-
随着工业和农业的快速发展,水环境问题中的硝酸盐污染日益严重,引起了世界各国的广泛重视。硝酸盐污染物的主要来源有垃圾渗滤液、牲畜粪便、生活和工业废水等[1]。水体中的硝酸盐含量高会破坏生态环境,造成水体富营养化,影响水生生物生存[2]。用于饮用的地表水或地下水中含有过高浓度的硝酸盐会严重危害人类的健康,导致婴儿出现蓝婴综合征(高铁血红蛋白血症)、肝损伤等,世界卫生组织的国际癌症研究机构将摄入硝酸盐归类为可能对人类致癌的一类物质,特别是在促进内源性亚硝化的条件下摄入硝酸盐,硝酸盐会在体内形成N-亚硝基化合物,增大了人类患胃癌等疾病的风险[3]。因此,世界卫生组织设定饮用水中硝酸盐的最大浓度为50 mg·L−1
${\rm{NO}}_3^- $ [4]。中国最新生活饮用水卫生标准(GB 5749—2006)设定硝酸盐氮浓度为10 mg·L−1,地下水源限制时标准可放宽至20 mg·L−1。由于水中硝酸盐污染的严重性和危害性,人们研究出各种方法致力于去除水中的硝酸盐,主要分为生物法[5]、物理分离法[6]、催化加氢法[7]、化学还原法[8]、电催化还原法[9]。其中生物法是利用微生物在生物反应器中的反硝化作用还原硝酸盐,释放出分子态氮或一氧化二氮。该方法由于微生物培养周期较长、反应器占地面积较大、出水可能存在细菌污染,其应用存在一定的限制性[10]。离子交换法和反渗透法等物理方法操作简单,但只是将硝酸盐从污水中分离出来,并没有将污染物从根本上去除。催化加氢法需要持续投加反应所需的氢气,而氢气的易燃易爆性质使工艺存在很大危险性。电催化还原法即电催化反硝化法,是在通电的条件下,硝酸盐离子在阴极上被还原生成氮气等产物的一种脱氮方法。具有不产生污泥,占地面积小,处理效率高,安全环保、操作易控等优点[11]。1980年代,人们就已经开始将硝酸盐电化学还原法用于水处理,HORÁNYI等[12]采用金属铂作为电极材料,探究了硝酸盐氮在碱性条件下的还原反应。近年来,水中硝酸盐的电催化还原研究受到广泛关注,被认为是很有前景的处理方法。在硝酸盐的电催化还原过程中,主要在阴极上进行硝酸盐吸附和电子传递等关键步骤。电极材料、支持电解质和施加电流等对反应的动力学和产物选择性都有影响,因此,开发一种性质稳定、电催化还原活性高的电极具有重要意义。
目前,许多研究人员已将Zn[13]、Fe[14]、Cu[15]、Pt[16]、Ti[17]、碳纸[18]等材料应用于硝酸盐还原电极的制备。由于不同材料间可能存在协同效应,关于双金属、三元金属或合金电极上的硝酸盐电还原也有广泛的研究[19]。Jonoush等[20]采用Ni、Ni-Fe0和Ni-Fe0@Fe3O4电极对硝酸盐氮的去除效果以及硝酸盐氮还原反应动力学进行探究,结果表明,Ni-Fe0@Fe3O4电极的电催化还原性能最好,在电解电流密度为5 mA·cm−2、pH=6.2的条件下,对50 mg·L−1的硝酸盐溶液进行电解,240 min后硝酸盐去除率达到90.19%。Zhou等[21]以3D Pd-Cu(OH)2/CF电极为阴极,对50 mg·L−1的硝酸盐溶液进行电解,能够在45 min内将
${\rm{NO}}_3^- $ 转化为${\rm{NH}}_4^+ $ ,转化率为98.8%,并且在60 min内${\rm{NH}}_4^+ $ 最终被彻底氧化为氮气,反应体系的总氮去除率为98.7%。泡沫镍是一种导电导热性良好、疏松多孔、比表面积大、成本较低的电极材料[22],本研究提出了Ni foam/Cu电极的制备方法,将其作为阴极分析电催化还原硝酸盐氮反应的影响因素,采用动力学模型对硝酸盐氮还原过程进行探究。并对Ni foam/Cu电极的电催化活性和运行稳定性进行评价。
-
实验所用的电源为直流稳压恒流型,泡沫镍购自天津艾维信化工科技有限公司,硫酸铜、硝酸钠、氯化钠、硫酸钠、亚硝酸钠、氯化铵均为分析纯,购自阿拉丁试剂有限公司。
-
在电极负载前进行电极基体的预处理,取尺寸为5 cm×5 cm的泡沫镍放入10 g·L−1的90℃ NaOH溶液中加热除油20 min,然后置于0.5 mol·L−1的硫酸溶液中酸洗5 min去除表面氧化物,在去离子水中超声10 min彻底冲洗。以经过预处理后的Ni foam为阴极,Ti/RuO2-Ir2O3为阳极,进行阴极电沉积实验。配置20 mmol·L−1的CuSO4·5H2O溶液,含有0.05 mol·L−1的Na2SO4和0.04 g·L−1的十二烷基硫酸钠(C12H25SO4Na)。采用恒电流电沉积一段时间后,用去离子水冲洗。把制备好的Ni foam/Cu电极放在恒温干燥箱中干燥12 h用于后续的电解实验。
-
室温下取硝酸盐氮溶液用于电解实验,在电解液中加入0.5 g·L−1的Na2SO4作为电解质以提高溶液的导电性。每次向电解槽中加入100 mL电解液,以Ni foam/Cu电极为阴极,Ti/RuO2-Ir2O3电极为阳极进行硝酸盐氮的电催化还原实验。两个电极在溶液中的有效反应面积均为25 cm2,极板间距设置为5 mm。采用直流稳压电源在恒流模式下进行电解实验。分别设定不同的电解电流密度(2 — 12 mA·cm−2)、添加到电解液中的NaCl浓度(0.5 — 1.25 g·L−1)、
${\rm{NO}}_3^- $ −N的初始浓度(50 — 200 mg·L−1)进行电解实验,探究电流大小、电解质浓度、${\rm{NO}}_3^- $ −N的初始浓度对硝酸盐氮去除效果的影响。硝酸盐氮溶液的电化学还原过程持续2.5 h,每隔一段时间取样,分析硝酸盐氮、亚硝酸盐氮和氨氮浓度。证实Ni foam/Cu电极处理硝酸盐氮废水的可行性。 -
用扫描电子显微镜(SEM, JEOLJSM 6500F,JEOL,日本)和能量色散x射线光谱仪(EDS, Genesis 7000 X,EDAX公司,美国)分析电极的微观形貌及元素组成。通过紫外分光光度计(U-3900,日立公司,日本)用分子吸收分光光度法测定处理液的
${\rm{NO}}_3^- $ −N浓度[23],用N-(1-萘基)-乙二胺二盐酸盐分光光度法测定处理液的${\rm{NO}}_2^- $ −N浓度[24],用纳氏试剂分光光度法测定处理液的${\rm{NH}}_4^+ $ −N浓度[25]。溶液中的镍离子和铜离子采用电感耦合等离子体原子发射光谱仪(ICP,Optima8300,PerkinElmer,美国)测定。 -
首先在20 mmol·L−1的CuSO4·5H2O电沉积溶液中,以7 mA·cm−2的电流密度沉积20 min制备Ni foam/Cu电极、不锈钢/Cu电极。对比Ni foam电极、不锈钢电极、Ni foam/Cu、不锈钢/Cu电极对100 mg·L−1硝酸盐氮电催化还原的影响。在电解电流密度为4 mA·cm−2的条件下,4个电极2.5 h的硝酸盐氮去除效果如图1(a)所示,Ni foam电极和不锈钢电极对硝酸盐氮没有明显的去除效果。与不锈钢/Cu电极的去除率相比,Ni foam/Cu电极在相同条件下的去除率提高了约34%。这是由于泡沫镍独特的三维立体结构具有较大的比表面积,为沉积金属组分Cu提供更多的活性位点,促进了硝酸盐氮电催化还原反应的进行。
然后在20 mmol·L−1的CuSO4·5H2O电沉积溶液中,以7 mA·cm−2的电流密度制备不同沉积时间的Ni foam/Cu电极。以Ni foam/Cu为阴极,Ti/RuO2-Ir2O3为阳极进行硝酸盐氮的电催化还原实验,探究电极沉积时间对溶液中100 mg·L−1的硝酸盐氮去除效果的影响。结果如图1(b)所示,随着沉积时间的增加,硝酸盐氮的去除率逐渐提高,当沉积时间超过20 min时硝酸盐氮去除率提高较为缓慢,因此选择沉积20 min作为Ni foam / Cu电极制备的条件,用于接下来的电解实验。
-
图2(a)和图2(b)表征了Ni foam / Cu电极的三维立体结构,可以看出电极具有不同大小五边形构成的网状结构,表面相对光滑。这种独特结构使电极具有较大的比表面积,提高了硝酸盐氮的电催化还原反应速率。图2(c)和图2(d)表明泡沫镍上负载了致密的Cu镀层,EDS结果显示泡沫镍上负载Cu后存在质量分数为16.71%的Cu和83.29%的Ni,证实活性金属组分铜成功地负载在泡沫镍基体上。
-
以Ni foam / Cu电极为阴极,Ti/RuO2-Ir2O3为阳极,在溶液的pH=7、电解质Na2SO4浓度为0.5 g·L−1、电解电流密度为4 mA·cm−2的条件下,分别对不同初始浓度的
${\rm{NO}}_3^- $ −N废水(50 — 200 mg·L−1)进行电催化还原处理。反应结果如图3(a)所示,随着硝酸盐氮初始浓度的升高,硝酸盐氮2.5 h的电解去除率先增大后降低,这与LIU等[26]的研究结果相似。50、100、150、200 mg·L−1的硝酸盐氮溶液去除率分别为71.36%、82.16%、74.72%、55.37%,溶液浓度为100 mg·L−1和150 mg·L−1时,硝酸盐氮去除率优于50 mg·L−1时硝酸盐氮的去除速率。溶液浓度为200 mg·L−1时,硝酸盐氮去除率又明显降低。这可能是由于硝酸盐氮浓度在一定范围内的增大促进硝酸盐离子在阴极表面的吸附,提高了反应速率和去除率,但是在较高的浓度下,阴极吸附量达到饱和,硝酸盐的传质被抑制,不能迅速到达电极表面,反应速率和去除率都有所降低。为了研究硝酸盐氮浓度变化的趋势,将一级动力学模型应用于硝酸盐氮还原过程,如下所述:
用拉普拉斯变换求解微分方程,得到硝酸盐氮浓度随时间的变化关系:
式中,
${C}_{t}{}_{,{{\rm{NO}}}_{3}^{-}{\text{-}}{\rm{N}}}$ 为电解t时间时硝酸盐氮的浓度,mg·L−1;${C}_{0}{}_{,{{\rm{NO}}}_{3}^{-}{\text{-}}{\rm{N}}}$ 为硝酸盐氮的初始浓度,mg·L−1;$ t $ 为反应时间,h;$ k $ 为一级动力学反应速率常数,h−1。根据上述方程对实验结果进行非线性回归,可以得出硝酸盐氮在Ni foam /Cu电极上的电催化还原反应符合一级动力学模型,结果如图3(b)所示,硝酸盐氮初始浓度为100 mg·L−1时的动力学常数最大,其值为0.677 h−1。该反应速率常数规律对于处理不同浓度硝酸盐氮废水的工艺条件控制具有一定指导意义。
-
以Ni foam / Cu电极为阴极,Ti/RuO2-Ir2O3为阳极,两电极板间距5 mm,在含有100 mL硝酸盐氮废水模拟体系(100 mg·L−1
${\rm{NO}}_3^- $ −N+0.5 g·L−1 Na2SO4,pH=7)内进行电催化还原实验,进一步探究电解电流密度对硝酸盐氮还原反应的影响。当电解电流密度为2 mA·cm−2时,电解2.5 h后硝酸盐氮的去除率仅有39.73%。当电流达到8 mA·cm−2以上时,电解2.5 h后硝酸盐氮的去除率均可达到95%以上。说明当电流增大,阴极电极电位更负,电子转移速率增大,有助于电催化还原硝酸盐氮反应的进行。此外产生了更多的还原氢也使硝酸盐氮的去除率逐渐增加。同时在电解的过程中可以观察到电极表面产生的气泡也随电流的增大而增多,这是电极表面副反应产生的气体,阴极发生析氢反应,阳极发生析氧反应。说明电流增大,反应时间增长,也促进了析氧等副反应的发生。因此,在该反应体系中电解电流密度应调节在4 — 8 mA·cm−2范围内,既能达到较高的硝酸盐氮去除率,也可以避免产生过多的副反应。图4(b)表明,在该体系中电催化还原硝酸盐氮反应符合一级动力学规律,随着电解电流增大,一级反应动力学常数由0.197 h−1增大至 1.547 h−1。 -
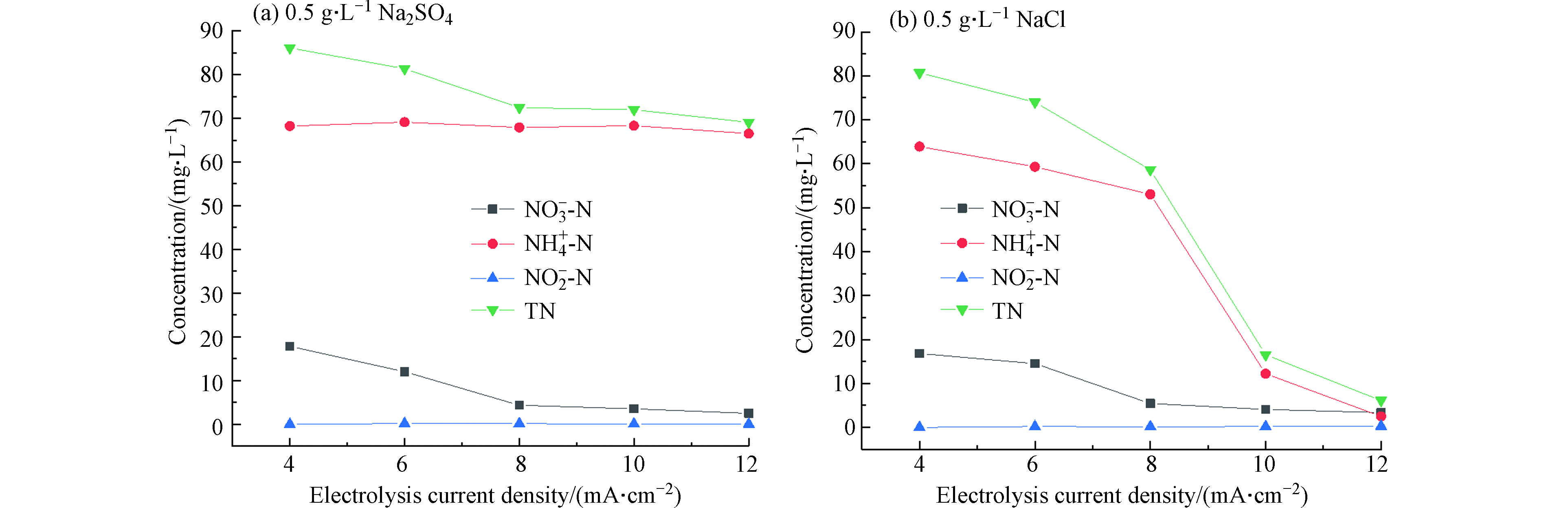
首先以不同大小的电流对100 mg·L−1的硝酸盐氮溶液电解2.5 h,探究电解质种类对硝酸盐氮还原产物的影响。图5说明当以0.5 g·L−1硫酸钠作为电解质时,电流对氨氮和亚硝酸盐氮的生成量几乎没有影响,同时随着电解电流的增大,溶液中总氮的去除效果提升并不显著,在电解电流密度为12 mA·cm−2时达到最大,约30.93%。但当以相同浓度的氯化钠作为电解质时,氨氮生成量随着电流的增大而减少,明显提高了电催化还原体系的总氮去除率,在电解电流密度为12 mA·cm−2时,总氮去除率可以达到93.88%。
为探究NaCl电解质对电催化还原体系总氮去除效果的影响,以Ni foam / Cu电极为阴极,Ti/RuO2-Ir2O3为阳极,在溶液的pH=7、电解电流密度为8 mA·cm−2的条件下,分别加入0.5、0.75、1、1.25 g·L−1的NaCl作为电解质对100 mL含有100 mg·L−1的
${\rm{NO}}_3^- $ −N废水进行电催化还原处理。结果如图6所示,NaCl浓度的变化对溶液中总氮的去除有较大影响,NaCl浓度为0.5 g·L−1时总氮去除率仅41.37%,但当NaCl浓度为1.25 g·L−1时,总氮去除率提高至79.47%。随着NaCl浓度的增加,电催化还原体系的总氮去除率增大,即提高了反应的氮气选择性。亚硝酸盐氮在整个电解过程中的生成量很低,不足1 mg·L−1,几乎都参与反应生成了其它含氮物质。硝酸盐氮和总氮随着电解时间的延长均逐渐降低,氨氮呈先增加后减少的趋势,且峰值随着NaCl浓度的增加而降低,这与加入的Cl−影响
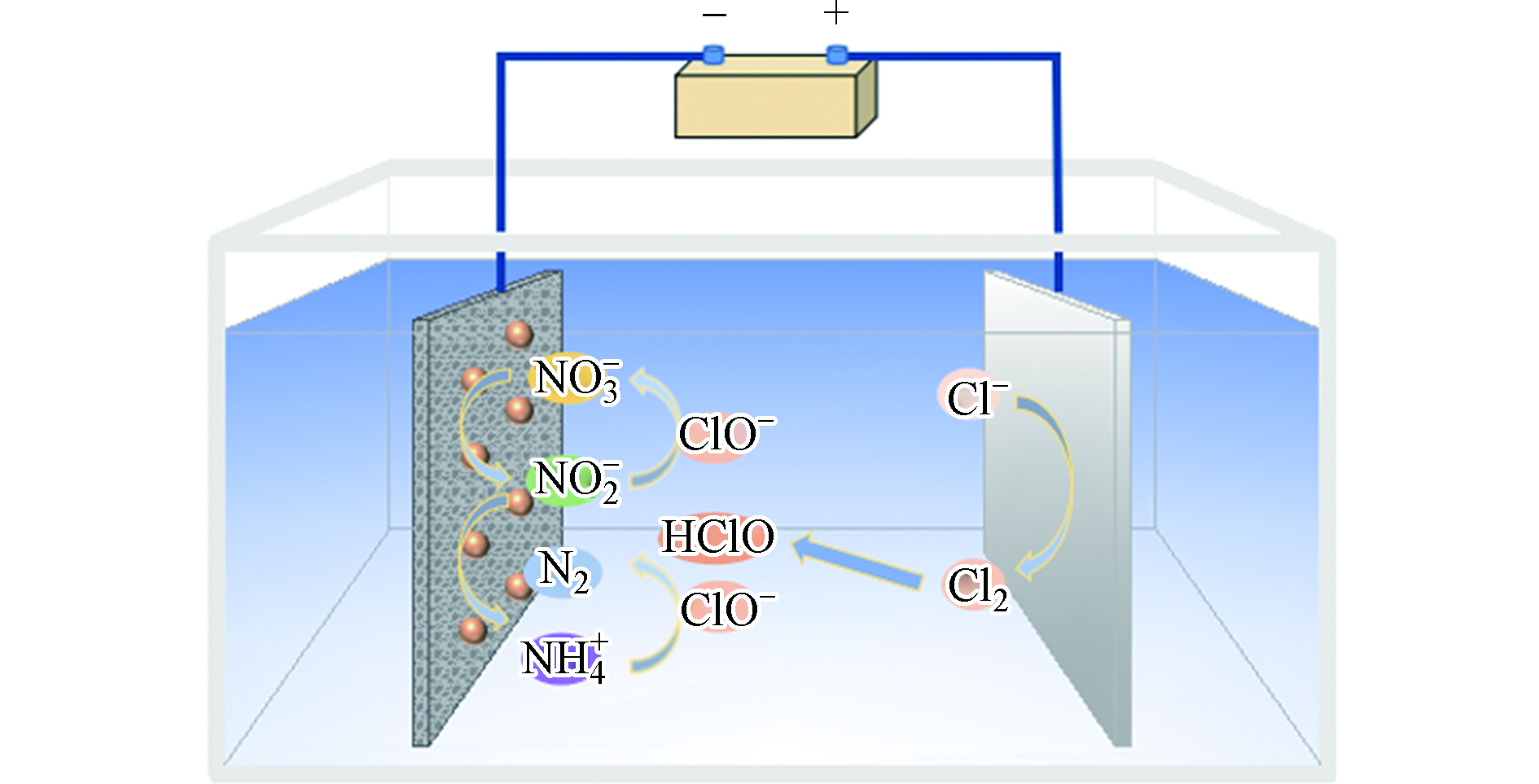
${\rm{NH}}_4^+ $ 的反应有关。向溶液中添加NaCl后,部分溶解的氯离子会被氧化为Cl2。氯气溶于水生成的HClO以及HClO电离生成ClO−均具有较强的氧化性,能够将${\rm{NO}}_3^- $ 还原的副产物${\rm{NH}}_4^+ $ 氧化成N2。但当溶液中NaCl的含量过低时,生成的HClO量将不足以氧化所有生成的氨氮。在NaCl浓度为1.25 g·L−1的条件下,对硝酸盐氮溶液电解2.5 h后,生成氨氮的含量为2.90 mg·L−1,硝酸盐氮的去除率为82.37%,与NaCl浓度为0.5 g·L−1时相比降低了12.21%,说明如果加入NaCl的含量过多,生成过量的ClO−可能会将${\rm{NO}}_2^- $ 氧化成${\rm{NO}}_3^- $ ,同时带负电荷的ClO−和Cl−与${\rm{NO}}_3^- $ 竞争吸附会抑制硝酸盐的去除[27],具体反应过程如图7和式1—9所示。 -
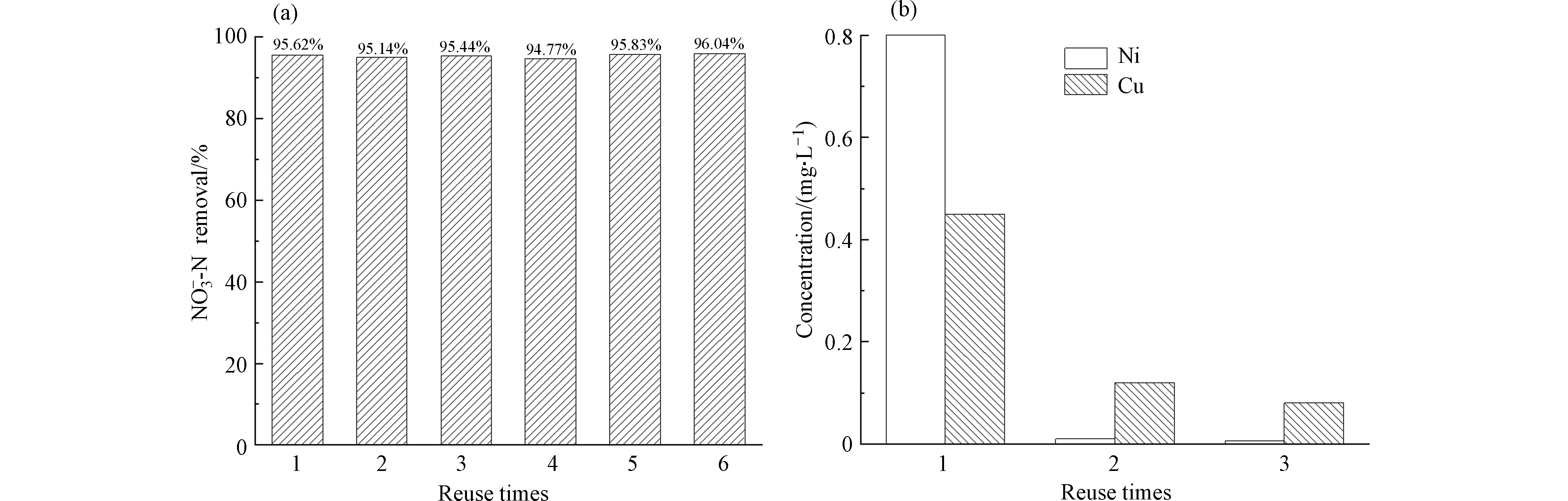
为了验证Ni foam/Cu电极电催化还原硝酸盐氮的稳定性,以Ni foam / Cu电极为阴极,Ti/RuO2-Ir2O3为阳极,在溶液pH=7、电解质NaCl浓度为0.5 g·L−1、电解电流密度为8 mA·cm−2的条件下,对100 mL含有100 mg·L−1的硝酸盐氮溶液进行了6次电解实验探究。实验结果如图8(a)所示,电解2.5 h后,硝酸盐氮的去除率均达到94.5%以上,没有呈下降趋势,并且电极表面无明显变化。
通过ICP测定3次电解反应结束后溶液中的Ni和Cu金属离子含量,结果如图8(b)所示,随着使用次数的增加,溶液中金属离子含量也随之减少,第三次电解后溶液中镍和铜的金属离子的浓度均低于0.1 mg·L−1,验证了Ni foam/Cu电极稳定性良好。
-
(1)采用恒流电沉积法制备的Ni foam / Cu电极在硝酸盐氮降解反应中具有良好的电催化还原性能和稳定性。在重复实验中硝酸盐氮的去除率均能达到94.5%以上。实验结束后溶液中镍和铜的金属离子浓度均低于1 mg·L−1,证明金属铜紧密地结合在泡沫镍基体上。
(2)增大电流有助于电催化还原硝酸盐氮反应的进行,在该电催化还原体系中电解电流密度为8 mA·cm−2时,即可以实现90%以上的硝酸盐氮去除率。
(3)在硝酸盐氮的电催化还原过程中,外加电解质NaCl浓度对产物的选择性有很大影响。在一定范围内,NaCl浓度适当增大有助于减少副产物的生成,提高总氮的去除率。当NaCl浓度为1.25 g·L−1时,氮气选择率接近100%,总氮去除率达到79.47%。
Ni foam/Cu电极电催化还原硝酸盐氮
Electrocatalytic reduction of nitrate nitrogen by Ni foam/Cu electrode
-
摘要: 采用恒流电沉积法制备了泡沫镍/铜(Ni foam/Cu)电极。以Ni foam/Cu电极为阴极、Ti/RuO2-Ir2O3电极为阳极构建电解体系,对水中硝酸盐氮进行电催化还原处理。并研究了电解质对总氮和氨氮去除率的影响和电极的稳定性。在一定范围内,增大电解质NaCl的浓度可以提高总氮和氨氮去除率。当NaCl浓度为0.5 g·L−1时,在电解电流密度为8 mA·cm−2的条件下对100 mg·L−1
${\rm{ NO}}_3^-$ −N溶液进行6次重复电解实验,2.5 h后硝酸盐氮去除率均可以达到94.5%以上。当NaCl浓度为1.25 g·L−1时,在电解电流密度为8 mA·cm−2的条件下对100 mg·L−1${\rm{NO}}_3^-$ −N溶液电解2.5 h,出水中氨氮浓度只有2.90 mg·L−1,总氮去除率达到79.47%。实验结果表明,Ni foam/Cu阴极具有较高的电催化还原活性和良好的稳定性。Abstract: The Ni foam/Cu electrode was prepared by galvanostatic electrodeposition method. The Ni foam/Cu and the Ti/RuO2-Ir2O3 electrode were used as the cathode and anode respectively to construct an electrolysis system to perform electrocatalytic reduction of nitrate nitrogen in water. The effect of the electrolyte on the removal rate of total nitrogen and ammonia nitrogen and the stability of the electrode were also investigated. In a certain range, the removal rate of total nitrogen and ammonia nitrogen can be improved by increasing the electrolyte NaCl concentration. When the concentration of electrolyte NaCl increased to 0.5 g·L−1, six repeated electrolysis experiments were performed in 100 mg·L−1${\rm{NO}}_3^- $ -N solution for 2.5 h under the condition of 8 mA·cm−2, and the removal rate of nitrate nitrogen could reach more than 94.5%. When the concentration of electrolyte NaCl increased to 1.25 g·L−1, the concentration of ammonia nitrogen was only 2.90 mg·L−1 after 2.5 h of electrolysis under the condition of 8 mA·cm−2, and the total nitrogen removal rate reached 79.47%. The experimental results show that the Ni foam/Cu cathode has high electrocatalytic reduction activity and good stability.-
Key words:
- nitrate nitrogen /
- electrocatalytic reduction /
- Ni foam/Cu
-

-
-
[1] 陈帅, 房宁, 唐亮. 光催化法还原水中硝酸盐氮研究进展 [J]. 环境与发展, 2018, 30(9): 105-106. CHEN S, FANG N, TANG L. Research progress in reduction of nitrate nitrogen in water by photocatalysis [J]. Environment and Development, 2018, 30(9): 105-106(in Chinese).
[2] DING Y J, SUN W Z, YANG W Y, et al. Formic acid as the in situ hydrogen source for catalytic reduction of nitrate in water by PdAg alloy nanoparticles supported on amine-functionalized SiO2 [J]. Applied Catalysis B:Environmental, 2017, 203: 372-380. doi: 10.1016/j.apcatb.2016.10.048 [3] MARTÍNEZ J, ORTIZ A, ORTIZ I. State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates [J]. Applied Catalysis B:Environmental, 2017, 207: 42-59. doi: 10.1016/j.apcatb.2017.02.016 [4] LEI X H, LIU F, LI M, et al. Fabrication and characterization of a Cu-Pd-TNPs polymetallic nanoelectrode for electrochemically removing nitrate from groundwater [J]. Chemosphere, 2018, 212: 237-244. doi: 10.1016/j.chemosphere.2018.08.082 [5] 余静, 蒲生彦, 王晓科, 等. 磁性壳聚糖凝胶微球固定反硝化菌去除地下水中硝酸盐氮 [J]. 环境化学, 2020, 39(2): 416-425. doi: 10.7524/j.issn.0254-6108.2019032002 YU J, PU S Y, WANG X K, et al. Removal of nitrate nitrogen in groundwater by denitrifying bacteria immobilized on magnetic chitosan gel microspheres [J]. Environmental Chemistry, 2020, 39(2): 416-425(in Chinese). doi: 10.7524/j.issn.0254-6108.2019032002
[6] CHABANI M, AMRANE A, BENSMAILI A. Kinetics of nitrates adsorption on Amberlite IRA 400 resin [J]. Desalination, 2007, 206(1/2/3): 560-567. [7] LUBPHOO Y, CHYAN J M, GRISDANURAK N, et al. Influence of Pd-Cu on nanoscale zero-valent iron supported for selective reduction of nitrate [J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 59: 285-294. doi: 10.1016/j.jtice.2015.08.005 [8] HAO S Z, ZHANG H W. High catalytic performance of nitrate reduction by synergistic effect of zero-valent iron (Fe0) and bimetallic composite carrier catalyst [J]. Journal of Cleaner Production, 2017, 167: 192-200. doi: 10.1016/j.jclepro.2017.07.255 [9] ZHANG Q, DING L, CUI H, et al. Electrodeposition of Cu-Pd alloys onto electrophoretic deposited carbon nanotubes for nitrate electroreduction [J]. Applied Surface Science, 2014, 308: 113-120. doi: 10.1016/j.apsusc.2014.04.119 [10] DUAN W J, LI G, LEI Z C, et al. Highly active and durable carbon electrocatalyst for nitrate reduction reaction [J]. Water Research, 2019, 161: 126-135. doi: 10.1016/j.watres.2019.05.104 [11] SONG Q N, LI M, WANG L L, et al. Mechanism and optimization of electrochemical system for simultaneous removal of nitrate and ammonia [J]. Journal of Hazardous Materials, 2019, 363: 119-126. doi: 10.1016/j.jhazmat.2018.09.046 [12] HORÁNYI G, RIZMAYER E M. Electrocatalytic reduction of NO2− and NO3− ions at a platinized platinum electrode in alkaline medium [J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1985, 188(1/2): 265-272. [13] FAN N W, LI Z K, ZHAO L, et al. Electrochemical denitrification and kinetics study using Ti/IrO2-TiO2-RuO2 as the anode and Cu/Zn as the cathode [J]. Chemical Engineering Journal, 2013, 214: 83-90. doi: 10.1016/j.cej.2012.10.026 [14] 陈西亮, 刘国, 高阳阳, 等. 零价纳米铁炭微电解体系去除水中硝酸盐 [J]. 环境化学, 2016, 35(8): 1670-1675. doi: 10.7524/j.issn.0254-6108.2016.08.2015123003 CHEN X L, LIU G, GAO Y Y, et al. Removal of nitrate from water by nano-zero-valent iron-carbon microelectrolysis system [J]. Environmental Chemistry, 2016, 35(8): 1670-1675(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.08.2015123003
[15] GAO W C, GAO L L, LI D, et al. Removal of nitrate from water by the electrocatalytic denitrification on the Cu-Bi electrode [J]. Journal of Electroanalytical Chemistry, 2018, 817: 202-209. doi: 10.1016/j.jelechem.2018.04.006 [16] URETA-ZAÑARTU S, YÁÑEZ C. Electroreduction of nitrate ion on Pt, Ir and on 70: 30 Pt: Ir alloy [J]. Electrochimica Acta, 1997, 42(11): 1725-1731. doi: 10.1016/S0013-4686(96)00372-6 [17] LI M, FENG C P, ZHANG Z Y, et al. Efficient electrochemical reduction of nitrate to nitrogen using Ti/IrO2-Pt anode and different cathodes [J]. Electrochimica Acta, 2009, 54(20): 4600-4606. doi: 10.1016/j.electacta.2009.03.064 [18] LI A Z, ZHAO X, HOU Y N, et al. The electrocatalytic dechlorination of chloroacetic acids at electrodeposited Pd/Fe-modified carbon paper electrode [J]. Applied Catalysis B:Environmental, 2012, 111/112: 628-635. doi: 10.1016/j.apcatb.2011.11.016 [19] GARCIA-SEGURA S, LANZARINI-LOPES M, HRISTOVSKI K, et al. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications [J]. Applied Catalysis B:Environmental, 2018, 236: 546-568. doi: 10.1016/j.apcatb.2018.05.041 [20] JONOUSH Z A, REZAEE A, GHAFFARINEJAD A. Electrocatalytic nitrate reduction using Fe0/Fe3O4 nanoparticles immobilized on nickel foam: Selectivity and energy consumption studies [J]. Journal of Cleaner Production, 2020, 242: 118569. doi: 10.1016/j.jclepro.2019.118569 [21] ZHOU C H, BAI J, ZHANG Y, et al. Novel 3D Pd-Cu(OH)2/CF cathode for rapid reduction of nitrate-N and simultaneous total nitrogen removal from wastewater [J]. Journal of Hazardous Materials, 2021, 401: 123232. doi: 10.1016/j.jhazmat.2020.123232 [22] PIEROZYNSKI B, MIKOLAJCZYK T, LUBA M, et al. Kinetics of oxygen evolution reaction on nickel foam and platinum-modified nickel foam materials in alkaline solution [J]. Journal of Electroanalytical Chemistry, 2019, 847: 113194. doi: 10.1016/j.jelechem.2019.113194 [23] 中华人民共和国国家环境保护总局. 中华人民共和国环保行业标准: 水质 硝酸盐氮的测定 紫外分光光度法(试行) HJ/T 346—2007[S]. 北京: 中国环境科学出版社, 2007. State Environmental Protection Administration of the People's Republic of China. Environmental Protection Standard of the People's Republic of China: Water quality-Determination of nitrate-nitrogen-Ultraviolet spectrophotometry. HJ/T 346—2007[S]. Beijing: China Environment Science Press, 2007(in Chinese).
[24] 中国国家环境保护局. 水质 亚硝酸盐氮的测定 分光光度法: GB/T7493—1987[S]. 北京: 中国标准出版社, 1987. State Environmental Protection Administration of China. Water quality-Determination of nitrogen(nitrite)-Spectrophotometric method: GB/T7493—1987[S]. China Standard Press, 1987(in Chinese).
[25] 环境保护部. 中华人民共和国环保行业标准: 水质 氨氮的测定 纳氏试剂分光光度法 HJ 535—2009[S]. 北京: 中国环境科学出版社, 2010. Ministry of Environmental Protection of the People's Republic of China. Environmental Protection Standard of the People's Republic of China: Water quality-Determinatiion of ammonia nitrogen-Nesslers reagent spectrophotometry. HJ 535—2009[S]. Beijing: China Environment Science Press, 2010(in Chinese).
[26] LIU F, LIU K W, LI M, et al. Fabrication and characterization of a Ni-TNTA bimetallic nanoelectrode to electrochemically remove nitrate from groundwater [J]. Chemosphere, 2019, 223: 560-568. doi: 10.1016/j.chemosphere.2019.02.028 [27] DING J, LI W, ZHAO Q L, et al. Electroreduction of nitrate in water: Role of cathode and cell configuration [J]. Chemical Engineering Journal, 2015, 271: 252-259. doi: 10.1016/j.cej.2015.03.001 -







 下载:
下载: