-
冰封是高纬度地区的常见且重要的水文特征,全球每年有超过5000万个湖泊会定期冻结[1]。水体结冰过程中的部分污染物会在冰体中临时存储,然后在春季温度回升时融化并释放到水中[2-5]。近年来,气候变暖引起了海洋与湖泊的异常变化[6-7],水体的冰封期缩短,甚至冰川也开始融化。冰融化过程中,若冰中的污染物集中释放就可能对生态环境产生破坏,因此有必要弄清冰消融过程中污染物的迁移规律。
近年来,冰封期的湖泊已不是“静止”的观点已经被湖沼专家所公认,因此开展了许多有关湖泊冰封期的研究[8-13],但这些研究主要集中在湖泊结冰的过程中。由于湖泊融冰过程中冰体强度骤降,难以开展现场实验,因此湖泊融冰过程中的污染物的迁移规律的研究大都采用室内模拟实验。李志军等[2]研究发现,融化过程中,冰内硝基苯“原位不动”,冰内的硝基苯只随冰的融化排出到水体中;薛爽等[14-15]通过室内模拟实验,发现了冰融化过程中溶解性有机物(DOM)有初期优先洗脱现象;Brimblecombe等[16-17]通过研究人工制冰融化过程中离子的洗脱现象,发现富含离子的溶液优先从最初的20%—30%的冰融水中获得。但有关湖泊冰封期农药迁移规律的研究尚未见报道。
本研究以化学性质稳定、水溶性强、难生物降解且易随降水、淋溶、径流、渗透等作用由土壤迁移至水体中的阿特拉津农药为研究对象[18],通过开展室内模拟实验,探索其在湖泊融冰过程的迁移规律,以期为湖泊融冰过程中的水环境管理提供参考。
-
主要药品与试剂:阿特拉津,纯度 > 98.8%;甲醇,HPLC级色谱纯;自制超纯水。
主要实验仪器:超高效液相色谱-电喷雾-三重四极杆质谱联用仪(UPLC-ESI-MS/MS),ACQUITYTM UPLC BEN C8色谱柱(2.1 mm×50 mm,1.7 μm)。
-
建立UPLC方法对阿特拉津进行检测,其中色谱质谱条件如下所示。
色谱条件:流动相为甲醇(A)和0.1%甲酸水(B);流速:5.0 μL·min−1;流量:0.2 mL·min−1;柱温:35 ℃;样品室温度:15 ℃;进样量:5 μL。
质谱条件:电喷雾离子源(ESI);正离子模式采集;多反应监测(MRM)模式;毛细管电压和锥孔电压分别为3.3 V和35 V;离子源温度和脱溶剂气温度分别为120 ℃和350 ℃;脱溶剂气流量和锥孔气(流量分别为500 L·h−1和30 L·h−1),其他质谱参数见表1。
经过标线检验,在0—100 μg·L−1范围内,阿特拉津的方法响应值与质量浓度线性良好,标准曲线为y=1337.6x+6314.3,相关系数R2为0.995。对选取浓度分别为2.5、10、25 μg·L−1的阿特拉津进行日内连续进样3次,日间连续进样3次,计算相对标准偏差(RSD),RSD值均小于1%,加标回收率在96%—112%之间,该方法线性良好且准确度高。
-
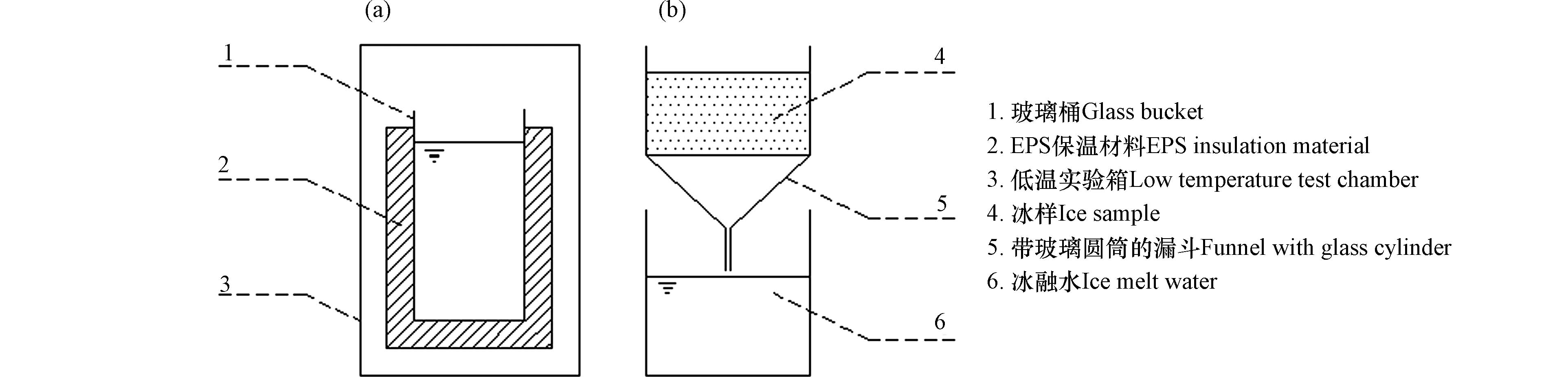
实验装置如图1所示,结冰装置采用圆柱形玻璃仪器(直径10 cm、高度29.5 cm),其四周及底部用聚苯乙烯泡沫(EPS)保温材料包裹以阻断玻璃仪器与外界的热量传递来模拟湖泊自上而下的结冰过程;冷冻过程在设有温度控制的低温实验箱中进行,温度控制的精度为0.5 ℃;融冰装置采用带有15 cm圆柱形上沿的玻璃漏斗,外部用铁架台固定,将冰样正向放入漏斗,底部用烧杯收集冰融水。
-
用超纯水配制A、B两组质量浓度分别为5、15、25、35、45 μg·L−1的阿特拉津溶液,在−10 ℃的低温箱中获得厚度为9 cm的A组冰样和B组冰样。将A组冰样每3 cm切片进行分层,由上而下分别记为冰1、冰2、冰3,测量冰下水和各层冰融水阿特拉津的浓度,探讨阿特拉津在冰水体系和冰中的分布;将B组冰样在20 ℃下等体积融化为5份,将0—20 %冰融水、20 %—40 %冰融水、40 %—60 %冰融水、60 %—80 %冰融水、80%—100%冰融水分别记为融1、融2、融3、融4、融5,检测冰融水中阿特拉津的浓度,以探讨融冰过程中阿特拉津的迁移规律。
-
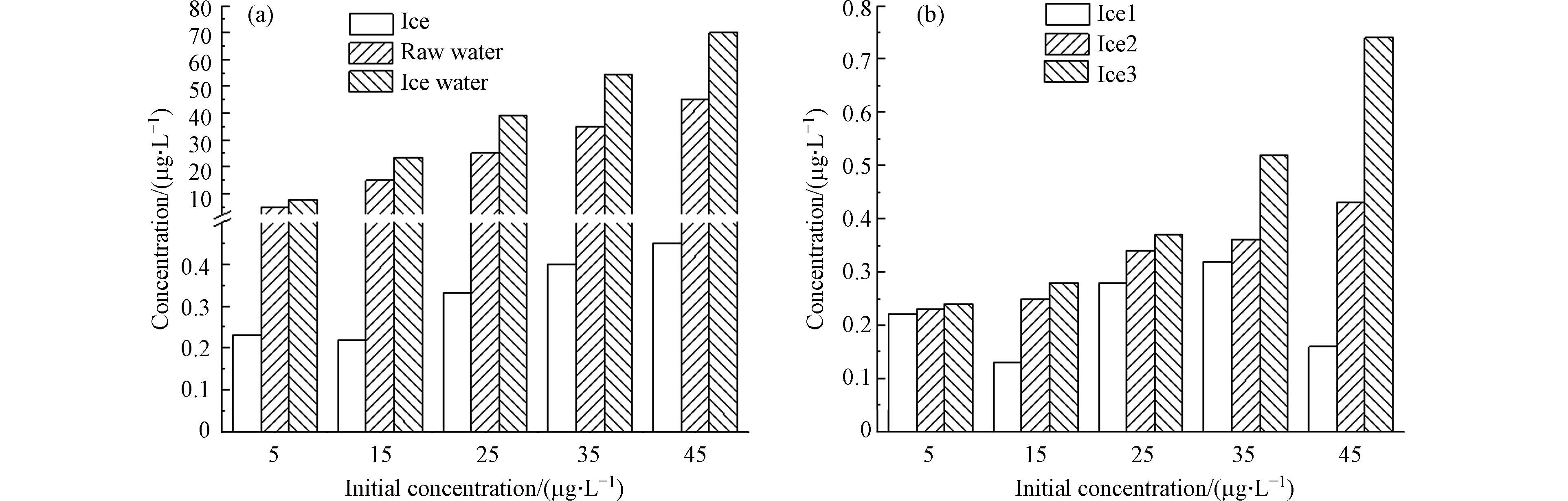
冰体内阿特拉津的分布会影响融冰过程中阿特拉津的迁移过程[19],因此需要探究阿特拉津在冰水体系和冰体内部的分布规律。通过分别检测不同初始浓度条件下,冰融水和冰下水的阿特拉津浓度可知,结冰厚度为9 cm时A组冰样冰融水阿特拉津的浓度分别为0.23、0.22、0.33、0.4、0.45 μg·L−1,冰下水中阿特拉津的浓度分别从5、15、25、35、45 μg·L−1增加至7.69、23.32、38.99、54.28、69.98 μg·L−1。在不同溶液初始浓度条件下,阿特拉津在各相的浓度关系均为:冰融水浓度<结冰前水浓度<冰下水浓度(图2a)。这是由于在结冰过程中,水分子会首先结合成一个巨大的集团,称之为冰晶[10],溶液中的冰晶数量随着溶液浓度的提高而增多[20],致使冰-水界面稳定性下降, 从而导致冰体中存留了部分阿特拉津[21]。但是冰中的阿特拉津存留量远小于结冰过程中对阿特拉津的排斥量,所以冰下水中阿特拉津含量相较结冰前水升高。
通过分别检测不同初始浓度条件下各层冰融水的阿特拉津浓度可知,冰1阿特拉津的浓度分别为0.22、0.13、0.28、0.32、0.16 μg·L−1,冰2阿特拉津的浓度分别为0.23、0.25、0.34、0.36、0.43 μg·L−1,冰3阿特拉津的浓度分别为0.24、0.28、0.37、0.52、0.74 μg·L−1,即冰中阿特拉津的浓度关系为:冰1<冰2<冰3(图2b),冰体中阿特拉津的含量呈现出由上层往下层增加的分布特点。在冰体内部,由于结冰初期结冰速率低,阿特拉津有足够的时间迁移到冰下水中,所以上层冰阿特拉津含量较少;随着结冰过程的进行,冰体为了更好散热而产生的树枝状分枝结构对阿特拉津具有吸附作用,使冰中阿特拉津含量开始增多;结冰过程中冰下水的阿特拉津浓度也在逐步增加,进而促使冰中阿特拉津含量增加[21];且冰体内还会形成类似于树枝分叉的迁移通道,在重力作用下被俘获在冰体内的阿特拉津会通过通道向下迁移,导致下层冰中阿特拉津的升高。冰厚的继续增加会阻碍热量传递,冰生长速率减缓,孔隙通道的密度减小[22],未排出的阿特拉津被冻结在下层孔隙通道中,而难以进一步迁移至冰下水体,最终形成冰中阿特拉津含量由上层往下层增多的分布规律。
-
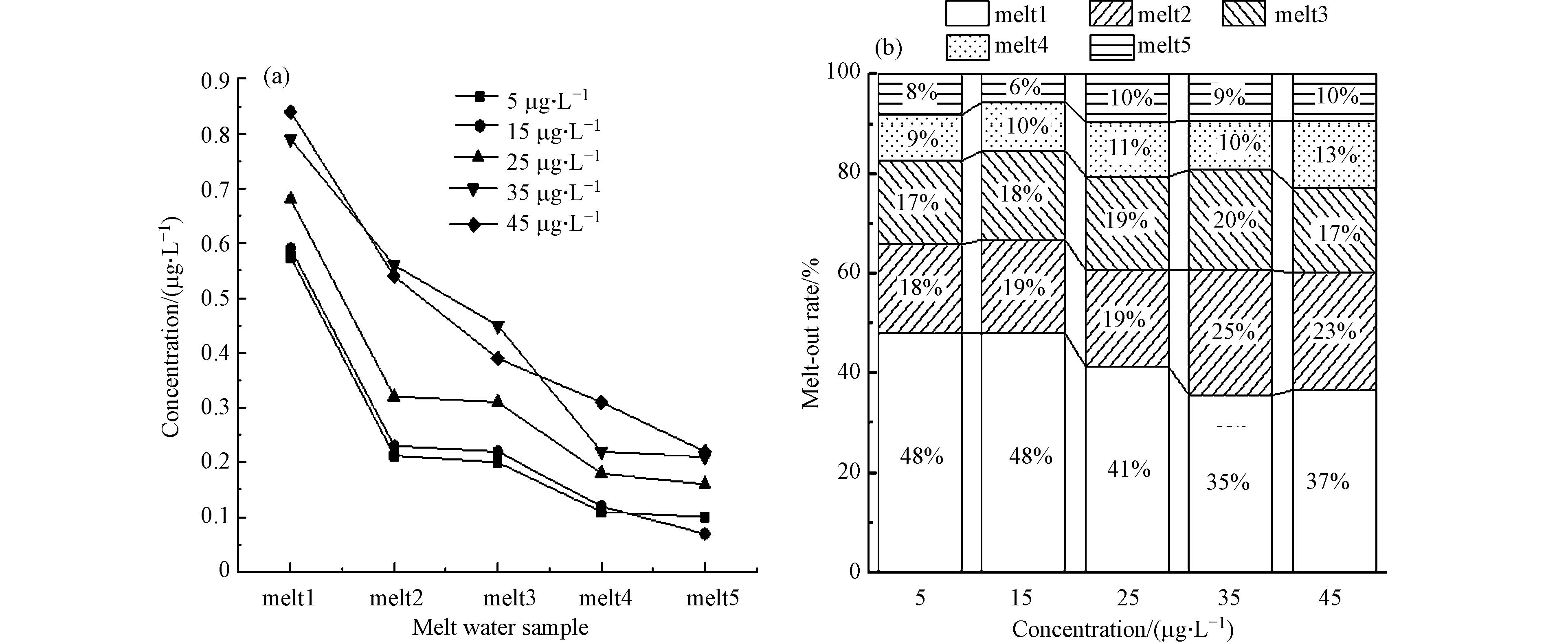
通过测定冰融化过程中,不同融出比例(融1—融5)的冰融水中阿特拉津的浓度可以总结其在融冰过程中的迁移规律。如图3a所示,融1中阿特拉津浓度分别为0.57、0.59、0.68、0.79、0.84 μg·L−1,融1中阿特拉津浓度随初始浓度的增大而增大。如图3b所示,B组冰样融1中阿特拉津含量占冰中总含量的比例分别为48%、48%、41%、35%、37%,且融1、融2、融3中阿特拉津即可达到冰中总含量的83%、84%、79%、81%、77%。即各浓度冰样融化过程中都呈现出初期大量释放,后期少量均匀释放的特点,阿特拉津在融冰初期迁移能力强于融冰后期。
融冰过程中的初期洗脱现象可以通过冰的局部融化、冰体微观结构和冰体中的气泡的三个方面来解释:在融冰过程中,冰最先在表面均匀融化,随后它将逐渐形成不同厚度的水层,经汇聚后形成水滴[23]。融化的水滴携带着融化冰体中的阿特拉津汇集到冰体底部,冰融水温度比冰体温度高,促进冰底部的融化。阿特拉津在底部冰中含量高(图2b),底部冰融化速率大于冰整体融化速率,形成了局部先融化的现象,使底部困在冰体缝隙中的阿特拉津更早的释放到冰融水水滴中[24],从而造成了初期洗脱的现象。
从冰体微观结构来看,冰中水分子间氢键断裂使存在于冰中的阿特拉津释放是造成初期洗脱现象的原因之一。在冰的分子结构中,1个氧原子与4个氢原子连接形成一个四面体,1个氢原子与2个氧原子连接,因此冰体形成许多空腔无法像水一样被水分子紧密堆积[25]。阿特拉津就像是“犯人”一样被关在由水分子组合成的“牢笼”中[26]。冰融化成水时,随着外部能量的不断供应,这5个水分子的四面体结构将逐渐破裂(图4a),原本存留在冰空隙中的阿特拉津从破裂的水分子缺口处集中释放,从而使冰中的阿特拉津在融化过程中随着冰融水大量释放[25—28]。
此外,结冰过程中冰体内部会产生气泡,且由于冰下水压力的增大使冰内气泡的压力也较高,这些气泡中会存留大量的阿特拉津[25]。如图4b所示,针对冰体内部物质的分布进行了示踪观察,采用高锰酸钾作为显色剂,高锰酸钾主要集中在冰中的孔隙和气泡中,且气泡与孔隙呈现出连同状,阿特拉津在冰中的分布与其相似。融冰过程中,由于气泡的比表面积很大,这会使气泡在融化过程中更容易接触冰融水,且在高压气泡的推动下促进阿特拉津向冰融水中迁移,即形成了初期洗脱现象。随着融冰过程的进行,融冰中形成的空隙增大并相互连接,冰层内部的气泡促使其上下贯通进而形成阿特拉津的迁移通道,这加速了冰中阿特拉津的集中释放。
-
(1)在不同初始浓度条件下,冰中阿特拉津的平均含量随初始浓度的增高而增大,阿特拉津在冰体中呈现由上层往下层逐渐增多的分布规律。
(2)在融冰过程中阿特拉津的迁移存在初期洗脱现象。融冰初期大量的阿特拉津瞬间释放到融水中,这可能破坏湖泊冰下水环境,甚至对冰体下水生态带来影响,因此湖冰的融化过程同样需引起研究者的关注和重视。
(3)阿特拉津在融冰过程中的迁移规律除受水体中阿特拉津初始浓度影响外还可能受到融冰温度、溶解氧、无机离子等其他影响因素的影响,这还有待进一步的研究。
模拟湖泊融冰过程中阿特拉津的迁移规律
The migration law of atrazine in the process of melting simulated lake ice
-
摘要: 为探究阿特拉津在湖泊冰融化过程中的迁移规律,配制2组不同初始浓度的阿特拉津水样,并通过模拟湖泊的自然结冰和融冰的过程,分析阿特拉津在冰水体系和冰体内部的分布及其在冰体融化过程中的融出浓度。研究结果表明,不同初始浓度的阿特拉津溶液在冰水两相中的分布规律均为冰融水浓度<结冰前水浓度<冰下水浓度,在冰中的分布表现均为上层浓度<中层浓度<下层浓度;阿特拉津在初期冰融水(融1)中含量最高,即融冰过程中阿特拉津存在初期洗脱现象;随着融冰过程的进行,阿特拉津会呈少量、有递减趋势的融出;融冰进程前段冰融水(融1、融2、融3)中阿特拉津含量大于原冰体含量的77%,阿特拉津在融冰初期向冰融水中的迁移能力大于融冰后期。Abstract: In order to explore the migration law of atrazine in the melting of lake ice, two groups of atrazine water samples with different initial concentrations were prepared, and the distribution of atrazine in the ice system and ice body and its melting concentration in the melting process were analyzed by simulating the natural freezing and melting process of the lake. The results showed that the distribution of atrazine in ice water two-phase is as follows: concentration of ice < initial concentration of water before freezing < concentration of under-ice water; the distribution in ice phase is in the order of the upper layer concentration < the middle layer concentration < the lower layer concentration. During the process of ice melting, the content of atrazine in the initial melt water (melt 1) was the highest. Atrazine was eluted at the initial stage, which was characterized by high concentration. With the process of ice melting, atrazine would melt out in a small amount with a decreasing trend. The content of atrazine in melt water (melt 1, melt 2, melt 3) in the initial stage of ice melting process was more than 77% of the original ice content, which means that more than 77% of atrazine in the ice body could be melted out of the first three melting water (melt 1, melt 2, melt 3), the migration of atrazine into ice melt water in the initial stage is stronger than that in the later stage.
-
Key words:
- lake /
- simulate icing /
- atrazine /
- ice melting process /
- migration law
-

-
表 1 质谱参数
Table 1. Mass spectrometry parameters
化合物
Compounds质荷比(mz) 碰撞能量/eV
Collision energy母离子
Parention子离子
Daughter ion阿特拉津
Atrazine215 95.8 25 103.73 28 173.9 17 -
[1] VERPOORTER C, KUTSER T, SEEKELL D A, et al. A global inventory of lakes based on high-resolution satellite imagery [J]. Geophysical Research Letters, 2014, 41(18): 6396-6402. doi: 10.1002/2014GL060641 [2] 李志军, 王昕, 李青山, 等. 不同条件下硝基苯在水-冰体系中的分配研究 [J]. 中国科学(E辑:技术科学), 2008, 38(7): 1131-1138. LI Z J, WANG X, LI Q S, et al. Distribution of nitrobenzene in water-ice system under different conditions [J]. Science in China (Series E:Technological Sciences), 2008, 38(7): 1131-1138(in Chinese).
[3] 张岩, 李畅游, SHEN Hung Tao, 等. 乌梁素海湖泊冰生长过程中总氮的迁移规律 [J]. 水科学进展, 2013, 24(5): 728-735. ZHANG Y, LI C Y, SHEN H T, et al. Total nitrogen migration in Wuliangsuhai Lake during ice growth process [J]. Advances in Water Science, 2013, 24(5): 728-735(in Chinese).
[4] JONES K C, de VOOGT P. Persistent organic pollutants (POPs): State of the science [J]. Environmental Pollution, 1999, 100(1/2/3): 209-221. [5] CABRERIZO A, GALBÁN-MALAGÓN C, del VENTO S, et al. Sources and fate of polycyclic aromatic hydrocarbons in the Antarctic and Southern Ocean atmosphere [J]. Global Biogeochemical Cycles, 2014, 28(12): 1424-1436. doi: 10.1002/2014GB004910 [6] 祁第, 陈立奇, 蔡卫君, 等. 北冰洋海洋酸化和碳循环的研究进展 [J]. 科学通报, 2018, 63(22): 2201-2213. doi: 10.1360/N972018-00334 QI D, CHEN L Q, CAI W J, et al. Review on ocean acidification and carbon cycling in the Arctic Ocean [J]. Chinese Science Bulletin, 2018, 63(22): 2201-2213(in Chinese). doi: 10.1360/N972018-00334
[7] 祝叶华. 全球变暖被预测进一步加重 [J]. 科技导报, 2017, 35(24): 9. ZHU Y H. Global warming is predicted to be further aggravated [J]. Science & Technology Review, 2017, 35(24): 9(in Chinese).
[8] WEYHENMEYER G A, LIVINGSTONE D M, MEILI M, et al. Large geographical differences in the sensitivity of ice-covered lakes and rivers in the Northern Hemisphere to temperature changes [J]. Global Change Biology, 2011, 17(1): 268-275. doi: 10.1111/j.1365-2486.2010.02249.x [9] MEYER T, LEI Y D, WANIA F. Measuring the release of organic contaminants from melting snow under controlled conditions [J]. Environmental Science & Technology, 2006, 40(10): 3320-3326. [10] NAKAGAWA K, NAGAHAMA H, MAEBASHI S, et al. Usefulness of solute elution from frozen matrix for freeze-concentration technique [J]. Chemical Engineering Research and Design, 2010, 88(5/6): 718-724. [11] NAKAGAWA K, MAEBASHI S, MAEDA K. Freeze-thawing as a path to concentrate aqueous solution [J]. Separation and Purification Technology, 2010, 73(3): 403-408. doi: 10.1016/j.seppur.2010.04.031 [12] HODGKINS R, TRANTER M, DOWDESWELL J A. Solute provenance, transport and denudation in a high arctic glacierized catchment [J]. Hydrological Processes, 1997, 11(14): 1813-1832. doi: 10.1002/(SICI)1099-1085(199711)11:14<1813::AID-HYP498>3.0.CO;2-C [13] SPENCER R G M, BOLTON L, BAKER A. Freeze/thaw and pH effects on freshwater dissolved organic matter fluorescence and absorbance properties from a number of UK locations [J]. Water Research, 2007, 41(13): 2941-2950. doi: 10.1016/j.watres.2007.04.012 [14] 薛爽, 陈静, 铁梅, 等. 水体冻结过程中卤乙酸前体物在水-冰体系中的分配研究 [J]. 中国环境科学, 2014, 34(11): 2773-2780. XUE S, CHEN J, TIE M, et al. Ratio of haloacetic acids precursor in water-ice system during the freezing processes of water [J]. China Environmental Science, 2014, 34(11): 2773-2780(in Chinese).
[15] XUE S, CHEN J, TIE M. Release of dissolved organic matter from melting ice [J]. Environmental Progress & Sustainable Energy, 2016, 35(5): 1458-1467. [16] BRIMBLECOMBE P, CLEGG S L, DAVIES T D, et al. Observations of the preferential loss of major ions from melting snow and laboratory ice [J]. Water Research, 1987, 21(10): 1279-1286. doi: 10.1016/0043-1354(87)90181-3 [17] BRIMBLECOMBE P, CLEGG S L, DAVIES T D, et al. The loss of halide and sulphate ions from melting ice [J]. Water Research, 1988, 22(6): 693-700. doi: 10.1016/0043-1354(88)90180-7 [18] 刘玉灿, 苏苗苗, 张岩, 等. 不同UV工艺中阿特拉津的降解效果与机理研究 [J]. 中国给水排水, 2019, 35(5): 60-66. LIU Y C, SU M M, ZHANG Y, et al. Degradation effect and mechanism of atrazine in UV-based oxidation processes [J]. China Water & Wastewater, 2019, 35(5): 60-66(in Chinese).
[19] GAO W, SMITH D W, SEGO D C. Release of contaminants from melting spray ice of industrial wastewaters [J]. Journal of Cold Regions Engineering, 2004, 18(1): 35-51. doi: 10.1061/(ASCE)0887-381X(2004)18:1(35) [20] TANG Y Q, ZHANG Y, ZHAO W L, et al. The migration law of iron during the process of water icing [J]. Water, 2020, 12(2): 441. doi: 10.3390/w12020441 [21] 甄志磊, 李畅游, 张生, 等. 冰封期达里诺尔湖主要离子特征 [J]. 环境化学, 2015, 34(10): 1901-1910. doi: 10.7524/j.issn.0254-6108.2015.10.2015050202 ZHEN Z L, LI C Y, ZHANG S, et al. Major ions in Dali Lake during the icebound season [J]. Environmental Chemistry, 2015, 34(10): 1901-1910(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.10.2015050202
[22] MARTIN S. A field study of brine drainage and oil entrainment in first-year sea ice [J]. Journal of Glaciology, 1979, 22(88): 473-502. doi: 10.1017/S0022143000014477 [23] KNIGHT C A. Observations of the morphology of melting snow [J]. Journal of the Atmospheric Sciences, 1979, 36(6): 1123-1130. doi: 10.1175/1520-0469(1979)036<1123:OOTMOM>2.0.CO;2 [24] NAKAGAWA K, MAEBASHI S, MAEDA K. Concentration of aqueous dye solution by freezing and thawing [J]. The Canadian Journal of Chemical Engineering, 2009, 87(5): 779-787. doi: 10.1002/cjce.20213 [25] XUE S, WEN Y, HUI X J, et al. The migration and transformation of dissolved organic matter during the freezing processes of water [J]. Journal of Environmental Sciences, 2015, 27: 168-178. doi: 10.1016/j.jes.2014.05.035 [26] 吕宏洲, 李畅游, 史小红, 等. 不同条件下乌梁素海污染物在冰-水体系中分布规律的模拟 [J]. 湖泊科学, 2015, 27(6): 1151-1158. doi: 10.18307/2015.0621 LV H Z, LI C Y, SHI X H, et al. Pollutant distribution under different conditions in Lake Ulansuhai ice-water system [J]. Journal of Lake Sciences, 2015, 27(6): 1151-1158(in Chinese). doi: 10.18307/2015.0621
[27] 薛洪海, 唐晓剑, 康春莉, 等. 六六六(α-HCH)在水、冰和雪中的光化学反应 [J]. 环境化学, 2014, 33(8): 1342-1346. doi: 10.7524/j.issn.0254-6108.2014.08.006 XUE H H, TANG X J, KANG C L, et al. The photochemistry of α-hexachlorocyclohexane(α-HCH)in water, ice and snow [J]. Environmental Chemistry, 2014, 33(8): 1342-1346(in Chinese). doi: 10.7524/j.issn.0254-6108.2014.08.006
[28] SÁNCHEZ J, RUIZ Y, RAVENTÓS M, et al. Progressive freeze concentration of orange juice in a pilot plant falling film [J]. Innovative Food Science & Emerging Technologies, 2010, 11(4): 644-651. -



 下载:
下载: