-
重金属因高毒性、不可降解性和生物累积性而威胁人类健康[1-2]。目前已有各种技术去除重金属离子,例如离子交换、化学沉淀、膜分离和吸附[3-5]。其中,吸附法有效、经济,因为它易于设计和操作,如活性炭、粘土、生物炭和聚合物被广泛用于废水处理[6-8]。然而,上述商业吸附剂可回收性差,去除效率低,成本高。因此,研究新型吸收剂是必要的。
气凝胶是高度互连的多孔固体材料,形状如海绵,具有超低密度、高表面积和强吸附能力,易于从水溶液中分离[9-10]。这些特质使其成为水处理理想材料。纤维素纳米纤维(cellulose nanofiber,CNF)由于高纵横比、丰富表面羟基和结构灵活性而适宜制备气凝胶[9]。众多研究表明CNF基材料对重金属有显著吸附作用[11-12],但单纯羟基吸附能力有限,CNF上引入羧基和氨基,可以提高重金属吸附能力[13]。传统物理交联气凝胶的力学性能较差[14],机械强度低,可再生性能差,严重限制其在重金属吸附领域的实际应用。
2,2,6,6-四甲基哌啶-1-氧自由基(2,2,6,6-tetramethylpiperidine -1- oxygen radical,TEMPO)改性是常用的CNF改性方法,改性能增加CNF中羧基含量[15]。本文将使用3-缩水甘油氧丙基三甲氧基硅烷(3- glycidoxypropyltrimethoxysilane,GPTMS)将支化聚乙烯亚胺(Branched polyethyleneimine,b-PEI)化学交联到TEMPO氧化的纤维素纳米纤维(TOCNF)上,制备得到CNF基复合海绵状吸附剂(TOCGP),并考察其对Cu2+、Cd2 +和Pb2+的吸附性能和自身循环再生性能。
-
GPTMS、b-PEI、TEMPO购于Sigma-Aldrich公司,分析纯;NaBr、NaClO、C2H5OH、HCl、NaOH、CuSO4∙5H2O、CdCl2∙1/2H2O、Pb(NO3)2、EDTA-Na2购于国药集团,分析纯;CNF(1%,直径:5 — 20 nm)购于浙江金加浩绿色纳米材料股份有限公司。
FD-780型大型仓式冷冻干燥机,上海一恒可序;Nexus670型傅里叶变换红外光谱仪(FTIR),美国Nicolet公司;SU8010型扫描电子显微镜/能谱仪(SEM/EDS),日本日立公司;ESCALAB 250Xi型X射线衍射仪(XRD),美国赛默飞公司;GZX-9146MBE型电热鼓风干燥箱,上海博讯实业有限公司;SG-3040型磁力搅拌器,中国硕光电子有限公司;Optima 5300DV型电感耦合等离子体发射质谱仪;Orion Star A215型pH计,美国赛默飞公司。
-
采用改进的TEMPO氧化法选择性氧化纤维素C6上的伯醇羟基为羧基[16]。将100 g 1% CNF悬浮液超声均质20 min,加入 0.016 g TEMPO 和0.1 g NaBr,搅拌至溶解。加入7.4 mL 10% NaClO 溶液,调整pH值保持 10。反应后期溶液pH下降,滴加 0.5% NaOH使 pH 仍维持在 10 左右,当 pH 保持恒定时,立即滴加乙醇溶液(5 mL)终止反应,调节溶液pH=7,60 ℃蒸发至溶液质量为100 g,标记为TOCNF冷藏备用。
室温磁力搅拌下,将GPTMS(0.3 g)滴加到TOCNF悬浮液(30 g,前期预实验得到最佳浓度1%)中,搅拌2 h。滴加 0.6 g 50%b-PEI水溶液搅拌30 min。加入去离子水使悬浮液总质量为45 g,倒入模具,每格1 mL,于−55 ℃真空冷冻干燥24 h。气凝胶于110 ℃真空烘箱中固化30 min,确保CNF、 GPTMS和b-PEI充分交联。得到数个海绵状吸附剂(TOCNFs/ GPTMS/ b-PEI),标为TOCGP。同样条件下,用未氧化的CNF制备CGP。为了后续进行物理表征对照,同时冻干相同浓度的CNF和TOCNF,得到海绵状气凝胶。
采用扫描电子显微镜(SEM)、能谱仪(EDS)对TOCGP形貌及元素进行表征;采用红外光谱仪(FT-IR)分析TOCGP中官能团;采用X 射线衍射仪(XRD)对TOCNF和TOCGP进行结晶度测定。
-
本文采用Cu2+、Cd2+和Pb2+作为TOCGP吸附性能研究对象。
-
将CuSO4∙5H2O溶于去离子水中配制初始浓度为 100 mg·L-1 的Cu2+溶液,并调节pH =3.0 — 7.0(梯度为1)。称取5份吸附剂TOCGP,置于5个50 mL试剂瓶,加入 30 mL Cu2+溶液,室温振荡吸附 24 h。吸附结束后用 0.45 μm 的滤头过滤,对滤液离心(7000 r·min−1,10 min)后进行ICP-MS分析。吸附量计算公式(1)为:
式中,Q 为单位吸附量(mg·g−1), C0 为吸附前溶液中 Cu2+初始浓度(mg·L−1), C 为吸附后溶液中 Cu2+ 浓度(mg·L−1), V为溶液体积(L), W为吸附剂用量(g)。
Cu2+、Pb2+也采用上述方法,计算不同pH下TOCGP对3种重金属的单位吸附量,得到最佳pH以及最大吸附量。
-
于3个250 mL锥形瓶中分别倒入1.3.1节中制备的Cu2+、Cd2+和Pb2+溶液各100 mL,分别加入0.04 gTOGGP与之混合均匀。调整溶液pH值,Cu2+和Cd2+溶液pH值均调整为5.0,Pb2+溶液pH值调整为 6.0。锥形瓶置于30 ℃恒温水浴振荡器中,以150 r·min−1转速振荡。在预定时间点(0、 1、 2、 3、 4、 8、 12、 18、 24 h)提取样品,离心(7000 r·min−1,10 min)处理,用ICPMS测量并计算TOCGP对Cu2+、Cd2+和Pb2+的吸附量,得到吸附量q随时间t的变化情况。
为进一步研究动力学规律,分别用准一级动力学模型和准二级动力学模型对上述吸附结果进行线性拟合。其中准二级动力学方程可用于描述发生在物理-化学复合过程的吸附动力学机制。上述两个模型分别由以下方程描述[17-18]。
式中, qe表示吸附平衡时吸附量,mg·g−1; qt 表示 t 时刻吸附量,mg·g−1; t 为吸附反应时间,h; k1、k2分别是准一级动力学模型、准二级动力学模型常数,qe、k 可由截距和直线斜率求得。
-
往一系列30 mL,不同Cu2+初始浓度(10、 20、 40、 80、 150、 250、 350、 500 mg·L−1)的溶液中加入0.008 g TOCGP,在pH= 5下,置于30℃恒温水浴振荡器24 h,测定TOCGP对Cu2+吸附量随Cu2+初始浓度的变化。Cd2+和Pb2+也采用上述方法,区别在于Cd2+溶液pH= 5,Pb2+溶液pH= 6。
以Langmuir和Freundlich等温吸附模型对上述数据进行拟合分析,描述平衡吸附量(qe,mg·g−1)与平衡吸附浓度(ce,mg·L−1)之间关系,探究 TOCGP 吸附机理、吸附容量。
(1)Langmuir等温吸附模型
Langmuir吸附等温线模型又称单层吸附模型,建立在吸附材料表面性质均匀基础上,其线性表达式如下[19]:
式中, ce表示吸附质在溶液中平衡浓度(mg·L−1),qe表示吸附质吸附量(mg·g−1), KL为 Langmuir 吸附平衡常数(L·mg−1), qm表示在吸附剂上单层形成的最大吸附能力(mg·g−1)。
另外,平衡常数RL值可以用来判断Langmuir模型所描述的吸附反应过程是否容易进行,计算公式如下[20]:
Freundlich模型用于描述非均相表面的经验方程,假设吸附剂表面性质不是均一的,其线性方程式如下[19]:
式中,KF为与最大吸附容量相关的 Freundlich 常数,吸附质浓度在一特定范围内,KF 值越大表示TOCGP的吸附容量越大; 1 /n 表示吸附指数,和吸附反应进行难易程度相关,n> 1 时有利于吸附,当 n< 1 时,吸附难以继续。
-
用EDTA-Na2对TOCGP进行5次Cu2+吸附和解吸循环,评估TOCGP的可重复使用性[15]。将1.3.1中配置的Cu2+溶液调节pH =5。称取3份吸附剂TOCGP作为平行,置于3个50 mL试剂瓶中,加入 30 mL Cu2+溶液,室温振荡吸附 24 h。吸附结束后过滤,通过ICP-MS测定溶液中金属离子浓度。将吸附Cu2+后得到的饱和TOCGP海绵浸入100 mL 0.1 mol·L−1 EDTA-Na2溶液中去除Cu2+,过程中观察到从亮蓝色到无色的清晰颜色转变。然后将TOCGP添加到1 mol·L−1 HCl溶液中,搅拌1 h后取出海绵。随后在超纯水中浸泡直到电导率变得恒定,用手挤压以除去多余液体,然后干燥。将再生海绵进行吸附过程,再生过程重复5个循环,再生率用公式(7)计算。
式中: qn表示TOCGP第n次吸附达到吸附平衡时吸附量,mg·g−1,η表示再生率,越大表示TOCGP再生性能越强。
-
SEM观察到气凝胶具有三维多孔结构,扫描电镜图见图1。
从图1(a)得到,纯CNF在网络中由原纤化和片状结构组成,原纤结构占主导地位[21]。纤维束松散地相互连接,形成多孔网络。相比之下,如图1(b),用未改性的纯CNF交联制得的CGP具有更致密的结构和更多纤维片,这与 GPTMS和 b-PEI的交联作用有关,它使纤维在冷冻过程中更接近形成薄片。片材以密集网络紧密连接,其中纤维束较少但明显,纤维片表面粗糙。从图1(b)可清晰发现一些细小纳米级单根纤维悬挂于膜状纤维外。
将图1(b)中CNF改为 TOCNF后形成的就是本实验吸附剂TOCGP(TOCNFs/ GPTMS/ b-PEI),图1(c)和图1(b)特征基本一致,但纤维素纤维胶粘在一起形成的膜状较图1(b)更加光滑,两者构成不同之处仅在 CNF是否氧化,所以推断TEMPO氧化后纤维尺寸明显变小,达到几十纳米级别,使氧化纤维素更加均匀和粘稠,形成的膜状更加细腻,粗糙感较图1(b)明显减弱。图1 (C1、C2、C3)分别是不同放大倍数的吸附剂 TOCGP的SEM图,可明显看出纤维片致密且表面光滑。
此外,气凝胶元素组成由能谱仪分析,如图1(a’-c’)所示。纯CNF由C、 H、 O组成,图1(a’)所示元素分析结果与预期C, O元素一致。用 GPTMS修饰和 b-PEI的进一步接枝后,硅信号出现并且氮信号增强,硅、氮信号分别占总元素 11.65%和 4.62%(见图1(b′)),同样结果出现在图1(c′)中。结果进一步证实交联反应在制备多孔气凝胶中是有效的。TOCGP气凝胶珠粒中氧含量增加到30.52%(见图1(c′)),高于 TOCGP 25.26%,氧含量增加是由于 CNF经 TEMPO氧化后变为 TOCNF。
-
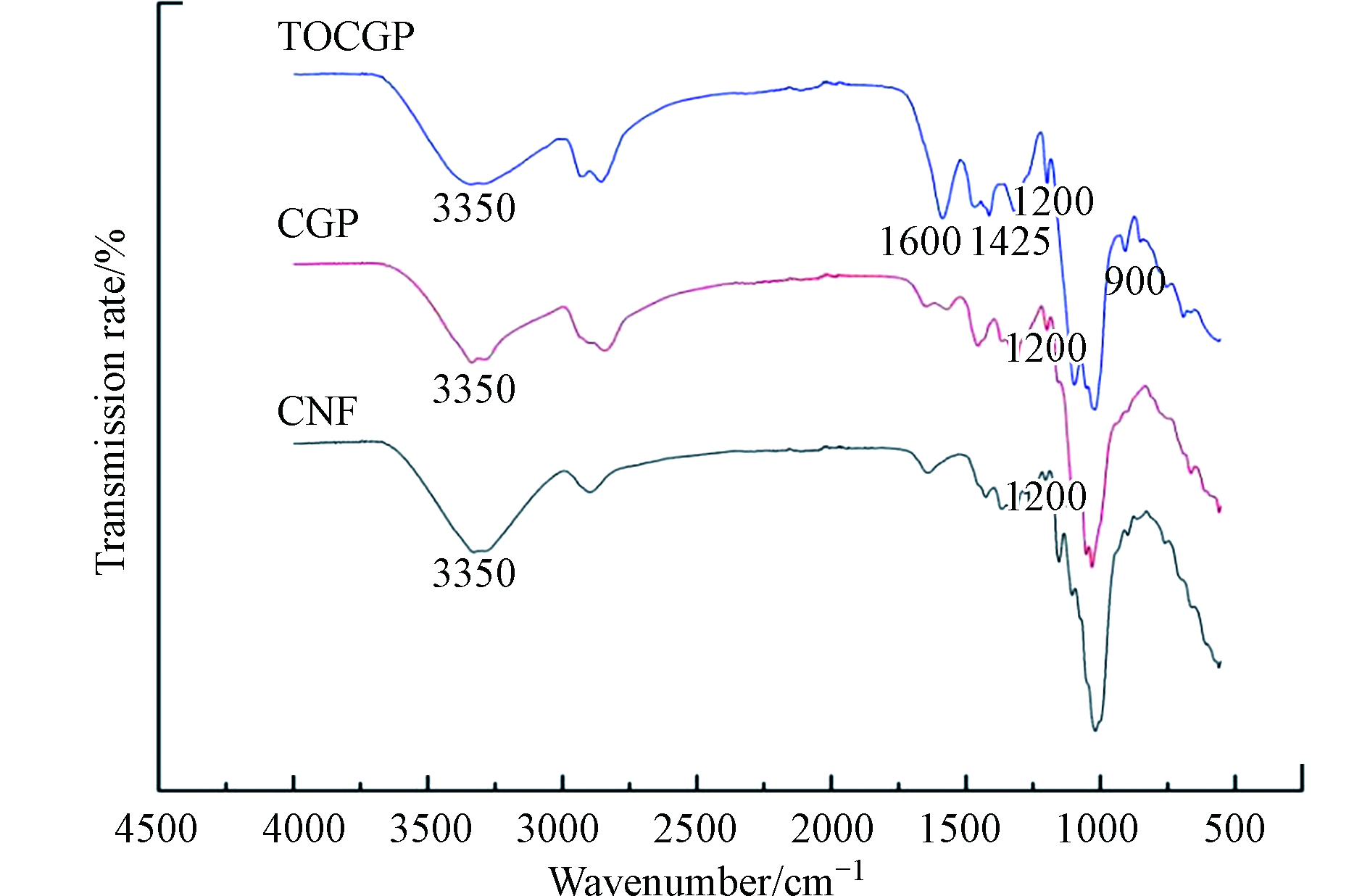
为进一步验证CNF、 GPTMS和 b-PEI的交联反应,用FTIR检测共价键特征峰,结果如图2所示。根据 Tang等[12]的研究,1200、 900 cm−1处显示的两个峰被指定为 GPTMS上环氧基团特征峰,从图上可以看出, CNF在 1200 cm−1和 900 cm−1处无特征峰而CGP和 TOCGP在此处有微弱特征峰。理论上用 b-PEI进一步修饰后上述两处峰应该因开环反应而完全消失,分析图中剩余微弱峰是由于 GPTMS未完全反应[22-23],CGP和 TOCGP中仍剩余部分环氧基团。CGP和 TOCGP在1425 cm−1处新峰归因于 N —H弯曲振动,新峰的出现和环氧基团的消失证实了交联反应的成功。
另外, TOCGP在 1600 cm−1左右出现了明显的羰基(C=O)吸收峰,证明CNF经 TEMPO氧化后引入了羧基。和 CGP相比, TOCGP在 3350 cm−1处羟基( —OH)伸缩振动峰变化不大,说明纤维中仍含有大量羟基, TEMPO氧化制备条件只有部分羟基被氧化。
-
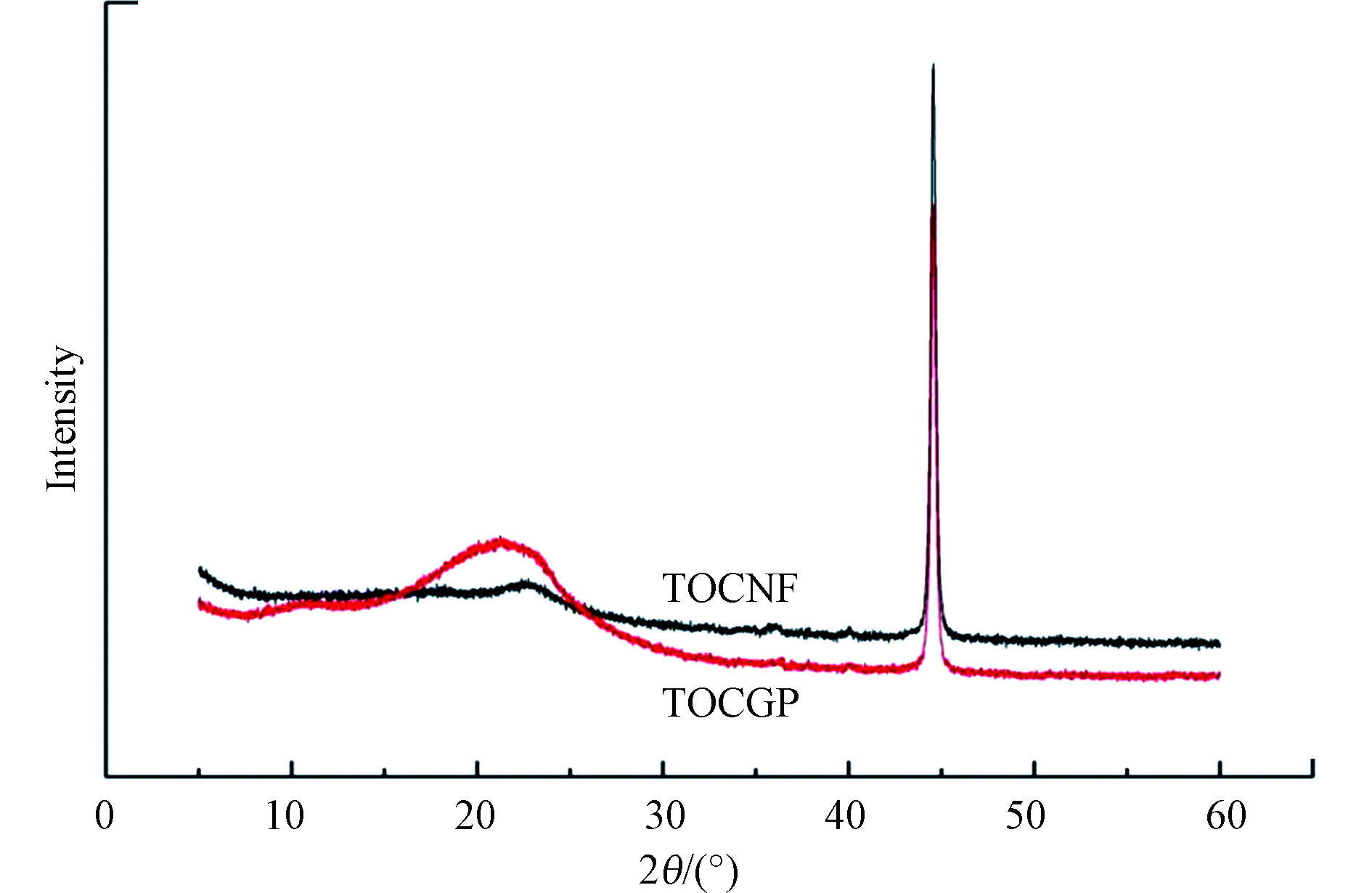
TOCGP及TOCNF的 XRD 谱图如图3 所示,可以看出 TOCNF和 TOCGP在 44°处有相同的特征峰,说明GPTMS和 b-PEI对 TOCNF的交联,并未彻底改变 TOCNF的晶体结构。除了 44°处尖锐衍射峰的减弱, TOCGP在 21°处增加了明显散射峰,原因可能是 GPTMS和 b-PEI的大量加入,因为这二者都具有非结晶结构,呈无序堆积状态。结晶度计算结果: I(TOCGP)=88.4%,I(TOCNF)=85.4%。TOCNF经交联处理后得到的TOCGP,其结晶度较纯TOCNF有所减小。
-
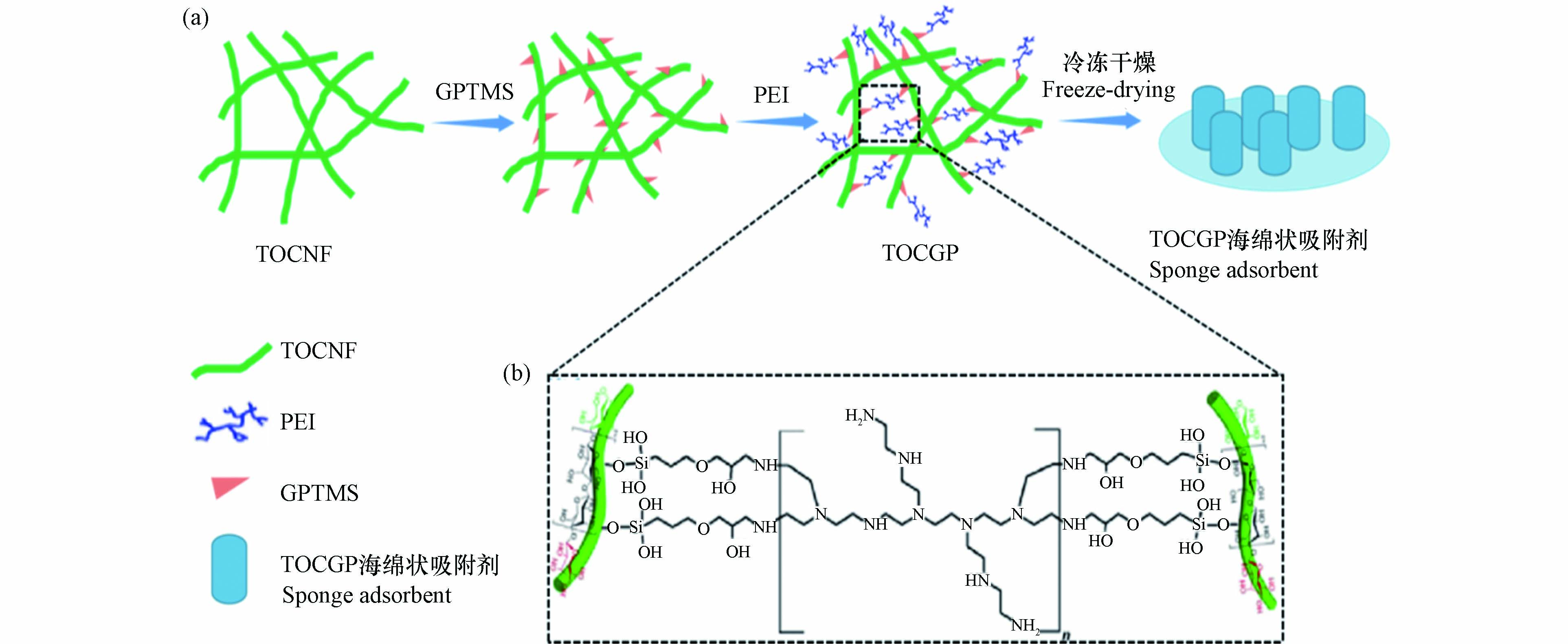
根据SEM / EDS、 XRD及 FTIR等物理表征初步推断, TOCGP复合海绵状吸附剂的制备过程和形成机理如图4(a)所示。在含水条件下, GPTMS水解形成硅羟基,通过缩合反应进一步与 TOCNF上羟基反应[24]。 GPTMS另一端环氧基团通过开环反应与 b-PEI上胺基团依次反应,产生稳定网状结构。网络化学结构如图4(b)所示。
-
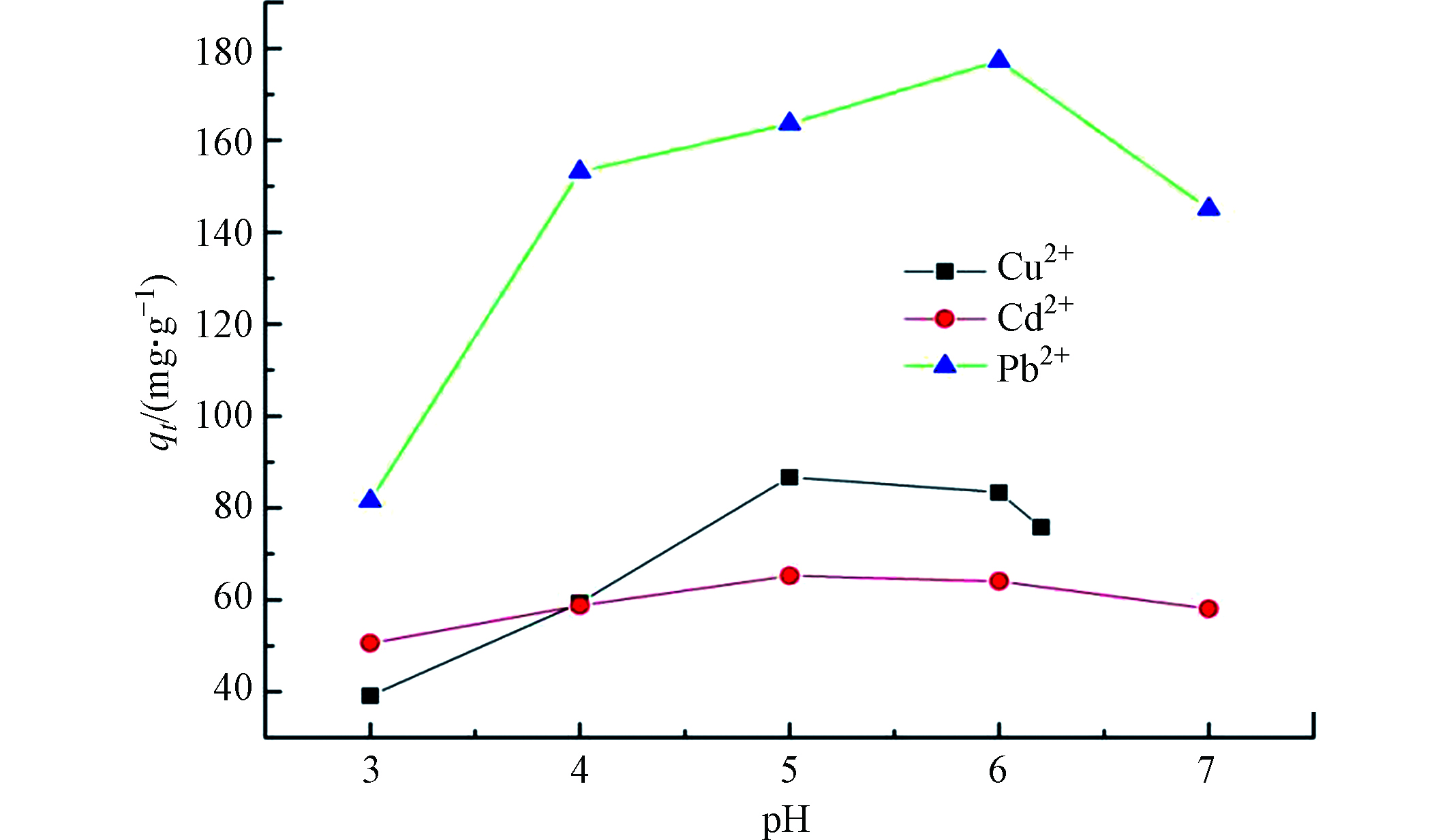
溶液 pH 值对 TOCGP吸附重金属离子的影响结果见图5。含 Cu2+的溶液在调节 pH 值达到 6.2 以上时出现明显沉淀,Cu2+转化为 Cu(OH)2。为避免氢氧化物沉淀对吸附作用的干扰,后续实验中Cu2+溶液 pH = 7时不进行吸附实验,为使3种离子的有效数据点都为5个,将产生沉淀的临界pH6.2作为最后一个取样点。从图5 中可以得出,3种重金属离子的吸附率都是先增加后减少,最佳吸附pH值分别为 5、 5和 6。主要原因是pH较小时,溶液中存在大量H+,TOCGP活性吸附位点结合H+并以NH3+、COOH和OH形式存在,质子化导致活性基团与Cu2+、Cd2+和Pb2+相互排斥,吸附能力变差。pH增大后,溶液中H+浓度不断减小,TOCGP表面负电荷不断增加,具有吸附能力的活性基团不断增多,吸附量增加。pH进一步增大后,溶液中OH-会和吸附剂活性基团竞争少许Cu2+、Cd2+和Pb2+,导致吸附容量开始下降。
-
吸附量 qt随时间 t的变化情况见图6。 TOCGP吸附量在最初四小时内迅速增加,随后增长速率逐渐减慢直到吸附平衡[25]。 TOCGP对 Cu2+和 Cd2+的吸附均在 12 h 左右达到平衡,对 Pb2+的吸附在 18 h 左右达到平衡。主要原因是吸附初期(0—4 h) TOCGP表面吸附点位较多,基本不存在竞争吸附,重金属离子快速被吸附,吸附量也随时间迅速增加。达到平衡后,吸附空间逐渐被占据,吸附剂活性位点减少,吸附点位逐渐饱和[26],金属离子进入吸附剂内部的速率逐渐变慢,吸附量仅缓慢增加。此外,将接触时间延长至24 h并不影响吸附容量,因为在此阶段几乎所有吸附位点都被占据,最佳吸附时间为18 h。
Cu2+、 Cd2+和 Pb2+的吸附容量分别为89.61、 72.80、 205.59 mg·g−1,即1.40、 0.65、 0.99 mmol·g−1。通常情况下金属离子和配位体之间所形成的配位键是一种静电作用力,因此组分的大小和电荷是重要影响因素。一般同类配体,金属离子半径越小,电离势能越大,形成的配合物稳定性越高,TOCGP对三种离子的吸附能力为Cu2+> Cd2+> Pb2+。另一方面,过渡金属元素的络合能力总体大于非过渡金属,所以Cu2+和Cd2+(过渡金属)>Pb2+(非过渡金属)。除此之外,金属活拨性越差,形成的配合物就越稳定,已知活动性 Cd> Pb> Cu,所以从这角度分析Cd2+络合能力最小。综合上便能解释TOCGP吸附能力:Cu2+> Pb2+> Cd2+。
-
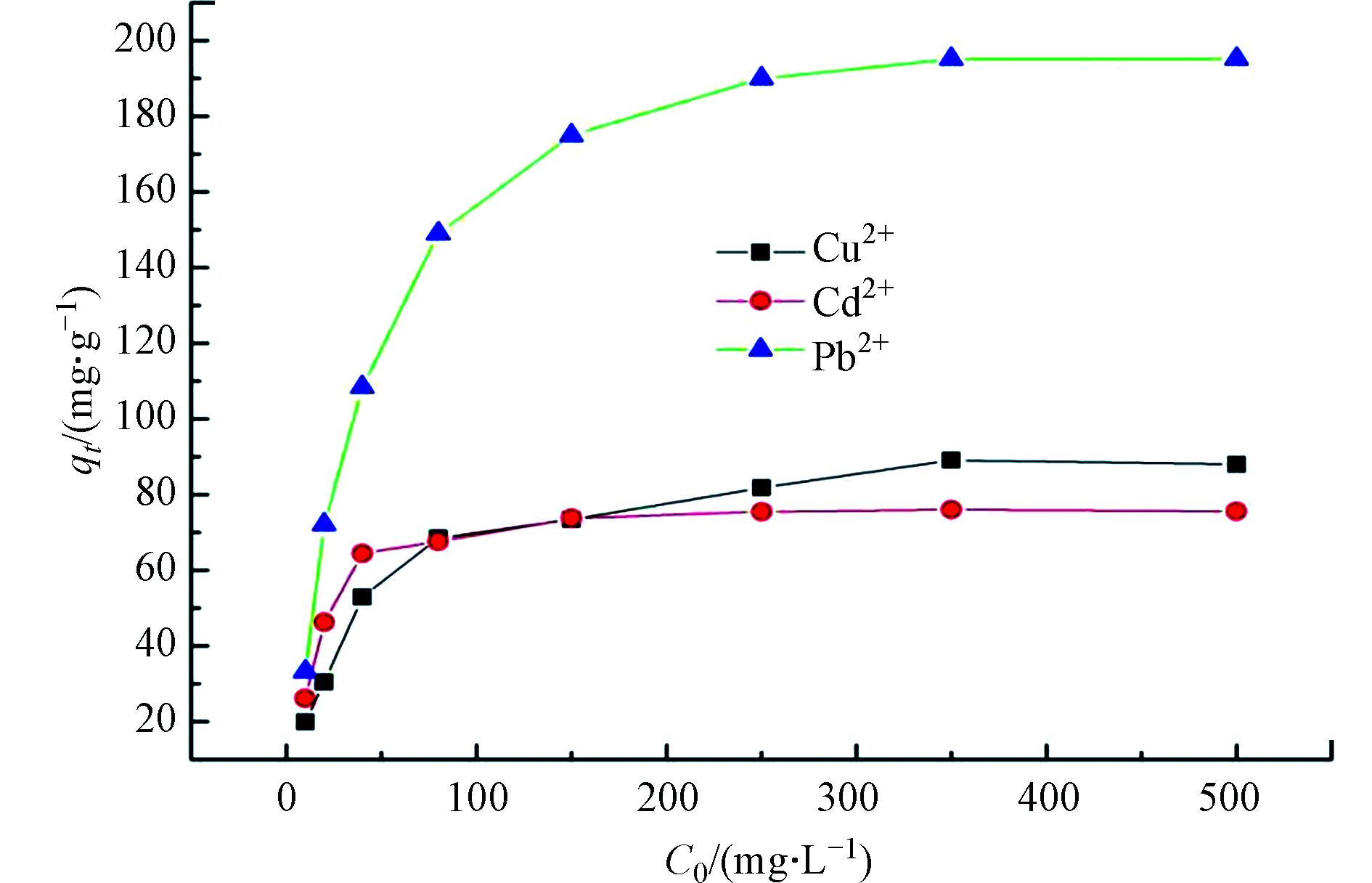
室温下, TOCGP的吸附容量与 Cu2+、 Cd2+和 Pb2+初始浓度之间关系如图7 所示, TOCGP的吸附容量随着初始浓度的增加而呈上升趋势。Cu2+初始浓度小于 80 mg·L−1时,吸附容量随浓度增加而明显增加,初始浓度处于 80 — 350 mg·L−1之间,吸附容量的增量受初始浓度变化的影响越来越小,初始浓度超过 350 mg·L−1时,吸附容量达到88.59 mg·L−1并达到平衡,说明吸附已经饱和。同样, Cd2+初始浓度小于 40 mg·L−1时,吸附容量随浓度增加而明显增加,初始浓度超过 350 mg·L−1时,吸附容量达到75.72 mg·L−1并达到平衡,吸附饱和。Pb2+初始浓度小于 80 mg·L−1时,吸附容量随浓度增加而明显增加,初始浓度超过 350 mg·L−1时,吸附达到饱和平衡,此时吸附容量达到195.15 mg·L−1。
在较低Cu2+、 Cd2+和 Pb2+浓度下吸附量随初始浓度的增加而呈线性增加,说明在此情况下 TOCGP中存在大量吸附位点, TOCGP吸附容量取决于重金属溶液输送到 TOCGP表面的 Cu2+、 Cd2+和 Pb2+的数量。而当 Cu2+、 Cd2+和 Pb2+初始浓度较高时,重金属离子数量多而吸附位点有限, TOCGP表面吸附位点数量限制了其吸附量。
-
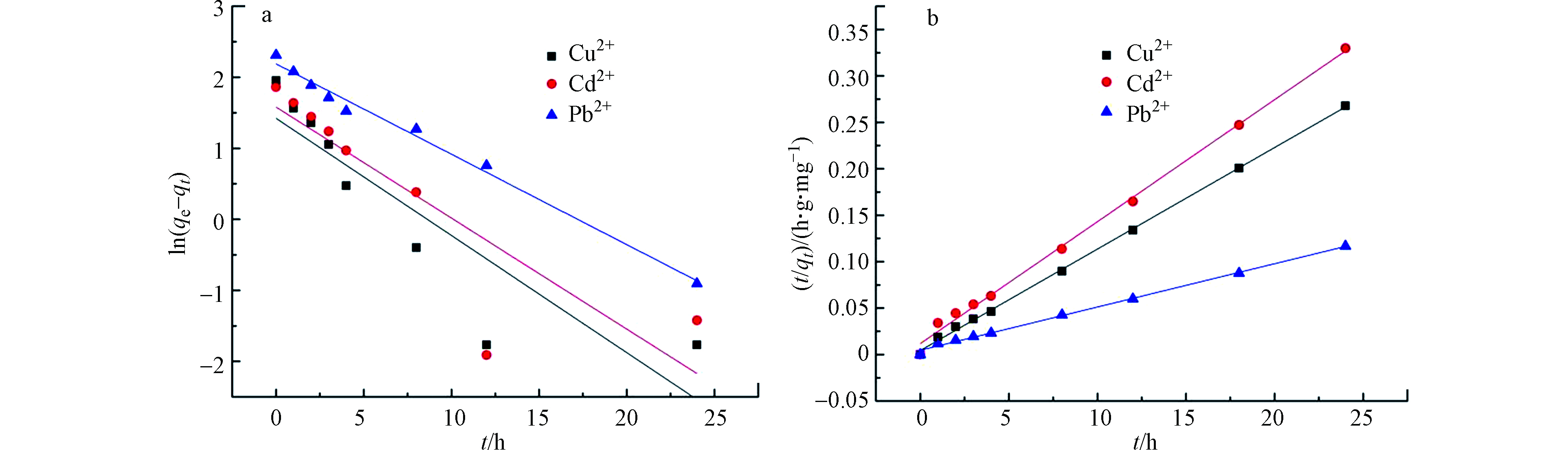
Cu2+、 Cd2+和 Pb2+在 TOCGP上吸附动力学[17-18]结果如图8所示。
由表1 可知,TOCGP吸附Cu2+、 Cd2+和 Pb2+的准二级动力学模型拟合参数更接近于1,理论平衡吸附量与相应的 89.61、 72.80、 205.6 mg·g−1实验值也十分符合。这表明TOCGP吸附Cu2+、 Cd2+和 Pb2+的过程,化学吸附占主要作用,包括化学键的形成以及电子的转移。
-
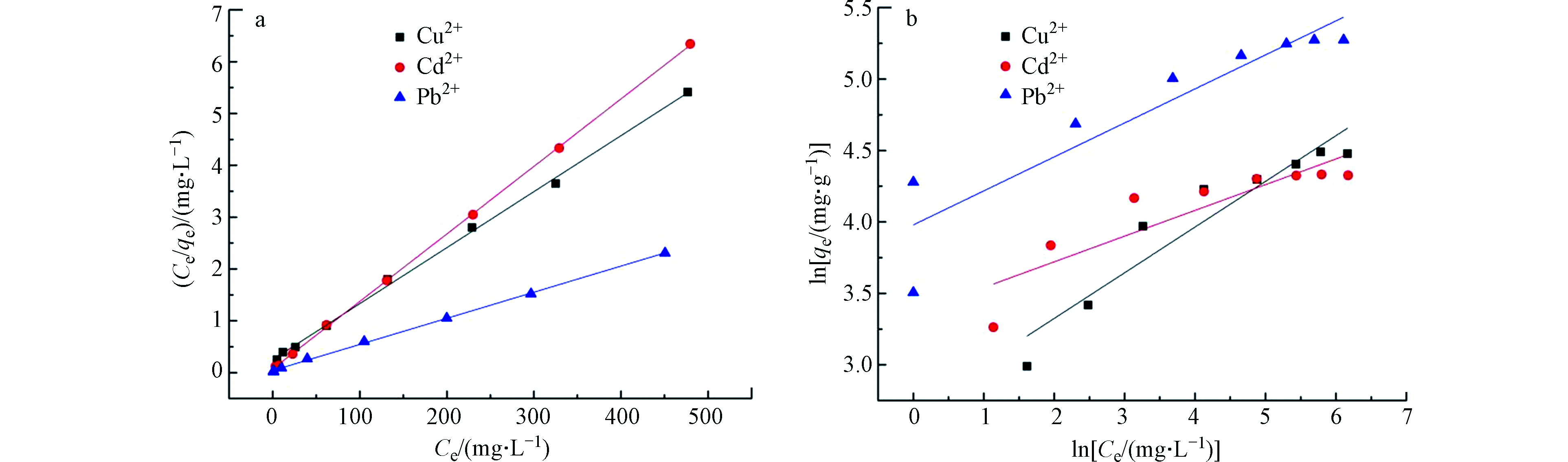
不同 Cu2+、 Cd2+和 Pb2+初始浓度条件下得到的吸附数据进行Langmuir和 Freundlich等温吸附模型拟合[27-29],拟合结果如图9 所示。
等温吸附拟合参数(表2)分析可知,Langmuir 方程比 Freundlich 方程更好地拟合 TOCGP 对 Cu2+、 Cd2+和 Pb2+的吸附过程。并且 Cu2+、 Cd2+和 Pb2+的最大吸附量(Qm)分别为 92.59 mg·g−1、 76.92 mg·g−1和 200.00 mg·g−1,与实验值 88.59 mg·g−1、 75.72 mg·g−1和 195.15 mg·g−1接近,说明 TOCGP 对Cu2+、 Cd2+和 Pb2+吸附以单分子层吸附为主[30]。
表2中列出了Langmuir模型中判断是否为优惠型吸附的常数RL,可以看到,RL值均在0到1之间,证明TOCGP吸附剂对Cu2+、Cd2+和Pb2+均为优惠型吸附。
表3 中列出了其他学者研究的改性CNF对Cu2+、 Cd2+和 Pb2+的吸附能力。可以看出,不管是Cu2+、Cd2+还是Pb2+,TOCGP的吸附都占有绝对优势,有非常可观的重金属吸附能力。
-
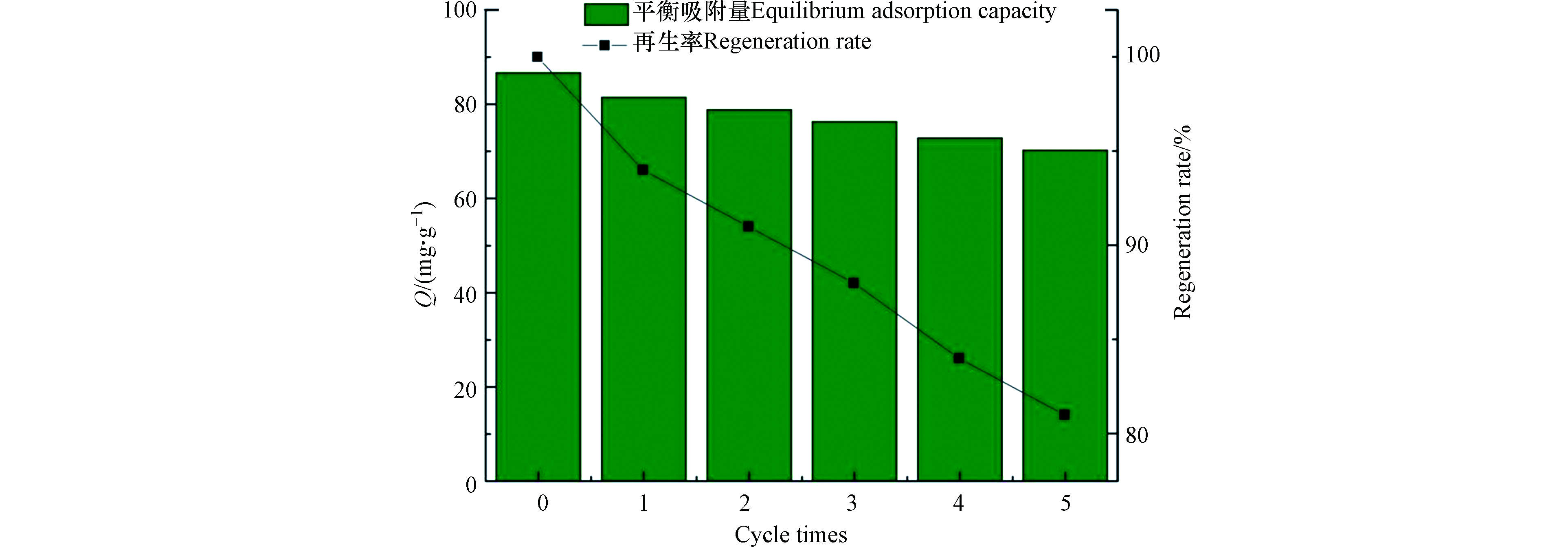
除高吸附能力外,优秀吸收剂应具有优秀可回收性,以满足可持续性和经济性的标准。图10 展示了 TOCGP再生和重复使用性结果,观察到 5 次再生循环后吸附能力没有明显降低,由最初的 86.67 mg·g−1降到 70.22 mg·g−1,吸附容量损失约为 16.45 mg·g−1,仍能达到初始吸附能力 80%以上,说明 TOCGP具有较好循环再生稳定性,这可能是 TOCGP的结构稳定性和优异螯合性能的结果。
-
本文以 TOCNF、 GPTMS和 b-PEI为原料,通过简单交联反应制备的一种可再生CNF基复合海绵状吸附剂TOCGP的方法。
(1)使用 SEM/ EDS、 XRD及 FTIR等物理表征分析得出,TOCGP表面已顺利引入氨基和羧基。
(2)TOCGP对 Cu2+、 Cd2+和 Pb2+的去除效果受 pH影响较大,最佳 pH值分别为 pHCu = 5、 pHCd= 5和 pHPb= 6,最大吸附容量分别为 86.67、65.22、177.27 mg·g−1。
(3)Cu2+、 Cd2+和 Pb2+在 TOCGP上的吸附更加符合准二级动力学,相关系数R2> 0.995。理论平衡吸附量与相应 89.61、72.80、205.60 mg·g−1实验值十分符合,接近于化学吸附过程。
(4)Langmuir 方程更好拟合 TOCGP 对 Cu2+、 Cd2+和 Pb2+的等温吸附过程,以单分子层吸附为主,实际最大吸附容量为 88.59、75.72、195.15 mg·g−1。TOCGP 的吸附结果和其他学者的改性CNF吸附剂吸附结果相比较得出,TOCGP在Cu2+、Cd2+和Pb2+吸附上占有较高优势。
(5)经过 5 次再生循环后吸附能力没有明显降低,仍能达到初始吸附能力的 80%以上,说明 TOCGP具有较好的循环再生稳定性。
本文研究结果表明, TOCGP海绵状吸附剂由于制备方法简单、室温反应、高吸附容量、快速去除速率以及优异再生性能使其成为一种很有前途的废水中重金属吸附剂。
纤维素纳米纤维基复合吸附剂的制备及吸附水中重金属离子
Preparation of cellulose nanofiber-based composite adsorbent and adsorption of heavy metal ions in water
-
摘要: 开发高效去除重金属生物基吸附剂是治理全球水污染的关键策略之一。纤维素纳米纤维(cellulose nanofiber,CNF)通过氧化,提高羧基含量可以增强对重金属的吸附能力。通过使用3-缩水甘油氧丙基三甲氧基硅烷(3- glycidoxypropyltrimethoxysilane,GPTMS)将支化聚乙烯亚胺(branched polyethyleneimine,b-PEI)交联到TEMPO氧化的TOCNF上,制备了CNF基复合海绵状吸附剂(TOCGP)。研究了TOCGP的物理表征及吸附性能。FTIR结果显示成功将氨基引入TOCGP,氨基和羧基都对重金属吸附有较大贡献。TOCGP对Cu2+、Cd2+和Pb2+有很高的吸附容量,在最佳pH和100 mg·L−1的初始离子浓度下,分别能达到89.61、 72.80、 205.59 mg·g−1。吸附过程符合准二级动力学和Langmuir等温吸附。TOCGP具有较强的再生性能,5次吸附-解吸循环后,吸附能力仍能达到最初的80%以上。
-
关键词:
- 纤维素纳米纤维(CNF) /
- 交联 /
- 海绵状吸附剂 /
- 重金属离子 /
- 吸附
Abstract: Developing bio-based adsorbents for removing heavy metals with high efficiency is one of the key strategies to control global water pollution. The adsorption capacity of heavy metals can be enhanced by oxidizing CNF and increasing carboxyl content. CNF-based composite sponge adsorbent(TOCGP) was prepared by crosslinking Branched polyethyleneimine(b-PEI) onto TOCNF oxidized by TEMPO with 3-glycidoxypropyltrimethoxysilane(GPTMS). The physical characterization and adsorption properties of TOCGP were studied. FTIR results showed that amino groups were successfully introduced into TOCGP, and both amino groups and carboxyl groups contributed greatly to the adsorption of heavy metals. TOCGP has a high adsorption capacity for Cu2+, Cd2+ and Pb2+, which can reach 89.61 mg·g−1, 72.80 mg·g−1 and 205.59 mg·g−1 under the optimal pH and initial ion concentration of 100 mg·L−1, respectively. The adsorption process accords with quasi-second-order kinetics and Langmuir isotherm adsorption. TOCGP has strong regeneration performance, and its adsorption capacity can still reach more than 80% of the original one after five adsorption-desorption cycles.-
Key words:
- CNF /
- crosslinking /
- sponge adsorbent /
- heavy metal ions /
- adsorb
-

-
表 1 TOCGP对 Cu2+、Cd2+和Pb2+的吸附动力学拟合参数
Table 1. Fitting parameters of adsorption kinetics of Cu2+,Cd2+ and Pb2+ by TOCGP
重金属离子
Heavy metal ion准一级动力学模型
Quasi-first-order dynamic model准二级动力学模型
Quasi-second order dynamic modelq1e /(mg·g−1) k1 /(min−1) R2 q2e /(mg·g−1) k2 /(g·mg−1·min−1) R2 Cu2+ 4.15 0.1649 0.8107 91.74 0.0276 0.9991 Cd2+ 4.86 0.1563 0.7663 76.34 0.0147 0.9968 Pb2+ 8.93 0.1272 0.9902 212.77 0.0048 0.9973 表 2 TOCGP对 Cu2+、Cd2+和Pb2+的等温吸附拟合参数
Table 2. Isothermal adsorption parameters of Cu2+, Cd2+ and Pb2+ by TOCGP
重金属离子
Heavy metal ionLangmuir等温吸附 Freundlich等温吸附 qm/(mg·g−1) KL/(L·mg−1) RL R2 KF/(L·mg−1) 1/n R2 Cu2+ 92.59 0.0431 0.0443 0.9984 14.664 0.3197 0.9088 Cd2+ 76.92 0.1841 0.0107 0.9999 28.806 0.1800 0.7881 Pb2+ 200.00 0.1241 0.0158 0.9994 53.485 0.2379 0.8578 表 3 CNF改性后对Cu2+、 Cd2+和 Pb2+的吸附能力
Table 3. Adsorption capacity of modified CNF for Cu2+,Cd2+ and Pb2+
重金属
heavy metal表面功能化材料
Surface functionalizationpH Qe//
(mg·g−1)Langmuir Freundlich 参考文献
referencesQm/
(mg·g−1)KL/
(mg·L−1)R2 KF/
(L·mg−1)1/n R2 Cd2+ 丝光化(-COO-) 5 5 2.06 691 0.923 - - - [31] 胺化作用 5 58.1 40.56 61.3 0.892 - - - [32] 聚甲基丙烯酸-共马来酸
接枝5 135 - - - - - - [33] 巯基化 4 45.9 45.9 0.011 0.995 2.17 0.6023 0.935 [34] TEMPO-氧化/GPTMS/b-PEI 5 75.72 76.92 0.1841 0.9999 28.806 0.18 0.7881 本研究 Cu2+ 胺化作用 5 - 18.9 0.17 0.975 6.71 0.24 0.903 [15] 5 50.6 55.6 4.072 0.77 - - - [32] 聚丙烯酸接枝 4.5 45.8 57.5 0.124 0.972 0.104 0.72 0.988 [35] 聚丙烯酸/腐植酸钠接枝 4.5 44.7 64.6 0.175 0.97 0.114 0.66 0.991 [35] TEMPO-氧化/聚乙烯亚胺
接枝5 - 52.3 0.17 0.985 31 10.4 0.919 [36] TEMPO-氧化/氧化石墨烯复合材料 5.7 63.5 - - - - - - [37] 接枝可再生腰果酚衍生的硅氧烷(CDA) 5 47.61 45.89 0.54 0.993 13.43 0.28 0.896 [38] TEMPO/高碘酸盐氧化 5.5 92.23 - - - - - - [39] Cu2+ TEMPO-氧化(-COO-) 5.1 67.2 - - - - - - [40] 巯基化 4 49 49 0.016 0.999 7.51 0.5284 0.926 [41] TEMPO-氧化/GPTMS/b-PEI 5 88.59 92.59 0.0431 0.9984 14.664 0.3197 0.9088 本研究 Pb2+ 磺化 5 123 251 0.69 0.97 72.8 0.66 0.93 [42] TEMPO-氧化(-COO-) 6 6 9.7 - - - - - [43] TEMPO-氧化(-COO-)/硫代化(-Si-SH) 5.5 133 137.7 0.783 0.998 - - - [44] 聚甲基丙烯酸-共马来酸
接枝5 165 - - - - - - [43] TEMPO/高碘酸盐氧化 5.5 97.34 - - - - - - [39] 巯基化 4 22 22 0.052 0.994 2.68 0.3937 0.822 [41] TEMPO-氧化/GPTMS/b-PEI 6 195.15 200 0.1241 0.9994 53.485 0.2379 0.8578 本研究 -
[1] HAN R R, ZHOU B H, HUANG Y Y, et al. Bibliometric overview of research trends on heavy metal health risks and impacts in 1989-2018 [J]. Journal of Cleaner Production, 2020, 276: 123249. doi: 10.1016/j.jclepro.2020.123249 [2] DUAN W W, XU C, LIU Q, et al. Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: A population-based cohort study [J]. Environmental Pollution, 2020, 263: 114630. doi: 10.1016/j.envpol.2020.114630 [3] JOSEPH L, JUN B M, FLORA J R V, et al. Removal of heavy metals from water sources in the developing world using low-cost materials: A review [J]. Chemosphere, 2019, 229: 142-159. doi: 10.1016/j.chemosphere.2019.04.198 [4] AHMED M J K, AHMARUZZAMAN M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions [J]. Journal of Water Process Engineering, 2016, 10: 39-47. doi: 10.1016/j.jwpe.2016.01.014 [5] GARBA Z N, RAHIM A A. Evaluation of optimal activated carbon from an agricultural waste for the removal of Para-chlorophenol and 2, 4-dichlorophenol [J]. Process Safety and Environmental Protection, 2016, 102: 54-63. doi: 10.1016/j.psep.2016.02.006 [6] LIU J, SU D H, YAO J R, et al. Soy protein-based polyethylenimine hydrogel and its high selectivity for copper ion removal in wastewater treatment [J]. Journal of Materials Chemistry A, 2017, 5(8): 4163-4171. doi: 10.1039/C6TA10814H [7] QIN H Q, HU T J, ZHAI Y B, et al. The improved methods of heavy metals removal by biosorbents: A review [J]. Environmental Pollution, 2020, 258: 113777. doi: 10.1016/j.envpol.2019.113777 [8] MAHMOODI N M. Photocatalytic ozonation of dyes using multiwalled carbon nanotube [J]. Journal of Molecular Catalysis A:Chemical, 2013, 366: 254-260. doi: 10.1016/j.molcata.2012.10.002 [9] de FRANCE K J, HOARE T, CRANSTON E D. Review of hydrogels and aerogels containing nanocellulose [J]. Chemistry of Materials, 2017, 29(11): 4609-4631. doi: 10.1021/acs.chemmater.7b00531 [10] ZHOU Z H, YANG Y B, HAN Y Y, et al. In situ doping enables the multifunctionalization of templately synthesized polyaniline@cellulose nanocomposites [J]. Carbohydrate Polymers, 2017, 177: 241-248. doi: 10.1016/j.carbpol.2017.08.136 [11] ZHAO H, OUYANG X K, YANG L Y. Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber-sodium alginate hydrogel beads [J]. Journal of Molecular Liquids, 2021, 324: 115122. doi: 10.1016/j.molliq.2020.115122 [12] TANG C X, BRODIE P, LI Y Z, et al. Shape recoverable and mechanically robust cellulose aerogel beads for efficient removal of copper ions [J]. Chemical Engineering Journal, 2020, 392: 124821. doi: 10.1016/j.cej.2020.124821 [13] MO L T, PANG H W, TAN Y, et al. 3D multi-wall perforated nanocellulose-based polyethylenimine aerogels for ultrahigh efficient and reversible removal of Cu(II) ions from water [J]. Chemical Engineering Journal, 2019, 378: 122157. doi: 10.1016/j.cej.2019.122157 [14] 李健, 张恩爽, 刘圆圆, 等. 超低密度气凝胶的制备及应用 [J]. 化学进展, 2020, 32(6): 713-726. LI J, ZHANG E S, LIU Y Y, et al. Preparation of the ultralow density aerogel and its application [J]. Progress in Chemistry, 2020, 32(6): 713-726(in Chinese).
[15] ZHANG N, ZANG G L, SHI C, et al. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal [J]. Journal of Hazardous Materials, 2016, 316: 11-18. doi: 10.1016/j.jhazmat.2016.05.018 [16] JRADI K, BIDEAU B, CHABOT B, et al. Characterization of conductive composite films based on TEMPO-oxidized cellulose nanofibers and polypyrrole [J]. Journal of Materials Science, 2012, 47(8): 3752-3762. doi: 10.1007/s10853-011-6226-9 [17] HO Y S, OFOMAJA A E. Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber [J]. Journal of Hazardous Materials, 2006, 129(1/2/3): 137-142. [18] MITTAL A, THAKUR V, GAJBE V. Evaluation of adsorption characteristics of an anionic azo dye Brilliant Yellow onto hen feathers in aqueous solutions [J]. Environmental Science and Pollution Research, 2012, 19(6): 2438-2447. doi: 10.1007/s11356-012-0756-9 [19] 杜明阳, 邹京, 豆俊峰, 等. 钾改性蒙脱石磁性微球对铯的吸附性能 [J]. 环境化学, 2021, 40(3): 779-789. doi: 10.7524/j.issn.0254-6108.2019110202 DU M Y, ZOU J, DOU J F, et al. Adsorption properties of potassium modified montmorillonite magnetic microspheres for cesium [J]. Environmental Chemistry, 2021, 40(3): 779-789(in Chinese). doi: 10.7524/j.issn.0254-6108.2019110202
[20] 侯璟玥, 马海红, 徐卫兵, 等. 改性羧甲基纤维素缓释肥包膜材料的制备与表征 [J]. 材料科学与工程学报, 2018, 36(1): 90-94. HOU J Y, MA H H, XU W B, et al. Preparation and characterization of modified carboxymethyl cellulose films coating materials of fertilizers [J]. Journal of Materials Science and Engineering, 2018, 36(1): 90-94(in Chinese).
[21] PAN Z Z, NISHIHARA H, IWAMURA S, et al. Cellulose nanofiber as a distinct structure-directing agent for xylem-like microhoneycomb monoliths by unidirectional freeze-drying [J]. ACS Nano, 2016, 10(12): 10689-10697. doi: 10.1021/acsnano.6b05808 [22] CHENG H, LI Y Z, WANG B J, et al. Chemical crosslinking reinforced flexible cellulose nanofiber-supported cryogel [J]. Cellulose, 2018, 25(1): 573-582. doi: 10.1007/s10570-017-1548-7 [23] DONG Z, ZHAO J, DU J F, et al. Radiation synthesis of spherical cellulose-based adsorbent for efficient adsorption and detoxification of Cr(VI) [J]. Radiation Physics and Chemistry, 2016, 126: 68-74. doi: 10.1016/j.radphyschem.2016.05.013 [24] LI Y Z, GRISHKEWICH N, LIU L L, et al. Construction of functional cellulose aerogels via atmospheric drying chemically cross-linked and solvent exchanged cellulose nanofibrils [J]. Chemical Engineering Journal, 2019, 366: 531-538. doi: 10.1016/j.cej.2019.02.111 [25] 周丹丹, 吴文卫, 赵婧, 等. 花生壳和松木屑制备的生物炭对Cu2+的吸附研究 [J]. 生态环境学报, 2016, 25(3): 523-530. ZHOU D D, WU W W, ZHAO J, et al. Study on the adsorption of Cu2+ to biochars produced from peanut shells and pine chips [J]. Ecology and Environmental Sciences, 2016, 25(3): 523-530(in Chinese).
[26] SARUCHI, KUMAR V. Adsorption kinetics and isotherms for the removal of rhodamine B dye and Pb+2 ions from aqueous solutions by a hybrid ion-exchanger [J]. Arabian Journal of Chemistry, 2019, 12(3): 316-329. doi: 10.1016/j.arabjc.2016.11.009 [27] GUSAIN D, SRIVASTAVA V, SHARMA Y C. Kinetic and thermodynamic studies on the removal of Cu(II) ions from aqueous solutions by adsorption on modified sand [J]. Journal of Industrial and Engineering Chemistry, 2014, 20(3): 841-847. doi: 10.1016/j.jiec.2013.06.014 [28] TRAN H N, YOU S J, CHAO H P. Insight into adsorption mechanism of cationic dye onto agricultural residues-derived hydrochars: Negligible role of π-π interaction [J]. Korean Journal of Chemical Engineering, 2017, 34(6): 1708-1720. doi: 10.1007/s11814-017-0056-7 [29] MITTAL A, AHMAD R, HASAN I. Iron oxide-impregnated dextrin nanocomposite: Synthesis and its application for the biosorption of Cr(VI) ions from aqueous solution [J]. Desalination and Water Treatment, 2016, 57(32): 15133-15145. doi: 10.1080/19443994.2015.1070764 [30] 王彤彤, 马江波, 曲东, 等. 两种木材生物炭对铜离子的吸附特性及其机制 [J]. 环境科学, 2017, 38(5): 2161-2171. WANG T T, MA J B, QU D, et al. Characteristics and mechanism of copper adsorption from aqueous solutions on biochar produced from sawdust and apple branch [J]. Environmental Science, 2017, 38(5): 2161-2171(in Chinese).
[31] HOKKANEN S, REPO E, SILLANPÄÄ M. Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose [J]. Chemical Engineering Journal, 2013, 223: 40-47. doi: 10.1016/j.cej.2013.02.054 [32] HOKKANEN S, REPO E, SUOPAJÄRVI T, et al. Adsorption of Ni(II), Cu(II) and Cd(II) from aqueous solutions by amino modified nanostructured microfibrillated cellulose [J]. Cellulose, 2014, 21(3): 1471-1487. doi: 10.1007/s10570-014-0240-4 [33] MAATAR W, BOUFI S. Poly(methacylic acid-co-maleic acid) grafted nanofibrillated cellulose as a reusable novel heavy metal ions adsorbent [J]. Carbohydrate Polymers, 2015, 126: 199-207. doi: 10.1016/j.carbpol.2015.03.015 [34] CHOI H Y, BAE J H, HASEGAWA Y, et al. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water [J]. Carbohydrate Polymers, 2020, 234: 115881. doi: 10.1016/j.carbpol.2020.115881 [35] ZHANG X F, ZHAO J Q, CHENG L, et al. Acrylic acid grafted and acrylic acid/sodium humate grafted bamboo cellulose nanofibers for Cu2+adsorption [J]. RSC Adv, 2014, 4(98): 55195-55201. doi: 10.1039/C4RA08307E [36] GENG B Y, WANG H Y, WU S, et al. Surface-tailored nanocellulose aerogels with thiol-functional moieties for highly efficient and selective removal of Hg(II) ions from water [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(12): 11715-11726. [37] ZHU C T, LIU P, MATHEW A P. Self-assembled TEMPO cellulose nanofibers: Graphene oxide-based biohybrids for water purification [J]. ACS Applied Materials & Interfaces, 2017, 9(24): 21048-21058. [38] JI Y, WEN Y Y, WANG Z, et al. Eco-friendly fabrication of a cost-effective cellulose nanofiber-based aerogel for multifunctional applications in Cu(II) and organic pollutants removal [J]. Journal of Cleaner Production, 2020, 255: 120276. doi: 10.1016/j.jclepro.2020.120276 [39] ABOU-ZEID R E, DACRORY S, ALI K A, et al. Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution [J]. International Journal of Biological Macromolecules, 2018, 119: 207-214. doi: 10.1016/j.ijbiomac.2018.07.127 [40] LIU P, OKSMAN K, MATHEW A P. Surface adsorption and self-assembly of Cu(II) ions on TEMPO-oxidized cellulose nanofibers in aqueous media [J]. Journal of Colloid and Interface Science, 2016, 464: 175-182. doi: 10.1016/j.jcis.2015.11.033 [41] SEHAQUI H, LARRAYA U P, LIU P, et al. Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment [J]. Cellulose, 2014, 21(4): 2831-2844. doi: 10.1007/s10570-014-0310-7 [42] SUOPAJÄRVI T, LIIMATAINEN H, KARJALAINEN M, et al. Lead adsorption with sulfonated wheat pulp nanocelluloses [J]. Journal of Water Process Engineering, 2015, 5: 136-142. doi: 10.1016/j.jwpe.2014.06.003 [43] SRIVASTAVA S, KARDAM A, RAJ K R. Nanotech reinforcement onto cellulosic fibers: Green remediation of toxic metals [J]. International Journal of Green Nanotechnology, 2012, 4(1): 46-53. doi: 10.1080/19430892.2012.654744 [44] YANG R, AUBRECHT K B, MA H Y, et al. Thiol-modified cellulose nanofibrous composite membranes for chromium (Ⅵ) and lead (Ⅱ) adsorption [J]. Polymer, 2014, 55(5): 1167-1176. doi: 10.1016/j.polymer.2014.01.043 -




 下载:
下载: