-
单电子氧化是指弱氧化反应体系中,从一个中性有机污染物分子中拿走一个电子的反应过程 [1]。单电子氧化虽然是污染物最简单的化学变化,却会对污染物分子的结构和反应性产生重要影响,也是自然界中广泛存在的一种污染物转化途径水环境自净化重要的环节[2]。
有机污染物首先经过单电子氧化以产生活性中间体,然后,通过自由基聚合和亲核偶联形成的低聚物可进一步沉淀。庞素艳等[3]发现KMnO4氧化降解四氯双酚A(TCBPA)的反应形成低聚物。Sun等[4]研究发现,纳米MnO2可以高效地与17β-雌二醇和三氯生发生单电子氧化反应形成二聚体、三聚体和四聚体等低聚物,并在加入腐殖酸(HA)后,推测腐殖酸与17β-雌二醇产生交叉偶联反应。
近几年来,定量构效关系(quantitative structure-activity relationships, QSAR)被广泛应用于有机污染物的生物毒性、致癌性等预测,通过建立物质活性与其分子特定结构的化学描述符之间的数学模型,不仅能够有效地预测未经过实验检测的物质的理化性质,而且能够帮助我们更好得验证和解释反应机理[5]。在实际应用中,通常使用逐步线性回归(MLR)及非线性回归来建立QSAR模型[6]。Kim等[7]通过MLR建立QSAR模型用于预测过氧乙酸环境中15种有机污染物的准一级速率常数与分子结构之间的关系,而Yang等[8]通过MLR建立QSAR模型,研究了41种含氮化合物在稳定超临界水氧化反应下的降解速率常数与17种分子结构特征的关系。现阶段对单电子氧化条件下废水中有机污染物的反应速率与其量化参数关系的QSAR模型研究较少,本论文对此进行探究。
许多研究报道了废水中污染物的单电子氧化反应,但由于对不同污染物进行单电子氧化反应的研究较为分散。本文首先通过文献收集氧化锰体系24种有机污染物的反应动力学速率常数,并基于DFT计算不同有机污染物的电子结构特征参数,进一步采用非线性回归及多元线性回归的方法将不同有机污染物的电子结构特性与单电子氧化的反应速率常数之间构建QSAR模型,最后采用留一法对构建的模型进行内部验证;并通过实验补充6种有机污染物的单电子氧化反应速率常数来验证QSAR模型的预测能力。模型为不同污染物结构发生单电子氧化活性的预测提供理论依据,进一步阐述单电子氧化反应发生的规律。
-
收集文献中不同污染物在不同反应条件下的准一级及二级反应动力学常数,实验补充了间苯二酚(上海麦克林生化科技有限公司)、间苯三酚(上海麦克林生化科技有限公司)、对苯二酚(上海麦克林生化科技有限公司)、苯酚(阿拉丁生化科技股份有限公司)、2,6二氯酚(阿拉丁生化科技股份有限公司)、对甲酚(国药集团化学试剂有限公司)等6种有机污染物的准一级及二级反应速率常数。通过P1201型液相色谱(大连依利特仪器分析有限公司)测定有机污染物在不同浓度下反应前后水中有机物浓度变化计算其反应速率常数,并进一步改变投加的二氧化锰(阿拉丁生化科技股份有限公司)浓度,计算其准一级及二级反应速率常数。
弱氧化反应:分别取50、100、200 μmol·L−1有机污染物溶液于100 mL玻璃瓶中,加入0.5 g·L−1二氧化锰,将反应瓶置于磁力搅拌机上搅拌,转速为500 r·min−1,每隔固定时间取样后进行液相色谱测定。再以相同的方法测定0.1、0.2、0.3、0.5、0.8 g·L−1二氧化锰浓度下100 μmol·L−1有机污染物浓度变化。
液相分析条件:C18反相色谱柱(2.1 mm×150 mm, 3.5 μm,安捷伦科技有限公司);流动相:甲醇:超纯水=40:60(V:V);流速:0.25 mL·min−1;进样量:20 μL;检测波长:间苯二酚272 nm、间苯三酚263 nm、对苯二酚286 nm、苯酚252 nm、2,6二氯酚274 nm、对甲酚278 nm。采用外标法定量。
反应动力学研究的是化学反应过程中由于化学物理等性质。弱氧化剂对邻苯二酚,2,6二氯酚等有机污染物进行降解反应。反应速率常数由不同时间检测水中有机物浓度变化测定。因此,可由以下方程得到反应速率常数[9-10]:
式中,t(min)为反应时间;ct、c0分别为反应进行到t时和反应刚开始时候的有机物浓度(μmol·L−1);ce则为氧化锰氧化有机物时的表面吸附量(μmol·L−1);k为准一级动力学反应常数(min−1)。
二级反应速率常数由准一级反应速率常数与氧化剂浓度呈线性关系,因此可有作图法得出二级反应速率常数k0(mol·L−1s−1)。
式中,k为准一级反应速率常数(min−1),C为氧化剂浓度(g·L−1)。
-
本文采用Gaussian 09软件中用密度泛函理论(DFT)的B3LYP/6-31G方法对物质结构进行优化,从Gaussian 09输出文件中可直接获得EHOMO等量子描述符。亲电亲核指数等概念密度泛函理论(Conceptual density functional theory,CDFT)参数的计算通过Multiwfn软件[11]得出。
计算所得的电子结构特征参数分别为:EB3LYP指在B3LYP方法下,分子的总能量;μ表示分子的偶极矩;EHOMO指已占有电子的能级最高的轨道称为最高占据分子轨道能量;ELUMO指最低未占据分子轨道能量;EGAP为分子的能隙值即EHOMO-ELUMO,表明电子从占据轨道向空轨道跃迁的能力;化学势为偏摩尔吉布斯函数;N指分子结构中容易发生反应的活性官能团数量对反应速率的影响。pH指溶液中氢离子的总数和总物质的量的比;C有机物即有机物投加量指体系中有机物浓度;CHA即腐殖酸(HA)投加量表示反应体系中腐殖酸浓度。
-
氧化锰氧化反应属于弱氧化反应中的金属氧化物氧化反应途径,有机物通过单电子氧化形成自由基,或存在易降解大分子有机物时,自由基间发生自偶联或与大分子交叉偶联反应生成分子量更大,极性更小的二聚物/多聚物。
氧化锰条件下,有机污染物发生准一级动力学反应。庞素艳等[12]发现KMnO4氧化降解三氯生符合准一级动力学规律,且准一级反应速率常数k (s−1)随KMnO4浓度的增加呈线性增加。庞素艳等[13]发现MnO2可以通过催化KMnO4氧化降解酚类化合物,该反应符合准一级动力学,且反应速率常数(k)随着MnO2浓度(30—180 μmol·L−1)的增加呈线性增加。反应遵循氧化耦合反应机制:酚类化合物吸附于MnO2表面,与表面活性位形成表面络合物,表面络合物发生单电子转移,导致酚被氧化形成酚氧自由基,形成的自由基在溶液中相互耦合形成聚合物。Lin等[14]通过伯氏石(δ-MnO2)对简单溴酚(BPS,如4-BP、2,4-DBP和2,4,6-TBP)的氧化转化实验发现,随着反应的进行,表观的速率常数逐渐降低,由反应产物的吸附引起的表面变化和表面部位分布向反应性较低的部位的偏移,δ-MnO2氧化BPS通过单电子氧化产生溴苯氧基自由基,并推测产物OH-PBDEs的形成可能是由溴苯氧基自由基的氧化偶联引起的。收集准一级反应速率常数数据共84组,并将其收集于表1;本研究用MnO2氧化间苯二酚、间苯三酚、对苯二酚、苯酚、2,6二氯酚、对甲酚等酚类有机物数据,pH=6.8,100 μmol·L−1有机物浓度条件下,测得准一级反应速率常数于表1。
氧化锰条件下,有机污染物反应速率与氧化剂投加量呈二级动力学关系。庞素艳等[3]发现,KMnO4氧化降解TCBPA的二级反应速率常数k0受pH影响较大,为40.1—981.7 L·(mol·s)−1,随着pH的升高先增加而后降低,在pH=8附近最大,TCBPA的pKa (7.5/8.5)时越大。高源[15]探究发现,加入HA可以加速KMnO4氧化降解TCBPA过程。且此过程受pH影响较大,酸性条件下明显可加速氧化降解过程,而当其处于弱碱性条件下时,又因HA将中间体还原为TCBPA从而抑制了该污染物的降解。收集二级反应速率常数数据共25组,并将其收集于表1;本研究通过添加不同浓度MnO2氧化间苯二酚、对苯二酚、2,6二氯酚、对甲酚等酚类有机物,pH=6.8,100 μmol·L−1有机物浓度条件下测得二级反应速率常数于表1。
综上所述,弱氧化反应是通过单电子氧化形成不同的不稳定自由基中间体,产生的自由基相互聚合形成稳定的二聚体或多聚体,在此过程中整个反应前期均呈现了准一级动力学反应的特征,且与各自氧化剂投加量形成二级动力学反应特征。因此,本研究根据不同动力学情况建立了24种污染物的QSAR模型。分别收集70组反应速率常数与14组实验数据作为准一级反应速率QSAR模型的训练集与测试集;收集21组反应速率常数与4组实验数据作为二级反应速率QSAR模型的训练集与测试集。
-
将文献中收集及实验中的不同污染物进行DFT电子结构特征参数的计算,所的结果列于表2中。
-
本研究将整体实验数据拆分为训练集和测试集,以训练集的lnk为因变量,电子结构特征参数为自变量,通过MLR进行QSAR模型的构建和分析。
相关分析可探究单元自变量和因变量的关系,判断自变量对因变量的影响是否显著,由此判断是否具有回归分析的意义[24]。皮尔逊相关系数通常以0.5为分界点,0.5 < | r | < 0.8 表示显著相关,0.8 < | r | < 1 时表示高度相关,| r | < 0.5 时相关性较低[25]。为了更准确的判断,通常会进行显著性检验,0.05 常被作为显著性F检验的(P值)判断临界值。当P< 0.05 时,变量间存在显著相关;当
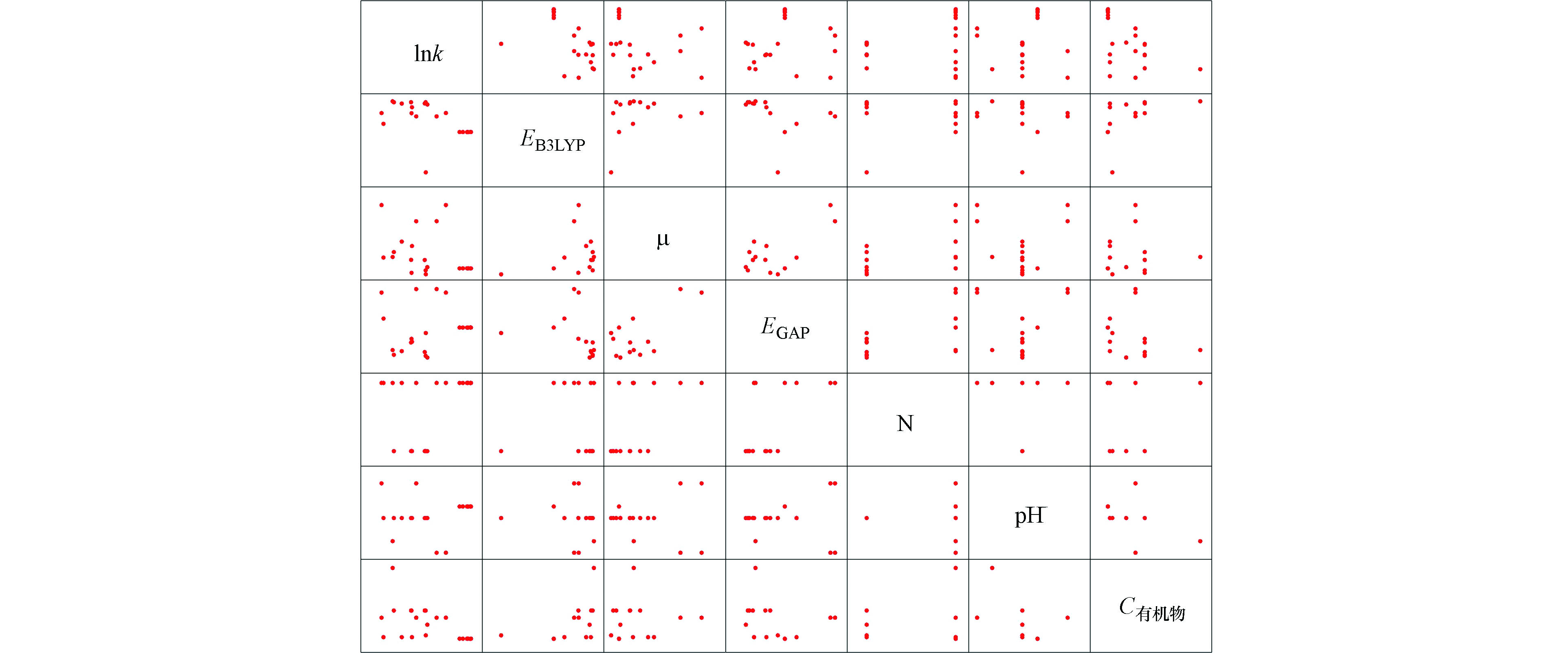
P < 0.01 时,变量间存在十分显著性相关。在分析结果中优先考虑显著性。 表3为氧化锰条件下准一级反应速率自变量与因变量皮尔逊相关系数表。自变量X2(μ)、X3(EHOMO)、X4(ELUMO)、X5(EGAP)、X9(N)、X11(C有机物)、X12(CHA)与因变量(lnk)的P值分别小于0.05 和 0.01,说明两个自变量与因变量存在显著相关关系和十分显著相关关系。所以,选择X2、X3、X4、X5、X9、X11和X12四个自变量与因变量建立模型。
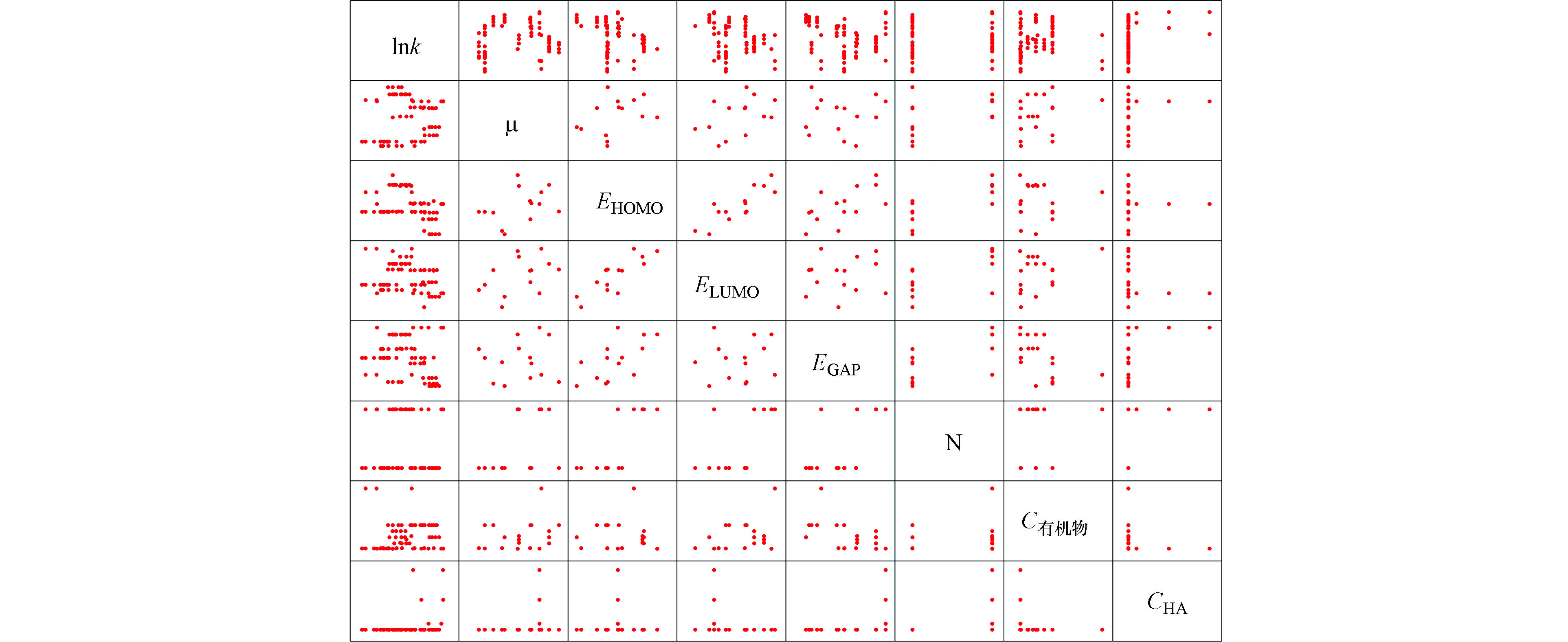
表4为氧化锰条件下二级反应速率自变量与因变量皮尔逊相关系数表。自变量X1(EB3LYP)、X2(μ)、X5(EGAP)、X9(N)、X10(pH)、X11(C有机物)与因变量lnk的P值分别小于0.05和0.01,说明两个自变量与因变量存在显著相关关系和十分显著相关关系。且自变量间相互的皮尔逊相关系数不高。
-
回归分析首要的是选择合适的非线性模型。非线性模型选择可以通过相关经验、专业知识和结合样本数据的散点图来确定。
图1为氧化锰条件下,准一级反应速率常数筛选出的相关自变量与因变量散点图。从图1中可看出,自变量与因变量的散点图呈曲线趋势。多项式函数模型是一个较为可靠的模型选择。结合图1趋势,选择二级多项式函数与指数函数进行数据拟合建立准一级反应速率常数模型。
建立了非线性回归模型:lnk=1343.888EHOMO−725.839ELUMO−0.266C有机物−0.001CHA+2.241μ2+104.472
$E_{\text{GAP}}^{2} $ −143.243N2−169917.9μ×EHOMO+169896.28μ×ELUMO+169923.54μ×EGAP +4.852μ×N−0.007μ×C有机物+32.944EHOMO×ELUMO −0.058 EHOMO×C有机物+58.662 ELUMO×EGAP +0.091 ELUMO×C有机物−71.681EGAP×N−4.165exp(EGAP×CHA)+4065.078模型的R2值为0.845。从R2值可以看出,模型可靠度高。其中,偶极矩反映了有机物的极性,偶极矩越大,有机物极性越大,反应速率越小;EHOMO指已占有电子的能级最高的轨道称为最高占据分子轨道能量,EHOMO越大,越不易失去电子,有机物被氧化能力降低;ELUMO指最低未占据分子轨道能量, ELUMO越大则氧化能力降低;能隙值表示电子从占据轨道向空轨道跃迁的能力,即分子参与化学反应的能力,能隙值越大,说明电子跃迁越不容易发生,那么此分子就越稳定,而能隙值越小,则说明电子跃迁较容易发生,分子就越容易参与化学反应;N代表分子结构中容易发生反应的活性官能团数量,数量越多,反应越容易发生;C有机物有机物投加量指体系中有机物浓度,能体现反应物浓度对反应的影响;CHA反映了外部添加较易降解且易聚合的大分子有机污染物时,对整个反应体系的影响。从模型中可以看出这7个主要影响因素呈非线性状态,呈现于模型中为二次项及指数函数的形式即需要两两因素间相互作用对准一级弱氧化反应有更明显的影响。
图2为氧化锰条件下,二级反应速率常数筛选出的相关自变量与因变量散点图。从图2可看出自变量与因变量的散点图呈曲线趋势。多项式函数模型是一个较为可靠的模型选择。
结合散点图趋势,选择非线性回归方法建立氧化锰条件下二级反应速率常数模型。量化参数对污染物弱氧化反应速率的影响呈现一定趋势。其中,模型lnk=−59.246+1.013C有机物−1.613
$E_{\text{B3LYP}}^{2} $ +1.767$E_{\text{GAP}}^{2} $ −0.286N2+0.618EB3LYP×EGAP−2.376EB3LYP×N+0.072EB3LYP×C有机物−0.122μ×EGAP+0.322μ×N−0.184μ×pH−0.277EGAP×pH+0.188EGAP×C有机物,模型的R2值达到0.928。从R2值可以看出,模型可靠度高。在模型中,EB3LYP反映了有机物在B3LYP条件下的总能量值;偶极矩反映了有机物的极性,偶极矩越大,有机物极性越大,反应速率越小;能隙值表示电子从占据轨道向空轨道跃迁的能力,即分子参与化学反应的能力,能隙值越大,说明电子跃迁越不容易发生,那么此分子就越稳定,而能隙值越小,则说明电子跃迁较容易发生,分子就越容易参与化学反应;N代表分子结构中容易发生反应的活性官能团数量,数量越多,反应越容易发生;pH反映了外部酸碱性环境对反应的进行存在着影响;C有机物指体系中有机物浓度,能体现反应物浓度对反应的影响。从模型中可以看出这六个主要影响因素呈非线性状态,呈现于模型中为二次项的形式即需要两两因素间相互作用对二级弱氧化反应有更明显的影响。综上所述,可以认为氧化锰氧化条件下,有机物反应速率与亲电亲核反应关系不大,大多数有机物弱氧化聚合通过单电子氧化方式进行。总的来说,不同因素两两间的相互影响对有机物能否采用氧化锰进行单电子氧化反应作用更为突出,有机物投加量C有机物、活性官能团数量N、能隙值EGAP等因素对能否采用氧化锰进行单电子氧化反应影响更大。在与相关性系数相对比后发现,两者所筛选出的自变量有高度一致性,则证明该模型选择是可靠的。
-
本文通过留一法(leave-one-out,LOO)交叉验证进行内部检验,以所建的QSAR模型的参数为自变量进行内部检验,所有样本中,依次剔除1个样本并重新建立方程,用新建的方程对剔除的样本进行预测,将预测结果与试验结果进行比较得到残差,计算得到交叉验证相关系数
$Q_{\text{LOO}}^{2} $ [26](表明模型内部稳健程度):式中,yi为各有机物单电子氧化的lnk;
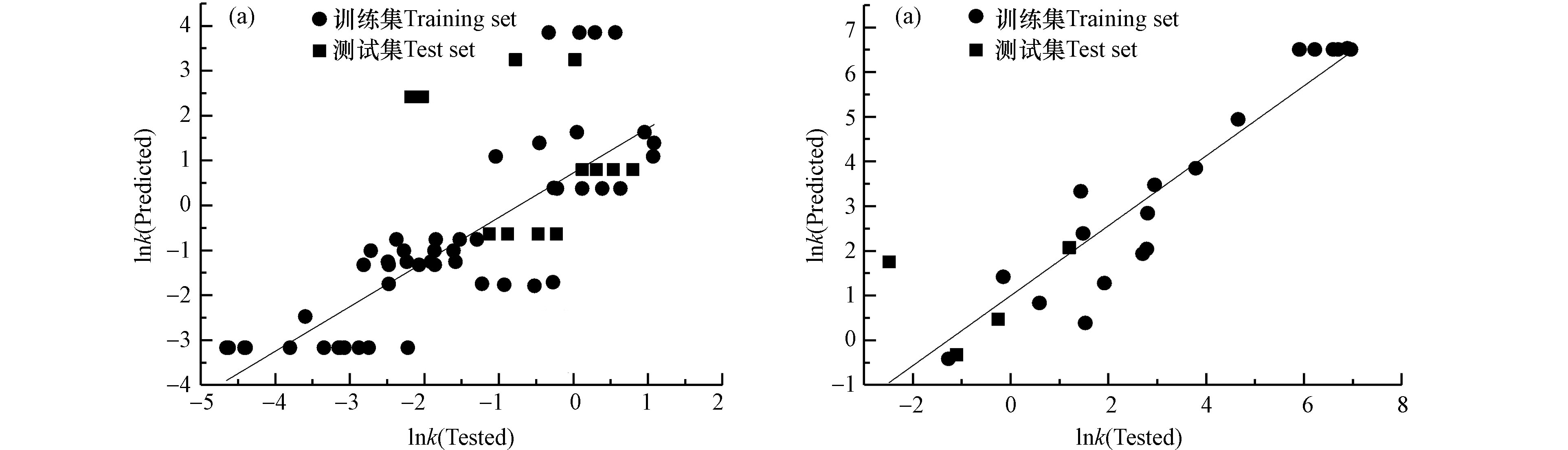
$ \bar y $ 为各有机物单电子氧化的lnk的平均值;$ {\hat y_i} $ 是新模型求得的预测值。R2和$Q_{\text{LOO}}^{2} $ 与$R_{\text{ext}}^{2} $ 差值远小于0.3,则表明模型具有良好的稳健性。通过文献总结收集的训练集与实验测得反应速率常数作为测试集的lnk作拟合图进行外部验证,如图3所示。判断其测试值与预测值一致性的参数为相关系数R2ext,R2ext越接近于1,模型的预测能力越好。
准一级反应动力学条件下所建立的模型:lnk=1343.888EHOMO−725.839ELUMO−0.266C有机物−0.001CHA+2.241μ2+104.472
$E_{\text{GAP}}^{2} $ −143.243N2−169917.9μ×EHOMO+169896.28μ×ELUMO+169923.54μ×EGAP +4.852μ×N−0.007μ×C有机物+32.944EHOMO×ELUMO −0.058EHOMO ×C有机物+58.662ELUMO ×EGAP +0.091 ELUMO × C有机物−71.681EGAP×N−4.165exp(EGAP× CHA)+4065.078$Q_{\text{LOO1}}^{2} $ =0.801,$R_{\text{ext1}}^{2} $ =0.813二级反应动力学条件下所建立的模型:lnk=−59.246+1.013C有机物−1.613
$E_{\text{B3LYP}}^{2} $ +1.767$E_{\text{GAP}}^{2} $ −0.286N2+0.618EB3LYP×EGAP−2.376EB3LYP×N+0.072EB3LYP×C有机物−0.122μ×EGAP+0.322μ×N−0.184μ×pH−0.277EGAP×pH+0.188EGAP×C有机物$Q_{\text{LOO2}}^{2}=0.990 $ ,$R_{\text{ext2}}^{2}=0.852 $ 经内外部验证,准一级和二级模型的R2和
$Q_{\text{LOO}}^{2} $ 与Rext2差值远小于 0.3,模型均具有良好的稳健性;且相关系数$R_{\text{ext}}^{2} $ 均接近于1,则两个模型均具有较好的预测能力。 -
在氧化锰单电子氧化条件下,分别对24种有机污染物进行了准一级动力学反应及二级动力学反应的定量构效关系研究。根据其不同的动力学反应常数与量子化学参数进行定量构效模型建立,筛选得出最优QSAR模型。
准一级反应动力学下,采用非线性回归方法得出: lnk=1343.888EHOMO−725.839ELUMO−0.266C有机物−0.001CHA+2.241μ2+104.472
$E_{\text{GAP}}^{2} $ −143.243N2−169917.9μ×EHOMO+169896.28μ×ELUMO+169923.54μ×EGAP +4.852μ×N−0.007μ×C有机物+32.944EHOMO×ELUMO −0.058EHOMO ×C有机物+58.662ELUMO ×EGAP +0.091 ELUMO × C有机物−71.681EGAP×N−4.165exp(EGAP× CHA)+4065.078(R2=0.845)经过内外部验证确认了其稳定性及较好的预测能力($Q_{\text{LOO1}}^{2}=0.801 $ ,$R_{\text{ext1}}^{2}=0.813 $ )。结果表明QSAR模型中代表有机物自身结构特性的量化参数,偶极矩μ、能隙值EGAP和存在活性基团个数N对氧化锰氧化有机污染物的影响较其他特征更为明显,同时,外部不同HA投加量越大,单电子氧化效率越差。在二级反应动力学下,筛选出的QSAR模型:lnk=−59.246+1.013C有机物−1.613
$E_{\text{B3LYP}}^{2} $ +1.767$E_{\text{GAP}}^{2} $ −0.286N2+0.618EB3LYP×EGAP−2.376EB3LYP×N+0.072EB3LYP×C有机物−0.122μ×EGAP+0.322μ×N−0.184μ×pH−0.277EGAP×pH+0.188EGAP×C有机物(R2=0.928)经过内外部验证确认了其稳定性及较好的预测能力($Q_{\text{LOO2}}^{2}=0.990 $ ,$R_{\text{ext2}}^{2}=0852 $ )。可以认为氧化锰氧化条件下,有机物反应速率与亲电亲核反应关系不大,大多数有机物弱氧化聚合通过单电子氧化方式进行。不同因素两两间的相互影响对有机物能否采用氧化锰进行单电子氧化反应作用更为突出,有机物投加量C有机物、活性官能团数量N、能隙值EGAP等因素对能否采用氧化锰进行单电子氧化反应影响更大。
有机污染物的单电子氧化结构-反应活性定量构效关系
Quantitative structure-activity relationship between single electron oxidation structure and reaction activity of organic ppollutants
-
摘要: 单电子氧化反应是自然界中普遍存在的一种弱氧化反应过程,但不同有机污染物的单电子氧化反应活性具有显著差异,实验筛选需要耗费大量时间和成本。而氧化体系中有机污染物的反应速率常数是衡量其单电子反应活性的一个重要参数。因此,本文首先通过文献系统总结氧化锰体系24种有机污染物的反应动力学速率常数,并基于密度泛函理论(DFT)计算不同有机污染物的电子结构特征参数,进一步采用非线性回归及多元线性回归的方法将不同有机污染物的电子结构特性与单电子氧化的反应速率常数之间构建定量构效关系(QSAR)模型,最后采用留一法对构建的模型进行内部验证;并通过实验补充有机污染物的单电子氧化反应速率常数来验证QSAR模型的预测能力。结果表明,根据文献总结91组氧化锰准一级和二级动力学反应体系构建并筛选得到的最优QSAR模型,均展现了较好的拟合度(
$R_{1}^{2}=0.845 $ ,$R_{2}^{2}=0.928 $ ),且经过实验总结18组数据进行内外部验证确认了其稳定性($Q_{\text{LOO1}}^{2}= 0.801$ ,$ Q_{\text{LOO2}}^{2}=0.990$ )及较好的预测能力($R_{\text{ext1}}^{2}= 0.813 $ ,$R_{\text{ext1}}^{2}=0.852 $ )。该研究结果也发现,不同因素两两间的相互影响对有机物能否采用氧化锰进行单电子氧化反应作用更为突出;其中,有机物投加量C有机物、活性官能团数量N、能隙值EGAP等因素对能否采用氧化锰进行单电子氧化反应影响更大。Abstract: The single-electron oxidation reaction is a kind of weak oxidation reaction process in nature, but the single-electron oxidation reaction activity of different organic pollutants has significant differences, and experimental screening requires a lot of time and cost. The reaction rate constant of organic pollutants in the oxidation system is an important parameter to measure its single-electron reaction activity. Therefore, this article first summarizes the reaction kinetic rate constants of 24 organic pollutants in the manganese oxide system through the literature system, and calculates the electronic structure characteristic parameters of different organic pollutants based on density functional theory (DFT), and further uses nonlinear regression and multiple linear regression method builds a quantitative structure-activity relationship (QSAR) model between the electronic structure characteristics of different organic pollutants and the reaction rate constant of single-electron oxidation, finally the leave-one-out method was used to internally verify the model; and the single-electron oxidation reaction rate constants of organic pollutants were supplemented by experiments to verify the predictive ability of the QSAR model. The optimal QSAR model constructed and screened based on 91 sets of manganese oxide quasi-first and second-stage kinetic reaction systems all show a good degree of fit ($R_{1}^{2}= 0.845$ ,$R_{2}^{2}=0.928 $ ), and 18 sets of experimental data Internal and external verification confirmed its stability ($Q_{\text{LOO1}}^{2}=0.801 $ ,$Q_{\text{LOO2}}^{2}=0.990 $ ) and good predictive ability ($R_{\text{ext1}}^{2}= $ $ 0.813$ ,$R_{\text{ext1}}^{2}=0.852 $ ). The research results also found that the mutual influence between two different factors on organic matter whether using single electron oxidation reaction of manganese oxide is more outstanding; Among them, organic additive amount of Corganic, active functional group number N, energy gap value EGAP on whether using single electron of manganese oxide oxidation reaction influence is greater. -

-
表 1 有机污染物反应动力学数据
Table 1. The data of organic micropollutant reaction kinetics
反应级数
Order of reaction有机污染物
Organic pollutants反应速率常数
k/min−1或k0/(L·g−1·min−1)pH C有机物/
(μmol·L−1)CHA /(μmol·L−1) 参考文献
References准一级反应 2,4-二氯酚(24DCP) 0.0095 —0.109 4—9 6 0 [13] 2,4-二溴酚(24DBP) 0.036—0.39 4.5—8.5 7.94 0 [14] 4-溴酚(4BP) 0.122—1.51 4.5 7.94 0 [14] 2,4,6-三溴酚(246TBP) 0.77 4.5 7.94 0 [14] 三氯生(TCS) 0.0035 —2.957 5 5 0—600 [15,17] 四氯双酚A(TCBPA) 0.535—0.977 8 1 0—10 [15] 雌二醇(E2) 0.000271 —0.056 5 4 0 [16] 2氯酚(2CP) 0.804—1.884 7 160 0 [18] 3氯酚(3CP) 0.06—0.156 7 160 0 [18] 4氯酚(4CP) 0.294—0.76 7 160 0 [18] 246三氯酚(246TCP) 1.128—2.226 7 80 0 [18] 对乙酰氨基酚(APAP) 0.066—0.9774 10 40—120 0 [19] 双酚A
(BPA)0.00215—0.015 6.8 50—200 0 [20] 邻苯二酚 0.0123—0.322 5 400 0 [21] 间甲酚 0.00061—0.0022 6.8 50—200 0 本研究 对苯二酚 0.186—0.359 6.8 50—200 0 本研究 间苯二酚 0.00244—0.0163 6.8 50—200 0 本研究 间苯三酚 0.0104—0.0218 6.8 50—200 0 本研究 苯酚
(BP)0.00741—0.00991 6.8 50—200 0 本研究 2,6二氯酚(26DCP) 0.0131—0.0313 6.8 50—200 0 本研究 对甲酚 0.00207—0.603 6.8 50—200 0 本研究 二级反应 四氯双酚A 368—1055 8 0.3 0 [3] 蛋氨酸(MET) 4.6 7 1020 0 [15] 组氨酸(HIS) 1.8 7 10120 0 [15] 2氯酚(2CP) 16.2 7 160 0 [18] 3氯酚(3CP) 0.86 7 160 0 [18] 4氯酚(4CP) 4.2 7 160 0 [18] 246三氯酚(246TCP) 19 7 80 0 [18] 邻苯二酚 0.774 5 400 0 [21] 2,4-二氯酚 0.5 7 20 0 [22] 磺胺噻唑 6.81—44.17 4—10 120 0 [23] 磺胺异恶唑 0.28—105.14 4—10 120 0 [23] 对甲酚 0.0831 6.8 100 0 本研究 对苯二酚 0.222 6.8 100 0 本研究 间苯二酚 3.278 6.8 100 0 本研究 2,6二氯酚 3.326 6.8 100 0 本研究 注:k为准一级反应速率常数(min−1),k0为二级反应速率常数(L·g−1·min−1)。
Note:k is the first order reaction rate constant (min -1), k0 is the second order reaction rate constant(L·g−1·min−1).表 2 不同有机污染物量化参数
Table 2. Quantitative parameters of different organic micropollutants
有机物 EB3LYP
/eVμ/debye EHOMO
/eVELUMO
/eVEGAP
/eV亲电指数
/eV
Electrophilicity
index亲核指数
/eV Nucleophilicity
index化学势
/eV
Chemical potentialN 17β-雌二醇
(E2)−850.89 2.64 −5.68 −0.37 −5.30 0.42 3.44 −2.76 2 三氯生
(TCS)−1992.77 3.625 −6.323 −1.093 −5.230 0.76 2. 80 −3.61 2 四氯双酚A (TCBPA) −0.62 1.878 −6.373 −1.001 −5.372 0.67 2.82 −3.41 2 2,4-二氯酚(24DCP) −1226.69 1.162 −6.497 −0.948 −5.549 0.73 2.27 −3.81 1 4-溴酚
(4BP)−2878.6291 3.201 −6.255 −0.708 −5.547 0.594 2.610 −3.474 1 2,4-二溴酚(2,4DBP) −5449.752 0.899 −6.495 −1.037 −5.458 3.613 2.423 −6.047 1 2,4,6-三溴酚(246TBP) −8020.871 1.939 −6.924 −1.329 −5.594 0.932 2.190 −4.131 1 磺胺噻唑 −1459.455 9.501 −6.171 −1.407 −4.765 0.773 3.084 −3.570 2 磺胺异恶唑 −1215.094 12.109 −6.154 −1.334 −4.820 0.781 2.846 −3.625 2 对乙酰氨基酚(APAP) −515.547 4.058 −5.896 −0.591 −5.305 0.018 7.915 −0.454 2 间甲酚 −9437.082 1.628 −6.236 −0.380 −5.856 0.428 3.237 −2.952 1 对甲酚 −9437.055 2.158 −6.080 −0.437 −5.643 0.415 3.357 −2.882 1 双酚A
(BPA)−731.748 2.700 −5.918 −0.468 −5.450 0.562 2.986 −3.193 2 2氯酚(2CP) −767.095 1.5493 −6.517 −0.6979 −5.819 0.614 2.403 −3.584 1 3氯酚(3CP) −767.098 4.498 −6.489 −0.687 −5.802 0.5654 2.7881 −3.3828 1 4氯酚(4CP) −767.099 3.257 −6.307 −0.699 −5.607 0.603 2.507 −3.532 1 26二氯酚
(26DCP)−1226.685 3.216 −6.664 −0.905 −5.759 0.5663 2.6137 −3.4064 1 246三氯酚(246TCP) −1686.271 2.054 −6.997 −1.153 −5.844 0.864 2.001 −4.112 1 表 3 氧化锰氧化条件下准一级反应速率常数相关系数
Table 3. The relationship of quasi-first order reaction rate constant under oxidation conditions of manganese oxide
P r X1 X2 X3 X4 X5 X6 X7 X8 X9 X10 X11 X12 X1 0.438 0.282 0.548 −0.211 −0.818 0.272 0.603 0.230 0.325 0.446 −0.095 X2 0 0.544 0.513 0.246 −0.567 0.604 0.700 0.653 0.300 0.337 0.198 X3 0.009 0 0.775 0.653 −0.368 0.751 0.711 0.807 0.294 0.095 0.023 X4 0 0 0 0.028 −0.473 0.537 0.631 0.480 0.265 0.556 −0.285 X5 0.04 0.02 0 0.411 −0.015 0.543 0.368 0.701 0.148 −0.516 0.377 X6 0 0 0.001 0 0.451 −0.425 −0.787 −0.401 −0.325 −0.308 −0.013 X7 0.011 0 0 0 0 0 0.891 0.683 0.713 0.039 −0.08 X8 0 0 0 0 0.001 0 0 0.671 0.639 0.204 −0.059 X9 0.028 0 0 0 0 0 0 0 0.152 0.047 0.323 X10 0.003 0.006 0.007 0.013 0.111 0.003 0 0 0.104 0.059 −0.242 X11 0 0.002 0.217 0 0 0.005 0.373 0.045 0.348 0.314 −0.219 X12 0.217 0.05 0.426 0.008 0.001 0.457 0.254 0.315 0.003 0.022 0.035 注:X1:EB3LYP;X2:μ;X3:EHOMO;X4:ELUMO;X5:EGAP;X6:Electrophilicity index;X7:Nucleophilicity index;X8:Chemical potential;X9:N;
X10:pH;X11:C有机物;X12:CHA.表 4 氧化锰氧化条件下二级反应速率常数相关系数
Table 4. The relationship of second order reaction rate constant under oxidation conditions of manganese oxide
P r X1 X2 X3 X4 X5 X6 X7 X8 X9 X10 X11 X12 X1 0.325 0.048 0.351 −0.27 −0.753 0.1 0.667 −0.232 −0.222 0.579 −0.295 X2 0.076 0.569 −0.421 0.706 −0.174 0.434 0.186 0.345 −0.124 0.201 −0.255 X3 0.418 0.004 0.095 0.530 −0.204 0.850 0.396 0.535 −0.105 0.129 −0.025 X4 0.059 0.029 0.341 −0.794 −0.238 0.211 0.371 −0.342 −0.169 0.283 −0.06 X5 0.118 0 0.007 0 0.079 0.34 −0.074 0.619 0.079 −0.162 0.036 X6 0 0.226 0.188 0.149 0.367 −0.335 −0.955 −0.249 0.006 −0.207 −0.07 X7 0.333 0.025 0 0.179 0.066 0.069 0.571 0.627 −0.112 0.153 0.056 X8 0 0.21 0.038 0.049 0.374 0 0.003 0.366 −0.034 0.212 0.099 X9 0.155 0.063 0.006 0.064 0.001 0.138 0.001 0.051 0.102 −0.222 0.288 X10 0.166 0.295 0.325 0.233 0.366 0.49 0.315 0.442 0.33 −0.383 0.202 X11 0.003 0.192 0.288 0.107 0.242 0.184 0.254 0.179 0.167 0.043 −0.325 X12 0.097 0.132 0.457 0.397 0.438 0.382 0.405 0.334 0.103 0.189 0.075 注:X1:EB3LYP;X2:μ;X3:EHOMO;X4:ELUMO;X5:EGAP;X6:Electrophilicity index;X7:Nucleophilicity index;X8:Chemical potential;X9:N;
X10:pH;X11: C有机物;X12:CHA. -
[1] 崔崇威, 黄君礼. 二氧化氯与苯酚的单电子转移反应机理 [J]. 环境化学, 2003, 22(6): 560-563. doi: 10.3321/j.issn:0254-6108.2003.06.007 CUI C W, HUANG J L. Mechanism of the single electron transfer between ClO2 and phenol [J]. Environmental Chemistry, 2003, 22(6): 560-563(in Chinese). doi: 10.3321/j.issn:0254-6108.2003.06.007
[2] ZHONG C, ZHAO H, CAO H B, et al. Polymerization of micropollutants in natural aquatic environments: A review [J]. The Science of the Total Environment, 2019, 693: 133751. doi: 10.1016/j.scitotenv.2019.133751 [3] 庞素艳, 段杰斌, 江进, 等. KMnO4氧化降解阻燃剂四氯双酚A的动力学、氧化产物及反应路径 [J]. 哈尔滨工业大学学报, 2018, 50(8): 20-26. doi: 10.11918/j.issn.0367-6234.201706036 PANG S Y, DUAN J B, JIANG J, et al. Degradation of flame retardant tetrachlorobisphenol A by potassium permanganate: Kinetics, oxidation products and reaction pathways [J]. Journal of Harbin Institute of Technology, 2018, 50(8): 20-26(in Chinese). doi: 10.11918/j.issn.0367-6234.201706036
[4] SUN K. , LIANG S T, KANG F X, et al. Transformation of 17β-estradiol in humic acid solution by epsilon-MnO2 nanorods as probed by high-resolution mass spectrometry combined with 13C labeling [J]. Environmental Pollution, 2016, 214(jula): 211-218. [5] BARUA N, SARMAH P, HUSSAIN I, et al. DFT-based QSAR models to predict the antimycobacterial activity of chalcones [J]. Chemical Biology & Drug Design, 2012, 79(4): 553-559. [6] SAXENA A K, PRATHIPATI P. Comparison of MLR, PLS and GA-MLR in QSAR analysis [J]. SAR and QSAR in Environmental Research, 2003, 14(5/6): 433-445. [7] KIM J, DU P H, LIU W, et al. Cobalt/peracetic acid: Advanced oxidation of aromatic organic compounds by acetylperoxyl radicals [J]. Environmental Science & Technology, 2020, 54(8): 5268-5278. [8] YANG B W, CHENG Z W, TANG Q L, et al. Nitrogen transformation of 41 organic compounds during SCWO: A study on TN degradation rate, N-containing species distribution and molecular characteristics[J].Water Research, 2018, 140: 167-180. [9] 李琬莹, 程治文, 陆丛蕊, 等. 紫外-双氧水复合氧化体系中有机污染物降解速率的定量构效关系研究 [J]. 计算机与应用化学, 2019, 36(2): 107-114. LI W Y, CHENG Z W, LU C R, et al. QSAR study on reaction kinetic constant of organic pollutants in UV/H2O2 process [J]. Computers and Applied Chemistry, 2019, 36(2): 107-114(in Chinese).
[10] 蒋艾. O3与H2O2氧化难降解有机物的对比及其定量构效关系研究[D]. 上海: 上海交通大学, 2018. JIANG A. Advanced oxidation of refractory organic pollutants with H2O2 and O3 and their quantitative structure-activity relationships[D]. Shanghai: Shanghai Jiaotong University, 2018(in Chinese).
[11] LU T, CHEN F. Multiwfn: A multifunctional wavefunction analyzer. [J]. Journal of Computational Chemistry, 2012, 33(5): 580-92. doi: 10.1002/jcc.22885 [12] 庞素艳, 王强, 鲁雪婷, 等. 中间价态锰强化KMnO4氧化降解三氯生 [J]. 哈尔滨工业大学学报, 2015, 47(2): 87-91. doi: 10.11918/j.issn.0367-6234.2015.02.016 PANG S Y, WANG Q, LU X T, et al. Oxidative removal of triclosan by potassium permanganate enhanced with manganese intermediates [J]. Journal of Harbin Institute of Technology, 2015, 47(2): 87-91(in Chinese). doi: 10.11918/j.issn.0367-6234.2015.02.016
[13] 庞素艳, 江进, 马军, 等. MnO2催化KMnO4氧化降解酚类化合物 [J]. 环境科学, 2010, 31(10): 2331-2335. PANG S Y, JIANG J, MA J, et al. Oxidation of phenolic compounds with permanganate catalyzed by manganese dioxide [J]. Chinese Journal of Environmental Science, 2010, 31(10): 2331-2335(in Chinese).
[14] LIN K D, YAN C, GAN J. Production of hydroxylated polybrominated diphenyl ethers (OH-PBDEs) from bromophenols by manganese dioxide [J]. Environmental Science & Technology, 2014, 48(1): 263-271. [15] 高源. KMnO4氧化降解酚类有机污染物的反应产物及低价态锰的作用[D]. 哈尔滨: 哈尔滨工业大学, 2018. GAO Y. Oxidative degradation of phenolic contaminants by permanganat: Products and effects of manganese intermediates in situ formed[D]. Harbin: Harbin Institute of Technology, 2018(in Chinese).
[16] 杜朋辉. 水中新兴有机污染物的聚合偶联机理研究[D]. 北京: 中国科学院大学(中国科学院过程工程研究所), 2018. DU P H. Mechanism for coupling and polymerization of emergin organic pollutants in waters[D]. Beijing:University of Chinese Academy of Sciences (Institute of Process Engineering Chinese Academy of Sciences), 2018(in Chinese).
[17] 徐勇鹏, 杨静琨, 王在刚. 高锰酸钾氧化去除水中三氯生动力学研究 [J]. 哈尔滨工业大学学报, 2011, 43(12): 48-52. XU Y P, YANG J K, WANG Z G. Kinetics on triclosan oxidation by potassium permanganate in drinking water [J]. Journal of Harbin Institute of Technology, 2011, 43(12): 48-52(in Chinese).
[18] HOSSAIN S , MCLAUGHLAN R G. Kinetic investigations of oxidation of chlorophenols by permanganate [J]. Journal of Environmental Chemistry and Ecotoxicology, 2013, 5(4): 81-89. [19] ZHONG C, ZHAO H, CAO H B, et al. Acidity induced fast transformation of acetaminophen by different MnO2 : Kinetics and pathways[J]. Chemical Engineering Journal, 2018, 359: 518-529. [20] 高娜, 于志强, 廖汝娥, 等. 二氧化锰氧化降解双酚A的动力学 [J]. 生态环境学报, 2009, 18(2): 431-434. doi: 10.3969/j.issn.1674-5906.2009.02.007 GAO N, YU Z Q, LIAO R E, et al. Oxidation kinetics of bisphenol A by manganese oxide [J]. Ecology and Environmental Sciences, 2009, 18(2): 431-434(in Chinese). doi: 10.3969/j.issn.1674-5906.2009.02.007
[21] 康秋红. 邻苯二酚类有机化合物的氧化降解研究[D]. 金华: 浙江师范大学, 2016. KANG Q H. Study on the Oxidative Degradation of Catechol Compounds[D]. Jinhua: Zhejiang Normal University, 2016(in Chinese).
[22] JIANG J, GAO Y, PANG S Y, et al. Oxidation of bromophenols and formation of brominated polymeric products of concern during water treatment with potassium permanganate [J]. Environmental Science & Technology, 2014, 48(18): 10850-10858. [23] 张旭, 高坡, 马军, 等. 高锰酸钾降解两种磺胺衍生物动力学方程的建立 [J]. 黑龙江大学自然科学学报, 2014, 31(3): 361-366. ZHANG X, GAO P, MA J, et al. Potassium permanganate degradation of two types of sulfonamide derivatives to establish dynamic equation [J]. Journal of Natural Science of Heilongjiang University, 2014, 31(3): 361-366(in Chinese).
[24] 岳佳鑫. 焦化废水处理过程集成优化[D]. 北京: 中国科学院大学(中国科学院过程工程研究所), 2020. YUE J X. Optimal synthesis and operation of coking wastewater treatment process[D]. Beijing: University of Chinese Academy of Sciences (Institute of Process Engineering Chinese Academy of Sciences), 2020(in Chinese).
[25] 袁亚茹. 臭氧氧化难降解有机物的定量构效关系研究[D]. 上海: 上海交通大学, 2017. YUAN Y R. Quantitative structure–activity relationships study for ozonation of refrectory organic componunds[D]. Shanghai: Shanghai Jiaotong University, 2017(in Chinese).
[26] 覃礼堂, 刘树深, 肖乾芬, 等. QSAR模型内部和外部验证方法综述 [J]. 环境化学, 2013, 32(7): 1205-1211. doi: 10.7524/j.issn.0254-6108.2013.07.012 QIN L T, LIU S S, XIAO Q F, et al. Internal and external validtions of QSAR model: Review [J]. Environmental Chemistry, 2013, 32(7): 1205-1211(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.07.012
-
















 下载:
下载: