-
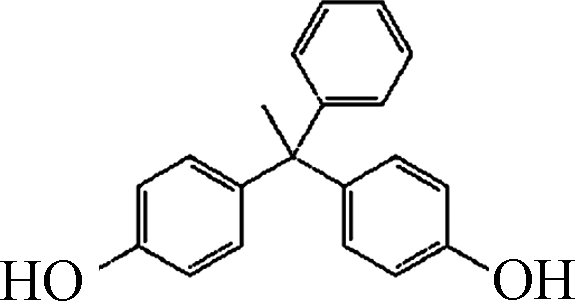
双酚类化合物(BPs)一般是具有两个酚基结构的一种典型的内分泌干扰物(EDCs),由于其具备良好的延展性,耐高温且性质稳定而被广泛的应用于工业生产中,常被用作塑料添加剂。此外,其在涂料、膜材料、电子产品制造、产品包装等领域也发挥着不可或缺的作用[1]。由于其在环境中的长期存在,这类污染物在一定的环境因素影响下,会从本体中析出,富集在土壤、灰尘、污泥和水体中,对人类健康和生态环境具有潜在危害,比如常用于食品包装的塑料添加剂双酚A(BPA)就会从塑料中释放出来[2]。余建龙建立了酒水饮料和谷物类食品中BPs的检测方法,发现BPA和双酚S(BPS)检出率较高,且BPs能显著诱导雌激素受体α下游基因的表达[3]。此外,研究者还发现BPs具有生物蓄积性、生殖毒性、遗传毒性,免疫毒性甚至心脏毒性等[4-6]。常见的BPs降解方法有物理吸附/过滤(颗粒活性炭和膜分离)、生物降解和高级氧化技术(AOPs,如臭氧氧化、芬顿氧化、氯化法、光催化降解和过硫酸盐氧化)[7-14],但传统的AOPs受到高昂的经济和能源成本限制,且在降解过程中易产生高毒副产物,例如氯化法产生高毒的氯化副产物,过硫酸盐氧化法产生硫酸根离子[10,14]。
四酰胺基六甲基苯基环铁(Fe(Ⅲ)-TAML))是一种功能类似于过氧化物酶和细胞色素P450酶的催化剂[15],是AOPs的一种简单而有效的绿色替代品,结构如下。
Fe(Ⅲ)-TAML分子结构:

较为常见的两种Fe(Ⅲ)-TAML为:M=Na, X=H, R=CH3;M=Li, X= Cl, R=F[16]。其在0.1—10.0 μmol·L−1的低剂量下就可发挥高效的催化作用[17]。这种催化剂已被广泛用于降解包括染料[18]、酚类[19]、雌激素[20]、有机磷农药[21]、药物[22]等在内的各种废水中的持久性芳香族有机污染物(ACs)。Fe(Ⅲ)-TAML需要先被过氧化物(如H2O2)氧化形成高价铁配合物(即Fe(Ⅳ)-TAML[16]和Fe(V)-TAML[15])。研究表明,这是一个非自由基控制过程[23]。据报道,经过Fe(Ⅲ)-TAML/H2O2处理后,废水的毒性显著降低,对ACs的氧化比AOP生成的自由基降解技术具有更高的活性,且残留的Fe(Ⅲ)-TAML对鱼类和微生物没有显著的毒性效应[24]。Liang等利用Fe(Ⅲ)-TAML/H2O2体系在几分钟内实现了对三氯生的高效去除,但对pH值有强烈的依赖性[25]。过一硫酸盐(PMS)相比于H2O2和过硫酸盐(PS)而言,在效果、稳定性和适用性方面的表现更加优越,这是由于硫酸根自由基(
${\rm{SO}}_4^{2-} \cdot $ )比羟基自由基(HO·)具有更长的半衰期,以及适用的pH值范围更为广泛,且${\rm{SO}}_4^{2-} \cdot $ 具有高氧化电势(2.5—3.1 V)[26]。Li等发现PMS可以在pH(6—11.5)的宽范围内被Fe(Ⅲ)-TAML有效激活,可以实现对氯苯酚的高效去除[27]。考虑体系反应速率受pH的影响较大,这是由于在产生反应中间体的过程中TAML是电子供体,过氧化氢是电子受体,[Fe(Ⅲ)-TAML (OH)]2−与[Fe(Ⅲ)-TAML (OH2)]−相比,前者电子云密度更大,是更好的电子供体,因此k2>>k1;同理分子态的H2O2电子云密度更低,是更好的电子受体,即k4<k2,如式(1)—(6)所示[19]。体系中各成分的质子化状态取决于溶液的pH值,同时决定了氧化态Fe(Ⅲ)-TAML的生成及污染物的降解。研究发现Fe(Ⅲ)-TAML的pKa约为10.5,H2O2的pKa约为11,考虑到PMS的pKa2为9.4,因此综合考虑pH=10时的降解效果最好[25,27-29]。
在这项工作中,探究了pH 10条件下Fe(Ⅲ)-TAML/PMS和Fe(Ⅲ)-TAML/H2O2体系对8种BPs的降解动力学,得到拟一级反应速率常数(Kobs)。建立Kobs与双酚类化合物的理论参数的定量活性结构相关性(QSAR)模型,得到的结果用于预测并进行相关性分析和适用性领域评估。建立一种可以快速预测双酚类物质Kobs的方法,并且获取速率限制机制的潜力,有望为其它BPs对系统的选择提供参考,以求为高价铁配合物通过非自由基途径有效降解有机污染物的机理提供有力支撑。
全文HTML
-
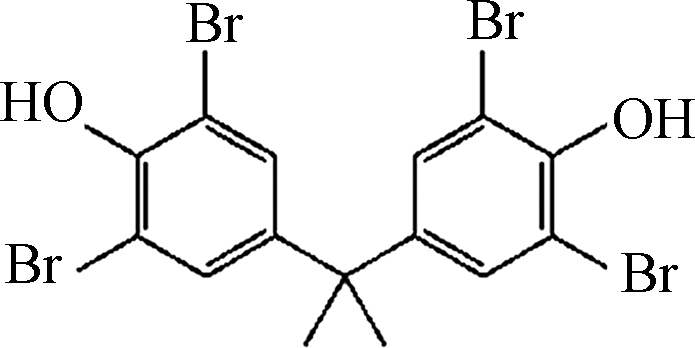
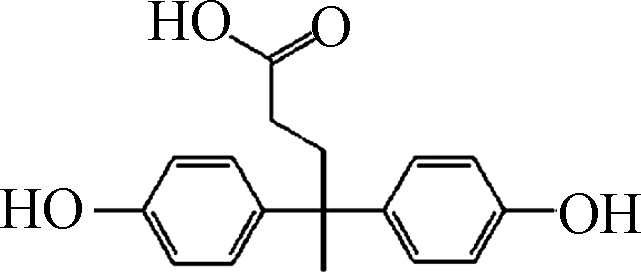
试剂:Fe(Ⅲ)-TAML来自GreenOx Catalysts, Inc.(Mellon Institute, Pittsburgh, PA, USA),通过紫外分光光度计在366 nm测量Fe(Ⅲ)-TAML的储备溶液浓度(摩尔吸光系数为6600(mol·L−1)−1 cm−1)。分析纯的BPA、双酚C(BPC)、双酚F(BPF)、双酚AF(BPAF)、双酚E(BPE)、双酚Z(BPZ)、BPS、四甲基双酚A(TMBPA)、双酚AP(BPAP)、四溴双酚A(TBBPA)、双酚酸(BP酸)和其他试剂均购自Sigma-Aldrich,由于BPs的水溶性有限,加入10 mmol·L−1的NaOH提高双酚类化合物的溶解度,配置100 mg L−1的BPs的标准储备溶液。H2O2(30% 质量分数)由Fisher Scientific提供。实验中用到的过一硫酸氢钾复合盐(PMS)来自Energy Chemical。实验使用Milli-Q水(18.2 MΩ·cm)。
-
在100 mL锥形瓶中进行降解实验。依次加入各反应物并补充Milli-Q水至100 mL,溶液中含有10.0 μmol·L−1的BPs和0.1 μmol·L−1的Fe(Ⅲ)-TAML。溶液采用碳酸盐缓冲液维持pH10的溶液条件。将锥形瓶置于磁力搅拌器上,加入1.0 mmol·L−1的H2O2启动反应。取样时间为0、0.25、0.5、0.75、1、1.5、2、3、5 min。取0.5 mL的反应液与含有10 μL 高氯酸(7 mol·L−1)的1 mL甲醇混合,终止反应。类似的实验操作同样应用于Fe(Ⅲ)-TAML/PMS体系中,加入1.0 mmol·L−1的PMS启动反应。本实验设置3组平行。
为验证体系主要是高价铁配合物而不是自由基引起的降解反应,进行了自由基淬灭实验,选取TMBPA作为反应物,向反应体系加入了乙醇(EtOH,100 mmol·L−1)作为自由基清除剂,测定TMBPA降解动力学并得到反应速率常数。
-
样品经0.22 μm聚四氟乙烯过滤器过滤后,用于高压液相色谱(HPLC)分析。HPLC包括紫外检测器和Eclipse XDB-C18色谱柱(5 μm,4.6 mm× 150 mm,Agilent)。流动相流速设置为1 mL ·min−1,柱温为(35 ± 1)℃。各双酚类化合物的具体HPLC检测方法如表1所示。
-
使用高斯09W软件中的B3LYP方法和6-311G(d,p)基组计算了双酚类化合物训练集的理论参数,包括总能量(TE)、最高占用分子轨道能(EHOMO)、最低未占用分子轨道能(ELUMO)、能量差(Egap,即EHOMO-ELUMO)、偶极矩(μ)、最正净电荷(q+)、最负净电荷(q−)、极化率(α)和分子体积(V)。此外还收集了各化合物的电离平衡常数(pKa)。Fe(Ⅲ)-TAML/PMS和Fe(Ⅲ)-TAML/H2O2体系对8种BPs的降解动力学,通过拟一级动力学公式(7),拟合得到Kobs。
式中,C0为BPs的初始浓度(μmol·L−1),Ct为BPs反应时间t(min)时的浓度(μmol·L−1)。为了计算Kobs,忽略了退化曲线平坦范围内的数据。采用多元线性逐步回归(MLR)方法建立Kobs与双酚类化合物的理论参数之间的QSAR模型。为测试模型的预测能力,选取3种双酚类化合物作为测试集,验证模型的可靠性和适用性。
1.1. 试剂
1.2. 降解动力学实验
1.3. BPs的检测
1.4. 理论计算和QSAR模型
-
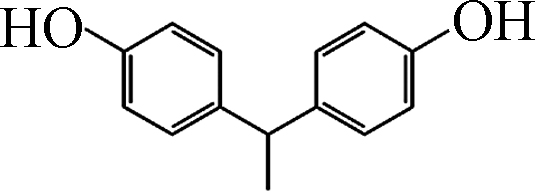
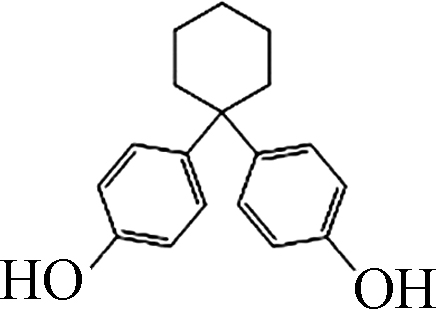
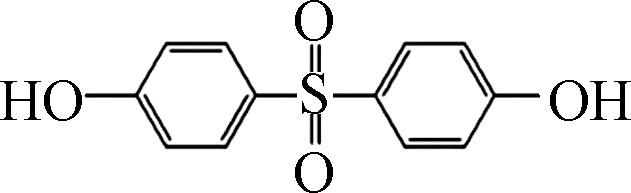
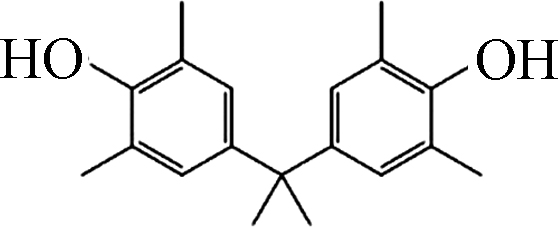
如图1所示,在pH10的条件下,Fe(Ⅲ)-TAML/H2O2和Fe(Ⅲ)-TAML/PMS体系可以快速降解除了BPAF和BPS之外的双酚类化合物。通过对BPs的降解进行拟一级动力学拟合,得到反应速率常数Kobs。BPA、BPC、BPF、BPAF、BPE、BPZ、BPS、TMBPA被Fe(Ⅲ)-TAML/PMS体系降解的Kobs分别为1.738、2.712、2.053、0.442、1.999、2.488、0.034、2.267 min−1,服从BPC>BPZ>TMBPA>BPF>BPE>BPA>BPAF>BPS;被Fe(Ⅲ)-TAML/H2O2体系催化氧化的Kobs分别为1.370、2.897、3.765、0.006、2.987、3.090、0.002、2.200 min−1,服从BPF>BPZ>BPE>BPC>TMBPA>BPA>BPAF>BPS(表2)。
根据拟合结果可以发现,在同种过氧化物条件下,不同种类的BPs降解速率不同;同一种BP在不同过氧化物体系中的降解速率也存在差异。有研究者认为导致双酚类化合物降解的关键活性物质,是高价铁配合物,即Fe(Ⅳ)-TAML,取代了单线态氧(1O2),超氧自由基(
${\rm{O}}_2^{-} \cdot $ ),${\rm{SO}}_4^{2-} \cdot $ 和HO·[27]。为了证实HO·和${\rm{SO}}_4^{2-} \cdot $ 在Fe(Ⅲ)-TAML/过氧化物体系中对BPs氧化的潜在作用,向反应体系加入了EtOH作为自由基清除剂,因为它对HO·和${\rm{SO}}_4^{2-} \cdot $ 具有高反应活性,二阶速率常数分别为1.2×109—2.8×109 mol−1·L·s−1和1.6×107—7.7×107 mol−1·L·s−1[30-31]。如图2所示,乙醇对TMBPA降解没有显著影响,表明HO·和${\rm{SO}}_4^{2-} \cdot $ 都不是导致BPs降解的主要反应活性物质。由此Fe(Ⅲ)-TAML的反应机理如式(8)—(10),Fe(Ⅲ)-TAML在过氧化物激发下生成氧化态的Fe-TAML(即Fe(Ⅳ)-TAML或Fe(Ⅴ)-TAML),氧化态的Fe-TAML迅速与有机污染物发生氧化还原反应,污染物被降解,氧化态的Fe-TAML被还原为Fe(Ⅲ)-TAML,达到催化氧化污染物的效果[16,29]。其中,kⅠ是TAML氧化的一阶速率常数,k-Ⅰ是逆反应的速率常数,kⅡ是BPs氧化的速率常数。
-
本研究选用的双酚类化合物的理论参数如表3所示,运用SPSS17.0统计软件包(SPSS Company, Chicago, IL,USA)建立QSAR模型。采用10个参数对8种双酚类化合物的Kobs数据进行MLR分析,得到单参数QSAR模型,可分别表示为方程(11)和(12)。并且得到相关系数(R2),Fisher检验(F),显著性水平(P)。R2(>0.6),F(>15)和P(<0.05)的结果表明构建的QSAR模型具有较好的拟合能力[36]。
n = 8,
$ {R} $ 2=0.831,$ {{R}}^{\text{'2}}=0.855 $ , F = 35.362, P < 0.001n = 8,
$ {R} $ 2= 0.691,$ {{R}}^{\text{'2}}=0.735 $ , F = 16.669, P < 0.006通过高斯软件计算出所需测试集的结构参数得到预测Kobs,降解动力学实验得到实测值Kobs,如表4所示。交叉验证结果如图3(a)显示,将8种BPs的EHOMO值代入公式
$ {{K}}_{\text{obs}}\text{=69.7}\text{2}\text{1}{{E}}_{\text{HOMO}}\text{+17.046} $ 中,得到预测值yp,实测值为ya。以ya为x轴,yp为y轴,线性拟合得到$ {R} $ 2=0.831。分别使用$ {{R}}_{0}^{\text{2}} $ 和$ {{R}}_{0}^{\text{ '2}} $ 表征通过原点的直线($y_{\rm{a}}^{{\rm{r0}}} = k{y_{\rm{p}}},\;y_{\rm{p}}^{{\rm{r0}}} = k'{y_{\rm{a}}} $ ),拟合结果显示$ R_0^2 = 0.964,k = 0.99979 $ ;$R_0^{'2} = 0.964,\;\;k' = 0.9687$ 。同理,如图3(b)显示,将μ代入公式$ {{K}}_{\text{obs}}=-\text{0.680} \mu \text{+3.745} $ 中,线性拟合得到$ {R} $ 2=0.691。过原点的拟合结果显示$R_0^2 = 0.90833,\;k = 0.9997 $ ;$R_0^{'2} = 0.90833,\;k' = 0.92006$ 。此外,$ {{R}}_{0}^{\text{2}} $ 和$ {{R}}_{0}^{\text{ '2}} $ 接近$ {R} $ 2,且$ {k} $ 和$ {{k}}^{\text{'}} $ 的值在0.85—1.15[37]。综上所述,构建的QSAR模型是具有良好预测性。
-
Fe(Ⅲ)-TAML/过氧化物体系在pH=10下可以在3min内完全降解除BPAF和BPS之外的双酚类化合物,从分子结构看(见表1),BPS的两个苯环之间有S和O,BPAF之间有6个F,存在较大的空间位阻,因此很难被氧化态的Fe-TAML进攻。在Fe(Ⅲ)-TAML/PMS体系中,Kobs与EHOMO呈高度正相关性。EHOMO反映的是BPs的供电子能力,具有较高EHOMO的化合物可以作为电子供体,数值越大,表示越容易受到亲电试剂的攻击失去电子,从而发生氧化反应。Su等建立了Fe(Ⅲ)-TAML/H2O2降解29种ACs的Kobs与ACs理论参数之间的QSAR模型,根据QSAR模型推断EHOMO是ACs在Fe(Ⅲ)-TAML/H2O2氧化体系中最重要的特征[29]。这一结果与我们的实验结论基本一致。
在Fe(Ⅲ)-TAML/H2O2体系中,Kobs与μ呈负相关。偶极矩描述了分子极性和电荷分离,是分子间相互作用强度的良好指标,这一结论与自由基降解途径中的结论恰好相反。Cheng等发现有机化合物在O3/H2O2中的降解常数与μ呈正相关,μ值大的化合物,它更可能接受来自氧化剂的电子,如O2·,
${\rm{O}}_2^- \cdot $ 和HO·[38]。本体系中,偶极矩大的分子通常电负性更强,BPS和BPAF两个苯环连接处分别存在O和F,具有较强的电负性,因此由于静电排斥作用,作为亲电试剂的氧化态的Fe-TAML难以进攻反应的活性位点。
2.1. 双酚类化合物的降解动力学
2.2. 理论计算结果
2.3. 反应活性相关性分析
-
通过测定8种BPs训练集和3种BPs测试集的降解动力学,可以得出以下结论:
(1)Fe(Ⅲ)-TAML/过氧化物体系可以在pH=10条件下快速降解除BPAF和BPS之外的双酚类化合物;
(2)根据Fe(Ⅲ)-TAML/PMS和Fe(Ⅲ)-TAML/H2O2两个体系降解BPs的速率常数与BPs的理论参数建立的QSAR模型,模型具有较好的拟合能力,可以快速预测其他BPs的反应速率常数;
(3)不同Fe(Ⅲ)-TAML/过氧化物体系降解BPs的决定因素不同,可以通过这一发现,为实际废水处理中降解体系的选择提供参考。




 下载:
下载: