-
聚二甲基硅氧烷(PDMS)是一类人工合成化学品,由于具有表面张力低、热稳定性强、润滑性能好等特点,被广泛应用于工业过程和消费产品中,如润滑剂、消泡剂、电气绝缘剂及个人护理品等[1-6]。低分子量的环挥发性甲基硅氧烷(cyclic volatile methylsiloxanes,cVMSs;一般用Dn表示,n表示硅原子的数目),如八甲基环四硅氧烷(D4)、十甲基环五硅氧烷(D5)和十二甲基环六硅氧烷(D6)(表1)等,通常是合成PDMS的重要单体[7]。近年来,由于其广泛的生产和应用,在水、沉积物、空气、土壤等多种环境基质乃至生物体内都能检测到cVMSs的存在[8-12]。cVMS的理化性质较为特殊,典型cVMSs的物理化学性质见表2。较高的辛醇水分配系数(lgKOW)和饱和蒸气压使cVMSs具有疏水性,又由于有机碳分配系数(lgKOC)较高以及其亲脂性较强且难以生物降解,导致其在水生生物体内积累并产生潜在的毒性作用[13-15]。因此,cVMSs作为潜在生物累积性和环境危害性有机污染物而广受关注。2017年,欧洲化学品管理局将D4列为持久性、生物累积性和毒性物质(PBT),将D5和D6列为高持久性和生物累积性(vPvB)物质,并建议限制其在个人护理产品中的浓度。
近年来全球硅氧烷的产能增长主要来自亚洲国家,尤其是中国。2009年我国环形硅氧烷总产量达10万吨,2010年的总产量上升至80万吨,超越美国成为全球第一生产大国,约占世界总产量的30%[19]。据统计,2018年中国硅氧烷产能达每年131万吨,年消费量约104万吨,同比增长7.4%。根据美国环境保护署(USEPA)和经济合作与发展组织(OECD)的信息,D4、D5和D6已被列为高产量化学品(年产量超过1000万吨)[19-20]。
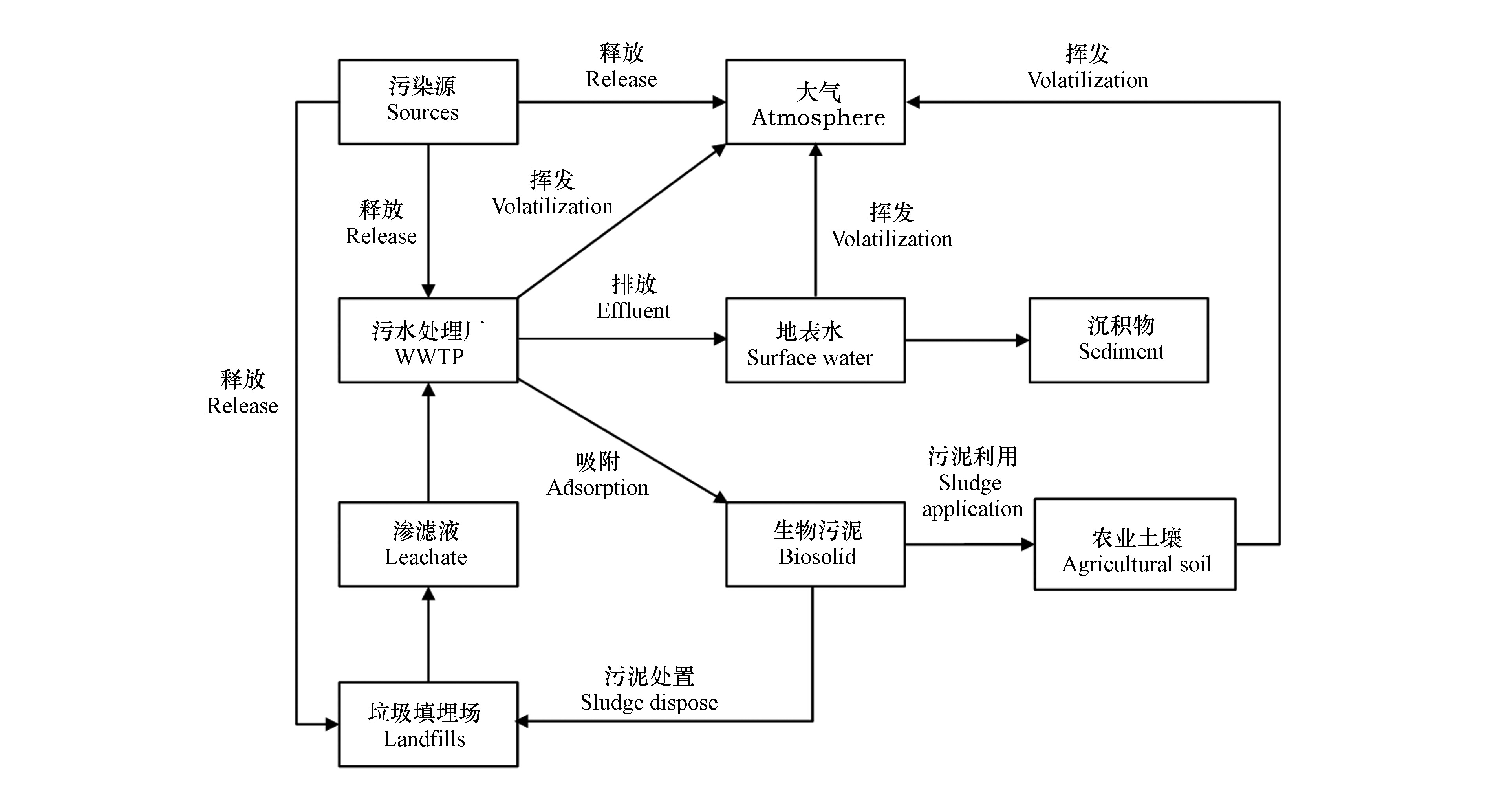
PDMS主要通过D4开链聚合生成,但由于聚合反应为平衡反应,这导致了低分子量D4以及其重排产物(D5和D6)等杂质的存在[21]。因此,PDMS产品在使用过程中D4-D6等cVMSs物质可排放进入各类环境介质。如图1所示,cVMSs通过挥发及吸附等诸多迁移途径可进入各种环境介质。由于其较高排放量和强挥发性,排放的cVMSs超过90%进入大气中,10%进入污水中[22-23]。挥发进入大气中的硅氧烷少部分可发生光解作用,大部分又经过干湿沉降迁入土壤等地球表层;排入污水厂的cVMSs,9.42%—48.2%挥发进入大气,48.2%—84.1%被污泥吸附,3.55%—6.48%排入地表水体中[24-26]。地表水体中的cVMSs又可通过吸附作用在沉积物中积累,最终达到动态平衡;污泥吸附的硅氧烷可通过在生物质改良迁入农业土壤。土壤中94.5%的cVMS挥发进入大气,0.8%随地表径流进入水体[27]。
由于其亲脂性较强,环境中的cVMSs易进入生物体的器官及组织中。近年来,已有相关文献陆续报道了cVMSs在生物体内的残留水平及归趋。本文综述了全球范围内3种典型cVMSs(D4、D5和D6)在生物及人体内的残留水平,并总结了3种cVMSs的生态毒性、生物效应、生物体内代谢行为等方面相关研究。
-
在加拿大工业污染区和污水处理厂下游采集到的鳄龟和鸬鹚的血浆中检出了cVMSs,D4和D6的平均浓度范围分别为0.051—0.122 ng·g−1 ww(wet weight,湿重)和0.125—0.967 ng·g−1 ww,在物种和采样点上没有明显的差异;D5的浓度较高,其范围为0.143—739 ng·g−1 ww[28]。Lu等[29]检测了从加拿大各地采集的欧洲椋鸟蛋样品,结果表明cVMS是欧洲椋鸟蛋中∑VMSs(Volatile methylsiloxanes,包括D3—D6、L4和L5)浓度的主要组成部分,在垃圾填埋场和城市工业场地的鸟蛋样品中D4、D5和D6的平均占比分别为1.1%、73%和22%。从垃圾填埋场收集的欧洲椋鸟蛋中D4、D5和D6的最大浓度分别为26.4、536、191 ng·g−1 ww,比城市工业区(5.6、248、29.4 ng·g−1 ww)和农村地区(3.35 ng·g−1 ww、<LOQ和6.81 ng·g−1 ww)高出1—2个数量级,说明垃圾填埋场是这些加拿大陆栖鸟类的VMSs的重要来源。此外,该研究还在14个海鸥蛋中检出了D4、D5、D6,其平均浓度分别为2.9、88、16 ng·g−1 ww。
-
KIERGAARD等报道了英国亨伯河口内,D4、D5和D6在沙蚕体内的最高含量分别为20、762、27 ng·g−1 ww,比目鱼肌肉样品中检测出D4,D5和D6的最高含量分别为10.4、299、4.7 ng·g−1 ww。对比发现,D5和D6在沙蚕和沉积物中的浓度之间存在明显的相关性[30]。在加拿大伊利湖浮游生物混合样品中发现D4和D6的浓度低于检出限(2 ng·g−1 ww),D5的浓度为5.2 ng·g−1 ww;在白眼鱼和淡水石首鱼肉混合样品中D4、D5和D6的平均浓度分别为9—3、15—36、7—14 ng·g−1 ww[31]。在挪威北部的Storvannet湖底收集的底栖动物摇蚊和豌豆蛤中检测到了cVMSs,其中摇蚊体内D4、D5和D6的浓度分别为(9.9±0.3)、(60±1.2)、(9.3±0.1) ng·g−1 ww,豌豆蛤体内D4、D5和D6的浓度分别为(4.7±0.4)、(107±4.5)、(11.8±0.2) ng·g−1 ww。然而需要指出的是由于这些底栖生物样品上附着的沉积物没有得到充分的清理,该论文报道的摇蚊体内的D4和豌豆蛤体内的D5浓度由于受到沉积物稀释[C底栖生物> C沉积物 (ng·g−1ww)]从而被低估;而摇蚊体内的D5、D6和豌豆蛤体内的D4、D6浓度由于受到沉积物的污染[C底栖生物≤C沉积物 (ng·g−1 ww)]从而被高估[32]。在McGoldrick等的另一项研究中,所有肉食性淡水鱼中检出了D4、D5和D6,其中湖鳟鱼体内D4—D6的浓度最高,分别为2.5—28 ng·g−1 ww、45—719 ng·g−1 ww和4.7—16 ng·g−1 ww[33]。Kierkegaard等发现在没有受到污水处理厂排水影响的湖泊中,鲈鱼体内D5的含量则低于检出限(0.12 ng·g−1 ww),而在污水处理厂纳污湖泊中鲈鱼体内检测到D5大多高于检出限[34]。
-
在中国渤海,底栖软体动物体内D6浓度较高,其浓度范围为<LOQ—90.4 ng·g−1 ww(平均值为(34.0±23.0) ng·g−1 ww),D4和D5的浓度范围分别为<LOQ—47.6 ng·g−1 ww((15.7 ± 12.3) ng·g−1 ww)和<LOQ—77.3 ng·g−1 ww((24.8 ± 15.8 )ng·g−1 ww),是大连湾无脊椎动物体内浓度(D4为(6.51 ± 2.90) ng·g−1 ww,D5为(8.92 ± 6.03) ng·g−1 ww,D6为(14.0 ± 8.48 )ng·g−1 ww)的2—3倍[35]。在一项关于挪威奥斯陆峡湾水生海洋食物网的研究中,贻贝中D4、D5和D6的浓度范围分别为2.66—3.77 ng·g−1 ww、27.8—252 ng·g−1 ww和1.35—8.69 ng·g−1 ww[36]。渤海底栖软体动物体内D5的浓度大约比挪威奥斯陆峡湾低1个数量级,而其D4的浓度要比挪威奥斯陆峡湾高出10倍左右。
瑞典鱼类肌肉样本中cVMSs(D4、D5和D6)的浓度水平均 <5 ng·g−1 ww,在北欧多个国家的海洋鱼类肝脏中检测到的D4—D6的浓度范围为 <5—100 ng·g−1 ww,其中挪威西海岸采集的鳕鱼肝脏样品中D5高达2.2×103 ng·g−1 ww[37-38]。在哺乳动物样品中,海豹和鲸鱼中D4、D5和D6的最高含量分别为12、24、7.9 ng·g−1 ww,但在海豚和鼠海豚样品中,cVMSs都低于LOQs[38]。Warner等对斯瓦尔巴群岛的生物样品进行了检测,在所有样本中都未检出D4,而在大西洋鳕鱼和杜父鱼中D5和D6的浓度范围分别为2—344 ng·g−1 lw(lipid weight,脂质重量)和2—16 ng·g−1 lw;海豹脂肪样品中检测到D6浓度分别为0.8 ng·g−1 lw和1.1 ng·g−1 lw,约为鱼体中浓度的十分之一,表明cVMSs在海豹体内的消除能力可能高于鱼类[39]。波罗的海鱼体内也检测到了cVMSs,鲱鱼肌肉中D4、D5、D6的浓度分别约为10、200、40 ng·g−1 lw,而灰海豹脂肪中检测到的cVMSs的浓度低于鲱鱼中的浓度, D5和D6浓度范围分别为9—24 ng·g−1 ww和4.4—9.5 ng·g−1 ww,D4的浓度范围为<3 ng·g−1 ww[40];其中灰海豹脂肪中D5和D6的浓度与Kaj等报道的丹麦海岸海豹脂肪中的浓度(17—24 ng·g−1 ww和<5—7.9 ng·g−1 ww)水平一致[38]。几种典型cVMSs在不同生物体中的浓度如表3所示。
-
近年来有文献报道在人体样本中检出了甲基硅氧烷。Xu等[41]在中国某生产硅氧烷工厂工人的血液样本中检出了较高浓度的D4、D5和D6(检出率为100%),平均浓度分别为206、215、88.7 ng·g−1,在该工厂下风向400—1000 m处的社区人群血液样品中D4、D5和D6(检出率为36%—100%),平均浓度分别为13.5、57.8、4.56 ng·g−1。在Xu等[42]的另外一项研究中,建筑、汽车和纺织行业的职业工人血浆中,D4、D5和D6的浓度分别为2.50—25.5、 3.44—38.0、4.40—10.1 ng·mL−1;工厂工人腹部脂肪中,D4、D5和D6的浓度范围分别为86.8—306 ng·g−1、35.8—170 ng·g−1和58.5—124 ng·g−1。
而普通对照人群血液样品中仅有少数检测到D4和D5,其浓度范围分别为1.2—3.6 ng·g−1和2.0—5.0 ng·g−1。结果分析发现,人体血液中cVMSs的浓度与人体暴露量(日摄入量)之间存在显著正相关(R2=0.90,P<0.05),但人体血液中cVMSs的浓度与他们的暴露时间没有显著的相关性。cVMSs在人体血液中的半衰期为2.34—3.15 d,且半衰期随着Si—O键数量的增加而增加[41]。另一项研究中在519份普通人群血浆样品中检测到D4、D5、D6的浓度范围分别为1.10—5.95 ng·mL−1(检出率为3.7%)、1.98—6.22 ng·mL−1(检出率为3.7%)、1.85—7.50 ng·mL−1(检出率为1.7%)。普通人群脂肪样品中D4、D5和D6的浓度分别为4.00—141、3.10—77.5、4.08—77.2 ng·g−1; D4、D5和D6在脂肪和血浆之间的分配比为5.3—241 mL·g−1[42],说明由于lg Kow较高,cVMSs趋向于从血浆转移到脂肪/组织中[42]。
Hanssen等检测了挪威孕期和绝经期女性血液样本中cVMSs的含量,血液中D4的最高浓度为 12.7 ng·mL−1,而D5和D6在绝大多数人的血液中未检出,该研究认为人体血液中甲基硅氧烷的浓度水平与个人护理品使用量并无明显关系[43]。Flassbeck等在植入过硅胶乳房假体的女性血浆样品中发现D4、D5和D6的最高浓度分别为50、28、17 ng·mL−1,硅胶乳房假体从女性体内移出后,在此类女性血液样品中仍能检测到cVMSs[44]。在Flassbeck另一项研究中发现,植入过硅胶乳房假体的女性体内的脂肪组织、纤维蛋白层和肌肉组织中也检测到了cVMSs,其检出的浓度范围为10—1.4×103 ng·g−1[45]。Rosendahl等[46]发现植入了硅胶乳房且假体破裂的女性血液样品中D4和D6浓度可以达到 0.57 ng·g−1和0.16 ng·g−1,而假体完好的女性血液样品中D4和D6的浓度低于定量限(0.18 ng·g−1和0.10 ng·g−1),血液中D4和D6的浓度可以成为判断假体是否破裂的标准,具有重要的医学价值。
-
近年来,鉴于其在生物体及人体内较高浓度水平的检出,cVMSs的毒理效应也逐渐引起了学者们的重视。相关研究结果表明,cVMSs对各种陆生哺乳动物具有直接或间接的毒性作用,例如生育能力受损[47-48],肝脏损害[49-50]和雌激素效应 [51-53]。目前,欧盟已将D4归为第3类生殖毒性物质,同时丹麦环保署将D4对生育能力的损害视为其关键影响[54]。D4能够在小鼠体内结合α受体从而对雌激素分泌水平产生影响,属于内分泌干扰物,可导致雌性生物的排卵率显著减少[47]。在D5的吸入[47,55]、口服[51]和两代生殖毒性试验[55]中,没有观察到D5具有雌激素效应和生殖毒性,其生殖毒性的最大无影响浓度(NOEC)为2400 μg·L−1。但有研究发现,在暴露于D5(浓度为3360 μg·L−1)的雄/雌性大鼠肺中观察到局部巨噬细胞积累和肺间质炎症加重[56]。Siddiqui等[55]做了关于小鼠的D5生殖毒性实验,发现小鼠肺血管矿化率明显的提升。Dekant等[57]做了关于小鼠D5暴露的生殖毒性实验,发现雌性小鼠子宫显著增大,表明D5对雌性小鼠具有潜在的致癌性。Burns-Naas 等[56]和McKim等[58]在鼻吸入实验中观察到,暴露过D5(46—224 μg·mL−1)后雌/雄性大鼠肝脏重量显着增加。Zhang等[59]在喂养研究中也观察到了D4或D5的肝脏效应,大鼠口服后肝体重比增加。
对于部分水生生物而言,较低浓度的D4就会对其产生危害。例如,小型虹鳟鱼急性毒性实验中,D4的最低不良效应浓度(LOAEC)和半数致死浓度(LC50)分别为6.9 μg·L−1和10 μg·L−1,在急性/慢性实验中D4对其的最大无影响浓度(NOEC)均为4.4 μg·L−1[60-61]。一项对水蚤的慢性毒性研究发现,当D4的浓度达到10 μg·L−1时,开始出现死亡现象,NOEC和LOAEC分别为7.9 μg·L−1和15 μg·L−1;在对糠虾和红鲈鱼的急性毒性实验中,D4的NOEC分别为9.1 μg·L−1和6.3 μg·L−1[60]。上述结果表明,D4对敏感的水生生物表现出比较明显的毒性,且已经达到了欧盟的毒性标准,即海洋或淡水生物的无效应浓度值<10 μg·L−1[62]。在黑头呆鱼的急性和慢性暴露毒性研究中,D5对鱼卵孵化、胚胎存活以及幼苗生长并没有产生明显影响[63],D5对鱼类可能没有明显的毒性。另一项针对黑头呆鱼的慢性实验研究发现,当D6浓度达到4.6 μg·L−1时(D6溶解度5 μg·L−1),没有观察到明显的不良影响[64],表明D6对鱼类的影响不显著。
由于D4,D5和D6具有较高的Koc(lgKoc>4,见表2),易吸附于有机质中,部分研究关注它们在沉积物及土壤中的生态效应。在一项沉积物毒性研究中发现,将蚊虫暴露于D4浓度为6.5—355 µg·g−1 dw的沉积物中,在最高浓度下其数量显著减少,蚊虫发育的NOEC和LOAEC分别为131和355 µg·g−1[65]。将蚊虫幼虫暴露于14C-D5标记的沉积物中的LC50为450 µg·g−1,NOEC和LOAEC分别为69和180 µg·g−1[66]。在D5对沉积物中底栖无脊椎动物端足虫的慢性毒性实验中,有机碳含量分别为0.5%和11%的沉积物中D5的LC50分别为191 µg·g−1 dw和857 µg·g−1 dw。上述结果表明,由于生物可利用性的提高,D5对端足虫的毒性随着沉积物中有机碳含量的下降而增加。因此,D5有可能对有机碳含量较低沉积物中生物产生负面影响[67]。在另一项对D5的慢性毒性研究中,当暴露浓度为160 µg·g−1时,蚊虫的发育能力显著下降,其NOEC和LOAEC分别为70 µg·g−1和160 µg·g−1[68-69]。目前还没有关于D6对底栖生物的毒性信息,但预测D6由于在有机质中吸附更强从而在沉积物中的生物利用度比D5更低,因此潜在毒性可能较低[70]。Velicogna 等测试了土壤中D5的生态毒性,发现 D5对植物大麦和陆生动物跳虫具有显著影响,其半数抑制浓度(IC50)分别为767 µg·g−1和209 µg·g−1[71],目前并没有D4和D6对土壤生物影响的相关研究。
-
对于水生生物,生物浓缩是指生物体通过与水的接触和呼吸作用(不包括消化吸收即食物暴露的途径)吸收水体中化学污染物质进入到体内,导致化学物质在生物体中的浓度大于水体中的浓度的过程。生物浓缩系数(bioconcentration factor,BCF)可以通过生物体内污染物浓度与水体内污染物浓度的比值获得,是评价污染物在环境介质与生物体之间分配的重要参数。根据欧盟标准,当BCF > 2000时,说明化合物具有潜在的生物富集性,当BCF > 5000时,则存在明显的生物富集性[62]。在水生生物暴露实验中,以虹鳟鱼估算D5的BCF值为3362 L·kg−1[72];对于呆头黑鱼,D4的BCF值为1875—10000 L·kg−1[72]或12400 L·kg−1[73],D5的BCF值为4450 L·kg−1[63]或13300 L·kg−1[74],与D4和D5相比,D6的BCF值较低,其BCF为1660 L·kg−1[75]或 >1000 L·kg−1[72]。总体来说,大部分文献中报道的D4和D5的BCF值 > 2000,说明两者具有潜在的生物富集性。
生物/沉积物富集系数(BSAF = COrganism tissue(mg·kg−1)/CSediment(mg·kg−1),biota-sediment accumulation factor,kg organic carbon·kg−1 lipid)是指沉积物中的生物体内某化学成分含量与沉积物中该成分浓度的比值,用以表征污染物质的积累程度。有文献指出,若BSAF值大于1.7,则表明生物对此化学物质有强烈的吸收和富集作用;若小于1.7,则表明生物对此化合物有较强的清除能力,不易在体内富集和积累[76]。在蚊虫亚慢性毒性研究中, D4对低、中和高有机碳含量沉积物中蚊虫的BSAF值分别为2.2、1.3和0.7[62]。Warner等[39]调查了欧洲北极斯瓦尔巴特群岛大西洋中鳕鱼和杜父鱼的生物富集情况,经测定BSAF值分别为2.1和1.5。在伊利湖和瑞斯特湖的沉积物中D5对淡水端足目动物的BSAF值分别为0.05和0.87,BSAF值均<1,说明无明显的生物积累[67]。然而,也有研究表明在挪威淡水湖中许多物种(尤其是端足类、蠕虫和摇蚊等底栖无脊椎动物)D4、D5和D6的BSAF值均 > 1,其中与沉积物密切接触的生物中BSAF值最大[77]。Hong等研究了中国渤海中cVMSs的生物富集情况,发现D4、D5和D6在大泷六线鱼中的BSAF值分别为0.716±0.456、0.103±0.0771和1.06±0.528[78]。
-
多介质生物累积系数mmBAFs(multimedia bioaccumulation factor)是指某种化学物质在生物生长后期体内与生长前期体内浓度的比值。KIERGAARD等[30]使用mmBAFs为评价标准,以沙蚕和比目鱼为生物研究对象量化评估了cVMSs的生物累积性。该研究以多氯联苯PCB-180(PCB-180是一种具有很强生物累积性的持久性有机污染物)为评价基准,结果发现沙蚕和比目鱼对D4的平均mmBAF值分别为PCB-180的6倍和14倍,对D5的mmBAF值均为PCB-180的2倍,而D6的mmBAF值比PCB-180低5—10倍。这表明D4和D5在水体多介质环境中具有相对较强的生物蓄积性,这是因为这两种化合物更倾向于分配到脂质中而非有机碳中(Kow/Koc > 100),而PCB-180在两种基质中的分配情况差不多(Kow/Koc≈1)[30]。实验室和现场研究都表明,通过沉积物暴露D4和D5或具有潜在的生物蓄积能力。
-
生物放大因子(biomagnification factors,BMF)即某一捕食者体内的浓度和一个特定的被捕食者体内浓度的比值,常用来定量评估生物放大效应的程度,通常BMF>1即具有生物放大作用。Woodburn等以虹鳟鱼为研究对象,得到D4和D5的动态BMFK分别是1.7和1.3。BMFK是指动态条件下吸收速率常数k1和清除速率常数k2的比值。BMFK均大于1,说明D4和D5可能存在生物放大效应[64]。Powell等[77]检测海洋食物网中,鲱鱼和虾中D4、D5、D6的BMF值分别为1.0和1.2、0.2和0.8、0.9和1.75,说明D4和D6具有潜在的生物放大性。
为更准确的描述污染物的生物放大效应,引入营养级放大因子(TMF,trophic magnification factor,通过计算生物体内化学物质浓度线性回归的斜率和判断此生物在食物网中的营养水平)来表示污染物随营养级的升高而增大的倍数。通常TMF > 1时,表明化合物具有生物放大效应,TMF < 1时,则表明存在营养级稀释作用。Powell等[77,36]测定了海洋食物网中不同营养级的cVMSs浓度,发现随着营养级升高不同物种体内cVMSs浓度随之降低。奥斯陆峡湾不同食物链内D4、D5和D6的TMF均<1.0(范围0.3至0.9),表明cVMSs存在营养级稀释作用。Borgåk等[79]研究挪威Mjøsa湖食物网中D4、D5和D6的生物放大性,发现D5和D6的TMF值分别为2.9和2.3,而D4在大多数样品中低于最低检出限,生物放大效应明显低于D5和D6。针对大连湾水生生物的一项研究也证明 D5存在显著的生物放大效应(TMF=1.77),并且其在不同物种中的浓度与营养级之间呈正相关关系[80]。而在日本东京湾海洋食物网中发现,D4、D5 和 D6存在营养级稀释效应[81]。以上研究结果存在矛盾,可能原因是:(1)样品来自不同的生态系统、不同的物种和不同的食物网,每种物种对目标化学物质敏感度不同。(2)一部分研究使用肌肉、肝脏和肺等部分组织样本来估计TMFs,另一部分研究则使用整个样本混合进行分析。因此,为了准确评估cVMSs的生物放大效应,有必要围绕典型区域进行长期的且技术方法相对统一的监测评价。
有研究者提出,cVMSs在低营养级生物体内有潜在的生物积累,但在高营养级生物体内没有显著的生物积累[82]。Cui等[83]研究发现渤海海浮游动物-无脊椎动物-鱼类-海鸟食物网中,D4、D5、D6的营养放大显著,其TMF值分别为1.7、3.5、1.8,但以浮游动物-无脊椎动物-鱼类为基础的食物网中,D5具有显著的营养放大作用(R2=0.16, P < 0.0001, TMF=3.0),但D4和D6的营养放大作用不显著(D4:R2=0.010, P=0.23, D6:R2=0.010, P = 0.23)。
根据鱼类和无脊椎动物的BMF和BSAF的研究,由于生物利用度降低,对于底栖生物来说,cVMSs的潜在生物累积性较弱。在许多研究中,D4的BASF值最大,D5的次之,D6的最小,说明沉积物中D4、D5和D6的生物可利用性大小顺序如下:D4 > D5 > D6。随着分子量的增大,lgKow增加,其与沉淀物有机质亲和性越强,越难被生物体吸收;同时分子量较大的同系物,往往分子体积更大,其生物膜透过性越差[70]。对于同一个物种而言,化学品BSAF值随着lgKow的增加而降低,意味着沉积物中D4、D5、D6的生物利用度随着lgKow的增加而降低,这可能与分子量较大同系物的消化道吸收效率降低或沉积物有机质亲和性增强有关。
-
Hutter等[84]建立生理药代动力学(PBPK)模型来预测D4通过静脉注射、吸入或植入等途径进入鼠和人体后的代谢过程。结果表明,在低剂量和高剂量暴露的所有途径下,脂肪是D4主要的储存库,其半衰期明显长于血浆。在大鼠中,D4在脂肪中的累积量最高,其次分别是肺、脑、血液、肝脏和肾脏。人体吸入D4后,模型预测其在血浆半衰期分别为1.7 h和7.4 h,脂肪半衰期为11.1 d。同样,该模型预测D4从人体乳房植入物解析后,血浆半衰期为7.8 h,脂肪半衰期为18.2 d。频繁吸入D4会使这种化合物在脂肪、肝脏和肾脏中大量积累,使其消除过程饱和。这种积累很可能发生在女性身上,因为从生理上讲,女性有大量的脂肪组织,同时新陈代谢比男性慢。Reddy等[85-86]运用PBPK模型对人体和老鼠吸入D4和D5进行药代动力学分析,由于具有较低的血液-空气分配系数和较高的脂肪-血液分配系数,两者优先在体内脂质中储存,而在其他组织器官中则迅速消除。与D4相比,组织器官对亲脂性更强的化合物D5的吸收较慢,这可能是由于D5难以穿透细胞脂膜进入多样化的组织器官中。Reddy等[87]在先前建立的生理药代动力学模型基础上,使用皮肤吸收模型来解释人体通过腋下皮肤吸收D4和D5的数据。模型计算表明,经皮肤暴露进入人体的D4和D5超过83%在24 h内通过呼吸去除。Andersen等[88]选取一种特别针对强亲脂性挥发性有机物的PBPK模型考察D4、D5的生物累积性,该模型将亲脂污染物的生物累积定义为其在频繁暴露条件下血液(或中央室)中浓度具有持续升高的趋势,认为导致血液和全身组织中挥发性有机物积累的主要原因不是其强亲脂性,而是全身清除速率慢;利用此模型对D4、D5的吸入进行分析发现其在血液中不具有生物累积性。而Hutter[84]和Tobin[89]等,研究发现D4、D5在脂肪组织中的消除速度远远低于血浆和其他组织,使D4、D5会储存在脂肪组织中,具有一定的生物累积性。
有文献指出,D4和D5主要通过肝脏氧化代谢和呼气从人体血液中去除[85-86,90]。由于D4的血气分配系数高,与D5相比,血液中D4的去除率较慢,但肝脏代谢速度较快[87],这与D4—D6半衰期趋势不完全一致。Andersen等[90]研究D4在大鼠体内的新陈代谢,发现D4在代谢过程中产生至少7种不同的中间产物,最初D4先由—CH3氧化为中间产物—CH2OH,随后通过水解和开环,进一步降解生成线性低聚体并最终形成硅醇类单体——一聚体。在另一项有关大鼠尿液中D5代谢产物的研究发现,常见的代谢产物有二甲基硅烷二醇[Me2Si-](OH)2)、存在多个羟基以及含有少量的D4D'CH2OH[91],表明其代谢途径与D4相似。
-
本文查阅文献时间点2020年4月份,关键词主要是硅氧烷、生物累积、生物代谢等,查阅了知网、万方数据库、Web of Science、Science Direct等。由于广泛应用于工业生产和居民生活中,cVMSs在全球环境中分布十分普遍。目前,虽然科研人员对cVMSs的生物效应有了初步的认识,但由于传统的营养级放大评价方法存在缺陷,对其生物放大效应的评估研究仍非常有限且相关结果存在较大争议。因此,开发科学合理的方法计算cVMS的营养级放大因子(TMF),结合对典型污染地区环境介质和食物链/网不同营养级生物长期深入的cVMSs残留监测,科学准确评估其生物放大具有重要意义。另外,虽然cVMSs在生物体内的分布和生物累积行为已有研究,但是对其内在驱动因素,如体内迁移、转化/代谢机制等研究较少,建议结合cVMS母体和代谢产物分析深入开展相关机制研究,为科学系统评估硅氧烷的生态效应提供理论技术基础和数据支撑。
生物体内环形挥发性甲基硅氧烷的分布、行为及效应研究进展
Research progress on the distribution, behavior and effects of cyclic volatile methylsiloxanes in organisms
-
摘要: 近年来,环形挥发性甲基硅氧烷(cVMSs)因其优异的物化特性而被广泛应用在工业生产和日常生活的各个方面,特别是经常被添加到个人护理品(PCPs)等各类消费品中。目前,人们对cVMSs在环境介质中的污染水平及其迁移转化行为有了一定的了解。另外,cVMSs兼有疏水性和挥发性,且难以进行生物降解,因此该类物质进入环境后潜在的生态风险,特别是其生物富集、生物累积、生物放大和毒性效应等已成为关注的热点。本文综述了cVMSs在全球生物体内的残留水平,并在此基础上总结了cVMSs生物效应等相关研究成果。总体上,众多研究表明cVMSs在某些水生生物体内呈现中等的生物富集性和累积性。然而,与其相对明确的生物富集/累积性相比,目前文献针对cVMSs生物放大效应的研究结果存在明显差异。建议后续的研究能够深入探索其生物效应,尤其是结合其在生物体内的降解过程以及中间产物的毒性数据,进一步评估其生态环境效应和人类健康风险。Abstract: Due to their excellent physicochemical properties, cyclic volatile methylsiloxanes (cVMSs) have been widely used in both industrial production and daily life, especially in various consumer products, such as personal care products (PCPs). At present, there have been some researches on distribution, migration and transformation behaviors of cVMSs in environmental matrices. In addition, cVMSs have both hydrophobicity and volatility, and were difficult to be biodegraded. Therefore, the potential ecological risks of cVMSs, especially their bioconcentration, bioaccumulation, biomagnificatioon and toxicity hadbecome a global concern. In this study, the residual levels of cVMS in organisms around the world were reviewed, and then research results on their ecological effects were summarized. In general, cVMSs underwent moderate bioconcentration and bioaccumulation in some aquatic organisms. However, compared with the relatively confirmed bioconcentration / bioaccumulatiion, biomagnification of cVMSs was still a controversial issue. The following research should further explore their biological effects. Especially, combing both their degradation process in organisms and toxic data of intermediate products, the ecological effects and human health risks of these compounds should be further evaluated.
-
Key words:
- cyclic volatile methylsiloxanes /
- toxicity effect /
- bioconcentration /
- bioaccumulation /
- biomagnification /
- metabolism
-

-
表 1 cVMS的分子式及结构信息
Table 1. Molecular formula and structure information of cVMS
化合物名称
Compound name缩写
AbbreviationCAS号
CAS Number分子式
Molecular formula分子量
Molecular mass化学结构
Chemical construction八甲基环四硅氧烷 D4 555-67-2 C8H24O4Si4 296.62 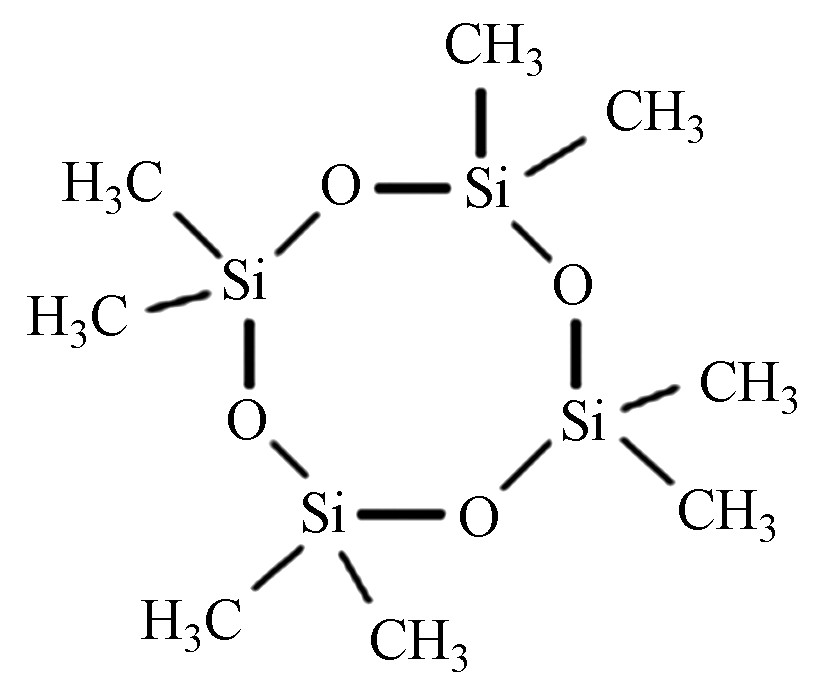
十甲基环五硅氧烷 D5 541-02-6 C10H30O5Si5 370.77 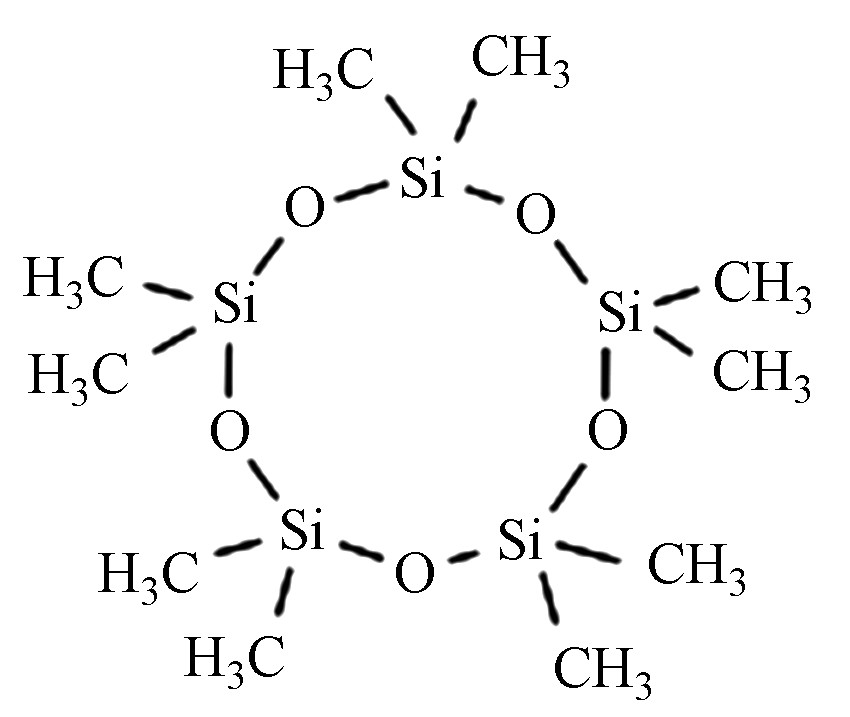
十二甲基环六硅氧烷 D6 540-97-6 C12H36O6Si6 444.92 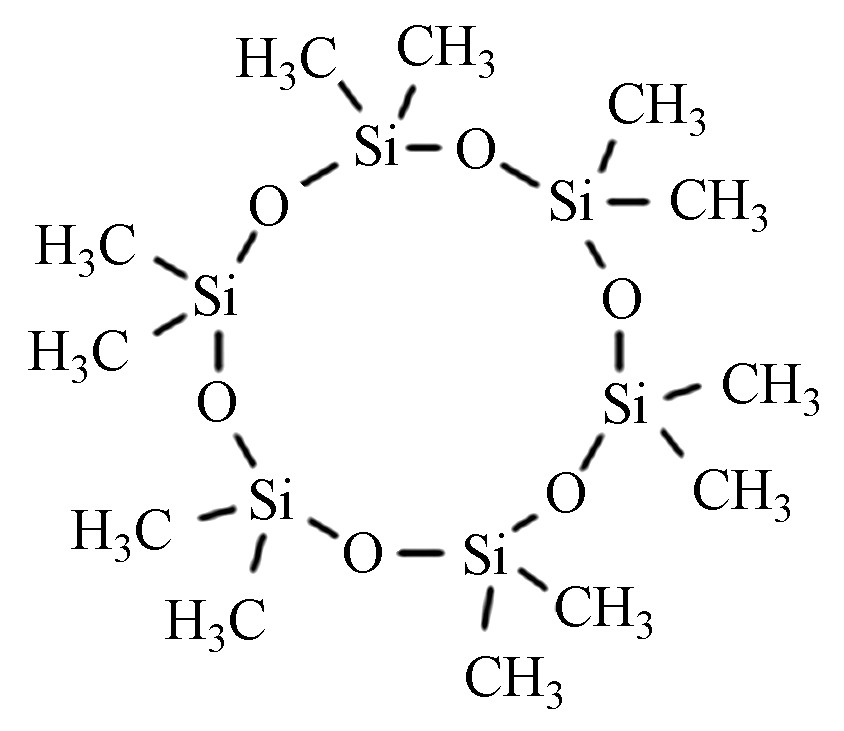
化合物
Compound饱和蒸汽压/Pa
Saturated vapor
pressure (25 ℃)溶解度/(mg·L−1)
Solubility亨利常数/ (Pa·m3 ·mol−1)
Henry constantlgKow lgKoc lgKoa lgKaw D4 140 0.056 1.21×106 6.49 4.22 4.34 2.69 D5 33.2 0.017 3.34×106 8.03 6.17 5.06 3.13 D6 4.60 0.005 4.94×106 9.06 6.10 5.76 3.30 表 3 生物体中典型cVMSs的浓度
Table 3. The concentrations of cVMS in different organisms
采样地区
Sampling area生物样品
Biological samplescVMSs/(ng·g−1ww) 参考文献
ReferenceD4 D5 D6 加拿大 鳄龟 0.077—0.122 0.143—3.59 0.125—0.458 [28] 鸬鹚 0.051—0.085 1.12—7.39 0.327—0.967 加拿大 欧洲椋鸟蛋 26.4 536 191 [29] 海鸥蛋 2.9 88 16 英国亨伯河口 沙蚕 <1.4—20 51—762 2.5—27 [30] 比目鱼肌肉 <0.8—10.4 12—299 0.12—4.7 加拿大伊利湖 浮游生物 <2 5.2 <2 [31] 钻穴浮游生物 7.0 11 5.7 白眼鱼和淡水石首鱼 9—13 15—36 7—14 加拿大 湖鳟鱼 2.5—28 45—719 4.7—16 [32] 中国渤海 软体动物 <LOQ—47.6 <LOQ—77.3 <LOQ—90.4 [35] 奥斯陆峡湾 贻贝 2.66—3.77 27.8—252 1.35—8.69 [36] 北欧地区① 海鱼 <5—70 <5—2200 <5—74 [38] 淡水鱼 <5—8.9 <5—84 <5 海洋哺乳动物 <5—12 <5—24 <5—7.9 北极地区 大西洋鳕鱼 <2.2—<10.8③ 12.7—358③ 5.3—52.8③ [39] 杜父鱼 <2.2—<10.8③ <1.5—2150③ <0.7—30.6③ 浮游动物② <8.7 <4.7 <4.8 海豹 — <1.5—1.9③ <0.7—1.1③ 波罗海域及
瑞典湖泊鲱鱼 <0.64—37 15—718 <0.3—128 [40] 灰海豹 <3 9—24 4.4—9.5 注:①包括丹麦、法罗群岛、芬兰、冰岛、挪威、瑞典;②主要包括水蚤、磷虾与浮游端足类动物;③单位为ng·g−1 lw.
Notes: ① Including Denmark, Falklands, Finland, Iceland, Norway and Sweden;② It mainly includes Daphnia, krill and planktonic Amphipoda;③ The unit is ng·g−1 lw (lipid weight). -
[1] 兰永超, 李娇敏, 钱程, 等. 污水处理厂进出水中环状挥发性甲基硅氧烷(cVMS)浓度季节性变化 [J]. 环境化学, 2019, 38(5): 1171-1179. LAN Y C, LI J M, QIAN C, et al. Seasonal variation of cVMS concentrations in the influents and effluents of wastewater treatment plants [J]. Environmental Chemistry, 2019, 38(5): 1171-1179(in Chinese).
[2] 周川琪, 袁涛. 挥发性甲基硅氧烷的环境分布与行为归趋研究进展 [J]. 环境化学, 2014, 33(3): 386-396. doi: 10.7524/j.issn.0254-6108.2014.03.003 ZHOU C Q, YUAN T. The environmental occurrences and behaviors of volatile methylsiloxanes: A review [J]. Environmental Chemistry, 2014, 33(3): 386-396(in Chinese). doi: 10.7524/j.issn.0254-6108.2014.03.003
[3] LU Y, YUAN T, WANG W H, et al. Concentrations and assessment of exposure to siloxanes and synthetic musks in personal care products from China [J]. Environmental Pollution, 2011, 159(12): 3522-3528. doi: 10.1016/j.envpol.2011.08.015 [4] GENUALDI S, HARNER T, CHENG Y, et al. Global distribution of linear and cyclic volatile methyl siloxanes in air [J]. Environmental Science & Technology, 2011, 45(8): 3349-54. [5] KROGSETH I S, KIERKEGAARD A, MCLACHLAN M S, et al. Occurrence and seasonality of cyclic volatile methyl siloxanes in arctic air [J]. Environmental Science & Technology, 2013, 47(1): 502-509. [6] LU Y, YUAN T, YUN S H, et al. Occurrence of Cyclic and linear siloxanes in indoor dust from china, and implications for human exposures [J]. Environmental Science & Technology, 2010, 44(16): 6081. [7] WANG R, MOODY R P, KONIECKI D, et al. Low molecular weight cyclic volatile methylsiloxanes in cosmetic products sold in Canada: Implication for dermal exposure [J]. Environment International, 2009, 35(6): 900-904. doi: 10.1016/j.envint.2009.03.009 [8] COMPANIONI-Damas E Y, SANTOS F J, GALCERAN M T. Linear and cyclic methylsiloxanes in air by concurrent solvent recondensation-large volume injection-gas chromatography-mass spectrometry [J]. Talanta, 2014, 118: 245-252. doi: 10.1016/j.talanta.2013.10.020 [9] DEWIL R, APPELS L, BAEYENS J, et al. The analysis of volatile siloxanes in waste activated sludge [J]. Talanta, 2008, 74(1): 14-19. [10] KIERKEGAARD A, ADOLFSSON-ERICI M, MCLACHLAN M S. Determination of cyclic volatile methylsiloxanes in biota with a purge and trap method [J]. Analytical Chemistry, 2010, 82(22): 9573-9578. doi: 10.1021/ac102406a [11] LEE S, MOON H B, SONG G J, et al. A nationwide survey and emission estimates of cyclic and linear siloxanes through sludge from wastewater treatment plants in Korea [J]. Science of the Total Environment, 2014, 497-498(Nov.1): 106-112. [12] WANG D G, AGGARWAL M, TAIT T, et al. Fate of anthropogenic cyclic volatile methylsiloxanes in a wastewater treatment plant [J]. Water Research, 2015, 72(apr.1): 209-217. [13] 吴婧娴, 栾晓新, 李清波, 等. 市政污水中环形挥发性甲基硅氧烷浓度水平与去除效率 [J]. 环境化学, 2016, 35(9): 1833-1841. WU J X, LUAN X X, LI Q B et al. Occurrence and removal efficiency of cyclic volatile methylsiloxanes in municipal wastewater [J]. Environmental Chemistry, 2016, 35(9): 1833-1841(in Chinese).
[14] WHELAN M J, SANDERS D, EGMOND R V. Effect of Aldrich humic acid on water-atmosphere transfer of decamethylcyclopentasiloxane [J]. Chemosphere, 2008, 74(8): 1111-1116. [15] KNOERR S M, DURHAM J A, MCNETT D A. Development of collection, storage and analysis procedures for the quantification of cyclic volatile methylsiloxanes in wastewater treatment plant effluent and influent [J]. Chemosphere, 2017, 182: 114-121. doi: 10.1016/j.chemosphere.2017.04.136 [16] SURITA S C, TANSEL B. A multiphase analysis of partitioning and hazard index characteristics of siloxanes in biosolids [J]. Ecotoxicology and Environmental Safety, 2014, 102: 79-83. doi: 10.1016/j.ecoenv.2014.01.012 [17] XU S, KOZERSKI G, MACKAY D. Critical review and interpretation of environmental data for volatile methylsiloxanes: Partition properties [J]. Environmental Science & Technology, 2014, 48(20): 11748-11759. [18] HOWARD P H, MUIR D C G. Identifying new persistent and bioaccumulative organics among chemicals in commerce [J]. Environmental Science & Technology, 2013, 47(10): 5259-5266. [19] DUDZINA T, VON GOETZ N, BOGDAL C, et al. Concentrations of cyclic volatile methylsiloxanes in European cosmetics and personal care products: prerequisite for human and environmental exposure assessment [J]. Environment International, 2014, 62(4): 86-94. [20] 曲垚, 徐琳, 蔡亚岐, 等. 山东某污水处理厂中环型和线型甲基硅氧烷的行为归趋 [J]. 环境化学, 2019, 38(02): 422-432. QU Y, XU L, CAI Y Q, et al. Occurrence and fate of cyclic and linear methylsiloxanes in a wastewater treatment plant in Shandong Province, China [J]. Environmental Chemistry, 2019, 38(02): 422-432(in Chinese).
[21] KENT B, WOODBURN R M, SESTON, J K, et al. Benthic invertebrate exposure and chronic toxicity risk analysis for cyclic volatile methylsiloxanes: Comparison of hazard quotient and probabilistic risk assessment approaches. 2018, 192: 337-347. [22] XU L, SHI Y, CAI Y. Occurrence and fate of volatile siloxanes in a municipal Wastewater Treatment Plant of Beijing, China [J]. Water Research, 2013, 47(2): 715-724. doi: 10.1016/j.watres.2012.10.046 [23] SHEN M, ZHANG Y, TIAN Y, et al. Recent advances in research on cyclic volatile methylsiloxanes in sediment, soil and biosolid: A review [J]. Chemistry and Ecology, 2018, 34(7): 675-695. doi: 10.1080/02757540.2018.1475561 [24] BROOK D N, CROOKES M J, GRAY D, et al. Environmental risk assessment report: Octamethylcyclotetrasiloxane[R]. Environment Agency of England and Wales, Bristol, UK, 2009. [25] BROOK D N, CROOKES M J, GRAY D, et al. Environmental risk assessment report: Decamethylcyclopentasiloxane[R]. Environment Agency of England and Wales: Bristol, UK, 2009. [26] BROOK D N, CROOKES M J, GRAY D, et al. Environmental risk assessment report: Dodecamethylcyclohexasiloxane[R]. Environment Agency of England and Wales: Bristol, UK, 2009. [27] MACKAY D, COWAN-ELLSBERRY C E, POWELL D E, et al. Decamethylcyclopentasiloxane (D5) environmental sources, fate, transport, and routes of exposure [J]. Environmental Toxicology & Chemistry, 2016, 34.(12): 2689-2702. [28] WANG D G, SOLLA S R D, LEBEUF M, et al. Determination of linear and cyclic volatile methylsiloxanes in blood of turtles, cormorants, and seals from Canada [J]. Science of the Total Environment, 2017, 574: 1254-1260. doi: 10.1016/j.scitotenv.2016.07.133 [29] LU Z, MARTIN PAMELA A, BURGESS NEIL M, et al. Volatile methylsiloxanes and organophosphate esters in the eggs of European starlings (Sturnus vulgaris) and congeneric gull species from locations across Canada[J[. Environmental Science and Technology , 2017, 51(17): 9836-9845. [30] KIERGAARD A, VAN EGMOND R, MCLACHLAN M S. Cyclic volatile methylsiloxane bioaccumulation in flounder and ragworm in the Humber Estuary [J]. Environmental Science and Technology, 2011, 45(14): 5936-5942. doi: 10.1021/es200707r [31] MCGOLDRICK D J, CHAN C, DROUILLARD K G, et al. Concentrations and trophic magnification of cyclic siloxanes in aquatic biota from the Western Basin of Lake Erie, Canada [J]. Environmental Pollution, 2014, 186(mar.): 141-148. [32] KROGSETH I S, UNDEMAN E M, EVENSET A, et al. Elucidating the behavior of cyclic volatile methylsiloxanes in a subarctic freshwater food web: A modeled and measured approach [J]. Environmental Science & Technology, 2017, 51(21): 12489. [33] MCGOLDRICK D J, LETCHER R J, BARRESI E, et al. Organophosphate flame retardants and organosiloxanes in predatory freshwater fish from locations across Canada [J]. Environmental Pollution, 2014, 193(oct.): 254-261. [34] KIERGAARD A, BIGNERT A, MCLACHLAN M S. Bioaccumulation of decamethylcyclopentasiloxane in perch in Swedish lakes [J]. Chemosphere, 2013, 93(5): 789-793. doi: 10.1016/j.chemosphere.2012.10.050 [35] ZHI L Q, XU L, HE X D, et al. Distribution of methylsiloxanes in benthic mollusks from the Chinese Bohai Sea [J]. Journal of Environmental Sciences, 2019, 76(02): 202-210. [36] POWELL D E, MERETE SCHØYEN, SIGURD ØXNEVAD, et al. Bioaccumulation and trophic transfer of cyclic volatile methylsiloxanes (cVMS) in the aquatic marine food webs of the Oslofjord, Norway [J]. Science of the Total Environment, 2018, 622/623(may1): 127-139. [37] KAJ L, ANDERSSON J, COUSINS A P, et al. Results from the Swedish national screening programme 2004. Subreport 4: Siloxanes [M]. Stockholm: IVL Swedish Environmental Research Institute Ltd. , 2005: 11. [38] KAJ L, SCHLABACH M, ANDERSSON J, PALM COUSINS A, et al. Siloxanes in the nordic environment[M]. Nordic Council of Ministers: Copenhagen, 2005. [39] WARNER N A, EVENSET A, CHRISTENSEN G, et al. Volatile siloxanes in the European arctic: assessment of sources and spatial distribution [J]. Environmental Science and Technology, 2010, 44(19): 7705-7710. doi: 10.1021/es101617k [40] KIERGAARD A, BIGNERT A, MCLACHLAN M S. Cyclic volatile methylsiloxanes in fish from the Baltic Sea [J]. Chemosphere, 2013, 93(5): 774-778. doi: 10.1016/j.chemosphere.2012.10.048 [41] XU L, SHI Y, WANGT, et al. Methyl siloxanes in environmental matrices around a siloxane production facility, and their distribution and elimination in plasma of exposed population [J]. Environmental Science and Technology, 2012, 46(21): 11718-211726. doi: 10.1021/es3023368 [42] XU L, SHI Y, LIU N, et al. Methyl siloxanes in environmental matrices and human plasma/fat from both general industries and residential areas in China [J]. Science of the Total Environment, 2015, 505(1): 454-463. [43] HANSSEN L, WARNER N A, BRAATHEN T, et al. Plasma concentrations of cyclic volatile methylsiloxanes (cVMS) in pregnant and postmenopausal Norwegian women and self-reported use of personal care products (PCPs) [J]. Environment International, 2013, 51: 82-87. doi: 10.1016/j.envint.2012.10.008 [44] FLASSBECK D, PFLEIDERER B, et al. Determination of low molecular weight silicones in plasma and blood of women after exposure to silicone breast implants by GC/MS [J]. Analytical Chemistry, 2001, 73(3): 606-611. doi: 10.1021/ac000738z [45] FLASSBECK D, PFLEIDERER B, KLEMENS P, et al. Determination of siloxanes, silicon, and platinum in tissues of women with silicone gel-filled implants [J]. Analytical & Bioanalytical Chemistry, 2003, 375(3): 356-362. [46] ROSENDAHL P, HIPPLER J, SCHMITZ O J, et al. Cyclic volatile methylsiloxanes in human blood as markers for ruptured silicone gel-filled breast implants. [J]. Analytical & Bioanalytical Chemistry, 2016, 408(12): 3309-3317. [47] QUINN A L, DALU A, MEEKER L S, et al. Effects of octamethyl- cyclotetrasiloxane (D4) on the luteinizing hormone (LH) surge and levels of various reproductive hormones in female Sprague-Dawley rats [J]. Reproductive Toxicology, 2007, 23(4): 532-540. doi: 10.1016/j.reprotox.2007.02.005 [48] SIDDIQUI W H, STUMP D G, PLOTZKE K P, et al. A two-generation reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in rats exposed by whole-body vapor inhalation [J]. Reproductive Toxicology, 2007, 23(2): 202-215. doi: 10.1016/j.reprotox.2006.11.011 [49] MCKIM J M, WILGA P C, KOLESAR G B, et al. Evaluation of octamethyl-cyclotetrasiloxane (D4) as an inducer of rat hepatic microsomal cytochrome P450, UDP-glucuronosyltransferase, and epoxide hydrolase: A 28-day inhalation study [J]. Toxicological Sciences, 1998, 41(1): 29-41. doi: 10.1093/toxsci/41.1.29 [50] MCKIM J M, KOLESAR G B, JEAN P A, et al. Repeated inhalation exposure to octamethylcyclotetrasiloxane produces hepatomegaly, transient hepatic hyperplasia, and sustained hypertrophy in female fischer 344 rats in a manner similar to phenobarbital [J]. Toxicology & Applied Pharmacology, 2001, 172(2): 83-92. [51] HE B, RHODES-BROWER S, MILLER M R, et al. Octamethylcyclotetrasiloxane exhibits estrogenic activity in mice via ERα [J]. Toxicology and Applied Pharmacology, 2003, 192(3): 254-261. doi: 10.1016/S0041-008X(03)00282-5 [52] QUINN A L, REGAN J M, TOBIN J M, et al. In vitro and in vivo evaluation of the estrogenic, andorgenic, and progestagenic potential of two cyclic siloxanes [J]. Toxicological Sciences, 2007, 96(1): 145-153. [53] MCKIM J M, WILGA P C, BRESLIN W J, et al. Potential estrogenic and antiestrogenic activity of the cyclic siloxane octamethylcyclotetrasiloxane (D4) and the linear siloxane hexamethyldisiloxane (HMDS) in immature rats using the uterotrophic assay [J]. Toxicological Sciences, 2001, 63(1): 37-46. doi: 10.1093/toxsci/63.1.37 [54] LASSEN C, HANSEN C L, MIKKELSEN S H, et al. Environmental Project No. 1031 – Siloxanes - Consumption, toxicity and alternatives [J]. Danish Environmental Protection Agency, 2005, 54(1): 1-5. [55] SIDDIQUI W H, STUMP D G, REYNOLDS V L, et al. A two-generation reproductive toxicity study of decamethylcyclopentasiloxane (D5) in rats exposed by whole-body vapor inhalation [J]. Reproductive Toxicology, 2007, 23(2): 216-225. doi: 10.1016/j.reprotox.2006.11.006 [56] BURNS-NAAS L A, MAST R W, MEEKS R G, et al. Inhalation toxicology of decamethylcyclopentasiloxane (D5) following a 3-month nose-only exposure in fischer 344 rats [J]. Toxicological Sciences An Official Journal of the Society of Toxicology, 1998, 43(2): 230-240. doi: 10.1093/toxsci/43.2.230 [57] DEKANT W, KLAUNIG J E. Toxicology of decamethylcyclopentasiloxane (D5) [J]. Regulatory Toxicology & Pharmacology, 2016, 74: S67-S76. [58] MCKIM J M, CHOUDHURI S, WILGA P C, et al. Induction of hepatic xenobiotic metabolizing enzymes in female Fischer- 344 rats following repeated inhalation exposure to decamethylcyclopentasiloxane (D5) [J]. Toxicological Sciences, 1999, 50(1): 10-19. doi: 10.1093/toxsci/50.1.10 [59] ZHANG J, FALANY J L, XIE X, et al. Induction of rat hepatic drug metabolizing enzymes by dimethylcyclosiloxanes [J]. Chemico-Biological Interactions, 2000, 124(2): 133-147. doi: 10.1016/S0009-2797(99)00153-2 [60] SOUSA J V, MCNAMARA P C, PUTT A E, et al. Effects of octamethylcyclotetrasiloxane (OMCTS) on freshwater and marine organisms [J]. Environmental Toxicology and Chemistry, 1995, 14: 1639-1647. doi: 10.1002/etc.5620141003 [61] KENT D J, MCNAMARA P C, PUTT A E, et al. Octamethylcyclotetrasiloxane in aquatic sediment: Toxicity and risk assessment [J]. Ecotoxicology and Environmental Safety, 1994, 29(3): 372-389. doi: 10.1016/0147-6513(94)90010-8 [62] European Commission. Amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Annex XIII[S]. [2020.04]. European Commission, 2010. http://register.consilium.europa.eu/pdf/en/10/st14/st14860.en10.pdf. [63] PARROTT J L, ALAEE M, WANG D, et al. Fathead minnow (Pimephales promelas) embryo to adult exposure to decamethylcyclopentasiloxane (D5) [J]. Chemosphere, 2013, 93(5): 813-818. doi: 10.1016/j.chemosphere.2012.10.053 [64] WOODBURN K, DROTTAR K, DOMORADZKI J, et al. Determination of the dietary biomagnification of octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane with the rainbow trout (Oncorhynchus mykiss) [J]. Chemosphere, 2012, 93(5): 779-788. [65] KRUEGER H O, THOMAS S T, KENDALL T Z, et al. D4: A prolonged sediment toxicity test with Chironomus riparius using spiked sediment. wildlife international, LTD[R]. Silicones Environmental, Health and Safety Council (SEHSC), 2008. [66] Springborn Smithers Laboratories. Decamethylcyclopentasiloxane (D5)-the Full Life-Cycle Toxicity to Midge (Chironomous riparius) under static conditions[R]. Silicones Environmental, Health and Safety Council (SEHSC), 2003. [67] NORWOOD W P, ALAEE M, SVERKO E, et al. Decamethylcyclopentasiloxane (D5) spiked sediment: Bioaccumulation and toxicity to the benthic invertebrate Hyalella azteca [J]. Chemosphere, 2013, 93(5): 805-812. doi: 10.1016/j.chemosphere.2012.10.052 [68] KRUEGER H O, THOMAS S T, KENDALL T Z. D5: A prolonged sediment toxicity test with Lumbriculus variegatus using spiked sediment. Wildlife international, LTD[R]. Centre Européen des Silicones (CES), 2007. [69] KRUEGER H O, THOMAS S T, KENDALL T Z. D5: A prolonged sediment toxicity test with Chironomus riparius using spiked sediment. wildlife international, LTD[R]. Silicones Environmental, Health and Safety Council (SEHSC), 2008. [70] WANG D G, NORWOOD W, ALAEE M, et al. Review of recent advances in research on the toxicity, detection, occurrence and fate of cyclic volatile methyl siloxanes in the environment [J]. Chemosphere, 2013, 93(5): 711-725. doi: 10.1016/j.chemosphere.2012.10.041 [71] VELICOGNA J, RITCHIE E, PRINCZ J, et al. Ecotoxicity of siloxane D5 in soil [J]. Chemosphere, 2012, 87(1): 77-83. doi: 10.1016/j.chemosphere.2011.11.064 [72] ANNELIN R B, FRYE C L. The piscine bioconcentration characteristics of cyclic and linear oligomeric permethylsiloxanes [J]. Science of the Total Environment, 1989, 83(1/2): 1-11. [73] KENT D, FACKLER P, HARTLEY D, et al. Interpretation of data from nonstandard studies: The fate of octamethylcyclotetrasiloxane in a sediment/water microcosm system [J]. Environmental Toxicology and Water Quality, 1996, 11(2): 145-149. doi: 10.1002/(SICI)1098-2256(1996)11:2<145::AID-TOX10>3.0.CO;2-C [74] DROTTAR K R. 14C-Decamethylcyclopentasiloxane (14C-D5): Bioconcentration in the fathead minnow (Pimephales Promelas) under flow-through test conditions[R]. Technical Dow Corning Corporation: Silicones Environment, Health and Safety Council (SEHSC), 2005. [75] DROTTAR K R. 14C-Dodecamethylcyclohexasiloxane (14C-D6): Bioconcentration in the fathead minnow (Pimephales Promelas) under flow-through test conditions[R]. Technical Dow Corning Corporation: Silicones Environment, Health and Safety Council (SEHSC), 2005. [76] OZKOC H B, BAKAN G E, ARIMAN S. Distribution and bioaccumulation of organochlorine pesticides along the Black Sea coast [J]. Environmental Geochemistry & Health, 2007, 29(1): 59-68. [77] POWELL D E, WOODBURN K B, DROTAR K D , et al. Trophic dilution of cyclic volatile methylsiloxane (cVMS) materials in a temperate freshwater lake[R]. Internal Report Conducted for Centre Européen des Sililcones, 2009, Report Number 2009-I0000-60988. [78] HONG W J, JIA H L, LIU C, et al. Distribution, source, fate and bioaccumulation of methyl siloxanes in marine environment [J]. Environmental Pollution, 2014, 191: 175-181. doi: 10.1016/j.envpol.2014.04.033 [79] BORGA K, FJELD E, KIERKEGAARD A, MICHAEL S, et al. Consistency in trophic magnification factors of cyclic methyl siloxanes in pelagic freshwater food webs leading to brown trout [J]. Environmental Science and Technology, 2013, 47: 14392-14402. [80] JIA H, ZHANG Z, WANG C, et al. Trophic transfer of methyl siloxanes in the marine food web from coastal area of northern China [J]. Environmental Science and Technology, 2015, 49(5): 2833-2840. doi: 10.1021/es505445e [81] POWELL D E, SUGANUMA N, KOBAYASHI K, et al. Trophic dilution of cyclic volatile methylsiloxanes (cVMS) in the pelagic marine food web of Tokyo Bay, Japan [J]. Science of the Total Environment, 2017, 578: 366-382. doi: 10.1016/j.scitotenv.2016.10.189 [82] MEEKS R G, STUMP D G, SIDDIQUI W H, et al. An inhalation reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in female rats using multiple and single day exposure regimens [J]. Reproductive Toxicology, 2007, 23(2): 192-201. doi: 10.1016/j.reprotox.2006.12.005 [83] CUI S, FU Q, AN L, et al. Trophic transfer of cyclic methyl siloxanes in the marine food web in the Bohai Sea, China [J]. Ecotoxicology and Environmental Safety, 2019, 178(AUG.): 86-93. [84] HUTTER L J C. Bioavailability of octamethylcyclotetrasiloxane (D(4)) after exposure to silicones by inhalation and implantation [J]. Environmental Health Perspectives, 2001, 109(11): 1095-1101. [85] REDDY M B, ANDERSEN M E, MORROW P E, et al. Physiological modeling of inhalation kinetics of octamethylcyclotetrasiloxane in humans during rest and exercise [J]. Toxicological Sciences, 2003, 72(1): 3-18. doi: 10.1093/toxsci/kfg001 [86] REDDY M B, DOBREV I D, MCNETT D A, et al. Inhalation dosimetry modeling with decamethylcyclopentasiloxane in rats and humans [J]. Toxicological Sciences, 2008(2): 275-285. [87] REDDY M B, LOONEY R J, UTELL M J, et al. Modeling of human dermal absorption of octamethylcyclotetrasiloxane (D4) and decamethylcyclopentasiloxane (D5) [J]. Toxicological Sciences, 2007, 99(2): 422-431. doi: 10.1093/toxsci/kfm174 [88] ANDERSEN M E, REDDY M B, PLOTZKE K P. Are highly lipophilic volatile compounds expected to bioaccumulate with repeated exposures? [J]. Toxicology Letters, 2008, 179(2): 85-92. doi: 10.1016/j.toxlet.2008.04.007 [89] TOBIN J M, MCNETT D A, DURHAM J A, et al. Disposition of decamethylcyclopentasiloxane in Fischer 344 rats following single or repeated inhalation exposure to 14C-decamethylcyclopentasiloxane (14C-D5) [J]. Inhalation Toxicology, 2008, 20(5): 513-531. doi: 10.1080/08958370801935075 [90] ANDERSEN M E, RAMESH S, REITZ R H, et al. Physiological modeling reveals novel pharmacokinetic behavior for inhaled octamethylcyclotetrasiloxane in rats [J]. Toxicological Sciences, 2001(2): 214-231. [91] VARAPRATH S. Metabolites of hexamethyldisiloxane and decamethylcyclopentasiloxane in Fischer 344 rat urine-a comparison of a linear and a cyclic siloxane [J]. Drug Metabolism & Disposition, 2003, 31(2): 206-214. -



 下载:
下载:




