-
硫酸根自由基(·
$\rm SO_4^- $ )氧化还原电位较高,相对于羟基自由基(·OH)具有更长的半衰期[1-3]. 因此基于·$\rm SO_4^- $ 的深度氧化处理技术已经广泛地运用于水体环境中有机污染物处理研究,然而基于·$\rm SO_4^- $ 的深度氧化处理技术对有机污染物进行降解过程中,存在多种活性氧化物种共存的情况,从而难以区分不同种类的活性氧物种对目标有机污染物的降解机制和降解贡献[4-6]. 非质子极性溶剂环境则有利于单一·$\rm SO_4^- $ 的稳定存在[7-8],因此,构建非质子极性溶剂中过硫酸盐活化反应体系对探索有机污染物和·$\rm SO_4^- $ 的反应机制和降解动力学具有重要的意义.茜素类染料是一种典型的媒介染料,也是印染废水常见组份之一. 其主要分为茜素类蒽醌染料、茜素类染料等[7]. 茜素类染料作为染料型配位剂广泛地运用于在分光光度分析、电化学分析等方面. 可以大大提高检测的灵敏度和选择性[9-10]. 随着茜素类染料在工业上广泛应用,其不可以避免的流入到水体环境中[11]. 然而关于茜素类染料在水体环境中降解动力学,特别是转化的共性机制还未见相关文献报道.
本文以茜素类染料为目标化合物,采用低压汞灯为光源,活化非质子极性溶剂中PDS. 使得有效产生单一的·
$\rm SO_4^- $ 和茜素类染料直接作用. 从而探索茜素类染料在UVC/PDS/非质子极性溶剂体系中单因素对其降解动力学的影响及其该体系中·$\rm SO_4^- $ 对茜素类染料反应机制和降解途径,试图为环境介质中茜素类染料在活性氧物种(ROSs)作用下的迁移、转化、归趋和光活化降解提供理论和实验依据. -
仪器与试剂主要参照本课题组前面系列研究[8],光催化反应仪配备主波长254 nm,功率15 W的低压汞灯为光源,反应器为双层夹空派克玻璃器皿并与光源距离为15 cm,高效液相色谱(岛津 HPLC-2030),LC/MS/MS联用仪器 (API 4000),PHS-3B 精密酸度计(上海雷磁仪器厂),茜素类染料(Sigma-Aldrich),K2S2O8为AR级(国药集团化学试剂有限公司),甲醇为GR级,去离子水(Millipore Milli-Q系统,保持TOC的总含量≤13 µg·L−1).
-
配制茜素类染料标准溶液的浓度为100 μmol·L−1,相关有机溶剂反应液则直接用有机溶剂做溶液,移取100 mL标准溶液于光反应器中. 紫外光源300 W汞灯下与反应器平行放置,实验中通过循环水装置连接光反应器控制反应器温度,误差在(±0.5 ℃),反应温度一般控制在室温( (25 ± 0.5) ℃)进行. 向100 mL茜素类染料溶液中加入一定量的K2S2O8,在磁力搅拌器作用下使溶液保持恒速搅拌. 在开灯反应之前,使溶液搅拌10 min,当打开汞灯时开始计时. 每隔一定时间用移液枪移取2 mL反应溶液于已放好等体积甲醇的离心管中. 反应液过0.22 μm滤膜,得样品用于HPLC分析,并通过HPLC分离及鉴定其降解过程中浓度变化情况. 降解中间产物的鉴定是通过LC/MS/MS完成.
-
茜素类染料的光活化过硫酸盐降解动力学分析采用岛津公司HPLC 2030(Shim-pack GIST C18,250 mm×4.60 mm,5 μm), 检测条件如下:柱温为30 ℃,进样量为 20 μL,流动相A (甲醇) : B (H2O)=55 : 45(体积比),λ=350 nm,流速为0.5 mL·min−1. 茜素类染料的降解产物的分析在质谱联用分析仪API 4000上进行,其中流速0.5 mL·min−1,流动相为ACN:H2O=50:50(体积比). 质谱条件为:电子喷雾界面作MS和MS-MS的测定,高纯氮做脱溶和喷雾碰撞气体. ESI电压为5. 5 kV,源模块为130 ℃,脱溶温度为400 ℃. 反应体系中ROSs种类的鉴定使用电子顺磁共振仪(德国EMX EPR/X-band)检测,100 mmol·L−1 5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)用作自旋捕获试剂. 不同介质中PDS在UVC光照10 min后直接取样,置于100 μL样品管中待测[8].
-
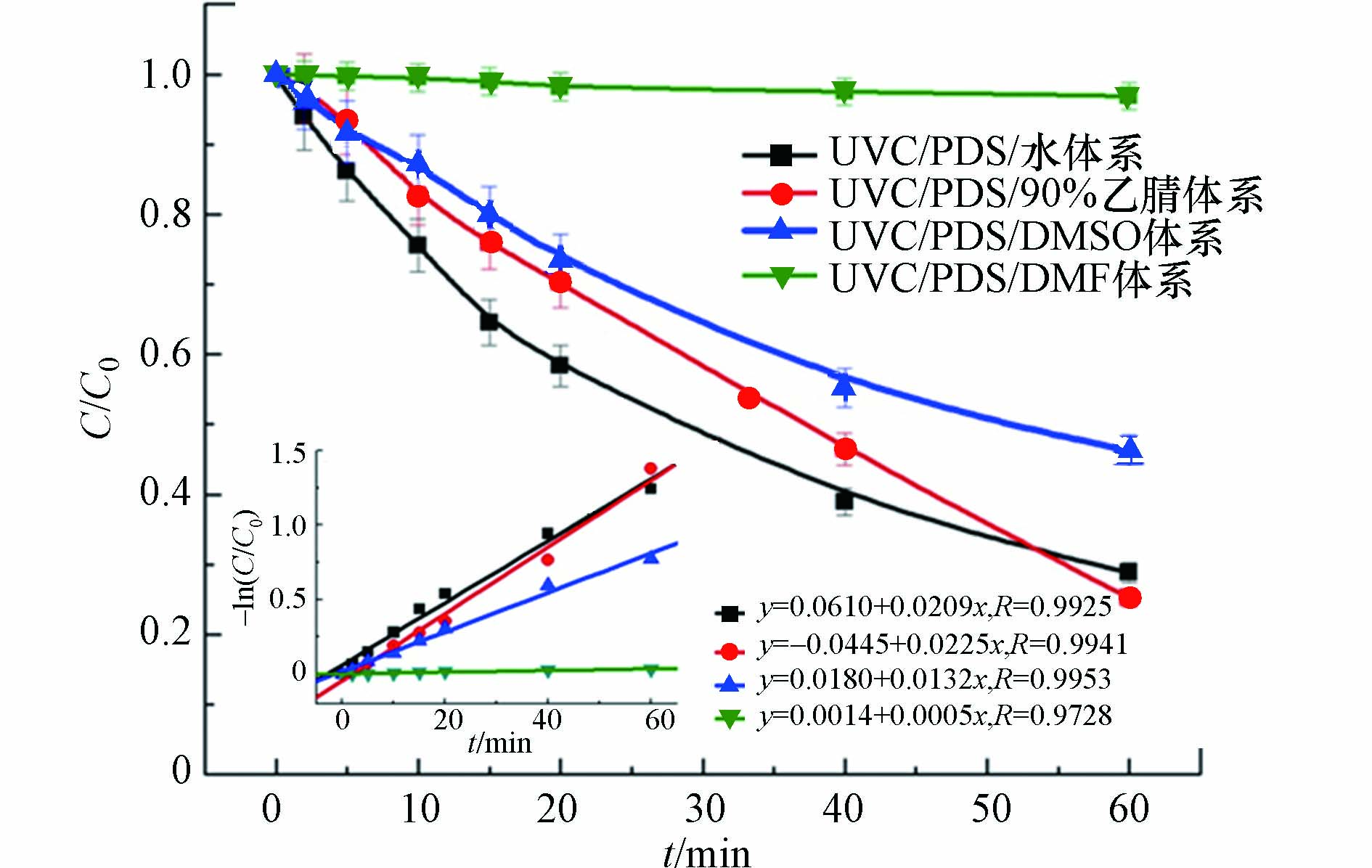
选用主波长为254 nm的UVC紫外光为光源,PDS为活化剂,媒染橙1为目标化合物. 探讨了其在不同溶剂中通过光活化降解效果的差异. 结果如图1所示,结果表明在初始浓度均为100 μmol,PDS用量为1.0 g·L−1,温度为25 ℃,光强为5.34 mW·cm−2的条件下,媒染橙1在4种不同溶剂体系中降解存在较大的差异. 其去除率呈现以下规律:(90%ACN +10%H2O)>100% H2O>100% DMSO>100% DMF.
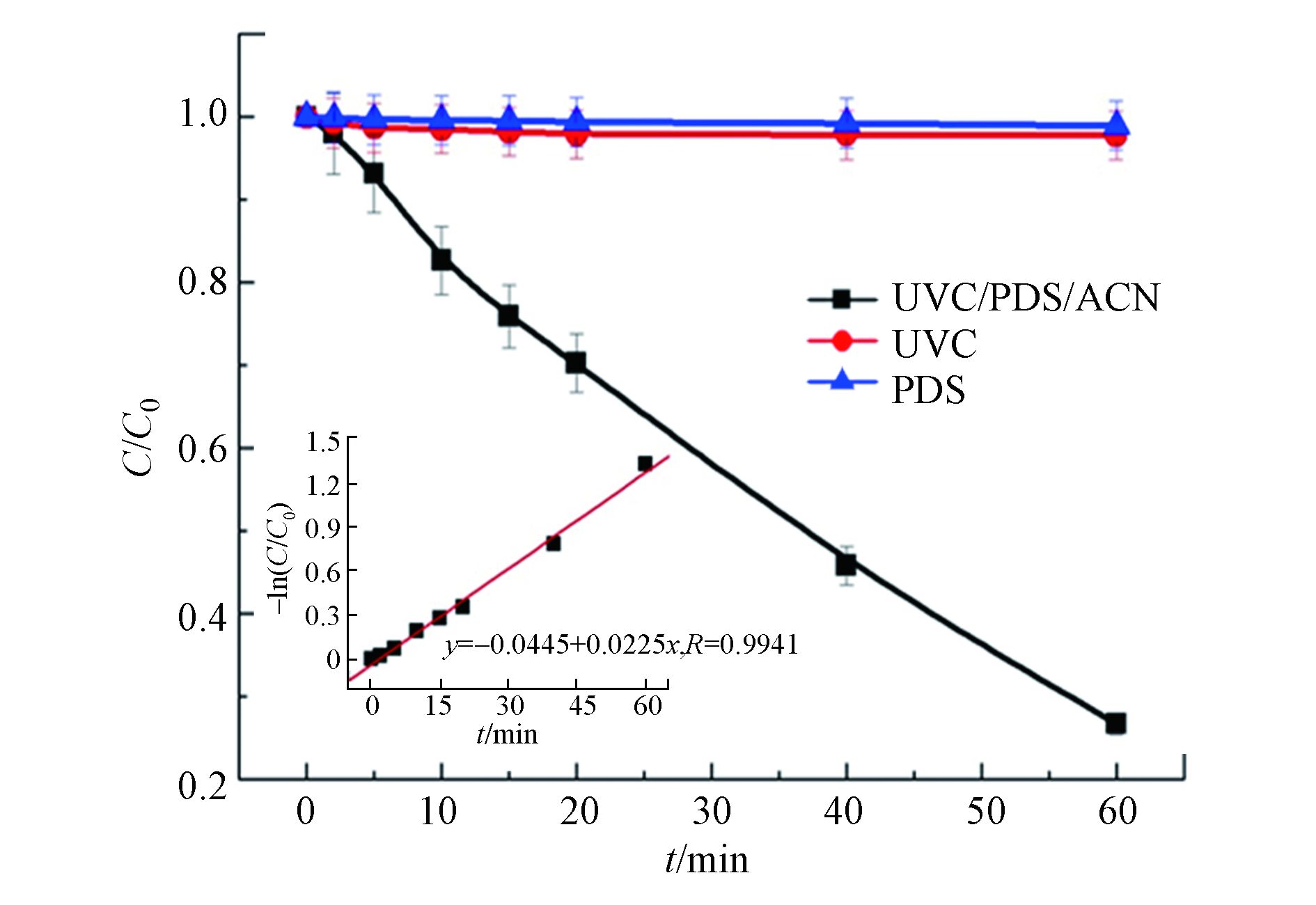
进一步利用Langmuir-Hinshelwood的简化方程式-dc/dt=kCt[12-13]对茜素类染料在乙腈体系中降解过程进行模拟. 式中k为假一级动力学常数,Ct为反应体系中媒染橙1溶液的浓度. 根据-ln(Ct/C0)对时间作图,如图2所示. 得出茜素类染料在乙腈溶剂体系中的降解符合假一级动力学. 反应60 min后,媒染橙1在(90%ACN+10%H2O)体系和100%H2O体系降解速度较快是由于这两种反应体系中有大量·
$\rm SO_4^- $ 和·OH等自由基离子存在. 然而在100%H2O体系中的前50 min,茜素类染料的去除率更高则可能是由于该体系中的自由基离子以·OH为主. ·OH相对于·$\rm SO_4^- $ 而言虽然半衰期较短,但具有更高的反应活性. 100%DMSO体系中,茜素类染料的降解速率居中则可能是由于该反应体系中以·$\rm SO_4^- $ 为主,其和·OH相比较反应活性稍低[14]. 100%DMF体系中媒染橙1基本不降解则是由于DMF的最大吸收波长为220 nm,刚好处在UVC紫外光波段. 因此,在该体系中UVC紫外光的透过率较低,活化溶液中的PDS效率较低,从而导致了DMF溶液中目标化合物较低地降解速率. 选定UVC/(90%ACN+10%H2O)/PDS体系,进一步考察单独的UVC或者PDS对媒染橙1的降解效果. 结果如图2所示,研究发现反应60 min,单独的UVC或者PDS对媒染橙1几乎没有降解效果. 而UVC/(90%ACN+10%H2O)/PDS体系中媒染橙1的去除效果则达到80%左右,因此本文进一步探索了茜素类染料在该体系中降解动力和转化机制. -
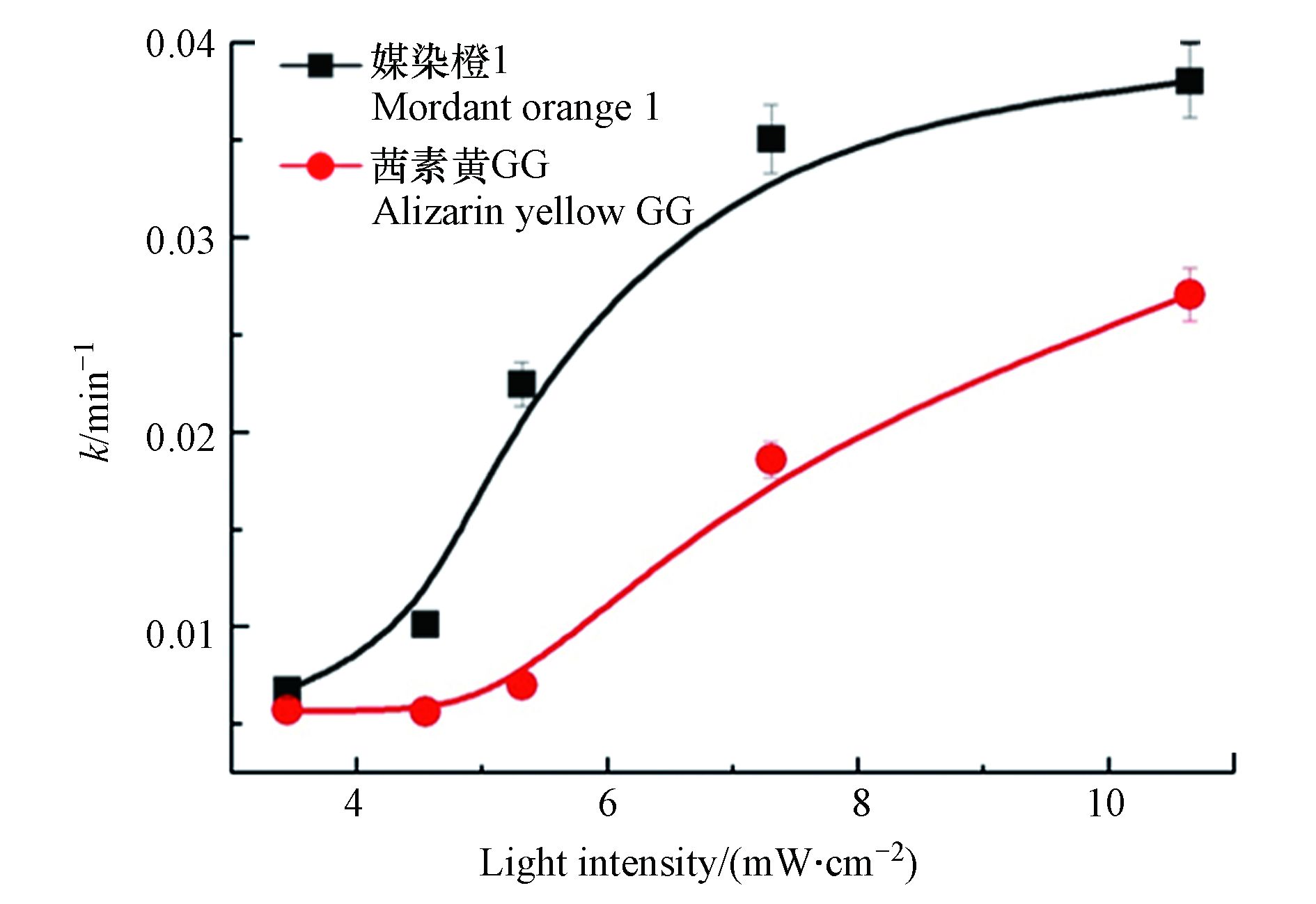
在底物初始浓度为100 μmol·L−1,PDS的浓度为1.0 g·L−1,温度为25 ℃的条件下,进一步探索了紫外光强对UVC/(90%ACN+10%H2O)/PDS体系中媒染橙1和茜素黄GG的降解速率常数的影响. 结果如图3所示,通过调节光源与反应器之间的距离来控制反应液面光强. 测得低压汞灯光源与反应液面之间的距离分别为3、5、7、9、11 cm时. 反应液面相应的光强则分别对应为10.63、7.31、5.32、4.55、3.45 mW·cm−2. 结果表明随着UVC光强的增强,两只染料的降解速率均显著提高. 当光强由3.45增加到10.63 mW·cm−2,媒染橙1和茜素黄GG降解速率分别由0.0067 min−1和0.0057 min−1增加到0.0380 min−1和0.0271 min−1. 光强的增强对两种茜素类染料降解效率均具有明显的促进作用,其主要原因是光强提高促进了PDS活化分解,产生了更多的活性氧物种[15].
-
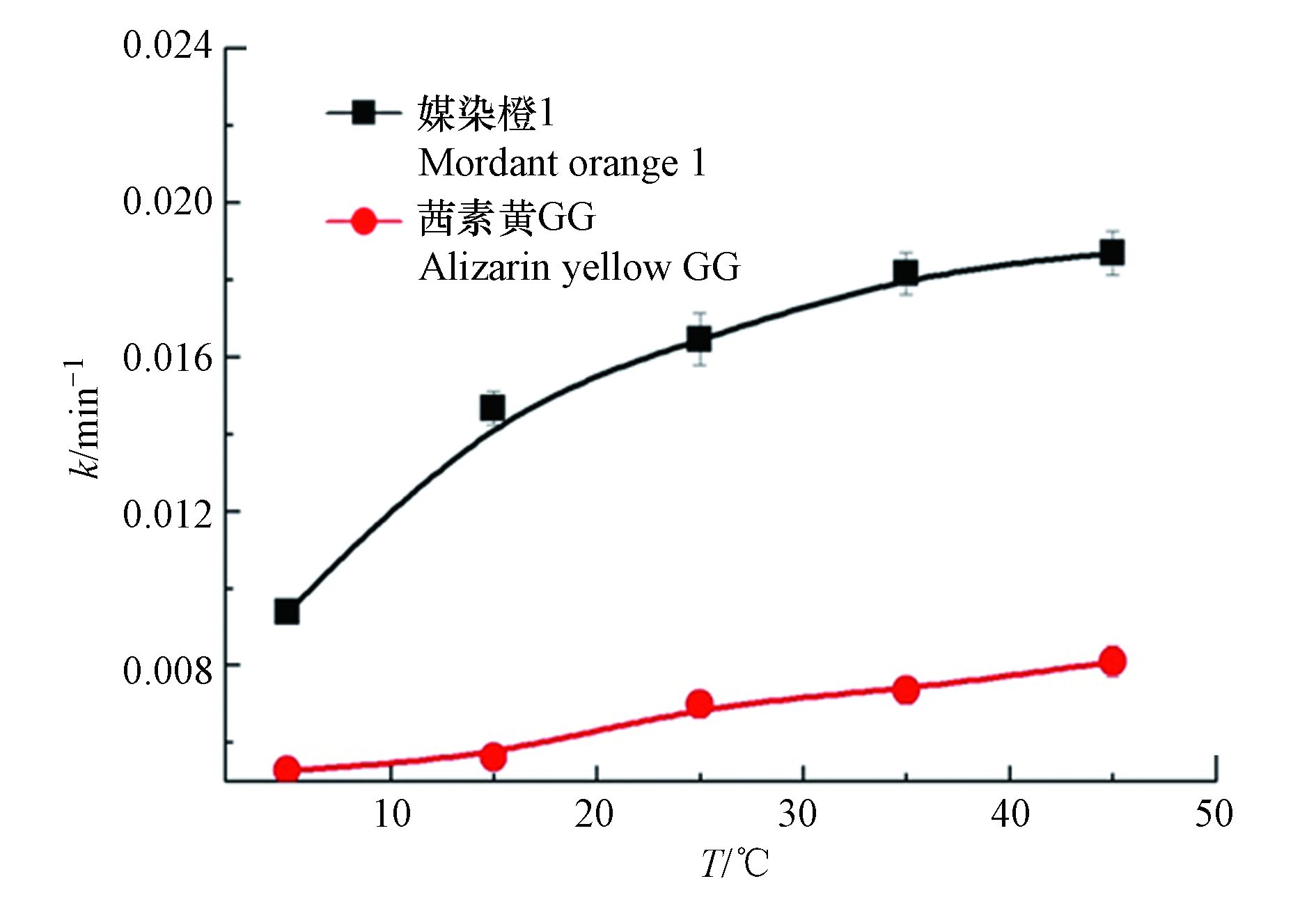
环境温度是影响PDS活化效率的一个重要因素,实验探讨了在反应温度为5、15、25、35、45 ℃对2种茜素类染料降解动力学的影响. 结果如图4所示,结果表明两种茜素类染料的降解速率常数随着温度的升高而增大,媒染橙1和茜素黄GG的降解反应速率分别由5 ℃的0.0096 min−1和0.0035 min−1升高至0.0192 min−1和0.0086 min−1,两者的反应速率均增加到原来的2倍多. 其可能是因为在高温条件下,UVC/(90%ACN+10%H2O)/PDS体系中PDS更容易被活化[16-17],UVC和高温对PDS起到协同活化作用,从而使得该体系中高效率的产生·
$\rm SO_4^- $ . 因此,高温条件下茜素类染料具有更高的降解速率常数. -
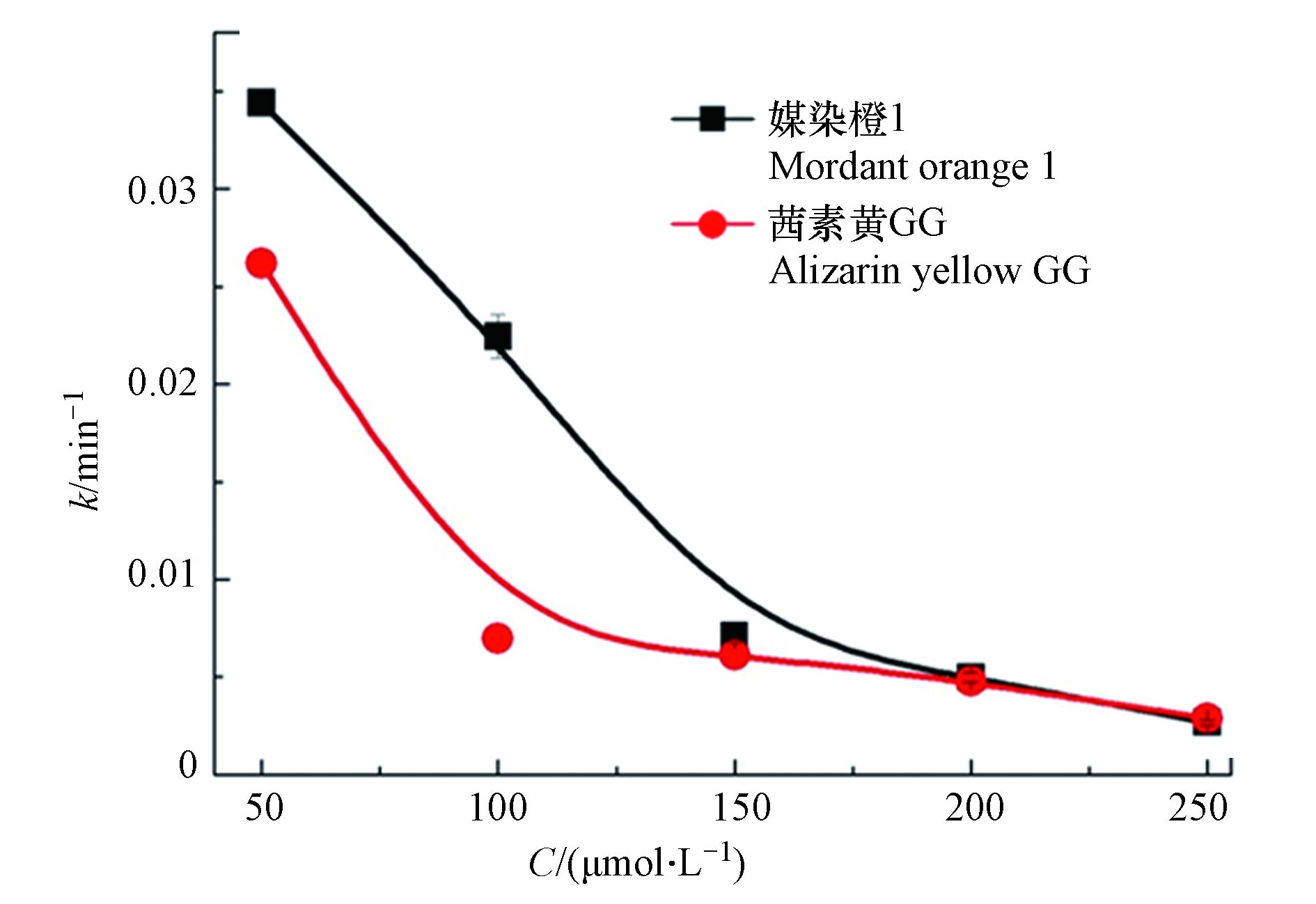
进一步探索了UVC/(90%ACN+10%H2O) /PDS体系中底物浓度对茜素类染料降解动力学的影响. 在PDS的浓度为1.0 g·L−1,光强为5.32 mW·cm−2和温度为25 ℃的条件下,探讨了媒染橙1和茜素黄GG的初始浓度在50、100、150、200、250 μmol·L−1条件下的降解动力学变化情况. 图5表明在目标化合物初始浓度为50 μmol·L−1时,媒染橙1和茜素黄GG的降解速率最大,分别为0.0351 min−1 和0.0273 min−1. 然而随着茜素类染料底物浓度的增加,其降解速率逐渐降低. 这是因为乙腈活化PDS体系中产生·SO4−等活性氧物种的量是一定的,过多的茜素类染料分子不能和·
$\rm SO_4^- $ 等活性氧物种发生有效的碰撞[18],从而降低了目标有机污染物的反应速率常数. -
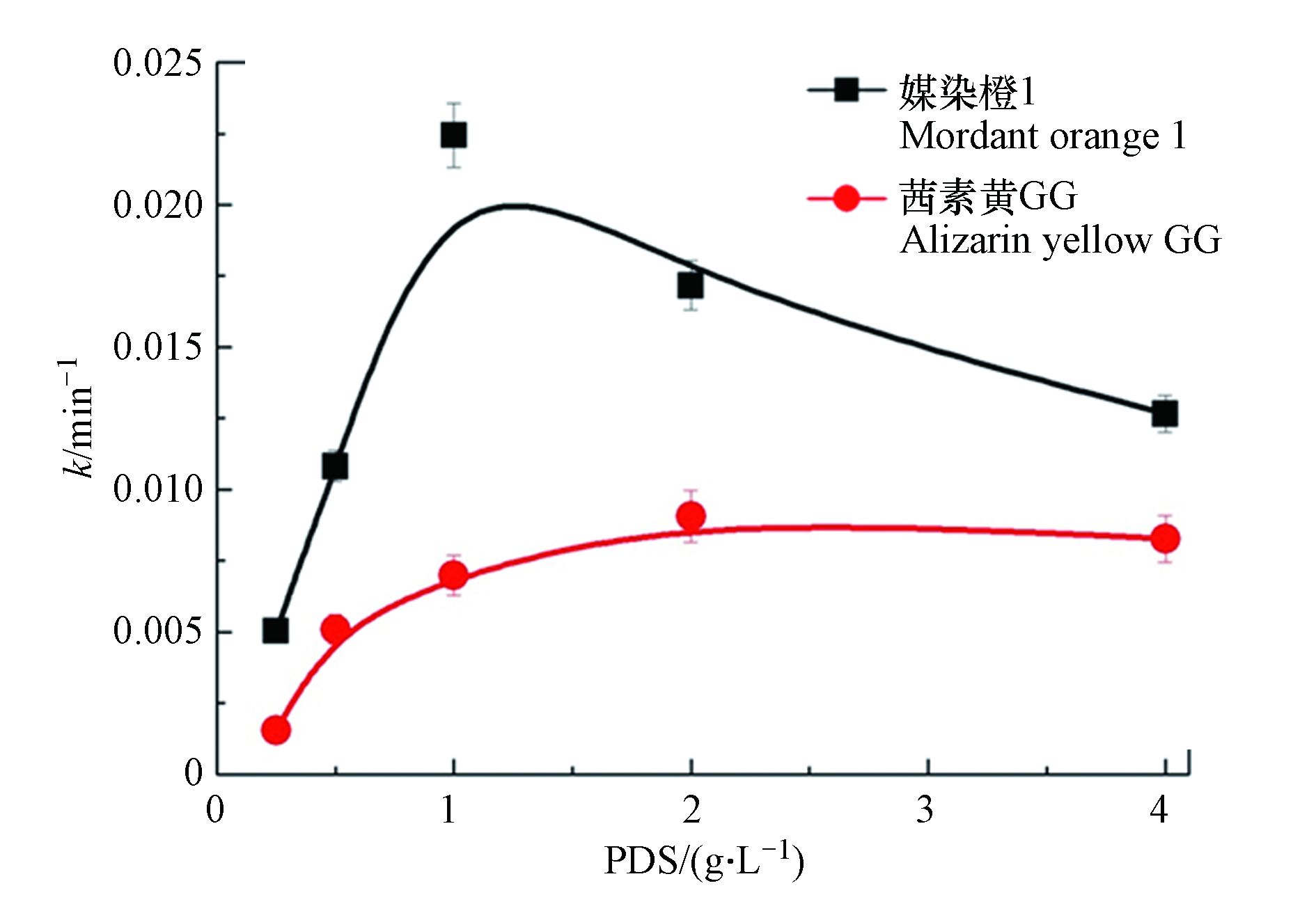
PDS浓度是影响有机污染物降解动力学的一个重要因素,图6探索了示在底物初始浓度为100 μmol·L−1,反应温度为25 ℃,反应光强为5.32 mW·cm−2, PDS浓度分别为0.25、0.5、1.0、2.0、4.0 g·L−1条件下,茜素类染料光活化降解动力学的变化情况. 茜素类染料随着PDS浓度的增大其降解速率先是逐渐增加. 当PDS浓度分别为1.0 g·L−1和2.0 g·L−1时,其降解速率常数达到最大值,分别为0.0225 min−1和0.0098 min−1. 然而进一步增加反应体系中PDS浓度,两种茜素类染料的降解速率常数均出现降低. 茜素类染料随着催化剂用量的增加其降解速率增加,可能是更多的PDS加入反应体系增加·
$\rm SO_4^- $ 等活性氧物种对目标化合物反应的参与度. 然而再进一步增大PDS的浓度则使得其在乙腈溶剂体系中逐渐趋于饱和,过多PDS以晶体的形式析出并没有参与反应中去. 另一方面当PDS浓度过高时,·$\rm SO_4^- $ 和过硫酸盐之间发生电子转移反应,从而减少了和目标化合物的碰撞几率,降低了反应速率[19]. -
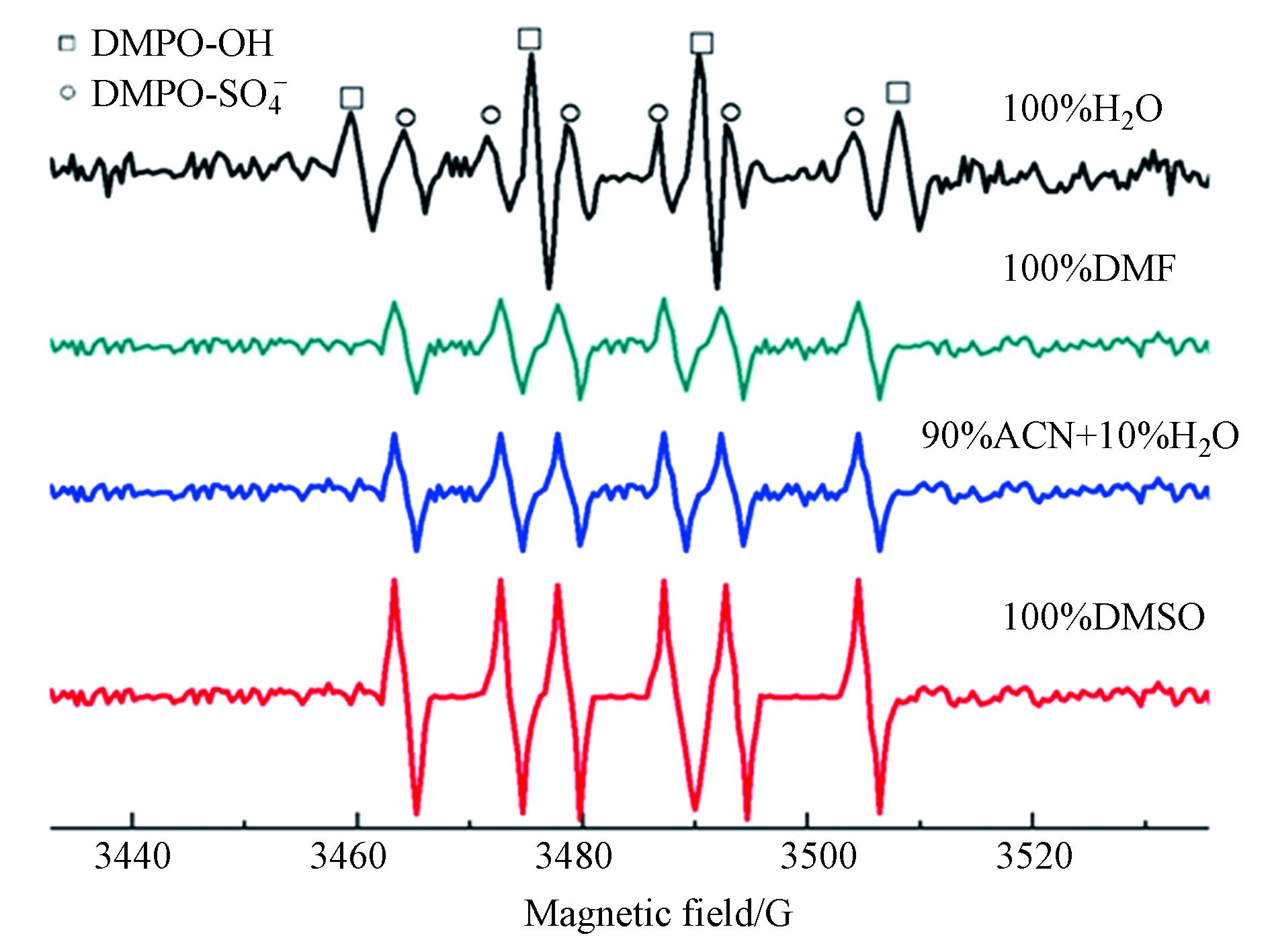
茜素类染料在不同的反应体系具有不同的降解速率. 为了探索不同反应体系中自由基离子的种类,向反应体系中分别加入自旋捕获试剂5,5-二甲基-1-吡咯啉-N-氧化物(DMPO),并通过电子顺磁共振仪对反应体系中的自由基离子进行检测,结果如图7. 从图7可以看出,UVC/100%H2O/K2S2O8体系中·
$\rm SO_4^- $ 和·OH两者共存[20]. 而100%DMF体系,(90%ACN+10%H2O)体系和100%DMSO体系中则主要是·$\rm SO_4^- $ 为主,100%DMSO体系中检测到·$\rm SO_4^- $ 的信号峰最强则说明DMSO溶剂有利于·$\rm SO_4^- $ 的稳定存在. (90%ACN+10%H2O)体系中检测到·$\rm SO_4^- $ 的信号峰次之则可能是由于该反应体系中·$\rm SO_4^- $ 在水分子等其它物质的作用下发了部分淬灭. 100%DMF体系中·$\rm SO_4^- $ 对应的信号峰最弱. EPR检测结果表明UVC/(90%ACN+10%H2O)/K2S2O8体系中以·$\rm SO_4^- $ 为主. 然而实际反应体系中·$\rm SO_4^- $ 有无进一步转化为其他的自由基离子,或者和有机物相互作用具体以哪种自由基离子为主仍然需要进一步的鉴别.乙醇(EtOH)与·
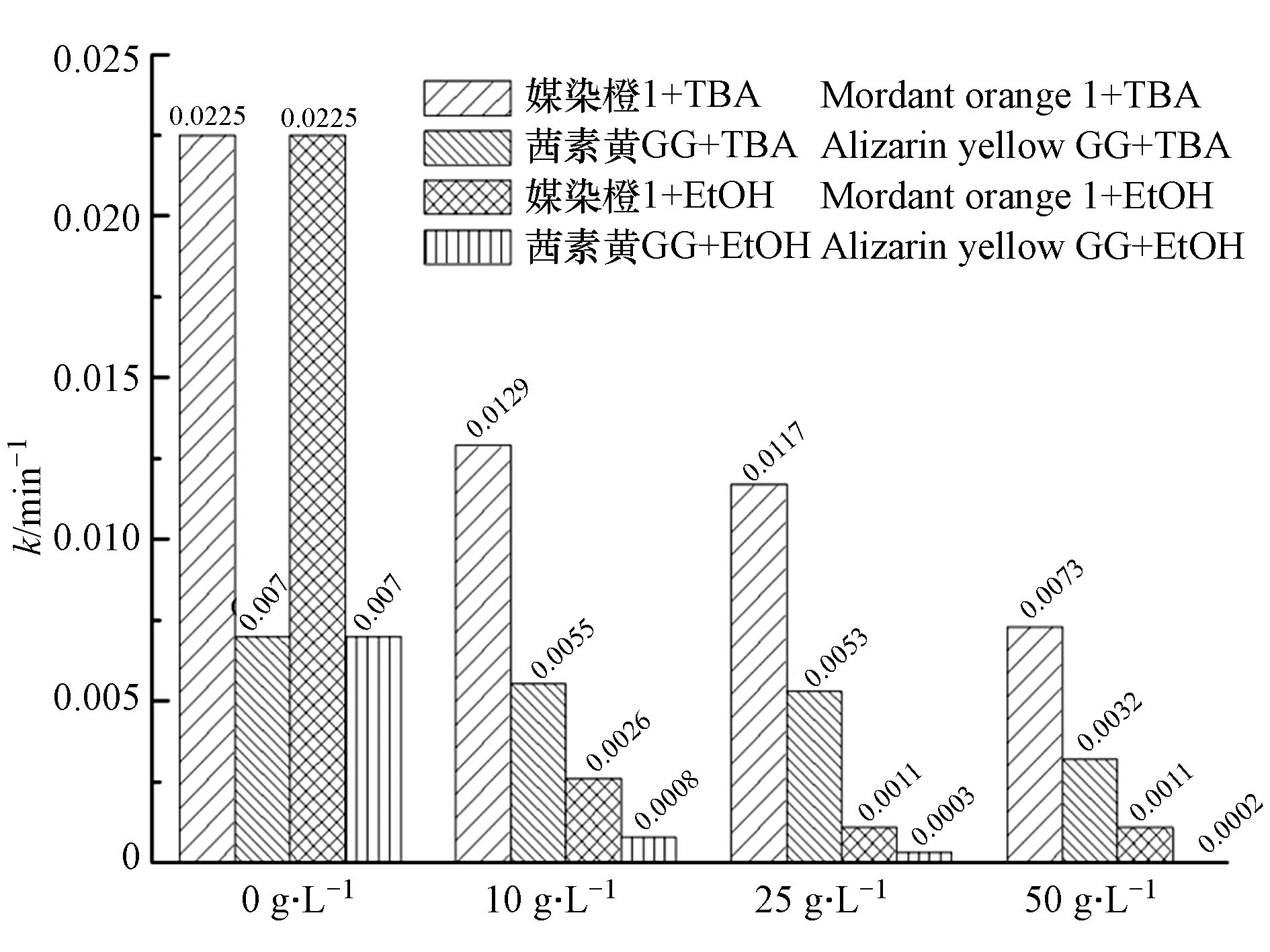
$\rm SO_4^- $ 和·OH都有较高的反应数率常数,对两者均具有良好的淬灭效果. 然而叔丁醇(TBA)中由于没有α氢的存在,其与·OH的反应速率常数则是·$\rm SO_4^- $ 的1000倍以上,其只对·OH具有较好的淬灭效果[21-23]. 为了区分UVC/(90%ACN+10%H2O)/PDS体系中不同活性氧物种对媒染橙1和茜素黄GG的降解贡献,本文选用EtOH和TBA作为分子探针进一步探索研究. 探索不同质量浓度的EtOH和TBA对媒染橙1和茜素黄GG的降解动力学的影响. 结果如图8所示,从图中可以看出,在0—50 g·L−1的范围内,随着EtOH质量浓度的增加,媒染橙1和茜素黄GG的降解速率均显著降低. 然而0—50 g·L−1的TBA加入则对媒染橙1和茜素黄GG的去除率的影响相对较小,仅当TBA质量浓度上升到50 g·L−1时对媒染橙1和茜素黄GG的去除率显示出其抑制作用. 进一步运用Langmuir-Hinshelwood动力学方程对其降解速率进行模拟,加入10、25、50 g·L−1 EtOH时,媒染橙1的降解速率常数由0.0225 min−1依次降低为0.0055、0.0053、0.0032 min−1. 茜素黄GG的降解速率常数由0.0070 min−1依次降低为0.0008、0.0003、0.0002 min−1. 而加入相同量的TBA时,媒染橙1的降解速率常数依次降低为0.0129、0.0117、0.0073 min−1. 茜素黄GG的降解速率常数依次降低为0.0026、0.0011、0.0011 min−1. 实验结果表明,UVC/(90%ACN+10%H2O)/PDS体系中有大量·$\rm SO_4^- $ 、·OH等ROSs的存在,而TBA对该体系中茜素类染料的降解没有明显的抑制作用. 则进一步说明该反应体系中·$\rm SO_4^- $ 对目标化合物的降解起主要作用,而·OH等其它活性样物种的贡献相对较小. 当TBA的量上升到50 g·L−1时茜素类染料的降解速率常数显著降低则可能是由于反应体系中高浓度的TBA和茜素类染料与·$\rm SO_4^- $ 起到竞争反应的作用,成而导致了茜素类染料较低降解速率. -
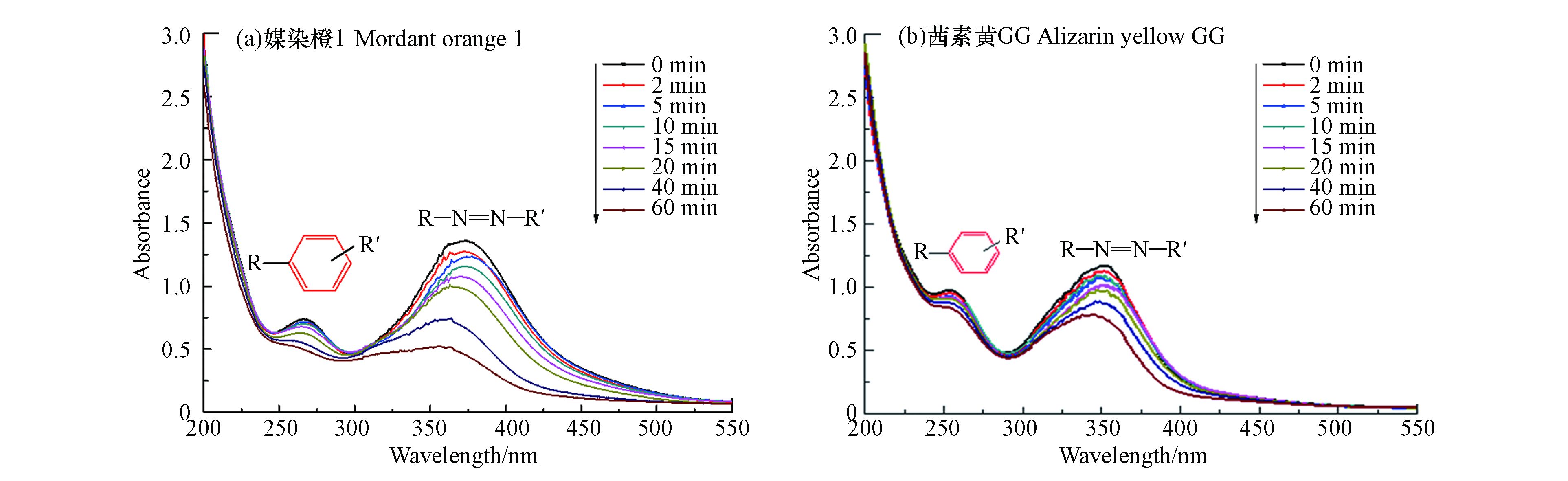
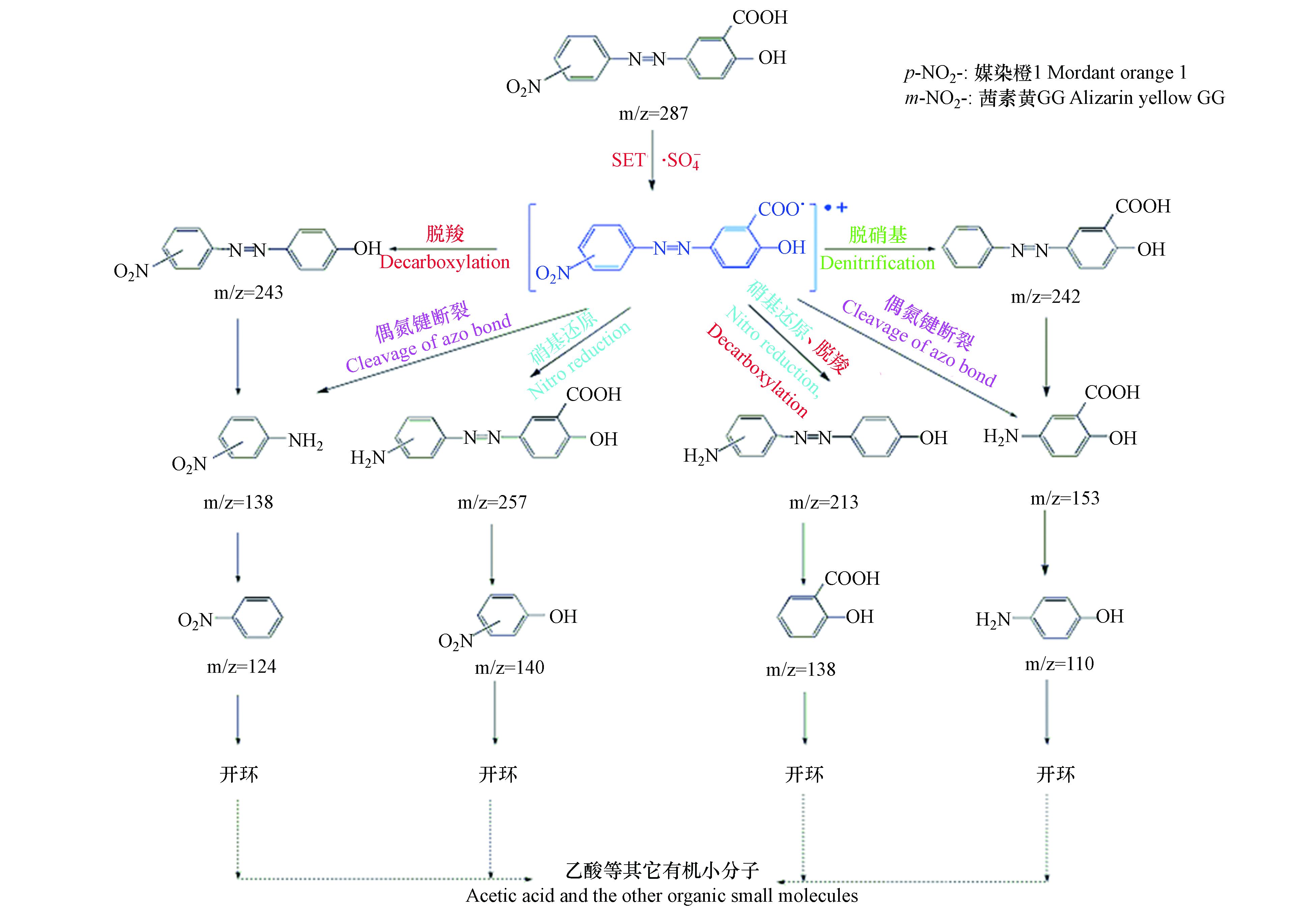
为了探索茜素类染料在·
$\rm SO_4^- $ 作用下的降解共性机制,以媒染橙1和茜素黄GG为目标化合物,比较UVC/(90%ACN+10%H2O)/PDS体系中这两种茜素类染料的降解产物异同. 图9中紫外吸收光谱图表明媒染橙1和茜素黄GG具有相似的吸收光谱,并且两者均具有两个较大的吸收峰. 其中260 nm左右的吸收峰对应茜素类染料中的芳香环结构[24],而360 nm左右的吸收峰则对应着分子中的偶氮键结构. 媒染橙1和茜素黄GG两种茜素类染料中偶氮键的吸收峰(355—375 nm)随着反应时间的增加,吸收峰的强度越来越弱. 但芳香结构的吸收峰(260 nm)强度则在反应的初始阶段并没有明显的降低,随着反应逐步进行时强度才出现慢慢减弱的趋势,因此,UVC/(90%ACN+10%H2O)/PDS体系中茜素类染料分子的降解是最先是由偶氮键的断裂引起的,而后随着反应时间的进一步推进才导致芳香环的裂解.利用HPLC/MS/MS对UVC/(90%ACN+10%H2O)/PDS体系中媒染橙1和茜素黄GG在反应初始阶段的降解中间产物进行进一步的检测. 结果如表1,从表中可以看出,根据降解中间产物的m/z、可能的分子式及其与标准样品的比对,推断出媒染橙1在乙腈体系中共有10种降解中间产物. 分别对应为硝基苯、单羟基化媒染橙1、2-羟基-5-氨基苯甲酸、2-羟基-5-(4-硝基偶氮苯)苯甲酸、2-羟基-5-(4-氨基偶氮苯)苯甲酸、对硝基苯胺、邻羟基苯甲酸、2-羟基-(4-偶氮苯)苯甲酸、4-(4-硝基偶氮苯) 苯酚和4-(4-氨基偶氮苯)苯酚. 茜素黄GG在乙腈体系中则检测到8种降解中间产物,分别对应为硝基苯、2-羟基-5-氨基苯甲酸、邻羟基苯甲酸、2-羟基-(4-偶氮苯)苯甲酸、4-(3-硝基偶氮苯)苯酚、2-羟基-5-(3-硝基偶氮苯)苯甲酸、间硝基苯胺和对氨基苯酚. 值得注意的是,在媒染橙1和茜素黄两种茜素类染料在UVC/(90%ACN+10%H2O)/PDS体系中均检测到了硝基苯、2-羟基-5-氨基苯甲酸、邻羟基苯甲酸和2-羟基-(4-偶氮苯)苯甲酸和4-(4-硝基偶氮苯)苯酚等五种降解中间产物.
结合两种茜素类染料降解中间产物的推导,并对媒染橙1和茜素黄GG降解中间产物的对比,发现茜素类染料在UVC/(90%ACN+10%H2O)/PDS体系中·
$\rm SO_4^- $ 的作用下主要以单电子转移反应(SET)为主[25-27],结果如图10所示.·
$\rm SO_4^- $ 在茜素类染料分子中得到一个电子,形成一个带正电荷的自由基离子. 带正电荷的自由基离子形成以后,在反应体系中其它自由基离子的作用下发生一系列氧化还原反应. 其主要有(1)偶氮键断裂反应,偶氮键断裂后形成的硝基苯和苯甲酸类化合物在两种茜素类染料的降解过程中均被检测到;(2)硝基还原反应,硝基还原后形成的降解中间产物m/z=257和m/z=213则分别对应着硝基直接还原产物和硝基还原之后的脱羧基产物;(3)脱羧基反应,茜素类染料的羧基上的硝基相对于其他官能团稳定然而当硝基被还原成氨基后,能进一步的发生氧化消除反应. 在两种目标化合物的乙腈反应体系中,均检测到了m/z=242的降解中间产物. 根据N律,该分子式不稳定,母体化合物形成自由基离子后,羧基能直接消除,对应降解中间产物m/z=243. 其在两种目标化合物的降解反应体系中均被检测到;(4)脱硝基反应,茜素类染料分子中芳香环中含有偶数个N原子,推断其分子式为C13H10N2O3. 因此,m/z=242的降解中间产物相对于母体化合物丢失了一个N原子. -
(1)短波紫外光活化有机溶剂中过硫酸盐体系中,媒染橙1在四种不同溶剂体系中降解存在较大的差异,其去除率呈现以下规律:(90%ACN +10%H2O)体系>100%H2O体系>100%DMSO体系>100%DMF体系.
(2)UVC/(90%ACN+10%H2O)/PDS体系中媒染橙1比茜素黄GG具有更高的反应速率. 不同PDS用量、底物浓度、反应温度和光照强度等因素对染料降解动力学均有明显的影响,高温和强的光强有利于茜素类染料的降解,而过高的PDS用量和底物浓度降低了茜素类染料的降解速率.
(3)UVC/(90%ACN+10%H2O)/PDS体系中硫酸根自由基对茜素类染料的降解占据主导作用. 而茜素类染料的初始降解途径主要包括单电子转移反应导致的脱磺酸基和偶氮键断裂,以及吸氢反应和取代反应等.
UVC/过硫酸盐/乙腈反应体系的构建及对茜素类染料的降解
Construction the reaction system of UVC/persulfate/aprotic solvents and its application on the degradation of alizarin dyes
-
摘要: 为探索水体环境中有机污染物在硫酸根自由基(·
$\rm SO_4^-$ )作用下的迁移转化规律,构建了UVC/过硫酸盐(PDS)/非质子极性溶剂反应体系,模拟了茜素类染料在·$\rm SO_4^- $ 作用下的降解动力学与反应机制.媒染橙1在不同溶剂体系中的降解动力学常数大小为:(90%乙腈(ACN)+10%水(H2O)) >100%H2O>100%二甲基亚砜(DMSO) >100%二甲基甲酰胺(DMF). 并且媒染橙1比茜素黄GG具有更高的降解反应速率. UVC/PDS/(90%ACN+10%H2O)体系中PDS用量、底物浓度、反应温度和光照强度等因素对茜素类染料降解动力学均有明显的影响.高温和强的光强有利于茜素类染料的降解,而过高PDS用量和底物浓度降低了茜素类染料的反应速率. ·$\rm SO_4^- $ 对茜素类染料的降解占据主导作用. 而茜素类染料的初始降解途径主要包括单电子转移反应导致的脱磺酸基和偶氮键断裂以及吸氢反应和取代反应等.Abstract: In order to investigate the transformation mechanism of organic pollutants under the attack of ·$\rm SO_4^- $ . The system of UVC/persulfates/aprotic solvents was constructed and applied to produce single ·$\rm SO_4^- $ , and then ·$\rm SO_4^- $ was used to react with alizarin dyes. The degradation kinetics and mechanism of azo dyes were also elucidated. The degradation kinetics of Mordant Orange 1 in different aprotic solvents followed this order: (90%ACN+10%H2O)>100%H2O>100%DMSO>100% DMF, and the degradation rate of Mordant Orange 1 was higher than the alizarin yellow GG. The PDS dosage substrate concentration, reaction temperature and light intensity have significantly effects on degradation kinetics of alizarin dyes. Higher temperature and stronger light intensity are favors for the degradation of alizarin dyes. While the higher persulfates dosage and substrate concentration hinder the degradation of alizarin dyes. EPR and reactive oxygen spices scavengers experiments indicated that •$\rm SO_4^- $ did the major contribution on the degradation of alizarin dyes. The single electron transfer was the main reason which resulted in the degradation of alizarin dyes. And the initial transformation mechanism of alizarin dyes includes 4 pathways, such as the loss of sulfonic acid group, cleavage of azo bonds, H-abstraction and substitution.-
Key words:
- mordant orange 1 /
- alizarin yellow GG /
- sulfate radical /
- degradation kinetics /
- reaction mechanism /
- aprotic solvents
-

-
表 1 媒染橙1和茜素黄GG可能的降解中间产物
Table 1. Proposed degradation intermediates for alizarin dyes
保留时间
Retention time质荷比m/z 分子式
Molecular formula产物结构式
Product structure备注
Remark实际值
Actual value理论值
Theoretical value0.84 122.92 123 C6H5NO2 硝基苯 A,B 0.93 302.16 303 C13H9N2O6 单羟基化媒染橙1 A 0.93 152.92 153 C7H7NO3 2-羟基-5-氨基苯甲酸 A,B 1.05 287.83 287 C13H9N2O5 2-羟基-5-(4-硝基偶氮苯)苯甲酸 A 4.94 257.64 257 C13H11N3O3 2-羟基-5-(4-氨基偶氮苯)苯甲酸 A 4.96 138.02 138 C6H6N2O2 对硝基苯胺 A 4.96 138.02 138 C7H6O3 邻羟基苯甲酸 A,B 5.93 242.18 242 C13H10N2O3 2-羟基-(4-偶氮苯)苯甲酸 A,B 5.93 244.19 243 C12H9N3O3 4-(4-硝基偶氮苯)苯酚 A,B 6.69 288.06 287 C13H9N2O5 2-羟基-5-(3-硝基偶氮苯)苯甲酸 B 7.51 212.84 213 C12H11N3O 4-(4-氨基偶氮苯)苯酚 A 8.44 139.66 138 C6H6N2O2 间硝基苯胺 B 8.52 109.23 109 C6H7NO 对氨基苯酚 B 注释: A是指媒染橙1乙腈反应体系,B为茜素黄GG乙腈反应体系. -
[1] REN H, SEDLAK J A, ELROD M J. General mechanism for sulfate radical addition to olefinic volatile organic compounds in secondary organic aerosol [J]. Environmental Science & Technology, 2021, 55(3): 1456-1465. [2] 胡倩, 阳海, 陶文杰, 等. 酸性红37在UV/K2S2O8体系中的降解动力学和转化机制 [J]. 环境化学, 2019, 38(12): 2869-2878. doi: 10.7524/j.issn.0254-6108.2019011703 HU Q, YANG H, TAO W J, et al. Degradation kinetic and transformation mechanism of acid red 37 in UV/K2S2O8 system [J]. Environmental Chemistry, 2019, 38(12): 2869-2878(in Chinese). doi: 10.7524/j.issn.0254-6108.2019011703
[3] 韩爽, 肖鹏飞. 过硫酸盐活化技术在四环素类抗生素降解中的应用进展 [J]. 环境化学, 2021, 40(9): 2873-2883. doi: 10.7524/j.issn.0254-6108.2020052401 HAN S, XIAO P F. Application progress of persulfate activation technology in degradation of tetracycline antibiotics [J]. Environmental Chemistry, 2021, 40(9): 2873-2883(in Chinese). doi: 10.7524/j.issn.0254-6108.2020052401
[4] AN T C, YANG H, LI G Y, et al. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water [J]. Applied Catalysis B:Environmental, 2010, 94(3/4): 288-294. [5] CAO Y, QIU W, LI J, et al. Sulfite enhanced transformation of iopamidol by UV photolysis in the presence of oxygen: Role of oxysulfur radicals [J]. Water Research, 2021, 189: 116625. doi: 10.1016/j.watres.2020.116625 [6] MA J, DING Y, CHI L P, et al. Degradation of benzotriazole by sulfate radical-based advanced oxidation process [J]. Environmental Technology, 2021, 42(2): 238-247. doi: 10.1080/09593330.2019.1625959 [7] CLÉMENT J L, FERRÉ N, SIRI D, et al. Assignment of the EPR spectrum of 5, 5-dimethyl-1-pyrroline N-oxide (DMPO) superoxide spin adduct [J]. The Journal of Organic Chemistry, 2005, 70(4): 1198-1203. doi: 10.1021/jo048518z [8] 庄帅, 阳海, 安继斌, 等. 硫酸根自由基对酸性红37的降解动力学与机制 [J]. 纺织学报, 2019, 40(11): 131-139. doi: 10.13475/j.fzxb.20181000409 ZHUANG S, YANG H, AN J B, et al. Degradation kinetics and mechanism of Acid Red 37 under attack of sulfate radicals [J]. Journal of Textile Research, 2019, 40(11): 131-139(in Chinese). doi: 10.13475/j.fzxb.20181000409
[9] ZHOU R W, ZHANG T Q, ZHOU R S, et al. Underwater microplasma bubbles for efficient and simultaneous degradation of mixed dye pollutants [J]. Science of the Total Environment, 2021, 750: 142295. doi: 10.1016/j.scitotenv.2020.142295 [10] RAO Y D, ZHANG Y X, LI A Q, et al. Photocatalytic activity of G-TiO2@Fe3O4 with persulfate for degradation of alizarin red S under visible light [J]. Chemosphere, 2021, 266: 129236. doi: 10.1016/j.chemosphere.2020.129236 [11] LI M L, LEI P, SONG S M, et al. Alizarin-based molecular probes for the detection of hydrogen peroxide and peroxynitrite [J]. The Analyst, 2021, 146(2): 509-514. doi: 10.1039/D0AN01805H [12] YANG H, ZHUANG S, HU Q, et al. Competitive reactions of hydroxyl and sulfate radicals with sulfonamides in Fe2+/S2O82− system: Reaction kinetics, degradation mechanism and acute toxicity [J]. Chemical Engineering Journal, 2018, 339: 32-41. doi: 10.1016/j.cej.2018.01.106 [13] 易兵, 胡倩, 杨辉琼, 等. 酸性红37光催化降解动力学的响应曲面法优化及其转化机制 [J]. 纺织学报, 2018, 39(6): 81-88. doi: 10.13475/j.fzxb.20170505108 YI B, HU Q, YANG H Q, et al. Photocatalytic degradation kinetics optimization of acid red 37 by response surface method and transformation mechanism thereof [J]. Journal of Textile Research, 2018, 39(6): 81-88(in Chinese). doi: 10.13475/j.fzxb.20170505108
[14] PADMAJA S, ALFASSI Z B, NETA P, et al. Rate constants for reactions of SO4??radicals in acetonitrile [J]. International Journal of Chemical Kinetics, 1993, 25(3): 193-198. doi: 10.1002/kin.550250307 [15] HE L Y, CHEN H, WU L, et al. Synergistic heat/UV activated persulfate for the treatment of nanofiltration concentrated leachate [J]. Ecotoxicology and Environmental Safety, 2021, 208: 111522. doi: 10.1016/j.ecoenv.2020.111522 [16] SHOKOOHI R, BAJALAN S, SALARI M, et al. Thermochemical degradation of furfural by sulfate radicals in aqueous solution: Optimization and synergistic effect studies [J]. Environmental Science and Pollution Research International, 2019, 26(9): 8914-8927. doi: 10.1007/s11356-019-04382-0 [17] KILIC M Y, ABDELRAHEEM W H, HE X X, et al. Photochemical treatment of tyrosol, a model phenolic compound present in olive mill wastewater, by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs) [J]. Journal of Hazardous Materials, 2019, 367: 734-742. doi: 10.1016/j.jhazmat.2018.06.062 [18] KHAN S, SOHAIL M, HAN C, et al. Degradation of highly chlorinated pesticide, lindane, in water using UV/persulfate: Kinetics and mechanism, toxicity evaluation, and synergism by H2O2 [J]. Journal of Hazardous Materials, 2021, 402: 123558. doi: 10.1016/j.jhazmat.2020.123558 [19] GABET A, MÉTIVIER H, de BRAUER C, et al. Hydrogen peroxide and persulfate activation using UVA-UVB radiation: Degradation of estrogenic compounds and application in sewage treatment plant waters [J]. Journal of Hazardous Materials, 2021, 405: 124693. doi: 10.1016/j.jhazmat.2020.124693 [20] CHEN L, XUE Y F, LUO T, et al. Electrolysis-assisted UV/sulfite oxidation for water treatment with automatic adjustments of solution pH and dissolved oxygen [J]. Chemical Engineering Journal, 2021, 403: 126278. doi: 10.1016/j.cej.2020.126278 [21] LIANG H Y, ZHANG Y Q, HUANG S B, et al. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate [J]. Chemical Engineering Journal, 2013, 218: 384-391. doi: 10.1016/j.cej.2012.11.093 [22] 戴慧旺, 陈建新, 苗笑增, 等. 醇类对UV-Fenton体系羟基自由基淬灭效率的影响 [J]. 中国环境科学, 2018, 38(1): 202-209. doi: 10.3969/j.issn.1000-6923.2018.01.024 DAI H W, CHEN J X, MIAO X Z, et al. Effect of alcohols on scavenging efficiencies to hydroxyl radical in UV-Fenton system [J]. China Environmental Science, 2018, 38(1): 202-209(in Chinese). doi: 10.3969/j.issn.1000-6923.2018.01.024
[23] ZHOU L, ZHENG W, JI Y F, et al. Ferrous-activated persulfate oxidation of arsenic(Ⅲ) and diuron in aquatic system [J]. Journal of Hazardous Materials, 2013, 263: 422-430. doi: 10.1016/j.jhazmat.2013.09.056 [24] PIELESZ A, BARANOWSKA I, RYBAK A, et al. Detection and determination of aromatic amines as products of reductive splitting from selected azo dyes [J]. Ecotoxicology and Environmental Safety, 2002, 53(1): 42-47. doi: 10.1006/eesa.2002.2191 [25] QIN W L, LIN Z, DONG H Y, et al. Kinetic and mechanistic insights into the abatement of clofibric acid by integrated UV/ozone/peroxydisulfate process: A modeling and theoretical study [J]. Water Research, 2020, 186: 116336. doi: 10.1016/j.watres.2020.116336 [26] HU J, CHEN H, DONG H Y, et al. Transformation of iopamidol and atrazine by peroxymonosulfate under catalysis of a composite iron corrosion product (Fe/Fe3O4): Electron transfer, active species and reaction pathways [J]. Journal of Hazardous Materials, 2021, 403: 123553. doi: 10.1016/j.jhazmat.2020.123553 [27] TONG X, WANG S N, WANG L M. Kinetics and mechanism of syringic acid degradation initiated by hydroxyl radical and sulphate radical in the aqueous phase [J]. Chemosphere, 2020, 256: 126997. doi: 10.1016/j.chemosphere.2020.126997 -










 下载:
下载: