-
目前,铜离子(Cu2+)已被美国环境保护署(EPA)列入需要优先控制的污染物,规定其在水体中浓度不得超过1.3 mg·L−1,世界卫生组织(WHO)标准中饮用水Cu2+的限制浓度为2.0 mg·L−1[1]。我国生活饮用水标准中总铜浓度为1.0 mg·L−1, 工业废水三级标准中总铜排放标准是1.0 mg·L−1[2]。为避免废水中Cu2+累积造成的污染,对废水中Cu2+污染的控制至关重要。
化学沉淀法、离子交换法、生物法和吸附法等是水体中重金属离子去除的主要方法[3]。其中, 吸附法因具备成本低、操作简便、工序简单、去除效果佳且无二次污染等优点备受关注。吸附剂的选择是吸附法成功实施的关键。活性炭、分子筛、硅胶、活性氧化铝等都是优异的吸附剂,但是成本较高限制上述吸附剂的广泛使用[4]。
近年来,生物炭作为吸附剂引起了研究者极大的关注[5-6]。然而,研究显示[7-11],原状生物炭对废水中污染物的吸附容量有限,需要进行定向的改性以进一步提升其吸附效果。生物炭改性的方法包括化学酸碱改性[12- 13]、金属改性[14-15]、物理改性[16]、矿物质负载改性[17]等。改性主要目的是增大生物炭的比表面积、制造更大孔隙、丰富表面的官能团、完备芳香结构和引入磁性组分等。
目前,氮改性生物炭逐渐引起了更多的研究,主要是用作催化剂降解废水中的污染物,而作为吸附剂的研究报道却较少。氮改性如何影响生物炭的表面形貌、孔隙结构、元素组成和官能团类型等仍有待进一步探究。Lian等[18]研究发现NH3改性玉米秸秆生物炭对酸性橙7和亚甲基蓝染料的吸附量可达292 mg·g−1和436 mg·g−1, 是原状生物炭吸附量的15—20倍。Yang等[19]研究发现氨基生物炭对铜离子的吸附量为(15.95±0.15) mg·g−1, 是原状生物炭吸附量的5倍多((4.14±0.11) mg·g−1),因为氨基的引入强化了生物炭对铜离子的络合作用。侯素珍等[20]研究发现在pH=3,温度为20 ℃, 投加量为0.5 g·L−1, Cr(Ⅵ) 初始浓度20 mg·L−1条件下, 反应5 min和60 min后氨基改性生物炭负载纳米零价铁对Cr(Ⅵ) 的去除率可分别达到87.1%和98.8%。然而上述研究存在制备成本较高、制备工艺复杂以及生物炭吸附机理探究不深等问题。因此,需要采取其它改性方法进一步提升生物炭对铜离子吸附效果。无毒、低成本、操作简便的尿素可作为生物炭改性的氮源选择,尿素改性不仅可增加生物炭的氮基团数量和电荷密度[21],优化生物炭对污染物的吸附性能,还可有效降低改性成本。
本文以水稻秸秆为对象,在700 ℃热解制备原状生物炭(RSBC)。同时,以尿素为氮改性剂,制备氮改性生物炭(N-RSBC)。通过仪器分析表征研究氮改性对生物炭理化性质的影响,考察pH、离子类型和强度对废水中Cu2+的吸附影响,并结合吸附等温线和吸附动力学实验,探究生物炭对废水中Cu2+的吸附机理。研究希冀为水稻秸秆的资源化和废水中Cu2+的去除提供理论依据和技术支撑。
-
水稻秸秆取自安徽省合肥市郊区某农田。将取回的水稻秸秆洗净去除表面杂质,烘干后粉碎,备用。称取一定量的秸秆于石英舟中,将石英舟放置于管式炉中热解,热解温度为700 ℃,升温速率为5 ℃·min−1,达到700℃后停留2 h,热解全程通氮气保护。冷却至室温后,取出原状生物炭,标记为RSBC。称取5 g秸秆和6 g尿素于烧杯中,加入100 mL纯水,置于磁力搅拌器中搅拌24 h,80℃烘干得到混合物后,将混合物置于马弗炉中,700℃热解,升温速率为5 ℃·min−1,保温2 h,冷却至室温后,取出氮改性生物炭,标记成N-RSBC。用纯水洗涤RSBC和N-RSBC至中性后烘干,球磨1 min后制得颗粒均匀的生物炭(粒径范围180—125 μm),用于后续的表征和吸附试验。
-
为研究氮改性对生物炭理化性质的影响,对生物炭进行了全面表征。利用扫描电子显微镜(SEM, SU-8010, Hitachi, Japan)表征秸秆生物炭的表面形貌。通过比表面积及孔径分布仪(Micromeritics, Tristar II 3020, USA)测定生物炭的比表面积和孔径分布。生物炭中C、H、N元素的含量采用元素分析仪 (Vario Micro Cube, Elementar, Germany) 进行测定,灰分含量根据700℃煅烧总量损失,O含量采用差减法,即100%-C(%)-H(%)-N(%)-ash(%)。采用zeta电位仪 (Zeta potentiometer, Delsa Max PRO, Beckman Coulter, USA) 测定生物炭的zeta电位。采用X射线衍射仪 (XRD, Bruker D8 Advance) 测定生物炭的矿物质及晶体结构。运用傅里叶红外光谱分析仪 (FTIR, Nicolette is50, Thermo Fourier, USA) 测定生物炭的官能团类型。通过X 射线光电子能谱 (XPS, Thermo-VG Scientific, ESCALAB250, USA) 测定生物炭表面的化学元素组成及价态。
为探究生物炭中官能团在吸附Cu2+中的作用,对吸附Cu2+后的生物炭进行FTIR和XPS表征(吸附动力学之后的样品冷冻干燥)。
-
吸附等温线。在50 mL离心管中加入20 mL Cu2+溶液(浓度范围10—60 mg·L−1),调至pH值为6.0,分别加入0.02 g RSBC和N-RSBC(固液比 1∶1 g·L−1, 固液比的选择基于预实验结果、吸附效果和经济性考虑),然后将离心管置于恒温摇床 (HZQ-X160, 金坛市杰瑞尔电器有限公司)中振荡24 h,摇床温度设定为25℃,振荡速度为150 r·min−1,之后离心(离心速度为4000 r·min−1,离心时间为5 min),取样品上清液,过0.45 μm滤膜后,采用原子吸收分光光度计(德国Jena,ZEEnit 700P, 乙炔-空气火焰,测定波长324.6 nm)测定上清液中的Cu2+浓度。
吸附动力学。在250 mL锥形瓶中放置150 mL Cu2+溶液(浓度为10、30、50 mg·L−1),调至pH 6.0,分别加入0.15 g RSBC和N-RSBC,然后将锥形瓶置于恒温摇床中振荡,摇床温度设定为25℃,振荡速度为150 r·min−1,动力学取样时间设定0、5、10、20、30、60、120、240、360、480、600 min,取出样品后过0.45 μm滤膜,利用原子吸收分光光度计测定溶液中的Cu2+浓度。
pH影响。在50 mL离心管中放入20 mL Cu2+溶液(浓度为30 mg·L−1),采用0.1 mol·L−1 HCl或0.1 mol·L−1 NaOH调节溶液pH值分别为2.0、3.0、4.0、5.0和6.0,之后分别加入0.02 g RSBC和N-RSBC,振荡、离心、过膜、测定同上。生物炭对Cu2+的去除率计算见公式(1)。
其中,R为去除率,%;C0、Ce为溶液初始和平衡时刻的Cu2+浓度(mg·L−1 )。
单一共存离子类型及强度影响。在50 mL离心管中放入20 mL Cu2+溶液(浓度为30 mg·L−1),分别加入不同类型和浓度的共存离子,分别选择Na+、K+、Ca2+、Mg2+作为单一共存离子,离子浓度分别为10、30、50 mg·L−1,之后分别加入0.02 g RSBC和N-RSBC,振荡、离心、过膜、测定步骤同上。
每个实验重复3次,数据给出的形式是平均值±标准偏差。
-
采用拟一级动力学方程[19](2)和拟二级动力学方程[22](3)对吸附过程进行的拟合,方程式如下所示:
式中,qt和qe分别为t时刻和吸附平衡时Cu2+的吸附量 (mg·g−1) ; t为吸附时间 (min) ; k1和k2分别为拟一级 (min−1) 和拟二级速率常数[g·( mg·min) −1]。
采用Langmuir模型[23](4)和Freundlich模型[24](5)进行吸附等温线进行拟合,方程式如下所示:
式中, bL为Langmuir 常数 (L·mg−1) ; Q0为吸附量 (mg·g−1) ; KF为Freundlich 常数; 1 /n是反映吸附亲和力的常数。
-
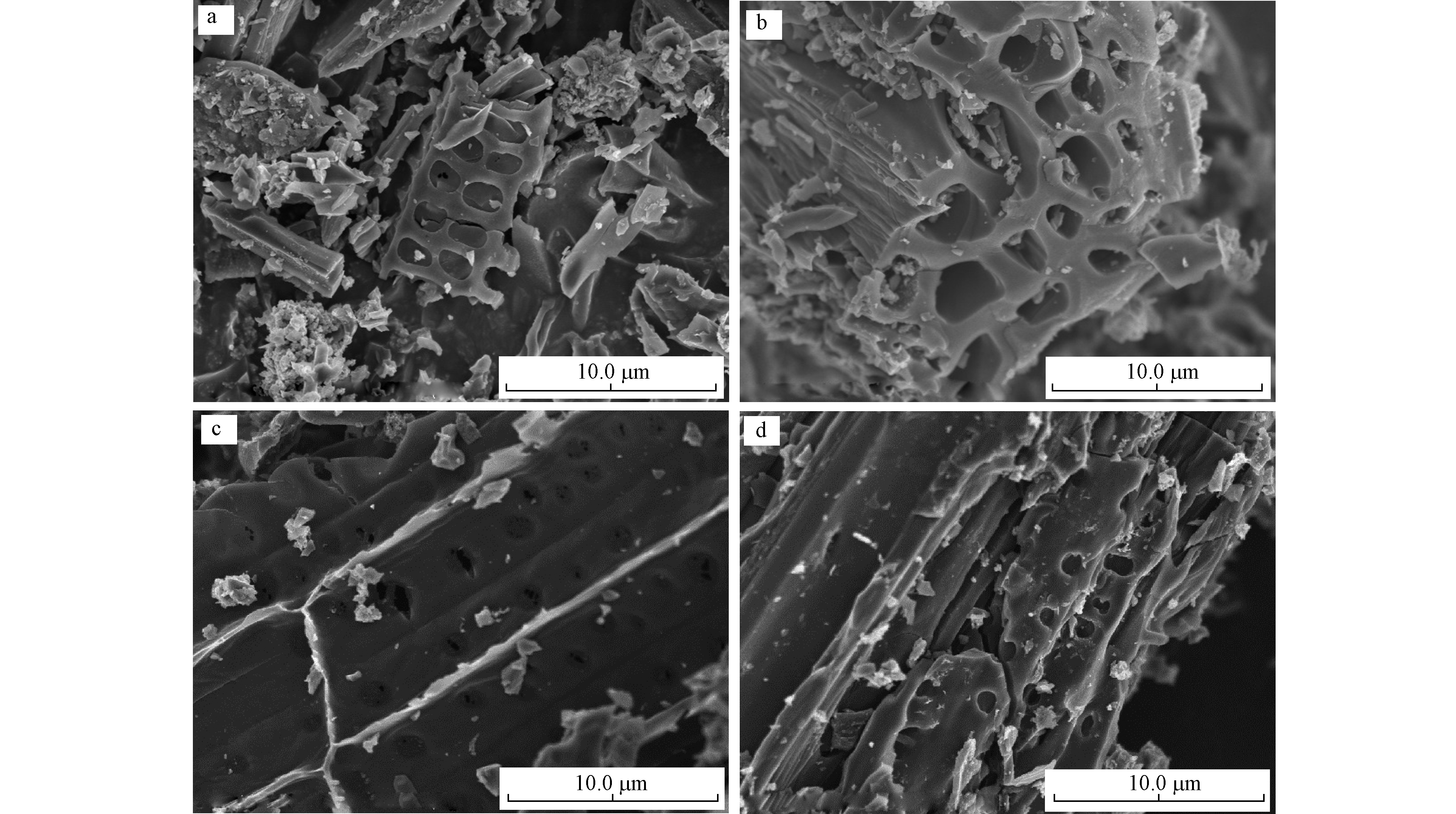
图1显示的是RSBC和N-RSBC的SEM图。由图1(a)和(b)可知,原状生物炭表面粗糙,不光滑,仍保留着大量碎片化的杆状、梗状组分,并含有大量颗粒,说明水稻秸秆已经炭化分解,并形成了较为发达的孔隙结构。与原状生物炭不同的是,氮改性生物炭的表面相对光滑,炭化固体之间粘结较为紧密,孔隙结构被堵塞(见图1(c)和(d))。可见,氮改性之后,生物炭的表面形貌和孔隙结构发生了改变。
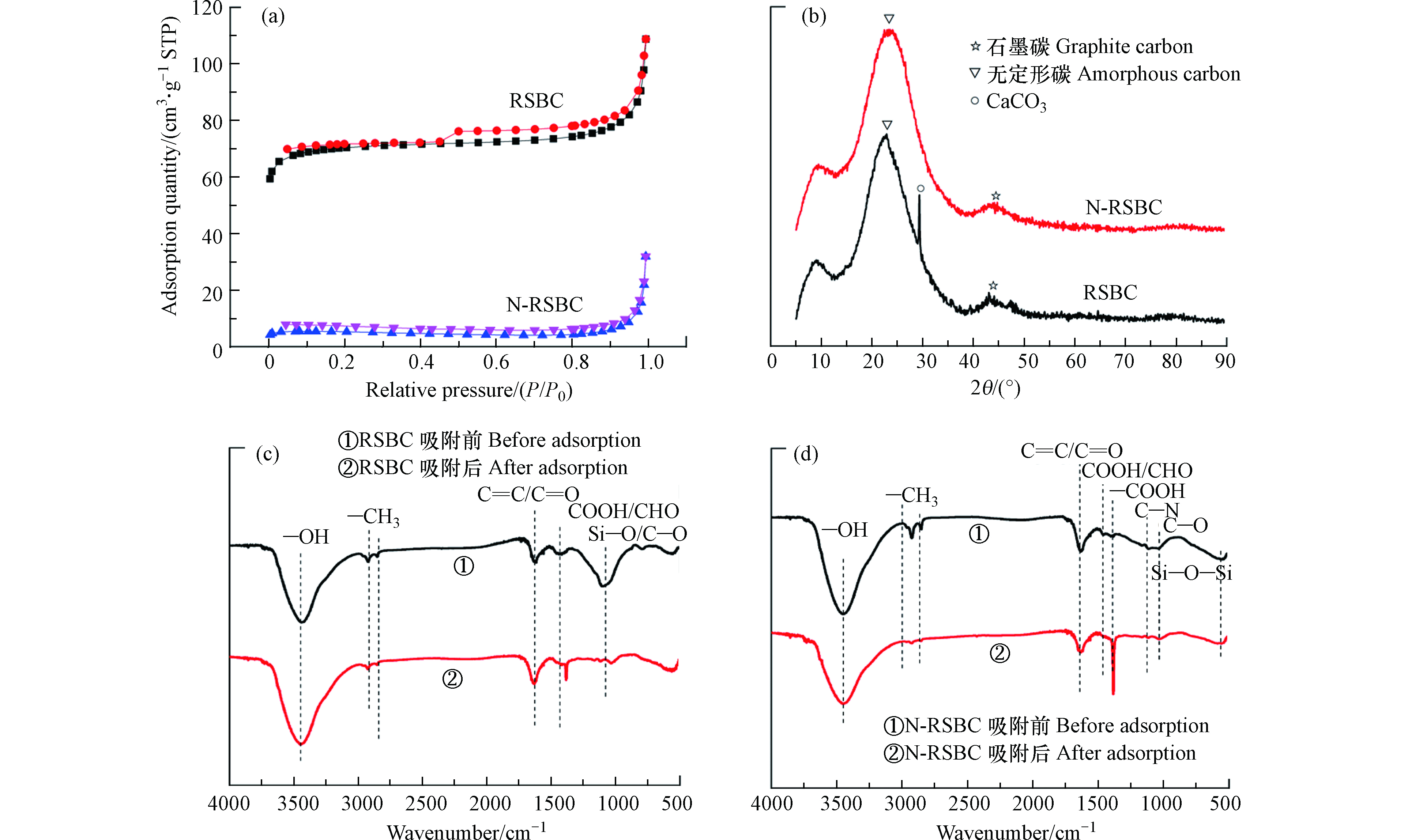
RSBC和N-RSBC的N2吸附-解吸等温线如图2(a)所示。RSBC的N2吸附-解吸等温线呈现明显的IV型吸附等温线,表明原状生物炭具有多孔结构,在吸附-解吸曲线之间形成了H4型闭合环,该型回滞环多出现在微孔和中孔混合的吸附剂上和含有狭窄的裂隙孔的固体中[21]。然而,N-RSBC的吸附-解吸等温线没有闭合,说明没有形成孔隙结构,这与SEM的观察结果是一致的。
表1显示的RSBC和N-RSBC的比表面积和孔径参数。由表1可知,改性之后,生物炭的比表面积、总孔体积和微孔体积分别从234.3874 m2·g−1、0.133822 cm3·g−1、0.094230 cm3·g−1降低到16.9736 m2·g−1、 0.019148 cm3·g−1、 0.009875 cm3·g−1。氮改性之后生物炭的平均孔径从2.28 nm增加到4.51 nm。可见,氮改性不利于生物炭比表面积的增大和孔隙结构的形成(与尿素作为改性剂和热解温度有关),主要是因为氮改性降低了生物炭中碳纤维之间的孔隙,或导致样品的剥落程度较低或氮的掺杂导致了生物炭结构高度无序和褶皱结构[25-26]。
RSBC和N-RSBC元素组成、原子比、灰分含量及zeta电位列于表2。由表2可知,生物炭的主要组成是C和O。灰分主要组成是秸秆生物炭中Ca、Mg、Na、K和Si等矿物质。氮改性之后生物炭中的氮含量从0.22%增加到7.33%,说明氮已经进入到生物炭的碳基质中,氮改性是可行和有效的。生物炭的原子比H/C、O/C和(N + O)/ C分别指示生物炭的芳香性、亲水性和极性大小。H/C比值越小则芳香性越高,O/C和(O+N)/C比值越大,则亲水性和极性越大[27]。由表2可知,氮改性增加了生物炭的亲水性和极性,但是却降低了生物炭的芳香结构,这种变化会影响生物炭对Cu2+的去除效果及机理。
氮改性会影响生物炭的zeta电位。氮改性之后生物炭的zeta电位从−43.89 mV变成−37.97 mV,zeta电位的变化可能与生物炭官能团的改变有关。
XRD可用于分析生物炭中的晶体矿物结构和类型。RSBC和N-RSBC的XRD见图2(b)。由图2可见,RSBC和N-RSNC在22°处出现了明显的无定形碳峰,在44°呈现明显的石墨碳的峰[28]。说明,RSBC和N-RSBC中碳有无定形碳为主,部分碳石墨化主要与热解温度有关(700℃)。XRD显示N改性之后生物炭中无定形碳的衍射峰从从22°偏移到22.6°, 说明N掺杂到了到生物炭的碳网格中[29]。在RSBC中有CaCO3衍射峰,但是N改性的N-RSBC的CaCO3衍射峰消失,说明在N改性促进了CaCO3的分解,可能与尿素参与热解反应造成的还原气氛有关[30]。
图2(c)及表3表示生物炭及N改性生物炭的FTIR及具体官能团类型。 RSBC中含有包括—OH、—CH3、C=O和C=C、COOH、CHO、Si—O—Si等官能团[31]。N改性导致生物炭中官能团发生明显变化,新出现的N—H、C—N,—COOH和C—O官能团表明N改性引入了含氮官能团和含氧官能团。C=C和C=O官能团从1617 cm−1偏移到1630 cm−1表示N改性改变生物炭中官能团类型,COOH和CHO从1437 cm−1偏移到1458 cm−1,表明生物炭中含氮、含氧官能团发生变化,这会变化也会影响到改性生物炭对Cu2+的去除效果。N改性生物炭中含氮和含氧官能团来自尿素的分解, 及尿素与碳组分之间的热化学反应。
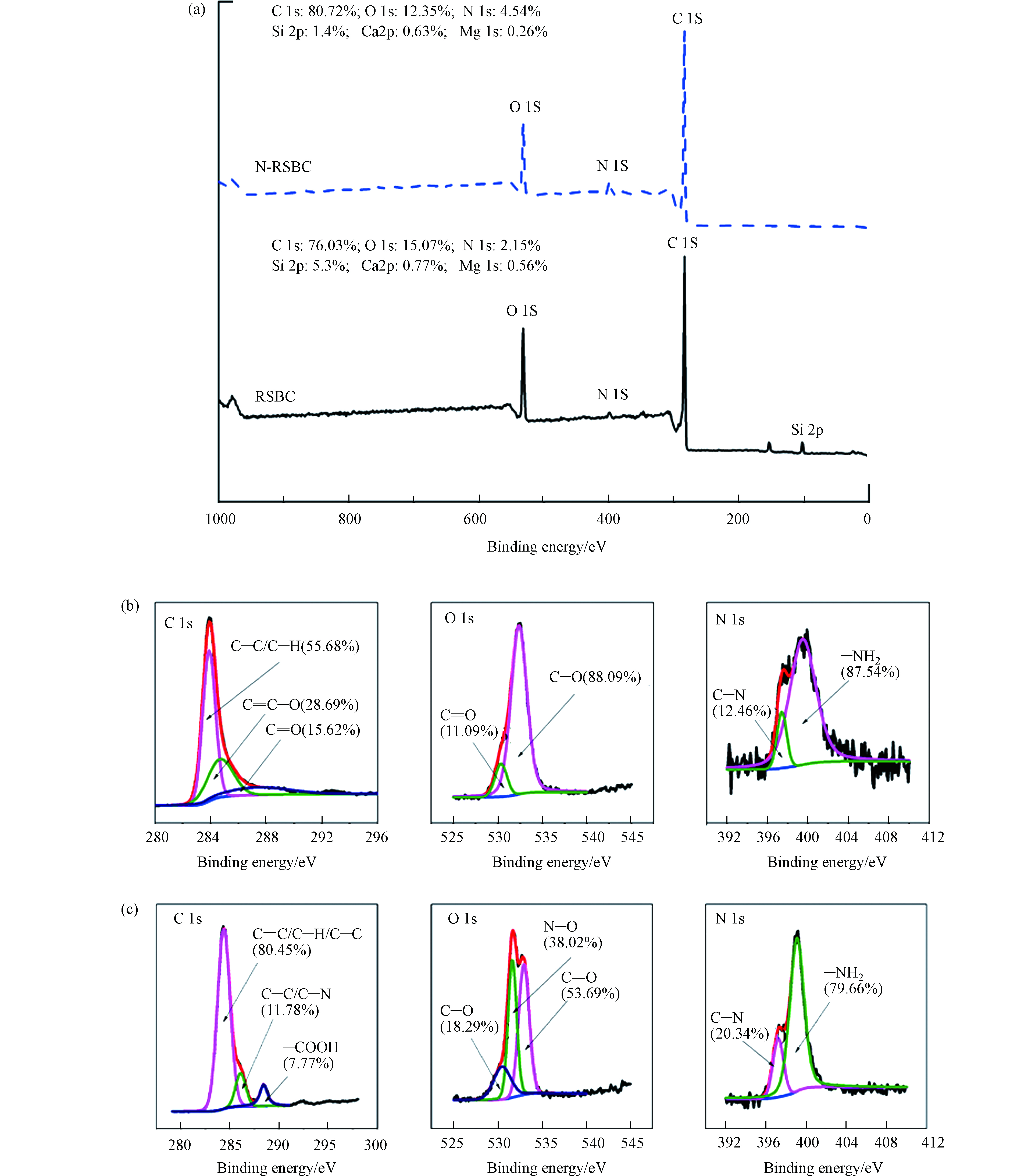
图3(a)显示的是RSBC及N-RSBC的XPS总峰图。由图3可见,生物炭表面主要含有C、O、N、Si、Ca、Mg等元素。N改性之后,生物炭表面的N相对含量从2.15%增加到4.54%,同时,C相对含量增加,O相对含量降低,说明N改性影响了生物炭表面元素的相对含量。
图3(b)和(c)显示的是RSBC及N-RSBC中C、O和N元素的分峰拟合。原状生物炭中C 1s的官能团为C—C/C—H、C=C/C—O和C=O [33],N改性之后,C—C/C—H的含量增加了,C—N的出现说明N进入到碳网格中,而—COOH的出现与FTIR表征结果一致。 N改性导致生物炭中O 1s的变化,C—O的含量改性前的88.09%降低至改性后的18.29%;而改性之后,C=O官能团含量从11.09%增加至18.29%;同时N—O的出现说明N改性影响了生物炭中的含氧官能团。对N 1s来说,RSBC中C—N官能团的比例为12.46%,经N改性之后,N-RSBC中C—N官能团含量增加了7.88%,而—NH2的相对含量降低了。因此,N改性明显改变了生物炭中含碳、含氧、含氮官能团的类型和含量,这种变化会影响到对污染物的去除。
-
pH是影响生物炭吸附重金属的关键因素之一,因为pH值能影响生物炭的表面电荷、电离度和重金属的形态。溶液初始pH值对RSBC和N-RSBC吸附Cu2+的影响如图4(a)所示。当溶液初始pH值从2.0增加到6.0 (pH<6为了防止形成沉淀),生物炭对于Cu2+的去除率逐渐增加。当pH值为6.0时,最大去除率达50%。当pH值在3.0-4.0范围内,生物炭对Cu2+的去除率急剧上升,之后上升速率缓慢。对比可知,N-RSBC对Cu2+的去除率大于RSBC,N-RSBC的最大去除率可达50%,RSBC的最大去除率达40%。当pH值<3.0时,溶液中含有的大量H+和Cu2+竞争生物炭表面的吸附位点形成静电斥力[36-37]。当溶液pH值上升时,静电斥力逐渐弱化,生物炭对Cu2+的去除率逐渐增加。相同pH值条件下,N-RSBC去除率大于RSBC,主要原因是N改性中引入的含氧以及含氮官能团提高了生物炭的静电引力。
选择Na+、K+、Ca2+、Mg2+为离子类型,探究单一共存离子存在对秸秆生物炭去除Cu2+的影响。离子类型及强度对生物炭去除Cu2+的影响见图4(b)。由图4(b)可知,共存离子存在能提高RSBC对Cu2+的去除率。如Mg2+为50 mg·L−1时,RSBC去除Cu2+效果最佳,去除率达64.8%,上升16.1%;N-RSBC对Cu2+去除具有抑制作用,其中Mg2+为30 mg·L−1时,N-RSBC对于去除Cu2+的抑制效果最大,去除率只有42.3%,下降了16.5%。离子强度导致生物炭对Cu2+去除率上升的原因可能是由于RSBC表面电荷的变化[38]。当Cu2+与生物炭之间存在静电排斥时,生物炭吸附量会随着离子强度的增加而增加[39]。生物炭与Cu2+形成内层表面络合物[40]或生物炭表面存在的有机物有利于造成生物炭吸附的疏水作用[41]也会导致生物炭对Cu2+去除率的上升。离子强度抑制N-RSBC吸附Cu2+的主要原因:随着离子浓度的增加,更多的金属离子与Cu2+竞争有效的吸附点位[37],产生离子交换竞争反应;吸附剂与吸附质之间静电相互作用引起的屏蔽效应[42];Cu2+与Na+、K+、Ca2+、Mg2+形成离子对,离子对会降低Cu2+浓度,进而导致生物炭对Cu2+吸附量降低[40]。
-
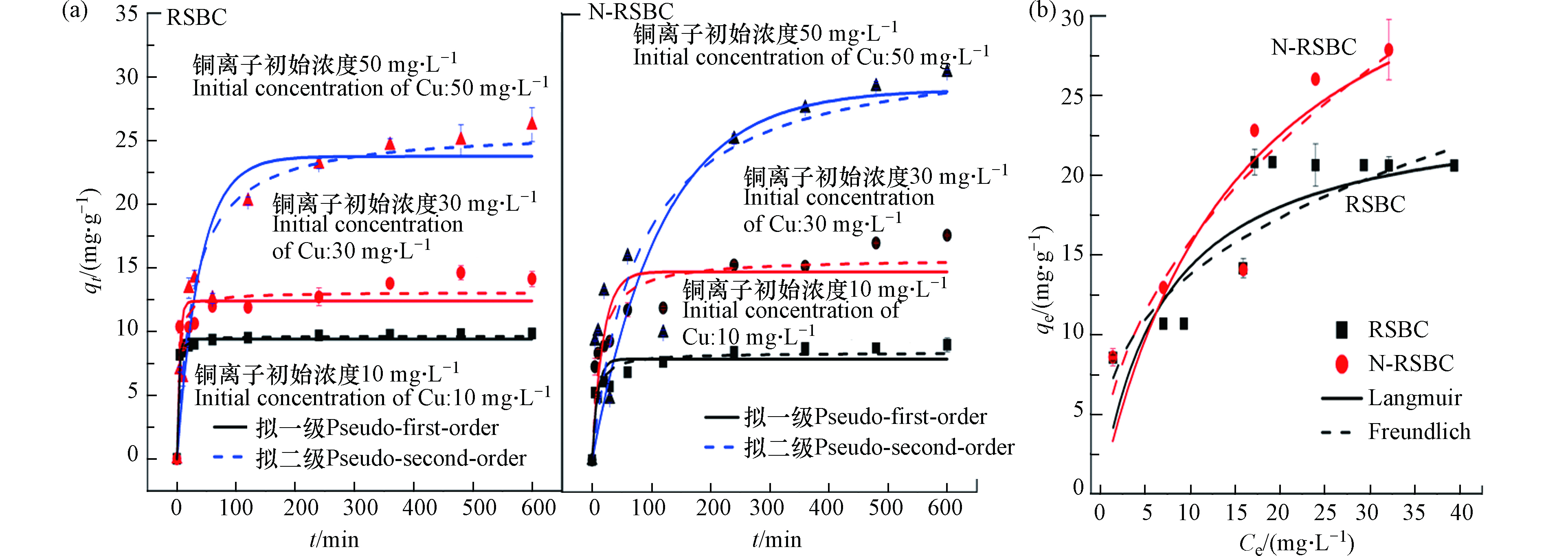
水稻秸秆生物炭对Cu2+的吸附动力学如图5(a)所示。随着吸附时间增加,生物炭对Cu2+的吸附量呈现先快速增加后趋于稳定的规律,因为在吸附初期,生物炭上的大量吸附位点有利于Cu2+的去除。
随着吸附时间的增加,吸附位点逐渐减少,导致吸附速率有所下降[43-44],直至吸附平衡。随着初始浓度的增加,生物炭对Cu2+的吸附量逐渐增加,因为高浓度有利于传质的进行。当Cu2+浓度为10 mg·L−1时,前5 min, RSBC和N-RSBC对Cu2+的去除率分别为81.86%和53.21%,而600 min时,RSBC和N-RSBC对Cu2+的去除率分别为98.38%和90.50%。当Cu2+浓度为30 mg·L−1时,前5 min, RSBC和N-RSBC对Cu2+的去除率分别为34.56%和24.46%,而600 min时,RSBC和N-RSBC对Cu2+的去除率分别为47.06%和58.83%。当Cu2+浓度为50 mg·L−1时,前5 min, RSBC和N-RSBC对Cu2+的去除率分别为14.04%和18.76%,而600 min时,RSBC和N-RSBC对Cu2+的去除率分别为52.47%和60.94%。对比可知,Cu2+浓度较低时,RSBC能更有效的去除Cu2+,吸附主要发生前期;当Cu2+浓度较高是,N-RSBC去除Cu2+的效果更好,吸附主要发生在中后期。对比可知,N-RSBC对Cu2+的吸附量高于RSBC,因为其能为Cu2+的去除提供更多的吸附位点。
拟一级和拟二级动力学模型对生物炭吸附动力学的拟合参数见表4。由表4可知,与拟一级动力学模型相比,拟二级动力学模型能更好的拟合RSBC和N-RSBC对Cu2+的吸附过程,说明生物炭吸附Cu2+的过程以化学吸附为主[7]。拟二级动力学模型包括了的吸附的所有过程,涉及外部液膜扩散、颗粒内扩散及表面吸附的所有过程[45]。对比不同Cu2+浓度的k2可知,浓度越高导致k2越高,因为Cu2+浓度差有利于吸附速率的提高。相同浓度下,RSBC的吸附速率大于N-RSBC,主要原因是N改性降低了生物炭比表面积的增大和孔隙结构的形成,不利于传质的进行(见表1)。
生物炭对Cu2+的吸附等温线如图5(b)所示。如图5所示,秸秆生物炭对Cu2+的吸附量呈现先增加后趋于稳定的规律。对比可知,N改性明显的提升了生物炭对Cu2+的吸附量,主要是归因于生物炭中官能团的络合和静电作用。
生物炭对Cu2+吸附等温线的拟合参数见表5。对比Langmuir而言,Freundlich方程所得R2均优于Langmuir方程所得R2,表明Freundlich可以更好的描述生物炭吸附Cu2+的过程,说明生物炭吸附Cu2+的异质性[17],因为生物炭物化性质的多样性。本研究中,RSBC和N-RSBC对Cu2+吸附的1/n小于0.5,说明生物炭对Cu2+的吸附易于进行[38]。对比Langmuir的最大吸附量可知,N改性之后的生物炭对Cu2+的吸附量从24.46 mg·g−1增加到40.56 mg·g−1。可见,N改性明显提升了生物炭对Cu2+的吸附量。
-
生物炭吸附废水中重金属的主要机理是络合作用、静电作用、离子交换和阳离子-π机制[46],具体主导机理取决于生物炭的理化性质和溶液的环境。本研究中生物炭及氮改性生物炭对Cu2+的吸附机理分析如下。
对比RSBC发现,N-RSBC的比表面积和孔体积有所降低,对Cu2+的吸附量却增加了,可见孔隙扩散不是生物炭吸附Cu2+的主要机理。生物炭及N改性生物炭的zeta电位为均为负值,有利于吸附带正电荷的Cu2+。此外,pH值的影响进一步说明静电作用是生物炭吸附Cu2+的重要机理,氮改性能促进生物炭对Cu2+的静电作用。结合XPS表面元素含量变化可知,生物炭吸附Cu2+之后,Ca和Mg离子的含量出现了降低,证实离子交换在生物炭吸附Cu2+的作用不容忽视。
为探究生物炭中官能团对Cu2+的吸附机理,对吸附Cu2+后的生物炭进行FTIR和XPS表征。图2和表6显示的是吸附Cu2+之后生物炭的FTIR官能团变化,对比可知,原状生物炭吸附Cu2+之后,3432 cm−1→3450 cm−1、1617 cm−1→1633 cm−1、1102 cm−1→1037 cm−1,而1437 cm−1峰却消失了,新出现了1384 cm−1、1235 cm−1、1168 cm−1、1116 cm−1峰,官能团的变化归因于表面络合和阳离子-π机制。N改性生物炭吸附Cu2+之后,1630 cm−1→1633 cm−1、1166 cm−1→1162 cm−1、1040 cm−1→1037 cm−1、424 cm−1→465 cm−1,1401cm−1消失了,新出现了1384 cm−1、1240 cm−1、1119 cm−1峰,官能团的变化涉及表面络合和阳离子-π机制。这些官能团的位移和峰强度的变化说明Cu2+与生物炭所形成的络合物或Cu2+−π作用会改变官能团周围的化学环境;此外,生物炭表面官能团的物理覆盖作用会减弱峰强度。
图6显示的是生物炭吸附Cu2+之后的XPS图。吸附Cu2+之后,生物炭的表面都检测到了Cu的存在,说明Cu已被吸附在生物炭的表面。与图3相比。吸附Cu2+之后,原状生物炭中,Ca和Mg的含量降低了,说明可能发生了离子交换作用。同时,N改性生物炭吸附铜离子之后表面的Ca和Mg含量也出现了降低了。
生物炭吸附Cu2+之后的XPS分峰拟合见图6(b)和(c)。对比图3可知,原状生物炭吸附Cu2+之后,出现了−COOH,N−O,说明含氧官能团与铜离子之间发生了络合作用;Cu-N的出现证实了铜离子与N之间发生络合作用;吸附后的铜离子主要以Cu 2p3/2、Cu 2p1/2的形态存在,以Cu 2p3/2为主[47]。N改性生物炭吸附铜离子之后,Cu-N的出现同样证实了铜离子与N之间发生络合作用。
综上分析可知,生物炭吸附铜离子的主要机理是络合作用和静电作用,离子交换和阳离子-π机制也能发挥一定作用。氮改性提高了生物炭对铜离子的吸附量主要归于生物炭上官能团影响的络合作用和静电作用。
-
研究发现尿素作为氮改性剂虽然降低了生物炭的比表面积和孔体积,但却可以丰富生物炭的官能团,进而提高了对Cu2+的吸附量。氮改性之后生物炭对Cu2+最大吸附量从24.46 mg·g−1增加到40.56 mg·g−1,主要是因为N改性可以提高生物炭对Cu2+的络合能力和静电作用。较低pH环境(pH<6.0)和单一共存离子(Na+、K+、Ca2+、Mg2+)的存在会降低N改性生物炭对铜离子的吸附效果。因此,尿素作为氮源添加剂对生物炭的改性是有效和可行的,氮改性生物炭有潜力应用于废水中Cu2+的去除。将来的研究方向应聚焦于优化氮源选择和添加比例,及氮改性生物炭的实际应用。
氮改性对生物炭理化性质的影响及其对废水中铜离子的吸附特性
Effect of nitrogen modification on the properties of biochars and their adsorption behavior on Cu2+ removal from wastewater
-
摘要: 原状生物炭对废水中污染物的去除效果有限,改性是提高其吸附能力的重要途径。本文以水稻秸秆为对象,尿素为改性剂,在700 ℃无氧热解条件下分别制备了原状秸秆生物炭(RSBC)和氮改性秸秆生物炭(N-RSBC),采用扫描电子显微镜(SEM)、比表面积分析仪(BET)、元素分析仪(EA)、Zeta电位、X射线衍射(XRD)、傅里叶红外光谱(FTIR)以及X射线光电子能谱(XPS)对RSBC和N-RSBC的形貌、比表面积、元素组成、矿物类型和官能团进行表征,考察溶液初始pH值、离子类型和离子强度对生物炭吸附Cu2+的影响,并结合吸附等温线和吸附动力学实验、吸附后表征结果探究生物炭对废水中Cu2+的吸附性能和机理。结果表明,氮改性导致了生物炭的比表面积和孔体积的降低,而生物炭的官能团类型却更加丰富,特别是含氮官能团。当溶液初始pH值从2.0增加到6.0,生物炭对于Cu2+的去除率逐渐增加。对RSBC而言,Na+、K+、Ca2+、Mg2+的存在能略微增加其对Cu2+的去除率。相反的是,Na+、K+、Ca2+、Mg2+的存在却降低了氮改性生物炭对Cu2+的去除率。拟二级动力学模型和Freundlich模型能较好的拟合生物炭吸附Cu2+的过程。RSBC和N-RSBC对Cu2+最大吸附量分别为24.46 mg·g−1和40.56 mg·g−1。络合作用、静电作用、离子交换和阳离子-π机制是生物炭吸附Cu2+的主要机理,氮改性可以提高生物炭对Cu2+的络合和静电作用。因此,氮改性生物炭有潜力应用于废水中Cu2+的去除。Abstract: The removal effect of pollutant by pristine biochar was limited. Modification is an important pathway to enhance its adsorption capacity. In this study, rice straw was pyrolyzed to prepare biochar at 700 °C (named as RSBC), and rice straw and urea was mixed to produce N-modified straw biochar (labelled as N-RSBC) under an oxygen-limited atmosphere. Biochars were characterized by scanning electron microscope (SEM), elemental analyzer (EA), surface area analyzer (BET), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Zeta potential and X-ray photoelectron spectroscopy (XPS). The influencing factor on Cu removal, such as solution pH, solid-to-liquid ratio, co-existing ions, were systematically investigated. The adsorption mechanisms were explored by combination adsorption kinetics, adsorption isotherms and instrumental analysis. The results showed that specific surface area and pore volume of biochar decreased after N modification, and the functional groups of N-RSBC became more abundant, especially the nitrogen-containing groups. When the pH value in solution increased from 2.0 to 6.0, the removal rate of Cu2+ ions on biochar increased. The present of co-existing Na+, K+, Ca2+, and Mg2+ promoted the Cu removal by RSBC, whereas inhibited the Cu removal by N-RSBC. The pseudo-second-order model and Freundlich model well fitted the Cu adsorption process onto biochar. The maximum adsorption capacity of Cu2+ on RSBC and N-RSBC were 24.46 mg·g−1 and 40.56 mg·g−1, respectively. The adsorption mechanisms of Cu2+ ions on biochar involved complexation, electrostatic interaction, ion exchange, and cation-π interaction. Nitrogen modification promoted the Cu2+ adsorption on N-RSBC via complexation and electrostatic interaction. Therefore, N-modified biochar has the potential to be used as a copper ions adsorbent in wastewater control.
-
Key words:
- rice straw /
- biochar /
- N-modification /
- copper ions /
- adsorption mechanism
-

-
表 1 生物炭的比表面积和孔径参数
Table 1. Specific surface area and pore diameter parameters of biochar
生物炭
Biochar比表面积/(m 2 ·g−1)
SBET总体积/(cm3·g-1)
Vtotal微孔体积/(cm3 ·g-1 )
Vmicro平均孔径/nm
Pore sizeRSBC 234.3874 0.133822 0.094230 2.28 N-RSBC 16.9736 0.019148 0.009875 4.51 表 2 生物炭的元素组成、元素比、灰分及zeta电位
Table 2. The element composition, element ratio, ash content and Zeta potential of biochar
生物炭
Biochar元素/%
ElementH/C O/C (N + O) / C 灰分/%
AshZeta电位/mV
Zeta potentialC N H O RSBC 55.76 0.22 0.843 9.187 0.01512 0.1648 0.1687 33.99 −43.89 N-RSBC 53.45 7.33 0.968 10.632 0.01811 0.1989 0.3361 27.62 −37.97 表 3 RSBC和N-RSBC的官能团类型
Table 3. Functional groups of RSBC and N-RSBC
RSBC N-RSBC 波数/cm−1
Wavenumber对应官能团
Corresponding functional groups波数/cm−1
Wavenumber对应官能团
Corresponding functional groups3432 —OH[31] 3450 —OH/N—H stretching[31] 2923 —CH3[31] 2923 —CH3[31] 2852 —CH3[31] 2852 —CH3[31] 1617 C=C和C=O[32] 1630 C=C 和C=O[32] 1437 COOH 和CHO[33] 1458 COOH 和 CHO[33] 1102 Si—O—Si/C—O[34] 1401 —COOH[34] 1166 C—N[35] 1040 C—O vibration[35] 424 Si—O—Si[31] 表 4 生物炭对Cu2+吸附动力学的拟合参数
Table 4. Fitting parameters of adsorption kinetic of Cu2+ onto RSBC and N-RSBC
生物炭
Biochar铜离子初始浓度/(mg·L−1)
Initial concentration of Cu拟一级模型
Pseudo-first-order model model拟二级模型
Pseudo-second-order modelqe/(mg·g−1) k1/min−1 R2 qe / (mg·g−1) k2/(g·(mg·min)−1) R2 RSBC 10 23.7563 0.02578 0.87613 25.91756 0.00139 0.934 30 12.39649 0.24405 0.82307 13.09237 0.0268 0.90195 50 9.41831 0.37022 0.97837 9.64357 0.09308 0.99452 N-RSBC 10 29.11295 0.00857 0.69686 32.62433 3.9091×10-4 0.73724 30 14.80378 0.05468 0.77431 15.85085 0.00511 0.88164 50 7.94829 0.11224 0.80149 8.45889 0.01957 0.90857 表 5 生物炭对Cu2+吸附等温线的拟合参数
Table 5. Fitting parameters of adsorption isotherms of Cu2+ onto RSBC and N-RSBC
生物炭
BiocharLangmuir Freundlich Q0/(mg·g−1) bL/(L·mg−1) R2 KF 1/n R2 RSBC 24.46 0.1391 0.6239 6.38683 0.33 0.76521 N-RSBC 40.56 0.0624 0.70909 5.29633 0.48 0.80967 表 6 生物炭吸附Cu2+后的FTIR官能团
Table 6. FTIR functional group of biochar after Cu2+ adsorption
RSBC+Cu N-RSBC+Cu 波数/cm−1
Wavenumber对应官能团
Corresponding functional groups波数/cm−1
Wavenumber对应官能团
Corresponding functional groups3450 —OH 3448 —OH 2924 —CH3 2924 —CH3 2854 —CH3 2854 —CH3 1633 C=C和C=O 1633 C=C 和C=O 1384 C—C 1384 C—C 1235 C—O 1240 C—O 1168 C—O和O—H 弯曲/拉伸 1162 C—N 1116 C—O和O—H弯曲/拉伸 1119 C—N 1037 C—O振动 1037 C—O 振动 465 Si—O—Si -
[1] 顾平, 武耘羽, 刘阳, 等. 废水中除铜研究的最新进展 [J]. 工业水处理, 2017, 37(5): 1-5. doi: 10.11894/1005-829x.2017.37(5).001 GU P, WU Y Y, LIU Y, et al. Latest research progress in the removal of copper from waste water [J]. Industrial Water Treatment, 2017, 37(5): 1-5(in Chinese). doi: 10.11894/1005-829x.2017.37(5).001
[2] BANG S, CHOI J W, CHO K, et al. Simultaneous reduction of copper and toxicity in semiconductor wastewater using protonated alginate beads [J]. Chemical Engineering Journal, 2015, 288: 525-531. [3] FU F L, WANG Q. Removal of heavy metal ions from wastewaters: A review [J]. Journal of Environmental Management, 2011, 92(3): 407-418. doi: 10.1016/j.jenvman.2010.11.011 [4] M NDEZ-D AZ J D, PRADOS-JOYA G, RIVERA-UTRILLA J, et al. Kinetic study of the adsorption of nitroimidazole antibiotics on activated carbons in aqueous phase [J]. Journal of Colloid and Interface Science, 2010, 345(2): 481-490. doi: 10.1016/j.jcis.2010.01.089 [5] LEHMANN J, RILLIG M C, THIES J, et al. Biochar effects on soil biota – A review [J]. Soil Biology and Biochemistry, 2011, 43(9): 1812-1836. doi: 10.1016/j.soilbio.2011.04.022 [6] 佟雪娇. 生物质炭对水体/红壤中Cu(Ⅱ)的去除和固定作用[D]. 南京: 南京农业大学, 2011. TONG X J. Removal of Cu(Ⅱ)from aqueous solutions and its fixation in red soil by biochars from crop straws[D]. Nanjing: Nanjing Agricultural University, 2011(in Chinese).
[7] CHEN X C, CHEN G C, CHEN L G, et al. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution [J]. Bioresource Technology, 2011, 102(19): 8877-8884. doi: 10.1016/j.biortech.2011.06.078 [8] 尹英杰, 朱司航, 徐东昊, 等. 生物炭和乙醇改性生物炭对铜的吸附研究 [J]. 农业环境科学学报, 2017, 36(9): 1877-1883. doi: 10.11654/jaes.2017-0269 YIN Y J, ZHU S H, XU D H, et al. Comparison of copper adsorption onto wheat biochar and ethanol-modified biochar [J]. Journal of Agro-Environment Science, 2017, 36(9): 1877-1883(in Chinese). doi: 10.11654/jaes.2017-0269
[9] PARK J H, OK Y S, KIM S H, et al. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions [J]. Chemosphere, 2016, 142: 77-83. doi: 10.1016/j.chemosphere.2015.05.093 [10] MEI Y L, LI B, FAN S S. Biochar from rice straw for Cu2+ removal from aqueous solutions: Mechanism and contribution made by acid-soluble minerals [J]. Water, Air, & Soil Pollution, 2020, 231(8): 1-13. [11] 周沈格颖. 秸秆生物质炭吸附处理含铜废水研究[D]. 温州: 温州大学, 2018. ZHOU S G Y. Study on adsorption treatment of Cu(Ⅱ) by rice straw-derived biochar from wastewater[D]. Wenzhou, China: Wenzhou University, 2018 (in Chinese).
[12] DENG J, LI X, WEI X, et al. Sulfamic acid modified hydrochar derived from sawdust for removal of benzotriazole and Cu(II) from aqueous solution: Adsorption behavior and mechanism [J]. Bioresource Technology, 2019, 290: 121765. doi: 10.1016/j.biortech.2019.121765 [13] GAO Y, ZHU X Z, YUE Q Y, et al. Facile one-step synthesis of functionalized biochar from sustainable prolifera-green-tide source for enhanced adsorption of copper ions [J]. Journal of Environmental Sciences (China), 2018, 73: 185-194. doi: 10.1016/j.jes.2018.02.012 [14] SON E B, POO K M, CHANG J S, et al. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass [J]. The Science of the Total Environment, 2018, 615: 161-168. doi: 10.1016/j.scitotenv.2017.09.171 [15] ZHOU Q, LIAO B, LIN L, et al. Adsorption of Cu(Ⅱ) and Cd(Ⅱ) from aqueous solutions by ferromanganese binary oxide-biochar composites [J]. Science of the Total Environment, 2018, 615(FEBa15): 115-122. [16] WEN R, YUAN B, WANG Y, et al. Improving Cu(Ⅱ) sorption by biochar via pyrolyzation under CO2: The importance of inherent inorganic species [J]. Environmental Science and Pollution Research, 2018, 25(6): 5105-5114. doi: 10.1007/s11356-017-9753-3 [17] WANG Y Y, LIU Y X, LU H H, et al. Competitive adsorption of Pb(Ⅱ), Cu(Ⅱ), and Zn(Ⅱ) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions [J]. Journal of Solid State Chemistry, 2018, 261: 53-61. doi: 10.1016/j.jssc.2018.02.010 [18] LIAN F, CUI G N, LIU Z Q, et al. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity [J]. Journal of Environmental Management, 2016, 176: 61-68. doi: 10.1016/j.jenvman.2016.03.043 [19] YANG G X, JIANG H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater [J]. Water Research, 2014, 48: 396-405. doi: 10.1016/j.watres.2013.09.050 [20] 侯素珍, 田浩然, 黄超, 等. 氨基改性生物炭负载纳米零价铁去除水中Cr(Ⅵ) [J]. 环境科学学报, 2020, 40(11): 3931-3938. HOU S Z, TIAN H R, HUANG C, et al. Removal of Cr (Ⅵ) from aqueous solution by amino-modified biochar supported nano zero-valent iron [J]. Acta Scientiae Circumstantiae, 2020, 40(11): 3931-3938(in Chinese).
[21] ZHAHG J J, SHAO J G, HUANG D R, et al. Influence of different precursors on the characteristic of nitrogen-enriched biochar and SO2 adsorption properties [J]. Chemical Engineering Journal, 2020, 285: 123932. [22] LI W, ZHANG L, GUAN Y, et al. A Slow pyrolysis biochar derived from tetrapanax papyriferum petiole as an effective sorbent for removing copper ions from aqueous solution [J]. Bioresources, 2019, 14(2): 4430-4453. doi: 10.15376/biores.14.2.4430-4453 [23] LI M, LIU Q, GUO L, et al. Cu(Ⅱ) removal from aqueous solution by Spartina alterniflora derived biochar [J]. Bioresource Technology, 2013, 141: 83-88. doi: 10.1016/j.biortech.2012.12.096 [24] SHIM T, YOO J, RYU C, et al. Effect of steam activation of biochar produced from a giant Miscanthus on copper sorption and toxicity [J]. Bioresource Technology, 2015, 197: 85-90. doi: 10.1016/j.biortech.2015.08.055 [25] DUAN X, O'DONNELL K, SUN H, et al. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions [J]. Small, 2015, 11(25): 3036-3044. doi: 10.1002/smll.201403715 [26] LIU S, ZHAO C, WANG Z, et al. Urea-assisted one-step fabrication of a novel nitrogen-doped carbon fiber aerogel from cotton as metal-free catalyst in peroxymonosulfate activation for efficient degradation of carbamazepine [J]. Chemical Engineering Journal, 2020, 386: 124015. doi: 10.1016/j.cej.2020.124015 [27] 王淑娟. 氨基磁化生物炭对水中铅、铜、铀的吸附性能研究[D]. 北京: 华北电力大学(北京), 2018. WANG S J. The removai of lead, copper and uranium by amnino modified magnetic biochar from aquecus solutions[D]. Beijing: North China Electric Power University, 2018(in Chinese).
[28] WU W, YANG M, FENG Q, et al. Chemical characterization of rice straw-derived biochar for soil amendment [J]. Biomass and Bioenergy, 2012, 47: 268-276. doi: 10.1016/j.biombioe.2012.09.034 [29] XU L, WU CX, LIU PH, et al. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants [J]. Chemical Engineering Journal, 2020, 387: 124056. doi: 10.1016/j.cej.2020.124056 [30] LI D P, ZHAO L, CAO X D, et al. Nickel-catalyzed formation of mesoporous carbon structure promoted capacitive performance of exhausted biochar [J]. Chemical Engineering Journal, 2021, 406: 126856. doi: 10.1016/j.cej.2020.126856 [31] XIAO X, CHEN B L, ZHU L Z. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures [J]. Environmental Science & Technology, 2014, 48(6): 3411-3419. [32] LENG L J, XU S Y, LIU R F, et al. Nitrogen containing functional groups of biochar: An overview [J]. Bioresource Technology, 2020, 298: 122286. doi: 10.1016/j.biortech.2019.122286 [33] SHAFEEYAN M S, DAUD W, HOUSHMAND A, et al. A review on surface modification of activated carbon for carbon dioxide adsorption [J]. Journal of Analytical and Applied Pyrolysis, 2010, 89: 143-151. doi: 10.1016/j.jaap.2010.07.006 [34] SHAFEEYAN M S, DAUD W, HOUSHMAND A, et al. Ammonia modification of activated carbon to enhance carbon dioxide adsorption: Effect of pre-oxidation', Applied Surface Science, 2011, 257: 3936-3942. [35] WANG D H, HUI S E, LIU C C. Mass loss and evolved gas analysis in thermal decomposition of solid urea [J]. Fuel, 2017, 207: 268-273. doi: 10.1016/j.fuel.2017.06.117 [36] 吴晴雯, 孟梁, 张志豪, 等. 芦苇秸秆生物炭对水体中重金属Ni2+的吸附特性 [J]. 环境化学, 2015, 34(9): 1703-1709. doi: 10.7524/j.issn.0254-6108.2015.09.2015031108 WU Q W, MENG L, ZHANG Z H, et al. Adsorption behaviors of Ni2+onto reed straw biochar in the aquatic solutions [J]. Environmental Chemistry, 2015, 34(9): 1703-1709(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.09.2015031108
[37] 黄柱坚, 朱子骜, 吴学深, 等. 皇竹草生物炭的结构特征及对重金属吸附作用机制 [J]. 环境化学, 2016, 35(4): 766-772. HUANG Z J, ZHU Z A, WU X S, et al. Adsorption of heavy metals by biochar derived from Pennisetum sinese Roxb [J]. Environmental Chemistry, 2016, 35(4): 766-772(in Chinese).
[38] 范世锁, 刘文浦, 王锦涛, 等. 茶渣生物炭制备及其对溶液中四环素的去除特性 [J]. 环境科学, 2020, 41(3): 1308-1318. FAN S S, LIU W P, WANG J T, et al. Preparation of tea waste biochar and its application in tetracycline removal from aqueous solution [J]. Environmental Science, 2020, 41(3): 1308-1318(in Chinese).
[39] SEPULVEDA L A, SANTANA C C. Effect of solution temperature, pH and ionic strength on dye adsorption onto Magellanic peat [J]. Environmental Technology, 2013, 34(8): 967-977. doi: 10.1080/09593330.2012.724251 [40] 吴志坚, 刘海宁, 张慧芳. 离子强度对吸附影响机理的研究进展 [J]. 环境化学, 2010, 29(6): 997-1003. WU Z J, LIU H N, ZHANG H F. Research progress on mechanisms about the effect of ionic strength on adsorption [J]. Environmental Chemistry, 2010, 29(6): 997-1003(in Chinese).
[41] CAMPINAS M, ROSA M J. The ionic strength effect on microcystin and natural organic matter surrogate adsorption onto PAC [J]. Journal of Colloid and Interface Science, 2006, 299(2): 520-529. doi: 10.1016/j.jcis.2006.02.042 [42] 陈尚龙. ATRP法制备羧基化生物吸附剂及其对重金属离子的吸附[D]. 徐州: 中国矿业大学(江苏), 2020. CHEN S L. Preparation of carboxylated biosorbents via atom transfer radical polymerization for adsorption of heavy metal ions[D]. Xuzhou: China University of Mining and Technology, 2020(in Chinese).
[43] 宋泽峰, 石晓倩, 刘卓, 等. 芦苇生物炭的制备、表征及其吸附铜离子与双酚A的性能 [J]. 环境化学, 2020, 39(8): 2196-2205. SONG Z F, SHI X Q, LIU Z, et al. Synthesis and characterization of reed-based biochar and its adsorption properties for Cu2+ and bisphenol A ( BPA) [J]. Environmental Chemistry, 2020, 39(8): 2196-2205(in Chinese).
[44] JIANG J, PENG Y, YUAN M, et al. Rice straw-derived biochar properties and functions as Cu(Ⅱ) and cyromazine sorbents as influenced by pyrolysis temperature [J]. An International Journal Pedosphere, 2015, 25(5): 781-789. doi: 10.1016/S1002-0160(15)30059-X [45] HO Y, MCKAY G. Pseudo-second order model for sorption processes [J]. Process Biochemistry, 1999, 34(5): 451-465. doi: 10.1016/S0032-9592(98)00112-5 [46] INYANG M I, GAO B, YAO Y, et al. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal [J]. Critical Reviews in Environmental Science and Technology, 2016, 46(4): 406-433. doi: 10.1080/10643389.2015.1096880 [47] TANG S, SHAO N, ZHENG C, et al. Amino-functionalized sewage sludge-derived biochar as sustainable efficient adsorbent for Cu(Ⅱ) removal [J]. Waste Management, 2019, 90(MAY): 17-28. -



 下载:
下载: