-
过氧化氢(H2O2)是当今世界100种重要的化学品之一[1],具有化学性质活泼、氧化能力强以及能量密度高等特点,在化工合成[2]、杀菌消毒[3]、废水处理[4]、纸张漂白[5]和燃料电池[6-7]等众多领域都有着广泛的应用。由于其分解产物只有水和氧气,符合可持续发展的需求,因此受到了广泛关注。
目前,H2O2的合成工艺主要有蒽醌法和氢氧直接化合法[8-9]。其中,蒽醌法是目前工业上生产H2O2的主要工艺,尽管通过该方法生产的H2O2占到了全球H2O2总产量的95%以上,但其生产过程中需经历蒽醌氢化、氧化以及后续的萃取、提纯、浓缩等一系列复杂工艺,具有能耗高、污染重、需要搭建大型生产设备等缺点[8]。此外,生产出的高浓度H2O2也为后续的储存和运输工作带来了极大的安全隐患。相较于传统的蒽醌法,氢氧直接化合法可在一定的反应条件下,利用贵金属催化H2和O2反应直接生成H2O2而不产生有毒有害的副产物,曾一度被认为是替代蒽醌法的理想工艺[10-12]。然而,由于该工艺存在氢氧直接接触带来的潜在爆炸风险以及氢气利用率低等问题,制约了其大规模工业应用。因此,开发节能环保且安全的新型H2O2合成技术仍然是H2O2合成领域的研究热点,具有非常重要的意义。
光催化法可在温和的反应条件下,利用太阳能驱动,将水和氧气转化为H2O2,因而被认为是实现安全、绿色和可持续性合成H2O2的一种潜在途径[13-15]。光催化合成H2O2的关键在于开发高效的光催化材料。目前,用于合成 H2O2的光催化材料主要包括TiO2[16-18]、BiVO4[19-20]、CdS[21]、rGO[22]等无机半导体和g-C3N4[23]、酚醛树脂(RF)[24]、三嗪共价有机框架(CTF)[25]等有机聚合物。其中,g-C3N4因具有独特的密勒胺(melem)结构,可在氧还原反应中催化氧气形成1, 4-内过氧化物中间态,利于O2分子按两电子路径被还原成H2O2而备受关注。研究表明,通过构建异质结[26-28]、修饰基团[29-30]、调控空间结构[31-32]、掺杂元素[33-34]以及制造缺陷[35]等方式进一步提升g-C3N4的两电子氧还原选择性和光生电荷分离性能后,获得的g-C3N4基材料会在氧还原反应中,对两电子产H2O2过程表现出更高的效率,从而在光催化产H2O2领域展现出更大的应用潜能。
本文以光催化产H2O2的基本原理为出发点,综述了g-C3N4 基材料在光催化产H2O2领域的研究进展,重点介绍了氧化还原双路径产H2O2的机理及提升g-C3N4光催化产H2O2性能的有效方法,旨在揭示该领域的机遇与挑战,为进一步推动光催化产H2O2技术的发展提供理论依据并指明新的方向。
-
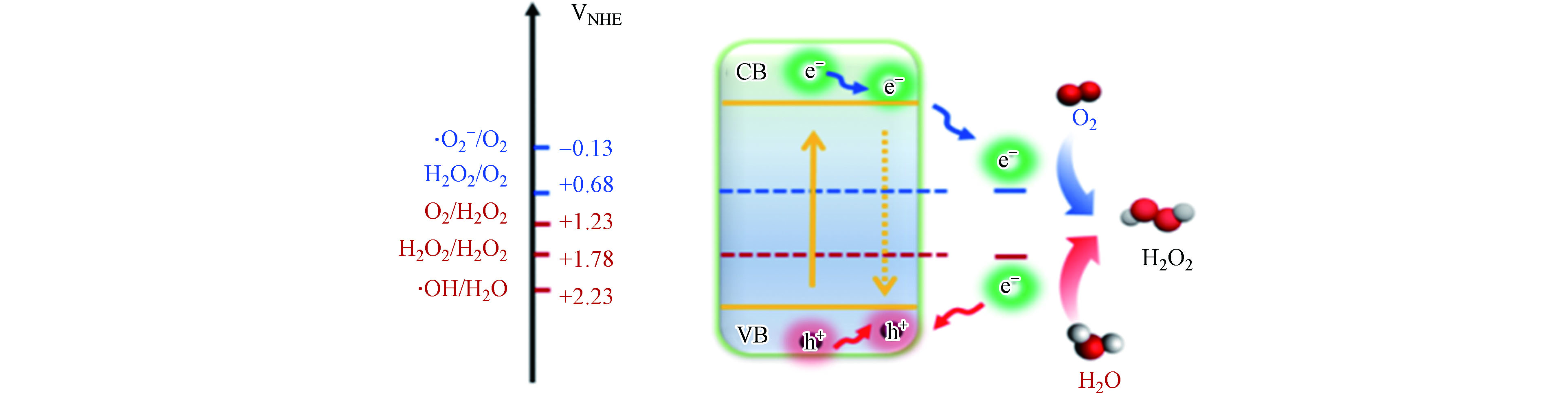
理论上,光催化产H2O2过程既可以通过两电子氧还原路径(式1)又可以通过两电子水氧化路径(式2)来实现。然而,无论是氧还原还是水氧化路径都存在相应的单电子和四电子路径与之竞争(式 3—6)。
相较于四电子氧还原/水氧化路径,单电子或两电子路径尽管在热力学上需要克服较高的氧化还原电势(−0.13 V/2.38 V或0.68 V /1.76 V vs 1.23 V/1.23 V),但其动力学效率更高。因此,只要光催化材料的氧化还原能力满足热力学上发生氧还原和水氧化反应的电势需求,即材料的导带和价带能够跨越单电子氧还原/水氧化的电势范围(图1),就会优先驱动单电子路径。此外,通过单电子路径生成的·
${\rm{O}}_2^- $ 和·OH还有机会进一步通过逐步单电子路径(${\mathrm{O}}_{2}\stackrel{{\mathrm{e}}^{-}}{\to }\rm{O}_2^{·-}\stackrel{{\mathrm{e}}^{-}}{\to }{\mathrm{H}}_{2}{\mathrm{O}}_{2}\mathrm{和}{\mathrm{H}}_{2}\mathrm{O}\stackrel{-{\mathrm{e}}^{-}}{\to }·\mathrm{O}\mathrm{H}\stackrel{-{\mathrm{e}}^{-}}{\to }{\mathrm{H}}_{2}{\mathrm{O}}_{2}$ )转化为H2O2,在促进光催化产H2O2方面较四电子路径更具优势。基于上述分析,为了实现高效产H2O2的目标,相应的光催化材料不但要有适宜的能带结构,还要能够有效抑制四电子路径的竞争、提高对产H2O2路径的选择性。近年来,相关研究人员的关注点已从如何提高逐步单电子或一步两电子氧还原产H2O2等还原路径的效率,逐渐转移到兼顾单电子水氧化产·OH替代传统的四电子产O2路径从而实现氧化还原双路径产H2O2方面[36-37]。尽管取得了一定的研究进展,如产量达到毫摩尔级、太阳能转化效率高于绿色植物光合作用过程(0.1%),但通过光催化技术产生的H2O2的量,距离满足水处理等实际应用的需求(浓度—30 mmol·L−1),还有近两个数量级的差距。因此,如何进一步提高光催化产H2O2反应的效率,仍然是该技术产业化应用前必须克服的一道重要难题。 -
为了进一步提升材料光催化产H2O2的效率,应充分了解光催化产H2O2过程所包含的具体步骤并掌握每一步的限制因素。总得来说,完整的光催化产H2O2过程可细化为光吸收、电荷分离和表面反应三步,而最终H2O2的生成速率又由上述步骤共同决定,提高其中任何一步的效率都有助于提升整个产H2O2反应的效率。
一方面,光吸收范围窄、光能利用率低以及光生载流子易复合是光催化材料普遍存在的问题。解决上述问题的经典方法:如构建异质结、掺杂元素、引入缺陷等,可借助窗口效应、内建电场以及中间带隙等作用,达到拓展材料光吸收范围以及促进其上光生电子和空穴有效分离的目的;而在应用于具体的光催化产H2O2过程时,引入的材料、元素或缺陷在氧还原/水氧化过程中若能发挥活性位点作用,上述方法还兼具提高表面反应效率的能力,因而具有普适性的研究价值。
另一方面,对于具体的光催化产H2O2过程而言,氧还原和水氧化两条路径的效率才是制约整个氧化还原产H2O2效率更直接的因素。而如何抑制四电子氧还原/水氧化路径的竞争,从而提高逐步单电子以及两电子路径的选择性无疑是解决上述问题的关键。相较于水氧化路径,目前关于氧还原产H2O2路径的研究较多、机理更加清晰,通过引入活性基团或贵金属等助催化剂修饰即可实现从O2到H2O2的高效转化;而四电子水氧化路径较高的动力学势垒仍然是制约水氧化反应乃至整个氧化还原产H2O2效率的主要因素。此外,生成的H2O2不能及时从材料表面脱离,不但会被光生空穴所消耗,还有引起材料中毒、导致活性降低的风险。因此,如何提高水氧化反应的效率、防止生成的H2O2被分解也是研究人员努力的方向。
-
为了便于比较不同光催化材料在产H2O2性能上的差异,需要引入恰当的评价指标。单就产H2O2过程而言,单位时间内H2O2的产生量(生成速率)无疑是最能直观反映材料产H2O2性能的指标,通过将材料在不同条件下产H2O2的量统一到标准的物质的量浓度(μmol·g−1·h−1)下比较,即可区分出优劣。而在光催化产H2O2过程中,由于涉及到光能的驱动,材料对入射光子的利用率(AQY)和对太阳能的转化效率(SCC)也是衡量其性能的重要指标,通过计算光催化材料吸收单位物质的量的光子后,有多少用于产H2O2过程即可判断光催化材料在产H2O2方面的应用潜能。然而,无论是哪种性能评价指标,都需要H2O2的准确定量作保障。目前,常用的H2O2检测方法主要有高锰酸钾法、碘量法和N, N-二乙基-1, 4-苯二胺/过氧化物酶法(DPD/POD)等。其中,前两种属于滴定法,定量结果精确但操作较为复杂;而后一种属于分光光度法,操作相对简便,但需要保证酶的活性。
-
利用H2O2在酸性溶液中易被KMnO4氧化生成氧气和水(式7)这一性质,根据滴定H2O2所消耗的KMnO4标准溶液的量可计算出H2O2的含量。
-
在酸性条件下,H2O2与过量的Br-反应生成Br2 (式8),再利用生成的Br2与定量的I−反应生成I2 (式9),最后用Na2S2O3标准溶液进行滴定I2 (式10)。根据所消耗的Na2S2O3标准溶液的量,可计算出H2O2的含量。
-
该方法的原理如下:H2O2在POD催化下可将DPD氧化成DPD+。该阳离子是一种稳定的粉红色的化合物,在510 nm和551 nm处有两个吸收峰。利用紫外-可见分光光度计在551 nm处检测DPD+的吸收峰即可对H2O2进行定量分析。
-
AQY的定义为单位时间内用于产H2O2的电子数与入射光子数的比值。AQY的计算公式为:
其中,N(H2O2)表示产生H2O2的分子数;N(photos)表示入射光子数。
-
利用太阳光模拟器在AM 1.5G[43]光谱下可计算光催化剂的太阳能转化效率。SCC效率的计算公式为:
其中,
${\Delta G}({\text{H}}_{\text{2}}{\text{O}}_{\text{2}})$ 表示H2O2标准生成吉布斯自由能(117 kJ·mol−1);n(H2O2)表示的物质的量;$ {{E}}_{\text{imput}} $ 表示入射光功率;T表示反应时间。 -
g-C3N4是由C、N元素共价结合成的一种具有类石墨烯π-π共轭电子结构的层状聚合物。其化学性质稳定、无毒无害、廉价易制备,已被广泛应用于光催化分解水产氢以及污染物降解等领域。g-C3N4的禁带宽度约为2.7 eV,可吸收波长小于460 nm的紫外及可见光。其导带位于−1.3 VNHE,比还原O2产H2O2所需的电位(E(O2/H2O2)=0.68 V)更负;而其价带位于+1.4 VNHE,理论上满足H2O氧化反应生成O2的势能要求(E(H2O/O2)=1.23 V)[44]。因此,当将其应用于光催化产H2O2过程时,所遵循的反应机理可通过2个半反应来表示:(1)光生空穴经四电子路径将H2O氧化成O2 (式6);(2)光生电子经两电子路径将O2还原成H2O2 (式1)。而g-C3N4参与的光催化产H2O2总反应可表示为:
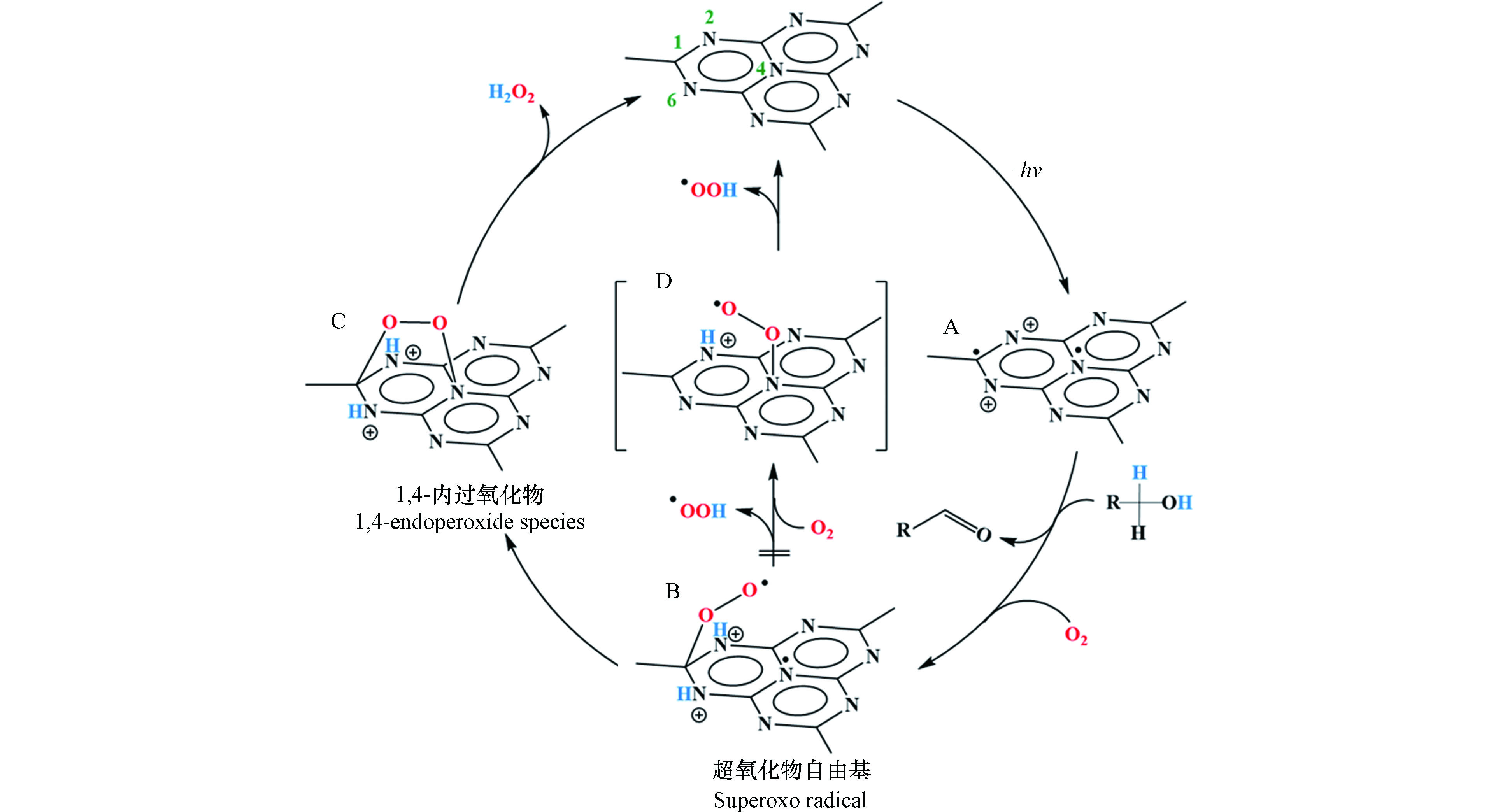
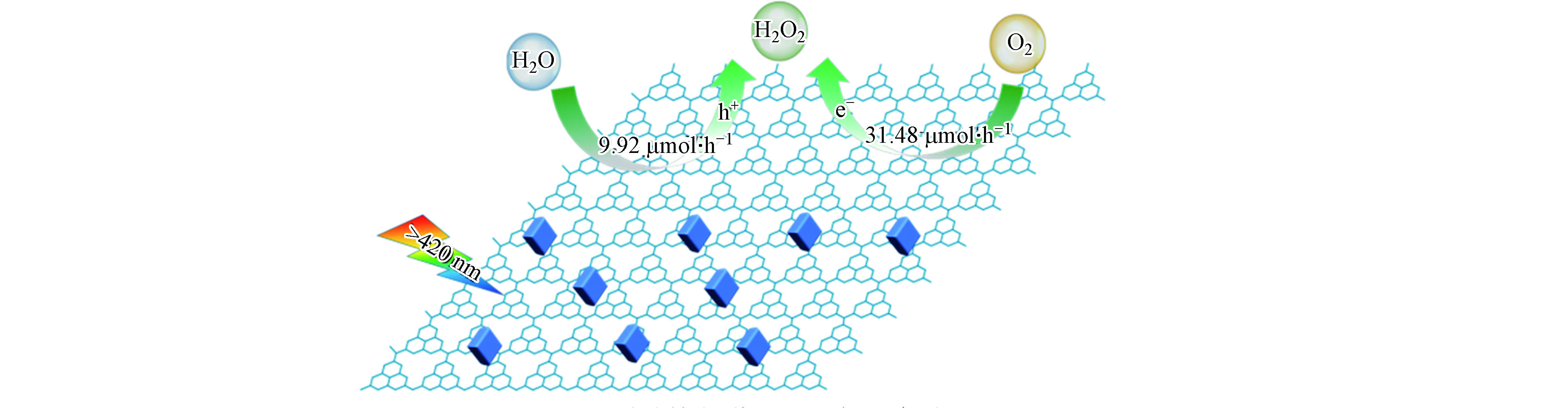
2014年Shiraishi等首次以g-C3N4为催化剂,实现光催化O2和H2O反应生成H2O2[23]。在该工作报道了一个重要发现,g-C3N4对两电子氧还原产H2O2路径表现出相当高的选择性(约为90%)。究其原因是g-C3N4所具有独特的三嗪结构,可在被光激发时将光生电子分布在1号位的C原子(C1)和4号位的N原子(N4)上,而当g-C3N4驱动氧还原过程时,O2分子可分别在其三嗪结构的C1和N4原子处各接收一个电子形成1,4-内过氧化物中间态,从而有效抑制单电子产·
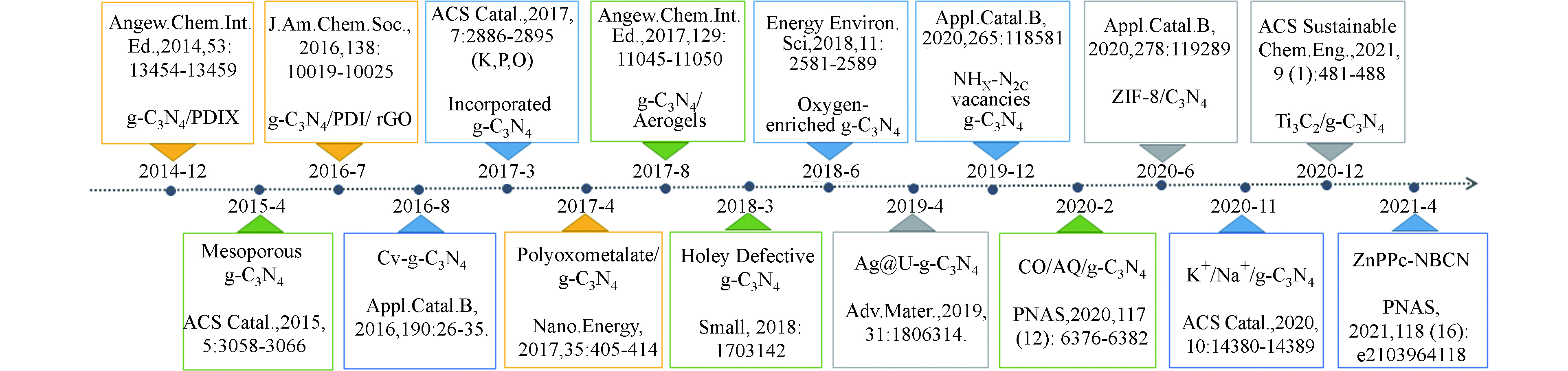
${\rm{O}}_2^- $ 和O—O键断裂后四电子产H2O路径的竞争,促进了O2到H2O2的选择性转化(图2)。随后的几年间,g-C3N4基催化材料在光催化产H2O2领域得到了蓬勃发展,图3以时间轴的形式记录了其发展历程中的重要节点、表1则更加全面地总结了g-C3N4基材料驱动氧还原产H2O2过程的具体反应条件和H2O2的生成效率。从中可以看出,构建异质结、修饰基团、调控空间结构、掺杂元素以及引入缺陷等方法是对g-C3N4材料的常见调控策略,而了解这些改性手段引起的深层次变化对于开发和设计新型的g-C3N4基光催化材料并进一步优化其产H2O2性能具有重要意义。
-
构建异质结是解决g-C3N4光吸收范围窄、光生电荷易复合等问题的有效手段。而应用于具体的光催化产H2O2反应时,还应考虑异质结对氧还原产H2O2路径的选择性以及驱动水氧化反应的效率。基于上述分析,在构建g-C3N4基异质结时主要从引入活性位点和提高价带氧化能力两个角度出发,得到的g-C3N4基异质结可归为(类)肖特基结和Z型异质结两类。
-
g-C3N4基(类)肖特基结是由g-C3N4与导电性良好且对氧还原产H2O2选择性高的材料,如贵金属Au[45]、Ag[26],过渡金属Sb[69],碳材料[46] (碳纳米管、石墨烯、多孔碳等)以及MXene [47-48]等构成的异质结。该类异质结在氧还原产H2O2过程中,g-C3N4上的光生电子会在内建电场作用下传递到金属(或碳材料和MXene)表面与O2发生反应。与此同时,金属表面通过等离子共振作用产生的热电子还能反向跃迁到g-C3N4的导带上,增加了参与氧还原反应的光生电子数、也拓宽了g-C3N4的光吸收范围,进而提高了异质结的光催化产H2O2性能。
具有代表性的工作包括:Lin等受贻贝结构的启发在超薄g-C3N4纳米片表面修饰Ag纳米颗粒制备出Ag@U-g-C3N4-NS异质结[26]。其中,U-g-C3N4-NS所具有的较大比表面积,为氧还原反应提供了更多的活性位点;而Ag纳米颗粒的引入又拓展了U-g-C3N4-NS的光吸收范围、促进了U-g-C3N4-NS中光生电荷的有效分离,进而提高了整个光催化产H2O2反应的效率。相应地,Ag@U-g-C3N4-NS在纯水体系中表现出优异的光催化产H2O2性能(118.5 μmol·g−1·h−1),与U-g-C3N4-NS (24.8 μmol·g−1·h−1)相比提高了4.77倍。
相较于金属材料,碳材料不仅在导电性方面能与之相媲美,还兼具来源广泛、价格低廉、安全无毒等优点,也常用作助催化剂以提高g-C3N4的光催化性能。近年来,由g-C3N4和碳材料构成的类肖特基结已被广泛应用于光催化产H2O2领域。例如,Zhao等通过酰胺化作用将酸化处理的CNTs与g-C3N4共价结合形成g-C3N4-CNTs异质结[46]。该异质结在5%(体积分数)的甲酸作牺牲剂、波长λ>400 nm的可见光照射下,还原O2产H2O2的效率为487 μmol·g−1·h−1,比单纯g-C3N4 (152 μmol·g−1·h−1)提高了3.2倍。深入研究发现,g-C3N4-CNTs在氧还原过程中转移电子数为1.96,比单纯g-C3N4(1.29)更接近两电子路径,说明引入的CNTs在提升g-C3N4 氧还原产H2O2选择性及最终产H2O2效率方面发挥了重要作用。由此得到启示,其它对氧还原产H2O2选择性高的碳基材料,如rGO、多孔碳和有序介孔碳等也是g-C3N4的潜在助催化材料。
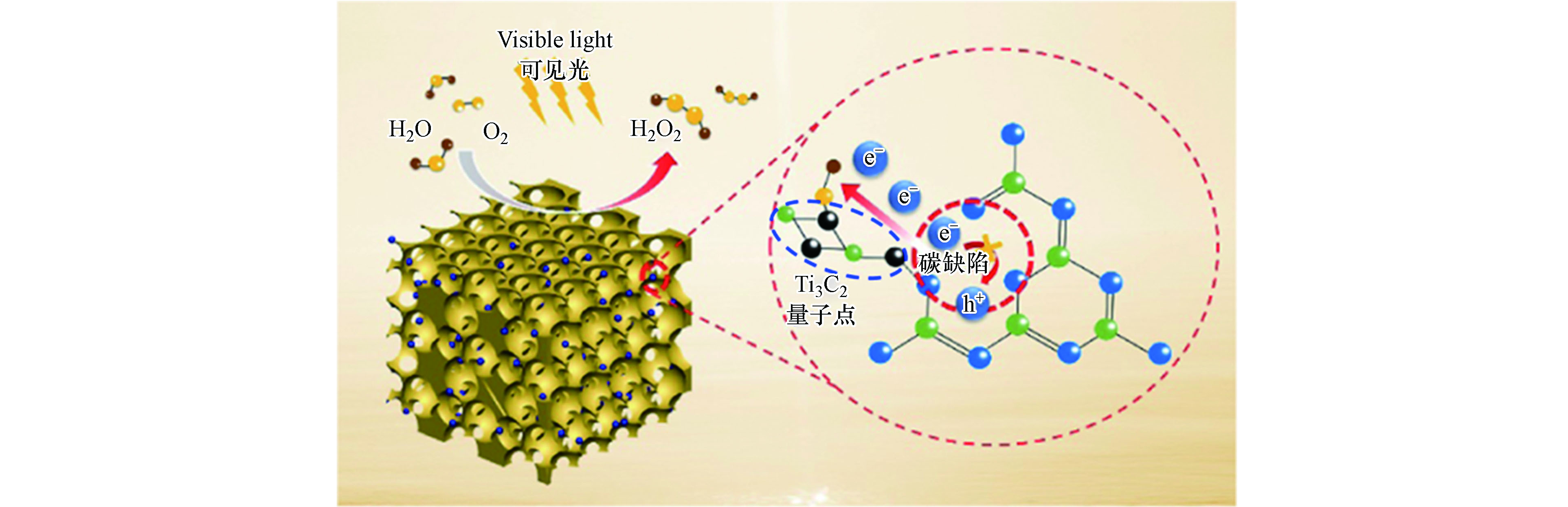
此外,具有导电性良好、片层结构易调控以及光吸收范围广等优点的过渡金属碳氮化物(MXene),也在光催化产H2O2领域逐渐引起关注。Zhang等通过静电自组装法将Ti3C2量子点与含碳缺陷的逆蛋白石g-C3N4相结合制备出TC/CN异质结[48],在该工作中,TC/CN-20表现出最优的光催化性能,在5%(体积分数)乙醇存在的体系中产H2O2效率为560.7 μmol·g−1·h−1比块体CN高出9.3倍,而在纯水体系中产H2O2效率也可达116.9 μmol·g−1·h−1与上文报道的Ag@U-g-C3N4-NS性能相当。引入Ti3C2后,g-C3N4 基光催化材料产H2O2性能的提升也可归功于二者间形成了类肖特基结,在其作用下界面处光生电荷得以有效分离,从而保留了更多的光生电子用于后续的光催化产H2O2反应(图4)。
基于上述研究,构建g-C3N4基(类)肖特基结的优势在于从拓展光吸收、促进电荷分离和提升氧还原选择性等多方面综合提升材料的光催化产H2O2性能;不足之处在于其参与水氧化反应效率受g-C3N4价带氧化能力不足以及水氧化生成的H2O2不能及时脱附会造成催化剂中毒等因素制约,导致最终产H2O2效率的提升幅度有限(10倍以下)。
-
构建Z型异质结是解决g-C3N4价带氧化能力不足的有效途径。在原有g-C3N4基础上引入另一价带氧化能力强的半导体与之复合,通过调控界面电荷定向迁移,即可在实现对g-C3N4基材料光吸收、电荷分离性能的优化,同时还能保留体系较强的氧化和还原能力。
Zhang等通过水诱导的自组装法制备了Bi4O5Br2/g-C3N4 Z型异质结[49]。在λ>420 nm的可见光激发下,Bi4O5Br2导带上还原能力较弱的光生电子与g-C3N4价带上氧化能力较弱的空穴在界面处复合,而g-C3N4导带上还原能力较强的光生电子和Bi4O5Br2价带上氧化能力较强的光生空穴得以保留下来,可分别用于还原氧气和氧化水,该异质结在纯水体系中产H2O2效率高达2.48 mmol·g−1·h−1,比g-C3N4和Bi4O5Br2分别高24.8倍和62倍。而在另一项工作中,Wu等借助金属磷酸盐修饰石墨相氮化碳合成了一种全光谱响应的Cu2(OH)PO4/g-C3N4 Z型异质结[27]。在模拟太阳光(AM 1.5G)照射下,当Cu2(OH)PO4的质量分数为20%时,Cu2(OH)PO4/g-C3N4异质结产H2O2效率最高,经6 h反应后体系中累积的H2O2浓度可达7.2 mmol·g−1,与单独g-C3N4和Cu2(OH)PO4 存在的体系相比分别提高了13倍和31.3倍。深入分析可知,同时解决g-C3N4基材料的光吸收范围窄、光生电荷易复合以及水氧化反应效率低等问题,是提高其光催化产H2O2效率的主要原因。
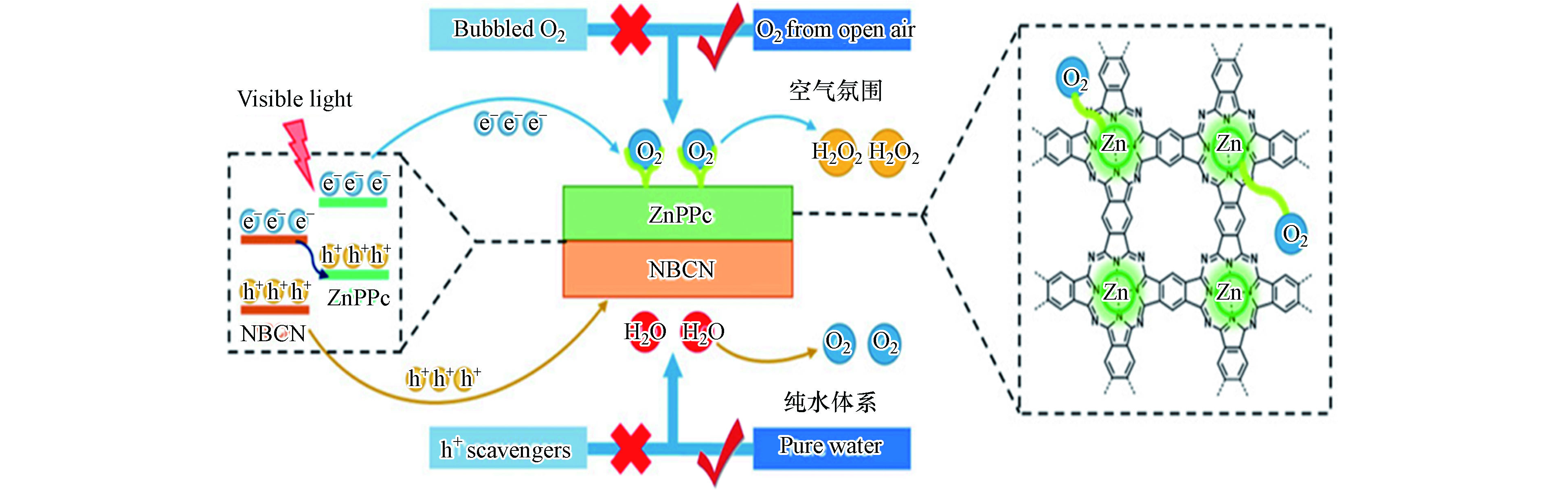
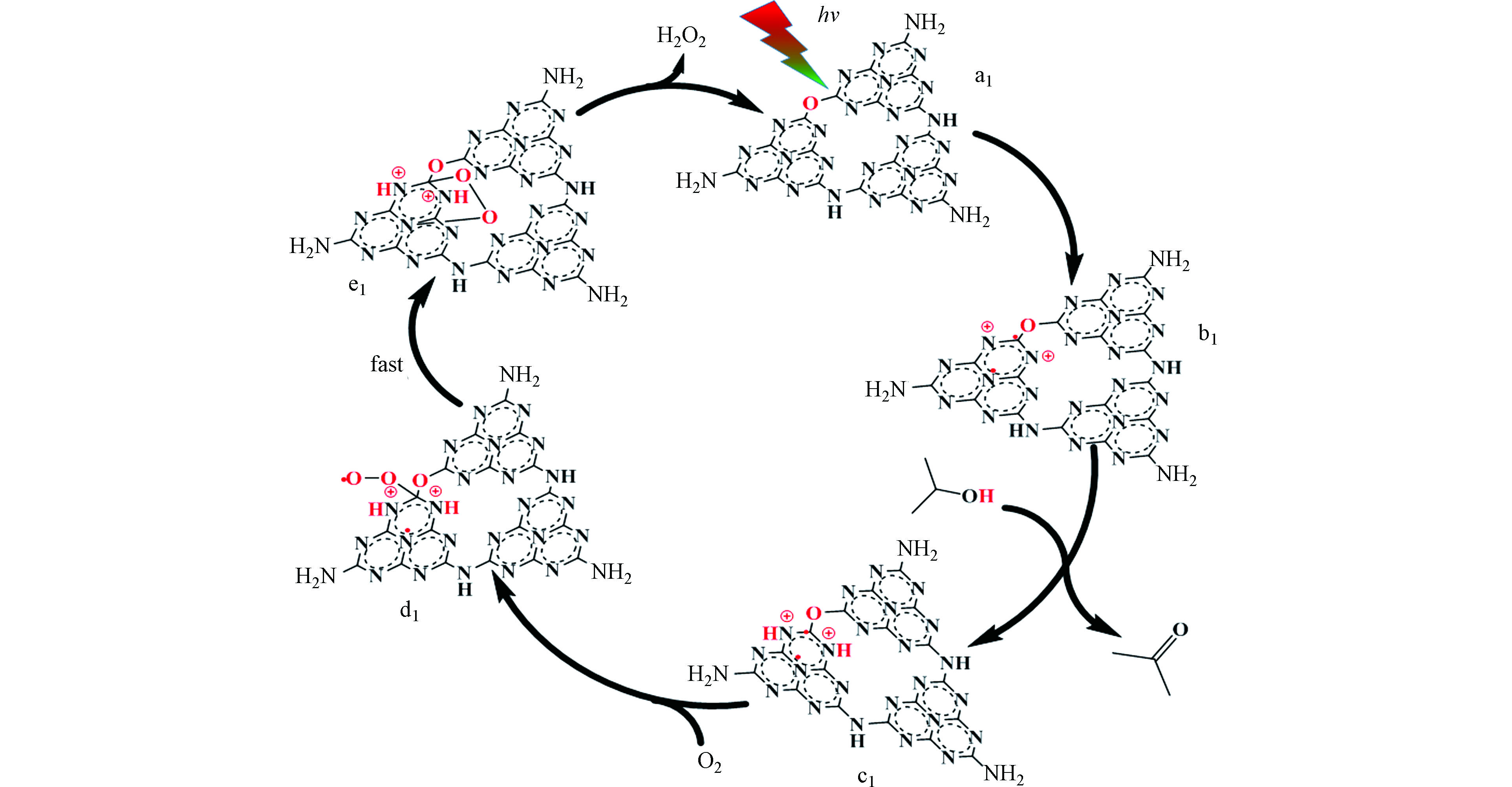
近日,Ouyang等在g-C3N4基Z型异质结光催化产H2O2相关研究中取得了重要进展。他们利用原位生长法将聚钛菁锌(ZnPPC)与B掺杂氮化碳(NBCN)有机结合,构建出能在400—800 nm可见光驱动下,暴露在空气中不通O2亦可高效产H2O2的ZnPPC-NBCN Z型异质结[50]。该异质结中Zn配位的PPC结构对两电子氧还原路径具有很高的选择性,是氧还原产H2O2的活性位点;而NBCN价带上光生空穴氧化水产O2效率很高,生成的O2可替代曝气供氧过程为氧还原产H2O2原位供给O2(图5)。其光催化产效率可达114 μmol·g−1·h−1,是相同条件下效果最好的材料,甚至可以与其它材料在通O2条件下产H2O2效率相媲美。该工作对推动光催化产H2O2技术向逐渐满足实际应用需求的方向发展意义重大。
上述实例充分展示了g-C3N4基Z型异质结在光催化产H2O2方面的巨大潜力。通过构建Z型异质结不但可以提高g-C3N4基材料参与水氧化反应的效率,还能开辟一条通过逐步单电子水氧化反应产H2O2的新路径,进而优化其光催化产H2O2性能。而如何有效调控其界面电荷定向迁移并优化分离效率则是决定能否成功构建高效Z型异质结的关键。
-
从另一个角度看,g-C3N4作为一种有机聚合物,具有分子结构易调控的天然优势。因此,在其固有的分子结构中,引入某些无机或有机基团以改变其相关物化性质,即可对其光电性能优化,进而提升其光催化产H2O2性能。
在众多无机纳米基团中,多金属氧酸盐(POMs)是颇具代表性的一类。它们通常由过渡金属与氧元素构成,具有尺寸可调,拓扑结构丰富多样等性质,常被用作光催化材料的改性基团。Zhao等在POMs改性g-C3N4应用于光催化产H2O2方面开展了大量研究[30, 51-52],通过有机连接策略将[PW11O39]7−(PW11)团簇与三维有序大孔石墨相氮化碳(3DOM g-C3N4)共价结合,所得的复合材料3DOM g-C3N4-PW11在模拟太阳光(320 nm < λ < 780 nm)驱动下,催化H2O和O2反应生成H2O2的效率为35 μmol·g−1·h−1。ESR测试表明,与3DOM g-C3N4相比,3DOM g-C3N4-PW11存在的体系中·
${\rm{O}}_2^- $ 的信号明显减弱,说明O2到·OOH转化这一单电子路径被有效抑制。后续的环盘电极测试结果表明3DOM g-C3N4-PW11在氧还原反应中转移电子数n=2.3,进一步印证了其主要通过两电子路径将O2还原成H2O2这一推论。在此基础上,该团队又通过相同的方法将另一种POM簇[SiW11O39]8−(SiW11)引入g-C3N4的分子结构中。由于SiW11比PW11的CB位置更负、还原能力更强,热力学上更利于O2到H2O2的转化。相应地,g-C3N4-SW11在5%(体积分数)甲醇作牺牲剂、AM 1.5G光照下,1 h产生17.8 μmol的H2O2,与g-C3N4 (8.7 μmol)和SW11(1.0 μmol)相比分别提高了2.0倍和17.8倍。此外,他们还通过将制备g-C3N4所需的前驱体双氰胺与多金属氧酸盐(NH4)3PW12O40 (NH4-PW12)先混合再煅烧,制备出g-C3N4-PWO复合材料。结构稳定的g-C3N4-POW4在λ > 420 nm的可见光照射下表现出优异的光催化产H2O2性能(2.9 μmol·h−1)。而在有机结构中,金属有机骨架材料(MOFs)因其超高的比表面积、丰富的拓扑结构以及结构易调控等特点,在催化领域也显示出巨大的应用前景。Kang等通过静电自组装结合后续的热处理法制备了ZIF-8/C3N4复合材料[53],热处理后ZIF-8与g-C3N4间形成共价键,ZIF-8所具有的多孔结构有利于O2的吸附、扩散以及H2O2生成后及时从g-C3N4表面脱离(图6)。该复合材料在可见光(420 nm < λ < 780 nm)驱动下产H2O2效率可效达2.64 mmol·g−1·h−1,换算成质量浓度为0.71% (质量分数),与工业上蒽醌法生产出的H2O2的浓度(1 %(质量分数))接近,标志着光催化产H2O2技术距离实际应用又近了一步。此外,工业上合成H2O2常用的催化剂——蒽醌(AQ)基团,由于对两电子氧还原过程具有超高的选择性,也被引入g-C3N4的分子结构中,以提高g-C3N4的光催化产H2O2效率[54]。综上所述,与贵金属修饰相类似,引入的有机/无机基团在g-C3N4基材料光催化产H2O2过程中主要发挥助催化剂作用。
另一方面,某些基团被引入到g-C3N4分子结构后还能起到调控其能带结构,进而提升其内部电荷分离以及参与氧化还原反应效率的作用。例如,Shiraishi等利用热缩合法将芳香族苝二酰亚胺(PDI)基团和还原氧化石墨烯(rGO)成功与g-C3N4复合,得到的g-C3N4/PDI/rGO材料价带氧化能力、光生电荷分离性能以及两电子氧还原选择性都有明显提升,产H2O2性能也大幅提高[55]。在模拟太阳光照射下,其还原O2产H2O2的效率为24.17 μmol·g−1·h−1,SCC达0.20%,超过了绿色植物光合作用的能量利用效率(约0.10%)。与此同时,其它芳香基团,如联苯二酰亚胺(BDI)[29]、三酰亚胺(MTI)[56]、苝酰亚胺(PI)[28]和聚乙酰亚胺(PEI)[58]等与g-C3N4 构建的复合材料也被应用于光催化产H2O2相关研究,并实现了与上文提到的g-C3N4/PDI体系类似的目标。
-
对g-C3N4基材料三维结构[31, 58]以及活性位点分布[59-60]等空间结构进行调控也是提升其光催化产H2O2性能的有效方法。目的是通过提高比表面积、增加活性位点暴露数量、促进反应物的传质/运输以及缩短光生电荷迁移到活性位点的路径等方式来提升其的光催化产H2O2效率。
Wang等通过水性溶胶-凝胶策略制备了具有3D网络结构的g-C3N4气凝胶[31]。在λ > 420 nm的可见光照射下,g-C3N4气凝胶在纯水体系中产H2O2的速率为28.8 μmol·g−1·h−1,而块体g-C3N4体系中几乎没有检测到H2O2。分析原因发现,在氧还原产H2O2反应中,g-C3N4气凝胶所具有的3D多孔结构及其结构中引入的—NHx,—C≡N和—OH等官能团利于O2分子在CN表面形成1, 4-内过氧化物中间态,抑制了O2到·
${\rm{O}}_2^- $ (单电子路径)和H2O(四电子路径)的转化,进而提高了O2还原成H2O2(两电子路径)的选择性。可见,在构建3D多孔结构g-C3N4时往往会引入杂元素与缺陷,其性能的提升也是多方面因素综合作用的结果。而在另一项工作中,Ye等利用一种新型的光辅助热处理工艺制备出富含缺陷的多孔洞g-C3N4材料[58]。这样的结构特性,不但暴露出更多的催化活性位点、为反应物提供了丰富的运输通道,而且缩短了光生电荷从体相迁移到O2活化位点处发生反应的距离,进而提高了光催化产H2O2过程的效率,结果表明,多孔洞的g-C3N4在2.5 h内产H2O2效率(242 μmol·g−1)比原始体g-C3N4高出10.1倍。此外,Kim等还报道了一种调控水氧化位点和氧还原位点空间分布,提高g-C3N4基材料光催化产H2O2性能的有效方法[59]。该工作以单原子Co作为水氧化活性位点、蒽醌基团作为选择性还原O2产H2O2的活性位点,通过将Co单原子配位锚定在g-C3N4的三角形空隙中心、同时将蒽醌配位修饰在g-C3N4纳米片的边缘,实现了氧化和还原位点的空间分离,具体原理如图7所示。理论计算和实验结果表明,在g-C3N4分子结构中引入空间分离的氧化还原位点对于增强表面电荷分离和实现高效产H2O2具有至关重要的作用,与单纯g-C3N4相比,Co单原子和AQ基团共同修饰的g-C3N4材料(CO/AQ/C3N4)产H2O2速率提高了7.3倍,充分说明合理调控g-C3N4基材料中氧还原和水氧化活性位点空间分布的必要性。
-
掺杂元素是调节g-C3N4能带结构的有效方法,当杂元素被引入g-C3N4的分子结构后,由于杂元素在吸电子或供电子性能方面与本征半导体存在差异,会在g-C3N4能带结构中靠近导带或价带区域形成杂质能级,进而起到拓宽光响应范围、促进电荷分离的作用。按掺杂元素的性质可分为金属掺杂和非金属掺杂两类。
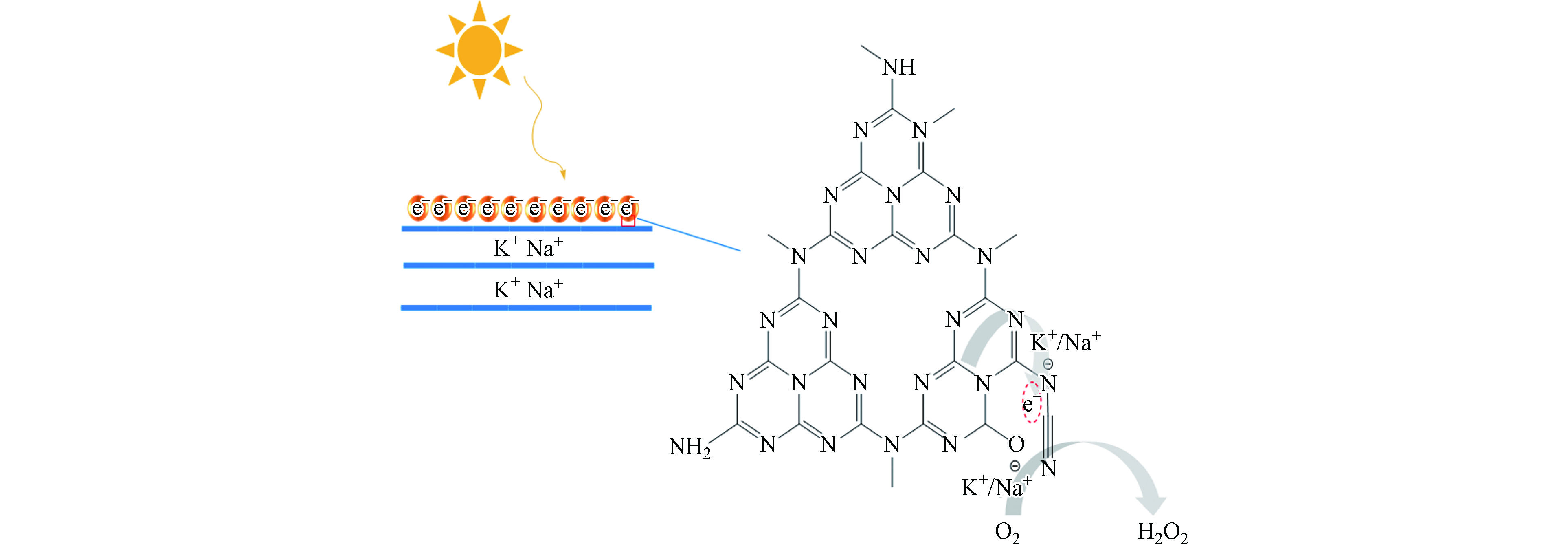
金属离子,如Fe3+、Co3+、Ni2+、Cu2+等过渡金属离子和K+、Na+、Li+等碱金属离子常用于掺杂改性g-C3N4。例如,Hu等通过模板辅助法制备了铜掺杂的g-C3N4空心微球[61]。铜掺杂后的Cu/g-C3N4复合材料光催化产H2O2效率为0.96 mmol·g−1·h−1与掺杂前单独g-C3N4 (0.09 mmol·g−1·h−1)相比提高了10倍。上述结果一方面归因于铜掺杂后在拓展g-C3N4材料光吸收范围及促进其内部电荷分离方面产生的积极作用;另一方面,DFT计算表明Cu与g-C3N4间形成的Cu-N键在氧还原产H2O2反应中同时发挥O2分子吸附活化位点和“电子转移桥”的作用,提高了光生电子参与的氧还原反应的效率。而在另一项工作中,他们又通过熔盐法将K+和Na+掺杂到g-C3N4中[62]。引入碱金属离子不但增加了材料对可见光的吸收强度,促进了其内部载流子的有效分离,而且拓展了g-C3N4的价带边界位置,使得掺杂了K+和Na+的g-C3N4 (MCN)不仅能够依靠导带上的光生电子将O2还原成H2O2,还能利用价带上的光生空穴通过逐步单电子过程先将OH−氧化成·OH,再将生成的·OH耦合成H2O2,从而大幅提升(从0.5 mmol·L−1增至4.6 mmol·L−1)其光催化产H2O2效率。
相较于金属掺杂,非金属掺杂在调控材料的能带结构、拓展其光吸收和电荷分离效率的同时,还兼具成本低廉的优势。据统计,包括C、B、N、P、O、S、F、Br、I等[39, 63]在内的多种非金属元素改性的氮化碳已被应用于光催化产H2O2相关研究。而在众多非金属掺杂的氮化碳材料中,最具代表性的要数Zhu等报道的富氧氮化碳聚合物(OCN)[34]。理论模拟和实验结果表明,OCN在氧还原过程中更容易活化O2分子形成1, 4-内过氧化物中间态,从而对两电子氧还原产H2O2路径表现出极高的选择性(图8)。OCN在纯水体系中、λ > 420 nm可见光照射下10 h产H2O2累积浓度可达1.06 mmol·g−1,比已报道的其它材料在相同条件下产H2O2 性能高出很多。此外,在λ = 420 nm处其产H2O2的表观量子产率AQY高达10.2%,是g-C3N4的3.5倍。相较于g-C3N4而言,OCN表现出更强的还原能力、更高的两电子氧还原选择性以及更高的光生电荷分离效率,这些都是促使其产H2O2效率提升的重要原因。
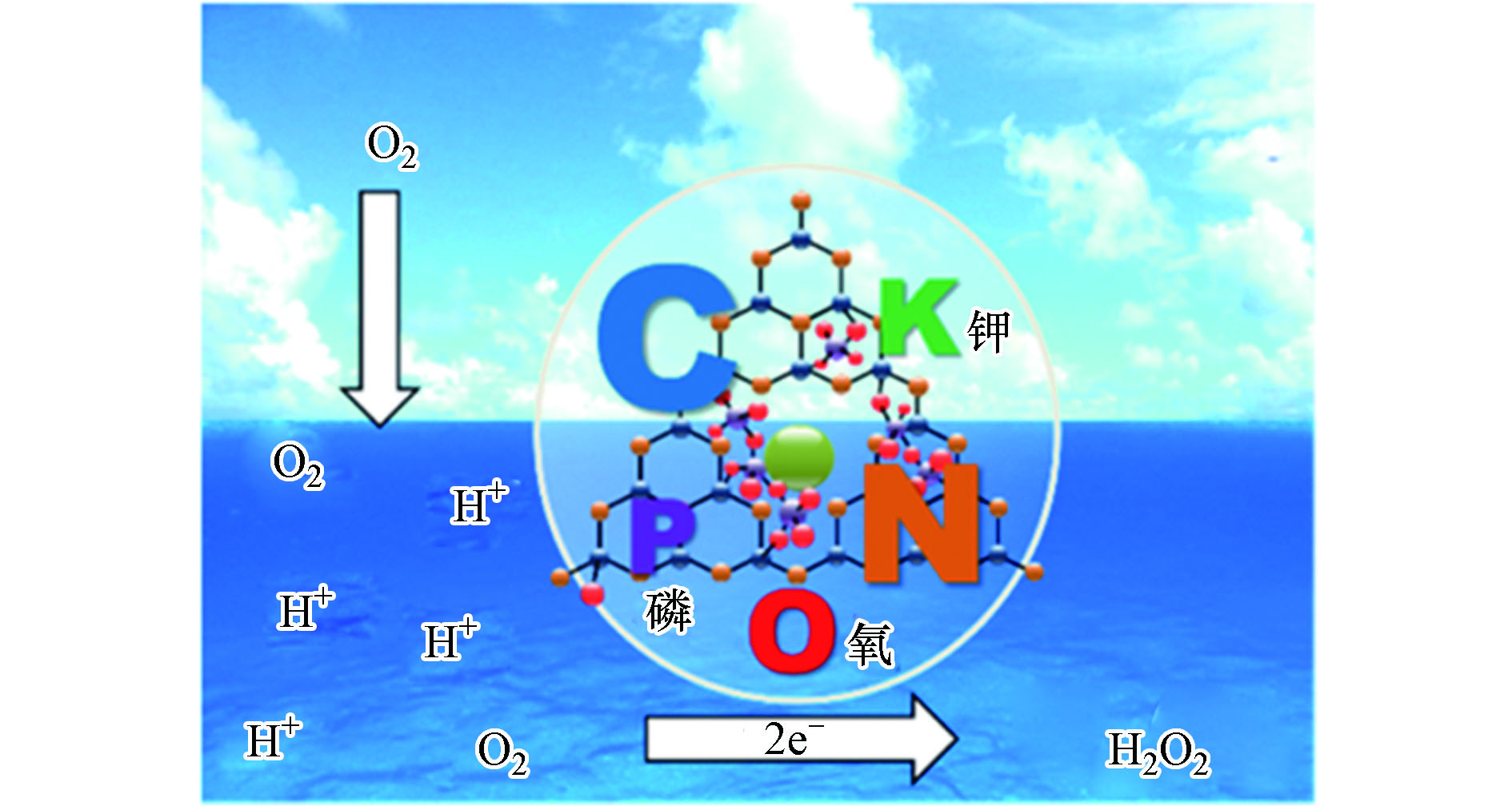
研究表明,与单一金属或非金属元素掺杂相比,金属和非金属元素共掺杂会产生协同效应,有望进一步提升g-C3N4的光电性能。Choi等在金属/非金属元素共掺杂方面开展了大量研究工作,并取得了丰厚的研究成果。他们通过原位热聚合法将KPF6引入氮化碳CN分子结构中制备出KPF-CN复合材料[64]。在该复合材料中,K+被N原子上孤对电子所稳定发挥O2活化位点作用、而PF6−被插入CN层间以促进质子传递。在K+和PF6−协同作用下KPF6-CN的光吸收、电荷分离以及氧还原产H2O2选择性等性能明显增强;同时,H2O2的原位分解现象得到了有效抑制。在420 nm的单色照射下,KPF6-CN的AQY为24.3%,是CN的26.1倍。在另一项工作中,Choi等又以K2HPO4为前驱体,在三聚氰胺的热聚合过程中,将K、P和O元素原位掺杂到CN基质中[33]。所制备的KPD-CN材料在λ = 420 nm处产H2O2的AQY比CN高25倍,而其单位时间对O2的转化效率TOF更是比CN高出73倍。其产H2O2效率提升的原因可归结为引入K、P、O等杂元素后,对CN表面电性、载流子寿命、电荷传递效率以及抑制H2O2分解等方面表现出积极作用,具体反应机理如图9所示。最近,又利用近似的策略制备了K和S元素共掺杂的氮化碳聚合物AKMT[70]。该材料3 h内产H2O2效率达4.3 mmol·L−1,比原始的氮化碳<0.1 mmol·L−1高出43倍。此外,其在波长360—450 nm光照下AQY接近100%,充分显示出金属和非金属共掺杂在提升材料产H2O2性能方面的优势。
-
除上述策略外,向g-C3N4中引入缺陷也是提升其光催化性能的有效方法。一方面,引入缺陷后会在其VB和CB间形成缺陷能级,拓展光吸收范围的同时、可促进其内部光生载流子有效分离;另一方面,引入的缺陷还能作为反应物的活化位点,起提高氧化还原反应效率的作用。对于g-C3N4而言,其分子结构中形成的缺陷主要有氮空位和碳空位两种形式。
Wang等通过在Ar氛围下热退火处理g-C3N4制备了含碳缺陷的g-C3N4(Cv-g-C3N4)[65]。在可见光照射下,Cv-g-C3N4的光催化产H2O2效率为900 μmol·g−1·h−1,比纯g-C3N4高出14倍。其光催化活性增强一方面可归因于引入碳空位后对材料光吸收范围的扩展以及光生载流子分离效率的提升。但更重要的是,碳空位周围形成的氨基位点会促使氧还原路径从逐步单电子向一步两电子路径转化,进而提升了氧还原产H2O2的选择性。
与碳空位类似,氮空位在提升g-C3N4光催化产H2O2效率方面也表现出优异的性能。Huang和Ye等合作在聚合物氮化碳的框架中引入了NHx和N2C两种氮空位[66]。研究发现,引入氮空位后的样品(CKCN)在模拟太阳光驱动下产H2O2效率提高了15倍,且经50 h连续反应后产H2O2效率并没有明显衰减,说明该材料具有很好的稳定性。在340 nm和420 nm处,其产H2O2的AQY分别达到26.78%和11.86%。理论计算与实验结果表明,NHx和N2C可在氧还原过程中分别起促进光生电荷分离和活化分子氧的作用,二者间存在协同效应。
基于上述分析可知,单独引入杂元素或缺陷虽然可在一定程度上提升g-C3N4光催化产H2O2性能,然而提升幅度有限、仅为原始g-C3N4的10—30倍。考虑到掺杂元素和制造缺陷都可以改变材料的电子结构,在掺杂元素的同时引入缺陷可协同增强光吸收、促进电荷分离。例如,Wu等通过熔盐法制备了碱金属和氮空位共掺杂的晶态氮化碳(ACNN)[67],同时增强了氮化碳的光吸收和电荷分离性能。引入碱金属和氮空位后,ACNN的带隙从2.85 eV减小到2.63 eV,拓展光吸收范围的同时极大地抑制了光生电荷的复合(图10)。
在碱金属掺杂和氮空位的协同作用下其光催化性能明显提高,H2O2产率为10.2 mmol·g−1·h−1,是原始C3N4的89.5倍。Tang等通过KBH4辅助热聚合的方法成功地在g-C3N4中引入了缺陷和硼掺杂位点[68]。通过控制KBH4的用量,可以方便有效地调节g-C3N4的光吸收和电荷分离性能。DFT计算证明缺陷与掺杂硼原子之间存在协同效应,可以大大提高g-C3N4的光催化性能。B元素和缺陷共掺杂的氮化碳BDCN材料在λ=420 nm处BDCN产H2O2的AQY达到27.8%,远高于许多其它现有光催化剂。上述工作充分说明了掺杂元素和引入缺陷协同作用在提升g-C3N4产H2O2性能方面所展现出的极大潜力。
-
本综述从光催化产H2O2的基本原理出发,结合氧化还原两方面提高g-C3N4光催化产H2O2的效率的有效途径,即构建异质结、修饰基团、空间结构调控、掺杂元素以及制造缺陷等,系统介绍了近年来该领域相关的重要研究成果。截至目前,尽管g-C3N4基材料在光催化产H2O2相关研究取得了一定的进展,但距实现工业化应用的目标仍存在很大差距。g-C3N4基材料光催化效率受光吸收范围窄和光生电荷复合易复合等制约的问题依然没有从根本上得到解决,两电子氧还原路径相对于单电子或四电子路径选择性低、产生的H2O2易分解以及水氧化动力学势垒高等问题仍是导致当前H2O2产生效率低下的主要原因。因此,可以从以下几个方面来进一步提高光催化剂的性能:
(1) 探索拓展g-C3N4光吸收范围的新思路。g-C3N4光吸收性能的优化主要体现在扩展光谱响应范围方面,尤其是对λ > 600 nm的可见光甚至红外光的吸收,以便更有效地利用太阳能,减缓紫外光对H2O2的分解;
(2) 寻找更好的方法来延长光生电子和空穴的寿命。在光催化反应过程中,必须有意引导光生载流子快速分离,以提升光催化产H2O2效率;
(3) 探索促进H2O2形成以及抑制其分解的新途径。已有的研究虽然在这方面做出了努力,然而效果并不令人满意。进一步的研究应集中在开发更合适的有机半导体方面,通过改变光催化材料的电子结构以及与其它改性手段相结合等方式进一步提升产H2O2性能;
(4) 加大对水氧化产H2O2过程的研究力度。截至目前,关于两电子氧还原产H2O2相关研究已经取得了一定进展,而对于两电子水氧化产H2O2的研究相对较少。在纯水和氧气共存的体系中,潜在的电子转移途径较为复杂,未来g-C3N4基材料通过氧化还原双路径制备H2O2将会是一个重点研究方向,提高水氧化产H2O2相关性能需要更深入的研究。
目前,g-C3N4在光催化产H2O2领域的研究热点依然主要集中在优化材料的形貌、结构和组成以提高其光催化效率。在未来的实际应用中,需综合考察g-C3N4基材料光催化产H2O2的效率、稳定性、成本和安全性等方面的性能。
g-C3N4基材料光催化产H2O2研究进展
Progress in photocatalytic production of H2O2 from g-C3N4-based materials
-
摘要: 开发节能环保的新型H2O2合成技术对解决蒽醌法存在的能耗高、污染重等问题具有重要意义,而基于半导体材料光催化水和氧气反应生成H2O2的方式被视为实现这一目标的潜在途径。本文从光催化产H2O2的基本原理出发,系统介绍了影响光催化材料产H2O2效率的因素及评价产H2O2性能的指标,并以g-C3N4为例重点解析了构建异质结、修饰基团、空间结构调控、掺杂元素以及制造缺陷等对提升光催化材料氧化还原产H2O2性能的积极影响,阐释了相关的作用机制并对g-C3N4基材料在光催化产H2O2领域的发展进行了总结和展望,旨在揭示光催化产H2O2领域的机遇与挑战,从而为推动该技术的发展提供理论和技术依据。Abstract: Development of new H2O2 synthesis technology with energy saving and environmental protection properties is of great significance for solving the problems of anthraquinone process, such as high energy cosumption and heavy pollution. Photocatalytic production of H2O2 from water and oxygen based on semiconductor is considered as a potential approach to achieve this goal. Herein, starting from the principle of phtocatalytic production of H2O2, we systematically introduced the impact factors that affecting the efficiency of photocatalysts for H2O2 production and the indexes for evaluating the performance of H2O2 production. Emphasis is followed by laid on analyzing the positive effects of constructing heterojunction, modifying groups, spatial structure regulation, doping elements and manufacturing defects on the performance of H2O2 production of g-C3N4 related materials. The relevant mechanism of reaction was illustrated, and the develoment of g-C3N4-based materials in the field of photocatalytic H2O2 production was also summarized and prospected, aiming to reveal the opportunities and challenges in the field of photocatalytic H2O2 production, so as to prvide theoretical and technical basis for promoting the development of the technology.
-
Key words:
- g-C3N4 /
- photocatalytic /
- H2O2 /
- oxygen reduction /
- water oxidation
-

-
表 1 g-C3N4基材料光催化产H2O2文献总结
Table 1. Summary of the photocatalytic H2O2 production with g-C3N4 based photocatalysts
材料
Material前驱体
Precursor牺牲剂
Sacrificial
Reagent催化剂浓度/
(mg·mL−1)
Concentration of photocatalyst照射条件
Irradiation
conditionsH2O2产量
H2O2 yields表观量子产率
AQY参考文献
ReferenceAu/g-C3N4 Dicyandiamide Ethanol 4.0 λ>420 nm 168.9 μmol·g−1·h−1 — [45] Ag@U-g-C3N4-NS Urea — 1.0 AM 1.5G 118.5 μmol·g−1·h−1 — [26] g-C3N4-CNTs Dicyandiamide Formic acid 1.0 λ≥420 nm 487 μmol·g−1·h−1 — [46] Ti3C2/g-C3N4 Urea Ethanol 1.0 λ>420 nm 560.7 mol·L−1·h−1 — [47] Ti3C2/porous g-C3N4 Dicyandiamide Isopropanol 1.0 λ>420 nm 131.7 μmol·g−1·h−1 — [48] Bi4O5Br2/g-C3N4 Melamine — 1.0 λ>420 nm 2.48 mmol·g−1·h−1 — [49] Cu2(OH)PO4/g-C3N4 Melamine — 1.0 AM 1.5G 1.2 mmol·g−1·h−1 — [27] ZnPPC-NBCN Melamine — 0.5 400-800 nm 114 mol·L−1·h−1 — [50] 3DOM g-C3N4-PW11 Cyanamide — 1.0 λ>320 nm 35 μmol·g−1·h−1 — [30] g-C3N4–SiW11 Cyanamide Methanol 1.0 AM 1.5G 152 μmol·g−1·h−1 6.5% at 420 nm [51] g-C3N4-PWO Dicyandiamide — 1.0 λ≥420 nm 630 μmol·g−1·h−1 — [52] ZIF-8/C3N4 Urea — 0.67 λ≥420 nm 2.46 mmol·g−1·h−1 19.57% at
420 nm[53] AQ/ g-C3N4 Melamine Isopropanol 0.5 AM 1.5G 361μmol·g−1·h−1 19.5% at 380 nm [54] g-C3N4/PDI/rGO Melamine — 1.67 λ>420 nm 24.17 μmol·g−1·h−1 6.1% at 420 nm [55] g-C3N4/BDI Melamine — 1.67 λ>420 nm 33.53 μmol·g−1·h−1 4.6% at 420 nm [29] g-C3N4/MTI Melamine — 1.67 λ>420 nm 22.47 μmol·g−1·h−1 6.1% at 420 nm [56] g-C3N4/PI Melamine — 1.0 λ>420 nm 1.24 mmol·g−1·h−1 — [28] g-C3N4/PEI Urea — 5.0 AM 1.5G 208.1μmol·g−1·h−1 2.12% at 420 nm [57] Mesoporous g-C3N4 Cyanamide Ethanol 4.0 λ>420 nm 183.5 μmol·g−1·h−1 — [35] g-C3N4 Aerogels Melamine — 1.67 λ>420 nm 28.24 μmol·g−1·h−1 — [31] Holey DCN Dicyandiamide Isopropanol 0.83 λ>420 nm 96.8 μmol·g−1·h−1 ~10% at
420 nm[58] g-C3N4/Co/AQ Melamine — 0.5 AM 1.5G 9.67 mmol·g−1·h−1 — [59] KTT g-C3N4 Melamine Isopropanol 1.0 λ>420 nm 40 mmol·g−1·h−1 20% at 400 nm [60] Cu-g-C3N4 Melamine — 1.0 λ>400 nm 1.35 mmol·g−1·h−1 — [61] MCN Melamine — 1.0 λ>400 nm 1.3 mmol·g−1·h−1 — [62] g-C3N4-Carbon Melamine Isopropanol 1.0 320—780 nm 10.6 mmol·g−1·h−1 — [39] Br-H/g-C3N4 Melamine EDTA 1.0 400—800 nm 1.99 mmol·g−1·h−1 — [63] OCN Dicyandiamide — 1.0 λ>420 nm 106 μmol·g−1·h−1 10.2% at 420 nm [34] KPF6/ g-C3N4 Melamine Ethanol 0.5 λ> 420 nm 600 μmol·g−1·h−1 24.3 % at
420 nm[64] KPD-CN Melamine Ethanol 0.5 λ>420 nm 6.0 mmol·g−1·h−1 8% at 420 nm [33] Cv-g-C3N4 Melamine — 1.0 λ>420 nm 900 μmol·g−1·h−1 — [65] CKCN Urea Ethanol 0.25 λ>420 nm 7.63 mol·g−1·h−1 11.8% at 420 nm [66] ACNN Urea Isopropanol 0.56 λ>420 nm 10.2 mmol·g−1·h−1 30.7% at 429 nm [67] BDCN Melamine Isopropanol 0.5 λ≥420 nm 5.74 mmol·g−1·h−1 27.8% at 420 nm [68] -
[1] XIA C, XIA Y, ZHU P, et al. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte [J]. Science, 2019, 366(6462): 226-231. doi: 10.1126/science.aay1844 [2] ZHAN W, JI L, GE Z M, et al. A continuous-flow synthesis of primary amides from hydrolysis of nitriles using hydrogen peroxide as oxidant [J]. Tetrahedron, 2018, 74(13): 1527-1532. doi: 10.1016/j.tet.2018.02.017 [3] RICHARDS T, HARRHY J H, LEWIS R J, et al. A residue-free approach to water disinfection using catalytic in situ generation of reactive oxygen species [J]. Nature Catalysis, 2021, 4(7): 575-585. doi: 10.1038/s41929-021-00642-w [4] BARAZESH J M, PRASSE C, WENK J, et al. Trace element removal in distributed drinking water treatment systems by cathodic H2O2 production and UV photolysis [J]. Environmental Science & Technology, 2018, 52(1): 195-204. [5] OROZCO S E, BISCHOF R H, BARBINI S, et al. Fate of lipophilic wood extractives in oxygen-based cellulose bleaching [J]. ACS Sustainable Chemistry & Engineering, 2021, 9(13): 4840-4849. [6] YANG Y, XUE Y S, HUANG F, et al. A facile microfluidic hydrogen peroxide fuel cell with high performance: Electrode interface and power-generation properties [J]. ACS Applied Energy Materials, 2018: acsaem.8b00943. doi: 10.1021/acsaem.8b00943 [7] YAN K, ZHU Y H, JI W H, et al. Visible light-driven membraneless photocatalytic fuel cell toward self-powered aptasensing of PCB77 [J]. Analytical Chemistry, 2018, 90(16): 9662-9666. doi: 10.1021/acs.analchem.8b02302 [8] GAO G H, TIAN Y N, GONG X X, et al. Advances in the production technology of hydrogen peroxide [J]. Chinese Journal of Catalysis, 2020, 41(7): 1039-1047. doi: 10.1016/S1872-2067(20)63562-8 [9] YANG S, VERDAGUER-CASADEVALL A, ARNARSON L, et al. Toward the decentralized electrochemical production of H2O2: A focus on the catalysis [J]. ACS Catalysis, 2018, 8(5): 4064-4081. doi: 10.1021/acscatal.8b00217 [10] RANGANATHAN S, SIEBER V. Recent advances in the direct synthesis of hydrogen peroxide using chemical catalysis—A review [J]. Catalysts, 2018, 8(9): 379. doi: 10.3390/catal8090379 [11] E FREITAS L F D L, PUÉRTOLAS B, ZHANG J, et al. Tunable catalytic performance of palladium nanoparticles for H2O2 direct synthesis via surface-bound ligands [J]. ACS Catalysis, 2020, 10(9): 5202-5207. doi: 10.1021/acscatal.0c01517 [12] LIU P, LIN Q, PAN H Y, et al. Direct synthesis of hydrogen peroxide from hydrogen and oxygen over yolk-shell nanocatalyst Pd@HCS with controlled Pd nanoparticle size [J]. Journal of Catalysis, 2019, 377: 511-523. doi: 10.1016/j.jcat.2019.07.044 [13] LIU J L, ZOU Y S, JIN B J, et al. Hydrogen peroxide production from solar water oxidation [J]. ACS Energy Letters, 2019, 4(12): 3018-3027. doi: 10.1021/acsenergylett.9b02199 [14] HAIDER Z, CHO H I, MOON G H, et al. Minireview: Selective production of hydrogen peroxide as a clean oxidant over structurally tailored carbon nitride photocatalysts [J]. Catalysis Today, 2019, 335: 55-64. doi: 10.1016/j.cattod.2018.11.067 [15] HOU H L, ZENG X K, ZHANG X W. Production of hydrogen peroxide by photocatalytic processes [J]. Angew. Chem. Int. Edit, 2020, 59(40): 17356-17376. doi: 10.1002/anie.201911609 [16] MOON G H, KIM W, BOKARE A D, et al. Solar production of H2O2on reduced graphene oxide–TiO2hybrid photocatalysts consisting of earth-abundant elements only [J]. Energy Environ Sci, 2014, 7(12): 4023-4028. doi: 10.1039/C4EE02757D [17] HONG Y, CHO Y, GO E M, et al. Unassisted photocatalytic H2O2 production under visible light by fluorinated polymer-TiO2 heterojunction [J]. Chemical Engineering Journal, 2021, 418: 129346. doi: 10.1016/j.cej.2021.129346 [18] CAO S, CHAN T S, LU Y R, et al. Photocatalytic pure water splitting with high efficiency and value by Pt/porous brookite TiO2 nanoflutes [J]. Nano Energy, 2020, 67: 104287. doi: 10.1016/j.nanoen.2019.104287 [19] FUKU K, TAKIOKA R, IWAMURA K, et al. Photocatalytic H2O2 production from O2 under visible light irradiation over phosphate ion-coated Pd nanoparticles-supported BiVO4 [J]. Applied Catalysis B:Environmental, 2020, 272: 119003. doi: 10.1016/j.apcatb.2020.119003 [20] SHI X J, ZHANG Y R, SIAHROSTAMI S, et al. Light-driven BiVO4 –C fuel cell with simultaneous production of H2O2 [J]. Advanced Energy Materials, 2018, 8(23): 1801158. doi: 10.1002/aenm.201801158 [21] GHOREISHIAN S M, RANJITH K S, PARK B, et al. Full-spectrum-responsive Bi2S3@CdS S-scheme heterostructure with intimated ultrathin RGO toward photocatalytic Cr(VI) reduction and H2O2 production: Experimental and DFT studies [J]. Chemical Engineering Journal, 2021, 419: 129530. doi: 10.1016/j.cej.2021.129530 [22] THAKUR S, KSHETRI T, KIM N H, et al. Sunlight-driven sustainable production of hydrogen peroxide using a CdS-graphene hybrid photocatalyst [J]. Journal of Catalysis, 2017, 345: 78-86. doi: 10.1016/j.jcat.2016.10.028 [23] SHIRAISHI Y, KANAZAWA S, KOFUJI Y, et al. Sunlight-driven hydrogen peroxide production from water and molecular oxygen by metal-free photocatalysts [J]. Angewandte Chemie (International Ed. in English), 2014, 53(49): 13454-13459. doi: 10.1002/anie.201407938 [24] SHIRAISHI Y, TAKII T, HAGI T, et al. Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion [J]. Nature Materials, 2019, 18(9): 985-993. doi: 10.1038/s41563-019-0398-0 [25] CHEN L, WANG L, WAN Y Y, et al. Acetylene and diacetylene functionalized covalent triazine frameworks as metal-free photocatalysts for hydrogen peroxide production: A new two-electron water oxidation pathway [J]. Advanced Materials (Deerfield Beach, Fla. ), 2020, 32(2): e1904433. doi: 10.1002/adma.201904433 [26] CAI J S, HUANG J Y, WANG S C, et al. Crafting mussel-inspired metal nanoparticle-decorated ultrathin graphitic carbon nitride for the degradation of chemical pollutants and production of chemical resources [J]. Advanced Materials (Deerfield Beach, Fla. ), 2019, 31(15): e1806314. doi: 10.1002/adma.201806314 [27] WANG X W, HAN Z, YU L H, et al. Synthesis of full-spectrum-response Cu2(OH)PO4/g-C3N4 photocatalyst with outstanding photocatalytic H2O2 production performance via a “two channel route” [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 14542-14553. [28] YANG L P, DONG G H, JACOBS D L, et al. Two-channel photocatalytic production of H2O2 over g-C3N4 nanosheets modified with perylene imides [J]. Journal of Catalysis, 2017, 352: 274-281. doi: 10.1016/j.jcat.2017.05.010 [29] KOFUJI Y, OHKITA S, SHIRAISHI Y, et al. Graphitic carbon nitride doped with biphenyl diimide: Efficient photocatalyst for hydrogen peroxide production from water and molecular oxygen by sunlight [J]. ACS Catalysis, 2016, 6(10): 7021-7029. doi: 10.1021/acscatal.6b02367 [30] ZHAO S, ZHAO X, ZHANG H, et al. Covalent combination of polyoxometalate and graphitic carbon nitride for light-driven hydrogen peroxide production [J]. Nano Energy, 2017, 35: 405-414. doi: 10.1016/j.nanoen.2017.04.017 [31] OU H H, YANG P J, LIN L H, et al. Carbon nitride aerogels for the photoredox conversion of water [J]. Angewandte Chemie (International Ed. in English), 2017, 56(36): 10905-10910. doi: 10.1002/anie.201705926 [32] ZHOU L, FENG J R, QIU B C, et al. Ultrathin g-C3N4 nanosheet with hierarchical pores and desirable energy band for highly efficient H2O2 production [J]. Applied Catalysis B:Environmental, 2020, 267: 118396. doi: 10.1016/j.apcatb.2019.118396 [33] MOON G H, FUJITSUKA M, KIM S, et al. Eco-friendly photochemical production of H2O2 through O2 reduction over carbon nitride frameworks incorporated with multiple heteroelements [J]. ACS Catalysis, 2017, 7(4): 2886-2895. doi: 10.1021/acscatal.6b03334 [34] WEI Z, LIU M L, ZHANG Z J, et al. Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers [J]. Energy & Environmental Science, 2018, 11(9): 2581-2589. [35] SHIRAISHI Y, KOFUJI Y, SAKAMOTO H, et al. Effects of surface defects on photocatalytic H2O2 production by mesoporous graphitic carbon nitride under visible light irradiation [J]. ACS Catalysis, 2015, 5(5): 3058-3066. doi: 10.1021/acscatal.5b00408 [36] ZHU C, ZHU M M, SUN Y, et al. Carbon-supported oxygen vacancy-rich Co3O4 for robust photocatalytic H2O2 production via coupled water oxidation and oxygen reduction reaction [J]. ACS Applied Energy Materials, 2019, 2(12): 8737-8746. doi: 10.1021/acsaem.9b01712 [37] CAO J J, WANG H, ZHAO Y J, et al. Phosphorus-doped porous carbon nitride for efficient sole production of hydrogen peroxide via photocatalytic water splitting with a two-channel pathway [J]. Journal of Materials Chemistry A, 2020, 8(7): 3701-3707. doi: 10.1039/C9TA13929J [38] LI X H, ZHANG J, ZHOU F, et al. Preparation of N-vacancy-doped g-C3N4 with outstanding photocatalytic H2O2 production ability by dielectric barrier discharge plasma treatment [J]. Chinese Journal of Catalysis, 2018, 39(6): 1090-1098. doi: 10.1016/S1872-2067(18)63046-3 [39] WANG R R, ZHANG X, LI F, et al. Energy-level dependent H2O2 production on metal-free, carbon-content tunable carbon nitride photocatalysts [J]. Journal of Energy Chemistry, 2018, 27(2): 343-350. doi: 10.1016/j.jechem.2017.12.014 [40] WANG R R, PAN K C, HAN D D, et al. Solar-driven H2O2 generation from H2O and O2 using earth-abundant mixed-metal Oxide@Carbon nitride photocatalysts [J]. ChemSusChem, 2016, 9(17): 2470-2479. doi: 10.1002/cssc.201600705 [41] KISCH H, BAHNEMANN D. Best practice in photocatalysis: Comparing rates or apparent quantum yields? [J]. The Journal of Physical Chemistry Letters, 2015, 6(10): 1907-1910. doi: 10.1021/acs.jpclett.5b00521 [42] SASAKI Y, NEMOTO H, SAITO K, et al. Solar water splitting using powdered photocatalysts driven by Z-schematic interparticle electron transfer without an electron mediator [J]. The Journal of Physical Chemistry C, 2009, 113(40): 17536-17542. doi: 10.1021/jp907128k [43] ASTM G173-03(2020), Standard tables for reference solar spectral irradiances: Direct normal and hemispherical on 37° tilted surface, ASTM International, West Conshohocken, PA, 2020, www. astm. org. [44] 张金水, 王博, 王心晨. 氮化碳聚合物半导体光催化 [J]. 化学进展, 2014, 26(1): 19-29. doi: 10.7536/PC130519 ZHANG J S, WANG B, WANG X C. Carbon nitride polymeric semiconductor for photocatalysis [J]. Progress in Chemistry, 2014, 26(1): 19-29(in Chinese). doi: 10.7536/PC130519
[45] ZUO G F, LIU S S, WANG L, et al. Finely dispersed Au nanoparticles on graphitic carbon nitride as highly active photocatalyst for hydrogen peroxide production [J]. Catalysis Communications, 2019, 123: 69-72. doi: 10.1016/j.catcom.2019.02.011 [46] ZHAO S, GUO T, LI X, et al. Carbon nanotubes covalent combined with graphitic carbon nitride for photocatalytic hydrogen peroxide production under visible light [J]. Applied Catalysis B:Environmental, 2018, 224: 725-732. doi: 10.1016/j.apcatb.2017.11.005 [47] YANG Y, ZENG Z T, ZENG G M, et al. Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production [J]. Applied Catalysis B:Environmental, 2019, 258: 117956. doi: 10.1016/j.apcatb.2019.117956 [48] LIN S F, ZHANG N, WANG F C, et al. Carbon vacancy mediated incorporation of Ti3C2 quantum dots in a 3D inverse opal g-C3N4 Schottky junction catalyst for photocatalytic H2O2 production [J]. ACS Sustainable Chemistry & Engineering, 2021, 9(1): 481-488. [49] ZHAO X S, YOU Y Y, HUANG S B, et al. Z-scheme photocatalytic production of hydrogen peroxide over Bi4O5Br2/g-C3N4 heterostructure under visible light [J]. Applied Catalysis B:Environmental, 2020, 278: 119251. doi: 10.1016/j.apcatb.2020.119251 [50] YE Y X, PAN J H, XIE F Y, et al. Highly efficient photosynthesis of hydrogen peroxide in ambient conditions [J]. PNAS, 2021, 118(16): e2103964118. doi: 10.1073/pnas.2103964118 [51] ZHAO S, ZHAO X, OUYANG S X, et al. Polyoxometalates covalently combined with graphitic carbon nitride for photocatalytic hydrogen peroxide production [J]. Catalysis Science & Technology, 2018, 8(6): 1686-1695. [52] ZHAO S, ZHAO X. Polyoxometalates-derived metal oxides incorporated into graphitic carbon nitride framework for photocatalytic hydrogen peroxide production under visible light [J]. Journal of Catalysis, 2018, 366: 98-106. doi: 10.1016/j.jcat.2018.08.003 [53] ZHAO Y J, LIU Y, CAO J J, et al. Efficient production of H2O2 via two-channel pathway over ZIF-8/C3N4 composite photocatalyst without any sacrificial agent [J]. Applied Catalysis B:Environmental, 2020, 278: 119289. doi: 10.1016/j.apcatb.2020.119289 [54] KIM H I, CHOI Y, HU S, et al. Photocatalytic hydrogen peroxide production by anthraquinone-augmented polymeric carbon nitride [J]. Applied Catalysis B:Environmental, 2018, 229: 121-129. doi: 10.1016/j.apcatb.2018.01.060 [55] KOFUJI Y, ISOBE Y, SHIRAISHI Y, et al. Carbon nitride-aromatic diimide-graphene nanohybrids: Metal-free photocatalysts for solar-to-hydrogen peroxide energy conversion with 0.2% efficiency [J]. Journal of the American Chemical Society, 2016, 138(31): 10019-10025. doi: 10.1021/jacs.6b05806 [56] KOFUJI Y, OHKITA S, SHIRAISHI Y, et al. Mellitic triimide-doped carbon nitride as sunlight-driven photocatalysts for hydrogen peroxide production [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 6478-6485. [57] ZENG X K, LIU Y, KANG Y, et al. Simultaneously tuning charge separation and oxygen reduction pathway on graphitic carbon nitride by polyethylenimine for boosted photocatalytic hydrogen peroxide production [J]. ACS Catalysis, 2020, 10(6): 3697-3706. doi: 10.1021/acscatal.9b05247 [58] SHI L, YANG L Q, ZHOU W, et al. Photoassisted construction of holey defective g-C3 N4 photocatalysts for efficient visible-light-driven H2 O2 production [J]. Small, 2018, 14(9): 1703142. doi: 10.1002/smll.201703142 [59] CHU C H, ZHU Q H, PAN Z H, et al. Spatially separating redox centers on 2D carbon nitride with cobalt single atom for photocatalytic H2O2 production [J]. PNAS, 2020, 117(12): 6376-6382. doi: 10.1073/pnas.1913403117 [60] ZHANG J Z, YU C Y, LANG J Y, et al. Modulation of Lewis acidic-basic sites for efficient photocatalytic H2O2 production over potassium intercalated tri-s-triazine materials [J]. Applied Catalysis B:Environmental, 2020, 277: 119225. doi: 10.1016/j.apcatb.2020.119225 [61] HU S Z, QU X Y, LI P, et al. Photocatalytic oxygen reduction to hydrogen peroxide over copper doped graphitic carbon nitride hollow microsphere: The effect of Cu(I)-N active sites [J]. Chemical Engineering Journal, 2018, 334: 410-418. doi: 10.1016/j.cej.2017.10.016 [62] QU X Y, HU S Z, BAI J, et al. Synthesis of band gap-tunable alkali metal modified graphitic carbon nitride with outstanding photocatalytic H2O2 production ability via molten salt method [J]. Journal of Materials Science & Technology, 2018, 34(10): 1932-1938. [63] ZHANG C L, BAI J, MA L, et al. Synthesis of halogen doped graphite carbon nitride nanorods with outstanding photocatalytic H2O2 production ability via saturated NH4X (X = Cl, Br) solution-hydrothermal post-treatment [J]. Diamond and Related Materials, 2018, 87: 215-222. doi: 10.1016/j.diamond.2018.06.013 [64] KIM S, MOON G H, KIM H, et al. Selective charge transfer to dioxygen on KPF6-modified carbon nitride for photocatalytic synthesis of H2O2 under visible light [J]. Journal of Catalysis, 2018, 357: 51-58. doi: 10.1016/j.jcat.2017.10.002 [65] LI S N, DONG G H, HAILILI R, et al. Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies [J]. Applied Catalysis B:Environmental, 2016, 190: 26-35. doi: 10.1016/j.apcatb.2016.03.004 [66] XIE Y, LI Y X, HUANG Z H, et al. Two types of cooperative nitrogen vacancies in polymeric carbon nitride for efficient solar-driven H2O2 evolution [J]. Applied Catalysis B:Environmental, 2020, 265: 118581. doi: 10.1016/j.apcatb.2019.118581 [67] WU S, YU H T, CHEN S, et al. Enhanced photocatalytic H2O2 production over carbon nitride by doping and defect engineering [J]. ACS Catalysis, 2020, 10(24): 14380-14389. doi: 10.1021/acscatal.0c03359 [68] FENG C Y, TANG L, DENG Y C, et al. Synthesis of leaf-vein-like g-C3 N4 with tunable band structures and charge transfer properties for selective photocatalytic H2O2 evolution [J]. Advanced Functional Materials, 2020, 30(39): 2001922. doi: 10.1002/adfm.202001922 [69] TENG Z Y, ZHANG Q T, YANG H B, et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide [J]. Nature Catalysis, 2021, 4(5): 374-384. doi: 10.1038/s41929-021-00605-1 [70] ZHANG P, TONG Y W, LIU Y, et al. Heteroatom dopants promote two-electron O2 reduction for photocatalytic production of H2O2 on polymeric carbon nitride [J]. Angewandte Chemie International Edition, 2020, 59(37): 16209-16217. doi: 10.1002/anie.202006747 -



 下载:
下载: