-
磷(P)对生命活动有重要的作用,但是磷资源正被污染,而且磷是有限且不可再生的资源[1],因此磷的回收至关重要。从城市污水厂中回收磷有助于磷资源的持续再生和生态平衡[2],而当前广泛应用的从城市污水中回收磷的方法是结晶法[3],它是通过添加额外的试剂形成不溶性磷酸盐。国际上常用的结晶法是鸟粪石法[4],但其产品价值低;而且此方法多适用于传统强化生物除磷工艺(EBPR),需对富磷污泥提取后回收磷,因此回收效率不理想(10%—50%) [5-7]。研究人员在污水处理厂的污泥中发现了蓝铁矿污泥,并且占了大部分Fe-P结合物[8-10];又因为蓝铁矿(Fe3(PO4)2 •8H2O)稳定易获得(Ksp = 10−36)和经济价值可观[11-13],因此在磷回收方面受到研究人员的广泛关注。
现有的以蓝铁矿的形式回收磷的研究中,一种是从污泥中回收:周健[14]在pH值为7.00—7.40、ORP(氧化还原电位)为−450—−400 mV、铁磷离子物质的量比2.5∶1的条件下对人工培养剩余污泥的磷回收率约90%,纯度为43.32%;Li 等[15]在pH值为5.00—5.80、ORP为−105 mV的条件下对厌氧消化污泥的磷回收率为90%左右,纯度为55.70%;程翔[16]在pH为6.00—9.00、ORP为−420 mV、铁磷离子物质的量比3∶1的条件下对化粪池的磷回收率在96%左右。这些研究中,回收得到的产物中除蓝铁矿外还有污泥,对后续的蓝铁矿分离纯化不利;另一方面,从污泥中进行提取磷再在上清液中进行磷回收,增加了磷损失,会导致回收效率降低[17]。另一种是从污水中回收:Priambodo等[18]在pH为5.00—6.00、铁磷离子物质的量比2.5∶1的条件下对TFT-LCD含磷废水进行了磷回收,磷回收率为93%,纯度为63%。这种方式虽然避免了与污泥分离困难的问题,但所得纯度不高,会使得其与其他沉淀固相难分离。
鉴于目前鸟粪石磷回收工艺存在污水处理流程长、污泥量大、回收效率不理想,本课题组采用生物膜法(好氧/厌氧交替运行模式)直接在主流工艺中富集磷酸盐,且富磷溶液的磷浓度已经达到179.5 mg·L−1[19-20],并将得到的富磷溶液进行蓝铁矿法磷回收。此工艺系统不仅避免了从污泥中回收磷的磷损失和与污泥难分离问题,还避免了在现有研究集中的厌氧消化、发酵过程中的pH(<5.00)与蓝铁矿生成的pH(6.00—9.00)不同(Fe(Ⅱ)-P会溶解)的问题[17]。由于蓝铁矿在自然条件下的形成需要较高浓度的磷铁元素、还原性环境和较丰富的有机质及适中的pH条件(6.00—9.00)[21],并且干扰因素如SS、Ca2+、Mg2+、NH4+、S2-、Al3+ [22-23]在本实验用水中的浓度几乎为0,因此本研究以生物膜法高倍富集的磷溶液为对象,通过探讨pH、ORP、铁磷离子物质的量比对蓝铁矿的磷回收率、纯度的影响,并对回收产物进行XRD、SEM-EDS定性表征,确定以蓝铁矿的形式回收生物膜法富集的磷溶液中的磷的最优反应条件,为建立新型磷回收工艺提供技术依据。
-
反应装置由一个1 L的血清瓶(密封)和一个磁力搅拌器组成,装置内放有pH/ORP在线监测仪,反应装置内为厌氧和还原环境。所用实验仪器为X-射线衍射仪(XRD)(Bruker Advanced D8,德国BRUKER—AXS公司)、电感耦合等离子体发射光谱仪(ICP—OES)(ICPE-9000,日本岛津公司)、真空冷冻干燥机(Labconco,Labconco公司)、扫描电子显微镜—能谱(SEM-EDS)(Quanta FEG 250,美国FEI公司 (能谱,英国牛津公司))、光学显微镜(DM2500,莱卡公司)、紫外分光光度计(DR2800,美国哈希公司)、离心机(低速,卢湘仪公司)、磁力搅拌器(ZNCL-DS 5点,巩义予华公司)、在线监测仪(Multi 9620 IDS、德国WTW公司)。
-
实验用水采用生物膜法富集的磷溶液,正磷酸盐浓度大于50 mg·L−1,其pH值为7.00—8.00,ORP为150 mV左右,SS约为2 mg·L−1,Ca2+、Mg2+、
${\rm{NH}}_4^+ $ 皆小于10 mg·L−1,S2−、Al3+分别为0.50 mg·L−1、0.20 mg·L−1,COD为40—50 mg·L−1。实验试剂为七水合硫酸亚铁(FeSO4·7H2O) 购于天津市科密欧公司,抗坏血酸购于上海展云公司,以上试剂皆为分析纯。 -
常规指标测定方法。pH与ORP采用玻璃电极法进行测定;正磷酸盐(OP)与总磷(TP)采用钼酸铵分光光度法进行测定;钙镁铁等金属离子采用ICP—OES法进行测定;氨氮采用纳氏试剂比色法进行测定。
磷回收率和蓝铁矿纯度依次按式(1)和式(2)计算。
式中:PR为磷回收率,%; cPs为反应初始时溶液磷浓度, mg·L−1;cPe为反应结束时溶液磷浓度, mg·L−1;Pu为纯度(每克沉淀物中蓝铁矿的质量),%;mp为提取后上清液中的磷酸盐质量,g;Mp为P的物质的量质量,g·mol−1;Mv为蓝铁矿的物质的量质量,g·mol−1;mr为所提取的回收物的量,g。
蓝铁矿的定性方法。XRD:将沉淀物离心干燥、充分研磨后测XRD,通过判断回收产物图谱与蓝铁矿标准图谱的出峰位置的吻合度,定性回收产物的晶相;SEM-EDS:将沉淀物离心干燥后测SEM-EDS,通过观察晶体晶型与元素占比进行回收产物定性。
蓝铁矿的定量方法(磷的分级提取)。在本实验过程中用Hupfer法对反应结束后的沉淀物进行分级提取[14]。在Hupfer法中主要对磷做了五个分类,分别为不稳定性磷(labile-P);碳酸盐吸附性磷(MCO3-P);Fe、Al结合的磷以及有机物中磷;与Ca结合磷;残渣态磷(residual-P),提取方法如表1所示。
-
通过单因素分析法,在富磷溶液中投加七水合硫酸亚铁进行反应。分别进行探究pH对蓝铁矿磷回收率和纯度的影响;探究ORP对蓝铁矿磷回收率和纯度的影响;探究铁磷离子物质的量比对蓝铁矿磷回收率和纯度的影响。所有实验组均设置3个平行样。反应结束后,进行离心分离,上清液测定磷、铁浓度,确定磷回收率(见式1),回收产物冷冻干燥后进行定性分析(X射线衍射仪(XRD)、扫描电镜(SEM)、能量分散谱仪(EDS))、取0.1 g回收产物进行定量分析(纯度,见式2)。各实验如表2所示。
-
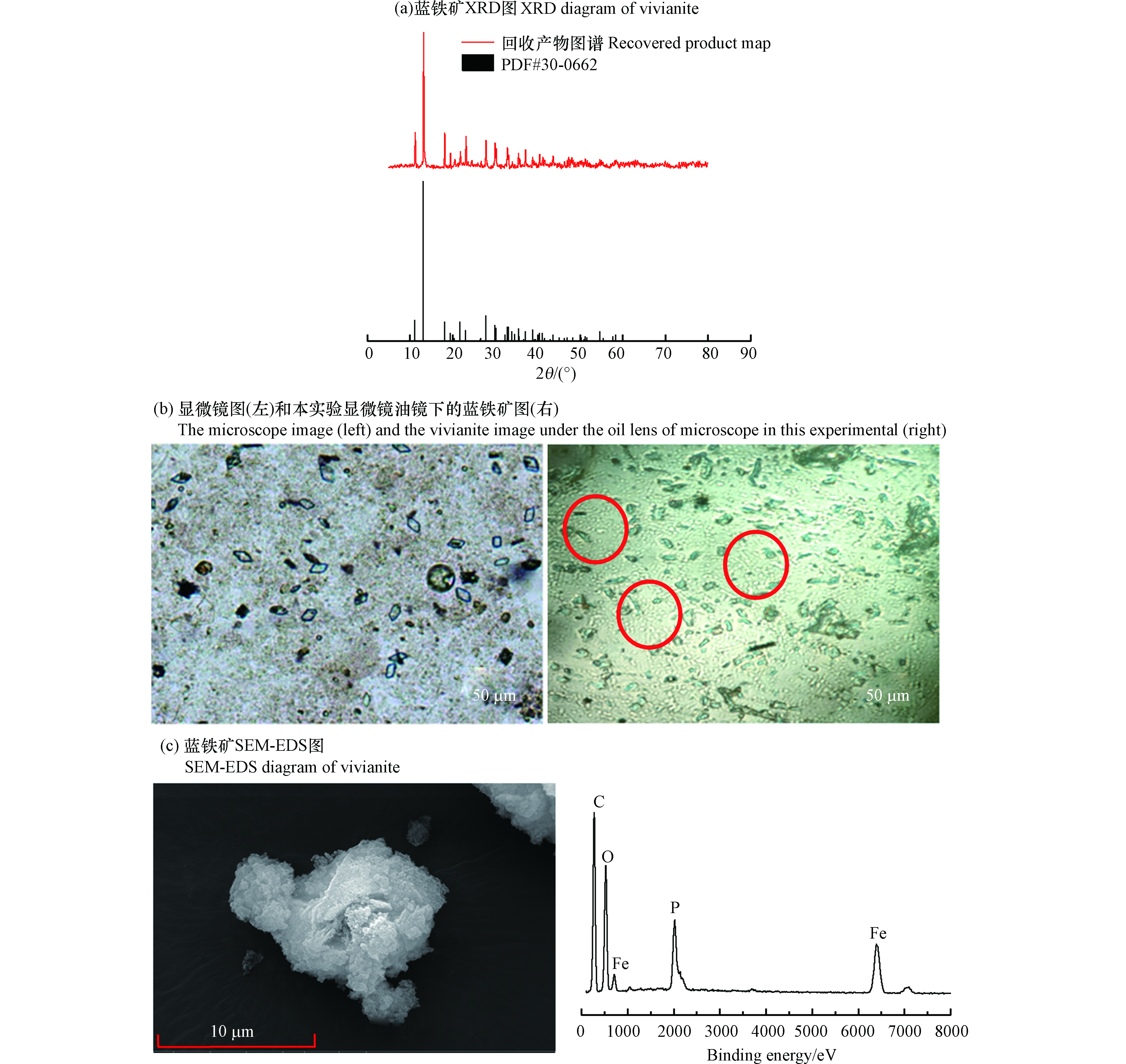
在原水的初始磷浓度为88.40 mg·L−1,反应体积1 L,ORP为−250— −100 mV,pH 7.00,n(Fe2+)∶n(
${\rm{PO}}_4^{3-} $ )=3∶1的条件下,研究了蓝铁矿法回收富磷溶液的有效性。结果发现,磷回收率为92%,这表明溶液中的磷大部分都转移到了沉淀物中,初步判断以蓝铁矿的形式回收生物膜法富集的磷是有效的。为了进一步判断沉淀物是否为蓝铁矿,本实验对得到的回收产物进行了XRD表征,结果如图1(a)所示。可以看到,回收产物图谱在衍射角为11.148°、13.144°等角度时出现了一系列的特征峰,经XRD专业分析软件jade分析发现,与蓝铁矿PDF标准图谱#30-0662比较吻合。该结果表明污泥粉末中生成了蓝铁矿晶体,且蓝铁矿晶体为该污泥粉末中唯一晶相。对回收产物进行了显微镜观察,如图1(b)所示。结果发现,本研究得到的显微镜图中的蓝色晶体呈棒状或菱形,与周健[14]所得到的蓝铁矿较为相近,为蓝铁矿的正常形状。通过对回收产物进行了SEM-EDS表征,如图1(c)所示,回收产物中的Fe、O、P含量在回收产物的占比都较高(除C外)。因为这3个元素是构成蓝铁矿的主要元素,所以从元素成分上来说,实验生成的沉淀物中的主要物质是蓝铁矿。综上所述,通过对回收产物的XRD、SEM、EDS表征,证实蓝铁矿法回收富磷溶液是有效的。
-
为进一步提高蓝铁矿的回收效果,需要对其生成条件进行优化。蓝铁矿法回收磷的首要作用是通过结晶的方式回收溶液中的磷,因此,高的磷回收率是本研究的前提。只有使蓝铁矿法回收磷的磷回收率处于较高水平,进一步优化蓝铁矿生成量才有意义。
-
从图2(a)中可以看出,磷回收率随pH的升高先增加再降低,在pH值为7.00—7.50时,磷回收率到达最高,为95%左右;蓝铁矿纯度随pH的增加先上升后下降,在pH 7.00附近时,蓝铁矿纯度达到最大,为87.60%,高于目前大多数现有报道[14-15,24-25]。由此可以看出,pH对蓝铁矿磷回收率和纯度的影响过程相同,并且将反应控制在pH7.00附近,可以同时使蓝铁矿磷回收率和纯度达到最高值。最佳pH值出现在7.00附近是因为在酸性条件下,生成的蓝铁矿会发生溶解,导致磷酸根离子重新溶于水中,从而使磷回收率和蓝铁矿纯度降低;碱性条件下氢氧根离子的增加会促进Fe(OH)2与Fe(OH)3的形成,减少亚铁离子与磷酸根离子的结合,从而使磷回收率和蓝铁矿纯度减少。本实验所得的最佳pH与周健[14]和Wilfert等[24]所得结论基本一致,但磷回收率(95%)大于周健(90%),蓝铁矿纯度也大于周健(43.32%)和Wilfert等(70%)的结果。由于本研究所用的富磷溶液的初始pH值为7.00—8.00,因此可以减少调pH需用的NaOH溶液和HCl溶液,降低了操作成本和复杂程度。
同时从图2(b)还可以看出,反应前后正磷酸盐和总磷浓度变化很接近,这说明参与反应的磷基本上是正磷酸盐,与周健[14]所说一致。而本实验用水中的正磷酸盐与总磷浓度接近,说明用蓝铁矿法回收本实验用水中的磷的磷回收率和蓝铁矿纯度理论上会很高。
-
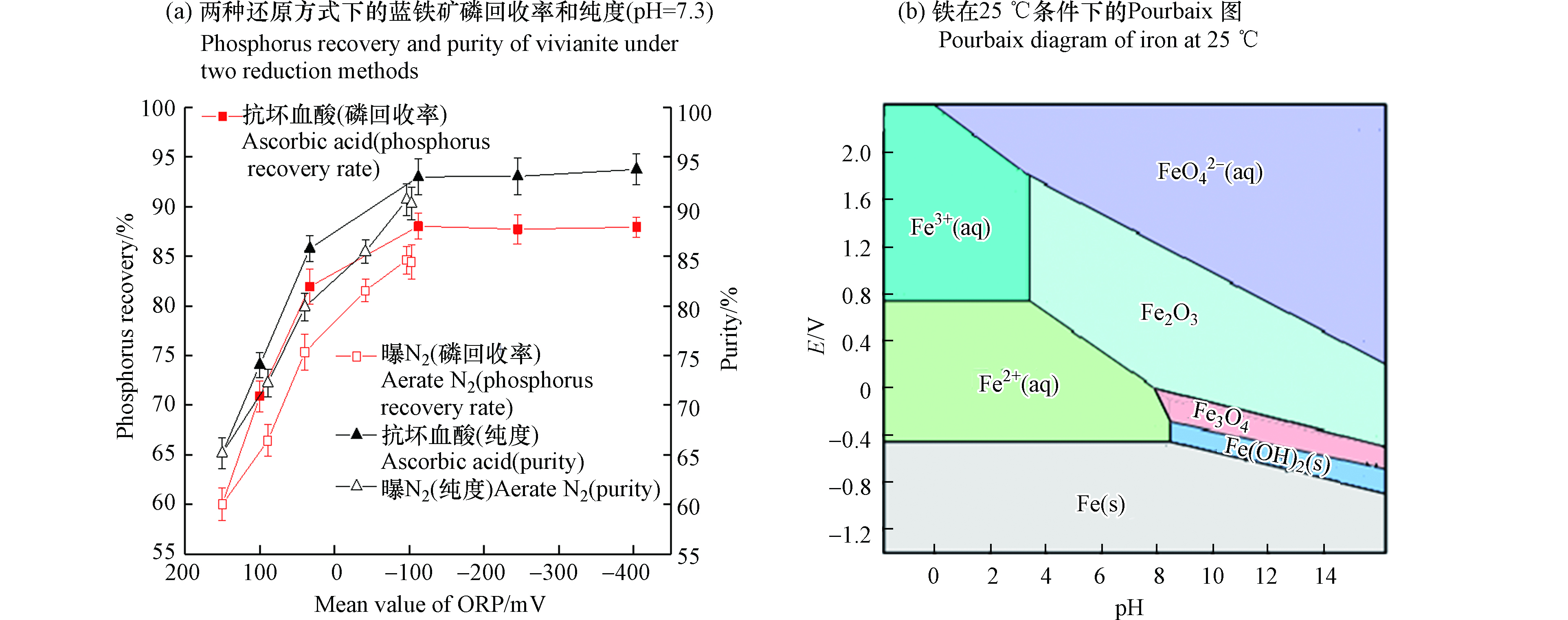
从图3(a)中可以看出,加抗坏血酸和曝N2方式较对照组(ORP为150 mV时)均能使磷回收率和蓝铁矿纯度随着ORP的降低升高。当ORP达到-100 mV时,达到最高值,分别为93%(加抗坏血酸)、90%(曝N2)和88%(加抗坏血酸)、85.60%(曝N2),两者相差较小,之后趋于稳定。加抗坏血酸比曝N2方式能使ORP降得更低。
综上所述,ORP的降低会使得蓝铁矿磷回收率和蓝铁矿纯度升高。结合铁的Pourbaix图(图3(b))可知,一定范围内,同样的pH波动强度,ORP越低,铁越能更好的保持在亚铁离子状态[26]。由于易与铁离子结合的S2-浓度很低,导致铁离子几乎只和磷酸根离子结合,所以将铁更好地保持在亚铁离子状态可以尽量避免FePO4沉淀的生成,更有利于生成蓝铁矿。因此,两种还原方式均能使磷回收率和蓝铁矿纯度增高且效果接近。但从工艺成本和工艺操作上来讲,曝N2的方法在能保证较高的磷回收率和蓝铁矿纯度的基础上,既能降低成本,又能降低操作难度,在后续的实际工艺研究中推荐使用曝N2的方法。
-
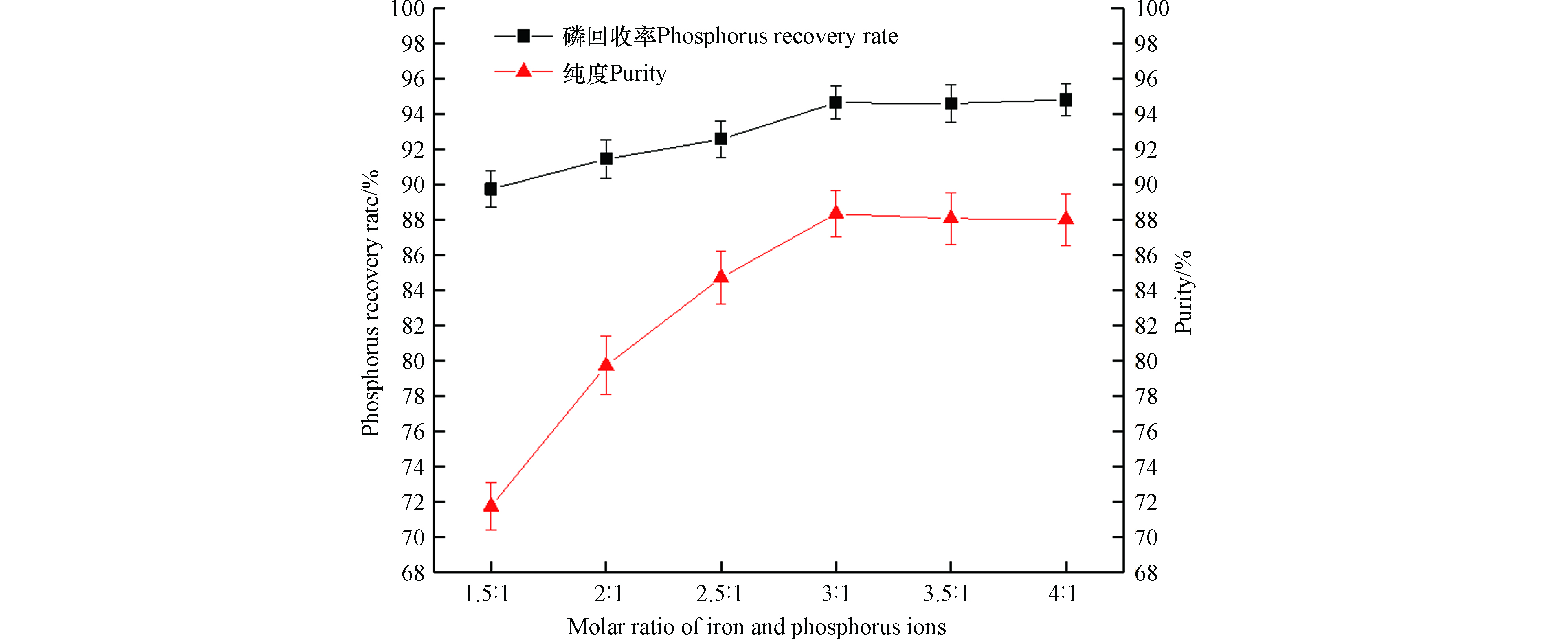
从图4中可以看出,磷回收率随铁磷离子物质的量比的增大而先增大后趋于稳定,当,铁磷离子物质的量比为3:1时磷回收率最大,接近95%;蓝铁矿纯度随铁磷离子物质的量比的增大而先增大后后趋于稳定,当铁磷离子物质的量为3∶1时蓝铁矿纯度最大,为88.35%。在同样的铁磷离子物质的量比(2.5∶1)下,本研究的磷回收率与Priambodo等[18] (93%)相近,蓝铁矿纯度(88.35%)远大于Priambodo等(63%)。结合磷回收率和蓝铁矿纯度的情况,可以得出,当铁磷离子物质的量比为3:1时,磷回收率和蓝铁矿纯度最大。
这种情况的出现可能跟过饱和度有关:由于蓝铁矿是一种晶体,因此它在水溶液中的析出与结晶过程有关,而结晶过程的推动力是过饱和度(大于1)。根据过饱和度定义—处于过饱和状态的离子在溶液中浓度与晶体浓度积常数之比[27-28],由于温度在水环境中变化范围较小[21],所以在蓝铁矿生成的溶液体系中溶液达到平衡状态时的蓝铁矿溶度积变化也较小,因此过饱和度主要受反应离子浓度的影响。所以实验中在磷浓度相同的情况下,提高亚铁离子浓度可以增大过饱和度,使结晶反应更多地向正方向进行,更多地回收磷。但是过饱和度过大,不仅会增加投药量,使得反应成本增加,药剂浪费,还会使晶体自发成核,形成更多的小晶体,引起晶体粒径不均匀[29],多余的铁的增加还会更多地形成氢氧化铁,通过吸附作用加大与蓝铁矿竞争磷,而且磷回收率和蓝铁矿纯度也不再增大。因此,铁磷物质的量比为3:1时可以更多地生成蓝铁矿。
-
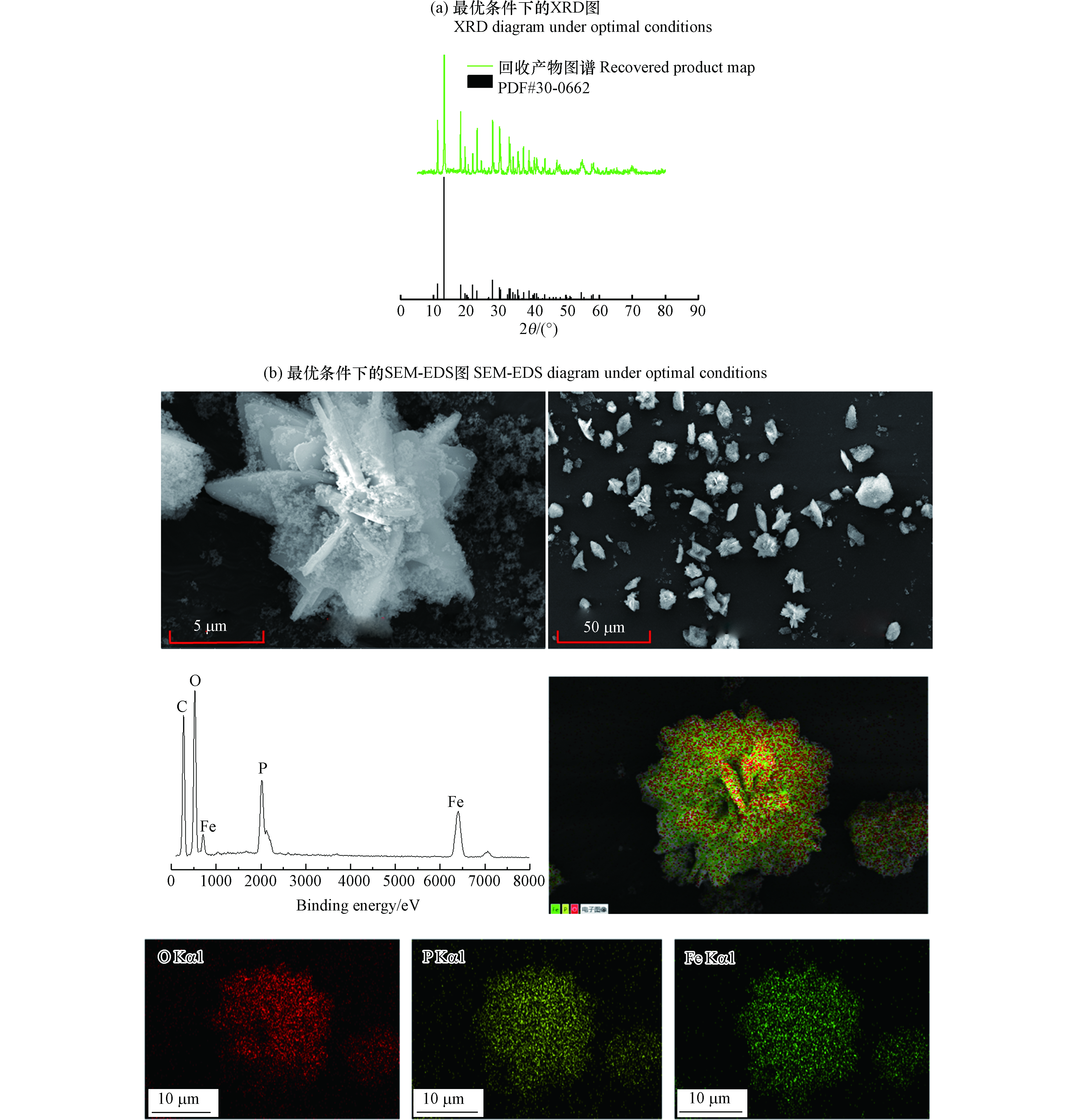
在上述实验得到的最优反应条件下,即pH值为7.00—7.50、ORP降到−100 mV左右、铁磷离子物质的量比为3∶1时,对生成的回收产物进行了XRD、SEM-EDS表征。
从图5(a)中可以看出,回收产物图谱在衍射角为11.148°、13.144°等角度时出现的一系列的特征峰与蓝铁矿PDF标准图谱#30-0662的出峰位置一致。该结果表明在此最优条件下生成的主要晶体相为蓝铁矿晶体。从图5(b)中的EDS点扫图可以看出,Fe、P、O构成蓝铁矿的主要元素的相对含量占总元素的比例较高。其次,分别对比5 μm和50 μm下的扫描电镜图发现,最优条件下生成的蓝铁矿晶体的晶型是公认的板状。从mapping图中可以看出,对晶体颗粒进行Fe、P、O等元素的面扫描结果的颜色图的形状接近于扫描对象,并且亮度较高,说明Fe、P、O元素在晶体上几乎处处分布,占比很高。
因此,结合上述表征方法可以得出,最佳条件下生成的回收产物中的主要晶相是蓝铁矿,且其晶型较好。
-
本研究通过探讨pH、ORP、铁磷离子物质的量比对蓝铁矿磷回收率与纯度的影响,得到磷回收新工艺后端的蓝铁矿法回收富磷溶液的工艺条件,结论如下:
(1)蓝铁矿法回收高倍富磷溶液的磷回收率达到了92%,对其回收产物的定性分析表明主要晶相为蓝铁矿,这说明以蓝铁矿的方式能有效回收富磷溶液。这推动了城市污水磷回收新工艺(先富集再回收)的建立。
(2)在pH值为7.00—7.50、ORP降到−100 mV左右、铁磷离子物质的量比为3∶1时,蓝铁矿法的磷回收率(约95%)和回收得到的蓝铁矿最多(88.35%),并且定性表征的结果也表明蓝铁矿生成情况较好。
(3)由于加抗坏血酸在磷回收率和蓝铁矿纯度上略大于曝N2,而且可以使溶液的ORP更低,更有利于蓝铁矿的生成,因此从实验最优化层面考虑,加抗坏血酸的方式更适合;从实际工艺运行层面考虑,曝N2的方法更为合适。这为后期建立磷回收工艺系统提供了研究思路与方向。
蓝铁矿法回收生物膜富集的城市污水中的磷
Vivianite crystallization method to recover phosphorus in municipal sewage enriched by biofilm method
-
摘要: 磷资源因具有不可再生性和污染性,需要进行磷回收。目前污水处理厂使用的传统磷回收方法多是鸟粪石法,但存在利用价值低、磷回收率不高等缺点。本课题组提出了一种新的磷回收工艺系统,即在生物膜法高倍富集城市污水中的磷的基础上,通过蓝铁矿的形式回收磷,具有经济价值高、磷回收率高等优势。本实验研究主要通过探讨蓝铁矿法回收富磷溶液的过程中获得高磷回收率和蓝铁矿纯度的反应条件,并进行X-射线衍射仪(XRD)、扫描电子显微镜—能谱(SEM-EDS)定性表征最优条件下的蓝铁矿。结果表明,最优反应条件是pH值为7.00—7.50、氧化还原电位(ORP)降到−100 mV左右、铁磷离子物质的量物质的量比为3∶1,此时蓝铁矿法的磷回收率约为95.00%,蓝铁矿纯度为88.35%,蓝铁矿为沉淀回收物中主要晶相且晶型较好。Abstract: Phosphorus resources need to be recycled due to their non-renewability and pollution. At present, the traditional phosphorus recovery method used in sewage treatment plants is mostly struvite method, but there are disadvantages such as low utilization value and low phosphorus recovery rate. Our research group proposed a new phosphorus recovery process system, which is based on the high enrichment of phosphorus in urban sewage by the biofilm method, and recovers phosphorus in the form of vivianite, which has high economic value and phosphorus recovery rate. Higher advantage. This experimental study mainly explores the reaction conditions for obtaining high phosphorus recovery rate and vivianite purity in the process of recovering phosphorus-rich solution by vivianite crystallization, and performs XRD and SEM-EDS to qualitatively characterize vivianite under optimal conditions. The results show that the optimal reaction conditions are pH 7.00—7.50, ORP drops to about −100 mV, and iron-phosphorus ion molar ratio of 3∶1. At this time, the phosphorus recovery rate of the vivianite crystallization is about 95.00%, the purity of vivianite is 88.35% and vivianite is the main crystal phase in the recovered precipitate and has a good crystal form.
-
Key words:
- phosphorus recovery /
- vivianite /
- phosphorus-rich solution /
- crystallization
-

-
表 1 Hupfer提取法
Table 1. Hupfer extraction method
磷组分
Phosphorus components提取剂Extractant 浓度/(mol·L−1)
Concentration提取时间/min
Extraction duration固液比(mL∶gDS)
Solid-to-liquid Ratiolabile-P 去离子水 — 20 60∶1 MCO3-P 醋酸 0.10 80 60∶1 Fe-P,Al-P,Org-P NaOH 1.00 1080 60∶1 Ca-P HCl 0.50 1080 60∶1 Residual-P HNO3 14.4 60 60∶1 表 2 实验方案表
Table 2. Experimental protocol table
影响因素
Influencing factor富磷溶液磷浓度/(mg·L−1)
Phosphorus concentration in phosphorus-rich solutionpH ORP/mV 铁磷离子物质的量比
Molar ratio of iron and phosphorus ionspH 86.5 6.00、6.50、7.00、7.50、8.00、8.50、9.00 −250— −100 3∶1 ORP 80.2 实验的最佳pH 加抗坏血酸和曝N2① 3∶1 铁磷离子物质的量比 86.8 实验的最佳pH ORP实验的最佳ORP 1.5∶1、2∶1、2.5∶1 、3∶1、3.5∶1、4∶1 注:①由于本实验用水的COD很低,很难维持金属还原菌的生长,使得无法通过金属还原菌维持反应时的还原环境。因此,本实验拟采用加抗坏血酸和曝N2两种物化手段使溶液ORP降低,保持还原环境。
Note: ① Because the COD of the water in this experiment is very low, it is difficult to maintain the growth of metal reducing bacteria, which makes it impossible to maintain the reduction environment during the reaction by metal reducing bacteria.Therefore, two physical and chemical means-adding ascorbic acid and blowing N2 gas, were adopted in this experiment to reduce the ORP of the solution and maintain the reduction environment. -
[1] SUN D Q, HALE L, KAR G, et al. Phosphorus recovery and reuse by pyrolysis: Applications for agriculture and environment [J]. Chemosphere, 2018, 194: 682-691. doi: 10.1016/j.chemosphere.2017.12.035 [2] MAYER B K, BAKER L A, BOYER T H, et al. Total value of phosphorus recovery [J]. Environmental Science & Technology, 2016, 50(13): 6606-6620. [3] HUANG H M, LIU J H, DING L. Recovery of phosphate and ammonia nitrogen from the anaerobic digestion supernatant of activated sludge by chemical precipitation [J]. Journal of Cleaner Production, 2015, 102: 437-446. doi: 10.1016/j.jclepro.2015.04.117 [4] 李子阳, 陆东亮, 华天予, 等. 蓝藻发酵液中氮磷回收及其作为反硝化碳源研究 [J]. 环境化学, 2020, 39(12): 3562-3573. LI Z Y, LU D L, HUA T Y, et al. Recovery of nitrogen and phosphorus from fermentation liquid of cyanobacteria and its application as a carbon source for denitrification [J]. Environmental Chemistry, 2020, 39(12): 3562-3573(in Chinese).
[5] HAO X D, WANG C C, van LOOSDRECHT M C M, et al. Looking beyond struvite for P-recovery [J]. Environmental Science & Technology, 2013, 47(10): 4965-4966. [6] HUANG H M, ZHANG D D, LI J, et al. Phosphate recovery from swine wastewater using plant ash in chemical crystallization [J]. Journal of Cleaner Production, 2017, 168: 338-345. doi: 10.1016/j.jclepro.2017.09.042 [7] LIN L, LI R H, LI Y, et al. Recovery of organic carbon and phosphorus from wastewater by Fe-enhanced primary sedimentation and sludge fermentation [J]. Process Biochemistry, 2017, 54: 135-139. doi: 10.1016/j.procbio.2016.12.016 [8] POFFET M S, KÄSER K, JENNY T A. Thermal runaway of dried sewage sludge granules in storage tanks [J]. Chimia International Journal for Chemistry, 2008, 62(1): 29-34. [9] RASMUSSEN H, NIELSEN P H. Iron reduction in activated sludge measured with different extraction techniques [J]. Water Research, 1996, 30(3): 551-558. doi: 10.1016/0043-1354(95)00203-0 [10] CABEZA R, STEINGROBE B, RÖMER W, et al. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments [J]. Nutrient Cycling in Agroecosystems, 2011, 91(2): 173-184. doi: 10.1007/s10705-011-9454-0 [11] JOHNSTON A E, RICHARDS I R. Effectiveness of different precipitated phosphates as phosphorus sources for plants [J]. Soil Use and Management, 2003, 19(1): 45-49. doi: 10.1111/j.1475-2743.2003.tb00278.x [12] LI Y C, GENG G W, HAO J H, et al. Optimized synthesis of LiFePO4 composites via rheological phase assisted method from FePO4 with acetic acid as dispersant [J]. Electrochimica Acta, 2015, 186: 157-164. doi: 10.1016/j.electacta.2015.10.121 [13] RAO S R, VARADARAJU U V. Hydrothermal synthesis of LiFePO4 nanorods composed of nanoparticles from vivianite precursor and its electrochemical performance for lithium ion battery applications [J]. Bulletin of Materials Science, 2015, 38(5): 1385-1388. doi: 10.1007/s12034-015-1025-6 [14] 周健. 从厌氧消化污泥中回收磷—蓝铁矿形成机制初探[D]. 北京: 北京建筑大学, 2018: 103. ZHOU J. Recovery of phosphorus from anaerobic digested sludge-a preliminary study on the formation mechanism of vivianite[D]. Beijing: Beijing University of Civil Engineering and Architecture, 2018: 103(in Chinese).
[15] LI R H, LI X Y. Recovery of phosphorus and volatile fatty acids from wastewater and food waste with an iron-flocculation sequencing batch reactor and acidogenic co-fermentation [J]. Bioresource Technology, 2017, 245: 615-624. doi: 10.1016/j.biortech.2017.08.199 [16] 程翔. 类水滑石吸附和蓝铁石沉淀回收污水中磷的研究[D]. 哈尔滨: 哈尔滨工业大学, 2010: 141. CHENG X. Phosphorus recovery from sewage water by layered double hydroxides as an adsorbent and by vivianite precipitation[D]. Harbin: Harbin Institute of Technology, 2010: 141(in Chinese).
[17] WU Y, LUO J Y, ZHANG Q, et al. Potentials and challenges of phosphorus recovery as vivianite from wastewater: A review [J]. Chemosphere, 2019, 226: 246-258. doi: 10.1016/j.chemosphere.2019.03.138 [18] PRIAMBODO R, SHIH Y J, HUANG Y H. Phosphorus recovery as ferrous phosphate (vivianite) from wastewater produced in manufacture of thin film transistor-liquid crystal displays (TFT-LCD) by a fluidized bed crystallizer (FBC) [J]. RSC Advances, 2017, 7(65): 40819-40828. doi: 10.1039/C7RA06308C [19] ZHANG H, BI Z, PAN Y, et al. Enhanced phosphorus storage in suspended biofilm by increasing dissolved oxygen [J]. Science of the Total Environment, 2020, 722: 137876. doi: 10.1016/j.scitotenv.2020.137876 [20] CORDELL D, DRANGERT J O, WHITE S. The story of phosphorus: Global food security and food for thought [J]. Global Environmental Change, 2009, 19(2): 292-305. doi: 10.1016/j.gloenvcha.2008.10.009 [21] 郝晓地, 周健, 王崇臣, 等. 污水磷回收新产物: 蓝铁矿 [J]. 环境科学学报, 2018, 38(11): 4223-4234. HAO X D, ZHOU J, WANG C C, et al. New product of phosphorus recovery—Vivianite [J]. Acta Scientiae Circumstantiae, 2018, 38(11): 4223-4234(in Chinese).
[22] 郝晓地, 周健, 王崇臣. 探究污泥厌氧消化系统中蓝铁矿生成的干扰因子 [J]. 中国给水排水, 2018, 34(23): 1-7. HAO X D, ZHOU J, WANG C C. Determination of interference factors of vivianite formation in anaerobic digestion of excess sludge [J]. China Water & Wastewater, 2018, 34(23): 1-7(in Chinese).
[23] ROTHE M, KLEEBERG A, HUPFER M. The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments [J]. Earth-Science Reviews, 2016, 158: 51-64. doi: 10.1016/j.earscirev.2016.04.008 [24] WILFERT P, DUGULAN A I, GOUBITZ K, et al. Vivianite as the main phosphate mineral in digested sewage sludge and its role for phosphate recovery [J]. Water Research, 2018, 144: 312-321. doi: 10.1016/j.watres.2018.07.020 [25] FROSSARD E, BAUER J P, LOTHE F. Evidence of vivianite in FeSO4-flocculated sludges [J]. Water Research, 1997, 31(10): 2449-2454. doi: 10.1016/S0043-1354(97)00101-2 [26] KOPELIOVICH D. Pourbaix diagrams [EB/OL]. [2012-5-31]. http:// www. substech. com/dokuwiki/doku. php?id=pourbaix_diagrams. [27] DOYLE J D, PARSONS S A. Struvite formation, control and recovery [J]. Water Research, 2002, 36(16): 3925-3940. doi: 10.1016/S0043-1354(02)00126-4 [28] REGY S, MANGIN D, KLEIN J P, et al. Phosphate recovery by stuvite precipitation in a stirred reactor [EB/OL]. [2009-10–9]. http://www. nhm. ac. uk/ research-curation/ research/ projects / phosphate-recovery/LagepReport. pdf , 2001. [29] 马晓迅, 夏素兰, 曾庆荣. 化工原理[M]. 北京: 化学工业出版社, 2010:456. MA X X, XIA S L, ZENG Q R. Principles of chemical engineering [M]. Beijing: Chemical Industry Press, 2010(in Chinese):456(in Chinese).
-



 下载:
下载: