-
Cr(Ⅵ)是广为关注的地下水和土壤污染物之一。目前,在酸性条件下加入还原剂将Cr(Ⅵ)还原为Cr(Ⅲ),随后生成Cr(OH)3沉淀来降低其毒性是修复铬污染环境介质的常用策略[1-3],如式(1)所示。此外,在自然环境中也存在Cr(Ⅵ)的还原过程,铁还原菌可以通过产生Fe(Ⅱ)抑制厌氧条件下Cr(Ⅵ)的产生[4]。还原生成的Cr(Ⅲ)主要以非晶态Cr(OH)3存在,其原子排列具有近程有序、长程无序状态特性,在热力学上属于非平衡的亚稳态[5]。
在对Cr(Ⅵ)污染土壤和地下水修复后,较长时间后会出现Cr(Ⅵ)再次超标现象。有研究[6]认为,Cr(Ⅲ)在一定环境条件下会被再次氧化生成Cr(Ⅵ),是土壤和地下水中Cr(Ⅵ)浓度升高的原因之一。人们认为锰氧化物是将还原生成的Cr(Ⅲ)重新氧化的氧化剂[7]。Mcclain等[8]发现矿物结合态的Cr(Ⅲ)会与结构类似水钠锰矿的生物性Mn(Ⅲ/Ⅳ)氧化物结合并生成Cr(Ⅵ)。Gonzalez等[9]认为加州圣克鲁斯县饮用水中所存在的大量Cr(Ⅵ)是由于Cr (Ⅲ)矿床被锰氧化物氧化。
研究表明[5],锰氧化物的类型和结构(包括δ-MnO2、水钠锰矿、水锰矿、钡镁锰矿、软锰矿等)对锰氧化物氧化Cr(Ⅲ)的速率和比率影响较大。由于晶性、价态和形态不同,氧化锰对Cr(Ⅲ)氧化能力有明显差异,顺序为δ-MnO2>α-MnO2>γ-MnOOH。相较于γ-MnOOH,δ-MnO2、α-MnO2中的Mn氧化度和活度更高,且δ-MnO2的晶性较差、比表面积大,故δ-MnO2对Cr(Ⅲ)的氧化能力强,γ-MnOOH相对较弱[10]。
当前研究中,对环境酸碱性在锰氧化物对Cr(Ⅲ)氧化过程的影响的认识尚存有争议。SEONYI等[11]研究了Cr(OH)3(s)和Mn(Ⅱ)共存时Cr(OH)3(s)的氧化反应,pH=7时未发现Cr(Ⅵ)的生成,pH≥8时,Cr(OH)3(s)被氧化并释放大量Cr(Ⅵ)。OZE等[12]探究了Cr(Ⅲ)与水钠锰矿反应19 d时Cr(Ⅵ)的生成速率,发现pH=7时,Cr(Ⅵ)的生成速率为0.001 μmol·d−1,pH=8时,反应速率为0,说明pH的升高会减小Cr(Ⅵ)的生成量。而PAN等[13]研究了δ-MnO2氧化铬铁氢氧化物的速率和机制,认为在Cr(Ⅲ)初始浓度为770 μmol·L−1、δ-MnO2初始浓度为436 μmol·L−1时,随着pH从5增大到9,Cr(Ⅵ)产生量从80 μmol·L−1逐渐减小至15 μmol·L−1。
本研究旨在探究含氧水环境中非晶态Cr(OH)3的氧化过程及环境酸碱性、有机质等环境因素对该过程的影响,具体包括:(1)实验观测δ-MnO2对水溶液中非晶态Cr(OH)3的氧化过程;(2)分析pH和DOM对δ-MnO2氧化非晶态Cr(OH)3的影响及潜在机理。
-
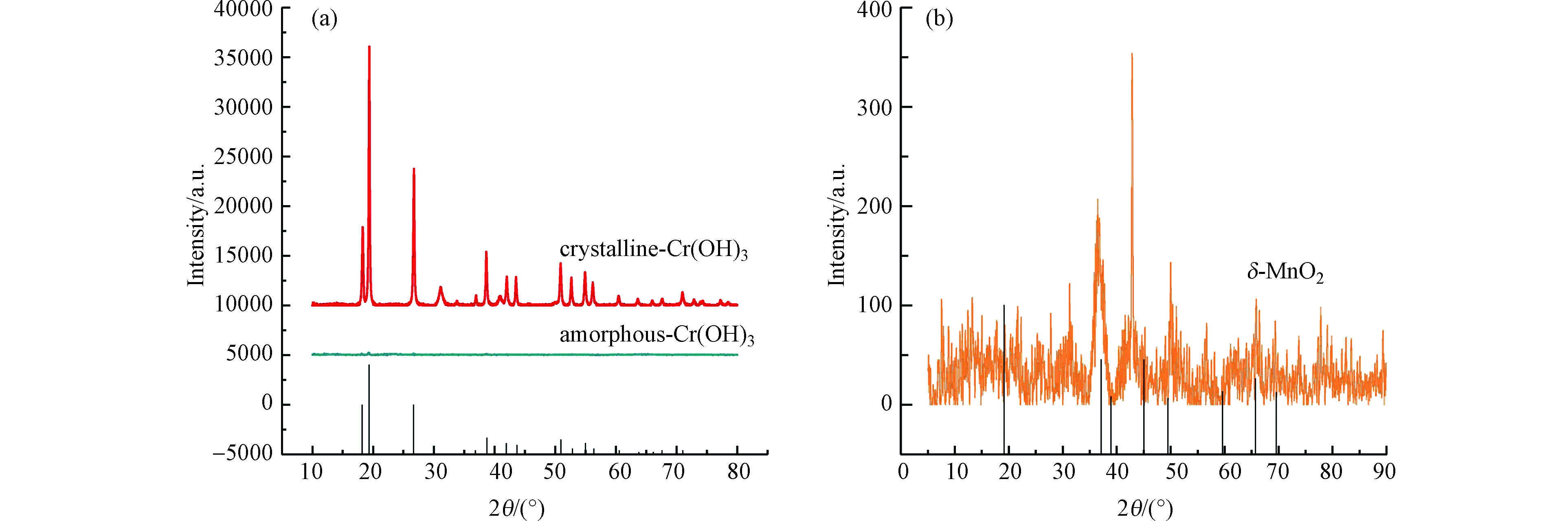
采用HUANG等[14]的方法制备非晶态Cr(OH)3,具体方法如下:25 ℃时,将0.2 mol·L−1氢氧化钠溶液以1 mL·min−1的速度加入至0.089 mol·L−1氯化铬溶液中并不断搅拌。当pH值达8.5时,停止加入氢氧化钠溶液并持续搅拌30 min。将悬浮液离心后倒去上清液,用蒸馏水洗涤3次,丙酮洗涤2次,乙醇洗涤1次。将固相材料置于25 ℃的真空干燥箱中干燥后研磨、过200目筛并充氮气密封保存。图1(a)为制备的Cr(OH)3的X射线衍射(X-ray diffraction,XRD)图谱,结果表明Cr(OH)3在18 °、19 °、26 °、38 °、50 °等位置均未发现明显的衍射峰,且谱线含有较多的毛刺,与HUANG等[14]制备的非晶态Cr(OH)3的表征结果相似,这表明实验制备的Cr(OH)3为非晶态。
采用VILLALOBOS等[15]的方法制备δ-MnO2。具体方法如下:250 mL的0.20 mol·L−1高锰酸钾溶液和250 mL的0.47 mol·L−1氯化锰溶液依次缓慢加入至270 mL的0.49 mol·L−1氢氧化钠溶液中,并不断搅拌。悬浮液静置分层,虹吸出上清液。剩余的悬浮液离心后倒掉上清液。离心后的物质与1 mol·L−1 NaCl混合振荡1 h后离心,倒去上清液,此过程重复5次,最后一次振荡持续12 h。将离心后物质置于透析袋,用去离子水透析整夜至pH=7.79。离心后的氧化物置于25 ℃的真空干燥箱内进行干燥、研磨,过200目筛后充氮气密封保存。图1(b)为δ-MnO2的X射线衍射图谱,与VILLALOBOS等[15]的研究结果相似,在37 °、45 °、49 °、66 °等位置有明显的衍射峰,在19 °、59 °的特征峰消失,且谱线含有较多的毛刺,这表明实验制备的δ-MnO2结晶度较差或者晶体呈无定型。
采用黄中林[16]的方法制备晶态Cr(OH)3,用K2Cr2O7和去离子水(18.25 MΩ·cm)配制Cr(Ⅵ)储存液,采用Na2HPO3、NaH2PO3和去离子水配制磷酸盐缓冲液;除了特殊说明外,均使用分析纯试剂。
-
在温度25 ℃、空气复氧、固定pH、特定DOM浓度等条件下,于1 L锥形瓶中加入50 mg·L−1非晶态Cr(Ⅲ)和50 mg·L−1 δ-MnO2,用NaCl控制体系离子强度为5 mmol·L−1,反应体系体积为700 mL。分别借助1 mmol·L−1 MES缓冲溶液和5 mmol·L−1 MOPS缓冲溶液控制体系pH为6和8;采用富里酸作为典型DOM观测溶解性有机质的影响,其浓度水平定为1、10 mg·L−1。为确保体系悬浮液的均匀混合以及其能与氧气充分接触,整个过程采用1000 r·min−1磁力搅拌。在反应进行至一定时间(1 d和2 d;取出后静置5、10、15、30、120、300、365 d)取样测定;取1 mL悬浮液立即用0.2 μm PES过滤器过滤后,分析滤液中Cr(Ⅵ)浓度;另取1 mL悬浮液,加入0.01 mL磷酸盐缓冲液,采用200 r·min−1振荡1 h后,用0.2 μm PES过滤器过滤,取滤液分析Total Cr(Ⅵ)浓度。每组实验均设置两个平行样。
-
采用X射线衍射仪(PANalytical X’Pert Powder,Spectris Pte. Ltd)对制备的Cr(OH)3和δ-MnO2进行物相分析,检测条件为:Cu靶;DS狭缝为1°;RS狭缝为0.3 mm;SS狭缝为1°;连续扫面;扫描速度为2(°)·min−1;采样间隔为0.02°;扫描范围5°—90°。采用X射线光电子能谱仪(ESCALAB250Xi,赛默飞世尔)对pH值为6和8、有氧条件下δ-MnO2氧化Cr(OH)3生成的反应产物进行表面分析。
使用梅特勒-托利多pH计测定pH值,采用二苯碳酰二肼分光光度法(GB/T 7467-1987)利用紫外-可见分光光度计(T6,北京普析)测定液相中水溶态Cr(Ⅵ)浓度,采用溶解氧仪(Seven2Go,METTLER TOLEDO)测定水溶液中溶解氧(Dissolved oxygen,DO)浓度,采用多参数水质分析仪(S220,梅特勒-托利多)测定反应体系的氧化还原电位(Electric potential of half cells,Eh)。
-
本研究所测得的吸光度由紫外可见分光光度计控制并由分析软件自动生成,所获得的实验数据均采用Excel数据分析软件进行分析,并用origin 2017软件进行制图;XRD数据均采用jade 6.6软件进行分析,并用origin 2017软件进行制图;X射线光电子能谱(X-ray photoelectron spectroscopy,XPS)数据均采用Avantage软件进行分析,并用origin 2017软件进行制图。
-
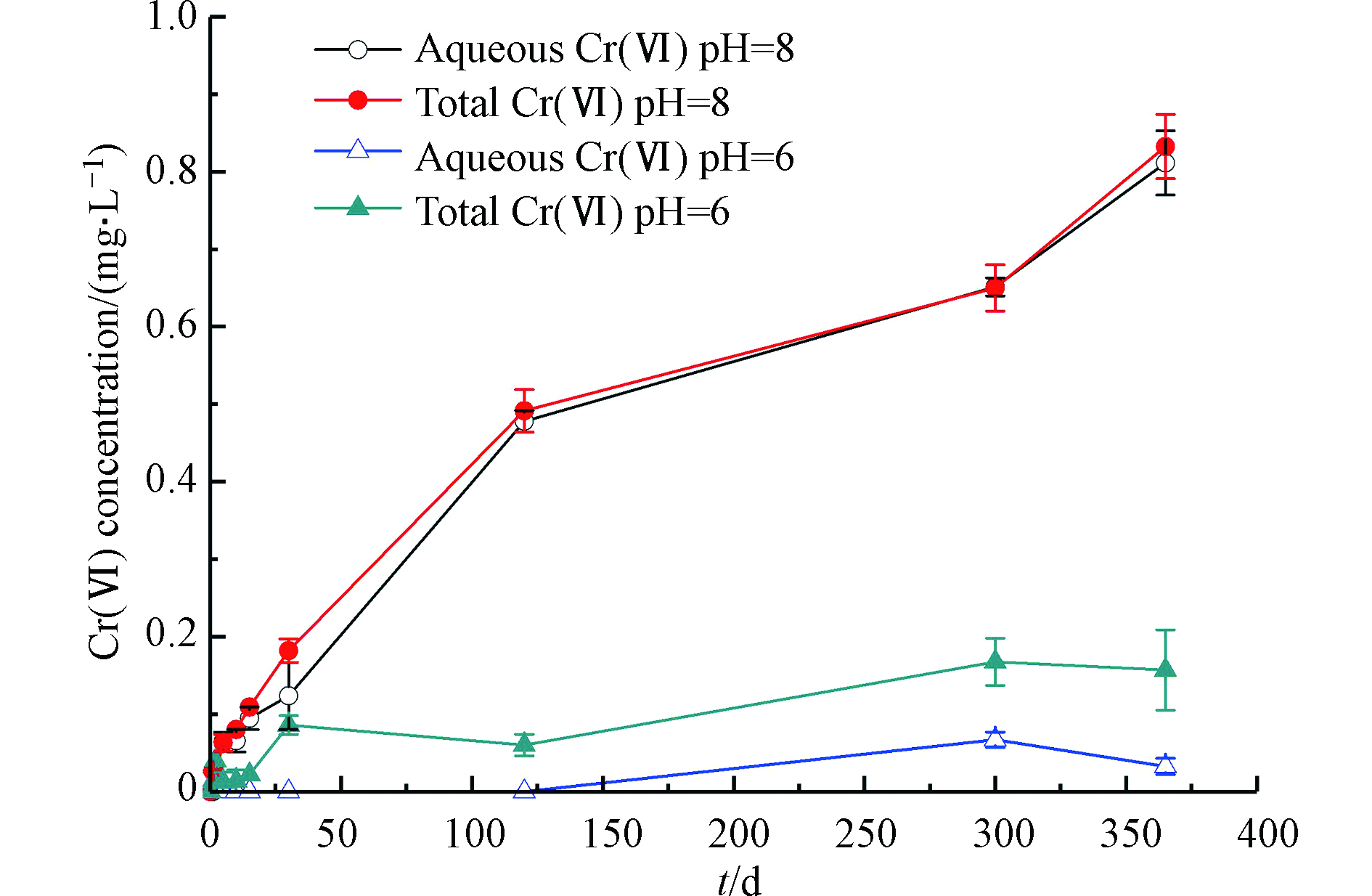
图2展示了δ-MnO2与非晶态Cr(OH)3反应过程中Cr(Ⅵ)浓度随时间的变化。由图2(a)可知,在pH=8时,反应300 d时可检测到0.02 mg·L−1的Cr(Ⅵ),说明氧气氧化Cr(OH)3的反应速率较慢,在整个检测的时间尺度内Cr(Ⅵ)浓度均低于Ⅴ类地下水质量标准(0.1 mg·L−1)[17];投加了δ-MnO2之后,水溶液中Cr(Ⅵ)浓度随时间呈上升趋势,反应至365 d,水溶液中总Cr(Ⅵ)浓度为0.83 mg·L−1,表明在弱碱条件(pH=8)下的含氧水溶液中,Cr(OH)3具有长期稳定性,而δ-MnO2会使Cr(OH)3的氧化量显著增大。
图2(b)为伪零级动力学模型对δ-MnO2对Cr(OH)3氧化过程的拟合结果。由图2(b)可知,δ-MnO2对Cr(OH)3的氧化过程较好的符合伪零级反应动力学,速率常数为0.0021 mg·L−1·d−1,R2=0.9465。呈现出Cr(Ⅵ)的生成量与Cr(Ⅵ)浓度无关,随时间线性增大的趋势,这与PAN等[13]发现的锰氧化物氧化CrxFe1−x(OH)3过程 Cr(Ⅵ)浓度随时间持续上升的趋势一致。
SCHROEDER等[18]研究了土壤中溶解氧对于三价铬氧化反应的影响,认为溶解氧在氧化三价铬过程中起到非常微小的作用,两周之后检测到有极其微小的三价铬被氧化。EARY等[19]研究发现在溶解氧浓度约为8 mg·L−1和pH=4—12.5条件下,浓度为19 μmol·L−1的三价铬溶液静置反应24 d后未检出Cr(Ⅵ)。PAN等[13]研究了MnO2对Cr0.23Fe0.77(OH)3氧化的影响,结果表明反应至400 h时检测到Cr(Ⅵ)的生成量为0.2 mg·L−1。本研究发现与前人的研究相符,有氧条件下,Cr(OH)3具有长期稳定性,δ-MnO2会显著增大Cr(OH)3的氧化量。
-
图3展示了典型环境酸碱条件下水溶液中δ-MnO2对非晶态Cr(OH)3氧化产生的Cr(Ⅵ)浓度随时间的变化。从图3可知,pH=8时,反应至365 d时总Cr(Ⅵ)浓度达到0.83 mg·L−1,明显高于pH=6条件下水溶液中Cr(Ⅵ)浓度,且溶液中溶解态Cr(Ⅵ)和总Cr(Ⅵ)浓度几乎相等,说明生成的Cr(Ⅵ)主要以水溶态存在。同时,观测到典型环境酸碱条件下反应体系的氧化还原电位随时间呈逐渐增大趋势;随着反应的进行,反应体系中Cr(Ⅵ)含量逐渐增大,根据能斯特方程,如式(3)所示,随着氧化性强的Cr(Ⅵ)浓度的增大,氧化还原电位也会逐渐增大。相较于pH=8(Eh=243 mV),pH=6条件下反应体系的初始状态氧化还原电位(Eh=332 mV)更高,说明pH值对氧化还原电位有较大影响。SEONYI等[11]研究发现在大气环境条件中,pH=7.0—9.0的情况下,Cr(OH)3(s)和溶解态Mn(Ⅱ)共存时,pH≥8时,Cr(OH)3(s)被氧化并释放大量Cr(Ⅵ),且Cr(Ⅵ)的生成量随着pH值升高而增大,与本研究在弱碱条件(pH=8)下得出的研究结果一致。pH=6时,30 d内总Cr(Ⅵ)浓度快速上升,之后上升速度趋于平缓,在300 d已达到平衡,总Cr(Ⅵ)的平衡浓度为0.15 mg·L−1;而溶解态Cr(Ⅵ)在反应300 d时才被检出,出现这种现象的原因可能是
CrO2−4 、HCrO−4 等形态的Cr(Ⅵ)在低pH下将完全吸附至Cr(OH)3(s)上,在较高pH下则会释放至溶液中[15]。 -
图4是pH=6和pH=8条件下δ-MnO2氧化非晶态Cr(OH)3生成固相产物的XPS全谱图和Cr 2p XPS图谱。由图4(a)可知,弱酸条件(pH=6)下产生的固相产物表面存在Mn、Cr、O等元素;由图4(b)可知,pH=6时,结合能为577.19、586.92 eV[20]处的峰代表的是Cr(Ⅲ)的2p3/2轨道,结合能为578.62、588.81eV[21]处的峰代表Cr(Ⅵ) 的2p3/2轨道,这表明固相产物中Cr以Cr(Ⅲ)和Cr(Ⅵ)形式存在,反应体系中产生的Cr(Ⅵ)部分为固相结合态。由图4(c)可知,弱碱条件(pH=8)下产生的固相产物表面存在Mn、Cr、O等元素;由图4(d)可知,pH=8时,结合能为576.61[22]、577.48[20]、586.64 eV[23]处的峰代表的是Cr(Ⅲ)的2p3/2轨道,未检测到Cr(Ⅵ)的结合能,这表明固相产物中Cr主要以Cr(Ⅲ)形式存在,反应体系中产生的Cr(Ⅵ)主要以溶解态存在,固相中Cr(Ⅵ)含量极低。这与2.2.1节中水溶态Cr(Ⅵ)和总Cr(Ⅵ)测试结果保持一致。这可能是由于生成的Cr(Ⅵ)在水溶液中主要以
HCrO−4 、CrO2−4 形态存在,Cr(OH)3的等电点为8.03[11],δ-MnO2的等电点为2.3,在pH=6时,Cr(OH)3带正电,Cr(Ⅵ)易吸附至非晶态Cr(OH)3上,成为吸附态Cr(Ⅵ);而在pH=8时δ-MnO2带负电,与HCrO−4 、CrO2−4 产生静电排斥,在δ-MnO2表面生成的Cr(Ⅵ)易于解吸。因此,在弱酸条件(pH=6)下,生成的Cr(Ⅵ)部分以溶解态存在,部分以固相结合态存在;弱碱条件(pH=8)下,生成的Cr(Ⅵ)主要以溶解态存在。PAN等[13]认为在初始Cr(Ⅲ)浓度为770 μmol·L−1和初始MnO2浓度为436 μmol·L−1条件下,pH=5—9时,CrxFe1−x(OH)3可被MnO2氧化,当pH=5—7时,生成的Cr(Ⅵ)部分以溶解态存在,部分以固相结合态存在;pH=8—9时,测得总Cr(Ⅵ)浓度远远大于溶解态Cr(Ⅵ)浓度,生成的Cr(Ⅵ)主要以固相结合态存在,这与本研究发现保持一致。 -
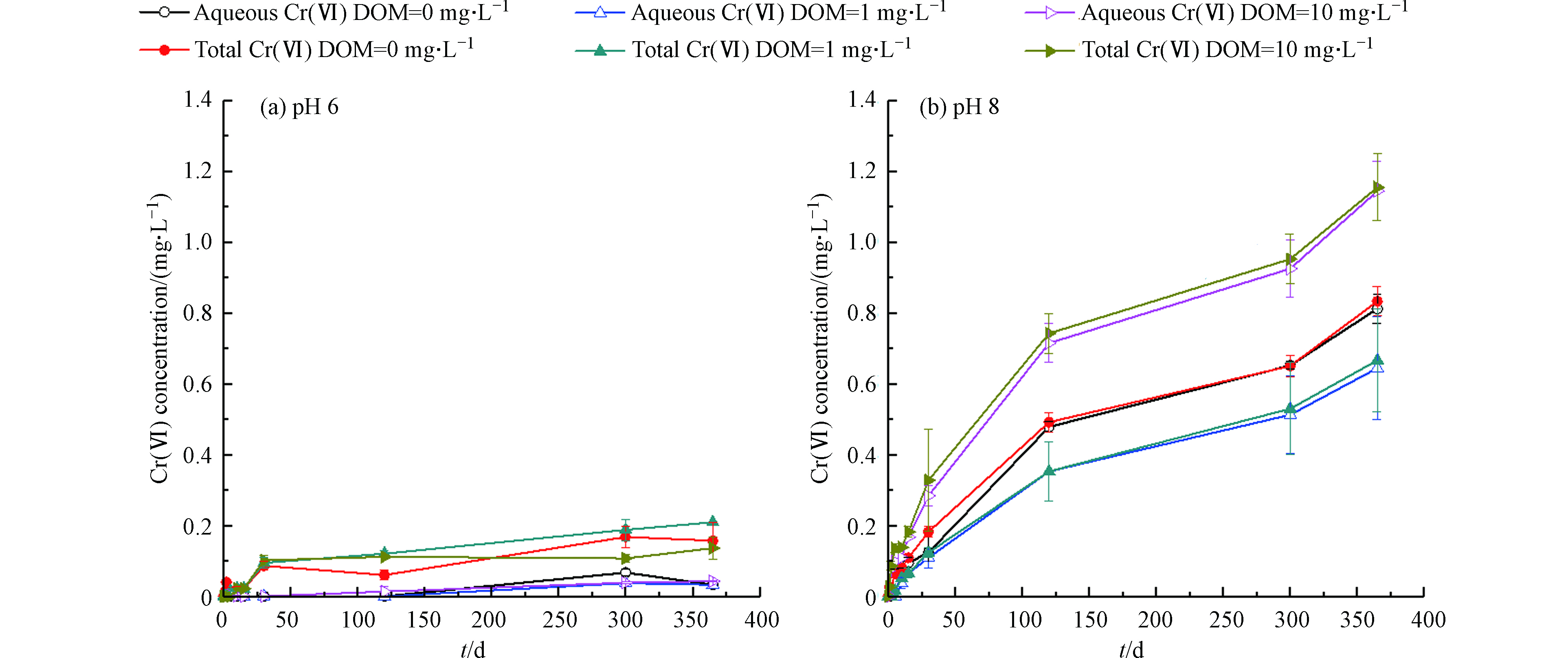
图5展示了DOM浓度为0、1、10 mg·L−1条件下,水溶液中δ-MnO2对非晶态Cr(OH)3氧化产生的Cr(Ⅵ)的浓度随时间的变化。由图5(a)可知,在pH=6时,溶解态Cr(Ⅵ)和总Cr(Ⅵ)浓度随时间呈上升趋势,但通过显著性检验,DOM对δ-MnO2氧化Cr(OH)3的影响不显著。可能是由于酸性条件下Mn(Ⅱ)被DO氧化为锰氧化物这一反应被抑制,使得锰氧化物氧化Cr(OH)3的反应处于被抑制的状态。从而,我们可以认为在弱酸条件(pH=6)下,DOM对δ-MnO2氧化Cr(OH)3的影响不显著。
由图5(b)可知,在pH=8时,溶解态Cr(Ⅵ)和总Cr(Ⅵ)浓度随时间呈上升趋势,反应至365 d时DOM=0、1、10 mg·L−1的条件下总Cr(Ⅵ)浓度分别为0.83、0.66、1.54 mg·L−1。通过显著性检验,低浓度DOM对δ-MnO2氧化Cr(OH)3的影响不显著(Ρ<0.05);而高浓度DOM则对δ-MnO2氧化Cr(OH)3具有协同作用。可能是高浓度DOM促进了Cr(OH)3的溶解,使更多Cr(Ⅲ)从固相转移到液相并与DOM反应生成可溶配合物[24-25]。相对于沉淀态Cr(OH)3,Cr(Ⅲ)-DOM络合物与δ-MnO2反应具有更高的氧化速率和更大的Cr(Ⅵ)生成量[7]。而低浓度DOM促进Cr(OH)3溶解的作用不显著,Cr(Ⅲ)-DOM络合物生成量较小。
MANDIWANA等[24]研究发现Cr(Ⅲ)在土壤中的溶解度取决于低分子量有机酸的存在,低分子量有机酸能促进Cr(Ⅲ)的浸出。GUSTAFSSON等[25]使用EXAFS光谱和批实验研究了Cr(Ⅲ)与富里酸的络合过程。EXAFS结果表明,Cr(Ⅲ)会与富里酸产生配合反应,在低pH(<5)下,单体Cr(Ⅲ)-NOM络合物占优势,而二聚体配合物(RO)3Cr2(OH)2+在较高的pH值下占主导地位。戴儒南[7]研究人工合成的水钠锰矿(δ-MnO2)对水溶态Cr(Ⅲ)、有机结合态Cr(Ⅲ)以及沉淀态Cr(Ⅲ)的氧化过程,发现相对于沉淀态Cr(Ⅲ),有机结合态Cr(Ⅲ)更易于被MnO2氧化。
-
相关研究[26]认为MnO2和Cr(Ⅲ)的氧化还原反应如式(4)所示。近年来研究[11]探究Mn(Ⅱ)氧化反应诱导Cr(OH)3(s)发生氧化机制发现,Mn(Ⅱ)在Cr(OH)3(s)表面被催化氧化形成锰氧化物,Cr(OH)3(s)溶解释放的溶解态Cr(Ⅲ)在锰氧化物表面被催化氧化生成Cr(Ⅵ),同时锰氧化物被还原为Mn(Ⅱ),如式(4)—(7)所示。
可从吸附和氧化还原两个过程来分析碱性条件更能促进 δ -MnO2氧化Cr(OH)3的原因。就吸附过程而言,Cr(OH)3的表面等电点为8.03[11],相较于pH=8的情况,在pH=6时Cr(OH)3所带正电荷更多,与Mn(Ⅱ)的静电排斥作用力更大,不利于Mn(Ⅱ)吸附至Cr(OH)3表面,从而抑制了≡Mn(Ⅱ)氧化为锰氧化物,进而抑制了Cr(OH)3与锰氧化物的反应。
从氧化还原过程的角度分析,由式(6)可知,Mn(Ⅱ)可被氧气氧化为锰氧化物,但酸性条件下不利于反应的进行。SEONYI [11]的研究表明,在pH≤8时,DO氧化溶解态Mn(Ⅱ)的速度比较慢。相比之下,在pH=9条件下,Mn(Ⅱ)可能通过被氧化形成锰氧化物而导致溶解的Mn(Ⅱ)被去除。因此,酸性条件会抑制Mn(Ⅱ)被DO氧化为锰氧化物,从而抑制了Cr(OH)3与锰氧化物的氧化反应。
-
(1)含氧水环境中非晶态Cr(OH)3具有较好的长期稳定性,但是,δ-MnO2会显著促进Cr(OH)3的氧化过程。
(2)弱碱条件(pH=8)相较于弱酸条件(pH=6)更能促进δ-MnO2对非晶态Cr(OH)3的氧化。弱酸条件(pH=6)下生成的Cr(Ⅵ)包含溶解态和固相结合态;弱碱条件(pH=8)下δ-MnO2氧化非晶态Cr(OH) 3生成的Cr(Ⅵ)主要以溶解态存在。
( 3)弱酸条件(pH=6)下,DOM对δ-MnO2氧化非晶态Cr(OH)3的影响不显著;弱碱条件(pH=8)下,低浓度DOM对δ-MnO2氧化非晶态Cr(OH)3的影响不显著,高浓度DOM则可显著增大非晶态Cr(OH)3的氧化量。
含氧水溶液中δ-MnO2对非晶态Cr(OH)3的化学氧化:影响因素和作用机理
Chemical oxidation of amorphous Cr(OH)3 in oxygen-containing aqueous solution: influencing factors and mechanisms
-
摘要: 对六价铬污染水体和土壤修复常采用化学还原方法,但是当前研究对还原产物非晶态Cr(OH)3的再氧化过程以及环境酸碱性、有机质等因素对该过程的影响仍有较多分歧。本研究通过批试验,观测了含氧水溶液体系中非晶态Cr(OH)3的长期稳定性及pH和溶解性有机质(Dissolved organic matter,DOM)对δ-MnO2氧化非晶态Cr(OH)3的影响。研究结果表明,在含氧水溶液中,Cr(OH)3具有较好的长期稳定性;δ-MnO2会显著增大Cr(OH)3的氧化量,Cr(Ⅵ)生成量从0.03 mg·L−1增大为0.83 mg·L−1。环境酸碱性可显著影响δ-MnO2对Cr(OH)3的氧化行为,碱性条件下Cr(Ⅵ)生成量更大。当pH=8,反应365 d时,总Cr(Ⅵ)浓度可达0.83 mg·L−1;pH=6时,反应365 d时,总Cr(Ⅵ)浓度为0.15 mg·L−1。弱酸条件(pH=6)下生成的Cr(VI)以溶解态和固相结合态存在;弱碱条件(pH=8)下δ-MnO2氧化Cr(OH)3生成的Cr(Ⅵ)主要以溶解态存在。DOM对δ-MnO2氧化Cr(OH)3的影响在弱酸条件(pH=6)下不显著;弱碱条件(pH=8)下,低浓度DOM (1 mg·L−1)对δ-MnO2氧化Cr(OH)3的影响不显著,高浓度DOM(10 mg·L−1)可显著增大Cr(OH)3的氧化量。Abstract: Chemical reduction methods are generally used to remediate hexavalent chromium-contaminated water and soil, but the reoxidation process of amorphous Cr(OH)3—the main reduction product—and the effects of pH, organic matter remain unclear. This study investigated the long-term stability of amorphous Cr(OH)3 and the effects of pH and dissolved organic matter (DOM) on the oxidation of amorphous Cr(OH)3 by δ-MnO2 in an oxygen-containing aqueous solution system through batch experiments. The results showed that Cr(OH)3 had long-term stability in an oxygen-containing aqueous solution. δ-MnO2 significantly increased the oxidation of Cr(OH)3, and the amount of generated Cr(Ⅵ) increased from 0.03 to 0.83 mg·L−1. pH significantly affected the oxidation of Cr(OH)3 by δ-MnO2, and more Cr(Ⅵ) was produced under alkaline conditions than acidic conditions. When the reaction time reached 365 d, the total Cr(Ⅵ) concentration reached 0.83 mg·L−1 at pH=8, whereas the total Cr(Ⅵ) concentration was 0.15 mg·L−1 at pH=6. At a weakly acidic pH (pH=6), the generated Cr(Ⅵ) mainly existed as dissolved ions and solid-binding species, whereas at a weakly alkaline pH (pH=8), the generated Cr(Ⅵ) mainly existed as dissolved ions. DOM had an insignificant effect on the oxidation of Cr(OH)3 by δ-MnO2 under weakly acidic (pH=6) conditions. Under weakly alkaline (pH=8) conditions, the effect of DOM on the oxidation of Cr(OH)3 by δ-MnO2 was not significant at a low concentration of 1 mg·L−1; however, a high DOM concentration of 10 mg·L−1 significantly increased the oxidation of Cr(OH)3.
-
Key words:
- chromium hydroxide /
- manganese dioxide /
- chemical oxidation /
- pH /
- dissolved organic matter
-
Cr(Ⅵ)是广为关注的地下水和土壤污染物之一。目前,在酸性条件下加入还原剂将Cr(Ⅵ)还原为Cr(Ⅲ),随后生成Cr(OH)3沉淀来降低其毒性是修复铬污染环境介质的常用策略[1-3],如式(1)所示。此外,在自然环境中也存在Cr(Ⅵ)的还原过程,铁还原菌可以通过产生Fe(Ⅱ)抑制厌氧条件下Cr(Ⅵ)的产生[4]。还原生成的Cr(Ⅲ)主要以非晶态Cr(OH)3存在,其原子排列具有近程有序、长程无序状态特性,在热力学上属于非平衡的亚稳态[5]。
stringUtils.convertMath(!{formula.content}) (1) 在对Cr(Ⅵ)污染土壤和地下水修复后,较长时间后会出现Cr(Ⅵ)再次超标现象。有研究[6]认为,Cr(Ⅲ)在一定环境条件下会被再次氧化生成Cr(Ⅵ),是土壤和地下水中Cr(Ⅵ)浓度升高的原因之一。人们认为锰氧化物是将还原生成的Cr(Ⅲ)重新氧化的氧化剂[7]。Mcclain等[8]发现矿物结合态的Cr(Ⅲ)会与结构类似水钠锰矿的生物性Mn(Ⅲ/Ⅳ)氧化物结合并生成Cr(Ⅵ)。Gonzalez等[9]认为加州圣克鲁斯县饮用水中所存在的大量Cr(Ⅵ)是由于Cr (Ⅲ)矿床被锰氧化物氧化。
stringUtils.convertMath(!{formula.content}) (2) 研究表明[5],锰氧化物的类型和结构(包括δ-MnO2、水钠锰矿、水锰矿、钡镁锰矿、软锰矿等)对锰氧化物氧化Cr(Ⅲ)的速率和比率影响较大。由于晶性、价态和形态不同,氧化锰对Cr(Ⅲ)氧化能力有明显差异,顺序为δ-MnO2>α-MnO2>γ-MnOOH。相较于γ-MnOOH,δ-MnO2、α-MnO2中的Mn氧化度和活度更高,且δ-MnO2的晶性较差、比表面积大,故δ-MnO2对Cr(Ⅲ)的氧化能力强,γ-MnOOH相对较弱[10]。
当前研究中,对环境酸碱性在锰氧化物对Cr(Ⅲ)氧化过程的影响的认识尚存有争议。SEONYI等[11]研究了Cr(OH)3(s)和Mn(Ⅱ)共存时Cr(OH)3(s)的氧化反应,pH=7时未发现Cr(Ⅵ)的生成,pH≥8时,Cr(OH)3(s)被氧化并释放大量Cr(Ⅵ)。OZE等[12]探究了Cr(Ⅲ)与水钠锰矿反应19 d时Cr(Ⅵ)的生成速率,发现pH=7时,Cr(Ⅵ)的生成速率为0.001 μmol·d−1,pH=8时,反应速率为0,说明pH的升高会减小Cr(Ⅵ)的生成量。而PAN等[13]研究了δ-MnO2氧化铬铁氢氧化物的速率和机制,认为在Cr(Ⅲ)初始浓度为770 μmol·L−1、δ-MnO2初始浓度为436 μmol·L−1时,随着pH从5增大到9,Cr(Ⅵ)产生量从80 μmol·L−1逐渐减小至15 μmol·L−1。
本研究旨在探究含氧水环境中非晶态Cr(OH)3的氧化过程及环境酸碱性、有机质等环境因素对该过程的影响,具体包括:(1)实验观测δ-MnO2对水溶液中非晶态Cr(OH)3的氧化过程;(2)分析pH和DOM对δ-MnO2氧化非晶态Cr(OH)3的影响及潜在机理。
1. 材料与方法(Materials and methods)
1.1 实验材料
采用HUANG等[14]的方法制备非晶态Cr(OH)3,具体方法如下:25 ℃时,将0.2 mol·L−1氢氧化钠溶液以1 mL·min−1的速度加入至0.089 mol·L−1氯化铬溶液中并不断搅拌。当pH值达8.5时,停止加入氢氧化钠溶液并持续搅拌30 min。将悬浮液离心后倒去上清液,用蒸馏水洗涤3次,丙酮洗涤2次,乙醇洗涤1次。将固相材料置于25 ℃的真空干燥箱中干燥后研磨、过200目筛并充氮气密封保存。图1(a)为制备的Cr(OH)3的X射线衍射(X-ray diffraction,XRD)图谱,结果表明Cr(OH)3在18 °、19 °、26 °、38 °、50 °等位置均未发现明显的衍射峰,且谱线含有较多的毛刺,与HUANG等[14]制备的非晶态Cr(OH)3的表征结果相似,这表明实验制备的Cr(OH)3为非晶态。
采用VILLALOBOS等[15]的方法制备δ-MnO2。具体方法如下:250 mL的0.20 mol·L−1高锰酸钾溶液和250 mL的0.47 mol·L−1氯化锰溶液依次缓慢加入至270 mL的0.49 mol·L−1氢氧化钠溶液中,并不断搅拌。悬浮液静置分层,虹吸出上清液。剩余的悬浮液离心后倒掉上清液。离心后的物质与1 mol·L−1 NaCl混合振荡1 h后离心,倒去上清液,此过程重复5次,最后一次振荡持续12 h。将离心后物质置于透析袋,用去离子水透析整夜至pH=7.79。离心后的氧化物置于25 ℃的真空干燥箱内进行干燥、研磨,过200目筛后充氮气密封保存。图1(b)为δ-MnO2的X射线衍射图谱,与VILLALOBOS等[15]的研究结果相似,在37 °、45 °、49 °、66 °等位置有明显的衍射峰,在19 °、59 °的特征峰消失,且谱线含有较多的毛刺,这表明实验制备的δ-MnO2结晶度较差或者晶体呈无定型。
采用黄中林[16]的方法制备晶态Cr(OH)3,用K2Cr2O7和去离子水(18.25 MΩ·cm)配制Cr(Ⅵ)储存液,采用Na2HPO3、NaH2PO3和去离子水配制磷酸盐缓冲液;除了特殊说明外,均使用分析纯试剂。
1.2 δ-MnO2对含氧水溶液中非晶态Cr(OH)3的氧化过程
在温度25 ℃、空气复氧、固定pH、特定DOM浓度等条件下,于1 L锥形瓶中加入50 mg·L−1非晶态Cr(Ⅲ)和50 mg·L−1 δ-MnO2,用NaCl控制体系离子强度为5 mmol·L−1,反应体系体积为700 mL。分别借助1 mmol·L−1 MES缓冲溶液和5 mmol·L−1 MOPS缓冲溶液控制体系pH为6和8;采用富里酸作为典型DOM观测溶解性有机质的影响,其浓度水平定为1、10 mg·L−1。为确保体系悬浮液的均匀混合以及其能与氧气充分接触,整个过程采用1000 r·min−1磁力搅拌。在反应进行至一定时间(1 d和2 d;取出后静置5、10、15、30、120、300、365 d)取样测定;取1 mL悬浮液立即用0.2 μm PES过滤器过滤后,分析滤液中Cr(Ⅵ)浓度;另取1 mL悬浮液,加入0.01 mL磷酸盐缓冲液,采用200 r·min−1振荡1 h后,用0.2 μm PES过滤器过滤,取滤液分析Total Cr(Ⅵ)浓度。每组实验均设置两个平行样。
1.3 表征和测定方法
采用X射线衍射仪(PANalytical X’Pert Powder,Spectris Pte. Ltd)对制备的Cr(OH)3和δ-MnO2进行物相分析,检测条件为:Cu靶;DS狭缝为1°;RS狭缝为0.3 mm;SS狭缝为1°;连续扫面;扫描速度为2(°)·min−1;采样间隔为0.02°;扫描范围5°—90°。采用X射线光电子能谱仪(ESCALAB250Xi,赛默飞世尔)对pH值为6和8、有氧条件下δ-MnO2氧化Cr(OH)3生成的反应产物进行表面分析。
使用梅特勒-托利多pH计测定pH值,采用二苯碳酰二肼分光光度法(GB/T 7467-1987)利用紫外-可见分光光度计(T6,北京普析)测定液相中水溶态Cr(Ⅵ)浓度,采用溶解氧仪(Seven2Go,METTLER TOLEDO)测定水溶液中溶解氧(Dissolved oxygen,DO)浓度,采用多参数水质分析仪(S220,梅特勒-托利多)测定反应体系的氧化还原电位(Electric potential of half cells,Eh)。
1.4 数据处理
本研究所测得的吸光度由紫外可见分光光度计控制并由分析软件自动生成,所获得的实验数据均采用Excel数据分析软件进行分析,并用origin 2017软件进行制图;XRD数据均采用jade 6.6软件进行分析,并用origin 2017软件进行制图;X射线光电子能谱(X-ray photoelectron spectroscopy,XPS)数据均采用Avantage软件进行分析,并用origin 2017软件进行制图。
2. 结果与讨论(Results and discussion)
2.1 δ-MnO2对非晶态Cr(OH)3氧化过程动力学
图2展示了δ-MnO2与非晶态Cr(OH)3反应过程中Cr(Ⅵ)浓度随时间的变化。由图2(a)可知,在pH=8时,反应300 d时可检测到0.02 mg·L−1的Cr(Ⅵ),说明氧气氧化Cr(OH)3的反应速率较慢,在整个检测的时间尺度内Cr(Ⅵ)浓度均低于Ⅴ类地下水质量标准(0.1 mg·L−1)[17];投加了δ-MnO2之后,水溶液中Cr(Ⅵ)浓度随时间呈上升趋势,反应至365 d,水溶液中总Cr(Ⅵ)浓度为0.83 mg·L−1,表明在弱碱条件(pH=8)下的含氧水溶液中,Cr(OH)3具有长期稳定性,而δ-MnO2会使Cr(OH)3的氧化量显著增大。
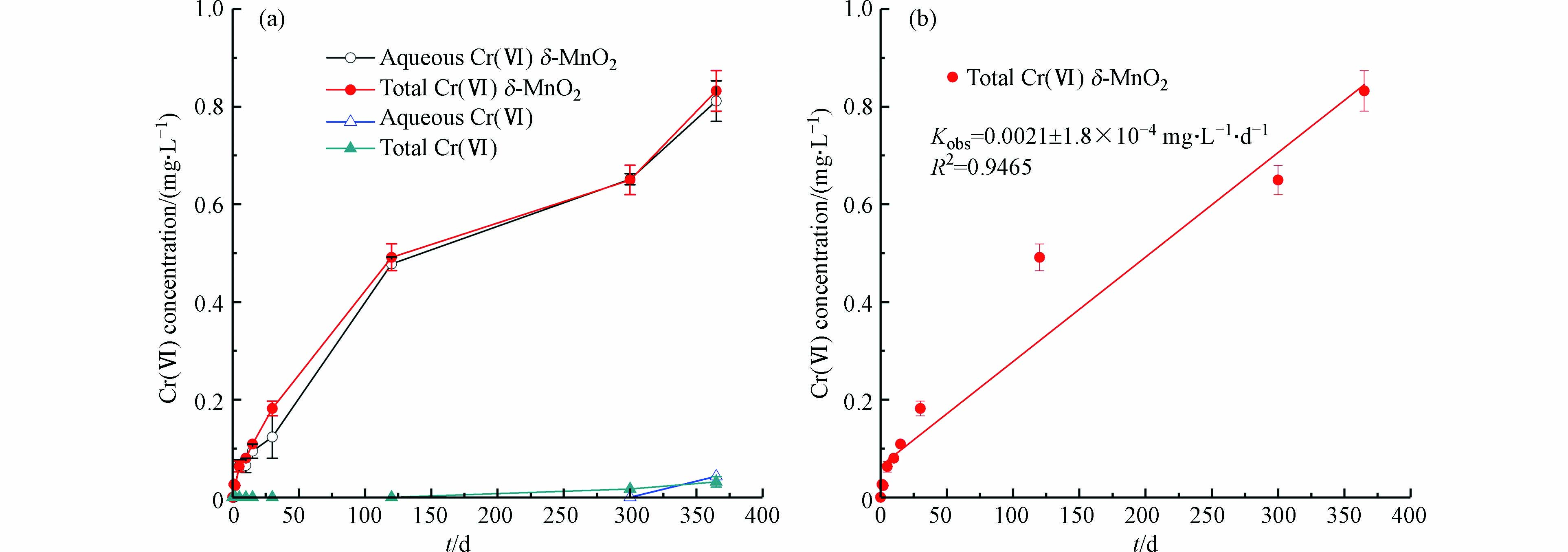 图 2 (a)含氧水溶液中δ-MnO2与Cr(OH)3反应体系中Cr(Ⅵ)浓度随时间的变化;(b)伪零级动力学拟合Figure 2. (a) The change in Cr(Ⅵ) concentration over time in the reaction system containing δ-MnO2 and Cr(OH)3 in an oxygen-containing aqueous solution; (b) Pseudo-zero-order dynamic fitting(空气复氧,pH 8,[Cr(Ⅲ)]0 = 50 mg·L−1,[δ-MnO2]0 = 50 mg·L−1,(25±3) ℃,1000 r·min−1,DO = 7.86—8.34 mg·L−1)
图 2 (a)含氧水溶液中δ-MnO2与Cr(OH)3反应体系中Cr(Ⅵ)浓度随时间的变化;(b)伪零级动力学拟合Figure 2. (a) The change in Cr(Ⅵ) concentration over time in the reaction system containing δ-MnO2 and Cr(OH)3 in an oxygen-containing aqueous solution; (b) Pseudo-zero-order dynamic fitting(空气复氧,pH 8,[Cr(Ⅲ)]0 = 50 mg·L−1,[δ-MnO2]0 = 50 mg·L−1,(25±3) ℃,1000 r·min−1,DO = 7.86—8.34 mg·L−1)图2(b)为伪零级动力学模型对δ-MnO2对Cr(OH)3氧化过程的拟合结果。由图2(b)可知,δ-MnO2对Cr(OH)3的氧化过程较好的符合伪零级反应动力学,速率常数为0.0021 mg·L−1·d−1,R2=0.9465。呈现出Cr(Ⅵ)的生成量与Cr(Ⅵ)浓度无关,随时间线性增大的趋势,这与PAN等[13]发现的锰氧化物氧化CrxFe1−x(OH)3过程 Cr(Ⅵ)浓度随时间持续上升的趋势一致。
SCHROEDER等[18]研究了土壤中溶解氧对于三价铬氧化反应的影响,认为溶解氧在氧化三价铬过程中起到非常微小的作用,两周之后检测到有极其微小的三价铬被氧化。EARY等[19]研究发现在溶解氧浓度约为8 mg·L−1和pH=4—12.5条件下,浓度为19 μmol·L−1的三价铬溶液静置反应24 d后未检出Cr(Ⅵ)。PAN等[13]研究了MnO2对Cr0.23Fe0.77(OH)3氧化的影响,结果表明反应至400 h时检测到Cr(Ⅵ)的生成量为0.2 mg·L−1。本研究发现与前人的研究相符,有氧条件下,Cr(OH)3具有长期稳定性,δ-MnO2会显著增大Cr(OH)3的氧化量。
2.2 环境酸碱性对δ-MnO2氧化非晶态Cr(OH)3的影响
2.2.1 Cr(Ⅵ)氧化生成量
图3展示了典型环境酸碱条件下水溶液中δ-MnO2对非晶态Cr(OH)3氧化产生的Cr(Ⅵ)浓度随时间的变化。从图3可知,pH=8时,反应至365 d时总Cr(Ⅵ)浓度达到0.83 mg·L−1,明显高于pH=6条件下水溶液中Cr(Ⅵ)浓度,且溶液中溶解态Cr(Ⅵ)和总Cr(Ⅵ)浓度几乎相等,说明生成的Cr(Ⅵ)主要以水溶态存在。同时,观测到典型环境酸碱条件下反应体系的氧化还原电位随时间呈逐渐增大趋势;随着反应的进行,反应体系中Cr(Ⅵ)含量逐渐增大,根据能斯特方程,如式(3)所示,随着氧化性强的Cr(Ⅵ)浓度的增大,氧化还原电位也会逐渐增大。相较于pH=8(Eh=243 mV),pH=6条件下反应体系的初始状态氧化还原电位(Eh=332 mV)更高,说明pH值对氧化还原电位有较大影响。SEONYI等[11]研究发现在大气环境条件中,pH=7.0—9.0的情况下,Cr(OH)3(s)和溶解态Mn(Ⅱ)共存时,pH≥8时,Cr(OH)3(s)被氧化并释放大量Cr(Ⅵ),且Cr(Ⅵ)的生成量随着pH值升高而增大,与本研究在弱碱条件(pH=8)下得出的研究结果一致。pH=6时,30 d内总Cr(Ⅵ)浓度快速上升,之后上升速度趋于平缓,在300 d已达到平衡,总Cr(Ⅵ)的平衡浓度为0.15 mg·L−1;而溶解态Cr(Ⅵ)在反应300 d时才被检出,出现这种现象的原因可能是
CrO2−4 HCrO−4 stringUtils.convertMath(!{formula.content}) (3) 2.2.2 固相产物分析
图4是pH=6和pH=8条件下δ-MnO2氧化非晶态Cr(OH)3生成固相产物的XPS全谱图和Cr 2p XPS图谱。由图4(a)可知,弱酸条件(pH=6)下产生的固相产物表面存在Mn、Cr、O等元素;由图4(b)可知,pH=6时,结合能为577.19、586.92 eV[20]处的峰代表的是Cr(Ⅲ)的2p3/2轨道,结合能为578.62、588.81eV[21]处的峰代表Cr(Ⅵ) 的2p3/2轨道,这表明固相产物中Cr以Cr(Ⅲ)和Cr(Ⅵ)形式存在,反应体系中产生的Cr(Ⅵ)部分为固相结合态。由图4(c)可知,弱碱条件(pH=8)下产生的固相产物表面存在Mn、Cr、O等元素;由图4(d)可知,pH=8时,结合能为576.61[22]、577.48[20]、586.64 eV[23]处的峰代表的是Cr(Ⅲ)的2p3/2轨道,未检测到Cr(Ⅵ)的结合能,这表明固相产物中Cr主要以Cr(Ⅲ)形式存在,反应体系中产生的Cr(Ⅵ)主要以溶解态存在,固相中Cr(Ⅵ)含量极低。这与2.2.1节中水溶态Cr(Ⅵ)和总Cr(Ⅵ)测试结果保持一致。这可能是由于生成的Cr(Ⅵ)在水溶液中主要以
HCrO−4 CrO2−4 HCrO−4 CrO2−4  图 4 δ-MnO2氧化Cr(OH)3生成固相产物的 (a) XPS全谱图(pH=6);(b) Cr 2p XPS图谱(pH=6);(c) XPS全谱图(pH=8);(d) Cr 2p XPS图谱(pH=8);Figure 4. (a) Survey XPS spectra (pH=6), (b) Cr 2p XPS spectra (pH=6), (c) survey XPS spectra (pH=8) and (d) Cr 2p XPS spectra (pH=8) of the solid phase product formed from the oxidation of Cr(OH)3 by δ-MnO2.
图 4 δ-MnO2氧化Cr(OH)3生成固相产物的 (a) XPS全谱图(pH=6);(b) Cr 2p XPS图谱(pH=6);(c) XPS全谱图(pH=8);(d) Cr 2p XPS图谱(pH=8);Figure 4. (a) Survey XPS spectra (pH=6), (b) Cr 2p XPS spectra (pH=6), (c) survey XPS spectra (pH=8) and (d) Cr 2p XPS spectra (pH=8) of the solid phase product formed from the oxidation of Cr(OH)3 by δ-MnO2.2.3 DOM对δ-MnO2氧化非晶态Cr(OH)3的影响
图5展示了DOM浓度为0、1、10 mg·L−1条件下,水溶液中δ-MnO2对非晶态Cr(OH)3氧化产生的Cr(Ⅵ)的浓度随时间的变化。由图5(a)可知,在pH=6时,溶解态Cr(Ⅵ)和总Cr(Ⅵ)浓度随时间呈上升趋势,但通过显著性检验,DOM对δ-MnO2氧化Cr(OH)3的影响不显著。可能是由于酸性条件下Mn(Ⅱ)被DO氧化为锰氧化物这一反应被抑制,使得锰氧化物氧化Cr(OH)3的反应处于被抑制的状态。从而,我们可以认为在弱酸条件(pH=6)下,DOM对δ-MnO2氧化Cr(OH)3的影响不显著。
由图5(b)可知,在pH=8时,溶解态Cr(Ⅵ)和总Cr(Ⅵ)浓度随时间呈上升趋势,反应至365 d时DOM=0、1、10 mg·L−1的条件下总Cr(Ⅵ)浓度分别为0.83、0.66、1.54 mg·L−1。通过显著性检验,低浓度DOM对δ-MnO2氧化Cr(OH)3的影响不显著(Ρ<0.05);而高浓度DOM则对δ-MnO2氧化Cr(OH)3具有协同作用。可能是高浓度DOM促进了Cr(OH)3的溶解,使更多Cr(Ⅲ)从固相转移到液相并与DOM反应生成可溶配合物[24-25]。相对于沉淀态Cr(OH)3,Cr(Ⅲ)-DOM络合物与δ-MnO2反应具有更高的氧化速率和更大的Cr(Ⅵ)生成量[7]。而低浓度DOM促进Cr(OH)3溶解的作用不显著,Cr(Ⅲ)-DOM络合物生成量较小。
MANDIWANA等[24]研究发现Cr(Ⅲ)在土壤中的溶解度取决于低分子量有机酸的存在,低分子量有机酸能促进Cr(Ⅲ)的浸出。GUSTAFSSON等[25]使用EXAFS光谱和批实验研究了Cr(Ⅲ)与富里酸的络合过程。EXAFS结果表明,Cr(Ⅲ)会与富里酸产生配合反应,在低pH(<5)下,单体Cr(Ⅲ)-NOM络合物占优势,而二聚体配合物(RO)3Cr2(OH)2+在较高的pH值下占主导地位。戴儒南[7]研究人工合成的水钠锰矿(δ-MnO2)对水溶态Cr(Ⅲ)、有机结合态Cr(Ⅲ)以及沉淀态Cr(Ⅲ)的氧化过程,发现相对于沉淀态Cr(Ⅲ),有机结合态Cr(Ⅲ)更易于被MnO2氧化。
2.4 非晶态Cr(OH)3被氧化产生Cr(Ⅵ)的主要机制
相关研究[26]认为MnO2和Cr(Ⅲ)的氧化还原反应如式(4)所示。近年来研究[11]探究Mn(Ⅱ)氧化反应诱导Cr(OH)3(s)发生氧化机制发现,Mn(Ⅱ)在Cr(OH)3(s)表面被催化氧化形成锰氧化物,Cr(OH)3(s)溶解释放的溶解态Cr(Ⅲ)在锰氧化物表面被催化氧化生成Cr(Ⅵ),同时锰氧化物被还原为Mn(Ⅱ),如式(4)—(7)所示。
stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) stringUtils.convertMath(!{formula.content}) (6) stringUtils.convertMath(!{formula.content}) (7) 可从吸附和氧化还原两个过程来分析碱性条件更能促进 δ -MnO2氧化Cr(OH)3的原因。就吸附过程而言,Cr(OH)3的表面等电点为8.03[11],相较于pH=8的情况,在pH=6时Cr(OH)3所带正电荷更多,与Mn(Ⅱ)的静电排斥作用力更大,不利于Mn(Ⅱ)吸附至Cr(OH)3表面,从而抑制了≡Mn(Ⅱ)氧化为锰氧化物,进而抑制了Cr(OH)3与锰氧化物的反应。
从氧化还原过程的角度分析,由式(6)可知,Mn(Ⅱ)可被氧气氧化为锰氧化物,但酸性条件下不利于反应的进行。SEONYI [11]的研究表明,在pH≤8时,DO氧化溶解态Mn(Ⅱ)的速度比较慢。相比之下,在pH=9条件下,Mn(Ⅱ)可能通过被氧化形成锰氧化物而导致溶解的Mn(Ⅱ)被去除。因此,酸性条件会抑制Mn(Ⅱ)被DO氧化为锰氧化物,从而抑制了Cr(OH)3与锰氧化物的氧化反应。
3. 结论(Conclusion)
(1)含氧水环境中非晶态Cr(OH)3具有较好的长期稳定性,但是,δ-MnO2会显著促进Cr(OH)3的氧化过程。
(2)弱碱条件(pH=8)相较于弱酸条件(pH=6)更能促进δ-MnO2对非晶态Cr(OH)3的氧化。弱酸条件(pH=6)下生成的Cr(Ⅵ)包含溶解态和固相结合态;弱碱条件(pH=8)下δ-MnO2氧化非晶态Cr(OH) 3生成的Cr(Ⅵ)主要以溶解态存在。
( 3)弱酸条件(pH=6)下,DOM对δ-MnO2氧化非晶态Cr(OH)3的影响不显著;弱碱条件(pH=8)下,低浓度DOM对δ-MnO2氧化非晶态Cr(OH)3的影响不显著,高浓度DOM则可显著增大非晶态Cr(OH)3的氧化量。
-
图 4 δ-MnO2氧化Cr(OH)3生成固相产物的 (a) XPS全谱图(pH=6);(b) Cr 2p XPS图谱(pH=6);(c) XPS全谱图(pH=8);(d) Cr 2p XPS图谱(pH=8);
Figure 4. (a) Survey XPS spectra (pH=6), (b) Cr 2p XPS spectra (pH=6), (c) survey XPS spectra (pH=8) and (d) Cr 2p XPS spectra (pH=8) of the solid phase product formed from the oxidation of Cr(OH)3 by δ-MnO2.
-
[1] 王兴润, 李磊, 颜湘华, 等. 铬污染场地修复技术进展 [J]. 环境工程, 2020, 38(6): 1-8,23. WANG X R, LI L, YAN X H, et al. Progress in remediation of chromium-contaminated sites [J]. Environmental Engineering, 2020, 38(6): 1-8,23(in Chinese).
[2] 刘涛, 祝方, 赵晋宇, 等. 降雨条件下纳米零价铁镍对污染土壤中六价铬迁移的影响 [J]. 环境化学, 2017, 36(4): 812-820. doi: 10.7524/j.issn.0254-6108.2017.04.2016080201 LIU T, ZHU F, ZHAO J Y, et al. Effect of nano zero valent iron/nickel under rain condition on the transportation of hexavalent chromium in contaminated soil [J]. Environmental Chemistry, 2017, 36(4): 812-820(in Chinese). doi: 10.7524/j.issn.0254-6108.2017.04.2016080201
[3] 高卫国, 钱林波, 韩璐, 等. 锰铁氧体吸附及催化柠檬酸还原六价铬的过程及机理 [J]. 环境化学, 2018, 37(7): 1525-1533. doi: 10.7524/j.issn.0254-6108.2017101302 GAO W G, QIAN L B, HAN L, et al. Iron manganese minerals catalyzed Cr(Ⅵ) reduction by citric acid and its mechanism [J]. Environmental Chemistry, 2018, 37(7): 1525-1533(in Chinese). doi: 10.7524/j.issn.0254-6108.2017101302
[4] MENG Y, ZHAO Z W, BURGOS W D, et al. Iron(Ⅲ) minerals and anthraquinone-2, 6-disulfonate (AQDS) synergistically enhance bioreduction of hexavalent chromium by Shewanella oneidensis MR-1 [J]. Science of the Total Environment, 2018, 640/641: 591-598. doi: 10.1016/j.scitotenv.2018.05.331 [5] TANG Y Z, WEBB S M, ESTES E R, et al. Chromium(III) oxidation by biogenic manganese oxides with varying structural ripening [J]. Environmental Science. Processes & Impacts, 2014, 16(9): 2127-2136. [6] 许维通, 苑文仪, 李培中, 等. 六价铬污染土壤还原处理后再氧化因素分析综述 [J]. 环境工程, 2018, 36(10): 130-134, 58. XU W T, YUAN W Y, LI P Z, et al. Analysis of re-oxidation factors in Cr(Ⅵ) contaminated soil after reduction treatment: A review [J]. Environmental Engineering, 2018, 36(10): 130-134, 58(in Chinese).
[7] 戴儒南. 环境中Cr(Ⅲ)的MnO2氧化以及光化学氧化研究[D]. 南京: 南京农业大学, 2010. DAI R N. The oxidation of Cr (Ⅲ) by MnO2 and its photooxidation in environments[D]. Nanjing: Nanjing Agricultural University, 2010(in Chinese).
[8] MCCLAIN C N, FENDORF S, WEBB S M, et al. Quantifying Cr(Ⅵ) production and export from serpentine soil of the California Coast range [J]. Environmental Science & Technology, 2017, 51(1): 141-149. [9] GONZALEZ A R, NDUNG'U K, FLEGAL A R. Natural occurrence of hexavalent chromium in the aromas red sands aquifer, California [J]. Environmental Science & Technology, 2005, 39(15): 5505-5511. [10] 孙亚乔, 校康, 段磊, 等. 粘土矿物作用下铬的迁移转化机理研究进展 [J]. 生态环境学报, 2019, 28(7): 1484-1491. SUN Y Q, XIAO K, DUAN L, et al. Chromium migration and transformation mechanism in the presence of clay minerals: A review [J]. Ecology and Environmental Sciences, 2019, 28(7): 1484-1491(in Chinese).
[11] NAMGUNG S, KWON M J, QAFOKU N P, et al. Cr(OH)3(s) oxidation induced by surface catalyzed Mn(Ⅱ) oxidation [J]. Environmental Science & Technology, 2014, 48(18): 10760-10768. [12] OZE C, BIRD D K, FENDORF S. Genesis of hexavalent chromium from natural sources in soil and groundwater [J]. PNAS, 2007, 104(16): 6544-6549. doi: 10.1073/pnas.0701085104 [13] PAN C, LIU H, CATALANO J G, et al. Rates of Cr(Ⅵ) generation from CrxFe1-x(OH)3 solids upon reaction with manganese oxide [J]. Environmental Science & Technology, 2017, 51(21): 12416-12423. [14] HUANG Z L, CHEN C G, XIE J Y, et al. The evolution of dehydration and thermal decomposition of nanocrystalline and amorphous chromium hydroxide [J]. Journal of Analytical and Applied Pyrolysis, 2016, 118: 225-230. doi: 10.1016/j.jaap.2016.02.006 [15] VILLALOBOS M, TONER B, BARGAR J, et al. Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1 [J]. Geochimica et Cosmochimica Acta, 2003, 67(14): 2649-2662. doi: 10.1016/S0016-7037(03)00217-5 [16] 黄中林. 氢氧化铬的基础及应用研究[D]. 重庆: 重庆大学, 2016. HUANG Z L. The investigation of fundamental and applications of chromium hydroxide[D]. Chongqing: Chongqing University, 2016(in Chinese).
[17] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 中华人民共和国推荐性国家标准: 地下水质量标准 GB/T 14848—2017[S]. 北京: 中国标准出版社, 2017. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People's Republic of China. National Standard (Recommended) of the People's Republic of China: Standard for groundwater quality. GB/T 14848—2017[S]. Beijing: Standards Press of China, 2017(in Chinese).
[18] SCHROEDER D C, LEE G F. Potential transformations of chromium in natural waters [J]. Water, Air, and Soil Pollution, 1975, 4(3/4): 355-365. [19] EARY L E, RAI D. Kinetics of chromium(Ⅲ) oxidation to chromium(Ⅵ) by reaction with manganese-dioxide [J]. Environmental Science & Technology, 1987, 21: 1187-1193. [20] 曾淦宁, 武晓, 郑林, 等. 负载纳米零价铁铜藻基活性炭的制备及其去除水中Cr(Ⅵ)的研究 [J]. 环境科学, 2015, 36(2): 530-536. ZENG G N, WU X, ZHENG L, et al. Preparation of nano zero-valent iron / Sargassum horneri based activated carbon for removal of Cr(Ⅵ) from aqueous solution [J]. Environmental Science, 2015, 36(2): 530-536(in Chinese).
[21] 孙西同. 磁性高分子复合材料的制备及对Cr(Ⅵ)的吸附性能研究[D]. 北京: 中国科学院研究生院(过程工程研究所), 2015. SUN X T. Preparation of magnetic polymer composite and its adsorption properties for Cr(Ⅵ)[D]. Beijing: University of Chinese Academy of Sciences, 2015(in Chinese).
[22] 肖俊杰. 改性海泡石对水体中Cu2+、Cd2+、Cr3+的吸附特性及机理研究[D]. 湘潭: 湘潭大学, 2018. XIAO J J. The adsorption characteristics and mechanism of Cu2+, Cd2+, Cr3+on modified sepiolite[D]. Xiangtan: Xiangtan University, 2018(in Chinese).
[23] 汪婷, 高滢, 金晓英, 等. 纳米四氧化三铁同步去除水中的Pb(Ⅱ)和Cr(Ⅲ)离子 [J]. 环境工程学报, 2013, 7(9): 3476-3482. WANG T, GAO Y, JIN X Y, et al. Simultaneous removal of Pb(Ⅱ) and Cr(Ⅲ) from wastewater by magnetite nanoparticles [J]. Chinese Journal of Environmental Engineering, 2013, 7(9): 3476-3482(in Chinese).
[24] MANDIWANA K L, PANICHEV N, KATAEVA M, et al. The solubility of Cr(Ⅲ) and Cr(Ⅵ) compounds in soil and their availability to plants [J]. Journal of Hazardous Materials, 2007, 147(1/2): 540-545. [25] GUSTAFSSON J P, PERSSON I, OROMIEH A G, et al. Chromium(Ⅲ) complexation to natural organic matter: Mechanisms and modeling [J]. Environmental Science & Technology, 2014, 48(3): 1753-1761. [26] 何振立. 污染及有益元素的土壤化学平衡[M]. 北京: 中国环境科学出版社, 1998. HE Z L. Soil-Chemical balances of pollution and beneficial elements[M]. Beijing: China Environment Science Press, 1998(in Chinese).
-






 下载:
下载: