-
作为一种新型的光催化材料,卤氧化铋(BiOX, X = Cl, Br, I)具有安全、无毒、稳定、高效等优点[1]。然而,由于BiOCl较大的禁带宽度(Eg=3.20—3.50 eV),仅对太阳光中的紫外光具有响应[2],BiOBr和BiOI虽然能够吸收可见光,但由于光生电子-空穴对的复合率高,其光催化活性不足以应对实际的环境污染[3-4]。BiOX的[Bi2O2]2+层与双卤离子层嵌套结构[1]可以形成BiOXxY1−x (X, Y = Cl, Br, I),如BiOBrxCl1−x[5]、BiOClxI1−x[6]、BiOBrxI1−x[7]。且研究发现,BiOXxY1−x固溶体的光催化活性普遍高于纯BiOX或BiOY。Yang等[1]通过乙二醇辅助水热法制备了花状BiOBrxCl1−x,发现BiOBr0.5Cl0.5光催化活性最高,结果显示50 mg BiOBr0.5Cl0.5在可见光照射50 min后完全降解50 mL 20 mg·L−1甲基橙溶液。因为BiOCl和BiOBr在水溶液中的热稳定性和化学稳定性比BiOI高,溶解度比BiOI低[8-9],所以本文选用BiOBrxCl1−x作为光催化剂。虽然BiOBrxCl1−x已成为可见光光催化剂的重要组成部分,但仍有必要通过增加反应活性位点、减少电子空穴复合率、增加可见光吸收率等手段提高其光催化效率。
近年来,为了提高Bi系材料的光催化效率,研究人员采用了很多研究方法。如调整结构或形态[10]、异质结的构建[11]、掺杂[12]等。其中,碳基材料是多相催化中具有前景的载体材料。生物炭(BC)具有大量的孔隙结构,因此其比表面积非常大,可为光催化剂提供负载位点,同时可以吸附目标污染物,使光催化产生的自由基与目标污染物充分接触[13]。生物炭的导电性能可以促进电子的分离和迁移,将低成本的生物炭与铋基光催化剂复合可以减少光生电子与空穴的复合率[14]。因此,光生电子的寿命延长,光催化效率得到提高。且生物炭可以由废弃生物资源制得,为农业废物的资源化提供了一条途径。在现有研究中,生物炭通常作为载体,很少有研究将其作为掺杂材料,Ji等[15]制备了负载苝二酰亚胺的生物炭二元光催化剂(PDI/BC, PB),其中PB-9 (PDI:BC = 1:9, W/W)在可见光照射2 h下可100%降解10 mg·L−1碘海醇,研究结果显示掺杂少量BC的二元光催化剂具有更高的光催化活性。
本文制备了不同Br、Cl比例的BiOBrxCl1−x,将竹叶生物炭掺入光催化效率最佳比例的BiOBr0.2Cl0.8中,并探究光催化活性最佳的复合材料。采用各种表征手段对制备的复合催化剂的形貌、结构及性质进行表征。在复合光催化剂可见光下降解罗丹明B溶液(RhB)的过程中设置不同催化剂投加量和反应pH,探究复合光催化剂的光催化性能,并探讨了生物炭/BiOBrxCl1−x在可见光下催化降解RhB的机理。
-
试剂:竹叶,购于河南南阳桐柏(山竹叶);硝酸铋(Bi(NO3)3·5H2O)、浓硝酸(HNO3)、溴化铵(NH4Br)、氯化铵(NH4Cl)、氯化锌(ZnCl2)均为分析纯,购于国药集团化学试剂有限公司;RhB,分析纯,购于天津市化学试剂研究所有限公司;实验用水为去离子水。
-
用去离子水洗去竹叶表面粉尘,放入烘箱60 ℃干燥后,使用粉碎机制成竹叶粉,过100目筛。称取8.00 g的竹叶粉溶解于350 mL 1 mol·L−1的ZnCl2溶液中,搅拌1 h后抽滤洗涤,放入60 ℃烘箱干燥。用研钵研磨均匀,将研磨后的粉末转移至50 mL的烧杯中,随后加入10 mL去离子水与8.00 g NaOH进行活化处理,密封保存60 ℃烘箱干燥。将产物平整的填进瓷舟并置于石英管式炉中实施裂解反应,其始末温度为30 ℃至750 ℃,上升速率为5 ℃·min−1,持续时间为2 h。之后将裂解的黑色粉末溶解于300 mL 1 mol·L−1的稀盐酸中搅拌2 h,水洗至中性,60 ℃干燥即可制得生物炭(BC)。
-
BiOBrxCl1-x的制备:将0.02 mol硝酸铋[Bi(NO)3)3·5H2O]和2 mL浓硝酸溶解在20 mL去离子水中,搅拌获得透明液体备用。另将0.02 mol卤铵(n(NH4Br)/n(总)=0、0.2、0.4、0.6、0.8、1.0)溶于20 mL去离子水中。迅速将两种溶液混合,搅拌2 h后,将获取的悬浮液5000 r·min−1离心,收集白色沉淀,用去离子水冲洗3次,在60 ℃空气中干燥,然后在300 ℃空气中煅烧1 h,获得BiOBrxCl1-x (x=0、0.2、0.4、0.6、0.8、1.0)。
生物炭掺杂BiOBrxCl1-x的制备:选取可见光降解效果效果最好的BiOBrxCl1-x (x=0.2的时候为最佳比例)与竹叶生物炭复合,具体步骤为:在制备BiOBr0.2Cl0.8的搅拌过程中分别加入质量为10、25、50、100、200 mg生物炭(分别标记为CBi-1、CBi-2、CBi-3、CBi-4、CBi-5)。
-
利用场发射扫描电子显微镜(SEM,日本JSM-7401F,JEOL Ltd.)对光催化剂的形貌进行表征;通过X射线衍射仪(XRD,日本D/max 2500/PC)对光催化剂的晶相结构进行表征;使用傅里叶变换红外光谱仪(FT-IR,美国Thermo,Nicolet IS5)对光催化剂的官能团进行分析;利用紫外-可见漫反射光谱仪(UV-Vis DRS,美国Varian,Cary 5000)确定光催化剂的光吸收范围;自由基信号由电子顺磁共振仪(EPR,德国Bruker BioSpin Corp.,EMX-8)捕获。
-
光催化剂的催化活性由可见光下降解RhB性能来评估。将25 mg光催化剂加入10 mg·L−1的RhB(50 mL)溶液中,在黑暗条件下反应30 min以达到吸附-脱附平衡,随后打开350 W氙灯进行可见光催化降解,加截留波长为420 nm的滤光片。每隔20 min取3 mL样品,用0.45 μm聚四氟乙烯滤头过滤以实现催化剂与溶液的分离。使用紫外分光光度计(UV759,上海奥普勒仪器有限公司)在552 nm下对滤后溶液进行吸光度测试。使用紫外可见分光光度计(全谱)(UV-5500pc,Metash)在200—800 nm下对滤后溶液进行吸光度测试。使用总有机碳分析仪(Multi N/C 3100,德国analytikjena)对滤后溶液进行总有机碳(TOC)测定。
RhB的去除率和TOC去除率计算如式(1)所示:
其中,C0(mg·L−1)为污染物的初始浓度,Ct(mg·L−1)反应t时间后取样的污染物浓度。
-
采用SEM对样品的表面形貌进行表征,图1a、1b和1c分别为BC、BiOBr0.2Cl0.8和CBi-4的SEM图。从图1a可以看出生物炭呈现不规则絮状结构,具有清晰的孔隙特征。如图1b所示,光催化剂BiOBr0.2Cl0.8呈现花状结构,直径约为3 μm,与文献报道一致[16]。如图1c所示,在CBi-4复合材料中,花状BiOBr0.2Cl0.8中嵌入了絮状生物炭,证明BC与BiOBr0.2Cl0.8复合成功。生物炭的掺入构建了电子传输通道,促进电子从BiOBr0.2Cl0.8到BC上的迁移,有利于阻碍BiOBr0.2Cl0.8中电子-空穴对的复合。
通过X射线衍射仪(XRD)对CBi-4、BiOBr0.2Cl0.8和BC的晶相结构进行表征,结果如图2a所示。与85-0861的标准卡号(001)、(101)、(110)、(102)、(112)、(200)、(211)和(212)相比较,CBi衍射峰在2θ为11.72°、25.80°、32.52°、33.18°、40.62°、46.66°、54.08°、58.42°与BiOCl特征峰相吻合。但难以观察到与BiOBr标准卡片(JCPDS No.09-0393)相对应的特征峰。这是因为制备的BiOBr0.2Cl0.8晶体并不是BiOCl和BiOBr相的混合物,它们是通过Cl和Br原子相互晶格取代形成的固溶体,且BiOBr0.2Cl0.8中Br的含量远少于Cl,因此衍射峰与BiOCl标准卡片更接近。由于生物炭是非晶态结构,其衍射峰强度很低,所以CBi-4中没有显示生物炭的衍射峰。通过FT-IR光谱进一步表征官能团。
利用FT-IR光谱探究BC、BiOBr0.2Cl0.8、CBi-4和回收后CBi-4(reCBi-4)的官能团,结果如图2b所示。在3420 cm−1处的峰为O-H的振动峰[17],在4种样品中均被检测出,说明样品表面均含结合水。521 cm−1处的峰属于Bi-O的振动峰[18],在BiOBr0.2Cl0.8、CBi-4和reCBi-4中均被检出。BC、CBi-4和reCBi-4检测出了在1621 cm−1处的C-O振动峰[19]和1090 cm−1处的Si-O反伸缩振动峰[20]。这说明竹叶生物炭含有C-O和Si-O官能团,且表明竹叶生物炭与光催化剂成功复合,重复使用后依然存在于体系中。另外可以看出与BiOBr0.2Cl0.8相比,reCBi-4在1621 cm−1处的C-O振动峰强度有所增强,这是因为reCBi-4表面吸附了RhB,导致C-O含量有所增加。
通过UV-Vis DRS分析BiOBr0.2Cl0.8和CBi-4的光学吸收性和禁带宽度,结果如图2c所示。可以看到单体BiOBr0.2Cl0.8的光吸收边缘在400 nm处,未达到可见光吸收范围。引入生物炭后,CBi-4的特征吸收发生一定程度的红移,光吸收边缘为420 nm,达到可见光吸收范围。根据式(2)计算样品的禁带宽度[20],得到BiOBr0.2Cl0.8、CBi-4的禁带宽度分别为3.10 eV和2.95 eV,表明生物炭的添加可以减小光催化剂的禁带宽度,增加可见光的吸收。
其中,Eg(eV)为半导体材料禁带宽度,λ(nm)为半导体材料吸收波长。
pH对CBi-4的Zeta电位的影响和中性条件下BC、BiOBr0.2Cl0.8和CBi-4的Zeta电位如图2d所示。BC、BiOBr0.2Cl0.8和CBi-4的Zeta电位值分别为-12.23 eV、-16.07 eV和-14.97 eV,均为负值,表明BC、BiOBr0.2Cl0.8和CBi-4呈负电荷。在pH=2时CBi-4体系的Zeta电位值为-0.39 eV,电位绝对值最小,且接近等电点。碱性条件下pH=9时,电位绝对值最大,为-20.66 eV。当pH值在2到9范围内,Zeta电位绝对值随pH增加而增加,这是由于CBi-4表明的H+吸附量不断降低及OH−的吸附量不断增高所致。在酸性条件下,CBi-4粒子间吸引和排斥的作用力较小,静电相互作用力较小,更有利于光催化降解。
-
不同Br、Cl比例的BiOBrxCl1-x对RhB的降解率如图3a所示,其中,RhB的初始浓度均为10 mg·L−1,材料的投加量均为0.5 g·L−1。可以看出BiOBr0.2Cl0.8的光催化性能最佳,在光照100 min后对RhB的降解率达到了86.0%。
纯BiOBr0.2Cl0.8和不同BC掺杂量复合材料对RhB的降解效果如图3b所示,可以看出BiOBr0.2Cl0.8和CBi对RhB均具有吸附效果,BiOBr0.2Cl0.8对RhB的吸附去除率为19.5%。复合材料中CBi-5的吸附效果最好,在30 min对RhB的吸附去除率达到28.7%。由于CBi-5中竹叶炭含量最高,具有更多的吸附位点,所以其吸附容量更大。在CBi体系中,不同竹叶炭掺杂量的CBi光催化效果存在差异。无BC掺杂时,BiOBr0.2Cl0.8对RhB的脱色率为85.0%。CBi-4的光催化活性最佳,100 min时对RhB的去除率达到96.1%。CBi-5、CBi-3、CBi-2和CBi-1对RhB的去除率分别为87.7%、66.9%、68.9%和65.4%。当竹叶生物炭掺杂量过低,对RhB的吸附富集能力有限,反应活性位点较少,且少量的生物炭可能会阻碍催化剂的光吸收,不利于光生载流子的分离。当竹叶生物炭掺杂量过高,无法保证BC的完全掺杂,可能有少量未掺杂BiOBr0.2Cl0.8的BC使得光催化效率降低。因此,后续实验均使用CBi-4作为光催化剂。BiOBr0.2Cl0.8和CBi-4的紫外全扫图如图3c所示,可以看到CBi-4对RhB的去除效率优于BiOBr0.2Cl0.8,且观察到全扫光谱的蓝移,表明RhB降解过程中发生脱乙基[14]。图3d为不同时间段CBi-4对RhB的矿化率,当反应时间为200 min,RhB的矿化率达到59.6%,表明CBi-4能破坏RhB发色基团,有效降解有机碳链和芳香基团并使之矿化。
-
不同投加量的CBi-4光催化剂对RhB的降解效果如图4a所示。其中,在质量浓度为10 mg·L−1的RhB溶液(50 mL)中,CBi-4的投加量分别为5、15、25、45 mg。结果显示,在5 mg到45 mg范围内,RhB的去除率由17.0%增加到99.7%,催化剂的光催化效果随投加量的增加而显著增强。以ln(C0/C)为纵坐标,以时间t为横坐标,对光催化降解过程进行动力学拟合,图4b为动力学拟合曲线,表1为相关参数。可以看到,不同投加量CBi-4对RhB的去除速率遵循准一级动力学方程,且不同投加量对反应速率的影响较大。从5 mg到45 mg,反应速率常数由0.002 min−1(R2=0.96)增加到0.054 min−1(R2=0.94)。随着光催化剂的增加,催化活性位点与吸附富集作用增加,进一步提高活性物种的产量,增强光催化效果,加速快光反应速率。其中,当催化剂投加量为15—25 mg时反应速率增加率最大,考虑到经济成本,本研究选用25 mg作为光催化剂投加量。
-
为探究不同pH条件下CBi-4对RhB光催化降解的影响,实验中以1 mol·L−1的盐酸和1 mol·L−1的氢氧化钠溶液调节溶液pH,使溶液pH分别为3、5、7、9、11,投加25 mg CBi-4降解10 mg·L−1的RhB溶液(50 mL),其降解曲线如图5a所示。在酸性条件下(pH=3,pH=5),光降解100 min时pH=3和pH=5条件下RhB的降解率均达到100%;中性条件下(pH=7)的降解率为91.6%;碱性条件下(pH=9,pH=11)的降解率分别为99.1%和3.9%。以ln(C0/C)为纵坐标,以时间t为横坐标,对光催化降解过程进行动力学拟合,图5b为动力学拟合曲线,表2为相关参数。可以看到,不同投加量CBi-4对RhB的降解速率遵循准一级动力学方程。且酸性条件下的反应速率常数远大于碱性条件下的速率常数,当pH=3时,反应速率常数最大为0.084 min−1;pH=11时,速率常数最小为0,表明强碱抑制催化反应的进行,RhB几乎无法降解。这是由于酸性条件可以促进光生电子和水中H+或O2反应,产生更多的光催化活性自由基(·O2−),提高光催化效率。然而,在碱性条件下,pH越大,离子团负电荷越多,而CBi-4带负电荷,RhB带正电荷,离子团易与RhB结合,较大的静电斥力将减少CBi-4与RhB碰撞的机会,抑制了CBi-4与RhB之间的相互作用[21],并且从吸附数据可以看出,强碱条件下大大抑制了CBi-4与RhB之间的接触。对应上文的pH对CBi-4的Zeta电位的影响可知,酸性条件下CBi-4的电位绝对值较小,且接近等电点。此时CBi-4粒子间吸引和排斥的作用力较小,静电相互作用力较小,更有利于光催化降解。
-
为探究CBi-4光催化剂的重复使用性和稳定性,在CBi-4光催化降解10 mg·L−1RhB后,收集光降解后的溶液,使用抽滤方法回收催化剂,洗净烘干后重复利用。循环利用3次后的罗丹明降解率如图6所示。从图6中可以看出,随着使用次数的增加,RhB的降解率分别为92.9%、79.7%、57.0%。在循环第3次后CBi-4对RhB与第一次相比,效率有所下降。对应上文的FT-IR光谱可知,回收后的CBi在1621 cm−1处的C-O振动峰强度有所增强,说明回收后的CBi表面吸附了RhB。即随着反应次数的增加,CBi吸附位点被大量RhB粒子和其降解产物占用,难以去除,导致吸附富集作用下降。一方面降低了CBi-4的吸附能力,另一方面部分RhB及其降解产物在CBi-4光催化剂上混合,减弱光催化剂的受光面积,进而减弱其催化效果。在后续实验中设置脱附、煅烧等实验,恢复光催化剂的活性。
-
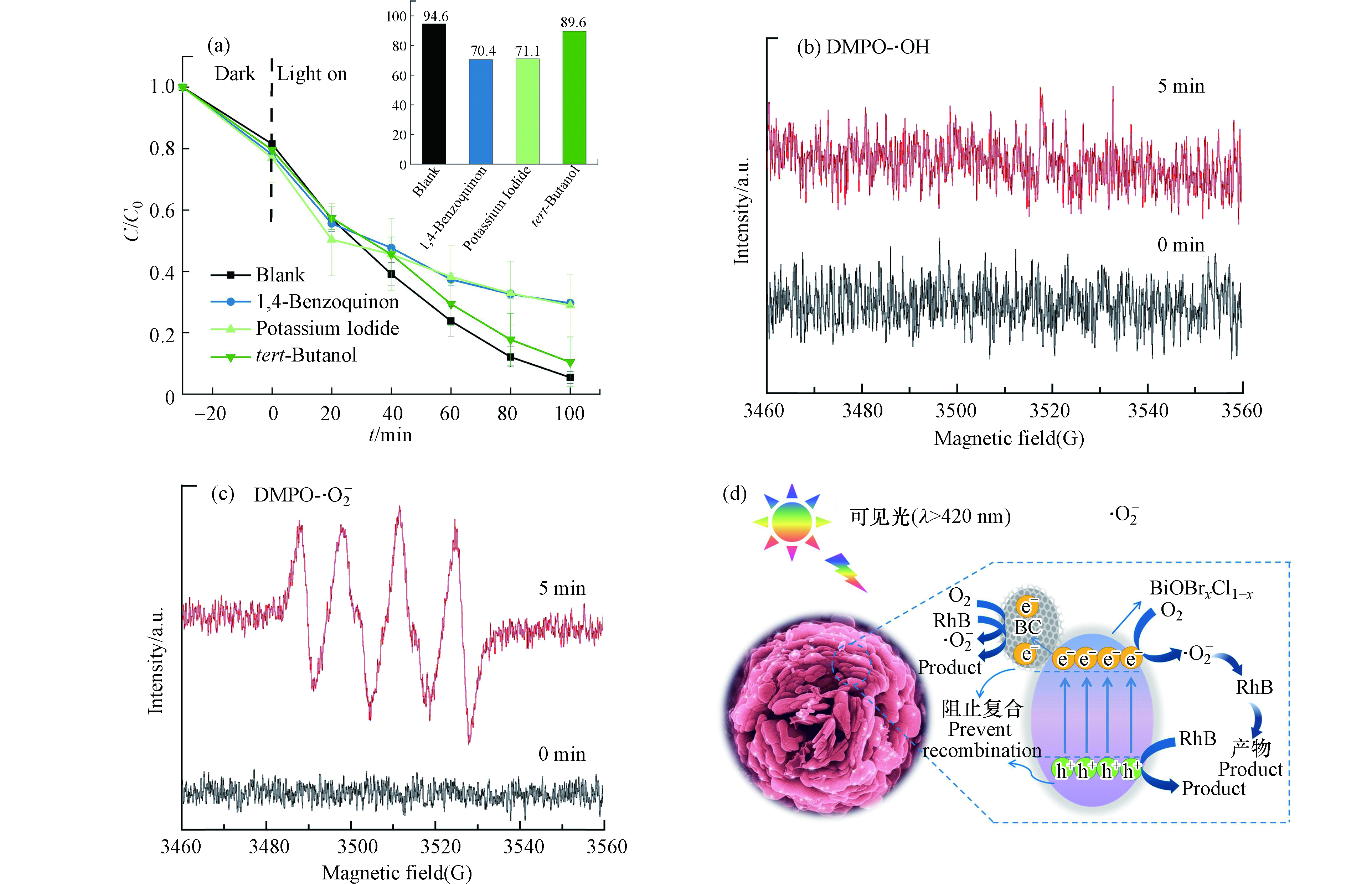
为研究CBi-4光催化降解中起主要降解作用的活性物种,在反应体系(25 mg CBi-4,10 mg·L−1的RhB)中分别加入0.3 mol叔丁醇(tert-Butanol)、0.005 mol对苯醌(1,4-Benzoquinon)和0.015 mol碘化钾(Potassium iodide)作为羟基自由基(·OH)、超氧自由基(·O2−)和空穴(h+)的捕获[15],结果如图7a所示。在不加任何捕获剂的情况下,光反应100 min时RhB的去除率达到94.6%。添加叔丁醇后,去除率下降至89.6%。在添加对苯醌和碘化钾的体系中,RhB的去除率分别下降至70.4%和71.1%。表明在CBi-4光催化降解体系中,·O2− 和h+的贡献较大,·OH的贡献较小,即在降解中起主要作用的是·O2− 和h+。
为证实活性物质的存在,通过EPR捕获了自由基信号。使用5,5-二甲基-1-吡咯啉N-氧化物(DMPO)作为自旋捕获剂分别捕获·OH和·O2−[22-23],结果如图7b-c所示。可见光照射后,DMPO-·OH未出现特征峰,而DMPO-·O2−有明显的加合信号特征峰。因此,在CBi-4光催化体系中不同自由基的贡献程度不同,再次证明·O2−的贡献较大,·OH的贡献较小。
由此可以推测出CBi-4的光催化降解机理(图7d)。在可见光激发下,价带电子跃迁至导带,形成光生电子(e−)和空穴(h+),导带的光生电子还原O2生成·O2−,价带的h+具有强氧化能力氧化RhB,部分光生电子迁移至导体生物炭中,在生物炭上发生还原反应形成·O2−,且生物炭的掺入构建电子传输通道,进一步阻碍了BiOBr0.2Cl0.8光生电子-空穴对的复合,·O2−和h+为主要的活性物种。
-
(1)采用共沉淀法合成一种新型光催化剂BC/BiOBr0.2Cl0.8。生物炭成功嵌入粒径3 μm左右的花状BiOBr0.2Cl0.8微粒中。生物炭掺杂BiOBr0.2Cl0.8后可以增加光催化剂的可见光吸收范围,减小其禁带宽度,明显提高光催化剂活性。
(2)100 mg竹叶生物炭掺杂0.02 mol BiOBr0.2Cl0.8(CBi-4)时光催化效果最佳,光催化100 min时可降解96.1%的10 mg·L−1 RhB溶液,光照120 min后RhB矿化率达到59.6%。CBi-4在重复利用3次之后对RhB的去除率仍可达到57.0%。
(3)CBi-4的光催化效率受到CBi-4投加量、pH影响。随着CBi-4投加量增加,光催化效率逐渐提高。在酸性条件下,CBi-4的光催化效率得到明显提高,强碱环境中光催化活性被明显抑制。CBi-4更适合在酸性条件下降解RhB。
(4)CBi-4降解过程中起主要作用的活性物种为·O2−和h+。生物炭的掺入构建了电子转移通道,阻碍了BiOBr0.2Cl0.8光生电子-空穴复合。
可见光下竹叶生物炭掺杂BiOBrxCl1-x光催化降解罗丹明B
Photocatalytic degradation of rhodamine B by bamboo leaf biochar doped with BiOBrxCl1-x under visible light
-
摘要: 利用共沉淀法合成不同Br、Cl比例的BiOBrxCl1−x,并掺杂不同质量的竹叶生物炭制备复合光催化剂。利用扫描电子显微镜、X射线衍射仪、傅里叶变换红外光谱仪、紫外-可见漫反射和Zeta电位对材料进行表征。结果表明,100 mg竹叶生物炭掺杂BiOBr0.2Cl0.8的光催化效果最佳,光催化100 min可降解96.1%的罗丹明B溶液(RhB, 10 mg·L-1),光照120 min后RhB的矿化率达到59.6%。探究了催化剂投加量和溶液pH等因素对复合光催化剂可见光下降解RhB的影响。自由基捕获实验和电子顺磁共振结果显示,在降解中起主要作用的活性物种为·O2-和h+。生物炭的掺杂能够减小光催化剂的禁带宽度,增加光吸收范围,构建电子传输通道,阻碍光生电子-空穴复合,进而提高光催化效率。
-
关键词:
- BiOBrxCl1-x /
- 竹叶生物炭 /
- 光催化 /
- 罗丹明B
Abstract: BiOBrxCl1−x photocatalysts with different ratios of Br and Cl were synthesized by a coprecipitation method, which were mixed with bamboo leaf biochar with different mass to prepare composite photocatalyst. The materials were characterized by scanning electron microscope, X-ray diffractometer, Fourier transform infrared spectrometer, UV-visible diffuse-reflectance spectra and Zeta potential. The results indicated that 100 mg bamboo leaf biochar doped BiOBr0.2Cl0.8 showed the best photocatalytic performance, which could degrade 96.1% of 10 mg·L-1 rhodamine B (RhB) solution in 100 min, and the mineralization rate of RhB reached 59.6% after 120 min illumination. The effects of catalyst dosage and pH of solution on the degradation of RhB under visible light were investigated. Based on the radical trapping experiment and electron paramagnetic resonance, it can be concluded that the main active species in the degradation process were ·O2- and h+. The doping of biochar can reduce the band gap of photocatalyst, increase the light absorption range, construct an electron transmission channel and hinder the photo-generated electron-hole recombination, then improve the photocatalytic efficiency.-
Key words:
- BiOBrxCl1-x /
- bamboo leaf biochar /
- photocatalysis /
- rhodamine B
-

-
图 3 不同Br、Cl比例的BiOBrxCl1-x对RhB的降解率(a);BiOBr0.2Cl0.8和CBi催化剂对RhB的降解曲线(b); 不同光照时间下BiOBr0.2Cl0.8、CBi-4的紫外全扫图谱(c); 不同时间段CBi-4对RhB的矿化率(d)
Figure 3. Removal rate of RhB by BiOBrxCl1-x with different ratios of Br and Cl(a); Degradation curves of RhB by BiOBr0.2Cl0.8 and CBi catalysts(b); Ultraviolet full scanning spectra of BiOBr0.2Cl0.8 and CBi-4 under different illumination time(c); Mineralization rate of CBi-4 to RhB in different time periods(d)
图 7 CBi-4可见光体系下不同猝灭剂对RhB降解的影响(a);DMPO-·OH(b)和DMPO-·O2-(c)的EPR信号;可见光下CBi-4对RhB的降解机理(d)
Figure 7. Effects of different inhibitors on the degradation of RhB in the visible light system of CBi-4(a); The EPR signals of DMPO-·OH(b)and DMPO-·O2-(c); The degradation mechanism of RhB by CBi-4 under visible light(d)
表 1 不同投加量的CBi-4催化剂对RhB降解的准一级动力学拟合参数
Table 1. Pseudo-first order kinetics fitting parameters of CBi-4 catalyst with different dosage for RhB degradation
投加量
Dosage/mg动力学拟合方程
First-order kinetic equationk/min−1 R2 5 y=0.00151x+0.03989 0.002 0.96 15 y=0.00811x+0.12643 0.008 1.00 25 y=0.02299x+0.16954 0.023 0.98 45 y=0.05432x-0.20754 0.054 0.94 表 2 不同pH下CBi-4催化剂对RhB降解的一级动力学拟合参数
Table 2. First-order kinetic fitting parameters of catalyst degradation of RhB under different pH conditions
pH 动力学拟合方程
First-order kinetic equationk/min−1 R2 3 y=0.08378x+0.1989 0.084 0.95 5 y=0.0691x-0.00715 0.069 0.98 7 y=0.00334x+0.0067 0.003 0.97 9 y=0.02207x+0.10236 0.022 0.97 11 y=0.00034x-0.0066 0.000 0.61 -
[1] YANG Y, ZHANG C, LAI C, et al. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management [J]. Advances in Colloid and Interface Science, 2018, 254: 76-93. doi: 10.1016/j.cis.2018.03.004 [2] PARE B, SARWAN B, JONNALAGADDA S B. Photocatalytic mineralization study of malachite green on the surface of Mn-doped BiOCl activated by visible light under ambient condition [J]. Applied Surface Science, 2011, 258(1): 247-253. doi: 10.1016/j.apsusc.2011.08.040 [3] FU J, TIAN Y L, CHANG B B, et al. BiOBr-carbon nitride heterojunctions: Synthesis, enhanced activity and photocatalytic mechanism [J]. Journal of Materials Chemistry, 2012, 22(39): 21159-21166. doi: 10.1039/c2jm34778d [4] XIAO X, ZHANG W D. Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity [J]. Journal of Materials Chemistry, 2010, 20(28): 5866-5870. doi: 10.1039/c0jm00333f [5] GU Y Y, XIONG Y Q, ZHANG X X, et al. Facile synthesis of hierarchical BiOClxBr1-x solid solution with enhanced photocatalytic activity [J]. Journal of Central South University, 2018, 25(7): 1619-1627. doi: 10.1007/s11771-018-3854-0 [6] XIAN D S, YANG J J, YU X, et al. In situ grown hierarchical 50%BiOCl/BiOI hollow flowerlike microspheres on reduced graphene oxide nanosheets for enhanced visible-light photocatalytic degradation of rhodamine B [J]. Applied Surface Science, 2018, 433: 502-512. doi: 10.1016/j.apsusc.2017.09.258 [7] CAI L, ZHANG G Q, ZHANG Y F, et al. Mediation of band structure for BiOBrxI1–x hierarchical microspheres of multiple defects with enhanced visible-light photocatalytic activity [J]. CrystEngComm, 2018, 20(26): 3647-3656. doi: 10.1039/C8CE00700D [8] ZHANG W D, ZHANG Q, DONG F. Visible-light photocatalytic removal of NO in air over BiOX (X = cl, br, I) single-crystal nanoplates prepared at room temperature [J]. Industrial & Engineering Chemistry Research, 2013, 52(20): 6740-6746. [9] KANDANAPITIYE M S, GAO M, MOLTER J, et al. Synthesis, characterization, and X-ray attenuation properties of ultrasmall BiOI nanoparticles: Toward renal clearable particulate CT contrast agents [J]. Inorganic Chemistry, 2014, 53(19): 10189-10194. doi: 10.1021/ic5011709 [10] YAN X D, ZHAO H M, LI T F, et al. In situ synthesis of BiOCl nanosheets on three-dimensional hierarchical structures for efficient photocatalysis under visible light [J]. Nanoscale, 2019, 11(21): 10203-10208. doi: 10.1039/C9NR02304F [11] LIU F Y, JIANG Y R, CHEN C C, et al. Novel synthesis of PbBiO2Cl/BiOCl nanocomposite with enhanced visible-driven-light photocatalytic activity [J]. Catalysis Today, 2018, 300: 112-123. doi: 10.1016/j.cattod.2017.04.030 [12] AGGARWAL R, SAINI D, SINGH B, et al. Bitter apple peel derived photoactive carbon dots for the sunlight induced photocatalytic degradation of crystal violet dye [J]. Solar Energy, 2020, 197: 326-331. doi: 10.1016/j.solener.2020.01.010 [13] MATOS I, BERNARDO M, FONSECA I. Porous carbon: A versatile material for catalysis [J]. Catalysis Today, 2017, 285: 194-203. doi: 10.1016/j.cattod.2017.01.039 [14] LI M, HUANG H W, YU S X, et al. Simultaneously promoting charge separation and photoabsorption of BiOX (X = Cl, Br) for efficient visible-light photocatalysis and photosensitization by compositing low-cost biochar [J]. Applied Surface Science, 2016, 386: 285-295. doi: 10.1016/j.apsusc.2016.05.171 [15] JI Q Y, CHENG X Y, SUN D Y, et al. Persulfate enhanced visible light photocatalytic degradation of iohexol by surface-loaded perylene diimide/acidified biochar [J]. Chemical Engineering Journal, 2021, 414: 128793. doi: 10.1016/j.cej.2021.128793 [16] NA Y, KIM Y I, WON CHO D, et al. Adsorption/photocatalytic performances of hierarchical flowerlike BiOBrxCl1−x nanostructures for methyl orange, Rhodamine B and methylene blue [J]. Materials Science in Semiconductor Processing, 2014, 27: 181-190. doi: 10.1016/j.mssp.2014.06.043 [17] ZHU N Y, YAN T M, QIAO J, et al. Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: Adsorption mechanism and depleted adsorbent utilization [J]. Chemosphere, 2016, 164: 32-40. doi: 10.1016/j.chemosphere.2016.08.036 [18] BERA K K, MAJUMDAR R, CHAKRABORTY M, et al. Phase control synthesis of α, β and α/β Bi2O3 hetero-junction with enhanced and synergistic photocatalytic activity on degradation of toxic dye, Rhodamine-B under natural sunlight [J]. Journal of Hazardous Materials, 2018, 352: 182-191. doi: 10.1016/j.jhazmat.2018.03.029 [19] AHMED I, JHUNG S H. Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions [J]. Chemical Engineering Journal, 2017, 310: 197-215. doi: 10.1016/j.cej.2016.10.115 [20] 卢涛, 刘国, 李春雪, 等. BiOCl/SiO2/Fe3O4复合材料可见光催化去除水中亚甲基蓝 [J]. 环境科学学报, 2019, 39(2): 352-358. LU T, LIU G, LI C X, et al. Photocatalytic degradation of methylene blue in water by BiOCl/SiO2/Fe3O4 composites [J]. Acta Scientiae Circumstantiae, 2019, 39(2): 352-358(in Chinese).
[21] 盛寒祯, 尤宏, 柳锋, 等. 可见光驱动下氧掺杂氮化碳活化过硫酸盐降解罗丹明B [J]. 环境科学学报, 2020, 40(8): 2708-2714. doi: 10.13671/j.hjkxxb.2020.0143 SHENG H Z, YOU H, LIU F, et al. Degradation of Rhodamine B by persulfate activated by oxygen-doped carbon nitride under visible light irradation [J]. Acta Scientiae Circumstantiae, 2020, 40(8): 2708-2714(in Chinese). doi: 10.13671/j.hjkxxb.2020.0143
[22] JIANG Y R, LIN H P, CHUNG W H, et al. Controlled hydrothermal synthesis of BiOxCly/BiOmIn composites exhibiting visible-light photocatalytic degradation of crystal violet [J]. Journal of Hazardous Materials, 2015, 283: 787-805. doi: 10.1016/j.jhazmat.2014.10.025 [23] XIAO X, XING C L, HE G P, et al. Solvothermal synthesis of novel hierarchical Bi4O5I2 nanoflakes with highly visible light photocatalytic performance for the degradation of 4-tert-butylphenol [J]. Applied Catalysis B:Environmental, 2014, 148/149: 154-163. doi: 10.1016/j.apcatb.2013.10.055 -




 下载:
下载: