-
金属镉(Cd)是一种生物蓄积性强,毒性作用持久的重金属元素[1-2]. 镉的大量使用造成了严重的生态环境污染,对公众健康构成一定的威胁[3]. 对一般人来说,饮食和吸烟是金属镉的主要暴露途径.镉可对肾脏、肝脏、肺、骨骼、心血管、内分泌、生殖系统造成危害,甚至产生基因毒性和致癌[4-6]. Cd毒性机制包括改变基因的正常表达,抑制受损DNA的修复,对细胞凋亡和自噬的干扰,诱导氧化应激的产生以及与生物元素的相互作用[7]. 多项研究表明,Cd毒性与ROS的过量产生相关[8]. 金属镉通过抑制氧化还原蛋白酶(NADPH、SOD、CAT)以及GST等抗氧化物的活性,打破细胞内氧化还原平衡态,导致脂质,蛋白质和DNA的氧化损伤[9-14].
目前检测细胞产生ROS的方法主要有荧光染料法[15]、化学发光法[16],电子自旋共振法[17]和流式细胞仪法[18]等. 荧光技术是研究细胞内ROS最广泛的技术,但荧光染料尚存在易发生荧光猝灭,信号不稳定,难于绝对定量的缺点. 化学发光法面临的问题是发光剂对活性氧物种的选择性差.电子自旋共振法和流式细胞术则存在预处理复杂,设备昂贵的缺点.
扫描电化学显微镜(SECM)是一种强大的电化学分析技术,近年来在生物、环境、材料研究中被广泛应用. SECM可通过氧化还原介质实现对生物样品的非侵入性检测[19-20]. SECM技术适用于一系列研究,包括反应动力学、表面和界面过程、微结构制造、膜转运、多药耐药性、神经细胞信号、细胞ROS和活性氮物种(RNS)检测以及细胞氧化还原过程.此外,SECM还可以用于单细胞形貌和膜通透性的快速检测[21].
本研究利用扫描电化学显微镜技术实时检测了经氯化镉孵育后MCF-10A细胞形态变化以及活性氧稳定产物(H2O2)的释放情况. 并通过测定金属镉对MCF-10A细胞内抗氧化物酶及非酶类抗氧化物质活性的影响,进一步分析了Cd诱导MCF-10A产生过量ROS的机制.
-
扫描电化学显微镜CHI920D(SECM),10 μm铂超微电极/探针,铂丝对电极,Ag/AgCl参比电极(上海辰华仪器有限公司,中国);倒置显微镜(奥林巴斯CKX41,日本);细胞培养箱(Thermo scientific, 3111);离心机(Eppendorf, Centrifuge 5415D);酶标仪(Molecular Devices, Spectra Max M4).
-
1× PBS(生工生物科技有限公司,上海);氯化镉(CdCl2)(天津市光复科技发展有限公司,99.0%);氯化六氨基合钌(Ru(NH3)6Cl3)(Sigma,美国, 98.0%);超纯水(1018 Ω, MilliQ超纯水系统,美国);MCF-10A细胞(ATCC);PBS缓冲液,胰酶(Invitrogen,美国);蛋白酶抑制剂(Roche,中国);DMEM-H培养基,SOD检测试剂盒,GSH检测试剂盒,CAT检测试剂盒,活性氧检测试剂盒(Genview,美国);NADPH检测试剂盒(Abcam,中国).
-
MCF-10A细胞培养在含10%胎牛血清,1%双抗的DMEM培养液中,37 °C,5%CO2的加湿培养箱(日本三洋)中培养. 在进行SECM活细胞实验前,将MCF-10A细胞接种到35 mm培养皿中,覆盖率约50%时,用1×PBS洗涤细胞2次,加入含有0、40、60、80、100 μmol·L−1CdCl2 溶液的培养基孵育2 h,以及使用60 μmol·L−1CdCl2溶液的培养基孵育MCF-10A细胞1、2、4、6 h.SECM分析前,取出细胞培养液,用1×PBS洗涤2次,然后用2 mL1×PBS代替培养液进行SECM实验.
-
将铺有细胞的培养皿,工作探针,银/氯化银参比电极和铂丝对电极放置在扫描平台上. 把探针移动到培养皿上方,设定工作电位为−0.5 V,以溶解氧作为氧化还原介质在PBS溶液中进行基底逼近,逼近后上抬8 μm. 借助倒置显微镜将工作电极移动到细胞附近,然后,在电势−0.5 V下,使用SECM恒高模式以5 μm·s−1的速度对MCF-10A细胞扫描成像. 确定细胞位置后,将探针移动到细胞正上方,使用恒高模式在不同电位下对细胞进行横向扫描实验.通过比较不同电位下检测到的细胞释放的ROS电流反馈情况来确定后续的检测电位.
-
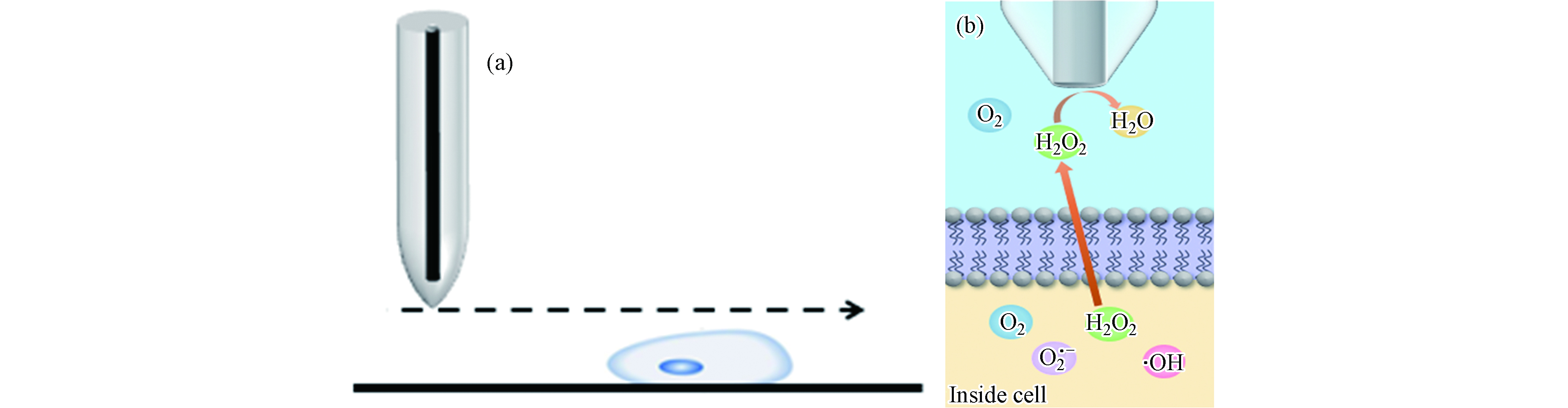
本研究利用H2O2 的电活性特点检测细胞形貌及ROS释放情况.正常生理条件下,细胞内ROS处于动态平衡状态,机体内的氧化还原体系会及时清除细胞产生的ROS. 如图1(a)所示,细胞相当于绝缘基底,当探针扫描到细胞上方时,探针与细胞表面的间距小于探针到培养皿的距离,空间距离的减小对物质扩散具有阻碍作用,导致电流减小. 在SECM恒高扫描模式下,探针的扫描高度保持不变,空间距离的减小只与细胞高度有关. 经CdCl2孵育后,细胞产生过量的ROS,H2O2作为ROS的稳定产物可以透过细胞膜到达探针表面. 如图1(b)所示,随着尖端到细胞表面距离的减小,由MCF-10A细胞产生的H2O2会在扩散到本体溶液之前到达探针,导致电流增加.通过对细胞上方等高扫描曲线电流变化情况的分析,实现对氯化镉作用下MCF-10A细胞形态和ROS释放情况的检测.
-
将细胞以6.0×104 个·μL−1的密度接种到96孔板中,在温度为37 ℃,CO2含量为5%的细胞培养箱中培养18 h之后,加入不同浓度的氯化镉和DCFH-DA共同孵育2 h. 孵育结束后,使用不含血清的DMEM清洗3遍. 每组设置4个平行样,同时设置正常细胞组和空白对照组,于酶标仪上测定DCF荧光强度数值,设置激发波长为488 nm,发射波长为525 nm.同理,对60 μmol·L−1氯化镉孵育1、4、6 h的MCF-10A细胞内活性氧产生情况进行测定.
-
细胞调整到对数生长期,胰酶消化后将细胞接种到6孔板中,密度约70%,继续培养18—24 h,分别加入40 μmol·L−1和60 μmol·L−1 CdCl2,将细胞于温度为37 ℃,CO2含量为5%的培养箱内孵育2 h. 胰酶消化处理完成后,将细胞进行沉淀,弃上清备用. 向细胞沉淀中加入细胞裂解液,冰置30 min后,12000 r·min−1下离心15 min.取上清液作为待测样品,准备二喹啉甲酸二钠盐(BCA)工作液,A液:B液=50:1,稀释好各个BSA标准品. 将待测样本溶于稀释缓冲溶液中,使用酶标仪在570 nm下测定OD值并进行浓度计算.
-
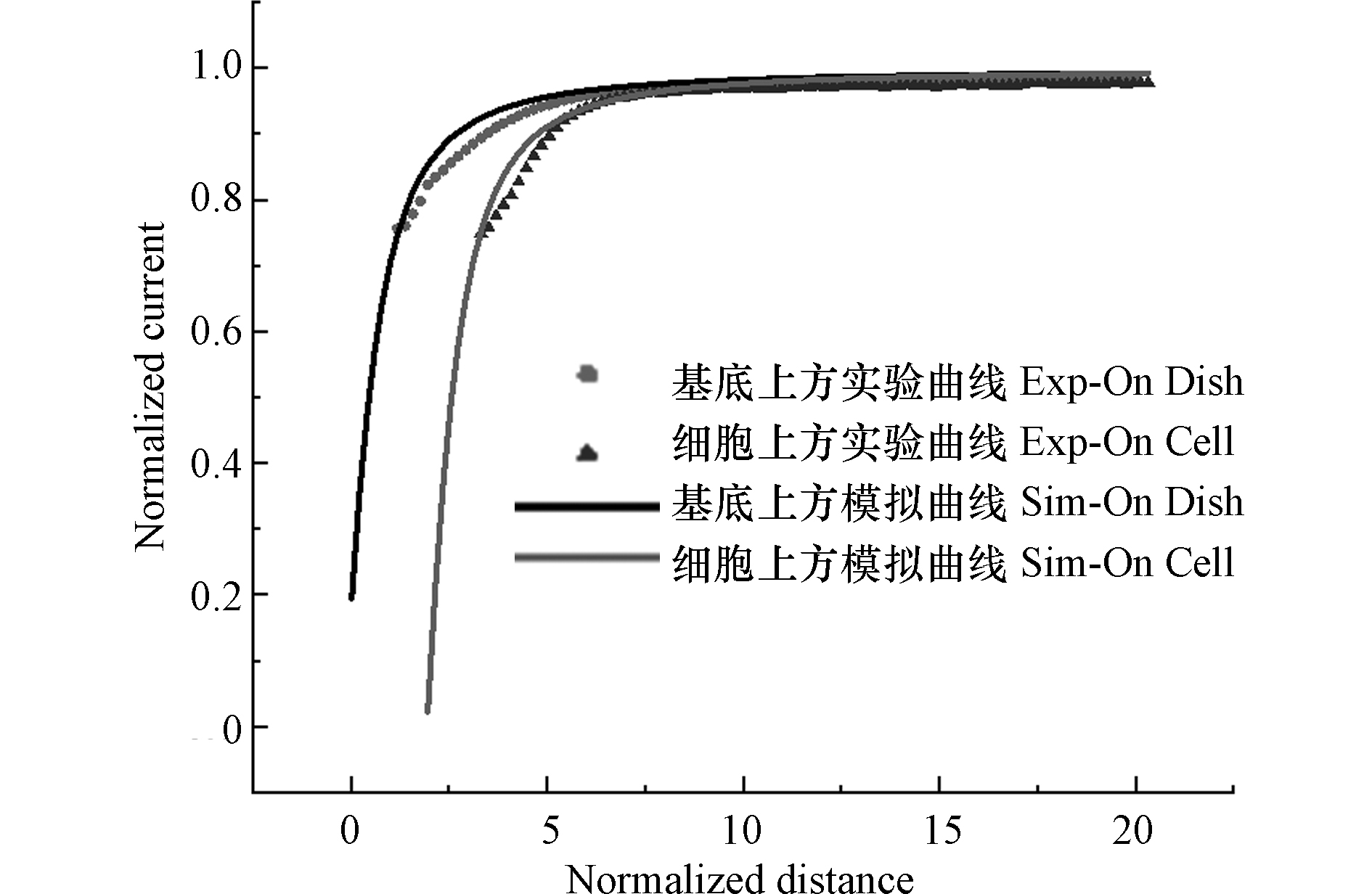
为了灵敏的对细胞表面电活性物质进行实时测定,探针与细胞的空间相对位置,显然是至关重要的. 本研究使用细胞膜非渗透性的Ru(NH3)6Cl3作为氧化还原分子,通过逼近曲线获取电极尖端与基底之间的距离,并分别以培养皿和细胞为基底做逼近曲线来确定细胞的高度[22]. 将培养皿置于扫描台上,通过SECM三维位移控制系统将探针浸入含有1 mmol·L−1 Ru(NH3)6Cl3的1×PBS溶液中,并将探针移到细胞附近. 以Ru(NH3)6Cl3的还原电位(−0.35 V)作为工作电位对培养皿底部逼近,在电极检测到电流I = 0. 75I∞时,停止逼近,获取探针的空间位置. 完成基底逼近后,使用SECM恒高工作模式以5 μm·s−1的速度对MCF-10A细胞进行原位扫描成像. 确定细胞位置后,把探针移至细胞上方,通过Z轴控杆将探针抬高相应的高度对细胞进行逼近. 根据公式(1)、(3)计算理论逼近曲线,式中,IT为探针尖端电流,IT,∞为探针距基底无穷远时探针尖端的电流,a为UME探针尖端半径,d为探针与基底之间的距离,do为从扫描第一点到基底表面的距离,d exp为探针距逼近起始点的距离.其中,公式(3)的参数参照表1[23]. 将实验测得数据值绘制为IT/I∞并与L函数进行拟合,通过更改do值,找到实验曲线和理论曲线间的良好拟合. 图2是IT/I∞与L函数的拟合图像,结合实验与模拟曲线拟合结果以及相应的理论计算[24],得到逼近结束后,探针尖端与基底之间的距离约为7 μm,MCF-10A细胞高度约为11 μm. 因此,以探针在基底逼近后,向上抬8 μm作为后续的检测距离.
-
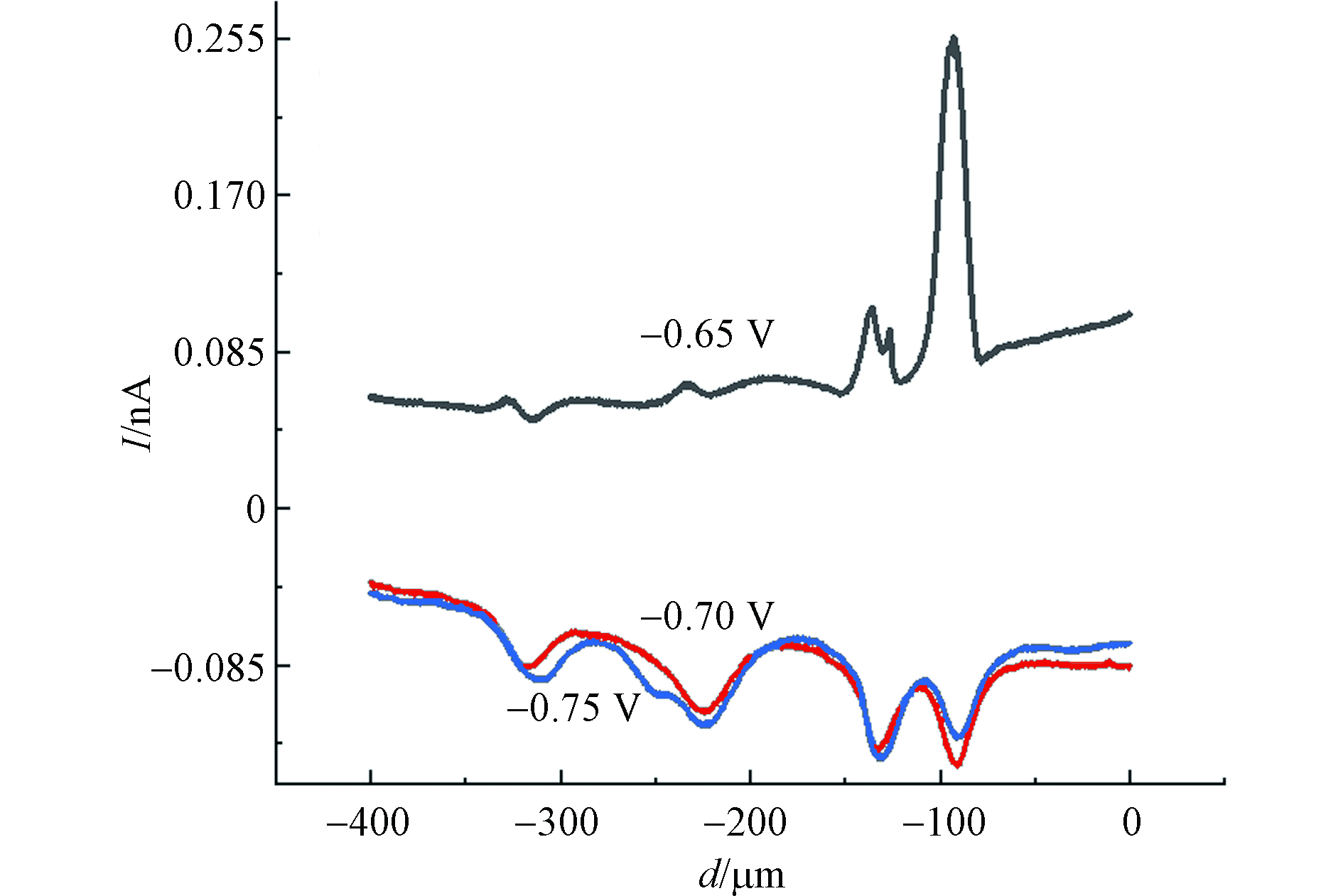
使用SECM恒高模式以5 μm·s−1的速度对MCF-10A细胞进行原位扫描成像,结果如图3所示. 图3中水平范围为 80 μm×80 μm,电流大小由不同颜色表示. 由于单个活细胞释放的H2O2量极少,准确设定工作电位对于H2O2灵敏检测是非常重要的. 依据文献选择工作电位分别为−0.65、−0.7、−0.75 V,检测在不同电位下MCF-10A细胞释放H2O2的情况[25]. 如图4所示,浓度为60 μmol·L−1的CdCl2孵育MCF-10A细胞2 h后,探针在不同电位下,细胞上方的横向扫描曲线. 图4中扫描曲线已扣除空白背景,通过比较不同电位下ROS电流反馈情况,可以得出当电位−0.65 V时,扫描过程中有IT的增加,探针对ROS电流信号反应最明显. 工作电位在−0.7 V和−0.75 V时,没有观察到IT的增加. 这是因为,当电位为−0.7 V和−0.75 V时,背景电流较大,严重影响ROS电流信号,因此,选择−0.65 V为ROS的检测电位.
-
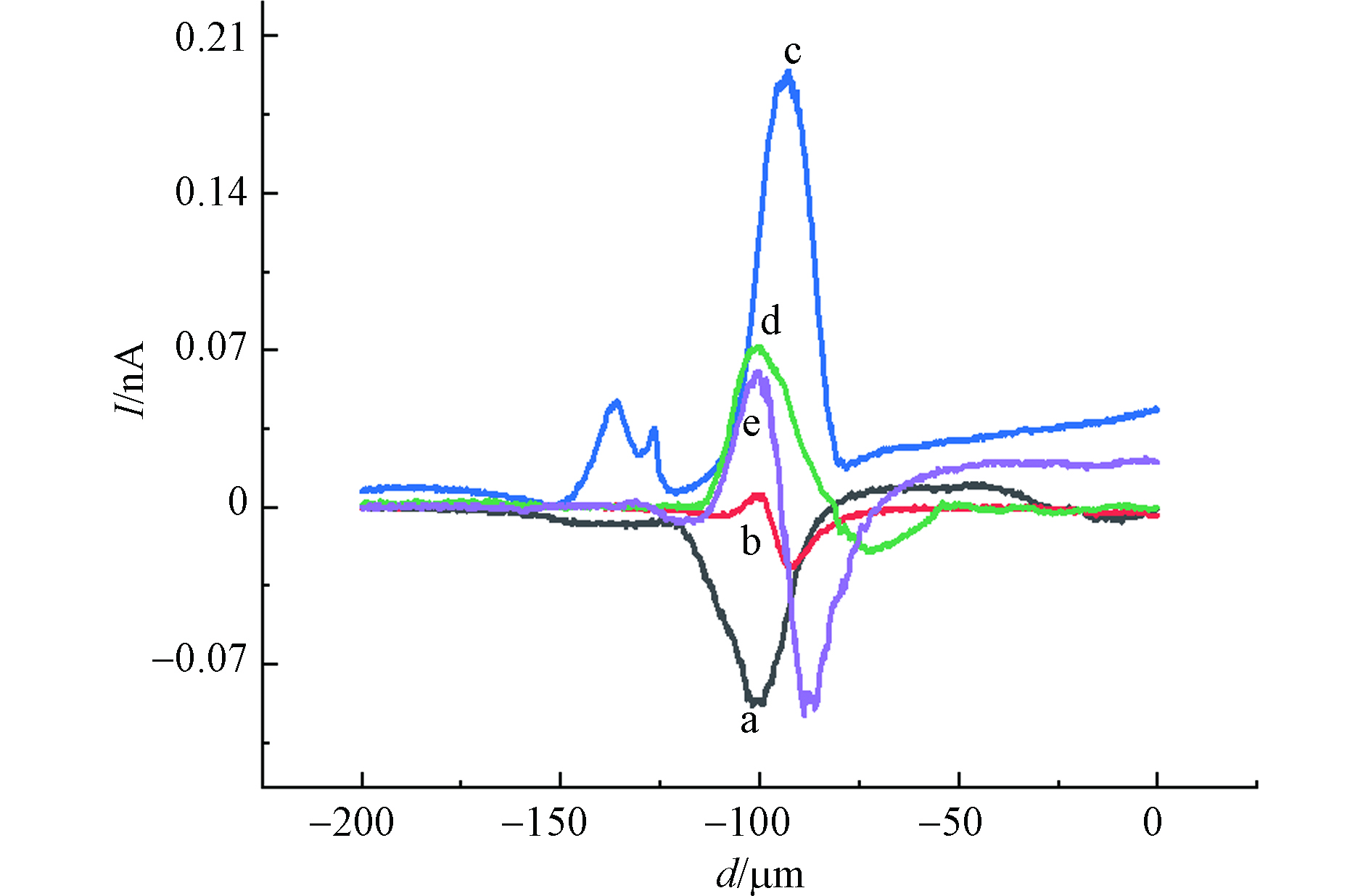
经不同浓度的CdCl2孵育2 h后,利用等高扫描模式得到MCF-10A细胞上方横向扫描曲线(曲线已扣除背景),如图5所示. 图5中a曲线为对照组细胞上方横向扫描曲线,可以看出探针扫描至某位置时电流减小.这是因为,当探针扫描到细胞上方时,探针尖端与细胞表面的间距小于探针尖端到基底之间的距离,电流显示出负反馈现象;图5中b曲线为40 μmol·L−1 CdCl2 孵育2 h后的MCF-10A细胞上方的电流曲线,扫描范围内既有电流降低的现象也有电流增大的现象,且在每次扫描时都是先出现电流下降,再出现电流升高的情况. 这种现象反映了基底形貌变化和MCF-10A细胞表面ROS释放情况对IT的综合影响[26]. 电流减小幅度取决于探针到细胞表面距离的变化,电流增大幅度则取决于细胞释放到溶液中ROS的多少.
比较不同浓度氯化镉孵育2 h后的MCF-10A细胞上方横向扫描曲线电流变化,可以得到MCF-10A细胞在不同浓度氯化镉作用下,细胞形态和氧化损伤情况.如表2所示,当氯化镉浓度从0增加到60 μmol·L−1,IT下降幅度逐渐减小,说明MCF-10A细胞在受到氯化镉的刺激时收缩,细胞这种受到有毒物种刺激后所表现出来的收缩是其防御功能的体现[27-28];而IT上升电流逐渐增加,说明MCF-10A细胞在受到氯化镉的刺激时发生氧化应激,释放出ROS,且ROS释放量随氯化镉浓度升高而增加,细胞受到的氧化损伤程度逐渐加强. 当氯化镉浓度从60 μmol·L−1增加到100 μmol·L−1,IT下降电流逐渐增大,说明此阶段细胞防御功能降低,细胞收缩能力下降;同时观察到IT上升电流逐渐降低,可能是高浓度氯化镉作用下,细胞氧化损伤严重,线粒体功能降低,产生ROS的量减少.
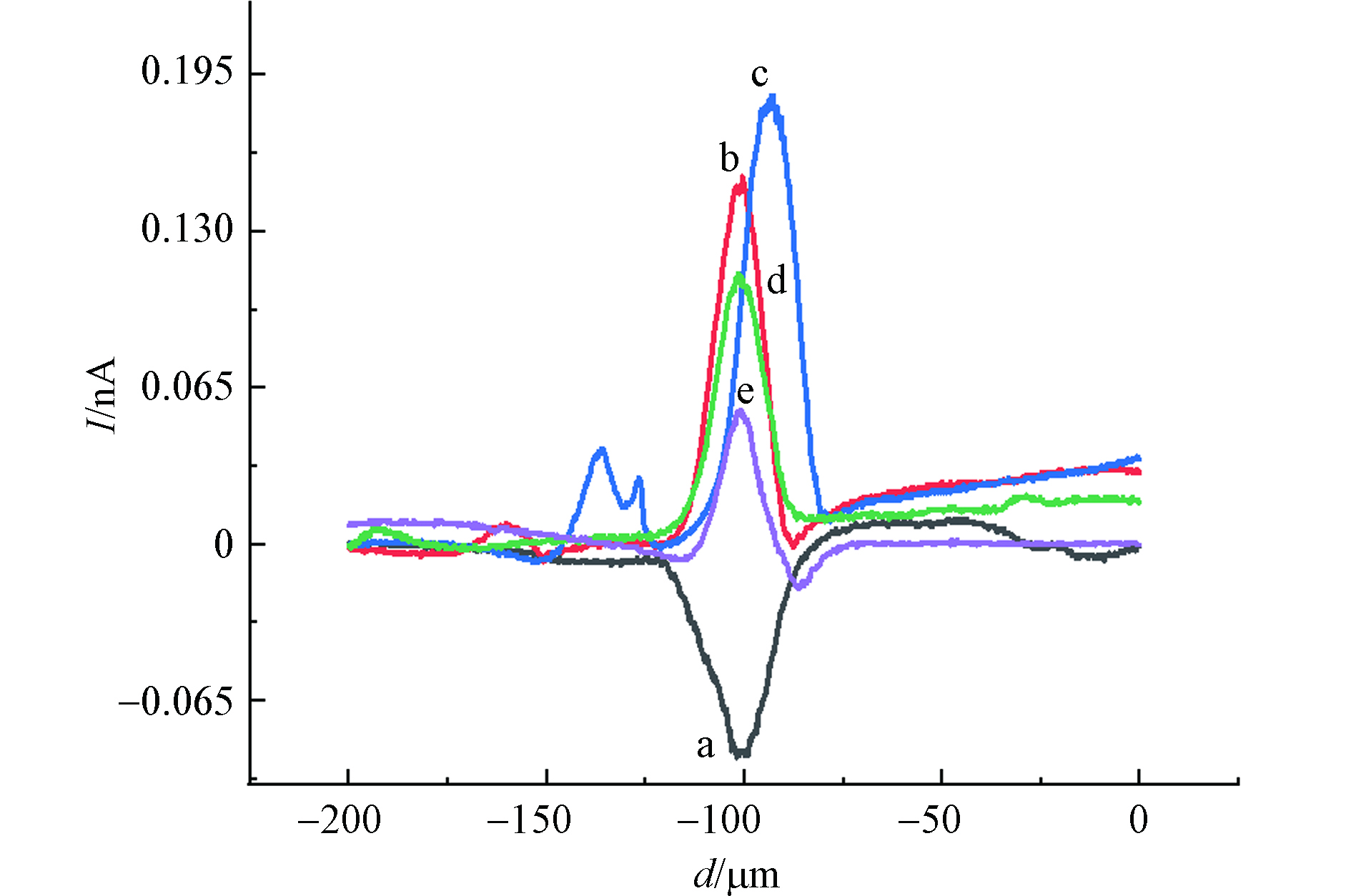
随后,考察了MCF-10A细胞在CdCl2不同孵育时间下的氧化应激响应,如图6所示. 从图6可以看出,在−0.65 V工作电位下,不同的孵育时间检测到的IT电流变化不同. 表3给出了不同孵育时间下,MCF-10A细胞上方IT变化情况,0-4 h时,IT下降电流逐渐减小,同样说明MCF-10A细胞在受到CdCl2的刺激时会收缩来抵御其毒性作用且这种防御功能在短期内随氯化镉作用时间的增加而表现的更强烈,当氯化镉作用时间为4-6 h时,MCF-10A细胞高度回升,收缩防御功能降低. 相比较于不同浓度氯化镉孵育下MCF-10A细胞上方横向扫描曲线IT上升电流变化情况,经60 μmol·L−1 CdCl2孵育不同时间后,MCF-10A细胞上方X扫描曲线IT上升电流同样是先升高后降低,且在60 μmol·L−1 CdCl2孵育MCF-10A细胞2 h时得到的IT上升值最大.
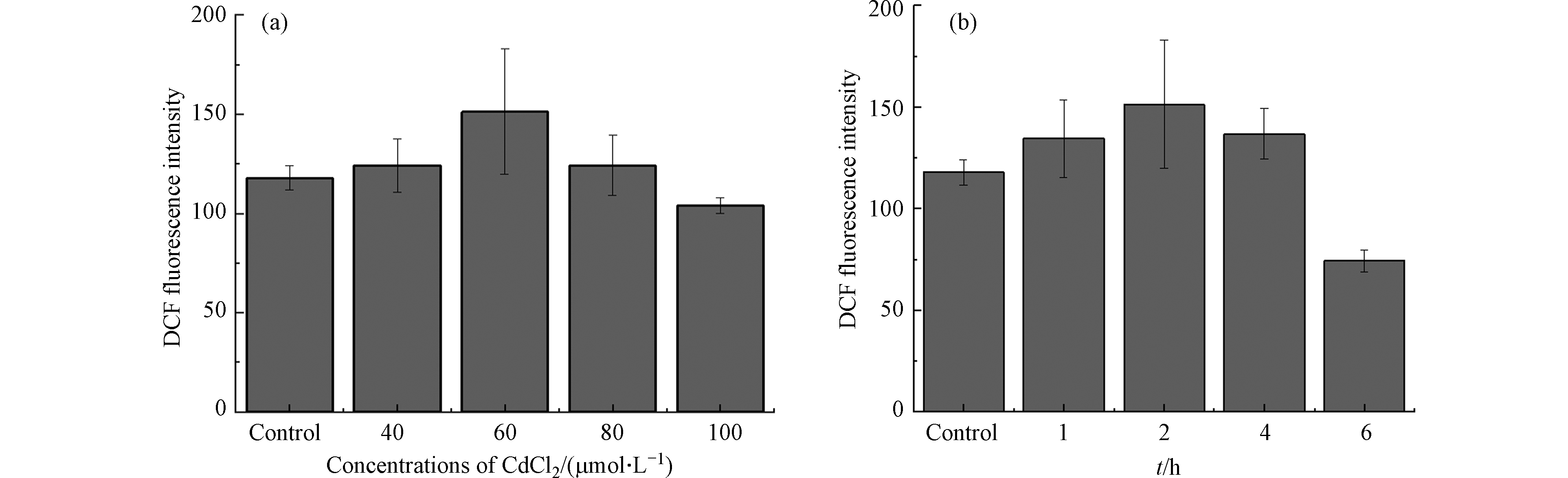
为了进一步验证扫描电化学显微镜实验现象的真实性和准确性,使用DCFH-DA法进行细胞活性氧检测,如图7(a)所示.不同浓度氯化镉(40、60、80、100 μmol·L−1)孵育2 h后的MCF-10A细胞内DCF荧光强度变化. 当氯化镉浓度从0增加到60 μmol·L−1,DCF荧光强度逐渐增强,说明氯化镉导致MCF-10A细胞ROS水平升高;当氯化镉浓度大于60 μmol·L−1时,DCF荧光强度逐渐降低,说明高浓度氯化镉作用下MCF-10A细胞中ROS水平下降. CUYPERS A等在锌对镉诱导的细胞凋亡和细胞培养中活性氧产生的抑制作用研究中,观察到镉诱导人宫颈癌细胞(HeLa)和牛主动脉内皮细胞(BAECs)产生的活性氧水平也是先升高后降低的情况,这可能与金属镉诱导的细胞凋亡有关[10]. 从7(b)中可以看出,Cd处理后的MCF-10A细胞中,ROS在2 h达到高峰,然而,当孵育时间达到6 h时, ROS下降至对照组的63.1%. 这可能是镉长时间孵育下,细胞膜通透性增强[8,29-30],导致更多的H2O2能够释放到细胞外. 荧光探针DCFH-DA法检测到金属镉诱导MCF-10A细胞内活性氧产生情况与用SECM技术检测到的细胞外H2O2含量变化相同.
-
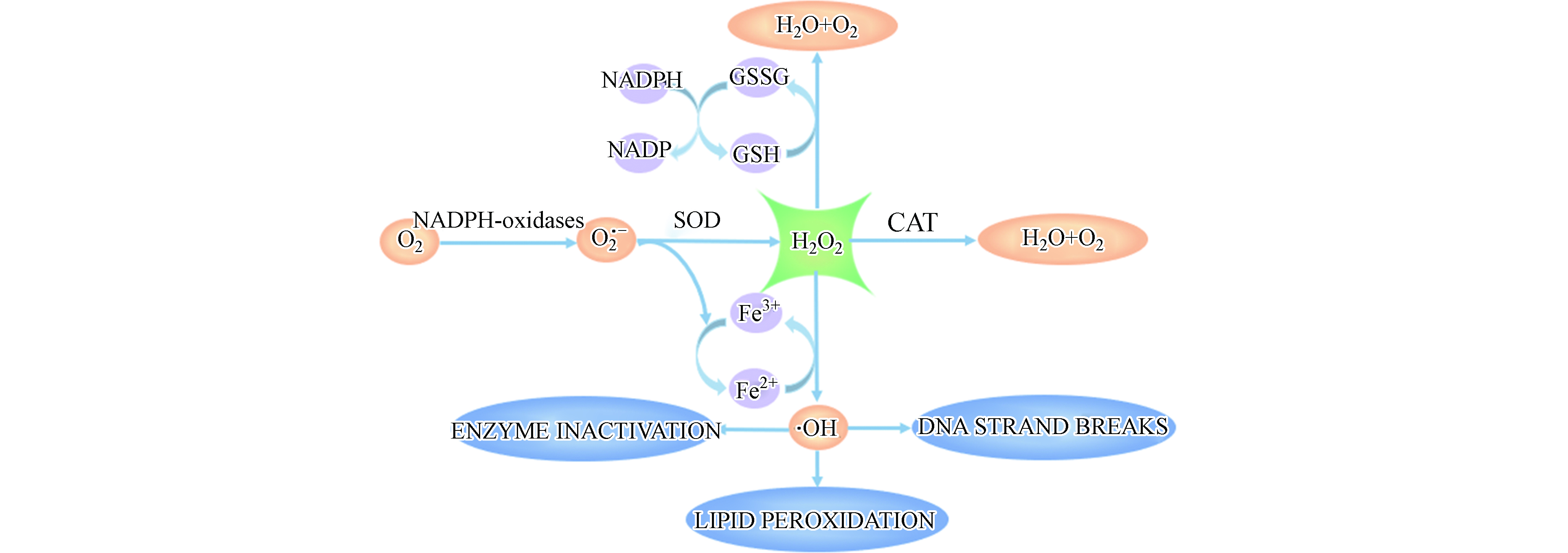
为进一步分析重金属镉诱导细胞氧化应激产生H2O2的机制,测定了镉对MCF-10A细胞氧化应激过程中相关酶活性的影响. 细胞内抗氧化系统主要由抗氧化物酶和非酶类抗氧化物质组成,其中,CAT和SOD是直接参与分解ROS的主要抗氧化物酶,GSH是机体内主要的非酶类抗氧化物质[27,31].
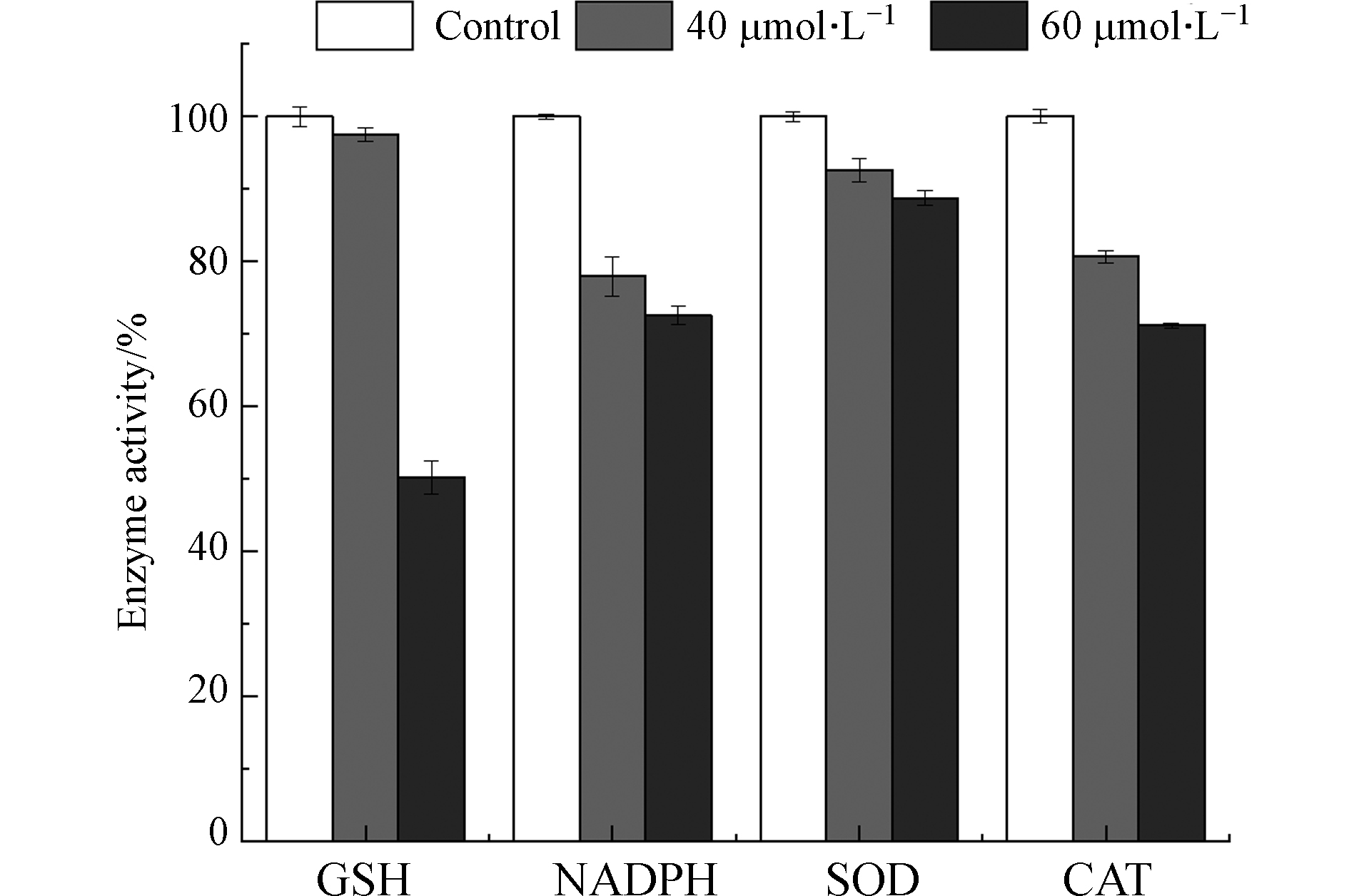
图8可见,NADPH氧化酶通过将NADPH中电子转移给O2产生O2·-[32]. 在正常生理情况下,适量的ROS作为重要信号分子,对维持细胞正常功能具有重要作用[33];然而,在病理情况下,NADPH氧化酶活性变化或者抗氧化系统受到破坏,造成细胞内 ROS 过度生成,从而对细胞造成损伤. 由图9可知,镉暴露剂量为40 μmol·L−1和60 μmol·L−1时,MCF-10A细胞中NADPH氧化酶含量降低到对照组的77.93%和72.59%. 研究表明,Cd2+通过改变HepG2细胞内游离和结合态NADPH的比例引起氧化应激[34].
如图8所示,SOD可以将O2·−歧化为H2O2 和O2,CAT进一步将H2O2催化分解为H2O和O2,机体内SOD和CAT活性改变会引起ROS的积累.如图9所示,当镉浓度为40 μmol·L−1和60 μmol·L−1时,SOD活力分别降低到对照组的92.62%和88.73%;CAT活力降低到对照组的80.66%和71.16%. MCF-10A细胞内CAT和SOD活性随镉暴露浓度的升高而降低. GSH是机体内主要的非酶类抗氧化物质,能够与ROS反应,还可以通过与镉离子结合,将有毒的离子态镉转化为没有毒性的化合态镉. 然而,GSH与镉结合后会丧失还原性,失去对细胞内ROS的清除能力,引起细胞内ROS过量聚集[35]. 如图9,与对照组相比,40 μmol·L−1和60 μmol·L−1镉暴露后,MCF-10A细胞中GSH含量降低到97.56%和50.20%. 结果说明,镉暴露消耗了MCF-10A细胞中的GSH. Qu等将鲫鱼暴露于镉(0.1 mg·L−1)12 d后也得到类似的结果[36]. 另外,他们在研究中指出这一现象还可能与GSH合成过程中相关转录基因的下调有关[37].
综上,镉暴露引起MCF-10A细胞氧化还原体系中主要成员的活力或含量发生变化,打破了MCF-10A内的氧化还原平衡态.氧化还原平衡一旦被破坏,多余的ROS会攻击机体内的脂质成分,包括氧化生物膜上的磷脂,酶和受体蛋白的不饱和脂肪酸及核酸等大分子物质,并形成脂质过氧化产物,引起氧化损伤[38].
-
本研究以MCF-10A细胞释放的H2O2作为目标测定物,利用扫描电化学显微镜技术实现了对MCF-10A细胞无侵害性的扫描成像以及金属镉刺激后细胞形态和ROS释放情况的实时检测. 针对目前利用荧光法研究细胞易发生荧光猝灭,信号不稳定等问题,SECM 技术检测ROS具有实时,原位,灵敏的优点,为研究生理条件下活细胞受环境污染物影响提供了新的,可靠的方法.
基于扫描电化学显微镜技术评价镉诱导MCF-10A细胞产生的过氧化氢水平
Hydrogen peroxide levels in cadmium-induced MCF-10A cells were evaluated by scanning electrochemical microscopy
-
摘要: 本研究利用扫描电化学显微镜(SECM)技术在生理条件下对人正常乳腺上皮细胞(MCF-10A)受氯化镉刺激的形态变化和活性氧物种(ROS)释放情况进行了实时检测. 结果表明,MCF-10A细胞在低浓度或短时间的氯化镉刺激下呈现收缩防御模式;但受到高浓度或长时间的氯化镉刺激时,细胞对氯化镉毒性的防御能力降低,细胞内氧化还原平衡态受到破坏,造成严重的氧化损伤. 并利用荧光探针DCFH-DA法证实了扫描电化学显微镜技术检测结果的正确性和可靠性. 此外,通过对镉作用下细胞内还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶,超氧化物歧化酶(SOD),过氧化氢酶(CAT)及谷胱甘肽(GST)活性的测定,进一步分析了金属镉诱导MCF-10A细胞的氧化应激机制.
-
关键词:
- 扫描电化学显微镜(SECM) /
- H2O2 /
- 氧化损伤
Abstract: In this study, scanning electrochemical microscopy (SECM) technology was used to achieve real-time detection of the morphological changes and the release of reactive oxygen species (ROS) of human normal breast epithelial cells (MCF-10A) stimulated by cadmium chloride under physiological conditions. The results showed that MCF-10A cells exhibited a contraction defense mode under the condition of a low concentration cadmium chloride stimulation or a short time stimulation; However, when dealt with a high concentration cadmium chloride stimulation or the stimulation time was long, the defense ability of the cells against the toxicity of cadmium chloride decreased, the internal redox balance of the cells was destroyed, causing a serious oxidative damage. And the correctness and reliability of the detection results of scanning electrochemical microscopy technology was verified by using the fluorescent probe DCFH-DA method. In addition, the mechanism of oxidative stress induced by metal cadmium in MCF-10A cells was further analyzed through the determination of activity of the reduction of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, superoxide dismutase (SOD), catalase (CAT), glutathione (GST) in the cell under the action of cadmium.-
Key words:
- scanning electrochemical microscopy (SECM) /
- H2O2 /
- oxidation damage
-

-
表 1 不同RG值下等式(3)(负反馈)的参数值
Table 1. Parameter Values for Equation(3)(Negative Feedback)at different RG Values
RG A B C D 10.2 0.40472 1.60185 0.58819 −2.37294 8.13 0.42676 1.46081 0.56874 −2.28548 5.09 0.48678 1.17706 0.51241 −2.07873 3.04 0.60478 0.86083 0.39569 −1.89455 2.03 0.76179 0.60983 0.23866 −2.03267 表 2 不同浓度CdCl2孵育2 h后的MCF-10A细胞上方电流变化量
Table 2. Changes of current above MCF-10A cells after incubation with different concentrations of CdCl2 for 2 h
氯化镉浓度/(μmol·L−1)
Concentrations of CdCl2IT下降/n A
The decline of ITIT上升/n A
The rise of ITControl 0.0957 0.0000 40 0.0267 0.0088 60 0.0069 0.1718 80 0.0202 0.0705 100 0.1052 0.0563 表 3 不同作用时间下MCF-10A细胞上方电流变化量
Table 3. Changes of current above MCF-10A cells at different time
孵育时间/h
Incubation timeIT下降/nA
The decline of ITIT上升/nA
The rise of ITControl 0.0957 0.0000 1 0.0258 0.1494 2 0.0069 0.1718 4 0.0000 0.0984 6 0.0180 0.0594 -
[1] ZHANG H, REYNOLDS M. Cadmium exposure in living organisms: A short review [J]. Science of the Total Environment, 2019, 678: 761-767. doi: 10.1016/j.scitotenv.2019.04.395 [2] 吴朝波, 王蕾, 郭建春, 等. 镉在海雀稗体内的分布及化学形态特征 [J]. 环境化学, 2016, 35(2): 330-336. doi: 10.7524/j.issn.0254-6108.2016.02.2015090201 WU C B, WANG L, GUO J C, et al. Distribution and chemical forms of Cd in PASPALUM VAGINATUM SW [J]. Environmental Chemistry, 2016, 35(2): 330-336(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.02.2015090201
[3] VANGRONSVELD J, ASSCHE F V, CLIJSTERS H. Reclamation of a bare industrial area contaminated by nonferrous metals: In situ metal immobilization and revegetation [J]. Environmental Pollution, 1995, 87(1): 51-59. doi: 10.1016/S0269-7491(99)80007-4 [4] BJRKLUND G, CRISPONI G, NURCHI V M, et al. A review on coordination properties of thiol-containing chelating agents towards mercury, cadmium, and lead[J]. Molecules, 2019, 24(18), 3247: 1-32. [5] 黄泽昊, 侯艳丽, 李君平, 等. 镉对小鼠骨生物力学及成骨细胞MG-63的影响 [J]. 环境化学, 2012, 31(12): 1835-1839. HUANG Z H, HOU Y L, LI J P, et al. Effects of cadmium on bone biomechanics in mice and osteoblast MG-63 [J]. Environmental Chemistry, 2012, 31(12): 1835-1839(in Chinese).
[6] SARKAR A, RAVINDRAN G, KRISHNAMURTHY V: A brief review on the effect of cadmium toxicity: from cellular to organ level. Int J Bio Technol Res 2013, 3: 17-36. [7] UKIOSI D, BARALI K, JAVORAC D, et al. An overview of molecular mechanisms in cadmium toxicity [J]. Current Opinion in Toxicology, 2020, 19: 56-62. doi: 10.1016/j.cotox.2019.12.002 [8] AGNIESZKA S C, ANNA S B, MARIA S B, et al. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures [J]. Toxicology, 2000, 145(2-3): 159-171. doi: 10.1016/S0300-483X(00)00144-X [9] LIU J, QU W, KADIISKA M B. Role of oxidative stress in cadmium toxicity and carcinogenesis [J]. Toxicol Appl Pharmacol, 2009, 238(3): 209-214. doi: 10.1016/j.taap.2009.01.029 [10] CUYPERS A, PLUSQUIN M, REMANS T, et al. Cadmium stress: an oxidative challenge [J]. BioMetals, 2010, 23(5): 927-940. doi: 10.1007/s10534-010-9329-x [11] BUHA A, BULAT Z, ZORICA, UKIOSI D, et al. Effects of oral and intraperitoneal magnesium treatment against cadmium-induced oxidative stress in plasma of rats [J]. Archives of Industrial Hygiene and Toxicology, 2012, 63(3): 247-254. doi: 10.2478/10004-1254-63-2012-2217 [12] MATOVI V, BUHA A, UKIOSI D, et al. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys [J]. Food and Chemical Toxicology, 2015, 78: 130-140. doi: 10.1016/j.fct.2015.02.011 [13] YANG H, SHU Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity [J]. International Journal of Molecular Sciences, 2015, 16(1): 1484-1494. doi: 10.3390/ijms16011484 [14] THEVENOD F, LEE W K. Cadmium and cellular signaling cascades: interactions between cell death and survival pathways [J]. Archives of toxicology, 2013, 87(10): 1743-1786. doi: 10.1007/s00204-013-1110-9 [15] SETSUKINAI K I, URANO Y, KAKINUMA K, et al. Development of novel fluorescence probes that Can reliably detect reactive oxygen species and distinguish specific species [J]. Journal of Biological Chemistry, 2003, 278(5): 3170-3175. doi: 10.1074/jbc.M209264200 [16] LU C, SONG G, LIN J M. Reactive oxygen species and their chemiluminescence-detection methods [J]. Trac Trends in Analytical Chemistry, 2006, 25(10): 985-995. doi: 10.1016/j.trac.2006.07.007 [17] KOHNO M. Applications of Electron spin resonance spectrometry for reactive oxygen species and reactive nitrogen species research [J]. Journal of Clinical Biochemistry & Nutrition, 2010, 47(1): 1-11. [18] WANG W, ZHAO F L, ZHANG J, et al. Luteolin induces apoptosis in mouse liver cancer cells through ROS mediated pathway: A mechanistic investigation[J]. Biomedical Research-India. 2017, 28(2): 839-845. [19] BEAULIEU I, KUSS S, MAUZEROLL J, et al. Biological scanning electrochemical microscopy and its application to live cell studies [J]. Analytical Chemistry, 2011, 83(5): 1485-1492. doi: 10.1021/ac101906a [20] FERNANDO C S, DMITRY M, HUBERT H, et al. Seeing big with scanning electrochemical microscopy [J]. Analytical Chemistry, 2011, 83(5): 1493-1499. doi: 10.1021/ac101931d [21] FILICE F P, HENDERSON J D, LI M S M, et al. Correlating live cell viability with membrane permeability disruption induced by trivalent chromium [J]. ACS Omega, 2019, 4(1): 2142-2151. doi: 10.1021/acsomega.8b02113 [22] LI M S M, FILICE F P, DING Z F. Determining live cell topography by scanning electrochemical microscopy[J]. Journal of Electroanalytical Chemistry. 2016, 779: 176-186. [23] BARD A J, MIRKIN M V. Scanning electrochemical microscopy, 2nd ed[M]. London: London CRC Rress. 2012: 75-85. [24] KOLEY D, BARD A J. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM) [J]. Proceedings of the National Academy of Sciences, 2010, 107(39): 16783-16787. doi: 10.1073/pnas.1011614107 [25] 樊孝银, 鲁理平, 康天放. 基于扫描电化学显微镜技术研究细胞实时释放ROS [J]. 分析测试学报, 2019, 38(4): 379-384. doi: 10.3969/j.issn.1004-4957.2019.04.001 FAN X Y, LU L P, KANG T F. Investigation on Real-time release of reactive oxygen species from cells based on scanning electrochemical microscopy [J]. Journal of Instrumental Analysis, 2019, 38(4): 379-384(in Chinese). doi: 10.3969/j.issn.1004-4957.2019.04.001
[26] SALAMIFAR S E, LAI R Y. Use of combined scanning electrochemical and fluorescence microscopy for detection of reactive oxygen species in prostate cancer cells [J]. Analytical Chemistry, 2013, 85(20): 9417-9421. doi: 10.1021/ac402367f [27] FILICE F P, LI M S M, HENDERSON J D, et al. Mapping Cd2+ induced membrane permeability changes of single live cells by means of scanning electrochemical microscopy [J]. Analytica Chemistry, 2016, 908: 85-94. doi: 10.1016/j.aca.2015.12.027 [28] HENDERSON J D, FILICE F P, LI M S M, et al. Tracking live cell response to cadmium (II) concentrations by scanning electrochemical microscopy [J]. Journal of Inorganic Biochemistry, 2016, 158: 92-98. doi: 10.1016/j.jinorgbio.2015.11.016 [29] LI M S M, FILICE F P, DING Z F. A time course study of cadmium effect on membrane permeability of single human bladder cancer cells using scanning electrochemical microscopy [J]. Journal of Inorganic Biochemistry, 2014, 136: 177-183. doi: 10.1016/j.jinorgbio.2014.02.009 [30] 崔建升, 高瑜, 王立新, 等. PCB77和Cd共同暴露诱导的线粒体损伤及其介导的联合毒性效应 [J]. 环境化学, 2016, 35(3): 590-596. doi: 10.7524/j.issn.0254-6108.2016.03.2015101501 CUI J S, GAO Y, WANG L X, et al. Mitochondrial damage induced by co-exposure of PCB77 and Cd and its mediated joint toxicity [J]. Environmental Chemistry, 2016, 35(3): 590-596(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.03.2015101501
[31] 张康保, 高倩, 张雅静, 等. 镉对PC12细胞的氧化损伤及α-硫辛酸的保护效应 [J]. 中国兽医学报, 2014, 34(8): 1349-1352. ZHANG K B, GAO Q, ZHANG Y J, et al. Oxidative damage of cadmium on PC12 cells and protective effect of α-lipoic acid [J]. Chinese Journal of Veterinary Science, 2014, 34(8): 1349-1352(in Chinese).
[32] VALKO M, LEIBFRITZ D, MONCOL J, et al. Free radicals and antioxidants in normal physiological functions and human disease[J]. International Journal of Biochemistry & Cell Biology. 2007, 39(1): 44-84. [33] NARUSZEWICZ M, JANKOWSKA E A, ZYMLINSKI R, et al. Hyperhomocysteinemia in patients with symptomatic chronic heart failure: Prevalence and prognostic importance-pilot study [J]. Atherosclerosis, 2007, 194(2): 408-414. doi: 10.1016/j.atherosclerosis.2006.08.014 [34] YANG M S, LI D, LIN T, et al. Increase in intracellular free/bound NADPH as a cause of Cd-induced oxidative stress in the HepG (2) cells [J]. Toxicology, 2008, 247(1): 6-10. doi: 10.1016/j.tox.2008.01.021 [35] 张鼎. 镉中毒及锌对镉中毒的作用机理研究[D]. 华中农业大学, 2015. ZHANG D. Cadmium poisoning and effect of zinc supplement on cadmium cytotoxicity[D]. Huazhong Agricultural University, 2015(in Chinese).
[36] QU R, WANG X, WANG Z, et al. Metal accumulation and antioxidant defenses in the freshwater fish Carassius auratus in response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes [J]. Journal of Hazardous Materials, 2014, 275: 89-98. doi: 10.1016/j.jhazmat.2014.04.051 [37] QU R, FENG M, WANG X, et al. Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values [J]. Aquatic Toxicology, 2014, 150: 9-16. doi: 10.1016/j.aquatox.2014.02.008 [38] WAISBERG M, JOSEPH P, HALE B, et al. Molecular and cellular mechanisms of cadmium carcinogenesis [J]. Toxicology, 2003, 192(2-3): 95-117. doi: 10.1016/S0300-483X(03)00305-6 -



 下载:
下载: