-
微塑料(MPs)指的是直径小于5 mm的塑料颗粒,纤维,薄膜和碎片[1],可分为原生MPs和次生MPs. 传统塑料对海洋生物以及对人类都构成巨大威胁,例如微塑料可作为三氯生的载体,对淡水微藻产生破坏[2]. 微米塑料和纳米塑料对哺乳动物会通过氧化应激,膜损伤,免疫反应和遗传毒性来诱导细胞毒性[3]. 暴露在微塑料中,会对男性生殖和精子质量存在有害影响[4]. 有研究者预计,直到2060年,MPs占塑料总重约13.2%[5],为了改变这一趋势,生物基、可生物降解塑料正在作为一种新的生态解决方案,以减少对环境的影响. 在各个领域,可生物降解塑料已被认为是不可生物降解塑料的替代品[6-8]. 然而,最近的研究表明,可生物降解MPs对有机污染物的吸附量显著高于不可生物降解塑料,从而具有更大的潜在环境风险[9-12]. 此外,相比于化学塑料聚合物,生物可降解MPs对植物生长可以构成更大的风险[13]. 现有的研究已经充分证明,塑料对环境的潜在风险,一方面是微小塑料的形成和添加剂的释放使塑料成为新的污染源;另一方面是其可以成为环境污染物的载体,参与有机和无机污染物的吸附、迁移、释放过程,甚至经食物和水进入人体,在人体组织中积聚,提高了人体暴露风险[14].
像不可生物降解塑料一样,可生物降解塑料在加工、贮存和使用过程中,由于受内外因素的综合作用发生老化反应,如光照、氧气、臭氧、热、水、机械应力、高能辐射等,这些反应往往伴随理化性质的改变. 老化行为除了可以加快添加剂的释放[15-16],还可以大大促进其污染物迁移能力的提高. 因此,老化过程塑料本身的变化需要引起研究者的重大关注. MPs的老化反应是塑料在环境中最为重要的转化过程,在这个过程中,MPs的形貌、表面特征以及微观结构会发生改变,从而影响MPs的环境行为[17]. 其中,光化学老化是最重要的一种自然老化过程[18]. MPs在太阳光,特别是太阳光中高能量UV-B(280—315 nm)和中能量UV-A(315 — 400 nm)照射下,会诱导MPs表面自由基的链式反应,从而发生加氧、脱氢、断键或者交联等反应[19].
目前,对可生物降解MPs的研究较少,且大部分文献都集中在对其毒性和污染物吸附解吸的研究上,有关可生物降解MPs老化研究鲜有报道[20]. 常见的可生物降解MPs有聚乳酸(PLA)、聚羟基脂肪酸酯(PHA)、聚丁二酸丁二醇酯(PBS)和聚对苯二甲酸-己二酸丁二酯(PBAT). 其中PLA和PHA是由自然物质生成的可生物降解塑料,PBS和PBAT是由石化原料生成的可生物降解塑料. PLA代表了最有希望的生物塑料之一,PLA和PBAT是可生物降解的塑料薄膜的常见成分[21],可以代替低密度聚乙烯(LDPE)薄膜;PHA常用于覆盖膜和包装材料,以最大程度地减少塑料浪费并减少土壤污染[22];PBS在可生物降解树脂的材料选择上最为成熟,产能也最大.
本研究选取了4种常见的可生物降解MPs为实验对象,利用傅立叶变换红外光谱(FTIR)和电子顺磁共振波谱(EPR)观察MPs在老化过程中旧键断裂和新键生成,同时采用高斯计算对四种图谱进行了计算和补充,旨在探究上述4种可生物降解MPs在自然光照下的老化过程和机理.
-
分析纯PLA、PHA、PBS和PBAT 等4种可生物降解MPs购自南京濯清环保科技有限公司(Nanjing, China),颗粒尺寸在100 — 150 μm之间.
-
老化实验的开展自2020年8月5日至2020年10月25日,样品位于中国南京大学环境学院顶楼光照充足的平台(经纬度:E118.948272°,N32.119549°,海拔高度:39.11 m),每天照射时长为(10 ± 0.5) h. 将每种样品分别铺满2个石英皿底部,每3 h振荡摇匀1次. 使用紫外辐照计(北京师范大学光电仪器厂,误差允许范围±10%)、手持式湿度计(VICTOR-231)和温度计(Fisher Scientific)分别记录3个时间点(9:00、13:00、16:00)的空气温度、空气湿度、地面温度以及365 nm波长的光强,并求取平均值. 采样时间为第0、13、28、56 d,将样品从两个石英皿中取出部分,在40 ℃下真空干燥30 min后避光保存,余下样品于老化平台继续老化.
-
待测样品使用衰减全反射/傅里叶变换显微红外光谱(Micro-ATR/FTIR)系统(Bruker HYPERION 2000, Germany)进行测试. 收集4种MPs不同老化时间下的红外光谱,扫描范围600 — 4000 cm−1.
-
使用电子顺磁共振波谱仪(EPR)(EMXmicro-6, Bruker, Germany)原位测定4种MPs光照条件下的EPR波谱. 称取20 mg可生物降解MPs加入毛细管中,然后放入样品仓中. EPR仪器参数调整为:中心场3500 G、微波功率6.325 mW、扫描宽度200 G、时间常数15.0 ms、扫描时间50 s、扫描次数5.
-
使用高斯16W软件中的M06-2X方法和6-311+G(d,p)基组,以opt freq为关键词,计算得到4种塑料的红外光谱.
-
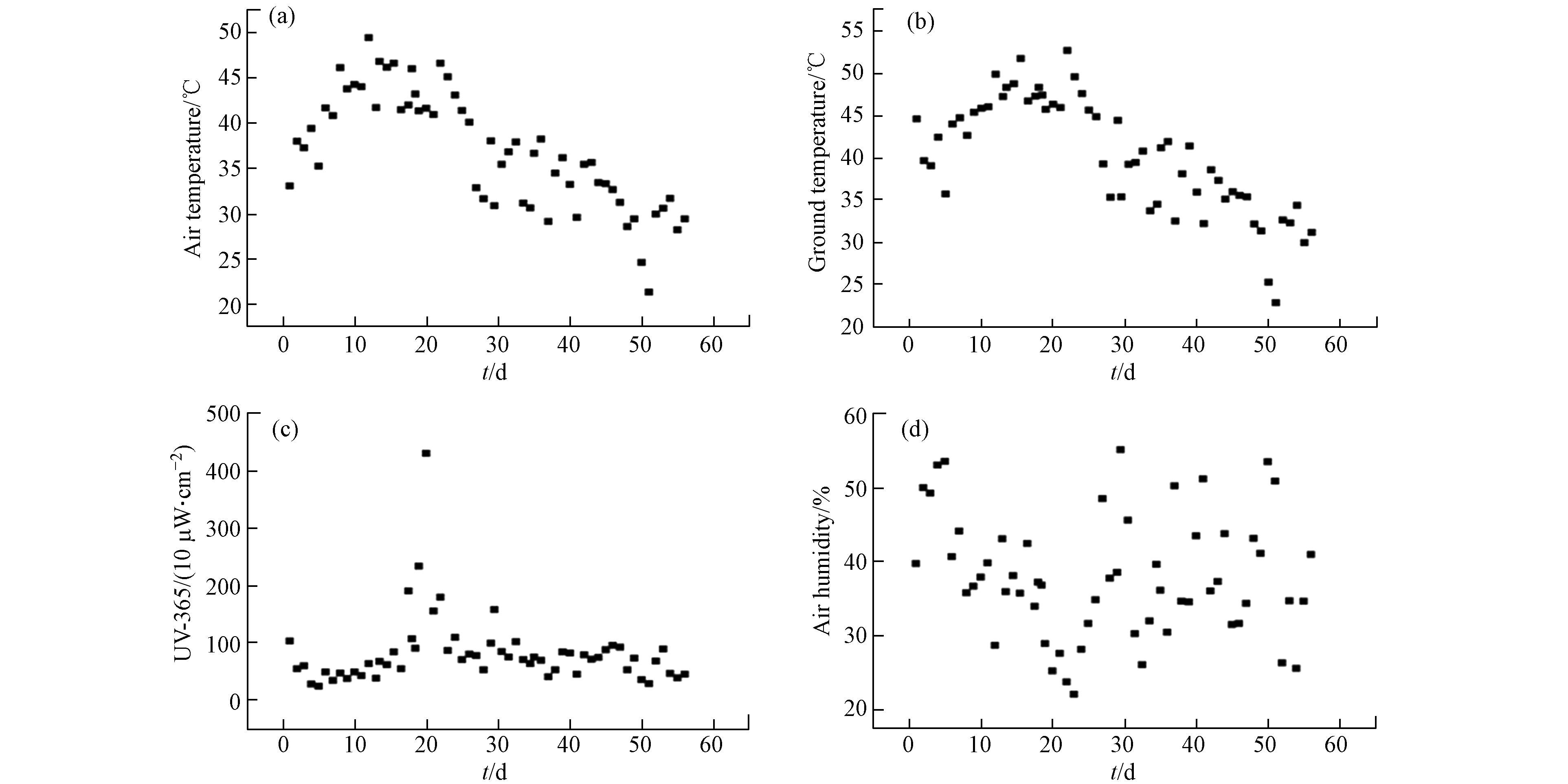
图1为4种可生物降解MPs自然光解老化过程的环境条件,即空气温度、地面温度、365 nm波长光强以及空气湿度. 在56 d的暴露时间中,空气最低温度为21.2 ℃,最高为49.5 ℃;地面温度变化范围为22.7 — 49.85 ℃;紫外强度(UV-365)从高到低为228.0 — 4299.5 μW·cm−2;空气湿度变化范围为25.2% — 55.1%. 如表1所示,为每个取样时间段内环境条件的平均值.
-
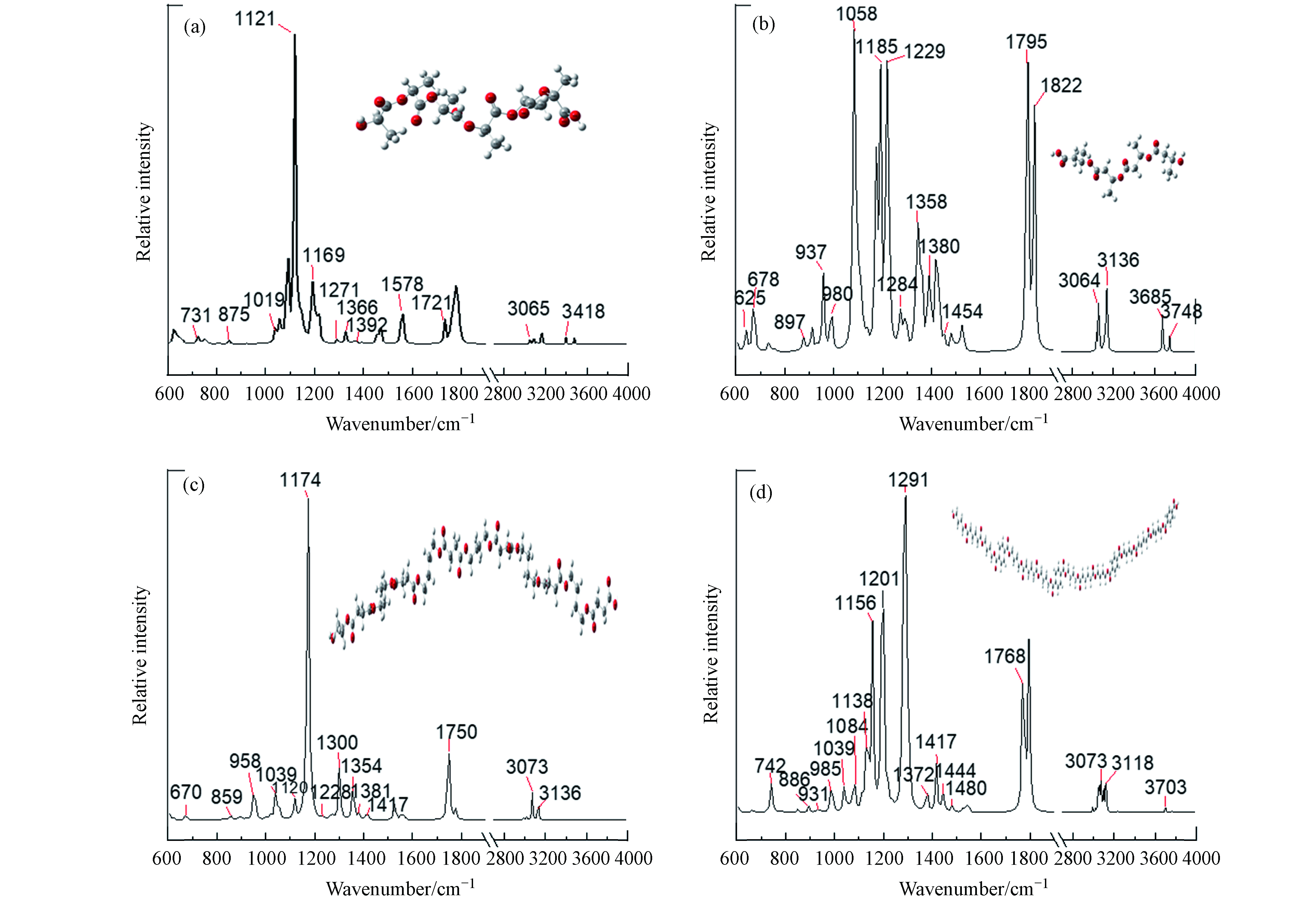
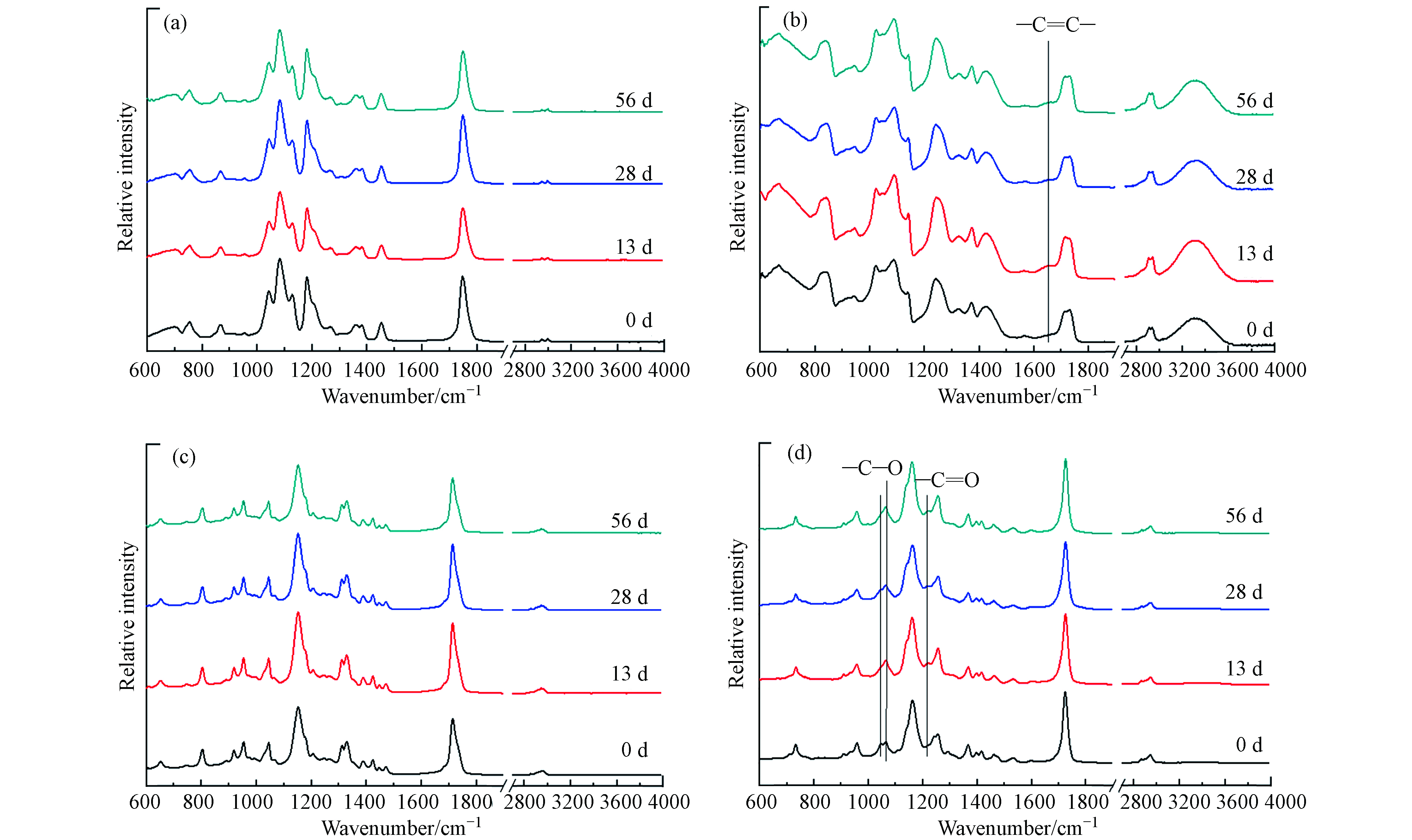
对照理论计算的红外光谱(图2(a)),PLA的红外光谱如图3(a)所示,推导出3065 cm−1和1721 cm−1的红外吸收峰分别对应—OH和C=O的伸缩振动[23],—CH—的不对称伸缩振动在2946 cm−1处的吸收峰[24-25],1271、1169、1121、1019 cm−1的红外吸收峰对应C—O—C的伸缩振动,以及—OCH2CH3的在875 cm−1 处的伸缩振动[26](表2). 随着光老化56 d的进行,—OH、C=O、C—O—C和—OCH2CH3的峰位置及峰强度均没有显著性变化.
对照理论计算的红外光谱(图2(b)),PHA的红外光谱如图3(b)所示,3685、1795 cm−1的红外吸收峰分别对应—OH和—C=O的伸缩振动,1651 cm−1的C=C的伸缩振动,1454 cm−1的—CH2—的弯曲振动,1380、1358 cm−1的红外吸收峰对应CH3的伸缩振动,980 cm−1的CH2的平面弯曲振动(表2). 随着光老化56 d的进行,—C=C的峰出现且峰强度增加.
对照理论计算的红外光谱(图2(c)),PBS的红外光谱如图3(c)所示,1750 cm –1处的强谱带是由于酯基的羰基引起的[27],C—H在1417 cm–1处弯曲,C—O在1174 cm–1和958 cm–1处伸展,1120 cm–1处信号是重复—CH2CH2单位中C—O—C拉伸振动的特征[28],—O(CH2)4O—在1039 cm-1处的拉伸振动,859 cm−1对应—CH2的弯曲振动,670 cm−1对应COO的伸缩振动(表2). 随着光老化56 d的进行,这些峰位置和峰强度没有显著性变化.
对照理论计算的红外光谱(图2(d)),PBAT的红外光谱如图3(d)所示,推导出3073 cm−1的红外吸收峰对应—CH—的伸缩振动,1444 cm−1和1372 cm−1的红外吸收峰对应—CH2—的平面内弯曲振动,1417 cm−1的红外吸收峰对应—OCH2—的伸缩振动,1291 cm−1和1084 cm−1的红外吸收峰分别对C=O和—C—O的伸缩振动,1039 cm−1处的=C—H苯环平面内弯曲振动,以及886 cm−1和742 cm−1处的=C—H苯环平面外弯曲振动(表2). 随着光老化56 d的进行,C—O和C=O的峰强度均有所增加. 说明PBAT经紫外线照射发生光氧老化降解时造成—CH含量减少,同时有C=O 和C—O生成.
-
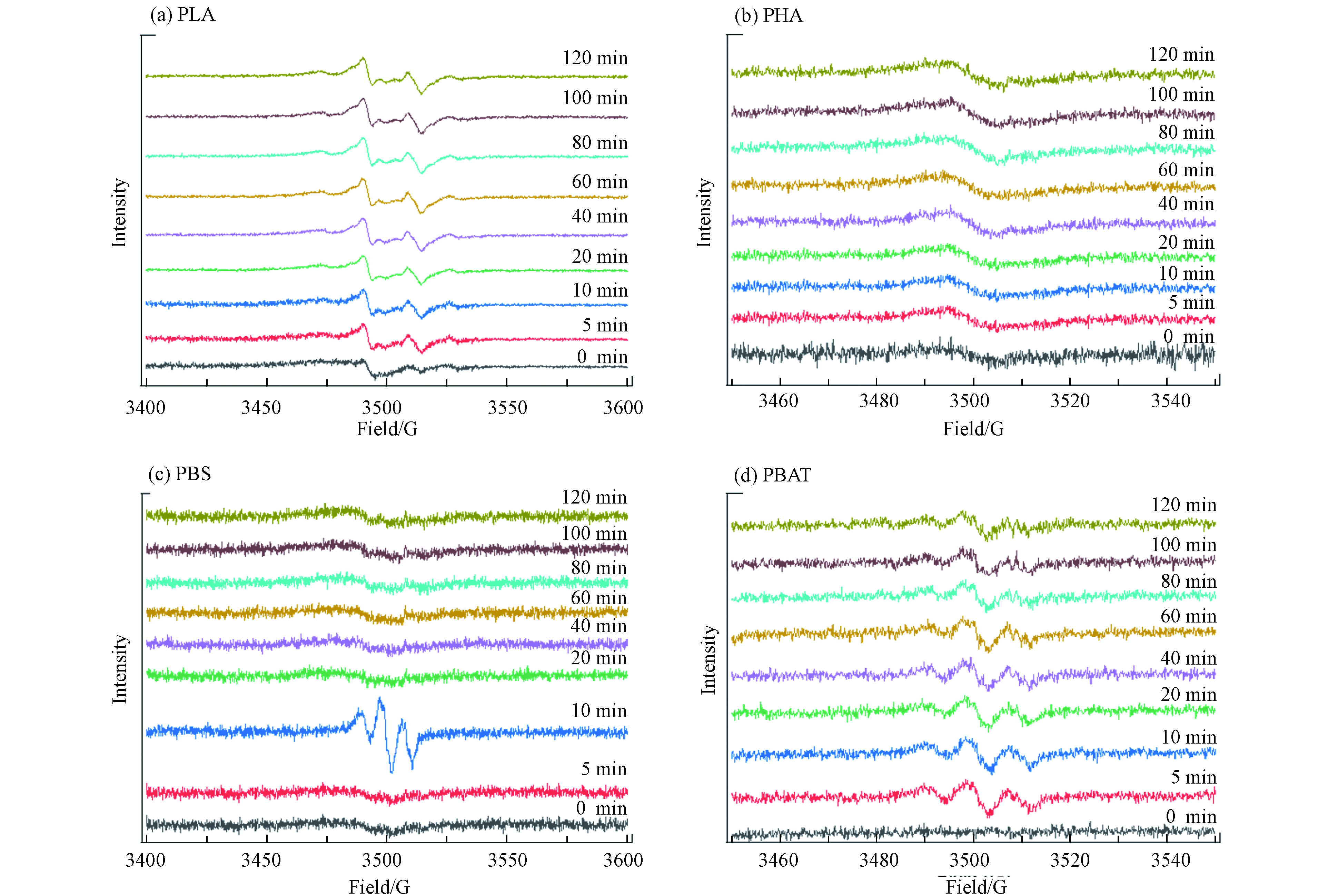
利用EPR检测环境持久性自由基(EPFRs)时, g 因子值是辨别EPFRs类型的重要波谱参数[29-30]. 以碳为中心的持久性自由基的 g 因子值一般小于 2.0030, 以氧为中心的持久性自由基的 g 因子值一般大于 2.0040[31].
如图4 所示,PLA在100 W汞灯光照后产生了新的以氧为中心的持久性自由基[32](g=2.00962),且随着光照时间的延长,自由基强度有增加的趋势.
PHA在100 W汞灯光照后和不加光照用EPR测试都显示有以氧为中心的自由基(g=2.00508),说明PHA光照后并没有EPFRs产生.
PBS在光照10 min后有新的以氧为中心的持久性自由基产生(g=2.01002),10 min 后自由基信号逐渐消失.
PBAT在光照后产生以氧为中心的持久性自由基(g=2.00451),随着光照时间的延长,自由基信号稳定存在,且信号强度并没有增加或者减少,PBAT光谱由两个中心单小波和弱侧峰组成. 通过EPR光谱分析发现,碳中心自由基很容易氧化为过氧自由基. 它们能够从碳氢化合物区分离氢原子,形成过氧化氢化合物和烷基/芳基自由基,从而引发进一步的氧化过程. 过氧化氢是老化过程中降解的前体,因为它可以分解成烷氧基/苯氧基和羟基自由基[33].
-
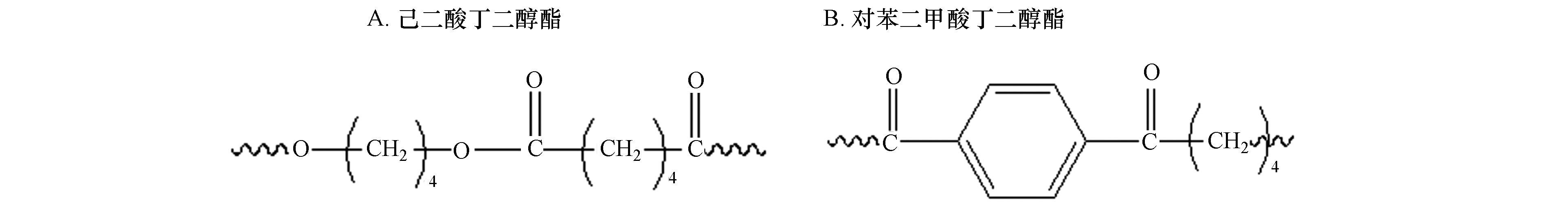
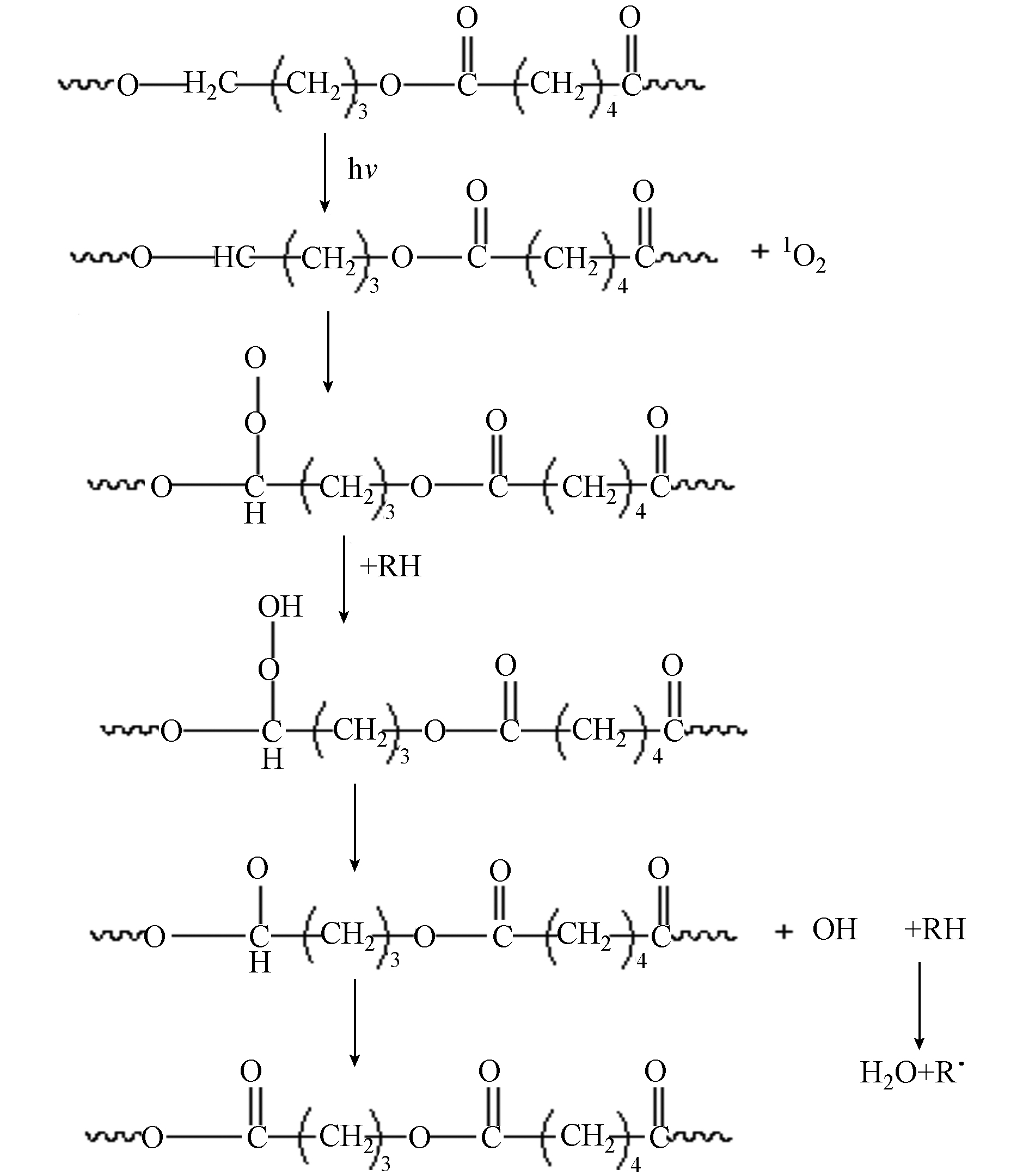
PLA,PHA,PBS和PBAT均为酯类聚合物,在本文中,它们都可以进行酯类的光氧老化降解,(具体反应机理以PBAT中的(A)结构己二酸丁二醇酯(图5)为例给出)[34]. 如图6所示,首先空气中3线态3O2吸收高能紫外光后跃迁变为活性很大的单线态1O2,然后单线态1O2和同样吸收光能后形成的聚合物自由基反应,生成带过氧键的自由基;而带过氧键的自由基由于活性很大可以从周围的链段中进行夺氢反应,生成带有—OH的结构;继续经紫外线照射,—OH脱落,生成羟基自由基和聚合物自由基;其中羟基自由基发生夺氢反应生成H2O.
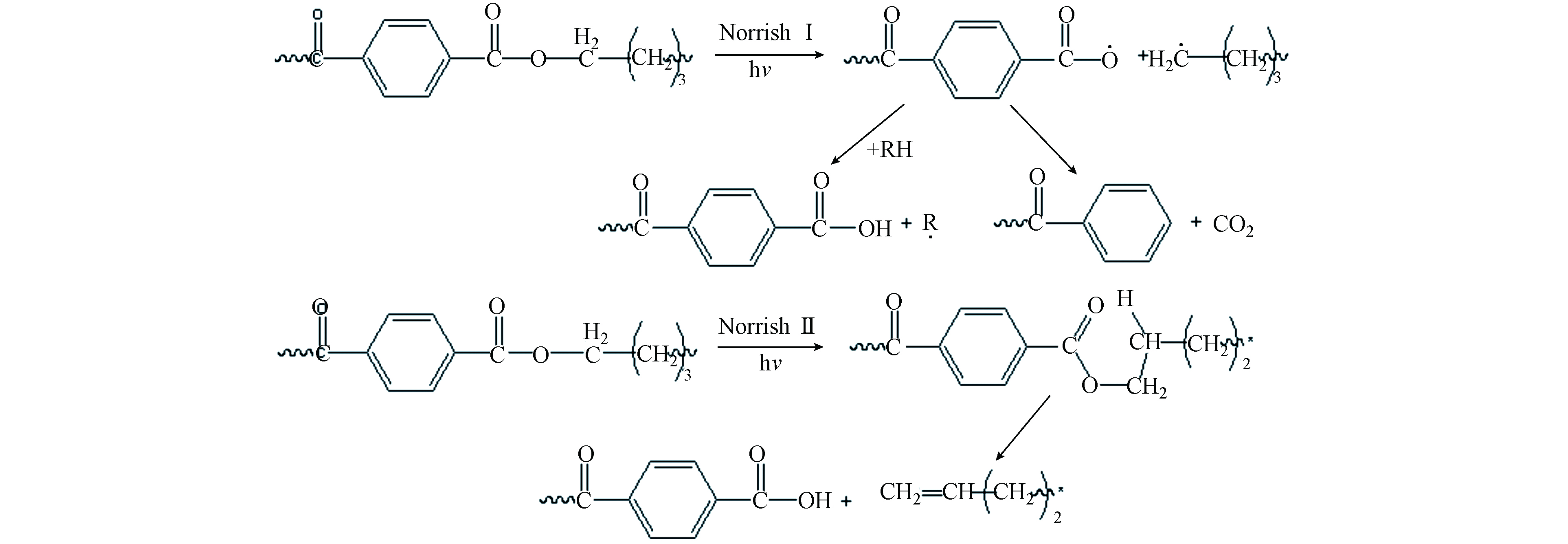
由于B结构对苯二甲酸丁二醇酯(图5)中含有苯环,属于芳香族酯类,能直接吸收波长范围在300—330 nm 之间的紫外光[35],因此除了可以发生图6所示的光氧老化降解外,还能进行Norrish反应(如图7所示). Norrish Ⅰ型反应的特点是光解时羰基与α-碳之间的键断裂,形成酰基自由基和烃基自由基,Norrish Ⅱ反应的特点是光化产物发生碳碳键的断裂生成烯烃或酮. B结构直接吸收紫外光后,既可以从酯键处断链生成两个初始自由基(Norrish Ⅰ型反应),也可以在光的作用下生成激发态羰基,然后按照Norrish Ⅱ反应进行断链.
-
本文使用了原位Micro-FTIR和EPR,表征了自然光照56 d过程中PLA、PHA、PBS和PBAT 四种MPs表面红外光谱和自由基的变化,同时采用高斯计算对4种图谱进行了计算和补充,研究得到的结论如下:
(1)在PLA-MPs的56 d光老化过程中,其表面的羰基数量并无明显变化. 在PHA-MPs的56 d光老化过程中,—C=C—的数量在增加. 在PBS-MPs的56 d光老化过程中,其表面并无发生旧键断裂、新键形成的现象. 在PBAT-MPs的56 d光老化过程中,—C—O和C=O的数量在增加.
(2)高斯计算图谱进一步说明了自然光照56 d后PLA和PBS两种MPs表面的官能团无显著变化;但PHA和PBAT两种MPs表面的官能团含量随老化时间的增加而增加.
(3)给出了PBAT中己二酸丁二醇酯和对苯二甲酸丁二醇酯较为详细的光氧老化降解机理以及对苯二甲酸丁二醇酯的Norrish型降解机理,为PBAT降解周期调控的研究奠定了一定的理论基础.
研究提供了对可生物降解MPs进行自然光照老化的思路,为揭示可生物降解MPs在气液界面的老化规律、评估可生物降解MPs的老化机理提供了参考.
可生物降解微塑料的自然光解老化
Natural photo-aging of biodegradable microplastics
-
摘要: 近年来,微塑料(MPs)污染问题受到人们的广泛关注. 可生物降解塑料被认为是常规的不可生物降解塑料的替代品,其产量和环境检出量显著增加. MPs的老化过程具有显著的环境行为和生态效应,而光照辐射在塑料老化和降解过程中起到主要作用. 然而,现有研究对可生物降解微塑料的自然光解老化过程及其机理的研究鲜有报道. 因此,本研究选取了4种常见的可生物降解微塑料为实验对象,分别为聚乳酸(PLA)、聚羟基脂肪酸酯(PHA)、聚丁二酸丁二醇酯(PBS)和聚对苯二甲酸-己二酸丁二酯(PBAT),旨在探究上述4种可生物降解微塑料在自然和模拟光照下的老化过程及潜在机理. 通过自然和模拟光解老化实验,利用傅立叶变换红外光谱(FTIR)和电子顺磁共振波谱(EPR)观察4种塑料在老化过程中旧键断裂和新键生成,发现在56 d的光老化过程中,PLA-MPs和PBS-MPs表面官能团含量并无显著变化,而随着老化时间的增加,PHA-MPs表面—C=C—的数量在增加,PBAT表面—C—O和C=O的数量在增加. 除了实验结果之外,分子模型构建和红外光谱计算等理论分析也为可降解MPs的自然光老化机理提供了理论支撑.
-
关键词:
- 可生物降解微塑料 /
- 光老化 /
- 原位红外光谱 /
- 原位电子顺磁共振波谱.
Abstract: Over the recent past years, microplastics (MPs) have raised widespread public attention due to their potential environmental and health risks. In order to eliminate the environmental burden of plastics, biodegradable plastics are industrially produced and recognized as an alternative to conventional non-biodegradable plastics. While, vast production and consumption inevitably led to their widespread distributions in various environmental matrices. The aging effect could significantly change physiochemical properties and subsequent environmental behaviors of MPs. Amongst various aging treatments, light radiation plays a dominant role in MP formation and degradation. However, few studies have been conducted to investigate the natural photo-aging process and mechanism of biodegradable MPs. Therefore, four commonly used biodegradable MPs were selected in the present study, i.e., polylactic acid (PLA), polyhydroxyalkanoate (PHA), polybutylene succinate (PBS) and polyparaben phthalic acid-butylene adipate (PBAT), which were employed to explore the aging process and underlying mechanism of biodegradable microplastics under natural and simulated solar irradiation. Moreover, Fourier transform infrared spectroscopy (FTIR) and electron paramagnetic resonance spectroscopy (EPR) were used to monitor the breakage and formation of bonds during the aging process. It was found that the content of functional groups on the surface of PLA-MPs and PBS-MPs did not change significantly during the 56 d photoaging process, while the amount of —C=C— on the surface of PHA-MPs and the amount of —C—O and C=O on the surface of PBAT increased with the lengthening irradiation time. In addition to experimental results, the theoretical analysis including molecular model construction and infrared spectra calculation were performed to support the photo-aging mechanism of degradable MPs.-
Key words:
- biodegradable microplastics /
- photo-aging /
- in-situ FTIR /
- in-situ EPR.
-

-
表 1 不同老化时间下环境条件的平均值.
Table 1. Average of environmental conditions at different aging times.
老化天数/d
Aging days空气温度/℃
Air temperature地面温度/℃
Ground temperatureUV-365 /(μW·cm−2)
Light intensity of UV-365空气湿度/%
Air humidity13 41.11 43.59 47.47 42.46 28 41.51 45.10 88.80 37.53 56 36.79 40.05 78.92 37.82 表 2 可生物降解微塑料的峰位置对应的官能团
Table 2. Functional groups corresponding to peak positions of biodegradable microplastics
波长/cm−1
Wavenumber官能团
Functional groupsPLA 3065 —OH 2946 —CH— 1721 —C=O 1271, 1169, 1121, 1019 C—O—C 875 O—CH—CH3 731 α—CH3 PHA 3685 —OH 1795 —C=O 1454 CH2弯曲模式 1380, 1358 CH3 980 CH2平面弯曲 PBS 1750 —C=O 1417 C-H弯曲 1174 C—O 1120 C—O—C 1039 O(CH2)4O 958 C-O 859 -CH2 670 COO PBAT 3073 —C—H伸缩 1444 CH2平面内弯曲 PBAT 1417 O—CH2 1372 CH2平面外弯曲 1291 C=O 1084 C—O 1039 =C—H苯环平面内弯曲 886, 742 =C—H苯环平面外弯曲 -
[1] THOMPSON R C, OLSEN Y, MITCHELL R P, et al. Lost at sea: Where is all the plastic? [J]. Science, 2004, 304(5672): 838. doi: 10.1126/science.1094559 [2] CHEN X, GU X N, BAO L J, et al. Comparison of adsorption and desorption of triclosan between microplastics and soil particles [J]. Chemosphere, 2021, 263: 127947. doi: 10.1016/j.chemosphere.2020.127947 [3] BANERJEE A, SHELVER W L. Micro- and nanoplastic induced cellular toxicity in mammals: A review[J]. The Science of the Total Environment, 2021, 755(Pt 2): 142518. [4] MAZUR A A, CHELOMIN V P, ZHURAVEL E V, et al. Genotoxicity of polystyrene (PS) microspheres in short-term exposure to gametes of the sand dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea) [J]. Journal of Marine Science and Engineering, 2021, 9(10): 1088. doi: 10.3390/jmse9101088 [5] SHARMA S, BASU S, SHETTI N P, et al. Microplastics in the environment: Occurrence, perils, and eradication [J]. Chemical Engineering Journal (Lausanne, Switzerland, 2021, 408: 127317. [6] 郭子耕, 苑静. 完全生物降解塑料的发展 [J]. 包装工程, 2010, 31(9): 126-130. doi: 10.19554/j.cnki.1001-3563.2010.09.035 GUO Z G, YUAN J. Development of biodegradable plastics [J]. Packaging Engineering, 2010, 31(9): 126-130(in Chinese). doi: 10.19554/j.cnki.1001-3563.2010.09.035
[7] WANG Y, DING K Q, REN L X, et al. Biodegradable microplastics: A review on the interaction with pollutants and influence to organisms [J]. Bulletin of Environmental Contamination and Toxicology, 2022, 108(6): 1006-1012. doi: 10.1007/s00128-022-03486-7 [8] QIN M, CHEN C, SONG B, et al. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments?[J] Journal of Cleaner Production, 2021, 312. [9] SCHÖPFER L, MENZEL R, SCHNEPF U, et al. Microplastics effects on reproduction and body length of the soil-dwelling nematode Caenorhabditis elegans [J]. Frontiers in Environmental Science, 2020, 8: 41. doi: 10.3389/fenvs.2020.00041 [10] JIANG M Y, HU L Y, LU A X, et al. Strong sorption of two fungicides onto biodegradable microplastics with emphasis on the negligible role of environmental factors [J]. Environmental Pollution (Barking, Essex:1987), 2020, 267: 115496. doi: 10.1016/j.envpol.2020.115496 [11] ZUO L Z, LI H X, LIN L, et al. Sorption and desorption of phenanthrene on biodegradable poly(butylene adipate co-terephtalate) microplastics [J]. Chemosphere, 2019, 215: 25-32. doi: 10.1016/j.chemosphere.2018.09.173 [12] GONG W W, JIANG M Y, HAN P, et al. Comparative analysis on the sorption kinetics and isotherms of fipronil on nondegradable and biodegradable microplastics [J]. Environmental Pollution, 2019, 254: 112927. doi: 10.1016/j.envpol.2019.07.095 [13] IQBAL S, XU J C, ALLEN S D, et al. Unraveling consequences of soil micro- and nano-plastic pollution on soil-plant system: Implications for nitrogen (N) cycling and soil microbial activity [J]. Chemosphere, 2020, 260: 127578. doi: 10.1016/j.chemosphere.2020.127578 [14] ANDERSON G, SHENKAR N. Potential effects of biodegradable single-use items in the sea: Polylactic acid (PLA) and solitary ascidians[J]. Environmental Pollution (Barking, Essex: 1987), 2021, 268(Pt A): 115364. [15] SHENG Y F, YE X Y, ZHOU Y, et al. Microplastics (MPs) act as sources and vector of pollutants-impact hazards and preventive measures [J]. Bulletin of Environmental Contamination and Toxicology, 2021, 107(4): 722-729. doi: 10.1007/s00128-021-03226-3 [16] 马思睿, 李舒行, 郭学涛. 微塑料的老化特性、机制及其对污染物吸附影响的研究进展 [J]. 中国环境科学, 2020, 40(9): 3992-4003. doi: 10.3969/j.issn.1000-6923.2020.09.032 MA S R, LI S X, GUO X T. A review on aging characteristics, mechanism of microplastics and their effects on the adsorption behaviors of pollutants [J]. China Environmental Science, 2020, 40(9): 3992-4003(in Chinese). doi: 10.3969/j.issn.1000-6923.2020.09.032
[17] LIU P, QIAN L, WANG H Y, et al. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes [J]. Environmental Science & Technology, 2019, 53(7): 3579-3588. [18] SINTIM H Y, BARY A I, HAYES D G, et al. Release of micro- and nanoparticles from biodegradable plastic during in situ composting [J]. The Science of the Total Environment, 2019, 675: 686-693. doi: 10.1016/j.scitotenv.2019.04.179 [19] GEWERT B, PLASSMANN M M, MACLEOD M. Pathways for degradation of plastic polymers floating in the marine environment [J]. Environmental Science. Processes & Impacts, 2015, 17(9): 1513-1521. [20] TONG H Y, HU X S, ZHONG X C, et al. Adsorption and desorption of triclosan on biodegradable polyhydroxybutyrate microplastics [J]. Environmental Toxicology and Chemistry, 2021, 40(1): 72-78. doi: 10.1002/etc.4902 [21] SUN Y, WANG X J, XIA S Q, et al. New insights into oxytetracycline (OTC) adsorption behavior on polylactic acid microplastics undergoing microbial adhesion and degradation [J]. Chemical Engineering Journal, 2021, 416: 129085. doi: 10.1016/j.cej.2021.129085 [22] 沈华艳, 陈明周, 刘海露, 等. PBAT/PLA全生物降解薄膜的紫外光老化行为 [J]. 合成树脂及塑料, 2020, 37(1): 13-16,20. doi: 10.19825/j.issn.1002-1396.2020.01.03 SHEN H Y, CHEN M Z, LIU H L, et al. Ultraviolet aging behavior of PBAT/PLA completely biodegradable films [J]. China Synthetic Resin and Plastics, 2020, 37(1): 13-16,20(in Chinese). doi: 10.19825/j.issn.1002-1396.2020.01.03
[23] 张鹏飞, 陈晓东. 贻贝启发蒙脱石-银/聚乳酸抗菌膜的制备及其性能 [J]. 精细化工, 2019, 36(2): 295-301. doi: 10.13550/j.jxhg.20180397 ZHANG P F, CHEN X D. Preparation and properties of mussel-inspired MMT-AgNPs/polylactic acid composite antibacterial film [J]. Fine Chemicals, 2019, 36(2): 295-301(in Chinese). doi: 10.13550/j.jxhg.20180397
[24] LV S S, LIU X J, GU J Y, et al. Microstructure analysis of polylactic acid-based composites during degradation in soil [J]. International Biodeterioration & Biodegradation, 2017, 122: 53-60. [25] 刘文龙, 雷英杰, 莫晓琴, 等. 三种添加物对聚乳酸复合膜性能影响 [J]. 包装工程, 2020, 41(17): 71-77. doi: 10.19554/j.cnki.1001-3563.2020.17.010 LIU W L, LEI Y J, MO X Q, et al. Effect of three additives on performance of polylactic acid composite film [J]. Packaging Engineering, 2020, 41(17): 71-77(in Chinese). doi: 10.19554/j.cnki.1001-3563.2020.17.010
[26] 冯诗艺, 蒋悦, 祁悦, 等. PLA/nano-TiO2复合膜的制备及性能研究 [J]. 塑料工业, 2019, 47(7): 82-87,116. doi: 10.3969/j.issn.1005-5770.2019.07.020 FENG S Y, JIANG Y, QI Y, et al. Preparation and properties of nanocomposites based on poly (lactic acid) and nano-TiO2 [J]. China Plastics Industry, 2019, 47(7): 82-87,116(in Chinese). doi: 10.3969/j.issn.1005-5770.2019.07.020
[27] 田小艳, 张敏, 王蕾, 等. PBS不同化学结构共聚物的性能 [J]. 塑料, 2009, 38(5): 27-30. TIAN X Y, ZHANG M, WANG L, et al. Properties of poly(butylene succinate)(PBS) with various chemical structures [J]. Plastics, 2009, 38(5): 27-30(in Chinese).
[28] 孙爱华, 张春花. 聚丁二酸丁二醇酯(PBS)的制备及特性黏数的测定 [J]. 山东化工, 2016, 45(9): 9-10,13. doi: 10.3969/j.issn.1008-021X.2016.09.004 SUN A H, ZHANG C H. The synthesis and intrinsic viscosity number determination of poly(butylene succinate) [J]. Shandong Chemical Industry, 2016, 45(9): 9-10,13(in Chinese). doi: 10.3969/j.issn.1008-021X.2016.09.004
[29] HALES B J, CASE E E. Immobilized radicals. IV. biological semiquinone anions and neutral semiquinones [J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1981, 637(2): 291-302. doi: 10.1016/0005-2728(81)90168-7 [30] di VALENTIN C, NEYMAN K M, RISSE T, et al. Density-functional model cluster studies of EPR g tensors of Fs+ centers on the surface of MgO [J]. The Journal of Chemical Physics, 2006, 124(4): 044708. doi: 10.1063/1.2161190 [31] DELA CRUZ A L, COOK R L, LOMNICKI S M, et al. Effect of low temperature thermal treatment on soils contaminated with pentachlorophenol and environmentally persistent free radicals [J]. Environmental Science & Technology, 2012, 46(11): 5971-5978. [32] 阮秀秀, 孙万雪, 程玲, 等. 环境持久性自由基的研究进展 [J]. 上海大学学报(自然科学版), 2016, 22(2): 114-121. RUAN X X, SUN W X, CHENG L, et al. Research progress of environmental persistent free radicals [J]. Journal of Shanghai University (Natural Science Edition), 2016, 22(2): 114-121(in Chinese).
[33] RZEPNA M, PRZYBYTNIAK G, SADŁO J. Radiation degradation and stability of PBAT: Copolymer of aromatic and aliphatic esters [J]. Journal of Applied Polymer Science, 2018, 135(37): 46682. doi: 10.1002/app.46682 [34] KIJCHAVENGKUL T, AURAS R, RUBINO M, et al. Atmospheric and soil degradation of aliphatic-aromatic polyester films [J]. Polymer Degradation and Stability, 2010, 95(2): 99-107. doi: 10.1016/j.polymdegradstab.2009.11.048 [35] RIVATON A. Photochemistry of poly(butyleneterephthalate): 2—identification of the IR-absorbing photooxidation products [J]. Polymer Degradation and Stability, 1993, 41(3): 297-310. doi: 10.1016/0141-3910(93)90076-U -



 下载:
下载: