-
由于干旱和半干旱地区地表水资源稀缺且具有间歇性,地下水往往是支撑中国西北煤矿地区用水需求的主要水源[1]。然而,煤炭开采与水资源管理之间很难达到平衡。此外,煤层上覆多层含水层的存在使得这种情况更加复杂。一方面,采矿可能会以各种方式影响地下水质量,这取决于水文地质环境和相邻含水层的化学性质[2-3]。另一方面,多层含水层以下的深部煤层,在开采过程中工作面或巷道附近可能发生突水,会对开采构成威胁[4-5]。为了支持矿山水资源的可持续利用和突水预测,有必要阐明地下水的来源、循环和水-岩作用对水化学演化过程的影响[6-7]。
近年来,人们对地下水系统的水化学演化进行了广泛的研究,其中水-岩作用是地下水系统水化学演化的主要机制。简单的散点图,多元统计分析,同位素等方法已被应用于调查地下水系统的水化学过程[8-10]。此外,不同离子之间的相关性同样为获取水化学演化过程中水岩相互作用信息提供了有效途径[11-12]。然而,这些方法仅利用部分现有数据定性描述可能的水化学演化过程,难以量化这些过程中各组分的形式和物质转移量。相对于这些定性方法,水化学反向模拟是在多种理论和技术方法基础上发展起来的定量方法[13]。重要的是,反演模型可以计算各种组分的形式、地下水系统中的物质转移量和水-岩平衡状态,从而揭示地下水系统中的水化学过程[14]。
本文选取的研究区为我国西北地区的大海则井田,通过水化学图、离子比例分析、饱和指数等方法探讨了多层含水层地下水的水化学特征和演化规律,并且通过水化学反向模拟的方法定量解释了控制不同含水层地下水化学演化的水文地球化学过程。
-
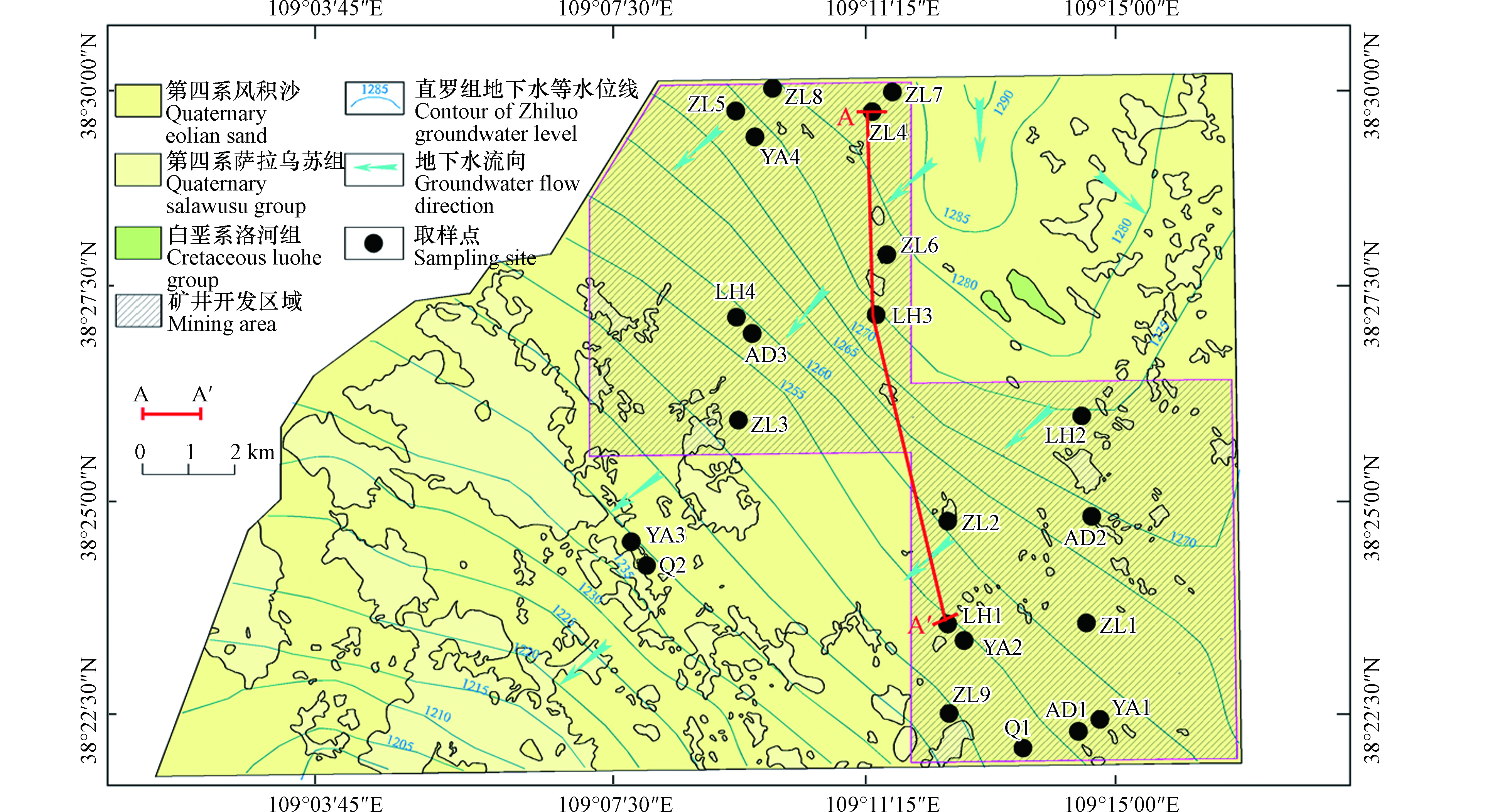
大海则井田位于中国陕西省榆林市西北方向50 km处,井田南北长14.7 km,东西宽12—22 km,占地面积280.03 km2。研究区的水文地质补勘工作于2013年12月完成。目前,井田的巷道钻打和基础设施建设工作已经完成,正式采煤工作计划于2021年年底开始。研究区位于毛乌素沙漠南缘,沙漠覆盖率达70%以上。主要地貌类型为沙漠洼地和沙丘,黄土梁地貌仅见于东南部地区。研究区地形较为平坦,最高海拔为1327 m,最低海拔为1210 m。
研究区位于鄂尔多斯盆地中部(图1),地表大部分被第四系风积沙覆盖,区内无大断层,也无岩浆活动。井田内发育有多个含水层,其中,第四系松散潜水含水层(单位涌水量q = 0.143—0.466 L·(s·m)−1)富水性中等-强;白垩系下统洛河组孔隙-裂隙含水层(单位涌水量q = 0.1—0.276 L·(s·m)−1)富水性中等;侏罗系中统安定组裂隙含水层(q = 0.003—0.02 L·(s·m)−1)富水性弱;侏罗系中统直罗组裂隙含水层(q = 0.033—0.271 L·(s·m)−1)富水性弱-中等;侏罗系中统延安组裂隙含水层(q = 0.0004—0.012 L·(s·m)−1)富水性极弱。研究区多层含水层系统地下水主要受降水补给,地下水流动主要受地形控制,流动方向由东北向西南。因此,多层含水层的地下水具有相同的补给面积和流向(图2)。此外,侧向径流排泄是地下水的主要排泄方式。
-
2011年10月,在大海则井田共采集地下水样24组(图1),其中包括第四系地下水样2组(Q1—Q2),洛河组地下水样4组(LH1—LH4),安定组地下水样3组(AD1—AD3),直罗组地下水样9组(ZL1—ZL9),延安组地下水样4组(YA1—YA4)以及矿井水水样2组(M1—M2)。
地下水样品的测试主要由陕西省核工业地质调查院进行的。结果如表1所示,现场参数(pH值、水温)通过便携式多参数监测仪进行测试。主要阳离子(Ca2+、Mg2+、Na++K+)采用电感耦合等离子体发射光谱仪(ICP-OES)测定,阴离子(SO42–、Cl–)采用离子色谱仪(ICS-1100)测定,HCO3–用HCl标准溶液(0.025 mol·L–1)滴定,可溶性SiO2用硅钼黄分光光度法测定。总溶解固体(TDS)是通过计算得到。除HCO3–外,阳离子和阴离子的精密度为0.01 mg·L–1;HCO3–精密度为0.60 mg·L–1。大部分样品电荷平衡误差小于5%,只有少数样品的电荷平衡误差较大,但仍小于10%,在合理范围内。
-
多层含水层地下水描述性统计分析结果见表2。第四系、洛河组和安定组地下水中阴阳离子以HCO3−和Ca2+为主,矿化度较低(TDS < 1000 mg·L–1)。直罗组和延安组地下水中阴阳离子以SO42−、Na++K+和Ca2+为主,为中等矿化度的微咸水(1000 mg·L–1 < TDS < 3000 mg·L–1)。然而,矿井水以SO42−和Na++K+为主,表现为高矿化度的咸水(TDS > 5000 mg·L–1)。《国家地下水质量标准》(GB/T 14848—2017)包含TDS等93项指标,将地下水质量标准分为5个等级。以TDS为依据,除第四系、洛河组、安定组和延安组地下水的YA4(428.1 mg·L–1)外,其他水样均超过Ⅲ型标准(500 mg·L–1 ≤ TDS ≤ 1000 mg·L–1),甚至部分地下水样属于Ⅴ型标准(TDS > 2000 mg·L–1)。说明安定组含水层以下的深层含水层的地下水不适宜饮用,但经适当处理后可用于工农业生产。
-
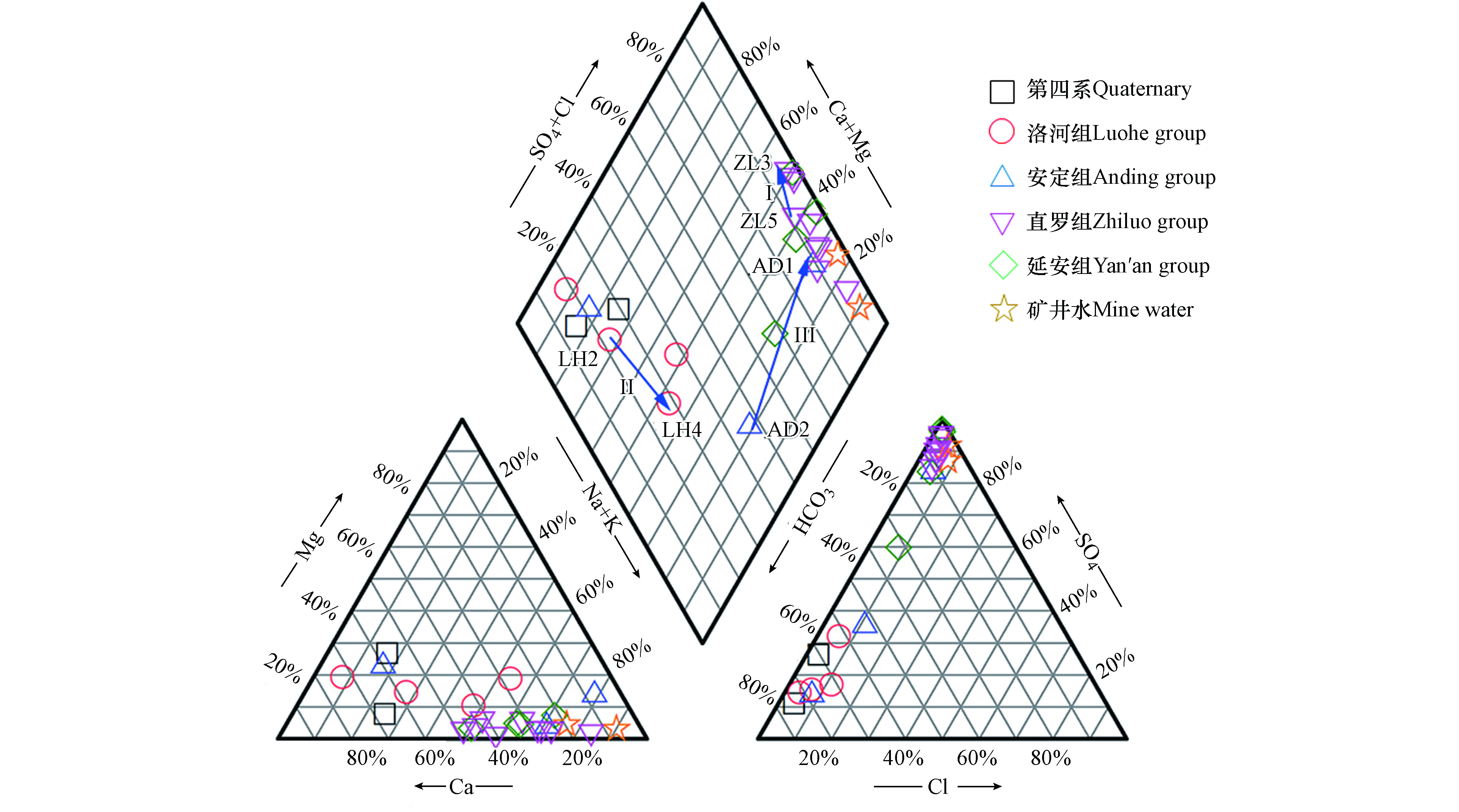
如图3所示,Piper图中多层含水层地下水样品分散:第四系地下水以HCO3·SO4-Ca和HCO3-Ca·Mg型为主;洛河组地下水类型为HCO3-Ca·Na+K、HCO3·SO4-Na+K·Ca和HCO3-Ca型;安定组地下水类型为SO4-Na+K·Ca、HCO3-Ca和HCO3·SO4-Na+K型;直罗组地下水类型为SO4-Ca·Na+K、SO4-Na+K和SO4-Na+K·Ca型;延安组地下水类型为SO4-Na+K·Ca和SO4·HCO3-Na+K型;矿井水的水化学类型为SO4-Na+K型,与直罗组地下水类似,说明直罗组地下水和矿井水之间存在水力联系,是煤矿开采过程中潜在的突水水源。
此外,Piper图可以用于表示地下水的水化学演化[15]。根据水文地质条件可知,多层含水层具有相同的补给区域和地下水流向(图1),这有助于研究不同含水层水化学演化的异同。此次研究主要调查了直罗组含水层、洛河组含水层和安定组含水层的水化学演化规律(图3)。
第一个演化路径(箭头Ⅰ:ZL5→ZL3)代表直罗组地下水的水化学演化,水化学类型从SO4-Na+K·Ca型变为SO4-Ca·Na+K,TDS沿着地下水流动方向明显增加。总体上,主要离子的变化规律是Ca2+、Na++K+、Mg2+和SO42−含量增加,HCO3−含量降低,而Cl−含量基本保持不变。其中,Ca2+和Mg2+同步增加,说明可能存在白云石的溶解(方程1);Ca2+和SO42−同步增加,这可能是由于石膏溶解导致(方程2),但SO42−增长幅度明显大于Ca2+,说明可能受到其他水岩作用的影响。除了石膏的溶解,黄铁矿的氧化可能是煤矿地下水中SO42−的另一个潜在来源(FeS2 + 7/2O2 + H2O = Fe2+ + 2SO42− + 2H+)[16]。但是研究区地下水总铁含量较低,且pH值呈中性(表1)。因此,SO42−的主要来源是石膏的溶解,而不是黄铁矿的氧化。由此可知,SO42−浓度逐渐高于Ca2+浓度是由于Ca2+的减少,而不是SO42−的增加,说明可能受到阳离子交换作用的影响(方程3)。此外,沿着流动路径Na++K+含量明显增大,而Cl−保持相对恒定,进一步验证了阳离子交换作用。
虽然从ZL5到ZL3的直罗组地下水中SiO2含量略有增加(表1),说明硅铝酸盐的溶解可能是Na+和/或K+增大的潜在来源(方程4—5)。但是地下水样品的可溶性SiO2含量较低,且pH值呈中性,因此硅铝酸盐的溶解不是Na+和/或K+的主要来源[17]。相比之下,HCO3−浓度沿着流动路径明显减小,而且大部分直罗组水样点方解石的饱和指数均大于零(表1),说明方解石呈过饱和状态,有可能沿流动路径析出沉淀。综上所述,直罗组地下水的演化路径(ZL5→ZL3)可能发生的水化学过程包括:白云石、石膏和少量硅铝酸盐的溶解,阳离子交换作用和方解石沉淀。
第二条演化路径(箭头Ⅱ:LH2→LH4)代表了洛河组地下水由HCO3-Ca·Na+K型向HCO3-Na+K·Ca型的水化学演化。水化学类型转变的实质是Na++K+、Mg2+、Cl−、SO42−和HCO3−含量增加,Ca2+含量减少。Na++K+和Cl−的增加表明岩盐的溶解,而Na++K+增加幅度明显大于Cl−,说明可能存在阳离子交换作用。Mg2+和SO42−的增加分别来自白云石和石膏的溶解作用。因此,第二条演化路径的主要水岩作用包括岩盐、白云岩和石膏的溶解并伴随阳离子交换。
第三条演化路径(箭头Ⅲ:AD2→AD1)代表安定组地下水由HCO3·SO4-Na+K型转化为SO4-Na+K·Ca型的演化过程。沿流动路径主要离子的变化是Na++K+、Ca2+、Cl−和SO42−含量增加,HCO3−含量下降,Mg2+含量略有下降。由表1可知,安定组地下水样方解石和白云石的饱和指数均大于零,说明方解石和白云石处于饱和/过饱和状态。因此,演化过程中HCO3−浓度的减小可能由方解石和白云石析出沉淀导致 [18]。此外,Ca2+与SO42−的浓度同步增大,说明Ca2+的主要来源是石膏的溶解。对于Na++K+和Cl−,二者浓度均发生了增大,说明了岩盐溶解的存在。但是,Na++K+增大幅度明显大于Cl−,说明可能受到了阳离子交换的影响。此外,硅铝酸盐的溶解(SiO2略有增加)和方解石和/或白云石的沉淀可能是导致Na++K+增加和Ca2+减少的其他原因。因此,第三条演化路径的水化学过程包括岩盐、石膏和硅铝酸盐的溶解,方解石和白云石的沉淀,并伴随阳离子交换作用。
-
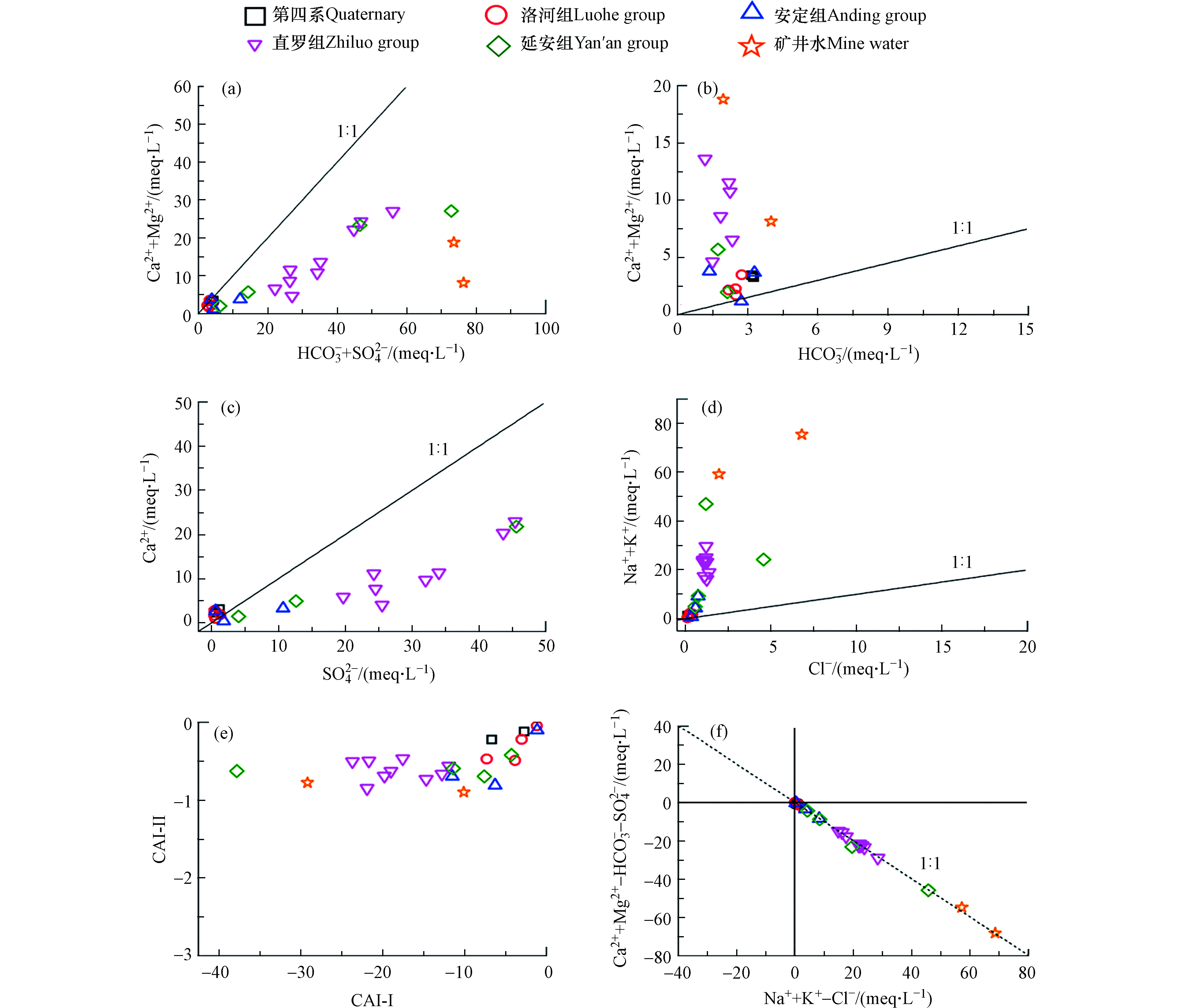
不同来源或条件下形成的地下水中离子的比值关系存在明显差异,因此常用不同离子的比值来识别地下水的来源或水化学演化过程[19]。(Ca + Mg)/(SO4 + HCO3)和(Ca + Mg)/HCO3值可以表明碳酸盐(方解石、白云石等)和硫酸盐(石膏)的溶解或沉淀对地下水化学成分的贡献[20]。第四系、洛河组和安定组地下水的(Ca + Mg)/(SO4 + HCO3),(Ca + Mg)/HCO3和Ca/SO4值均接近于1:1线(图4a—c),说明存在碳酸盐岩的溶解和石膏的溶解。直罗组、延安组地下水和矿井水的(Ca + Mg)/(SO4 + HCO3)低于1:1线,(Ca + Mg)/HCO3值高于1:1线,一方面说明石膏的溶解是Ca2+的主要来源,另一方面说明可能受到了碳酸盐沉淀的影响。此外,由图4c可知,直罗组、延安组地下水和矿井水的SO42–含量是要明显高于Ca2+的,这说明石膏的溶解过程还有可能受到了阳离子交换或者碳酸盐沉淀的影响。
如果盐岩和钾盐的溶解是Na+和K+的唯一来源,那么(Na + K)/Cl的值应是1。然而,在大多数情况下,Na+和K+可能有其他来源(如硅酸盐风化和阳离子交换),从而导致(Na + K)/Cl大于1[21]。如图4d所示,第四系、洛河组和安定组地下水的(Na + K)/Cl值接近于1,说明Na+和K+主要来源于岩盐和钾盐的溶解。相比之下,直罗组、延安组地下水和矿井水的水样点的分布明显高于1:1线,即(Na + K)/Cl >> 1,表明存在Na+和K+的其他来源,如硅铝酸盐的溶解。而水样中的SiO2含量证明了该趋势的可能性(表1),与上述规律一致。此外,阳离子交换作用是造成Na++K+过量的常见原因。通常可利用地下水的Schoeller指数表征地下水化学演化过程中阳离子交换的强度(方程6—7)。如果 Schoeller 指数得到是负的,说明 Na+、K+从含水层矿物中析出,Ca2+、Mg2+取代了它们的位置;如果指数是正的,说明发生了相反的交换[22]。如图4e可知,研究区水样点的Schoeller 指数均为负值,且研究区的含水层中富含黏土矿物[23],含有丰富的Na+和K+,因此,可能发生了含水层矿物中 Na+和K+取代水溶液中 Ca2+和Mg2+的阳离子交换作用。
根据(Na++K+–Cl–)与(Ca2++Mg2+–HCO3––SO42–)关系同样可以判断阳离子交换是否为主导的水文地球化学作用[24]。(Na++K+–Cl–)表示除去岩盐和钾盐溶解后剩余的 Na++K+,(Ca2++Mg2+–HCO3––SO42–)表示除去碳酸盐矿物和石膏溶解后剩余的(Ca2++Mg2+)。若样点落在第Ⅳ 象限内,说明样点中 Na+、K+除了盐岩和钾盐溶解有额外的增加,而碳酸盐矿物和石膏溶解产生的Ca2+、Mg2+有减少,可能是由于正向阳离子交换反应释放了Na+、K+,减少了水中的 Ca2+、Mg2+;若样点落在第Ⅱ 象限,说明样点中 Ca2+、Mg2+除了碳酸盐矿物和石膏溶解有额外的增加,而岩盐和钾盐溶解产生的 Na+、K+有减少,可能是含水介质中的 Ca2+、Mg2+离子与水中 Na+、K+离子发生反向阳离子交换反应。从图4f可知,几乎所有地下水样点在斜率为-1的线附近分布,并沿右侧分布,表明地下水中 Na+增多而 Ca2++Mg2+减少,表明正向阳离子交换作用在研究区广泛发生,与Schoeller指数得出的结论一致。
-
水化学反向模拟能够定量解释水化学成分的变化和水化学演化的主要水岩反应[25]。本研究采用NETPATH程序对上述水化学演化过程进行了定量模拟和定性分析。为了验证和量化这些路径中的水化学过程,本研究选择前文分析提到的直罗组含水层的ZL5→ZL3、洛河组含水层的LH2→LH4和安定组含水层的AD2→AD1作为模拟路径。
-
根据前文的分析,可能的矿物相包括岩盐、石膏、白云石、方解石和硅铝酸盐(如钠长石)。同时,阳离子交换是本研究中必不可少的水化学过程,也应考虑到模型中。根据3个模拟路径不同组分的饱和指数变化可知(表3),3条路径中岩盐、石膏均发生溶解,方解石趋于沉淀,直罗组和洛河组地下水中白云石均发生溶解,而安定组地下水中的白云石趋于沉淀。除了可能矿物相,约束变量是在质量平衡模型中要考虑的水化学元素[26]。根据水化学测试结果,设定Na++K+、Ca2+、Mg2+、HCO3–、SO42–、Cl–和SiO2为模拟约束变量。
-
由表4可知,直罗组地下水的演化路径(ZL5→ZL3)主要反应包括石膏、白云岩、岩盐、钠长石和CO2的溶解,方解石的沉淀和阳离子交换。直罗组含水层与含煤地层延安组含水层相邻(图2),所以当延安组含水层进行巷道掘进工作时,直罗组含水层极容易生成导水裂隙。所以,CO2可以通过掘进产生的裂隙进入地下水,从而促进白云石和钠长石的溶解(方程1和5)。岩盐的溶解和阳离子交换作用产生Na++K+,使得Na++K+不断进入地下水。Ca2+主要受到石膏和白云石的溶解、方解石的沉淀和阳离子交换的影响。虽然阳离子交换可以导致Ca2+的降低,但由于石膏的大量溶解,使得Ca2+的浓度仍呈现出持续升高的趋势。由于Ca2+的增加,地下水水化学类型由ZL5的SO4-Na+K·Ca型转化为ZL3的SO4-Ca·Na+K型。
沿着演化路径LH2→LH4,洛河组地下水的水化学演化经历了石膏、白云岩、岩盐和CO2的溶解;方解石的沉淀与阳离子交换。根据地层剖面图可知(图2),洛河组含水层与第四系含水层相邻,洛河组地下水为潜水,属于开放系统。CO2可以直接进入地下水从而加速白云石的溶解(方程1),另外,阳离子交换作用和岩盐的溶解可以不断产生Na+,且导致Ca2+的减少。虽然白云石和石膏的溶解会产生新的Ca2+,但是方解石的沉淀和阳离子交换的程度更大,从而使得Ca2+总浓度减小。总体上,由于Na+的增加和Ca2+的减少,导致演化路径上水化学类型由LH2的HCO3-Ca·Na+K型转化为LH4的HCO3-Na+K·Ca型。
安定组地下水演化路径(AD2→AD1)的主要水化学过程包括石膏、岩盐和钠长石的溶解;方解石、白云石的沉淀与阳离子交换作用。少量的岩盐、钠长石的溶解和阳离子交换作用使得Na+不断进入地下水。而石膏的溶解、方解石、白云石的沉淀和阳离子交换是控制Ca2+的水化学过程。虽然碳酸盐岩(方解石和白云石)的沉淀和阳离子交换可以降低Ca2+浓度,但石膏的大量溶解导致Ca2+总浓度的增加。同时,石膏的溶解、方解石和白云石的沉淀会导致SO42–的升高和HCO3–的降低。最终,由于Ca2+、SO42–的增加和HCO3–的减少,AD2的水化学类型从HCO3·SO4-Na+K型转化为AD1的SO4-Na+K·Ca型。上述3种演化路径的水化学过程可由下式表示(单位:mmol·L–1):
综上所述,利用水化学反向模拟可以识别出3个不同含水层的水化学演化路径,并且与定性分析的结果相符。研究发现,不同含水层的演化路径中水岩作用和物质转移量都不同,从而导致水化学类型的差异。根据研究区水文地质背景,由于无明显断层,含水层之间较弱的水力联系是造成多层含水层系统水化学类型和水化学演化差异的主要原因。虽然目前相邻含水层之间的水力联系相对较差,但后续的煤矿开采会产生导水裂隙,进而促进相邻含水层之间的水力联系。因此,需要对开采过程中和开采后的水化学演化进行连续监测,预防高矿化度矿井水对主要供水含水层的污染。
-
(1)研究区多层含水层地下水的主要水化学类型为:第四系地下水为HCO3·SO4-Ca型和HCO3-Ca·Mg型;洛河组地下水为HCO3-Ca·Na+K、HCO3·SO4-Na+K·Ca和HCO3-Ca型;安定组地下水为SO4-Na+K·Ca、HCO3-Ca和HCO3·SO4-Na+K型;直罗组地下水为SO4-Ca·Na+K、SO4-Na+K和SO4-Na+K·Ca型;延安组地下水为SO4-Na+K·Ca和SO4·HCO3-Na+K型;矿井水水化学类型以SO4-Na+K型为主,与直罗组地下水类似,说明直罗组地下水是潜在的突水水源,应予以特别重视。
(2)主要离子关系和水化学模拟结果表明,不同矿物的溶解和沉淀以及阳离子交换作用是控制水化学类型的主要因素。然而,来自不同含水层的演化途径表现出了完全不同的水化学过程,这是研究区缺乏断层,相邻含水层之间的水力联系相对较差所导致。
大海则井田多层含水层系统水化学特征及演化规律
Hydrochemical characteristics and evolution of groundwater in multi-layer aquifer system in the Dahaize Coalfield
-
摘要: 研究多层含水层系统地下水化学特征及演化规律对于矿区水资源保护和突水灾害防治具有重要意义。本文运用Piper图解法、离子比值法和反向地球化学模拟研究了我国西北地区大海则井田多层含水层的水化学过程和水质特征。结果表明,不同含水层地下水的水化学类型差异明显,主要表现为浅层地下水水呈以HCO3型为主的淡水,而深层地下水呈以SO4型为主的微咸水和咸水。其中,矿井水和直罗组地下水的水化学类型接近,说明直罗组地下水是矿井水的主要水源,也是潜在的突水水源。此外,通过离子关系和水化学模拟得出,不同矿物的溶解和沉淀以及阳离子交换作用是导致不同含水层地下水水化学类型差异性特征的主要因素。Abstract: It is of great significance for water resources protection and water inrush prevention to study hydrochemical characteristics and evolution of coalfield groundwater in multi-layer aquifer system. The groundwater in Dahaize Coalfield in Northwest China was stored in typical multi-layer aquifers. To get better understanding of its hydrochemical characteristics, hydrogeological field investigations and tests were carried out in the Coalfield. The Piper diagram, ion ratios and inverse geochemical simulation were applied to identify the hydrochemical process and water quality in the multi-layer aquifer. The results showed that the hydrochemical types of the groundwater in the different layers of the aquifer system were significant different. The groundwater in the shallow layers was fresh water with a dominated hydrochemical type of HCO3. However, it was brackish or salty water in the deep layers with a dominated hydrochemical type of SO4. Meanwhile, the hydrochemical type of the mine water was approximately the same with that of the groundwater in Zhiluo group. This indicated that the groundwater in Zhiluo group could be the main source of the mine drainage and water inrush of the Coalfield. As a result of this, together with the ion relationships and hydrochemical simulation results, the dissolution and precipitation of the minerals and cation exchange could be the main factors that influenced the Coalfield hydrochemical types of the groundwater in the different layers of the aquifer system.
-
Key words:
- multi-layer aquifer /
- hydrochemical evolution /
- inverse modeling /
- Dahaize Coalfield
-

-
表 1 研究区水化学数据
Table 1. Hydrochemical data from the study area
编号
ID浓度(mg·L−1) 平衡
误差/%
Balance
errorTDS/
(mg·L−1)水温/℃
TemperaturepH SiO2/
(mg·L−1)总铁/
(mg·L−1)
Total
FeSI Na++K+ Ca2+ Mg2+ Cl– HCO3– SO42– 方解石
Calcite白云石
Dolomite石膏
GypsumQ1 26.4 61.10 4.30 5.30 195.3 57.60 0.29 252.35 14 7.5 10.7 <0.08 –0.04 –1.04 –1.83 Q2 14.5 44.04 12.6 6.04 199.2 20.37 0.45 197.15 13 7.47 7.2 0 –0.2 –0.76 –2.39 LH1 6.81 54.65 8.8 4.92 168.13 23.46 10.58 182.71 14 7.81 10. 6 0.17 0.18 –0.24 –2.24 LH2 18.54 33.95 5.2 6.89 133.98 21 2.76 152.57 15 8.2 10. 6 0.06 0.29 –0.02 –2.44 LH3 47.8 36.1 5.5 8.9 158.6 64.8 5.72 242.4 10 7.7 14.3 <0.08 –0.21 –1.12 –1.97 LH4 44.68 19.95 8.28 14.27 153.51 28.81 3.04 192.75 18 7.7 5 0.1 –0.34 –0.8 –2.55 AD1 211.78 65.41 6 26.02 83.52 513.15 1.07 864.12 13 8.57 14.08 0.18 0.46 0.07 –1.08 AD2 98.81 7.88 9 20.82 164.67 87.26 5.50 306.11 14 8.73 10.6 0.8 0.18 0.62 –2.53 AD3 17.5 53.1 12.2 12.4 210.5 28.8 3.96 229.25 16 7.8 5.4 0.4 0.27 0.12 –2.19 ZL1 539.27 405.5 22 36.43 66.12 2095.57 –0.63 3131.83 16 7.63 15.8 0.04 0.03 –0.99 –0.09 ZL2 537.81 226.46 27.24 41.33 110.56 1631.79 1.71 2519.91 19 7.08 18 0.08 –0.46 –1.58 –0.38 ZL3 535.70 457.88 17.00 44.40 87.00 2180.13 –1.25 3278.61 17 7.73 17.6 0.79 –0.32 –1.8 –0.05 ZL4 436.10 153.30 10.9 48.90 112.90 1176.70 –0.71 1882.35 17 7.9 17 <0.08 0.22 –0.46 –0.58 ZL5 371.80 222.40 4.9 44.30 134.20 1164.70 –0.04 1875.2 19 7.8 15.2 0 0.39 –0.61 –0.44 ZL6 574.80 192.40 13.40 42.50 137.30 1537.00 0.63 2428.75 22 8.5 — 0.17 0.98 1.1 –0.47 ZL7 554.00 80.20 7.30 37.20 91.50 1224.80 2.16 1949.25 23 8.77 — 0.06 0.73 0.73 –0.86 ZL8 398.00 116.20 8.50 39.00 143.40 946.20 2.67 1579.6 18 8.58 — <0.08 0.9 0.92 –0.75 ZL9 682.00 468.90 42.60 42.50 88.50 2617.60 –0.98 3897.85 18 8.49 — 0.1 1 1.22 –0.01 YA1 1086.17 473.24 40.80 42.80 71.34 3442.00 –0.09 5120.68 15 8.52 7.04 0.18 0.84 0.84 0.04 YA2 556.71 435.11 18.8 35.42 55.68 2190.12 –7.29 3264 17 7.7 10.6 0.8 0.05 –1.01 –0.06 YA3 215.94 98.78 8.91 26.67 106.14 605.88 –0.94 1009.25 15 8.18 10.6 0.4 0.37 –0.09 –0.89 YA4 112.6 29.1 6.1 20.2 131.2 194.5 1.29 428.1 13 7.8 4.8 0.04 –0.32 –1.14 –1.68 M1 1368.17 304.8 42.65 161.5 119.6 3436.9 3.18 5373.82 18 7.78 — 0.08 0.21 –0.17 –0.16 M2 1734.4 110.2 31.6 241.1 244.1 3472.6 0.52 5711.95 18 8.22 11.8 0.79 0.5 0.71 –0.6 表 2 不同含水层水化学组分统计(mg·L−1)
Table 2. Statistics of hydrochemical compositions in different aquifers (mg·L−1)
含水层
Aquifer统计量
StatisticsNa++K+ Ca2+ Mg2+ Cl− HCO3− SO42− TDS 第四系
Quaternary最大值 26.40 61.10 12.60 6.04 199.20 57.60 263.34 最小值 14.50 44.04 4.30 5.30 195.30 20.37 186.16 第四系
Quaternary平均值 20.45 52.57 8.45 5.67 197.25 38.99 224.75 标准差 5.95 8.53 4.15 0.37 1.95 18.62 38.59 洛河组
Luohe
group最大值 47.80 54.65 8.80 14.27 168.13 64.80 242.40 最小值 6.81 19.95 5.20 4.92 133.98 21.00 152.57 平均值 29.46 36.16 6.95 8.75 153.56 34.52 192.61 标准差 17.32 12.34 1.61 3.49 12.46 17.71 32.33 安定组
Anding
group最大值 211.78 65.41 12.20 26.02 210.50 513.15 864.12 最小值 17.50 7.88 6.00 12.40 83.52 28.80 229.25 平均值 109.36 42.13 9.07 19.75 153.63 209.74 466.86 标准差 79.66 24.73 2.53 5.61 52.68 215.87 282.70 直罗组
Zhiluo group最大值 682.00 468.90 42.60 48.90 143.40 2617.60 3897.85 最小值 371.80 80.20 4.90 36.43 66.12 946.20 1579.60 平均值 514.39 258.14 17.09 41.32 107.94 1619.39 2504.29 标准差 91.25 139.62 11.29 3.71 25.11 532.93 736.86
延安组
Yan’an group最大值 1086.17 473.24 40.80 42.80 131.20 3442.00 5120.68 最小值 112.60 29.10 6.10 20.20 55.68 194.50 428.10 平均值 492.86 259.06 18.65 31.27 91.09 1608.13 2455.51 标准差 379.93 197.13 13.63 8.57 29.49 1294.67 1868.08
矿井水
Mine water最大值 1734.40 304.80 42.65 241.10 244.10 3472.60 5711.95 最小值 1368.17 110.20 31.60 161.50 119.60 3436.90 5373.82 平均值 1551.29 207.50 37.13 201.30 181.85 3454.75 5542.89 标准差 183.12 97.30 5.53 39.80 62.25 17.85 169.07 表 3 不同演化路径的主要矿物饱和指数
Table 3. Main mineral saturation index in different evolution paths
样品编号Sample ID 路径ⅠPath I 路径II Path II 路径III Path III ZL5 ZL3 LH2 LH4 AD2 AD1 SI(石膏Gypsum) −0.44 −0.05 −2.44 −2.55 −2.53 −1.08 SI(方解石Calcite) 0.39 0.32 0.29 0.34 0.18 0.46 SI(白云石Dolomite) −0.61 −1.8 −0.02 −0.8 0.62 0.07 SI(岩盐Halite) −6.43 −6.31 −8.42 −7.74 −7.23 −6.84 SI(CO2) −2.77 −2.25 −3.14 −2.56 −3.6 −3.77 SI(钠长石Albite) −0.68 −0.28 −2.69 −2.79 −2.78 −1.33 表 4 矿物转移量(mmol·L−1)
Table 4. Mineral transfer amount (mmol·L−1)
路径Path ZL5→ZL3 LH2→LH4 AD2→AD1 白云石Dolomite 0.48 0.13 –0.12 方解石Calcite –1.79 –0.09 –1.08 石膏Gypsum 10.62 0.08 4.44 CO2 0.06 0.15 — 岩盐Halite 0.005 0.2 0.13 钠长石Albite 0.26 — 1.18 阳离子交换Cation exchange 6.86 0.94 3.6 注:正值表示矿物发生溶解,进入地下水;负值表示矿物在地下水中沉淀析出离开地下水.
Note: positive value indicate mineral dissolution and enter groundwater; negative value indicate mineral precipitation and leave groundwater. -
[1] 柳凤霞, 史紫薇, 钱会, 等. 银川地区地下水水化学特征演化规律及水质评价 [J]. 环境化学, 2019, 38(9): 2055-2066. doi: 10.7524/j.issn.0254-6108.2019043003 LIU F X, SHI Z W, QIAN H, et al. Evolution of groundwater hydrochemical characteristics and water quality evaluation in Yinchuan area [J]. Environmental Chemistry, 2019, 38(9): 2055-2066(in Chinese). doi: 10.7524/j.issn.0254-6108.2019043003
[2] LIU P, HOTH N, DREBENSTEDT C, et al. Hydro-geochemical paths of multi-layer groundwater system in coal mining regions—Using multivariate statistics and geochemical modeling approaches [J]. Science of the Total Environment, 2017, 601/602: 1-14. doi: 10.1016/j.scitotenv.2017.05.146 [3] 吴耀国, 沈照理, 钟佐, 等. 淄博煤矿区矿井水的化学形成及其模拟 [J]. 环境科学学报, 2000, 20(4): 401-405. doi: 10.3321/j.issn:0253-2468.2000.04.004 WU Y G, SHEN Z L, ZHONG Z S, et al. Chemical origin of mine drainage and its simulation for Zibo coal mining district [J]. Acta Scientiae Circumstantiae, 2000, 20(4): 401-405(in Chinese). doi: 10.3321/j.issn:0253-2468.2000.04.004
[4] QIAO X J, LI G M, LI M, et al. Influence of coal mining on regional Karst groundwater system: A case study in West Mountain area of Taiyuan City, Northern China [J]. Environmental Earth Sciences, 2011, 64(6): 1525-1535. doi: 10.1007/s12665-010-0586-3 [5] SINGH A K, MAHATO M K, NEOGI B, et al. Hydrogeochemistry, elemental flux, and quality assessment of mine water in the pootkee-balihari mining area, jharia coalfield, India [J]. Mine Water and the Environment, 2011, 30(3): 197-207. doi: 10.1007/s10230-011-0143-7 [6] QU S, WANG G C, SHI Z M, et al. Using stable isotopes (δD, δ18O, δ34S and 87Sr/86Sr) to identify sources of water in abandoned mines in the Fengfeng coal mining district, Northern China [J]. Hydrogeology Journal, 2018, 26(5): 1443-1453. doi: 10.1007/s10040-018-1803-5 [7] 孙芳强, 侯光才, 窦妍, 等. 鄂尔多斯盆地白垩系地下水循环特征的水化学证据: 以查布水源地为例 [J]. 吉林大学学报(地球科学版), 2009, 39(2): 269-275,293. SUN F Q, HOU G C, DOU Y, et al. Hydrogeochemistry evidence of groundwater circulation features in Ordos Cretaceous basin—A case study in chabu well field [J]. Journal of Jilin University (Earth Science Edition), 2009, 39(2): 269-275,293(in Chinese).
[8] 陈晨, 高宗军, 李伟, 等. 泰莱盆地地下水化学特征及其控制因素 [J]. 环境化学, 2019, 38(6): 1339-1347. doi: 10.7524/j.issn.0254-6108.2018090504 CHEN C, GAO Z J, LI W, et al. Characteristics and possible factors of hydrochemistry in the groundwater in Tailai basin [J]. Environmental Chemistry, 2019, 38(6): 1339-1347(in Chinese). doi: 10.7524/j.issn.0254-6108.2018090504
[9] 韩佳君, 周训, 姜长龙, 等. 柴达木盆地西部地下卤水水化学特征及其起源演化 [J]. 现代地质, 2013, 27(6): 1454-1464. doi: 10.3969/j.issn.1000-8527.2013.06.025 HAN J J, ZHOU X, JIANG C L, et al. Hydrochemical characteristics, origin and evolution of the subsurface brines in western Qaidam basin [J]. Geoscience, 2013, 27(6): 1454-1464(in Chinese). doi: 10.3969/j.issn.1000-8527.2013.06.025
[10] 华琨, 李洲, 李志. 黄土区长武塬地下水水化学特征及控制因素分析 [J]. 环境化学, 2020, 39(8): 2065-2073. doi: 10.7524/j.issn.0254-6108.2019052703 HUA K, LI Z, LI Z. The hydrochemical characteristics and controlling factors of groundwater in the Changwu loess tableland [J]. Environmental Chemistry, 2020, 39(8): 2065-2073(in Chinese). doi: 10.7524/j.issn.0254-6108.2019052703
[11] ANDRÉ L, FRANCESCHI M, POUCHAN P, et al. Using geochemical data and modelling to enhance the understanding of groundwater flow in a regional deep aquifer, Aquitaine Basin, south-west of France [J]. Journal of Hydrology, 2005, 305(1/2/3/4): 40-62. [12] 林永生, 裴建国, 杜毓超, 等. 基于多元统计方法的岩溶地下水化学特征及影响因素分析 [J]. 环境化学, 2016, 35(11): 2394-2401. doi: 10.7524/j.issn.0254-6108.2016.11.2016032801 LIN Y S, PEI J G, DU Y C, et al. Hydrochemical characteristics of Karst groundwater and their influencing factors based on multiple statistical analysis [J]. Environmental Chemistry, 2016, 35(11): 2394-2401(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.11.2016032801
[13] SOUMYA B S, SEKHAR M, RIOTTE J, et al. Inverse models to analyze the spatiotemporal variations of chemical weathering fluxes in a granito-gneissic watershed: Mule Hole, South India [J]. Geoderma, 2011, 165(1): 12-24. doi: 10.1016/j.geoderma.2011.06.015 [14] SHARIF M U, DAVIS R K, STEELE K F, et al. Inverse geochemical modeling of groundwater evolution with emphasis on arsenic in the Mississippi River Valley alluvial aquifer, Arkansas (USA) [J]. Journal of Hydrology, 2008, 350(1/2): 41-55. [15] 张丽, 陈永金, 刘加珍, 等. 东平湖水化学特征及成因分析 [J]. 环境化学, 2021, 40(5): 1490-1502. doi: 10.7524/j.issn.0254-6108.2019122502 ZHANG L, CHEN Y J, LIU J Z, et al. Analysis on hydrochemical characteristics and causes of Dongping Lake [J]. Environmental Chemistry, 2021, 40(5): 1490-1502(in Chinese). doi: 10.7524/j.issn.0254-6108.2019122502
[16] GAMMONS C H, BROWN A, POULSON S R, et al. Using stable isotopes (S, O) of sulfate to track local contamination of the Madison Karst aquifer, Montana, from abandoned coal mine drainage [J]. Applied Geochemistry, 2013, 31: 228-238. doi: 10.1016/j.apgeochem.2013.01.008 [17] HAN Y, WANG G C, CRAVOTTA C A III, et al. Hydrogeochemical evolution of Ordovician limestone groundwater in Yanzhou, North China [J]. Hydrological Processes, 2013, 27(16): 2247-2257. doi: 10.1002/hyp.9297 [18] HUANG X J, WANG G C, LIANG X Y, et al. Hydrochemical and stable isotope (δD and δ18O) characteristics of groundwater and hydrogeochemical processes in the ningtiaota coalfield, northwest China [J]. Mine Water and the Environment, 2018, 37(1): 119-136. doi: 10.1007/s10230-017-0477-x [19] LI P Y, WU J H, TIAN R, et al. Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the hongdunzi coal mine, northwest China [J]. Mine Water and the Environment, 2018, 37(2): 222-237. doi: 10.1007/s10230-017-0507-8 [20] 沈照理. 水文地球化学基础[M]. 北京: 地质出版社, 1993. SHEN Z L. Fundamental hydrogeochemistry [M]. Beijing: Geological Publishing House, 1993(in Chinese).
[21] EDMUNDS W M, GUENDOUZ A H, MAMOU A, et al. Groundwater evolution in the continental intercalaire aquifer of southern Algeria and Tunisia: Trace element and isotopic indicators [J]. Applied Geochemistry, 2003, 18(6): 805-822. doi: 10.1016/S0883-2927(02)00189-0 [22] 栾风娇, 周金龙, 贾瑞亮, 等. 新疆巴里坤-伊吾盆地地下水水化学特征及成因 [J]. 环境化学, 2017, 36(2): 380-389. doi: 10.7524/j.issn.0254-6108.2017.02.2016062001 LUAN F J, ZHOU J L, JIA R L, et al. Hydrochemical characteristicsand formation mechanism of groundwater in plain areas of Barkol-Yiwu Basin, Xinjiang [J]. Environmental Chemistry, 2017, 36(2): 380-389(in Chinese). doi: 10.7524/j.issn.0254-6108.2017.02.2016062001
[23] 马庆伟, 杨晨光. 陕北地区砂岩的技术指标特性 [J]. 筑路机械与施工机械化, 2019, 36(2): 83-86. doi: 10.3969/j.issn.1000-033X.2019.02.015 MA Q W, YANG C G. Technical characteristics of sandstone in northern Shaanxi [J]. Road Machinery & Construction Mechanization, 2019, 36(2): 83-86(in Chinese). doi: 10.3969/j.issn.1000-033X.2019.02.015
[24] QU S, SHI Z M, LIANG X Y, et al. Multiple factors control groundwater chemistry and quality of multi-layer groundwater system in Northwest China coalfield—Using self-organizing maps (SOM) [J]. Journal of Geochemical Exploration, 2021, 227: 106795. doi: 10.1016/j.gexplo.2021.106795 [25] 刘瑞平, 徐友宁, 亢文婷. 基于phreeqci和netpath联合反演水文地球化学过程: 以小秦岭太峪水库为例 [J]. 西北地质, 2019, 52(1): 239-243. LIU R P, XU Y N, KANG W T. Based on phreeqci and netpath joint inversion hydrology geochemistry process: Example from the Xiaoqinling Tianyu reservoir [J]. Northwestern Geology, 2019, 52(1): 239-243(in Chinese).
[26] GASTMANS D, HUTCHEON I, MENEGÁRIO A A, et al. Geochemical evolution of groundwater in a basaltic aquifer based on chemical and stable isotopic data: Case study from the Northeastern portion of Serra Geral Aquifer, São Paulo state (Brazil) [J]. Journal of Hydrology, 2016, 535: 598-611. doi: 10.1016/j.jhydrol.2016.02.016 -



 下载:
下载: