-
由于船舶压载水导致的外来生物入侵对海洋生态环境构成了巨大威胁[1-3],2004年2月在伦敦国际海事总部通过了《2004年国际船舶压载水和沉积物控制和管理公约》[4]。公约要求处理后压载水中的微生物活体数量应满足D-2排放标准,因此港口国需对压载水中的存活生物进行监督检查,从根源解决外来生物入侵等问题。2020年2月国际海事组织(IMO)污染预防与应急分委会召开的第七次会议中建议无需对指示细菌进行指示性分析,因此微藻的活死判定是港口国检查的一项核心任务[5]。
目前,有一些方法可以判定微藻的死活,如染色法[6]、三磷酸腺苷(ATP)法[7]及叶绿素自发荧光法[8]等。这些方法自身都存在不同的缺点,如染色法使用场景单一,仅能对特定微藻染色,在使用中容易造成检测结果偏低[9];三磷酸腺苷(ATP)仅存在于活生物体中,然而有报道称紫外辐照处理后的微藻ATP含量并没有显著降低[10],并且游离ATP也将导致过高估计检测到的存活微藻数量[11];叶绿素a是一种光合色素,通过测定叶绿素a的荧光强度,可估算出存活微藻数量[12],但在检测中存在微藻死亡但叶绿素仍可发生荧光和其他光合色素的荧光光谱与叶绿素荧光光谱重合等问题。这将使压载水检测可靠性降低,甚至出现误判。
不难看出,上述方法在判定浮游植物活性方面均存在一些不足之处,海洋微生物以及船舶压载水处理系统的多样性也对发展新的检测技术提出了需求。水生微生物表面大都有一些羧基、氨基、羟基和磷酸盐[13],从而会使其表面带负电,并且表面电荷受微生物的种类[14]和生命过程的影响[15]。微生物表面带电特性已广泛用于微藻的浮选[14,16-17]。此外,Martinez等[18]发现蓝藻死亡后,其表面电荷会明显减少。近期,有研究者基于死活微藻表面电荷的差异,实现了模拟电解和UV辐照处理后微藻活性的判定[19]。应当注意的是,微藻表面电荷与其生命活动的过程有关,光照是影响微藻生命活动的一个重要因素,如能获得微藻表面电荷随光照时间的变化规律,则可为基于微藻表面电荷活性判定方法确定最佳的检测窗口。
本文选取了在船舶压载水中常见的等鞭金藻(Isochrysis galbana)和小球藻(Chlorella vulgaris)两种微藻,其中等鞭金藻为金藻门,具有体积小,无细胞壁等特点;小球藻属于绿藻门,是地球上最早的生命之一,在全球范围内分布极广且生命力顽强。微藻在电场下的运动速度可以表征其表面电荷的数量和极性(即活性),因此本文通过在微流控芯片上探究紫外线灭活处理前后,经不同时间白光光照后,微藻在电场下的运动速度,分析了白光光照时间对微藻表面电荷的影响规律。研究结果为基于微藻表面电荷活性判定方法提供了选择合理光照时间的依据,以期为压载水中微藻的指示性分析提供一种新的研究方法。
-
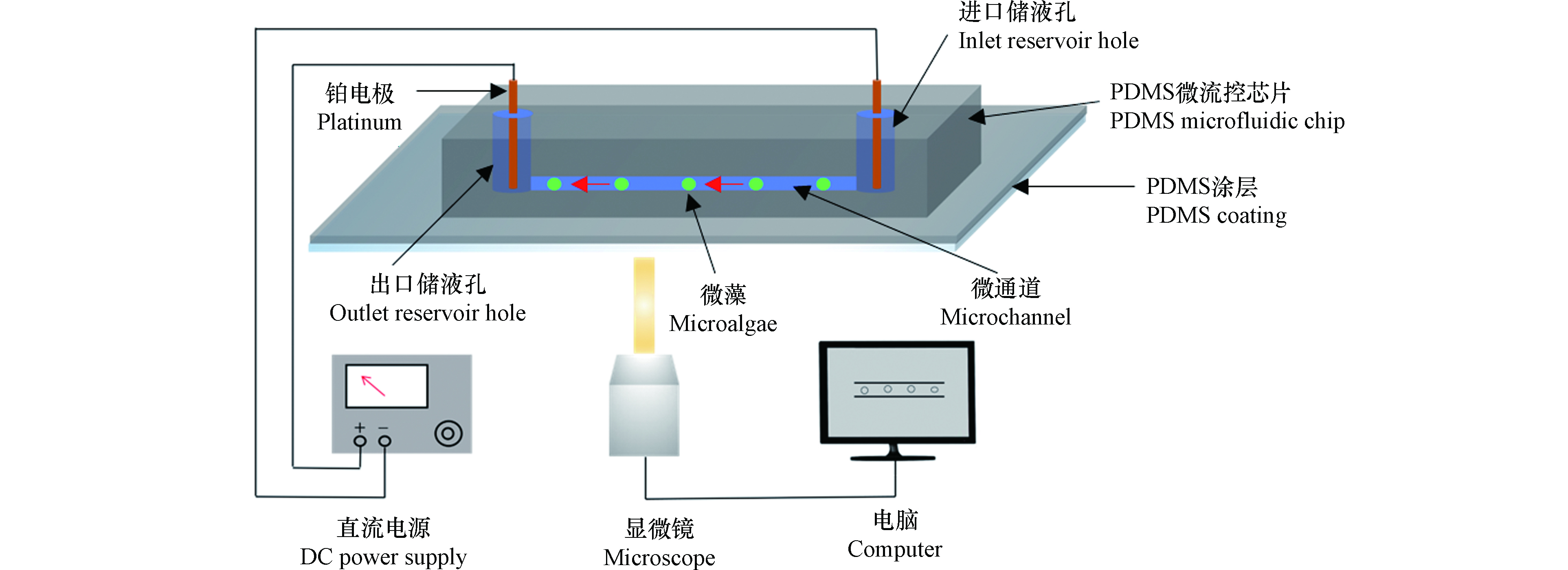
图1示出了微藻电动运动速度测量系统组成,主要由直通道微流控芯片、直流电源和显微成像系统组成。两个电极与直流电源连接,分别插入通道的两端储液孔中。
对于直通道中的微藻,当两端储液孔无液位高度差时,在电场作用下,微藻沿微通道运动是通道溶液的电渗流(VEOF)和微藻自身的电泳(VEP)运动的合运动[20-21],其净速度(V)可表示为:
溶液电渗流和微藻的电泳速度分别由下式给出:
式中,E为电场强度,µ为溶液的黏度,ε0为真空中的介电常数,εr为相对介电常数,ζW和ζP分别为微通道和微藻的zeta电位(与微通道的材质、溶液种类以及微藻表面电荷有关)。
实验过程中使用相同材质的微流控芯片,并且微通道中充满相同的溶液,因此ζW值不变,当外加电场强度相同时,
$ {V}_{{\rm{EOF}}} $ 保持不变。由公式(1)可知,微藻的最终运动速度仅与微藻的zeta电势,即表面电荷有关。一般来说,微藻表面带负电,其zeta电位为负,并且其大小随生存状态的不同而不同[18]。 -
实验所用小球藻和等鞭金藻购自上海光语生物科技有限公司(中国,上海)。先将微藻置于锥形瓶中,暗适应8 h,做同一化处理,记为对照组(0 h)。然后取同一化处理后的微藻140 mL等分为7份置于不同烧杯中,并对其进行不同时间的白光辐照(光强为160 fc)。实验时,取1.5 mL辐照后的微藻于离心管,用离心机(Centrifuger 5424,Eppendorf,GER)以4000 r·min−1转速离心5 min,去除上清液,加入适量PBS缓冲液(pH=7.5)于离心管中,然后再次离心清洗,避免将样品中残留的培养基带入微流控芯片。
为灭活处理微藻,在黑暗条件下使用紫外灯(YG-BX1,Cotton Konight,中国)辐照微藻。然后采用相同的离心处理步骤对灭活后的样品进行浓缩处理,以便用于电动运动实验。缓冲溶液由0.02 g·L−1的Na2CO3(Sigma,USA)、2.0 g·L−1的NaNO3(Sigma,USA)、0.02 g·L−1的KH2PO4(Sigma,USA)和0.8 g·L−1的CH4N2O(Sigma,USA)在pH=6.0的环境下配置。
-
采用软光刻法[22]加工获得硅基芯片模具后,将液态聚二甲基硅氧烷(PDMS)和固化剂按照10:1的质量比混合均匀、抽真空1 h后,倒在模具上,再将模具置于80 ℃烘箱(Isotemp model 280A,Fisher Scientific,Pittsburgh,PA,USA)30 min待PDMS固化后获得带有凹通道的微流控芯片。为在载玻片上涂敷PDMS涂层,首先将载有液态PDMS的载玻片以1000 r·min−1转速旋涂40 s,旋涂完成后置于80 ℃烘箱30 min 固化PDMS。最后将PDMS芯片和载玻片在等离子清洗机(HARRICK PLASMA,Ithaca,NY,USA)内清洗,完成芯片的封接。
-
实验时,首先将10 μL缓冲溶液滴加到微通道(1 cm×100 μm×30 μm)的出口储液孔中,然后将20 μL微藻样品溶液注入进口储液孔。接着用移液枪在样品出口加入适量缓冲溶液,平衡进出口的液位,然后在两个孔中插入铂电极并施加50 V·cm−1的电场。同时,利用倒置显微镜(Ti-E, Nikon)成像系统的CCD摄像机(DS-Qi1Mc, Nikon)记录微藻的电动运动,利用成像系统测量单位微藻在一定时间内的移动距离,从而获得运动速度。重复测量至少20个微藻,然后获得平均的尺寸速度,使用imageJ软件测量微藻的直径。
-
判定处理后微藻的死活是本研究的重要前提,本研究采用荧光素二乙酸酯(FDA)染色法对灭活处理后的微藻活性进行判定,检测不同紫外辐照剂量条件下(0、25、50、100、200、300 、400 mJ·cm−2)微藻的活性。按照参考文献[23]所述的步骤,对微藻样品进行FDA染色处理,染色时,将5 mg FDA粉末(Sigma,USA)溶解于1 mL丙酮(天津大茂化学试剂厂,中国)中制备FDA溶液,在4 ℃下保存。最后使用PBS缓冲溶液重新悬浮,在Ti-E显微镜下使用波长为440 nm的激发光照射,若微藻呈绿色,则表明其仍具有活性,反之则死亡。
-
实验使用SPPS19.0软件对实验数据进行分析。采用单因子方差分析(one way ANOVA)对数据进行比较分析,利用Tukey post hoc表征数据之间的差异性,ANOVA的显著性水平为P<0.05。
-
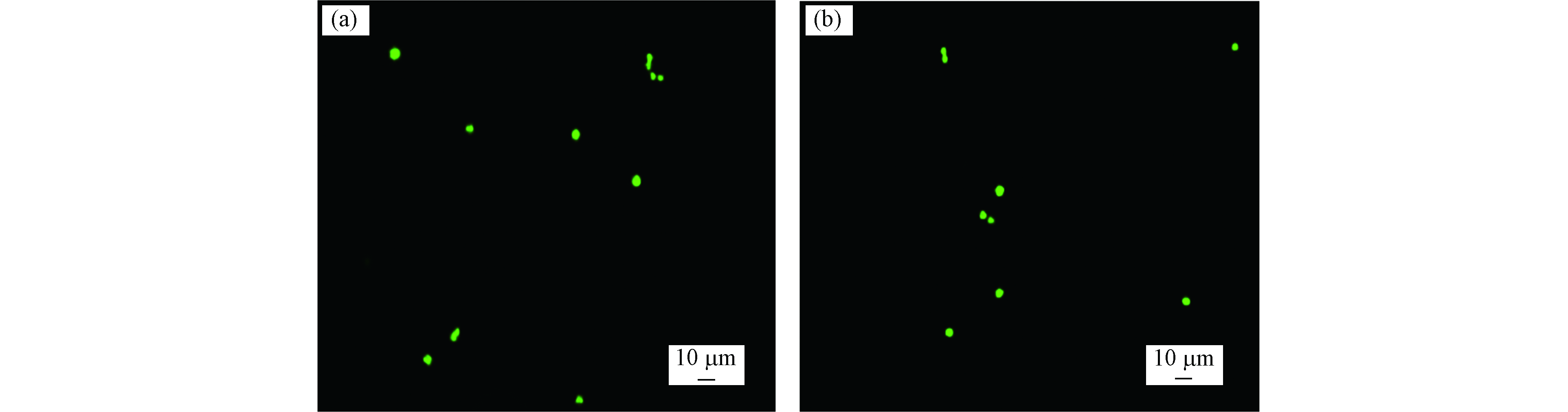
图2示出了5 mJ·m−2紫外线照射250 s后的样品经FDA染色后,在显微镜下的典型观察结果。由图2可以看出,等鞭金藻和小球藻在440 nm激发光照射下显绿色,即辐照后小球藻和等鞭金藻都能被FDA染色,说明这两种微藻没有被辐照致死。据此原理和操作步骤,表1总结了不同剂量紫外线处理后微藻的存活状态。由表1可以看出,当辐照剂量为25 mJ·cm−2时,直径大于4 μm的微藻存活下来;而当辐照剂量增加至50 mJ·cm−2时,小球藻和等鞭金藻均死亡。因此,实验中使用剂量不小于50 mJ·cm−2的紫外线辐照微藻,保证微藻全部死亡。
-
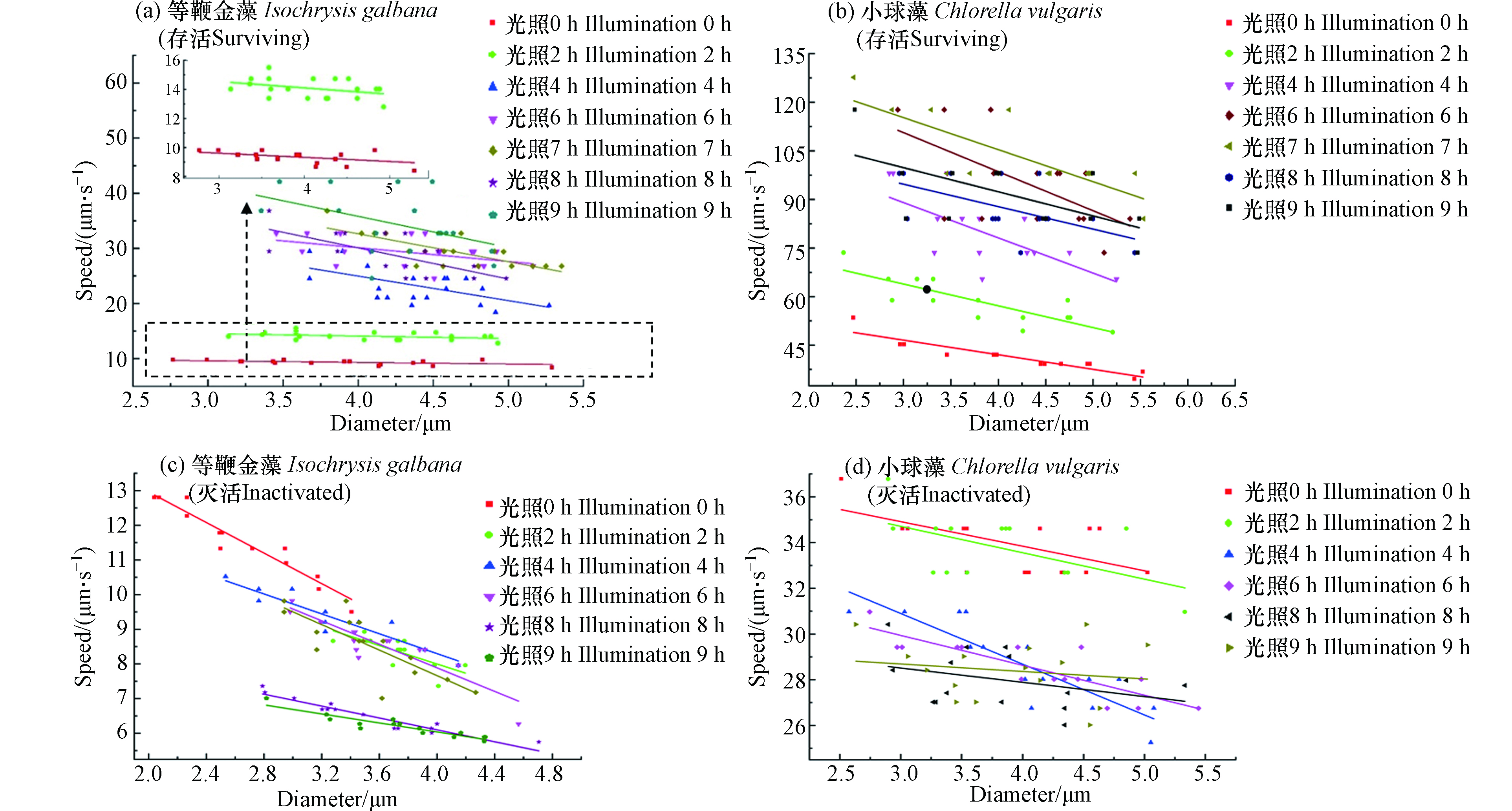
由于同种微藻的尺寸有差异,因此探究微藻的电动运动速度随尺寸的变化显得尤为必要,图3(a,b)示出了白光照射下,存活等鞭金藻和小球藻的电动运动速度随其直径和光照时间的变化关系。由图3(a,b)可以看出:
(1)不同组别微藻的平均运动速度随光照时间的增加而变大。对于等鞭金藻来说,光照0 h组的平均速度为8 μm·s−1,而当光照时间增加至9 h时,其平均速度为35 μm·s−1,平均速度增加了337.5%。对于小球藻,光照0 h组的平均速度为45 μm·s−1,当光照时间增加至6 h时,其平均速度为105 μm·s−1,平均速度增加了133.3%,此外,由表2可知,两种活体微藻的拟合方程中的一次系数的绝对值随着光照时间的增加而增加,在光照6 h内尤其明显(P<0.05)。这表明随着光照时间的增加,微藻的电动速度不断增加,微藻的直径对速度的影响越来越大,这与上面的结论相一致。
(2)相同光照时间条件下,微藻的运动速度随直径的增加而变小。具体来看,等鞭金藻在光照时间为0 h的实验组中,直径2.8 μm的微藻速度为9 μm·s−1,而直径5.3 μm的微藻速度为5 μm·s−1,速度减小了44.4%。在光照时间为9 h的实验组中,直径3.2 μm和4.9 μm的微藻速度分别为42 μm·s−1和28 μm·s−1,速度减小了33.3%。小球藻和等鞭金藻具有相似的变化规律。在光照时间为0 h的实验组中,直径为5.5 μm和直径为2.5 μm的微藻相比,速度减小了30.2%。在光照时间为6 h的实验组中,速度减小了33.1%。
(3)相同直径下,微藻的运动速度随光照时间的增加而变大。以直径为4.2 μm的等鞭金藻为例,光照时间0 h时的速度为7 μm·s−1,光照时间达到9 h时速度增加到37 μm·s−1,速度增加了285.7%。直径为4.0 μm的小球藻在光照时间为0 h时的速度为42 μm·s−1,光照时间达到6 h时速度增加到105 μm·s−1,速度增加了150%。
应当注意的是,个别微藻的运动现象和上述规律不符,如光照时间为9 h的等鞭金藻实验组中,直径为4.8 μm和3.3 μm的微藻电动运动速度相同;直径为3.8 μm的小球藻在光照时间为3 h和4 h时的速度均为65 μm·s−1。这主要是由于实际中微藻作为一个生命体,其生命状态受很多因素影响,个体之间有差异,此外还有可能是由于存在测量误差,但上述少量异常现象不影响实验结论。
图3(c,d)示出了紫外线灭活后等鞭金藻和小球藻的电动运动速度随其直径和光照时间的变化关系(在这里规定高电势方向为微藻运动的正方向)。由图3(c,d)可以看出:
(1)不同光照时间组别的平均运动速度随光照时间的增加而变小。对于等鞭金藻来说,光照0 h组的平均速度为11.5 μm·s−1,光照9 h组的平均速度为6.5 μm·s−1,平均速度减小了43.5%。对于小球藻来说,光照0 h组的平均速度为34 μm·s−1,光照6 h组的平均速度为29 μm·s−1,平均速度减小了14.7%。表2中死亡微藻的速度和直径关系的拟合曲线一次项系数的绝对值随着光照时间的增加而减小,这表明随着死亡时间的增加,微藻的电动速度不断减小,直径对速度的影响不断减小,这与上面的结论是一致的。
(2)相同光照时间条件下,微藻的运动速度随直径的增加而变小。具体来看,等鞭金藻在光照时间为0 h的实验组中,直径2.1 μm的微藻速度为12.7 μm·s−1,而直径3.4 μm的微藻速度为9.5 μm·s−1,速度减小了25.2%。在光照时间为9 h的实验组中,直径2.8 μm和直径4.3 μm的微藻速度分别为7 μm·s−1和5.8 μm·s−1,速度减小了14.1%。小球藻和等鞭金藻具有相似的变化规律。在光照时间为0 h的实验组中,直径为2.5 μm和5.3 μm的微藻相比,速度减小了16.2%。在光照时间为6 h的实验组中,速度减小了12.9%。
(3)相同直径下,微藻的运动速度随光照时间的增加而变小。以直径为3.2 μm的等鞭金藻为例,光照时间0 h时的速度为10.5 μm·s−1,光照时间达到9 h时速度减小到6.5 μm·s−1,速度减小了38.1%。直径为4.0 μm的小球藻在光照时间为0 h时的速度为34 μm·s−1,光照时间达到6 h时速度减小到28 μm·s−1,速度减小了17.6%。
值得注意的是,紫外线灭活后的个别微藻的运动行为也有和上述规律不符的现象。如光照时间为2 h的等鞭金藻实验组中,直径为3.7 μm和直径为4.2 μm的微藻电动运动速度相同;而直径为4.0 μm的小球藻在光照时间为4 h和6 h时的速度均为28 μm·s−1。这主要与微藻灭活后表面结构状态和测量误差有关,个别微藻出现异常行为,符合生物学中个体差异现象。
-
由图3的实验结果可知,微藻的速度存在着尺寸效应,即运动速度随直径的增加而变小。在实际中,处理后压载水中可能含有不同尺寸、不同存活状态的微藻。在同一电场下,尺寸较大的存活微藻的速度和尺寸较小的死亡微藻的速度会相同,从而对微藻活性判定带来较大的误差。由于微藻的运动速度与其直径大小成反比,那么是否可以使用“尺寸速度”(速度乘以直径)来消除上述“尺寸效应”。为验证上述观点,本文探究了微藻尺寸速度和光照时间的关系。
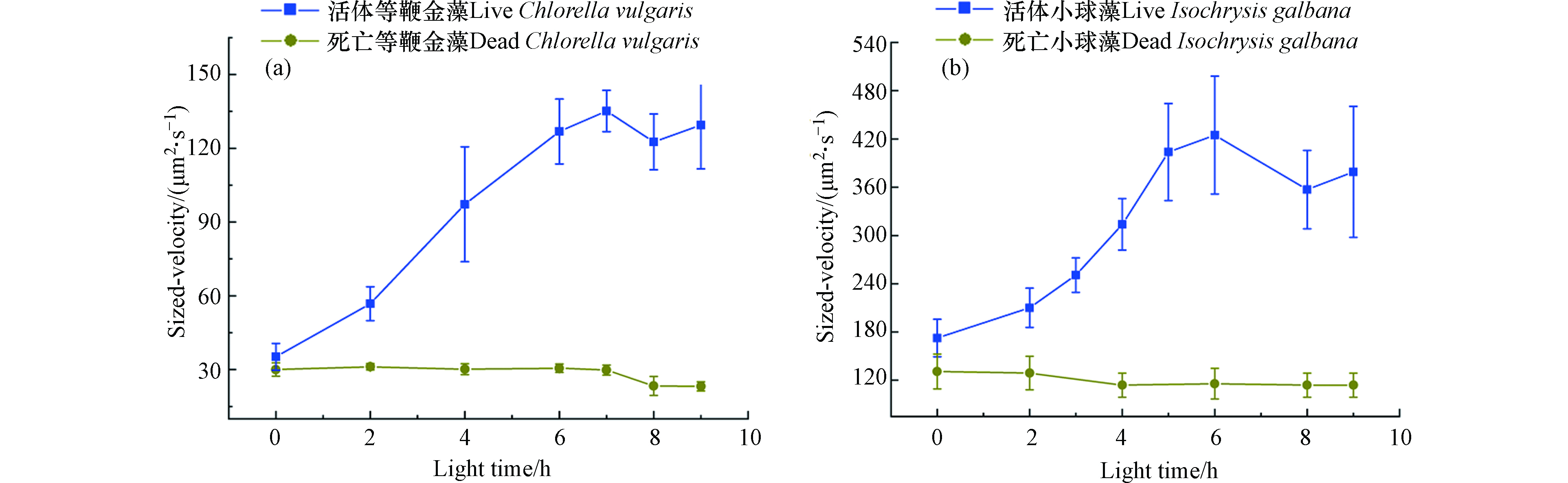
图4示出了等鞭金藻和小球藻的尺寸速度随光照时间的变化关系。图中的每个数据点通过如下步骤获得:首先计算单个微藻的尺寸速度(速度乘以直径),每个实验组中至少测量20个微藻,然后获得平均的尺寸速度。由图4可以看出:
(1)活藻的尺寸速度明显大于死藻的尺寸速度,并且尺寸速度差异随光照时间的增加而变大。由图4(a)可知,活体等鞭金藻最小的尺寸速度为0 h的36 μm2·s−1,灭活等鞭金藻最大的尺寸速度为0 h的29 μm2·s−1;两者的尺寸速度差异随光照时间的增加而增加,在6 h时差异最大。由0 h时的6 μm2·s−1增加到7 h的100 μm2·s−1,增加了1328%。由图4(b)可知,活体小球藻和灭活小球藻的最小及最大尺寸速度分别为175 μm2·s−1和130 μm2·s−1;两者的尺寸速度差异在6 h时达到最大,增加了566.7%。
(2)活藻的尺寸速度在6 h内随光照时间的增加而增加,死藻的尺寸速度随光照时间的增加而减小。由图4(a)可知,活体等鞭金藻的尺寸速度由0 h的36 μm2·s−1增加到6 h的130 μm2·s−1,尺寸速度增加了261.1%;对于死亡等鞭金藻,其尺寸速度在4 h内随光照时间的增加而减小,4 h内由30 μm2·s−1减小到24 μm2·s−1,减小了20%。由图4(b)可知,活体小球藻的尺寸速度最小值和最大值分别为175 μm2·s−1和420 μm2·s−1,增加了140%;死亡小球藻的尺寸速度最小值和最大值分别130 μm2·s−1和110 μm2·s−1,减小了15.4%。
应当注意的是,死藻在微通道中也有一定的向正极运动的速度,这是由于死亡微藻表面仍能吸附溶液中一定的离子,从而带有一定的负电荷。
-
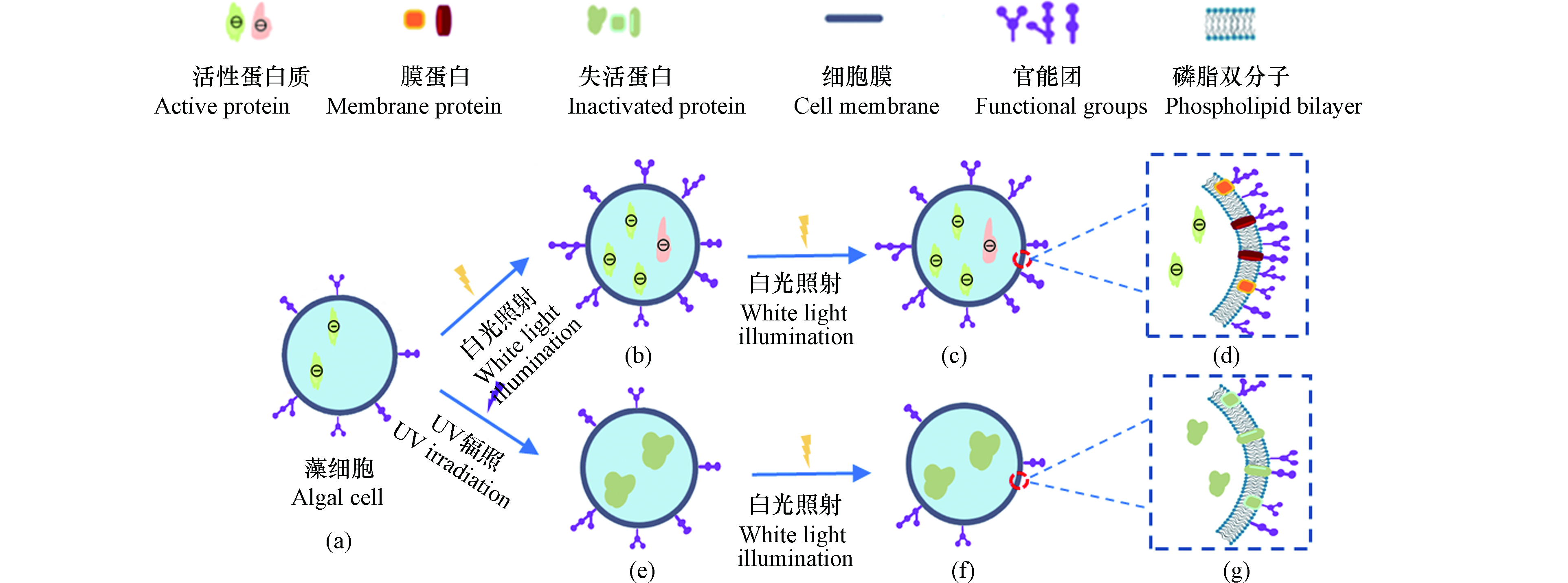
上述实验中微藻的电动运动速度和尺寸速度随光照时间变化的现象是由于微藻表面带电并且带电量与其活性有关造成的,可用图5所示的微藻细胞活性变化模型进行解释。
微藻表面电荷在维持细胞功能方面起着关键作用,其受最外层官能团(如羧基、氨基及羟基)和内部蛋白质的影响。这些官能团均和蛋白质表现为负电性[24]。McConnaughey等[25]研究表明,微藻表面电荷受代谢过程和细胞膜结构特征的控制,通过电荷吸引光合作用所需的离子以及维持细胞功能所需营养物质。郝媛等[26]在分析微藻在不同光照条件下形成的生物膜结构中发现,不同的光源照射微藻,其生物膜的孔隙率和粗糙度有较大的差异。较高的生物膜孔隙率有利于生物膜的渗透性进而促进营养物质在生物膜中的有效扩散,养分扩散的增加促进生物膜的增长和生物量的积累。灭活微藻表面电荷只受最外层官能团结构的影响,不再受代谢活性的影响。Martinez等[18]通过酸碱滴定法测定得到灭活蓝藻的表面OH−比活性蓝藻表面要低约0.2×10−4 mol·L−1。
活性微藻在同一化处理后(图5(a)),因为在较长时间处于黑暗条件下,无法进行光合作用,生命状态不活跃。此时微藻内部的蛋白质和膜外官能团数量较低,微藻表现为较低负电性,微藻的电动运动速度也较小。在经过一段时间光照后(图5(b)),由于光合作用,微藻生命活动变强,此时微藻内部产生更多带负电的蛋白质,膜外官能团也增多,从而使得微藻表面电荷增加,从而使得微藻的电动运动速度随光照时间的增加而变大。继续光照微藻足够长时间后,由于微藻已是最活跃的生命状态,膜内蛋白质(图5(c))和膜外官能团(图5(d))不再增多。此时微藻的表面电荷也趋于稳定,微藻尺寸的运动速度也趋于稳定,不再有明显变化,这与实验结果是吻合的。
微藻经过紫外线灭活后,其生命活动停止,但微藻的细胞壁结构还保持着较完整的状态。此时微藻内部蛋白质(图5(e))在紫外线照射后失去活性[27],但因膜外官能团(图5(g))的存在,微藻仍呈现负电性,因此在电场下仍有正向电动运动速度。随着死亡时间的增加(图5(f)),膜外官能团不断脱落,负电数量随之降低,其运动速度也会随死亡时间的增加而减小。
-
本文主要探究了白光光照时间对微藻电动运动速度的影响。研究结果表明:活藻的电动运动速度在一定光照时间内,随着光照时间的增加而增加,这是因为光照改变了活藻的表面电荷。死藻的电动运动速度随死亡时间的增加不断减小,这是由于死微藻表面活性基团不断脱落导致。活死微藻的尺寸速度差随着光照时间的增加而增加,在光照6 h后基本达到最大值。本研究结果为后续发展基于微藻电动运动速度差异的活性判定技术奠定了重要基础。
白光光照时间对船舶压载水中微藻电动运动速度的影响
The effect of white light illumination on the electrokinetic velocity of algae in ship’s ballast water
-
摘要: 判定处理后船舶压载水中微藻的活性是压载水指示性检查的重要任务,死活微藻的表面电荷量不同,并且可由其电动运动速度来表征。本文选取等鞭金藻和小球藻开展实验,实验研究了紫外线灭活处理前后,经不同时间白光光照后,微藻在微通道中的电动运动速度。实验结果表明,在紫外辐照剂量为50 mJ·cm−2处理下,微藻全部死亡;活死微藻表面均带负电,向电源正极运动。此外,在一定培养时间内,活体微藻的电动运动速度随光照时间的增加而增大,死亡微藻的速度随死亡时间的增加而降低。光照6 h后死活微藻的尺寸速度(速度乘以直径)差最大。本研究利用白光光照使微藻表面带电而产生的电动运动速度来表征微藻的死活情况,为压载水处理后判定微藻活性提供了一种新思路。Abstract: Determining the activity of microalgae in treated ship ballast water is an important task in ballast water indicative examination. The surface charge of dead and live microalgae varies and can be characterised by their electrokinetic velocity. In this paper, experiments were carried out with Isochrysis galbana and Chlorella vulgaris to investigate the electrokinetic velocity of microalgae in the microchannel before and after UV inactivation treatment and after different times of white light exposure. The results showed that all microalgae died under the treatment of UV irradiation dose of 50 mJ·cm−2; all the living and dead microalgae were negatively charged on the surface and moved towards the positive pole of the power supply. In addition, the electrokinetic velocity of live microalgae increased with increasing light time and the velocity of dead microalgae decreased with increasing death time within a certain incubation time. The difference in size velocity (velocity times diameter) between dead and living microalgae was greatest after 6 h of light. In this study, the velocity of electrokinetic movement of microalgae due to the electrification of their surface by white light illumination was used to characterize the dead and alive microalgae, providing a new idea for determining the activity of microalgae after ballast water treatment.
-
Key words:
- ship’s ballast water /
- algae viability /
- illumination time /
- surface charge /
- electrokinetic movement
-

-
图 5 微藻细胞活性变化示意图(a)活藻同一化后状态;(b)活藻光照后状态;(c)活体微藻稳定后状态;(d)活藻细胞膜结构;(e)微藻死亡初始状态;(f)微藻死亡变化状态;(g)死藻细胞膜结构
Figure 5. Schematic diagram of changes in microalgal cell activity (a)state of live algae after homogenisation;(b)state of live algae after light exposure;(c)state of live microalgae after stabilisation;(d)cell membrane structure of live algae;(e)initial state of microalgal death;(f)state of change in microalgal death;(g)cell membrane structure of dead algae
表 1 不同辐照剂量下微藻的存活状态
Table 1. Survival status of algae under different irradiation doses
紫外线剂量/(mJ·cm−2)
UV dose0 25 50 100 200 300 400 等鞭金藻(4.5 μm)
Isochrysis galbana√ √ × × × × × 小球藻(3.5 μm)
Chlorella vulgaris√ × × × × × × 注:“√”表示微藻存活;“×”表示微藻死亡.
Note: “√” indicates that the microalgae are alive; “×” indicates that the microalgae are dead.表 2 不同光照条件下微藻运动速度与细胞直径的拟合曲线
Table 2. Fitting curves of the velocity of movement of microalgae versus cell diameter under different light conditions
光照时间/h
Light hours等鞭金藻 Isochrysis galbana 小球藻 Chlorella vulgaris 拟合方程
Fitting equation相关系数
Correlation Coefficient拟合方程
Fitting equation相关系数
Correlation Coefficient0 Y=−0.28X+10.46 0.77 Y=−4.5X+60.10 0.84 2 Y=−0.44X+15.84 0.87 Y=−6.7X+84.05 0.68 4 Y=−4.45X+42.76 0.32 Y=−10.89X+121.71 0.39 活体 6 Y=−2.51X+40.14 0.21 Y=−12.05X+146.78 0.32 7 Y=−5.08X+52.96 0.57 Y=−9.89X+144.92 0.49 8 Y=−5.66X+52.75 0.40 Y=−6.93X+115.49 0.28 9 Y=−5.65X+58.41 0.21 Y=−7.45X+122.16 0.36 0 Y=−2.21X+17.37 0.61 Y=−1.07X+38.14 0.33 2 Y=−1.34X+13.34 0.63 Y=−1.15X+38.17 0.62 4 Y=−1.44X+14.04 0.86 Y=−1.03X+32.92 0.73 灭活 6 Y=−1.69X+14.69 0.80 Y=−1.31X+33.87 0.71 7 n.d. n.d. n.d. n.d. 8 Y=−0.86X+52.96 0.89 Y=−2.22X+27.56 0.74 9 Y=−0.65X+52.75 0.85 Y=−0.33X+29.67 0.64 -
[1] RUIZ G M, RAWLINGS T K, DOBBS F C, et al. Global spread of microorganisms by ships [J]. Nature, 2000, 408(6808): 49-50. doi: 10.1038/35040695 [2] DRAKE J M, LODGE D M. Global hot spots of biological invasions: Evaluating options for ballast-water management [J]. Proceedings. Biological Sciences, 2004, 271(1539): 575-580. doi: 10.1098/rspb.2003.2629 [3] KELLER R P, DRAKE J M, DREW M B, et al. Linking environmental conditions and ship movements to estimate invasive species transport across the global shipping network [J]. Diversity and Distributions, 2011, 17(1): 93-102. doi: 10.1111/j.1472-4642.2010.00696.x [4] REN J Z. Technology selection for ballast water treatment by multi-stakeholders: A multi-attribute decision analysis approach based on the combined weights and extension theory [J]. Chemosphere, 2018, 191: 747-760. doi: 10.1016/j.chemosphere.2017.10.053 [5] WANG Z J, SAEBI M, CORBETT J J, et al. Integrated biological risk and cost model analysis supports a geopolitical shift in ballast water management[J]. Environmental Science & Technology, 2021, 30: 1c04009. [6] OLSEN R O, HESS-ERGA O K, LARSEN A, et al. Flow cytometric applicability to evaluate UV inactivation of phytoplankton in marine water samples [J]. Marine Pollution Bulletin, 2015, 96(1): 279-285. [7] MARANDA Y, LACROIX G. Temporal variability of zooplankton biomass (ATP content and dry weight) in the St. Lawrence Estuary: Advective phenomena during neap tide [J]. Marine Biology, 1983, 73(3): 247-255. doi: 10.1007/BF00392250 [8] VANDEN BYLLAARDT J, ADAMS J K, CASAS-MONROY O, et al. Examination of an indicative tool for rapidly estimating viable organism abundance in ballast water [J]. Journal of Sea Research, 2018, 133: 29-35. doi: 10.1016/j.seares.2017.02.002 [9] CASAS-MONROY O, CHAN P S, LINLEY R D, et al. Comparison of three techniques to evaluate the number of viable phytoplankton cells in ballast water after ultraviolet irradiation treatment [J]. Journal of Applied Phycology, 2016, 28(5): 2821-2830. doi: 10.1007/s10811-016-0798-3 [10] SHANNON T, HATCH W I, FITT W K. Evidence of photosynthate translocation in an algal-acoel symbiotic system: An in vivo, qualitative approach [J]. Journal of Experimental Marine Biology and Ecology, 2009, 382(1): 69-75. doi: 10.1016/j.jembe.2009.10.011 [11] SUDHAHARAN T, REDDY A R. Metal ion mediated inhibition of firefly bioluminescence: A possibility via a quaternary complex [J]. Indian Journal of Biochemistry & Biophysics, 2000, 37(4): 256-267. [12] DRAKE L A, TAMBURRI M N, FIRST M R, et al. How many organisms are in ballast water discharge?A framework for validating and selecting compliance monitoring tools [J]. Marine Pollution Bulletin, 2014, 86(2): 122-128. [13] MOZES N, MARCHAL F, HERMESSE M P, et al. Immobilization of microorganisms by adhesion: Interplay of electrostatic and nonelectrostatic interactions [J]. Biotechnology and Bioengineering, 1987, 30(3): 439-450. doi: 10.1002/bit.260300315 [14] IVES K J. The significance of surface electric charge on algae in water purification [J]. Journal of Biochemical and Microbiological Technology and Engineering, 1959, 1(1): 37-47. doi: 10.1002/jbmte.390010105 [15] MYERS R E. Four patterns of perinatal brain damâge and their occurrence in Primates [J]. Advances in Neurology, 1975, 10: 223-234. [16] EWERTS H, BARNARD S, SWANEPOEL A. The impact of Zeta potential changes on Ceratium hirundinella cell removal and the ability of cells to restore its natural surface charge during drinking water purification [J]. RSC Advances, 2017, 7(36): 22433-22440. doi: 10.1039/C7RA01185G [17] TAKI K, SEKI T, MONONOBE S, et al. Zeta potential measurement on the surface of blue-green algae particles for micro-bubble process [J]. Water Science and Technology, 2008, 57(1): 19-25. doi: 10.2166/wst.2008.787 [18] MARTINEZ R E, POKROVSKY O S, SCHOTT J, et al. Surface charge and Zeta-potential of metabolically active and dead cyanobacteria [J]. Journal of Colloid and Interface Science, 2008, 323(2): 317-325. doi: 10.1016/j.jcis.2008.04.041 [19] SONG Y X, LI Z, FENG A R, et al. Electrokinetic detection and separation of living algae in a microfluidic chip: Implication for ship's ballast water analysis [J]. Environmental Science and Pollution Research, 2021, 28(18): 22853-22863. doi: 10.1007/s11356-020-12315-5 [20] MANZ A, HARRISON D J, VERPOORTE E M J, et al. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems: Capillary electrophoresis on a chip [J]. Journal of Chromatography A, 1992, 593(1/2): 253-258. [21] PALINSKA K A, KRUMBEIN W E. Electrophoretic separation of two unicyanobacterial strains leading to purification [J]. Journal of Microbiological Methods, 1995, 24(1): 41-48. doi: 10.1016/0167-7012(95)00052-6 [22] 夏飞. PDMS微流控芯片的制备工艺研究[D]. 南京: 南京理工大学, 2010. XIA F. Studies on the fabrication technology of PDMS microfluidic chips[D]. Nanjing: Nanjing University of Science and Technology, 2010(in Chinese).
[23] LEE J, CHOI E J, RHIE K. Validation of algal viability treated with total residual oxidant and organic matter by flow cytometry [J]. Marine Pollution Bulletin, 2015, 97(1/2): 95-104. [24] 高春芳. 高产油小球藻基因组测序和蛋白质组学研究[D]. 北京: 清华大学, 2012. GAO C F. Genome sequencing and proteomic research of the oleaginous microalga Chlorella protothecoides[D]. Beijing: Tsinghua University, 2012(in Chinese).
[25] MCCONNAUGHEY T A, WHELAN J F. Calcification generates protons for nutrient and bicarbonate uptake [J]. Earth-Science Reviews, 1997, 42(1/2): 95-117. [26] YUAN H, WANG Y, LAI Z J, et al. Analyzing microalgal biofilm structures formed under different light conditions by evaluating cell-cell interactions [J]. Journal of Colloid and Interface Science, 2021, 583: 563-570. doi: 10.1016/j.jcis.2020.09.057 [27] LU Z, ZHANG K, LIU X L, et al. High efficiency inactivation of microalgae in ballast water by a new proposed dual-wave UV-photocatalysis system (UVA/UVC-TiO2) [J]. Environmental Science and Pollution Research, 2019, 26(8): 7785-7792. doi: 10.1007/s11356-019-04268-1 -



 下载:
下载: