-
日益严格的环境管理政策及污染物排放标准的实施,持续推动我国污水处理从常规二级处理向污水深度处理(尤其是总氮)的转变。反硝化生物滤池作为生物法深度脱氮的主流工艺,在污水处理厂的提标改造中应用较广[1]。然而,目前在我国,污水处理厂二级生化尾水等污水的一大特征是低碳氮比,反硝化将被抑制,造成亚硝酸盐的累积以及反硝化效率低等问题,导致出水难以达标[2-3]。目前,为实现对低碳氮废水的深度脱氮,反硝化滤池主要通过进水处直接添加甲醇、乙酸钠、乙醇等外加碳源作为电子供体,但会造成运行成本高和出水COD超标的问题[4]。因此,采用缓释碳源材料作为滤池滤料成为反硝化脱氮的研究热点之一,其主要特点是无需持续投加碳源且可提供微生物附着位点。
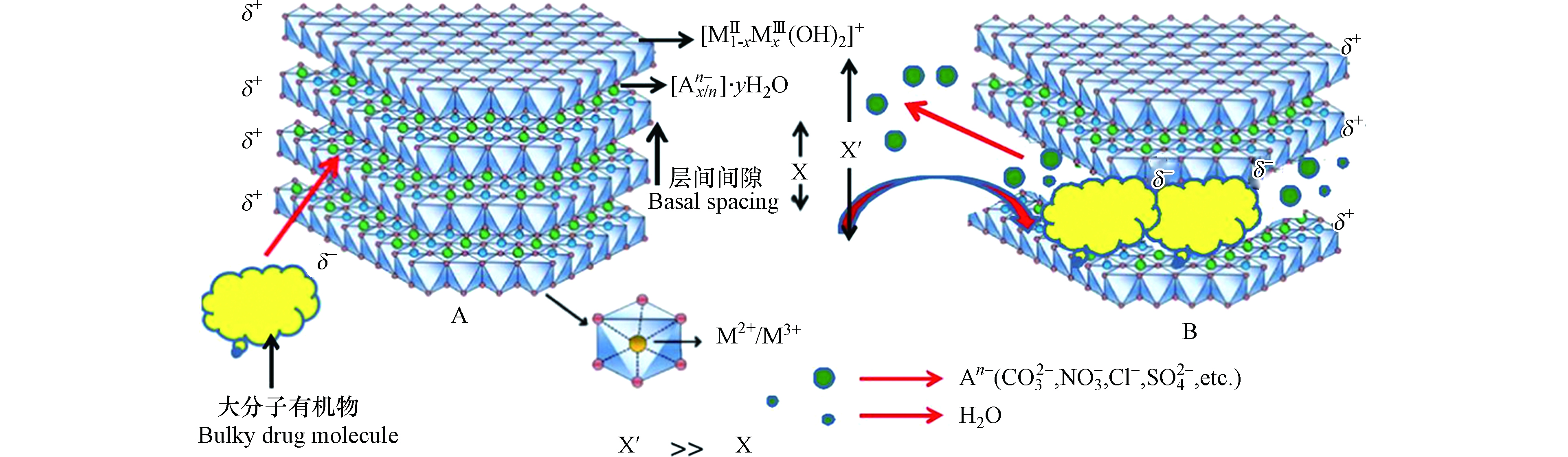
释碳材料包括天然释碳材料(秸秆、稻壳、木屑等[5-7])和可生物降解的聚合物(聚乳酸、聚乙烯醇、聚丁二酸丁二醇酯等[8-11])。使用天然释碳材料虽然价格低廉,但其脱氮效果不佳,反硝化负荷较低,用聚合物作为碳源可实现较理想的脱氮效果,但是其成本相对较高。层状双金属化合物(LDHs)结构示意图如图1,LDHs具有双金属(三价金属离子和二价金属离子)层状结构,层间结合负电离子,层间存在氢键,紧密结合,结构稳定,具有良好的机械性能和缓释性能[12]。若将LDHs用于制备释碳材料的骨架、并将其与较为廉价的有机碳源混合制备反硝化滤料,既可实现缓释碳效果,又能适当降低运行成本。如Islam等[13]合成Zn-Al-Cl-LDH碳源,使用0.3 g的碳源置于100 mL初始浓度为10 mg·L−1的硝酸盐溶液中,硝酸盐去除率为85.5%。Fang等[14]制备Bio-ceramic/Zn-LDHs,将其用于去除模拟废水的氨氮,最高去除率可达50%。Jiang等[15]针对微量金属(铁、锰、铜、锌、钼)对反硝化活性的影响研究,发现铁可显著提高生物反硝化速率。Chen等[16]将含金属镍的层状双金属纳米颗粒用于反硝化以改善微生物脱氮性能。Mg2+和Al3+是合成LDHs最常用的二价和三价金属离子,Ni2+和Fe3+可以赋予LDHs更好的释放性能,并能促进反硝化活性和反硝化速率[17]。同时,有机物羟甲基纤维素钠(CMC)具有黏合性且较为廉价。故使用CMC作为LDHs缓释碳源的有机碳源和包埋法制备滤料中的黏合剂,选用Fe3+、Ni2+等金属离子制备LDHs缓释碳源的方法具有潜在有效性,且此方案在国内外鲜见报道。
因此,本研究选择Fe3+、Al3+、Ni2+、Mg2+等4种金属离子,率先选用CMC为负电离子用于制备基于层状双金属化合物的缓释碳源材料,并初步筛选出碳源FeNi-LDH-CMC用于LDHs基滤料的制备。对制得的LDHs基滤料进行系统研究,确定了各组成物质最优质量分数及释碳性能。选用最优LDHs基滤料,构建反硝化滤池反应器,以低碳氮比模拟废水作为处理对象,综合分析不同水力停留时间下反硝化滤池对废水的处理效果并探究其机理,结果表明,新型LDHs基缓释碳源滤料可提高反硝化滤池对低碳氮比废水的深度脱氮效果,具有较强的推广应用前景。
-
实验试剂:氢氧化钠、六水三氯化铁、六水三氯化铝、六水氯化镍、六水氯化镁、羧甲基纤维素钠(CMC)、聚乙烯醇(PVA)、硼酸、无水氯化钙、乙酸钠、硝酸钾、磷酸二氢钾、碳酸氢钠、高纯氮气等,所用试剂纯度均在98%及以上。
实验仪器:总有机碳测定仪(Multi+N/C 3000)、电子分析天平(AL104)、紫外可见分光光度计(UV-2700)、电热恒温鼓风干燥箱(DHG-9070A)、恒温加热搅拌器(DF-101S)、低温冷冻离心机(Centrifuge5810R)、pH仪(SG7型)、COD快速消解仪(DRB2000)、傅里叶变换红外光谱仪(NEXUS870)、X射线光电子能谱仪(ESCALAB 250)、超纯水系统(Milli-Q)、荧光定量PCR仪(ABI 7500)、Nanodrop分光光度计(ND-100)等。
-
LDHs基缓释碳源材料采用共沉淀法制备而成[18],具体步骤如下:称量一定的FeCl3·6H2O和NiCl2·6H2O,使二者的物质的量之比为2:3,溶于一定体积的去离子水中,将含有Fe3+、Ni2+的溶液置于1 L的三口瓶中。三口瓶置于恒温搅拌器中,在氮气保护环境下持续搅拌,然后滴加羟甲基纤维素钠溶液,用2 mol·L−1的NaOH溶液调节溶液的pH值为9.0左右。加热三口瓶使温度维持在75 ℃左右,搅拌12 h,需添加冷凝装置。对得到的混合液进行冷却、离心,并用去离子水洗涤至少3次,在65 ℃的烘箱中烘干48 h。再经研磨,不同目数的筛网过筛,得到3种粒径的FeNi-LDH-CMC碳源。实验中所用的去离子水需提前氮吹30 min,除去其中的氧气。以此方法也可得到FeMg-LDH-CMC、AlNi-LDH-CMC和AlMg-LDH-CMC等 3种碳源材料,此3种碳源材料在制备过程中三价与二价金属离子的物质的量之比为2:3。
基滤料采用包埋法制备而成[19],具体步骤如下:称量一定量的PVA,在90 ℃条件下溶解于去离子水中,然后下调温度至60 ℃。称量一定量的CMC完全溶解其中,再称量本研究中已制备好的LDHs-CMC缓释碳源材料,加入到混合液中。充分混合之后,待混合液冷却至40 ℃左右,将混合液以100—300 μL·s−1的速度滴加到质量分数为4%的CaCl2饱和硼酸溶液中,交联24 h,得到粒径为3—5 mm的颗粒,在65 ℃条件下干燥48 h,即得LDHs基滤料。
-
取不同粒径的4种碳源材料FeNi-LDH-CMC、FeMg-LDH-CMC、AlNi-LDH-CMC和AlMg-LDH-CMC各0.5 g,溶于250 mL超纯水中,进行快速静态释碳实验[20]。在1、2、4、6、8、10、12、24、48、72、96、120、144、168 h取样,经过0.45 μm醋酸纤维素滤膜过滤后置于4 ℃冰箱内保存至测定COD。
-
使用Cu-Kα辐射(λ=0.15418 nm)以4 min−1的扫描速度重新采集5°—80°之间的粉末X射线衍射(XRD)数据。仪器在40 kV和40 mA下运行,用于确定低密度脂蛋白样品的相结构。使用PHI5000 VersaProbe使用带有AlKa射线源的X射线光电子能谱(XPS,Thermo Scientific K-Alpha+)分析释碳材料的不同价态元素所占比例。
-
根据单因素实验分析,已初步判断了PVA、CMC、FeNi-LDH-CMC的较佳配比,为响应面实验提供范围依据。响应面实验采用Design-Expert.V8.0.6.1软件设计,共17组相关设计见表1。
LDHs基滤料用于投加至反硝化生物滤池中为缓慢释放碳源,需要绘制LDHs基滤料的释碳曲线,操作步骤为:称量不同配比的LDHs基滤料、PVA、CMC各2 g,溶于1 L超纯水中,在1、2、4、6、8、10、12、24、48、72、96、120、144、168 h取样,经过0.45 μm醋酸纤维素滤膜过滤后,置于4 ℃冰箱内保存至测定COD。相关评价的方程为:K=cm/t1/2,其中,K为释碳系数,表示碳源释放过程受到的阻力,K值越低,缓释碳源滤料释碳性能越好,cm为单位缓释碳源滤料在溶液中释放的饱和COD值,t1/2为单位缓释碳源滤料在溶液中释放的COD达饱和浓度一半时所用时间[21]。
-
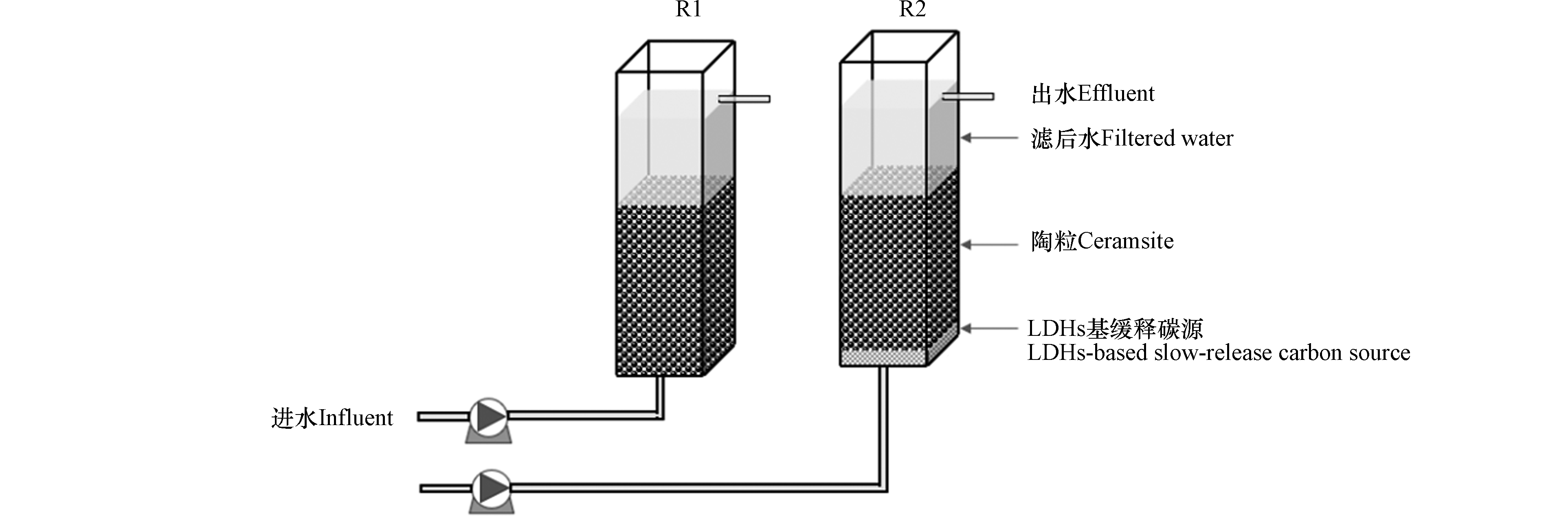
本研究共设置2个实验室规模的反硝化滤池,装置示意图如图2 所示。
反应器由有机玻璃制成,总有效容积为3 L,长和宽均为10 cm,总高度35 cm,滤料填充层的高度为20 cm,水位高度为10 cm左右。其中陶粒滤料购买自安徽华祺环保,直径为3—5 mm,有效比表面积为500—600 m2·m−3,密度为1.5—1.6 g·cm−3。反应器温度为25 ℃,基于反硝化滤池实际运行时间和脱氮负荷,水力停留时间设置为6 h和3 h。R1-1、R2-1代表HRT为6 h时的反应器,R1-2、R2-2代表HRT为3 h时的反应器。R2反应器中FeNi-LDH-CMC碳源滤料投加的位置在反应器的最下端(反应器的进口处),用纱布包裹,便于更换。
LDHs基滤料的投加量可根据模拟进水组分及TN低于5 mg·L−1的处理目标确定。根据前期调研相关数据,反硝化生物滤池进水中的碳氮比为5:1时,可实现处理目标,故本实验需投加LDHs基滤料补充COD值为100 mg·L−1。经过实际测算,此反硝化生物滤池静态运行过程中,废水的容积约为1.6 L,日处理量约为6.4 L,则LDHs基滤料投加补充的有机碳值为0.64 g,本研究中滤料的最大释碳量为320.96 mg·g−1,则每天需要的LDHs基滤料质量约为2 g,LDHs基滤料的密度为0.83 g·cm−3,则每天所需LDHs基滤料的体积为2.4 cm3,为满足预期的脱氮效果,拟运行60 d需要投加的LDHs滤料的理论体积为144 cm3,其与陶粒的体积比约为1:12.9。但实际操作中,考虑碳源流失等情况,适当增加LDHs基滤料与陶粒的体积比至1:10。
本研究使用人工合成低碳氮比废水作为进水,进水中COD为150 mg·L−1,硝态氮为50 mg·L−1,碳氮比为3:1,pH值在8.10±0.15。模拟废水详细组分见表2。
本研究反硝化滤池的挂膜方式采用接种挂膜法,接种污泥取自南京市某市政污水处理厂氧化沟工艺缺氧段,混合液悬浮固体浓度(mixed liquor suspended solids, MLSS)在3000 mg·L−1左右,活性污泥倒入反硝化滤池内至淹没滤料,闷曝24 h后将接种活性污泥全部排出并开始连续通入模拟废水,反硝化滤池采用上流式连续运行2个月。实验进水溶解氧浓度为0.29—0.41 mg·L−1,低于0.5 mg·L−1。
在反应器运行期间,每2—3 d采集反应器出水1次并测定水质指标:NO3–-N、NO2–-N、TFe,测定方法参考国家环保总局颁布的水和废水监测和分析方法,TOC和TN采用总有机碳测定仪进行测定。
-
在反应器连续运行的第30天和第60天,对反应器内滤料表面生物膜进行取样,在8000 r·min−1下离心5 min,使用Fast DNA Soil Kit(MP Biomedicals,CA,USA)将离心后的生物质用于DNA提取。对提取得到DNA产物采用Nanodrop分光光度计进行纯度和浓度的测定,符合要求后送至上海美吉生物医药科技有限公司进行琼脂凝胶电泳检测、PCR扩增、扩增纯化和测序,其中PCR扩增区域为16S rRNA基因V3-V4区域,所用的正向引物序列为341F (CCCTACACGACGCTCTTCCGATCTG),反向引物序列为805R(GACTGGAGTTCCTTGGCACCCGAGAATTCCA)。
-
对1.7节中提取得到的DNA样品进行实时荧光定量PCR(qPCR)扩增,选取微生物反硝化脱氮过程中氮素转化的重要基因:硝酸盐还原酶(nitrate reductase, narG)、周质硝酸盐还原酶(pericytic nitrate reductase, napA)、亚硝酸盐还原酶(nitrite reductase, nirK和nirS)和一氧化二氮还原酶(nitrous oxide reductase, nosZ)。在NCBI数据库中搜索并确定各基因片段序列,并委托上海捷瑞生物工程有限公司进行质粒标准品及正反引物的构建,根据质粒标准品绘制标准曲线,并计算出各功能基因的拷贝数进行分析[22]。实验过程设计的引物序列、基因片段长度和扩增实验条件见表3.
-
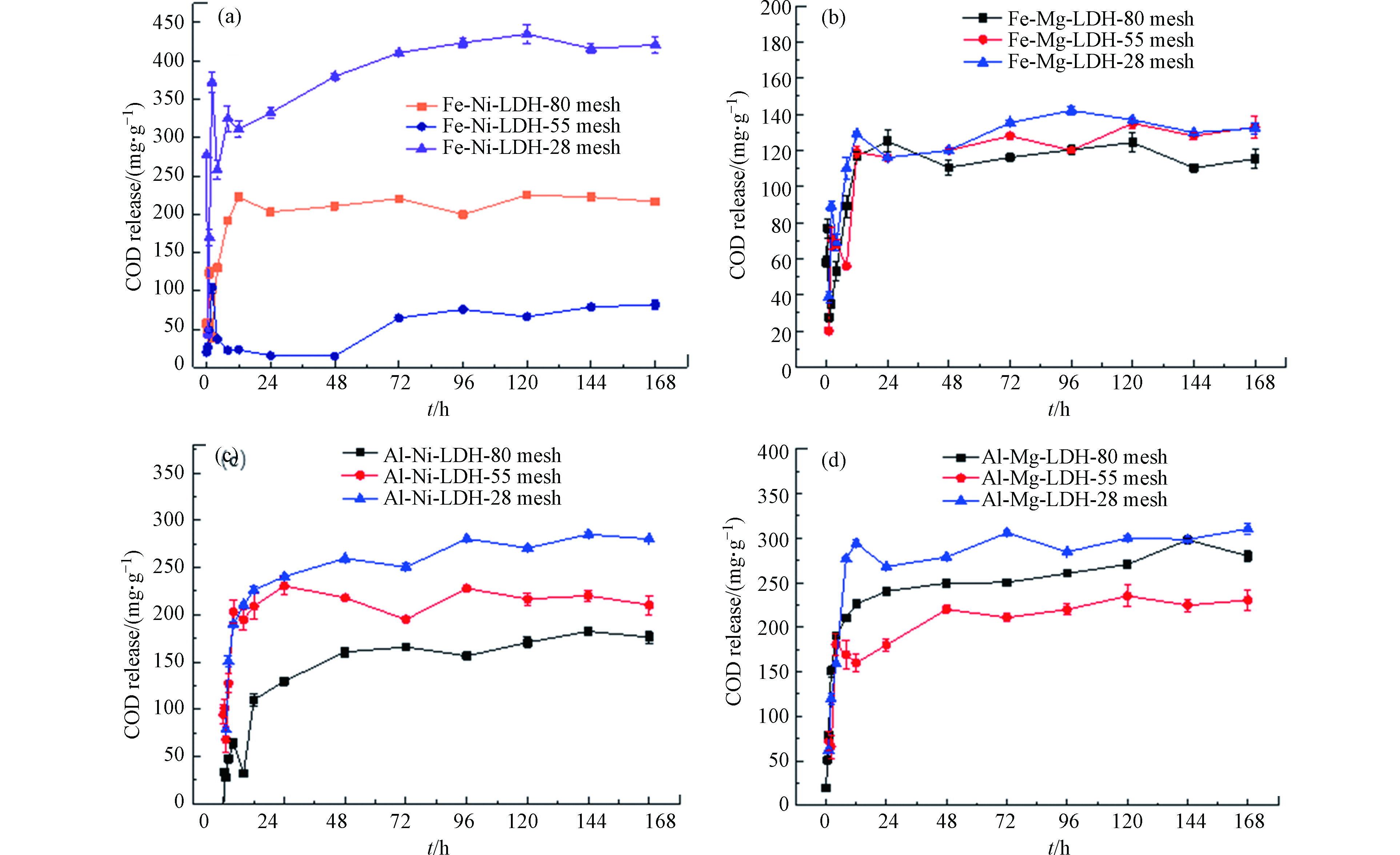
对制得的FeNi-LDH-CMC、FeMg-LDH-CMC、AlNi-LDH-CMC和AlMg-LDH-CMC等4种缓释碳源进行释碳性能研究,比较不同种类、不同粒径的缓释碳源之间的差异。结果如图3所示。从图3可知,缓释碳源的粒径对释碳性能有一定的影响,且不同金属种类合成的碳源,存在较大差异。
释碳量最大的碳源材料是28目的FeNi-LDH-CMC,其最大COD释放量为(420.36±5.36) mg·g−1;对于FeMg-LDH-CMC碳源,其释碳量随粒径的改变并不明显变化,最大COD释放量在(115.32—136.94) mg·g−1。在实验涉及到的范围内,AlNi-LDH-CMC碳源粒径越大,释碳量越大;对于FeNi-LDH-CMC、AlMg-LDH-CMC这两种碳源,释碳量均存在28目>80目>55目的规律。这与Jiang等[15]的结论不一致,可能是因为对于含铁的层状双金属碳源,Ni2+和Mg2+对其晶型结构的改变情况不同。
通过对比不同碳源的释碳量及缓释速率可知,最优碳源28目的FeNi-LDH-CMC碳源,其释碳能力最强,且释碳速率较为缓慢。这可能是因为Fe3+的电负性高于Al3+,Ni2+的电负性高于Mg2+,金属阳离子和阴离子之间的静电吸引力总是随着金属电荷密度的增加而增强,故FeNi-LDH-CMC碳源的正电荷密度高于其他离子,其中的Fe3+与Ni2+之间静电吸引力最大,结构更稳定,缓释速率也最慢[15]。
-
FeNi-LDH-CMC、AlMg-LDH-CMC、AlNi-LDH-CMC、FeMg-LDH-CMC等4种缓释碳源和CMC的XRD和XPS如图4和图5所示。
XRD研究了缓释碳源的结晶特征。CMC在20°处有峰,而4种碳源均无,说明CMC与金属离子并非简单的混合,而是进行了晶态结合。FeNi-LDH-CMC、AlMg-LDH-CMC、AlNi-LDH-CMC的 3种碳源晶态良好,FeMg-LDH-CMC碳源未观察到明显的峰,说明结晶状态不好,结构不稳定,这也解释了在实验周期内其释碳速率较快,且释碳量随粒径变化不大。碳源材料AlNi-LDH-CMC与AlMg-LDH-CMC的图谱类似,峰数一致,而峰值有所差异,说明在同一种金属阳离子存在时,阴离子对晶体的结构仅表现在峰值,而不影响特征峰。
表4所示为根据XPS数据计算出的4种碳源材料中主要元素含量,AlNi-LDH-CMC、AlMg-LDH-CMC、FeNi-LDH-CMC、FeMg-LDH-CMC的4种碳源由于掺杂了金属元素,其含碳量(25.1%、21.18%、33.14%、33.33%)均低于CMC(53.39%),FeMg-LDH-CMC碳源的碳元素相对含量最高,FeNi-LDH-CMC碳源略低。值得关注的是,根据实验设计,投加的三价与二价金属物质的量之比为2:3,而FeNi-LDH-CMC碳源中,元素Fe与Ni的物质的量之比约为0.38:1,其余碳源三价与二价金属元素比例均小于1:10,仅FeNi-LDH-CMC碳源中三价与二价金属的物质的量之比最接近实验设计投加的比例,说明FeNi-LDH-CMC碳源中金属离子利用率更高,结合更紧密,结构更稳定,也进一步证明了其良好的缓释性能。故本实验采用FeNi-LDH-CMC碳源材料制备LDHs基缓释碳源,用于反硝化滤池处理低碳氮比废水。另外,4种碳源的氧含量均大于CMC,可能是由于层状双金属结构之间存在大量羟基[12]。
-
响应面实验结果见表5,软件拟合的方程式为:K=30.51950−1.58888×PVA−4.59338×LDH−9.92300×CMC−0.076875×PVA×LDH−0.98500×PVA×CMC+0.32000×LDH×CMC+0.39681×PVA2+0.29431×LDH2+5.57900×CMC2。其中,K代表释碳系数;PVA、LDH、CMC分别代表PVA、FeNi-LDH-CMC、CMC的质量分数。
由图6可知,在某个因素不变的情况下,其它两个因素的交互作用对释碳系数K的影响。
图6(a)中,当CMC质量分数为1%时,随着FeNi-LDH-CMC碳源质量分数和PVA的升高,K值先减小后增大,存在最佳的含量值;图6(b)中,随着CMC质量分数的增加,释碳系数K呈现先降低后升高的规律,说明CMC也存在一个最佳浓度;由图6(c)可知,释碳系数K随FeNi-LDH-CMC碳源的质量分数变化不大。因为PVA的用量影响着载体本身的强度和牢固程度,超过最佳浓度后由于PVA本身密度增大,导致孔隙变小,固定CMC的量减少,释放变快。同时,由于CMC与交联剂溶液中的氯化钙发生交联反应,能够增强材料的强度,对材料的牢固程度有影响,最佳浓度时材料的释碳阻力较大,释碳过程更缓慢[23]。
由响应面软件模拟结果可知,当PVA、CMC和FeNi-LDH-CMC碳源的质量分数分别为4.02%、1.02%、7.77%时,K值最小,为4.40,由实际实验验证,此时LDHs基缓释碳源最大COD释放量为320.96 mg·g−1,接近FeNi-LDH-CMC碳源材料的最大COD释放量。
-
各反应器对有机物(以TOC计)、总氮(TN)、NO3–-N、NO2–-N的去除效果如图7所示。由于LDHs基滤料的投加,R2反应器的TOC值(>40 mg·L−1)明显大于R1(<20 mg·L−1)。且R2中出水TN明显低于R1(P<0.05),说明LDHs基滤料投加后,促进了异养反硝化菌的反硝化作用[24]。LDHs基滤料的投加使TN去除率提高了35.32%—38.46%(P<0.05),一方面,LDHs基滤料投加使得反应器中的有机碳含量升高,促进反硝化作用,另一方面,LDHs基滤料中的碳源中含有少量的Fe3+,对反硝化也起到了一定的促进作用。在HRT为6 h时,投加LDHs基滤料之后,出水TN可达中国污水排放一级A标准(≤15 mg·L−1)。在HRT为3 h时,R2中反应器出水的TN并未随HRT的改变而产生很大波动,说明投加LDHs基滤料之后,异养反应器的受冲击能力更强。在HRT为3 h时,由图7(c)和(d)可知,出水的NO3−-N与TN的波动较为同步,同时说明普通异养反应器TN去除率较低,关键在于NO3−-N并未去除。所有反应器的NO2−-N基本在10 mg·L−1以内波动,且在R1中最高,这是因为废水中碳源较低容易导致反硝化不完全,进而造成亚硝酸盐的累积[25]。在HRT变为3 h后,R1出水中NO2−-N进一步提高,而投加缓释碳源的R2反应器NO2−-N并没有明显变化,反硝化效果较为稳定。
反硝化生物滤池稳定阶段的出水水质及氮负荷如表6所示,在HRT为3 h时,LDHs基滤料的投加使反硝化滤池氮负荷提高了(0.118±0.0092)kg·m−3·d−1 N,氮负荷最大为(0.2140±0.001) kg·m−3·d−1 N,通过对比各反应器不同HRT时的氮负荷可知,HRT的缩短引起氮负荷的升高,但氮负荷并非计算公式中的成倍增加,说明HRT的缩短对反应器具有一定的冲击,一定程度上限制了反硝化效率[26]。在反硝化生物滤池中,投加LDHs基滤料可明显提高氮负荷,可能是由于LDHs基滤料缓释出的有机碳被异养反硝化菌利用,促进了异养反硝化菌的活性,提高了系统反硝化性能[27]。
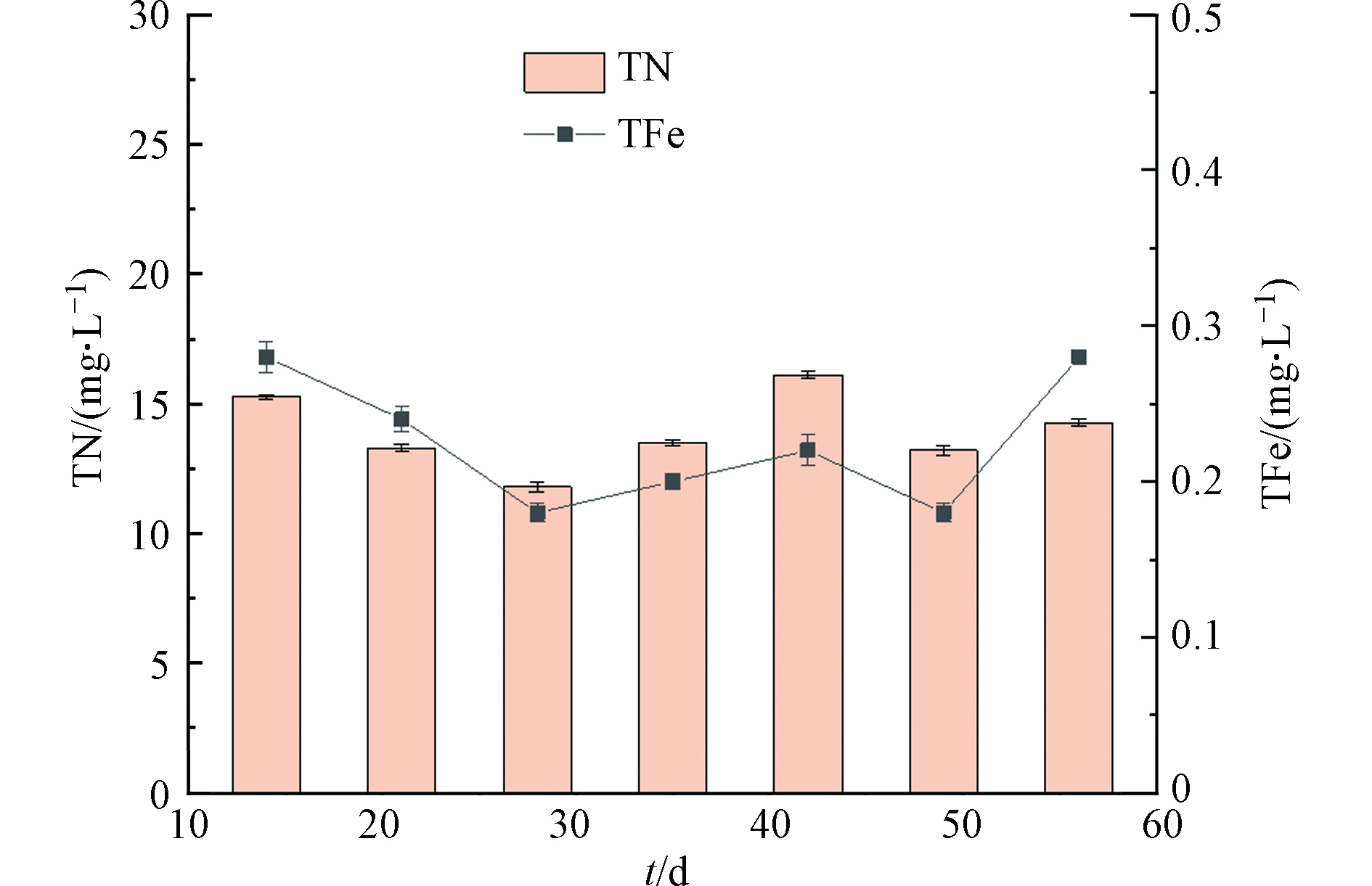
对各反应器出水的总铁(TFe)和TN进行了显著性差异分析,结果如图8所示。TFe与TN的Pearson相关系数为0.575,P=0.177>0.05,说明在LDHs滤料投加的反硝化滤池中,TFe的释放促进反硝化作用,提高TN的去除率,但是影响不显著。各反应器的出水中TFe浓度均低于0.3 mg·L−1,满足城市污水再生利用标准(GB/T 18920—2020,GB/T 19923—2005)。
-
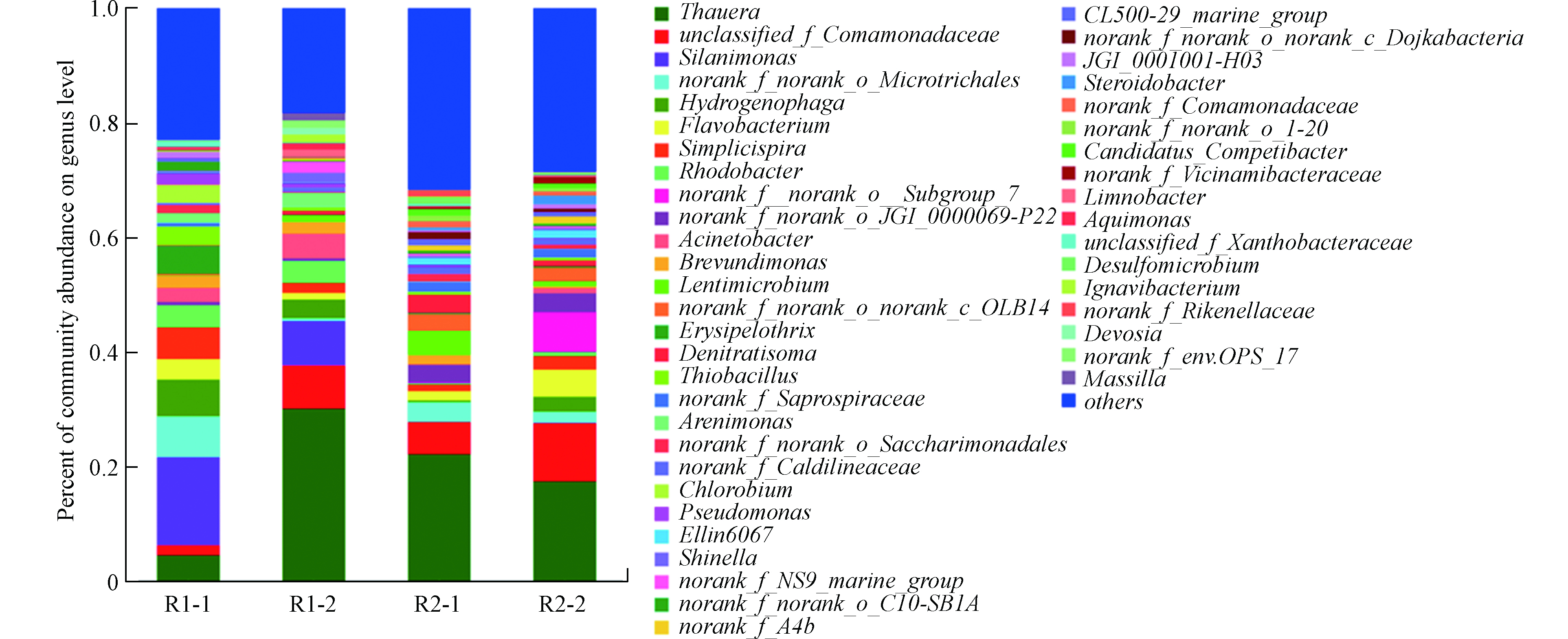
采用16S rRNA基因测序分析了生物膜的微生物结构,结果表明在反应器之间存在明显差异。其结果如图9所示。
在属水平上,Thauera和unclassified_f_Comamondacea是异养反硝化滤池中的主要菌属,但Silanimonas仅存在于R1,且丰度范围在8%—15.3%。其中Thauera属于Betoproteobacteria门,是能在有氧条件和厌氧条件生存并进行反硝化的菌属[28]。R2中的Simplicispira最高可达5.5%,Rhodocyclaceae丰度为11.8%。其中Simplicispira为一种固相反硝化菌,很容易使用生物聚合物作为碳源[29]。Rhodocyclaceae是一种异养反硝化菌,且被发现在聚己内酯固相反硝化滤池中[30]。两者均受投加的LDHs基滤料相关。因此,两者可能为降解LDHs基滤料中PVA的功能菌。Silanimonas和Hydrogenophaga的丰度均明显受HRT的影响,随着HRT的缩短,其丰度明显下降(P < 0.05),可能是因为其易受冲击负荷的影响。
-
为了进一步探究LDHs基滤料对反硝化作用的影响,对主要的反硝化功能基因napA、narG、nirS、nirK和nosZ进行测定,结果如图10所示。
napA和narG反硝化过程中编码硝酸盐还原酶的主要基因[31]。结果表明,随着HRT的缩短,两种基因的丰度有所降低。但当HRT为6 h时,LDHs基滤料的投加明显提高了两种基因的丰度(P<0.05),且napA基因的丰度超过对照组的2倍。当HRT为3 h时,LDHs基滤料的投加明显提高了narG基因的丰度(P<0.05),而napA基因的丰度变化不明显。且通过Pearson相关性分析,出水的TFe与基因narG的丰度呈显著正相关(P<0.05),说明LDHs基滤料溶出的TFe有利于硝酸盐的还原,进一步促进反硝化作用。
亚硝酸盐还原酶编码基因nirS和nirK基因都被认为是反硝化过程中亚硝酸盐还原的标志物[32],在反硝化生物滤池中,LDHs基滤料的投加明显提高了这两种基因的丰度(P<0.05)。nirK基因丰度在105—106 copies·µg−1 DNA水平,而nirS的基因丰度在104—105 copies·µg−1 DNA水平,这表明nirK基因的表达可能在亚硝酸盐还原中起更加关键的作用,并且能更有效地促进反硝化的另一关键过程——NO气体的产生[33]。已有的研究表明,废水处理工艺中微生物的nirS基因一般比nirK基因更加丰富[32, 34],但nirK型反硝化细菌对环境的剧烈波动具有更好的耐受性,例如受氧和pH的波动影响不大[35].
一氧化二氮还原酶编码基因(nosZ)是气态氮还原过程中的主要功能基因[36],在反硝化滤池中,其丰度均保持在105 copies·µg−1 DNA。作为整个反硝化过程的最后一步,nosZ基因在反硝化途径中起着重要作用。综合考虑以上5种基因丰度的变化,在投加LDHs基滤料后,这五种基因的拷贝数均有明显增加(P<0.05),说明滤料的投加在一定程度上有利于反硝化功能基因的富集,从反硝化的各个阶段提高脱氮效率。
为了综合分析LDHs基滤料添加的反硝化生物滤池深度脱氮机理,对反硝化滤池稳定运行期间出水水质、微生物群落结构以及运行条件进行了相关性分析,结果如图11所示。
其中“C”代表LDHs基滤料的投加。可以看出LDHs基滤料的投加与TN和Silanimonas负相关,说明LDHs基滤料添加有利于去除TN,但不利于Silanimonas的生存,HRT对TN的去除没有做出太大的贡献。为了进一步确定主要的相关细菌,去除TN和LDHs基滤料投加的关系,应用Pearson相关分析法研究了它们之间的相关性。C与norank_f_norank_o_JGI-0000069-P22和norank_f_norank_o_norank_c_OLB14呈正相关(P < 0.05),说明LDHs基滤料投加可能通过促进norank_f_norank_o_JGI-0000069-P22和norank_f_norank_o_norank_c_OLB14的生长提高对TN的去除率。
-
本研究率先选用CMC用于制备基于层状双金属化合物的缓释碳源,并通过释碳性能及化学表征筛选出最佳碳源FeNi-LDH-CMC用于LDHs基滤料的制备,确定了LDHs基滤料的最佳配比并进行实验室规模反硝化滤池深度脱氮应用和机理研究,验证了LDHs基滤料在反硝化生物滤池中的可行性,具有较强的应用推广场景。研究主要结论如下:
(1) 选用Fe3+、Al3+、Mg2+、Ni2+等金属离子为正电子来源,并率先选用CMC作为负电离子来源,通过共沉淀法制备出4种碳源,其中FeNi-LDH-CMC碳源释碳能力最强,COD释放量最大为(420.36±5.36) mg·g−1,且释碳速率较为缓慢、结构稳定、金属离子利用率高,可作为后续制备释碳滤料的碳源;
(2) 确定了FeNi-LDH-CMC碳源、PVA、CMC的最佳配比质量分数分别为7.77%、4.02%、1.02%,此时制备的LDHs基滤料的最大COD释放值为320.96 mg·g−1,缓释碳源系数K=4.40;
(3) LDHs基滤料使反硝化滤池的TN去除率提高了35.32%—38.46%,脱氮负荷最大提高到(0.2140±0.001) kg·m−3·d−1 N,表明LDHs基缓释碳源可提高反硝化滤池低碳氮比废水深度脱氮效果,具有较强的推广应用前景。
(4) 溶出的TFe与基因narG的丰度呈正相关(P<0.05),且在投加LDHs基滤料后,5种反硝化功能基因的丰度均明显增加(P<0.05),从反硝化过程的各个阶段提高脱氮效率。
新型LDHs基缓释碳源的制备及应用
Preparation and application of a novel LDHs-based slow-release carbon source
-
摘要: 以羟甲基纤维素钠(CMC)为负电离子,选用4种金属离子(Fe3+、Al3+、Ni2+、Mg2+),以共沉淀法制备4种基于层状双金属化合物(LDHs)的缓释碳源材料。采用X射线衍射(XRD)、X射线光电子能谱(XPS)和缓释性能分析,筛选出释碳量大、稳定缓释的FeNi-LDH-CMC碳源材料。通过响应面实验确定了FeNi-LDH-CMC、聚乙烯醇(PVA)、CMC的最佳质量分数为7.77%、4.02%、1.02%,此时制备的LDHs基滤料释碳系数为4.40,最大COD释放值为320.96 mg·g−1。最后构建反硝化生物滤池,对模拟低碳氮比废水进行脱氮处理。结果表明,HRT=3 h时,反硝化滤池对TN平均去除率仅为32.4%,氮负荷为(0.0960±0.0082)kg·m−3·d−1 N,而投加LDHs基滤料使滤池TN去除率提高了35.32%—38.46%,氮负荷提高了(0.118±0.0092)kg·m-3·d−1 N;在投加LDHs基滤料后,5种反硝化功能基因(napA、narG、nirS、nirK、nosZ)的丰度均有显著增加(P<0.05),提高了反硝化的各个阶段脱氮效率。研究结果表明LDHs基缓释碳源在反硝化滤池中可强化低碳氮比废水深度脱氮,是一种潜在应用于废水深度脱氮的新型材料。
-
关键词:
- 羟甲基纤维素钠(CMC) /
- 层状双金属化合物(LDHs) /
- 缓释碳源 /
- 深度脱氮
Abstract: This study took the lead in using sodium hydroxymethyl cellulose (CMC) as the negative ion and four kinds of metal ions (Fe3+, Al3+, Ni2+ and Mg2+) to prepare layered bimetallic compounds (LDHs)-based slow-release carbon source materials by coprecipitation method. FeNi-LDH-CMC with the charecteristics of large amount and stable slow-release of carbon was screened out through X-ray diffraction (XRD), X ray photoelectron spectroscopy (XPS) and slow-release performance analyses. The optimum mass fraction of FeNi-LDH-CMC, polyvinyl alcohol (PVA) and CMC was determined as 7.77%:4.02%:1.02% by response surface experiment, to achieve the carbon release coefficient of 4.40 and the maximum chemical oxygen demand (COD) release of 320.96 mg·g-1 of the prepared LDHs-based filter media. Finally, denitrification biological filter was constructed for denitrogenation of synthetic wastewater with low C/N ratio. Results showed that under HRT of 3 h, the average removal rate of total nitrogen (TN) was only 32.4% and the nitrogen load was (0.0960±0.0082) kg·m-3·d-1 N. By contrast, TN removal rate and nitrogen load were increased by 35.32%—38.46% and (0.118±0.0092) kg·m-3·d-1 N, respectively, after the addition of LDHs-based filter media. The abundances of five denitrifying functional genes (napA, narG, nirS, nirK and nosZ) were also significantly increased (P<0.05), which improved the denitrogenation efficiency at each stage of denitrification. It demonstrated that LDHs-based slow-release carbon source could strengthen the deep denitrogenation of wastewater with low C/N ratio in denitrification biological filter, and could be employed as a potentially novel material for advanced denitrogenation of wastewater. -

-
表 1 响应面实验设计
Table 1. The experimental design of Response Surface Experiment
实验组别
Experimental groupPVA质量分数/%
PVA mass fractionCMC质量分数/%
CMC mass fractionFeNi-LDH-CMC质量分数/%
FeNi-LDH-CMC mass fraction1 4.0 1.5 6.0 2 4.0 1.0 8.0 3 4.0 0.5 10.0 4 4.0 1.0 8.0 5 2.0 0.5 8.0 6 6.0 1.0 6.0 7 6.0 0.5 8.0 8 6.0 1.5 8.0 9 4.0 0.5 6.0 10 4.0 1.0 8.0 11 2.0 1.5 8.0 12 4.0 1.5 10.0 13 2.0 1.0 8.0 14 2.0 1.0 6.0 15 6.0 1.0 10.0 16 4.0 1.0 8.0 17 4.0 1.0 8.0 表 2 人工合成废水组分
Table 2. Components of synthetic wastewater
主要组分
Essential components浓度/(mg·L−1)
Concentration微量元素组分
Trace element components浓度/(mg·L−1)
ConcentrationCOD 150.0 MgCl2·6H2O 24.0 NO3−-N 50.0 CaCl2·2H2O 24.0 KH2PO4 25.0 ZnSO4 0.4 NaHCO3 250.0 CoCl2·6H2O 0.5 (NH4)6Mo7O24·4H2O 0.5 MnCl2·4H2O 0.3 EDTA-2Na 5.0 表 3 功能基因的引物序列及实验条件
Table 3. Primers of functional genes and corresponding experiment conditions
功能基因
Functional gene引物名称
Primer name引物序列
Primer sequence (5′-3′)退火温度/℃
Annealing temperature基因长度/bp
Amplification sizenirS nirS-F ATGCAGGAAGCGGGAGGT 54 410 nirS-R TGAAGGAAACGGGCAAGGT nirK nirK583F TCATGGTGCTGCCGCGKGACGG 60 350 nirK909R GAACTTGCCGGTKGCCCAGAC napA napA-F AGCGAGAATGGGCTGTTG 52 152 napA-R TGGACGATGGGCTTCAAC narG narGF TCGCCSATYCCGGCSATGTC 61 173 narGR GAGTTGTACCAGTCRGCSGAYTCSG nosZ nosZ-F TGTCCTTGCCGTCCTTGC 55 392 nosZ-R CGACTGGGTGGTGGTGTTC 表 4 CMC和4种碳源的元素组成
Table 4. Element composition of CMC and four carbon sources
碳源
Carbon sources元素
Elements含量/%
Percentage compositionCMC C1s 53.39 O1s 38.69 Na1s 7.92 AlNi-LDH-CMC C1s 25.10 O1s 48.27 Al2p 18.20 Ni2p 4.35 Cl2p 4.05 AlMg-LDH-CMC C1s 21.18 O1s 51.31 Al2p 11.18 Mg1s 12.18 Cl2p 4.16 FeNi-LDH-CMC C1s 33.14 O1s 55.92 Fe2p 1.96 Ni2p 5.35 Cl2p 3.63 FeMg-LDH-CMC C1s 33.33 O1s 49.50 Fe2p 2.23 Mg1s 11.76 Cl2p 3.17 表 5 各组分含量对K值的影响
Table 5. Effect of each component content on K value
实验组别
Experimental groupPVA质量分数/%
PVA mass fractionCMC质量分数/%
CMC mass fractionFeNi-LDH-CMC质量分数/%
FeNi-LDH-CMC mass fraction释碳系数K
Carbon release coefficient K1 4.0 1.5 6.0 5.69 2 4.0 1.0 8.0 4.26 3 4.0 0.5 10.0 7.65 4 4.0 1.0 8.0 4.66 5 2.0 0.5 8.0 5.98 6 6.0 1.0 6.0 7.32 7 6.0 0.5 8.0 8.11 8 6.0 1.5 8.0 6.85 9 4.0 0.5 6.0 7.39 10 4.0 1.0 8.0 4.68 11 2.0 1.5 8.0 8.64 12 4.0 1.5 10.0 7.29 13 2.0 1.0 8.0 7.66 14 2.0 1.0 6.0 6.98 15 6.0 1.0 10.0 6.77 16 4.0 1.0 8.0 4.16 17 4.0 1.0 8.0 4.33 表 6 反硝化生物滤池稳定阶段出水污染物浓度
Table 6. Concentration of effluent contaminants in stable stage of denitrification biofilters
实验组别
Experimental groupTOC/(mg·L−1) TN/(mg·L−1) NO3−N/(mg·L−1) NO2−N/(mg·L−1) 反硝化负荷/(kg·m−3·d−1 N)
Denitrification loadR1-1 17.94±0.11 29.82±1.17 16.23±1.76 8.10±3.04 0.0630±0.0030 R1-2 12.73±0.92 33.98±3.84 23.2±2.97 11.03±0.40 0.0960±0.0082 R2-1 38.10±2.96 12.16±1.2 8.50±0.56 2.60±0.31 0.1130±0.0016 R2-2 42.16±1.6 14.26±0.66 9.90±4.30 3.60±1.10 0.2140±0.001 -
[1] HU H D, LIAO K W, SHI Y J, et al. Effect of solids retention time on effluent dissolved organic nitrogen in the activated sludge process: Studies on bioavailability, fluorescent components, and molecular characteristics [J]. Environmental Science & Technology, 2018, 52(6): 3449-3455. [2] CAO S B, DU R, LI B K, et al. Nitrite production from partial-denitrification process fed with low carbon/nitrogen (C/N) domestic wastewater: Performance, kinetics and microbial community [J]. Chemical Engineering Journal, 2017, 326: 1186-1196. doi: 10.1016/j.cej.2017.06.066 [3] 张蕊, 韩志英, 陈重军, 等. 生物膜型污水脱氮系统中膜结构及微生物生态研究进展 [J]. 生态学杂志, 2011, 30(11): 2628-2636. doi: 10.13292/j.1000-4890.2011.0381 ZHANG R, HAN Z Y, CHEN C J, et al. Microstructure and microbial ecology of biofilm in the bioreactor for nitrogen removing from wastewater: A review [J]. Chinese Journal of Ecology, 2011, 30(11): 2628-2636(in Chinese). doi: 10.13292/j.1000-4890.2011.0381
[4] HAN F, WEI D, NGO H H, et al. Performance, microbial community and fluorescent characteristic of microbial products in a solid-phase denitrification biofilm reactor for WWTP effluent treatment [J]. Journal of Environmental Management, 2018, 227: 375-385. [5] HER J J, HUANG J S. Influences of carbon source and C/N ratio on nitrate/nitrite denitrification and carbon breakthrough [J]. Bioresource Technology, 1995, 54(1): 45-51. doi: 10.1016/0960-8524(95)00113-1 [6] COSTA D D, GOMES A A, FERNANDES M, et al. Using natural biomass microorganisms for drinking water denitrification [J]. Journal of Environmental Management, 2018, 217: 520-530. [7] LIANG X Q, LIN L M, YE Y S, et al. Nutrient removal efficiency in a rice-straw denitrifying bioreactor [J]. Bioresource Technology, 2015, 198: 746-754. doi: 10.1016/j.biortech.2015.09.083 [8] MARTÍNEZ N B, TEJEDA A, del TORO A, et al. Nitrogen removal in pilot-scale partially saturated vertical wetlands with and without an internal source of carbon [J]. The Science of the Total Environment, 2018, 645: 524-532. doi: 10.1016/j.scitotenv.2018.07.147 [9] ALI M, SHAW D R, ZHANG L, et al. Aggregation ability of three phylogenetically distant anammox bacterial species [J]. Water Research, 2018, 143: 10-18. doi: 10.1016/j.watres.2018.06.007 [10] LI P, ZUO J E, WANG Y J, et al. Tertiary nitrogen removal for municipal wastewater using a solid-phase denitrifying biofilter with polycaprolactone as the carbon source and filtration medium [J]. Water Research, 2016, 93: 74-83. doi: 10.1016/j.watres.2016.02.009 [11] HUANG G X, FALLOWFIELD H, GUAN H D, et al. Remediation of nitrate-nitrogen contaminated groundwater by a heterotrophic-autotrophic denitrification approach in an aerobic environment [J]. Water, Air, & Soil Pollution, 2012, 223(7): 4029-4038. [12] SAHA S M, RAY S, ACHARYA R, et al. Magnesium, zinc and calcium aluminium layered double hydroxide-drug nanohybrids: A comprehensive study [J]. Applied Clay Science, 2017, 135: 493-509. doi: 10.1016/j.clay.2016.09.030 [13] ISLAM M, PATEL R. Synthesis and physicochemical characterization of Zn/Al chloride layered double hydroxide and evaluation of its nitrate removal efficiency [J]. Desalination, 2010, 256(1/2/3): 120-128. [14] FANG C J, ZHANG X L, LEI Y, et al. Nitrogen removal via core-shell bio-ceramic/Zn-layer double hydroxides synthesized with different composites for domestic wastewater treatment [J]. Journal of Cleaner Production, 2018, 181: 618-630. doi: 10.1016/j.jclepro.2018.01.249 [15] JIANG L, LIU J Y, ZUO K, et al. Performance of layered double hydroxides intercalated with acetate as biodenitrification carbon source: The effects of metal ions and particle size [J]. Bioresource Technology, 2018, 259: 99-103. doi: 10.1016/j.biortech.2018.03.032 [16] CHEN M, BI R, ZHANG R, et al. Tunable surface charge and hydrophilicity of sodium polyacrylate intercalated layered double hydroxide for efficient removal of dyes and heavy metal ions [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2021, 617: 126384. doi: 10.1016/j.colsurfa.2021.126384 [17] LABBÉ N, PARENT S, VILLEMUR R. Addition of trace metals increases denitrification rate in closed marine systems [J]. Water Research, 2003, 37(4): 914-920. doi: 10.1016/S0043-1354(02)00383-4 [18] JIANG L, LIU J Y, ZHANG C, et al. Synthesis of layered double hydroxides with fermentation liquid of organic waste to extract short-chain fatty acids as a biodenitrification carbon source [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(10): 9095-9101. [19] NARAYAN K D, SABAT S C, DAS S K. Mechanism of electron transport during thiosulfate oxidation in an obligately mixotrophic bacterium Thiomonas bhubaneswarensis strain S10 (DSM 18181^T) [J]. Applied Microbiology and Biotechnology, 2017, 101(3): 1239-1252. doi: 10.1007/s00253-016-7958-x [20] XU Z X, LIU S H, YING H L, et al. Biological denitrification using corncobs as a carbon source and biofilm carrier [J]. Water Environment Research, 2009, 81: 242-247. [21] 邵留, 徐祖信, 王晟, 等. 新型反硝化固体碳源释碳性能研究 [J]. 环境科学, 2011, 32(8): 2323-2327. doi: 10.13227/j.hjkx.2011.08.048 SHAO L, XU Z X, WANG S, et al. Performance of new solid carbon source materials for denitrification [J]. Environmental Science, 2011, 32(8): 2323-2327(in Chinese). doi: 10.13227/j.hjkx.2011.08.048
[22] PENG C, HUANG H, GAO Y L, et al. A novel start-up strategy for mixotrophic denitrification biofilters by rhamnolipid and its performance on denitrification of low C/N wastewater [J]. Chemosphere, 2020, 239: 124726. doi: 10.1016/j.chemosphere.2019.124726 [23] 闫续, 许柯, 耿金菊, 等. 两种释碳材料的制备及其性能研究 [J]. 中国环境科学, 2012, 32(11): 1984-1990. doi: 10.3969/j.issn.1000-6923.2012.11.009 YAN X, XU K, GENG J J, et al. Preparation and properties of two kinds of carbon releasing material [J]. China Environmental Science, 2012, 32(11): 1984-1990(in Chinese). doi: 10.3969/j.issn.1000-6923.2012.11.009
[24] XIONG R, YU X X, YU L J, et al. Biological denitrification using polycaprolactone-peanut shell as slow-release carbon source treating drainage of municipal WWTP [J]. Chemosphere, 2019, 235: 434-439. doi: 10.1016/j.chemosphere.2019.06.198 [25] XU Z S, DAI X H, CHAI X L. Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes [J]. Science of the Total Environment, 2018, 634: 195-204. doi: 10.1016/j.scitotenv.2018.03.348 [26] WU H, ZHANG Q, CHEN X, et al. Effect of HRT and BDPs types on nitrogen removal and microbial community of solid carbon source SND process treating low carbon/nitrogen domestic wastewater [J]. Journal of Water Process Engineering, 2021, 40: 101854. doi: 10.1016/j.jwpe.2020.101854 [27] YANG M, WANG X N, LIU S, et al. Carbon release behaviour of polylactic acid/starch-based solid carbon and its influence on biodenitrification [J]. Biochemical Engineering Journal, 2020, 155: 107468. doi: 10.1016/j.bej.2019.107468 [28] YANG N, ZHAN G Q, LI D P, et al. Complete nitrogen removal and electricity production in Thauera-dominated air-cathode single chambered microbial fuel cell [J]. Chemical Engineering Journal, 2019, 356: 506-515. doi: 10.1016/j.cej.2018.08.161 [29] ZHANG Y S, JIANG J Q, ZHAO Q L, et al. Accelerating anodic biofilms formation and electron transfer in microbial fuel cells: Role of anionic biosurfactants and mechanism [J]. Bioelectrochemistry, 2017, 117: 48-56. doi: 10.1016/j.bioelechem.2017.06.002 [30] PENG P C, HUANG H, REN H Q. Effect of adding low-concentration of rhamnolipid on reactor performances and microbial community evolution in MBBRs for low C/N ratio and antibiotic wastewater treatment [J]. Bioresource Technology, 2018, 256: 557-561. doi: 10.1016/j.biortech.2018.02.035 [31] van DOAN T, LEE T K, SHUKLA S K, et al. Increased nitrous oxide accumulation by bioelectrochemical denitrification under autotrophic conditions: Kinetics and expression of denitrification pathway genes [J]. Water Research, 2013, 47(19): 7087-7097. doi: 10.1016/j.watres.2013.08.041 [32] ZHI W, JI G D. Quantitative response relationships between nitrogen transformation rates and nitrogen functional genes in a tidal flow constructed wetland under C/N ratio constraints [J]. Water Research, 2014, 64: 32-41. doi: 10.1016/j.watres.2014.06.035 [33] XU H, LIN C S, CHEN W, et al. Effects of pipe material on nitrogen transformation, microbial communities and functional genes in raw water transportation [J]. Water Research, 2018, 143: 188-197. doi: 10.1016/j.watres.2018.06.040 [34] XING W, LI J L, CONG Y, et al. Identification of the autotrophic denitrifying community in nitrate removal reactors by DNA-stable isotope probing [J]. Bioresource Technology, 2017, 229: 134-142. doi: 10.1016/j.biortech.2017.01.010 [35] DESNUES C, MICHOTEY V D, WIELAND A, et al. Seasonal and diel distributions of denitrifying and bacterial communities in a hypersaline microbial mat (Camargue, France) [J]. Water Research, 2007, 41(15): 3407-3419. doi: 10.1016/j.watres.2007.04.018 [36] LI M Y, REN L H, ZHANG J C, et al. Population characteristics and influential factors of nitrogen cycling functional genes in heavy metal contaminated soil remediated by biochar and compost [J]. Science of the Total Environment, 2019, 651: 2166-2174. doi: 10.1016/j.scitotenv.2018.10.152 -



 下载:
下载: