循环试验后的XPS图谱. 如图14a所示,RNCN-3中C1s和O1s在催化剂中的比例有所提升,这是因为BPA及其降解产物吸附在了NCN-3的表面,观察图14d也可发现,除了在532.0 eV催化剂表面上吸附的二氧化碳(C=O)和水中的O原子的峰之外,在533.1 eV处出现了一个新峰,这可能是因为BPA或者其中间产物上的O导致的. 以上结果与RNCN-3的红外表征的结论相吻合,说明BPA或者其中间产物吸附在了NCN-3的表面. 图14c中,石墨N的比例减少,是因为经过电子转移以及催化反应后,一部分石墨N转化成了吡啶N. 由上述分析,可知石墨N的邻C是反应活性位点.
-
(1)以尿素和2-萘甲醛为前驱体一步热聚合制备的氮掺杂的碳催化剂大大提高了石墨N的含量,能够有效活化PMS降解BPA,并避免金属离子的二次污染和能源消耗.
(2)NCN-3、PMS的浓度均与BPA的降解效率呈正相关;催化剂NCN-3的活化能为36.2 kJ·mol−1,在较广的pH范围内均可有效降解BPA,且Cl−的存在对BPA降解影响并不明显,有一定的实际应用前景.
(3)捕获实验表明,单线态氧是反应体系中的主要活性物种;表明BPA主要通过非自由基途径降解,PMS吸附在NCN-3上形成复合体,污染物的电子通过催化剂转移至PMS,通过电子转移与产生的1O2共同作用降解BPA;降解过程中也存在自由基途径,但做出的贡献较小.
基于席夫碱反应合成氮掺杂的碳活化PMS降解双酚A
Degradation of BPA by activating PMS over N-doped carbon synthesized based on Schiff base reaction
-
摘要: 基于石墨相氮化碳(g-C3N4)的热聚合形成过程和席夫碱反应,本文将尿素和2-萘甲醛作为前驱体,经一步热聚合反应制备了氮掺杂的碳材料(NCN-x),并以其作为催化剂活化过一硫酸盐(PMS)来降解双酚A(BPA). 在催化剂特性研究部分,利用SEM、XRD、FTIR以及XPS对其表面形貌、结构与元素组成进行分析. 结果表明,2-萘甲醛的加入使NCN-x催化剂的表面更加粗糙,提供更多活性位点;由XRD和FTIR的图谱可知,对应于g-C3N4 中(100)晶面的峰强随着2-萘甲醛加入比例的增加而逐渐降低,表明NCN-x中的七嗪单元逐渐被破坏;由XPS表征结果可知,NCN-3中石墨N含量是g-C3N4中的20倍左右,使邻近碳原子带正电,并激活了碳原子上的π电子,提高对PMS的吸附性能. 通过研究催化剂的加入量、PMS添加量、反应温度和初始pH值等对BPA降解的影响,发现催化剂和PMS的添加量均与降解效率呈正相关,催化剂的活化能为36.2 kJ·mol−1;初始pH值对反应体系的影响不大,说明催化剂有较强的抗pH波动干扰的能力. 自由基猝灭实验表明,单线态氧是主要活性物种,且水中共存的氯离子对BPA降解的影响并不明显.Abstract: Based on the thermal polymerization formation process of graphitic carbon nitride (g-C3N4) and the Schiff base reaction, urea and 2-naphthaldehyde were used as precursors to prepare N-doped carbon catalysts (NCN-x). The obtained catalysts were used to activate peroxymonosulfate (PMS) to degradate bisphenol A (BPA). The characterization methods of SEM, XRD, FTIR and XPS were used to analyze the surface morphology, structure and elements composition of catalysts. The acquired results showed that the addition of 2-naphthaldehyde maked the surface of the NCN-x catalysts rougher, providing more activation sites. The analysis of the XRD and FTIR spectra showed that the (100) facet peak intensity of g-C3N4 gradually decreased with the increase of the ratio of 2-naphthaldehyde during the preparation process of NCN-x, indicating that the heptazine unit was gradually destroyed. The results of XPS characterization indicated that the graphite N content of NCN-3 was about 20 times of that in g-C3N4. The graphite N made the adjacent carbon atoms positively charged and π electrons on the carbon atoms were activated to improve the adsorption performance of PMS. Further study concerning the effect of catalyst concentration, PMS concentration, reaction temperature and initial pH on the degradation of BPA indicated that the addition amount of the catalyst and PMS was positively related to the degradation efficiency of BPA. The Ea of NCN-3 was calculated to be 36.2 kJ·mol−1, and the initial pH had little effect on the reaction system, indicating that NCN-3 had a strong ability to resist the interference of pH fluctuations. Free radical quenching experiments indicated that the main active species was singlet oxygen, and the coexisting Cl− in water had little effect on the degradation of BPA.
-
Key words:
- N-doped carbon /
- peroxymonosulfate /
- Bisphenol A /
- singlet oxygen
-

-
表 1 主要实验试剂
Table 1. Main experimental reagents
药品 Reagent 纯度 Purity 生产厂家 Manufacturer 尿素 分析纯 国药集团化学试剂有限公司 双酚A 99.0% 上海麦克林生化科技有限公司 甲醇 色谱级 默克股份两合公司 过硫酸氢钾 ≥47% 上海阿拉丁生化科技股份有限公司 叔丁醇 ≥99.5% 上海阿拉丁生化科技股份有限公司 L-组氨酸 ≥99% 上海阿拉丁生化科技股份有限公司 表 2 主要实验仪器
Table 2. Main experimental equipment
仪器 Instrument 型号 Model 生产厂家 Manufacturer 数显恒温磁力搅拌器 HJ-4A 江苏省金坛市荣华仪器制造有限公司 pH计 PB-10 德国赛多利斯仪器有限公司 马弗炉 YFX7/10Q-GC 上海意丰电炉有限公司 高效液相色谱仪 e2685 Waters公司 超纯水发生器 EPED-20TJ 南京易谱达科技发展有限公司 表 3 催化剂中不同类型的N的含量
Table 3. The content of different types of N in the catalysts
吡啶 N 吡咯 N 石墨 N NOx g-C3N4 65.6% 25.3% 1.0% 8.1% NCN-3 42.4% 21.7% 22.7% 13.2% 表 4 N原子在催化剂中的浓度
Table 4. The concentration of N atoms in catalysts
g-C3N4 NCN-1 NCN-2 NCN-3 NCN-4 NCN-5 N/% 35.8 43.0 40.4 27.3 25.7 24.2 表 5 猝灭剂与自由基的反应速率常数
Table 5. The rate constant of the reaction between quencher agent and free radicals
捕获剂 Capture reagent 活性物种 Active species 反应速率常数 Reaction rate constant 甲醇 ${\rm{SO}}_4^{ \cdot - } $ 3.2×106 L·mol−1·s−1 ·OH 9.7×108 L·mol−1·s−1 叔丁醇 ·OH 3.8×108–7.6×108 L·mol−1·s−1 L-组氨酸 1O2 3.2×107 L·mol−1·s−1 -
[1] CHEN D, KANNAN K, TAN H L, et al. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review [J]. Environmental Science & Technology, 2016, 50(11): 5438-5453. [2] VANDENBERGL N, HAUSER R, MARCUS M, et al. Human exposure to bisphenol A (BPA) [J]. Reproductive Toxicology, 2007, 24(2): 139-177. doi: 10.1016/j.reprotox.2007.07.010 [3] 邓茂先, 吴德生, 詹立. 环境雌激素双酚A的生殖毒理研究 [J]. 环境与健康杂志, 2001, 18(3): 134-136,150. doi: 10.3969/j.issn.1001-5914.2001.03.002 DENG M X, WU D S, ZHAN L. Study on mechanism of reproduction toxicity of to estrogic Bisphenol-A related to environment [J]. Journal of Environment and Health, 2001, 18(3): 134-136,150(in Chinese). doi: 10.3969/j.issn.1001-5914.2001.03.002
[4] DU J K, BAO J G, LIU Y, et al. Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A [J]. Journal of Hazardous Materials, 2016, 320: 150-159. doi: 10.1016/j.jhazmat.2016.08.021 [5] LIANG C J, WANG Z, MOHANTY N, et al. Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 ℃ [J]. Science of The Total Environment, 2006, 370(2): 271-277. [6] GONG Y, ZHAO X, ZHANG H, et al. MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation [J]. Applied Catalysis B-environmental, 2018, 233: 35-45. doi: 10.1016/j.apcatb.2018.03.077 [7] LI X N, WANG Z H, ZHANG B, et al. FexCo3-xO4 nanocages derived from nanoscale metal–organic frameworks for removal of bisphenol A by activation of peroxymonosulfate [J]. Applied Catalysis B-environmental, 2016, 181(181): 788-799. [8] JI Y F, FAN Y, LIU K, et al. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds [J]. Water Research, 2015, 87: 1-9. doi: 10.1016/j.watres.2015.09.005 [9] LIN Y T, LIANG C J, CHEN J H, et al. Feasibility study of ultraviolet activated persulfate oxidation of phenol [J]. Chemosphere, 2011, 82(8): 1168-1172. doi: 10.1016/j.chemosphere.2010.12.027 [10] LUO C W, MA J, JIANG J, et al. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2, UV/HSO5− and UV/S2O82− [J]. Water Research, 2015, 80: 99-108. doi: 10.1016/j.watres.2015.05.019 [11] HU L X, YANG X P, DANG S T, et al. An easily recyclable Co/SBA-15 catalyst: Heterogeneous activation of peroxymonosulfate for the degradation of phenol in water [J]. Applied Catalysis B-environmental, 2011, 102(1): 19-26. [12] ZOU J, MA J, CHEN L W, et al. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine [J]. Environmental Science & Technology, 2013, 47(20): 11685-11691. [13] FAN J H, QIN H H, JIANG S M, et al. Mn-doped g-C3N4 composite to activate peroxymonosulfate for acetaminophen degradation: The role of superoxide anion and singlet oxygen [J]. Chemical Engineering Journal, 2019, 359: 723-732. doi: 10.1016/j.cej.2018.11.165 [14] ZHANG J, SHAO X T, SHI C, et al. Decolorization of acid orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon [J]. Chemical Engineering Journal, 2013, 232: 259-265. doi: 10.1016/j.cej.2013.07.108 [15] CHEN J B, ZHANG L M, HUANG T Y, et al. Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: Radical versus non-radical mechanism [J]. Journal of Hazardous Materials, 2016, 320: 571-580. doi: 10.1016/j.jhazmat.2016.07.038 [16] WANG G, CHEN S, QUAN X, et al. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants [J]. Carbon, 2017, 115: 730-739. doi: 10.1016/j.carbon.2017.01.060 [17] DUAN X G, SUN H Q, AO Z M, et al. Unveiling the active sites of graphene-catalyzed peroxymonosulfate activation [J]. Carbon, 2016, 107: 371-378. doi: 10.1016/j.carbon.2016.06.016 [18] GAO Y W, ZHU Y, LYU L, et al. Electronic structure modulation of graphitic carbon nitride by oxygen doping for enhanced catalytic degradation of organic pollutants through peroxymonosulfate activation [J]. Environmental Science & Technology, 2018, 52(24): 14371-14380. [19] LIN K A, ZHANG Z. Degradation of Bisphenol A using peroxymonosulfate activated by one-step prepared sulfur-doped carbon nitride as a metal-free heterogeneous catalyst [J]. Chemical Engineering Journal, 2017, 313: 1320-1327. doi: 10.1016/j.cej.2016.11.025 [20] WANG S Z, XU L J, WANG J L, et al. Nitrogen-doped graphene as peroxymonosulfate activator and electron transfer mediator for the enhanced degradation of sulfamethoxazole [J]. Chemical Engineering Journal, 2019, 375: 122041. doi: 10.1016/j.cej.2019.122041 [21] QU L T, LIU Y, BAEK J, et al. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells [J]. ACS Nano, 2010, 4(3): 1321-1326. doi: 10.1021/nn901850u [22] PARAKNOWTTSCH J P, THOMAS A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications [J]. Energy and Environmental Science, 2013, 6(10): 2839-2855. doi: 10.1039/c3ee41444b [23] XUE W L, CAO S H, LIU R, et al. Preparation of nitrogen-containing carbon using a one-step thermal polymerization method for activation of peroxymonosulfate to degrade bisphenol A [J]. Chemosphere, 2020, 248: 126053. doi: 10.1016/j.chemosphere.2020.126053 [24] HO W K, ZHANG Z Z, LIN W, et al. Copolymerization with 2, 4, 6-Triaminopyrimidine for the Rolling-up the Layer Structure, Tunable Electronic Properties, and Photocatalysis of g-C3N4 [J]. ACS Applied Materials & Interfaces, 2015, 7(9): 5497-5505. [25] ZHAO Y H, LIU M X, DENG X X, et al. Nitrogen-functionalized microporous carbon nanoparticles for high performance supercapacitor electrode [J]. Electrochimica Acta, 2015, 153(153): 448-455. [26] CHIDHAMBARAM N, RAVICHANDRAN K. Single step transformation of urea into metal-free g-C3N4 nanoflakes for visible light photocatalytic applications [J]. Materials Letters, 2017, 207: 44-48. doi: 10.1016/j.matlet.2017.07.040 [27] CHEN H, YAO J H, QIU P X, et al. Facile surfactant assistant synthesis of porous oxygen-doped graphitic carbon nitride nanosheets with enhanced visible light photocatalytic activity [J]. Materials Research Bulletin, 2017, 91: 42-48. doi: 10.1016/j.materresbull.2017.02.042 [28] GU J Y, CHEN H, JIANG F, et al. Visible light photocatalytic mineralization of bisphenol A by carbon and oxygen dual-doped graphitic carbon nitride [J]. Journal of Colloid and Interface Science, 2019, 540: 97-106. doi: 10.1016/j.jcis.2019.01.023 [29] QIU P X, CHEN H, JIANG F, et al. Cobalt modified mesoporous graphitic carbon nitride with enhanced visible-light photocatalytic activity [J]. RSC Advances, 2014, 4(75): 39969-39977. doi: 10.1039/C4RA06451H [30] DONG G H, YANG L P, WANG F, et al. Removal of nitric oxide through visible light photocatalysis by g-C3N4 modified with perylene imides [J]. ACS Catalysis, 2016, 6(10): 6511-6519. doi: 10.1021/acscatal.6b01657 [31] QIU P X, XU C M, CHEN H, et al. One step synthesis of oxygen doped porous graphitic carbon nitride with remarkable improvement of photo-oxidation activity: Role of oxygen on visible light photocatalytic activity [J]. Applied Catalysis B-environmental, 2017, 206: 319-327. doi: 10.1016/j.apcatb.2017.01.058 [32] CAO S H, ZHOU N, GAO F H, et al. All-solid-state Z-scheme 3, 4-dihydroxybenzaldehyde-functionalized Ga2O3/graphitic carbon nitride photocatalyst with aromatic rings as electron mediators for visible-light photocatalytic nitrogen fixation [J]. Applied Catalysis B-environmental, 2017, 218: 600-610. doi: 10.1016/j.apcatb.2017.07.013 [33] OH W, VEKSHA A, CHEN X, et al. Catalytically active nitrogen-doped porous carbon derived from biowastes for organics removal via peroxymonosulfate activation [J]. Chemical Engineering Journal, 2019, 374: 947-957. doi: 10.1016/j.cej.2019.06.001 [34] MA W J, WANG N, FAN Y N, et al. Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate [J]. Chemical Engineering Journal, 2018, 336: 721-731. doi: 10.1016/j.cej.2017.11.164 [35] LIU C, CHEN L W, DING D H, et al. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor [J]. Applied Catalysis B-environmental, 2019, 254: 312-320. doi: 10.1016/j.apcatb.2019.05.014 [36] LUO R, LIU C, LI J S, et al. Nanostructured CoP: An efficient catalyst for degradation of organic pollutants by activating peroxymonosulfate [J]. Journal of Hazardous Materials, 2017, 329: 92-101. doi: 10.1016/j.jhazmat.2017.01.032 [37] LUO R, LI M Q, WANG C H, et al. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition [J]. Water Research, 2019, 148: 416-424. doi: 10.1016/j.watres.2018.10.087 [38] HU L M, ZHANG G S, WANG Q, et al. Facile synthesis of novel Co3O4-Bi2O3 catalysts and their catalytic activity on bisphenol A by peroxymonosulfate activation [J]. Chemical Engineering Journal, 2017, 326: 1095-1104. doi: 10.1016/j.cej.2017.05.168 [39] WANG Y B, LIU M, ZHAO X, et al. Insights into heterogeneous catalysis of peroxymonosulfate activation by boron-doped ordered mesoporous carbon [J]. Carbon, 2018, 135: 238-247. doi: 10.1016/j.carbon.2018.01.106 [40] BOKARE A D, CHOI W Y. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes [J]. Journal of Hazardous Materials, 2014, 275: 121-135. doi: 10.1016/j.jhazmat.2014.04.054 [41] PAN X X, CHEN J, WU N N, et al. Degradation of aqueous 2, 4, 4'-Trihydroxybenzophenone by persulfate activated with nitrogen doped carbonaceous materials and the formation of dimer products [J]. Water Research, 2018, 143: 176-187. doi: 10.1016/j.watres.2018.06.038 [42] REN W J, GAO J K, LEI C, et al. Recyclable metal-organic framework/cellulose aerogels for activating peroxymonosulfate to degrade organic pollutants [J]. Chemical Engineering Journal, 2018, 349: 766-774. doi: 10.1016/j.cej.2018.05.143 [43] YIN R L, GUO W Q, WANG H Z, et al. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism [J]. Chemical Engineering Journal, 2019, 357: 589-599. doi: 10.1016/j.cej.2018.09.184 [44] HU W R, TONG W H, LI Y L, et al. Hydrothermal route-enabled synthesis of sludge-derived carbon with oxygen functional groups for bisphenol A degradation through activation of peroxymonosulfate[J]. Journal of Hazardous Materials, 388. [45] HOU J F, YANG S S, WAN H Q, et al. Highly effective catalytic peroxymonosulfate activation on N-doped mesoporous carbon for o -phenylphenol degradation [J]. Chemosphere, 2018, 197: 485-493. doi: 10.1016/j.chemosphere.2018.01.031 [46] GUAN Y H, MA J, REN Y M, et al. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals [J]. Water Research, 2013, 47(14): 5431-5438. doi: 10.1016/j.watres.2013.06.023 [47] REN W, NIE G, ZHOU P, et al. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis [J]. Environmental Science and Technology, 2020, 54(10): 6438-6447. doi: 10.1021/acs.est.0c01161 [48] WANG N, MA W J, REN Z Q, et al. Prussian blue analogues derived porous nitrogen-doped carbon microspheres as high-performance metal-free peroxymonosulfate activators for non-radical-dominated degradation of organic pollutants [J]. Journal of Materials Chemistry, 2018, 6(3): 884-895. doi: 10.1039/C7TA08472B [49] CHEN X, OH W D, HU Z T, et al. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes[J]. Applied Catalysis B: Environmental, 2018, 225: 243-257. -



 下载:
下载: