-
有机磷酸酯阻燃剂(OPFRs)作为一种替代传统的新型溴化阻燃剂,随着使用量的增加已在环境中普遍存在。它们被添加到各种材料(如塑料、纺织品、橡胶等)中,用于阻燃或塑化[1] 。有研究报告OPFRs进入环境后,在大气中会被长距离输送,甚至到达偏远地区[2] 。OPFRs存在于废水[3-4] 、污泥[5] 、土壤[6-7] 、大气[8] 、粉尘[9-11] 、沉积物[12] 等,甚至在人的血浆[13] 中。由于OPFRs的亲油性和疏水性,其倾向在大气颗粒物[14] 上积累。人体可通过呼吸、摄入和直接皮肤接触等暴露途径接触OPFRs[15-18] ,虽然OPFRs比传统阻燃剂更环保,但大多数还是具有神经毒性、致癌性和胎儿毒性等[19-21] 。建立大气颗粒物中OPFRs的预处理方法,便于深入分析大气中OPFRs的来源、浓度水平和人体暴露情况[22] 。同时,也可以为相关部门提供检测大气颗粒物中有机磷酸酯阻燃剂的相关技术。
目前,国内外大气颗粒物样品的预处理方法主要有索氏提取[23] 、微波辅助提取[8] 、加压溶剂提取[24-25] 、超声提取[26] 等。索氏提取时间长,微波辅助提取和加速溶剂提取成本高,超声波装置成本低,操作方便。大气颗粒物基质复杂,存在痕量的有机污染物,提取后需要进一步提纯浓缩以达到更高的灵敏度。常用的固相萃取盒、层析纯化柱或其他纯化技术,会消耗较多的有机溶剂。分散液液微萃取(DLLME)以纯水为底物进行净化,用微量溶剂萃取,有机溶剂的消耗最小化并可作为一个整体进行净化浓缩。在分散剂的作用下,当萃取剂与目标分析物的接触面积增加时,可以立即达到反应平衡。无需额外的设备,节省大量的预处理时间,达到较高的萃取效率。与其他提取纯化方法相比,该技术简单、高效[27] 。
为了进一步提高该方法的灵敏度,本文建立了超声提取与分散液液微萃取相结合的预处理方法[28] . 样品经超声提取,分散液液微萃取提纯、浓缩,最后经气相色谱串联质谱仪(GC-MS/MS)检测。
-
三重四极杆气质联用仪(德国布鲁克SCION TQ),KQ-500DE数控超声清洗器(昆山市超声仪器有限公司),TTL-DCI型氮吹仪(北京同泰联科技公司),SK-1快速混匀器(金坛市盛蓝仪器制造公司),800型离心沉淀机(上海精科实业有限公司),雷博2030智能大气综合采样器(青岛高科技工业园雷博电子仪器厂),90 mm玻璃纤维滤膜,7种有机磷酸酯阻燃剂标准品的性质如表1,用甲醇定容至1000 μg·L−1标准储备液,置于冰箱中−22 ℃下保存;回收率指示物氚代磷酸三丁酯(TnBP-D27),纯度为98%以上,美国剑桥同位素实验室生产;二氯甲烷、正己烷、丙酮、无水乙醇、乙酸乙酯、正己烷、无水乙醇、四氯化碳、甲苯、1,2-二氯苯、1,1,2-三氯乙烷、氯苯、氯化钠均为分析纯;甲醇、乙腈为色谱纯;实验用水为超纯水。
-
色谱条件 色谱柱:BR-5MS毛细管色谱柱(30 m×0.25 mm×0.5 μm);载气:高纯氦气;进样口温度:270 ℃;柱箱温度:90 ℃;不分流进样;柱流量:1 mL·min−1;进样口和传输线温度均为270 ℃。升温程序:初始温度90 ℃保持1 min,以25 ℃·min−1升至255 ℃,保持2 min,再以2 ℃·min−1升至270 ℃,保持2 min;溶剂延迟时间:4.5 min。
质谱条件 离子源:EI源;电离电压:−20 eV;碰撞压力为0.266 Pa;离子源温度:230 ℃;检测模式:MRM模式。
-
取15 cm2(1/4份)玻璃纤维滤膜样品,均匀滴加20 μL有机磷酸酯阻燃剂标准溶(1000 μg·L−1),将加标滤膜(采样滤膜)剪碎,加入乙酸乙酯和丙酮(3∶2,V/V)混合液9 mL,30 ℃下超声提取20 min,离心后取上清液,过0.45 μm有机相滤头,将过滤液转移至棕色玻璃尖底离心管中;重复提取1次,合并提取液,之后氮吹至近干。迅速加入500 μL无水乙醇和40 μL四氯化碳混合液于离心管中,混匀。最后在离心管中加入5 mL的超纯水,混匀后形成乳浊液,以3500 r·min−1离心5 min,用微量进样针于离心管底部取出沉积相放至玻璃内插管中,最后取1 μL的样品进 GC-MS/MS 进行分析测定。如图1所示。
-
有机磷酸酯阻燃剂具有阻燃和增塑的双重功能,部分属于增塑剂,塑料、橡胶等材料加工过程中会被加入,存在于大部分塑料制品中。实验过程中实验用品要求全部使用玻璃制品,每次使用前都要用超纯水和甲醇依次进行超声波清洗。实验前将玻璃纤维滤膜放在马弗炉中450 ℃灼烧12 h。实验进行了每个环节的空白验证实验,空白实验中目标物(TiBP、TnBP、TDCPP)检出含量为实际样品中检出含量的1%。因其检出含量较低,实验结果数据均已扣除空白实验数据。实验使用外标法定量,其中所有样品进行萃取前,均加入已知量的TnBP-D27,其回收率为88%±15%。
-
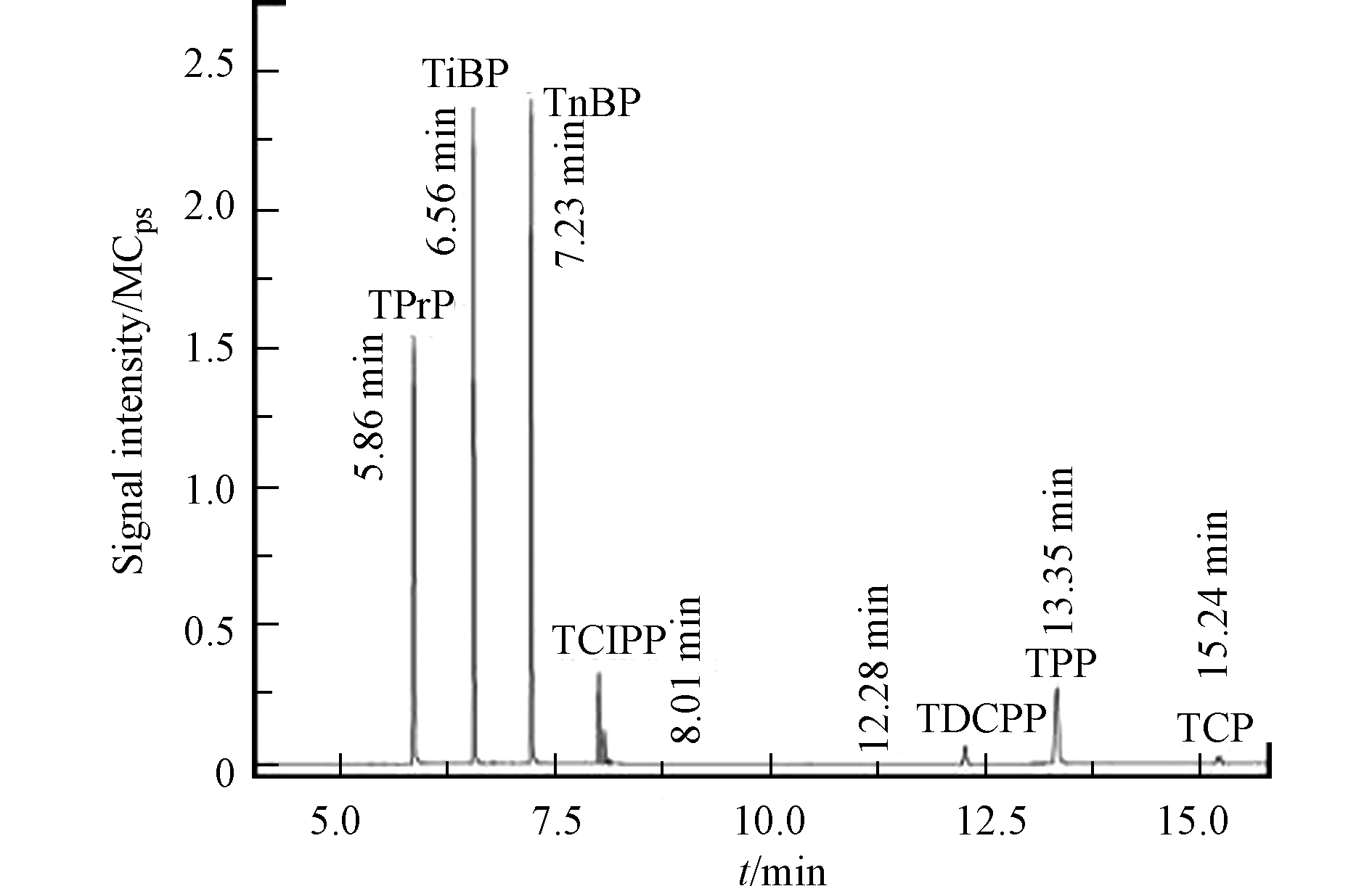
将7种有机磷酸酯标准混合溶液(10000 μg·L−1)进行m/z范围为50—600的全扫描(Full scan)后得到目标物各自的保留时间和母离子。在设置碰撞电压为10 eV的情况下,用其母离子及各自的保留时间进行子离子扫描(Daughter scan)。选取母离子碎片的最强子离子峰作为目标物的子离子。为了能够更加灵敏的检测出目标化合物,本文对每个目标物的碰撞能量进行了优化。在优化的MRM模式下,对大气颗粒物中的7种有机磷酸酯阻燃剂以及替代物进行分析鉴定。7种有机磷酸酯阻燃剂和替代物的保留时间、碰撞能量见表2,7种有机磷酸酯阻燃剂总离子流图如图2所示。
-
本文考察了丙酮、二氯甲烷、乙酸乙酯、丙酮∶正己烷(1∶1,V/V)、乙酸乙酯:丙酮(3∶2,V/V)作为超声提取剂的提取效果。如图3(a)所示,当乙酸乙酯∶丙酮(3∶2,V/V)混合溶液与乙酸乙酯作为萃取剂时,萃取效率较高。当化合物种类较多时,往往选择提取效果较好的混合溶液。综合考虑萃取效率后,选择乙酸乙酯∶丙酮(3∶2,V/V)作为超声提取剂。超声波提取中加入的萃取剂的总体积以及超声时间也是影响实验的重要因素。优化的提取剂总体积分别为12、15、18、20、24 mL时,如图3(b)中当乙酸乙酯和丙酮的混合溶液体积大于18 mL,萃取效率趋于稳定。图3(c)中在超声时间为5、10、20、30 min的情况下,当超声时间大于20 min时提取效果较好。为了减少有机溶剂的使用,节省预处理时间,最终萃取剂的总体积选择为18 mL,两次超声提取时间均为20 min。
-
根据前期单因子优化的实验结果(萃取剂:四氯化碳、分散剂:无水乙醇、萃取剂体积:40 μL、分散剂体积500 μL、不加盐)以及文献研究报道,选取萃取剂体积(A)、分散剂体积(B)和pH值(C)作为BBD实验设计研究影响DLLME的重要实验参数。BBD实验设计在3因素3水平下进行,共17组实验。其中12组为分析因子,共有5组作为中心点估计误差。所研究变量范围为:萃取剂体积为30—50 μL,分散剂体为400—600 μL,pH值为5—9。实验设计以目标物平均回收率为响应值。响应面实验方案及结果见表3。
由表4方差分析可知,模型的F=178.95,模型非常显著。模型的概率P < 0.0001表示只有噪声引起的“模型F值”出现的可能性为0.01%,表示该模型具有显著性统计意义。失拟项(lack of fit)用来表示所用模型与实验拟合的程度,即二者差异的程度。本实验P值为0.6324﹥0.05,表示失拟项不显著,模型可靠。信噪比为37.613,表明信号足够。因此可用该回归方程代替试验真实点对实验结果进行分析。AB、AC、BC、A2、B2、C2的P值均小于0.05,说明AB、AC、BC、A2、B2、C2对化合物回收率均有显著影响。用Desk-Expert 8.0.6软件对结果进行多元回归拟合得到:
相关系数R2=0.9957,调整后R2=0.9901。该模型能准确反映各因素与平均回收率之间的关系。
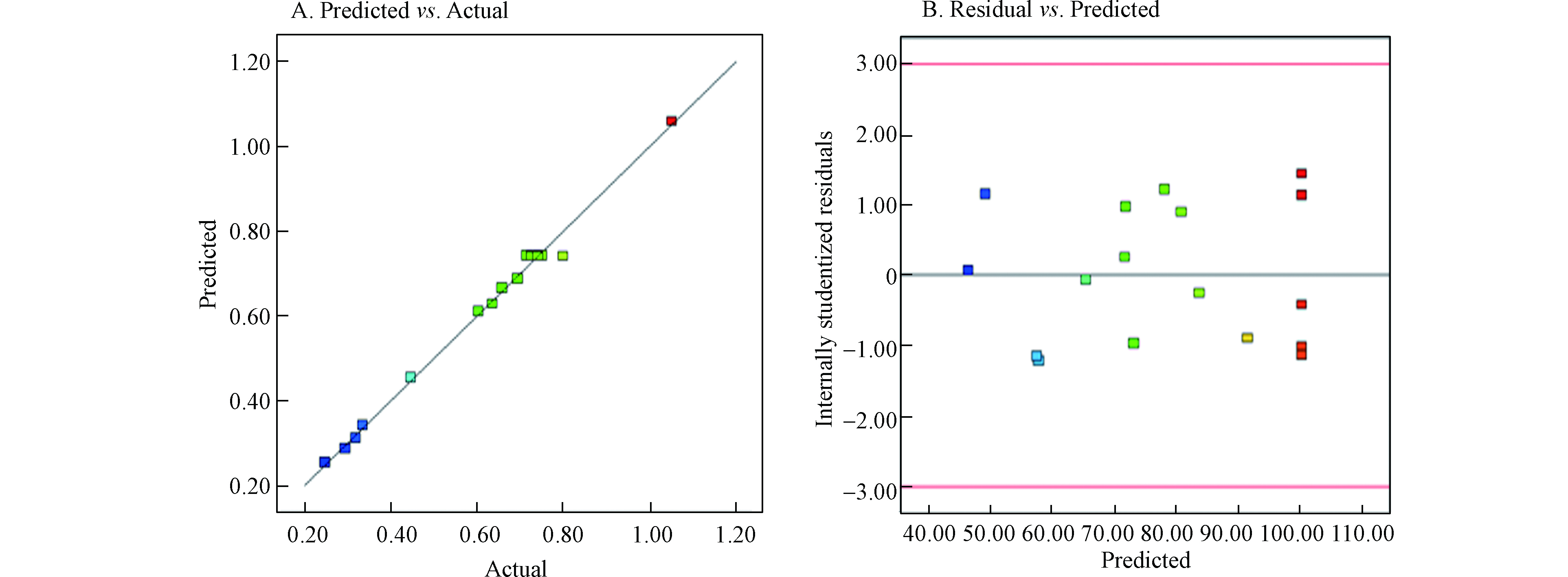
图4(A)中数据的预测点以及实测点都基本位于回归线附近,表明预测响应值和实际响应值之间线性关系良好。图4(B)中的残差值是随机分布的,这表明实验的方差对于所有实验结果都是恒定的。
为了考察这3个因素(萃取剂体积、分散剂体积、pH)与目标物平均回收率之间的关系,绘制了3D响应表面和轮廓线(图5)。从图5可以看出,在萃取剂和分散剂固定的情况下,设置参数量程内响应面上最大值均出现在图中红色部分(萃取剂用量44.54 μL,分散剂用量495.76 μL,pH为7.12)。结合单因子实验结果以及3种因素之间的交互作用,最佳实验条件总结如下:萃取剂体积为40 μL,分散剂体积为500 μL,pH7。
-
配制浓度为1、2、10、20、50、100、200、500、1000 μg·L−1系列标准浓度,以化合物浓度为横坐标(x,μg·L−1),对应峰面积为横坐标(y)进行线性拟合,检出限按信噪比(S/N=3)、定量下限按信噪比(S/N=10)计算,实验结果表明7种有机磷酸酯阻燃剂在表中所述浓度之间具有良好的线性关系,检出限为0.0049—0.3310 pg·m−3,定量下限为0.0158—1.0835 pg·m−3,结果见表5。
取不同浓度的有机磷酸酯阻燃剂标准溶液(50、250、1000 μg·L−1)滴加至空白样品基质中,以高、中、低不同浓度的加标基质按照1.3节步骤对样品进行预处理,并连续测定6次,计算实验加标回收率及相对标准偏差。如表6所示,加标回收率为70.0%—119.7%,相对标准偏差为0.2%—9.4%。
-
对苏州地区学校、建筑工地、交通路口、室内环境大气中的PM10进行采样分析,4个大气颗粒物样品采样时间均为8 h,采样流量为100 L·min−1,将采样体积换算为标准大气压下体积。加入回收率指示物,用已经建立的方法对样品进行分析测定。如表7所示,实际大气中目标物的选择离子色谱图中有大部分有机磷酸酯阻燃剂检出。检测浓度范围为0—7.177 ng·m−3。
-
本文建立了超声提取-分散液液微萃取-气相色谱-质谱联用法测定大气颗粒物中7种OPFRs的方法。采用单因子优化法和正交实验设计法(BBD)确定了最佳实验条件,并应用于大气颗粒物中OPFRs的分析检测。超声提取法与分散液-液微萃取相结合,超声提取后待测物质包含于提取液,经分散液液微萃取被富集在萃取剂中,提高了方法的灵敏度,实现浓缩和纯化的一体化。该方法便捷、高效、绿色,应用于大气样品PM10的有机磷酸酯阻燃剂分析,具有耗费有机溶剂少,成本低廉,节省时间,灵敏度高等优点。
超声提取-分散液液微萃取-气相色谱串联质谱法测定大气PM10中的7种有机磷酸酯阻燃剂
Determination of seven organophosphate flame retardants in atmospheric particulate matter (PM10) by ultrasonic extraction-dispersed liquid-liquid microextraction combined with gas chromatography tandem mass spectrometry
-
摘要: 建立了超声提取-分散液液微萃取联合气相色谱串联三重四极杆质谱测定大气中7种有机磷酸酯阻燃剂(磷酸三丙酯(TPrP)、磷酸三异丁酯(TiBP)、磷酸三正丁酯(TnBP)、磷酸三(1-氯-2-丙基)酯(TCIPP)、磷酸三(1,3-二氯-2-丙基)酯(TDCPP)、磷酸三苯酯(TPP)、磷酸三甲苯酯(TCP))的方法。本实验采用单因素优化法结合BBD实验设计法对超声波提取以及分散液-液微萃取的工艺参数进行优化后确定实验的最佳条件。样品以乙酸乙酯和丙酮(3∶2,V/V)混合溶液为提取剂进行超声提取,再采用分散液-液微萃取技术(纯水为纯化基质,四氯化碳为萃取剂,无水乙醇为分散剂)进一步提纯浓缩。7种OPFRs的检出限为0.0049—0.3310 pg·m−3,定量下限为0.0158—1.0835 pg·m−3,加标回收率为70.0%—119.7%,相对标准偏差为0.2%—9.4%。将此方法用于苏州地区实际样品大气PM10的检测,检测显示当地大气PM10中存在大部分有机磷酸酯阻燃剂。Abstract: A method of ultrasonic extraction-dispersive liquid-liquid microextraction combined with gas chromatography-triple quadrupole tandem mass spectrometry (GC-MS/MS) was established for the determination of 7 organophosphate flame retardants (OPFRs) (tripropyl phosphate (TPrP), triisobutyl phosphate(TiBP), tri-n-butyl phosphate (TnBP), tris (1-chloro-2-propyl) phosphate (TCIPP), tris (1,3-dichloro-2) ester (TDCPP), triphenyl phosphate (TPP), tricresyl phosphate (TCP). In this experiment, the single factor optimization method combined with BBD experimental design method was adopted to optimize the technological parameters of ultrasonic extraction and dispersed liquid-liquid microextraction. Finally, the optimal conditions for the experiment were determined. The samples were ultrasonic extracted with ethyl acetate and acetone (3∶2, V/V) as the extraction agent, and then further purified and concentrated with the dispersive liquid-liquid microextraction technology (pure water as the purification matrix, carbon tetrachloride as the extraction agent, anhydrous ethanol as the dispersant). The results showed that the limits of detection (LOD) ranged from 0.0049 pg·m−3 to 0.3310 pg·m−3, and the limits of quantitative (LOQ) ranged from 0.0158 pg·m−3 to1.0835 pg·m−3.The spiked recoveries ranged from 70.0% to 119.7%, and the RSDs ranged from 0.2% to 9.4%. The method was applied to the actual atmospheric PM10 samples in Suzhou area, the results showed that most of the organophosphate flame retardants existed in the local atmospheric PM10.
-

-
表 1 化合物性质
Table 1. compound properties
化合物
Compounds简称
Abbreviation化学式
Molecular formula分子量
Molecular weightCAS号
CAS number生产商
Manufacturer沸点/℃
Boiling point极性
lgKow磷酸三丙酯 TPrP C9H21O4P 224.24 513-08-6 Sigma-Aldrich 120—122 1.87 磷酸三异丁酯 TiBP C12H27O4P 266.31 126-71-6 Fluoro Chem 205 3.60 磷酸三正丁酯 TnBP C12H27O4P 266.31 126-73-8 aladdin 289 4.00 磷酸三(1-氯-2-丙基)酯 TCIPP C9H18Cl3O4P 327.57 13674-84-5 Adamas 270 2.59 磷酸三(1,3-二氯-2-丙基)酯 TDCPP C9H15Cl6O4P 430.90 13674-87-8 Adamas 315 3.65 磷酸三苯酯 TPP C18H15O4P 326.28 115-86-8 Adamas 370 4.59 磷酸三甲苯酯 TCP C21H21O4P 368.36 563-04-2 Adamas 410 5.11 表 2 目标物的多反应监测条件
Table 2. Multi-reaction monitoring conditions of the target
化合物
Compounds保留时间/min
Retention time子母离子对(m/z)
Parent ion pair碰撞能量/eV
Collision energyTPrP 5.87 99>81、141>99、141>125 5 TiBP 6.57 99>81、139>99、155>139 5 TnBP-D27 7.12 103>102、103>83、167>103 10 TnBP 7.24 99>81、125>99、155>99 5 TCIPP 8.02 99>91、125>99、157>99 10 TDCPP 12.30 191>75、191>99、209>99 10 TPP 13.37 326>233、326>325、327>326 5 TCP 15.25 92>91、92>91、368>251 20 表 3 响应面试验方案及结果
Table 3. Response surface test plan and results
运行
Run萃取剂用量/μL
Extractant volume分散剂用量/μL
Dispersant volumepH 回收率/%
Recovery1 40 600 5 56.5 2 40 500 7 102.2 3 50 600 7 79.3 4 30 600 7 81.6 5 40 500 7 102.7 6 40 400 5 72.8 7 30 400 7 56.7 8 30 500 5 72.0 9 40 500 7 98.6 10 50 400 7 90.7 11 40 500 7 98.4 12 50 500 9 83.5 13 30 500 9 46.5 14 40 600 9 72.3 15 50 500 5 65.4 16 40 500 7 99.6 17 40 400 9 50.3 表 4 BBD设计的方差分析
Table 4. Analysis of variance for BBD design
变量
Source平方和
Squares自由度
df均方
SquareF值
F ValueP值
Prob﹥FModel 5614.50 9 623.83 178.95 ﹤0.001 significant A-Extractant volume 480.50 1 480.50 137.83 ﹤0.001 B-Dispersant volume 46.56 1 46.56 13.36 0.0081 C-pH 24.85 1 24.85 7.13 0.0320 AB 331.24 1 331.24 95.02 ﹤0.001 AC 475.24 1 475.24 136.33 ﹤0.001 BC 366.72 1 366.72 105.20 ﹤0.001 A2 393.11 1 393.11 112.77 ﹤0.001 B2 771.64 1 771.64 221.35 ﹤0.001 C2 2382.51 1 2382.51 683.44 ﹤0.001 Residual 24.40 7 3.49 Lack of fit 8.04 3 2.68 0.66 0.6205 not significant Pure Error 16.36 4 4.09 Cor Total 5638.90 16 表 5 7种有机磷酸酯阻燃剂的选择离子、线性方程、检出限及定量下限
Table 5. Selected ions, linear equations, detection limits and lower limit of quantification of seven OPFRs
化合物
Compounds线性方程
Linear equation线性范围/(μg·L−1)
Linearity range相关系数
Correlation coefficients检出限/(pg·m−3)
LOD定量下限/(pg·m−3)
LOQTPrP y=1.03×102x+8.36×102 1—200 0.9993 0.0049 0.0158 TiBP y=6.19×102x+1.52×102 1—200 0.9996 0.0056 0.0186 TnBP y=6.44×102x+1.23×102 1—200 0.9992 0.0133 0.0433 TCIPP y=7.62×102x+4.94×102 1—200 0.9993 0.0049 0.0158 TDCPP y=5.65×102x−1.54×103 1—200 0.9999 0.0574 0.1878 TPP y=2.58×103x+1.25×104 1—200 0.9993 0.0399 0.1307 TCP y=0.15×102x+11.93×102 2—1000 0.9960 0.3310 1.0835 表 6 7种有机磷酸酯阻燃剂的加标回收率、相对标准偏差
Table 6. The recovery rate and relative standard deviation of seven OPFRs
化合物
Compounds1000 μg·L−1 250 μg·L−1 50 μg·L−1 加标回收率/%
Recovery相对标准偏差/%
RSD(n=6)加标回收率/%
Recovery相对标准偏差/%
RSD(n=6)加标回收率/%
Recovery相对标准偏差/%
RSD(n=6)TPrP 94.9 5.8 82.3 6.6 70.0 4.6 TiBP 79.5 9.4 94.1 1.3 83.9 7.3 TnBP 89.0 2.7 92.7 4.0 87.2 2.3 TCIPP 119.7 8.5 117.5 7.7 117.6 2.4 TDCPP 93.8 2.1 102.7 2.3 94.0 4.8 TPP 88.2 0.5 83.4 0.6 85.8 5.8 TCP 86.7 0.2 84.9 3.1 85.3 2.9 表 7 样品分析结果(ng·m−3)
Table 7. Sample analysis results
采样地点
Sampling position建筑工地
Construction site室内环境
Indoor environment交通道路
Traffic route学校
SchoolTPrP ND. 0.188 ND. 0.246 TiBP 0.613 ND. 0.841 0.348 TnBP 0.734 ND. 0.210 0.120 TCIPP 7.177 1.911 4.646 3.240 TDCPP 5.406 ND. ND. 0.559 TPP 1.530 ND. ND. 0.108 TCP 1.229 0.437 ND. ND. ∑OPFRs 16.689 2.536 5.697 4.621 注:ND.表示未检出. ND. means not detected. -
[1] CHEN Y Y, CHEN Y J, ZHANG Y H, et al. Determination of HFRs and OPFRs in PM2.5 by ultrasonic-assisted extraction combined with multi-segment column purification and GC-MS/MS [J]. Talanta, 2019, 194: 320-328. doi: 10.1016/j.talanta.2018.10.025 [2] MÖLLER A, STURM R, XIE Z Y, et al. Organophosphorus flame retardants and plasticizers in airborne particles over the northern Pacific and Indian ocean toward the polar regions: Evidence for global occurrence [J]. Environmental Science & Technology, 2012, 46(6): 3127-3134. [3] HAO C Y, HELM P A, MORSE D, et al. Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent [J]. Chemosphere, 2018, 191: 288-295. doi: 10.1016/j.chemosphere.2017.10.060 [4] PANTELAKI I, VOUTSA D. Organophosphate flame retardants (OPFRs): A review on analytical methods and occurrence in wastewater and aquatic environment [J]. Science of the Total Environment, 2019, 649: 247-263. doi: 10.1016/j.scitotenv.2018.08.286 [5] PANG L, YUAN Y T, HE H, et al. Occurrence, distribution, and potential affecting factors of organophosphate flame retardants in sewage sludge of wastewater treatment plants in Henan Province, Central China [J]. Chemosphere, 2016, 152: 245-251. doi: 10.1016/j.chemosphere.2016.02.104 [6] LORENZO M, CAMPO J, PICÓ Y. Determination of organophosphate flame retardants in soil and fish using ultrasound-assisted extraction, solid-phase clean-up, and liquid chromatography with tandem mass spectrometry [J]. Journal of Separation Science, 2018, 41(12): 2595-2603. doi: 10.1002/jssc.201701461 [7] LUO Q, SHAN Y, MUHAMMAD A, et al. Levels, distribution, and sources of organophosphate flame retardants and plasticizers in urban soils of Shenyang, China [J]. Environmental Science and Pollution Research, 2018, 25(31): 31752-31761. doi: 10.1007/s11356-018-3156-y [8] NACCARATO A, TASSONE A, MORETTI S, et al. A green approach for organophosphate ester determination in airborne particulate matter: Microwave-assisted extraction using hydroalcoholic mixture coupled with solid-phase microextraction gas chromatography-tandem mass spectrometry [J]. Talant, 2018, 189: 657-665. doi: 10.1016/j.talanta.2018.07.077 [9] THU H T, DUC C N, THI H L, et al. Determination of organophosphate ester flame retardants in indoor dust and their potential health exposure risk [J]. Vietnam Journal of Chemistry, 2020, 58(6): 723-730. [10] GILL R, HURLEY S, BROWN R, et al. Polybrominated diphenyl ether and organophosphate flame retardants in canadian fire station dust [J]. Chemosphere, 2020, 253: 126669. doi: 10.1016/j.chemosphere.2020.126669 [11] de la TORRE A, NAVARRO I, SANZ P, et al. Organophosphate compounds, polybrominated diphenyl ethers and novel brominated flame retardants in European indoor house dust: Use, evidence for replacements and assessment of human exposure [J]. Journal of Hazardous Materials, 2020, 382: 121009. doi: 10.1016/j.jhazmat.2019.121009 [12] GAO X Z, XU Y P, MA M, et al. Distribution, sources and transport of organophosphorus flame retardants in the water and sediment of Ny- Ålesund;lesund, Svalbard, the Arctic [J]. Environmental Pollution, 2020, 264: 114792. doi: 10.1016/j.envpol.2020.114792 [13] LI P, JIN J, WANG Y, et al. Concentrations of organophosphorus, polybromobenzene, and polybrominated diphenyl ether flame retardants in human serum, and relationships between concentrations and donor ages [J]. Chemosphere, 2017, 171: 654-660. doi: 10.1016/j.chemosphere.2016.12.126 [14] VEEN I V D, BOER J D. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis [J]. Chemosphere, 2012, 88(10): 1119-1153. doi: 10.1016/j.chemosphere.2012.03.067 [15] CHEN Y X, LIU Q Y, MA J, et al. A review on organophosphate flame retardants in indoor dust from China: Implications for human exposure [J]. Chemosphere, 2020, 260: 127633. doi: 10.1016/j.chemosphere.2020.127633 [16] LI M Q, YAO Y M, WANG Y, et al. Organophosphate ester flame retardants and plasticizers in a Chinese population: Significance of hydroxylated metabolites and implication for human exposure [J]. Environmental Pollution, 2020, 257: 113633. doi: 10.1016/j.envpol.2019.113633 [17] SANCHEZ-PINERO J, BOWERBANK S L, MOREDA-PINEIOR J, et al. The occurrence and distribution of polycyclic aromatic hydrocarbons, bisphenol A and organophosphate flame retardants in indoor dust and soils from public open spaces: Implications for human exposure [J]. Environmental Pollution, 2020, 266(Pt1): 115372. [18] van den EEDE N, DIRTU A C, NEELS H, et al. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust [J]. Environment International, 2011, 37(2): 454-461. doi: 10.1016/j.envint.2010.11.010 [19] LUO D, LIU W, WU W X, et al. Trimester-specific effects of maternal exposure to organophosphate flame retardants on offspring size at birth: A prospective cohort study in China [J]. Journal of Hazardous Materials, 2020, 406: 124754. [20] YAO Y M, LI M Q, PAN L Y, et al. Exposure to organophosphate ester flame retardants and plasticizers during pregnancy: Thyroid endocrine disruption and mediation role of oxidative stress [J]. Environment International, 2021, 146: 106215. doi: 10.1016/j.envint.2020.106215 [21] AL-SALEM A M, SAQUIB Q, SIDDIQUI M A, et al. Organophosphorus flame retardant (tricresyl phosphate) trigger apoptosis in HepG2 cells: Transcriptomic evidence on activation of human cancer pathways [J]. Chemosphere, 2019, 237: 124519. doi: 10.1016/j.chemosphere.2019.124519 [22] 李锦, 张占恩, 陈鑫, 等. 超声提取-分散液相微萃取-气相色谱质谱法测定大气PM2.5中15种邻苯二甲酸酯 [J]. 环境化学, 2017, 36(1): 183-189. doi: 10.7524/j.issn.0254-6108.2017.01.2016051302 LI J, ZHANG Z N, CHEN X, et al. Determination of fifteen phthalate esters in air particulate matter (PM2.5) by ultrasonic extraction-dispersive liquid-liquid microextraction combined with gas chromatography-mass spectrometry [J]. Environmental Chemistry, 2017, 36(1): 183-189(in Chinese). doi: 10.7524/j.issn.0254-6108.2017.01.2016051302
[23] COCHRAN R E, KUBÁTOVÁ A. Pressurised fluid extraction of polycyclic aromatic hydrocarbons and their polar oxidation products from atmospheric particles [J]. International Journal of Environmental Analytical Chemistry, 2015, 95(5): 434-452. doi: 10.1080/03067319.2015.1025225 [24] RAMOS-CONTRERAS C, CONCHA-GRAÑA E, LÓPEZ-MAHÍA P, et al. Determination of atmospheric particle-bound polycyclic aromatic hydrocarbons using subcritical water extraction coupled with membrane microextraction [J]. Journal of Chromatography. A, 2019, 1606: 460381. doi: 10.1016/j.chroma.2019.460381 [25] COSCOLLÀ C, YUSÀ V, MARTÍ P, et al. Analysis of currently used pesticides in fine airborne particulate matter (PM2.5) by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry [J]. Journal of Chromatography. A, 2008, 1200(2): 100-107. doi: 10.1016/j.chroma.2008.05.075 [26] FERNÁNDEZ-AMADO M, PRIETO-BLANCO M C, LÓPEZ-MAHÍA P, et al. Ion-pair in-tube solid phase microextraction for the simultaneous determination of phthalates and their degradation products in atmospheric particulate matter [J]. Journal of Chromatography. A, 2017, 1520: 35-47. doi: 10.1016/j.chroma.2017.09.010 [27] GARCÍA-LÓPEZ M, RODRÍGUEZ I, CELA R. Development of a dispersive liquid-liquid microextraction method for organophosphorus flame retardants and plastizicers determination in water samples [J]. Journal of Chromatography. A, 2007, 1166(1/2): 9-15. [28] YAN H Y, WANG H, QIN X Y, et al. Ultrasound-assisted dispersive liquid-liquid microextraction for determination of fluoroquinolones in pharmaceutical wastewater [J]. Journal of Pharmaceutical and Biomedical Analysis, 2011, 54(1): 53-57. doi: 10.1016/j.jpba.2010.08.007 -




 下载:
下载: