-
挥发性有机物是主要的大气污染物之一,特别是其中的含氯挥发性有机物(chlorinated volatile organic compounds, CVOCs),被大多数国家列为高危害目标排放物. 该类污染物具有排放量大、毒性强、稳定性高和不易降解的特点. CVOCs主要来源于石化、医药、农药、轻工业等生产过程中,其大量排放对人类健康和生态环境造成危害[1-4]。鉴于此,世界各地展开了治理CVOCs废气的技术研究,探索出多种处理方法,主要包括[5-11]:直接燃烧法、吸收法、催化氧化法、生物法、低温等离子净化法等. 其中,催化氧化技术具有反应温度低、适用范围广、净化效率高以及二次污染物少等优点,已作为比较成熟的环保技术广泛地应用于CVOCs治理领域.
众所周知,催化剂是催化氧化反应过程中的关键因素之一,因此探索和开发高效、廉价、无污染的催化剂是CVOCs催化氧化研究中不可或缺的一部分. 目前,用于催化氧化CVOCs的催化剂主要包括贵金属、非贵金属和分子筛三类[12-18]. 贵金属催化剂如Pt(铂)、Pd(钯)、Ru(钌)、Au(金)、Rh(铑)等对CVOCs的催化活性相对较高,但价格昂贵,且容易与产物中含氯物种发生反应,形成含氯的金属有机物,造成催化剂中毒失活. 分子筛催化剂包括H-Y、HZSM-5、ZSM-5、SAPO-34、KIT-6、MCM-41以及各种沸石,其比表面积较大、耐久性优异,但容易产生积碳从而导致催化剂活性降低. 非贵金属氧化物包括过渡金属V、Cr、Mn、Fe、Co、Ni、Cu等金属,还包括镧系的Ce、La等. 尽管非贵金属催化剂催化效率不如贵金属,但仍具有足够的活性,并可通过掺杂改性得到进一步提高,且还具备成本低,来源广泛的优势. 其中,CeO2作为一种无毒且廉价的材料,因具有较高的储氧能力和丰富的氧空位而受到广泛关注[19- 20]. 最近的研究表明,与纯氧化铈相比,掺铜氧化铈具有更好的氧化还原性能和储氧能力,从而使得CuO@CeO2在催化反应中的活性甚至与贵金属催化剂相当[21-22]. 因此,铜掺杂氧化铈有望成为CVOCs催化降解的良好候选材料.
据报道,多孔CeO2基材料因其高比表面积和纳米孔隙率而显示出良好的氧化还原性能和优异的催化活性. 因此,CuO@CeO2材料中多孔结构的设计被认为是获得高催化活性和稳定性的重要策略. 金属有机骨架(Metal-organic frameworks,MOFs)材料由于其柔性和可控的组成而表现出多样性,并且作为多孔结构制备的前驱体极具吸引力[23-26]. 以MOFs为前驱体制备的金属氧化物不仅继承了母体MOFs的多孔结构和大的比表面积,克服了母体的不稳定性,并且,相比于其他制备方法活性组分分散更均匀,从而促进反应物分子的吸附和活化,有利于污染物的降解. 因此,本文采用了一种简单方法合成Ce-BTC,并通过负载、煅烧,制备出多孔CuO@CeO2双金属氧化物催化剂. 由于1,2-二氯苯的分子结构类似于剧毒的2,3,7,8-四氯二苯并二恶英(TCDD),且毒性相对较低、检测方便,因此常被用作CVOCs的模型化合物[27]. 本文便以1,2-二氯苯为目标污染物,采用微型固定床装置对催化剂低温催化降解CVOCs性能进行评价.
-
硝酸铈(III)六水合物(Ce(NO3)3∙6H2O,99.5%)购自北京迈瑞达科技有限公司;硝酸铜(II)三水合物(Cu(NO3)2·3H2O,99%),1,3,5-苯三甲酸(C6H3(CO2H)3,98%),石英棉(SiO2,1—3 μm,元素分析仪专用),弗罗里硅土(2MgO·3SiO2,60–100目,农残级)均购自上海阿拉丁试剂有限公司;1,2-二氯苯(C6H4Cl2,99.0%)购自北京化学试剂厂;甲醇(CH4O,99.5%),乙醇(C2H6O,99.5%)均购自天津科密欧化学试剂有限公司;正己烷(C6H14,农残级)购自美国J. T. Baker公司.
-
Ce-BTC模板的制备:将4.34 g Ce(NO3)3·6H2O 溶解于适量去离子水中,另外称取2.10 g 1,3,5-苯三甲酸溶解在水-乙醇(V/V = 1:1)溶液中,混合上述两种溶液,于60 oC搅拌1 h. 将混合液离心后所得的固体先后用去离子水和甲醇各洗涤3次,70 oC真空干燥12 h,制备出Ce-BTC模板.
CuO@CeO2的制备:分别称取0.60 g,1.51 g Cu(NO3)3·3H2O溶于去离子水-乙醇(V/V = 1:5)溶液中,搅拌过程中加入Ce-BTC,于50 oC搅拌至全干,得到两种负载不同Cu2+量的Ce-BTC,再置于马弗炉中,700 oC煅烧6 h,制备出25%-CuO@CeO2和62%-CuO@CeO2两种双金属氧化物. 为了进行对比,采用相同的方法在700 oC分别煅烧了Ce-BTC和Cu(NO3)2·6H2O,分别制备出CeO2和CuO.
-
XRD (X-ray difraction) 测试采用荷兰PANalytical X`Pert Pro型粉末衍射仪;SEM (Scanning electron microscopy) 分析在JEOL JSM-7800F高分辨场发射扫描电子显微镜进行,对催化剂样品进行结构和形貌表征;BET (Brunauer-Emmett-Teller) 比表面积分析采用美国Quantachrome公司生产的型号为Nova 4000分析仪;H2-TPR (H2-temperature programmed reduction) 分析由Autochem II 2920型程序升温吸附仪测定.
-
1,2-二氯苯催化降解实验是在固定床微反应装置中进行. 自下而上,在吸附区和反应区内分别装填0.60 g弗罗里硅土和0.05 g制备的催化剂,二者用石英棉隔开. 待加热器的温度达到设定温度后,持续加热反应床层3 min,随后注入2 μL的1,2-二氯苯,由流量为6 mL·min−1的载气吹扫至反应床层,反应20 s后,立即取出反应管放入冰水混合物终止反应,未降解的1,2-二氯苯吸附在弗罗里硅土上.反应结束后,将反应装置中的催化剂和弗罗里硅土一同转移至玻璃离心管中,用正己烷淋洗反应管内壁3次,并将淋洗液收集在离心管中. 用正己烷超声20 min,再进行离心,离心后上清液转移至容量瓶中,重复3次,收集液定容冷藏待测. 采用Agilent 7890气相色谱/电子俘获检测器 (Gas chromatography/electron-capture detector,GC/ECD),DB-5毛细管柱(30 m × 0.32 mm × 0.25 µm)测定1,2-二氯苯.
催化剂对1,2-二氯苯的降解效率(Degradation efficiency)按照公式(1)计算:
其中,IDCB为初始加入1,2-二氯苯的量;RDCB为降解剩余1,2-二氯苯的量.
-
收集反应后的催化剂,用正己烷洗涤3次,凉干后于马弗炉中700 oC煅烧6 h,再次进行催化实验,考察其循环使用效果.
-
每次反应前,反应管均经过彻底清洗,并于650 oC马弗炉中烘烤1 h. 所有玻璃器皿使用前均经过正己烷3次润洗. 每个催化降解反应都进行3次重复,用不注入底物1,2-二氯苯的反应做空白对照. 系统过程中1,2-二氯苯的方法回收率在95% —101%之间.
-
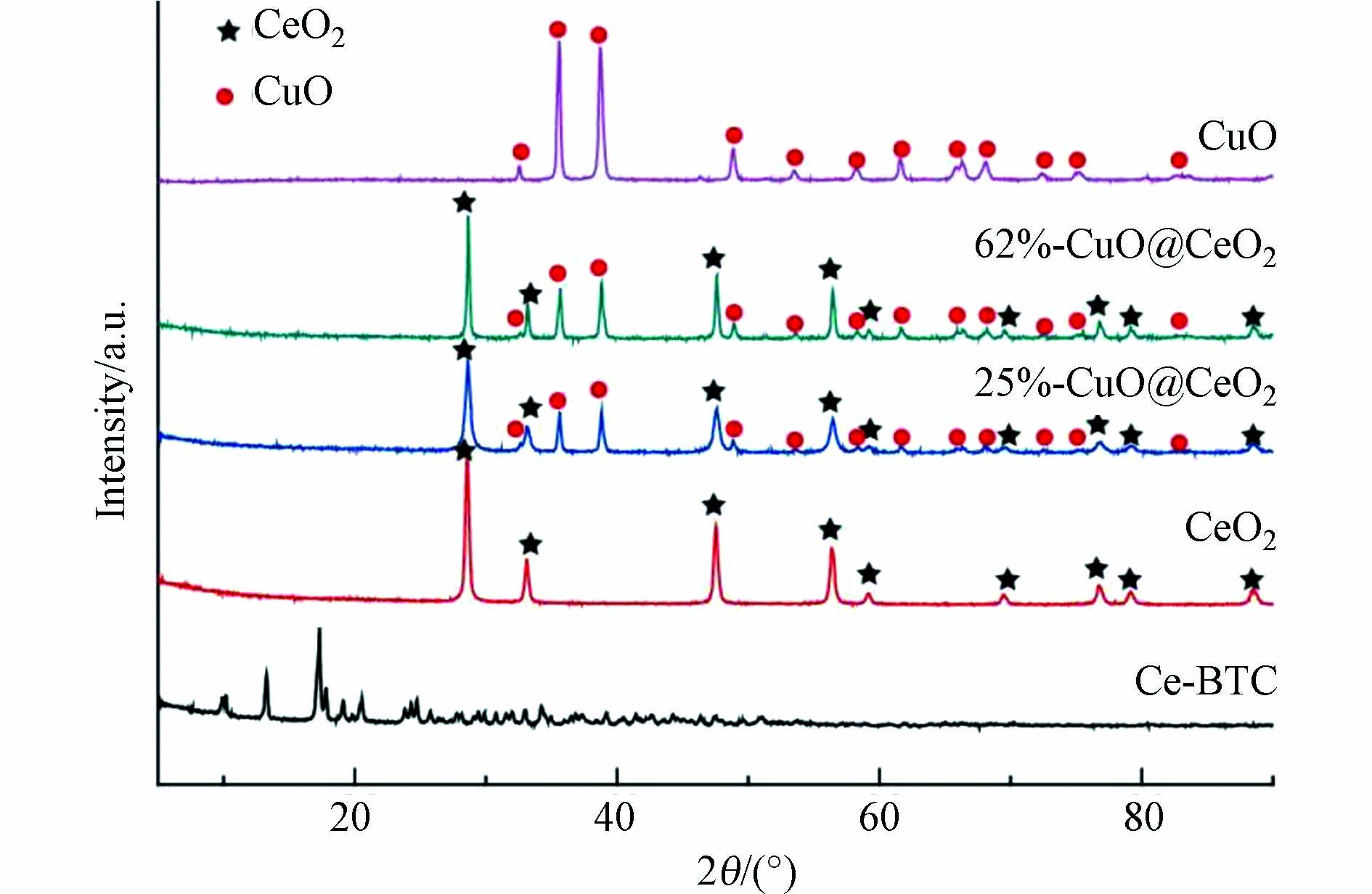
图1为Ce-BTC和担载摩尔含量分别为25%、62% CuO的CuO@CeO2双金属氧化物及采用相同方式制备的CeO2和CuO的XRD的表征结果. 从图1可以看出,通过简单的搅拌加热法成功制备出Ce-BTC.通过煅烧得到的CeO2,在其XRD图谱中,2θ = 28.6o、33.1o、47.5o、56.3o、59.1o、69.4o、76.7o、79.1o和88.4o处的衍射峰全部归属于立方萤石结构的CeO2(JCPDS No.34─0394),未发现母体Ce-BTC的衍射峰,说明Ce-BTC经过700 oC煅烧完全转化成了CeO2. 采用相同的煅烧方式,硝酸铜也全部转化成CuO,其中,2θ = 32.6o、35.6o、38.8o、48.8o、53.5o、58.3o、61.6o、65.9o、66.3o、68.2o、72.4o、75.0o和82.4o均归属于单斜相的CuO(JCPDS No.48─1548). 催化剂25%-CuO@CeO2和62%-CuO@CeO2二者的XRD图谱较为相似,二者均含有CuO和CeO2的衍射峰,并且衍射峰的强度和宽度也相似,说明CuO的负载量不会影响CuO@CeO2双金属氧化物的晶粒尺寸和分散度.
-
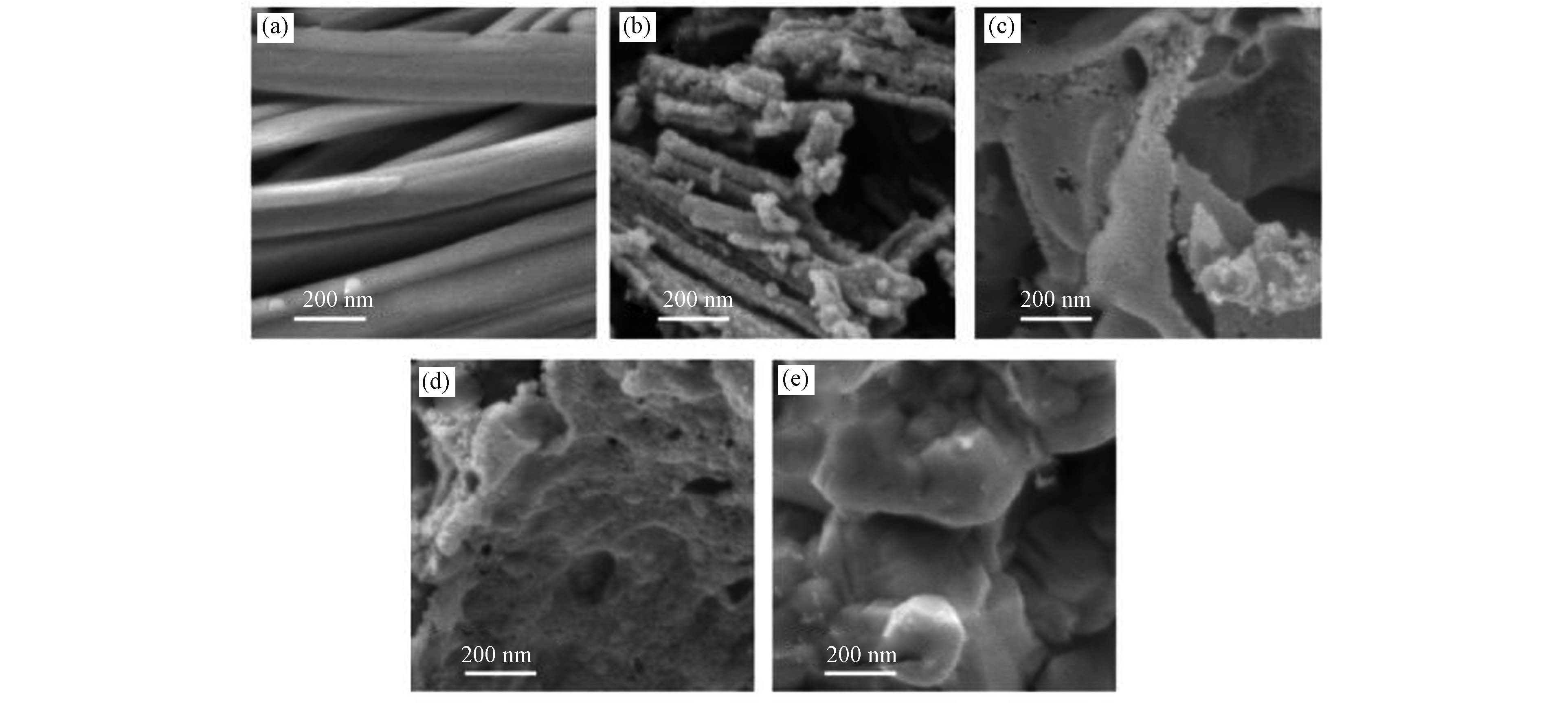
采用SEM对样品的形貌和结构进行研究. 图2是Ce-BTC、CeO2、25%-CuO@CeO2、62%-CuO@CeO2以及CuO的5种材料的SEM图像. 从图2(a)可以清楚地观察到,Ce-BTC呈表面光滑的纳米棒状,经过煅烧后得到的CeO2仍能保持棒状结构(如图2b),但表面变得粗燥. 图2(c)显示,负载Cu2+后的Ce-BTC经过煅烧后得到的25%-CuO@CeO2,结构发生完全变化,呈蓬松的多孔片状结构,当增加Cu2+的负载量,煅烧后得到的62%-CuO@CeO2(图2d)表面出现颗粒物,同CuO(图2e)表面相似,说明CuO的负载量可以改变催化材料的表面形态.
-
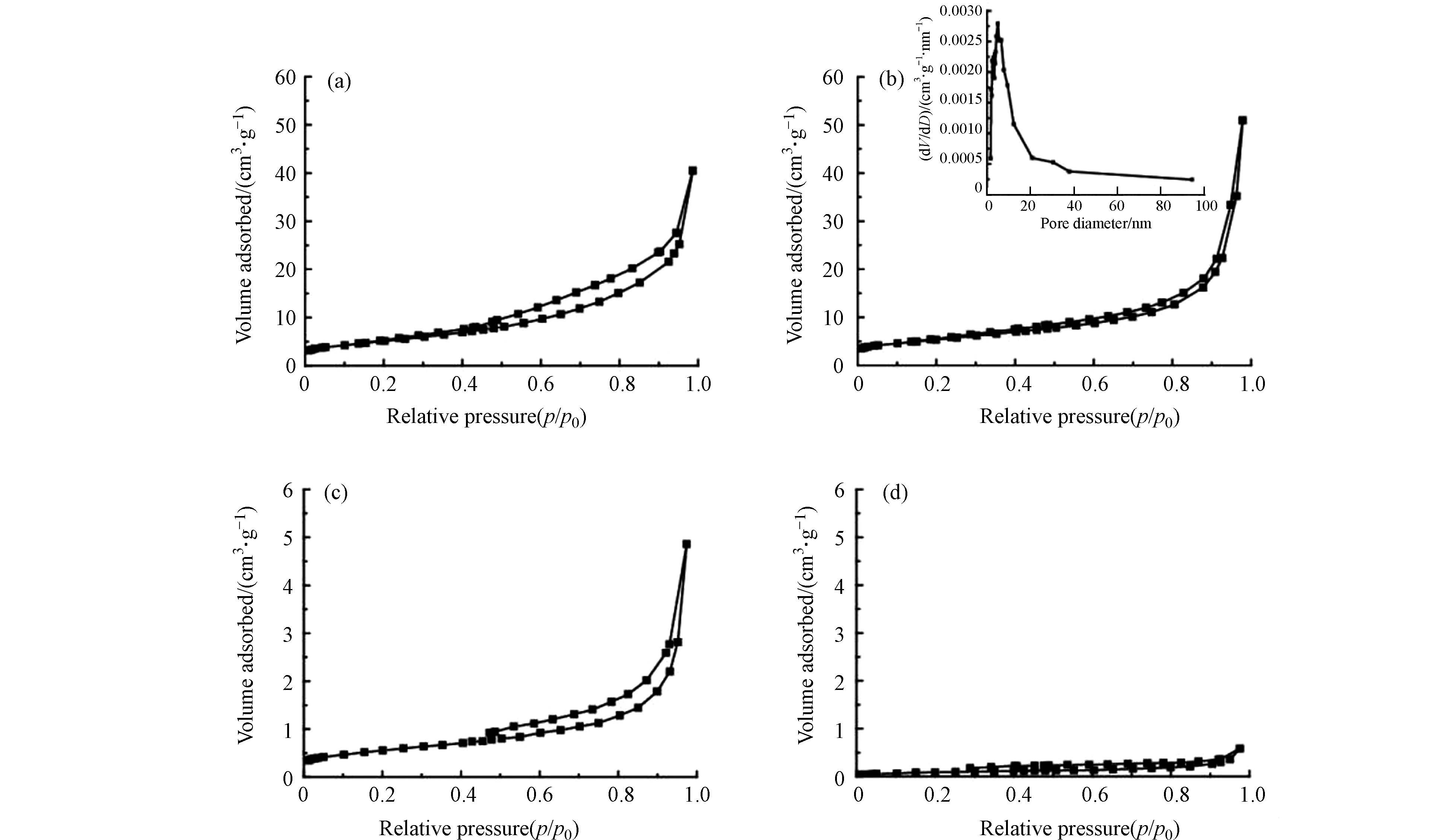
催化剂的比表面积和孔道性质对于催化剂的活性同样有着一定的影响. 图3给出了催化剂的N2吸附-脱附等温线. 其中,CeO2和两种CuO@CeO2催化剂的等温线变化趋势相同,在相对压力较高的范围内出现了明显的滞回环,此类吸附等温线属于IV等温线,表明3种材料具有典型的介孔结构[28]. 图3(b)插图中显示了催化剂25%-CuO@CeO2的BJH孔径分布曲线,该曲线在以4.54 nm为中心的中孔范围内显示了一个单一的主峰,表明了该催化剂的孔结构具有良好的均质性.
4种材料的BET分析结果见表1,根据吸附-脱附曲线,计算出了4种不同成分含量和形貌的催化材料的比表面积、总孔容和平均孔径. 4种材料的比表面积和总孔容大小顺序为:CeO2 > 25%-CuO@CeO2 > 62%-CuO@CeO2 > CuO;平均孔径大小顺序为:25%-CuO@CeO2 > CeO2 ≈ 62%-CuO@CeO2 > CuO,结果发现通过MOFs煅烧制备的金属氧化物为介孔材料,并且25%-CuO@CeO2的比表面积为18.8 m2·g−1,高于62%-CuO@CeO2的1.99 m2·g−1,且平均孔径和孔体积也相对较高. 较高的比表面积和孔径能更好的吸附污染物,为催化反应提供充足的反应空间和活性位点[29],因此推测25%-CuO@CeO2催化剂的活性较高.
-
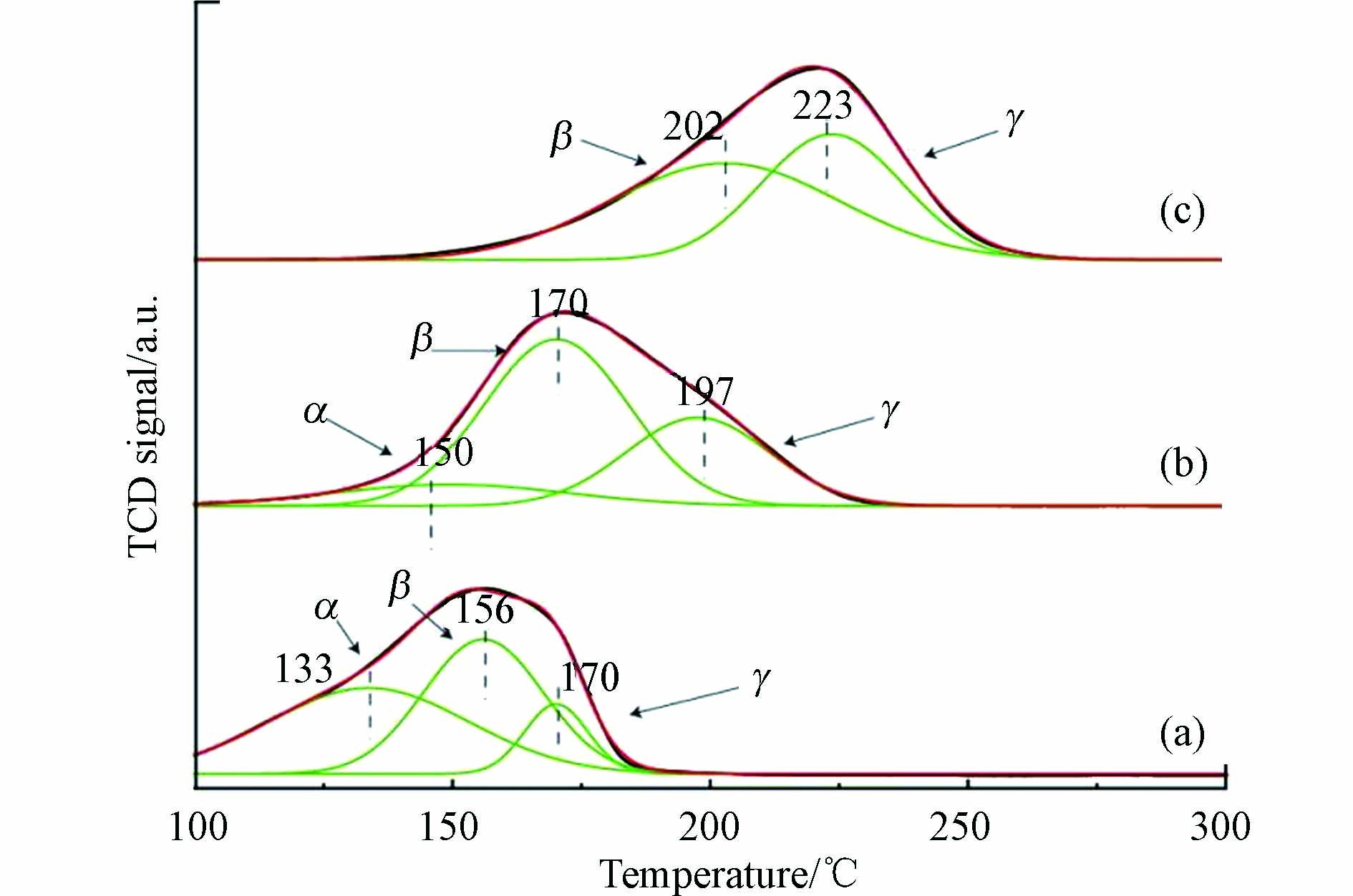
为了进一步考察催化材料中铜物种的分布,采用氢气程序升温还原(H2-TPR)表征来辨析不同铜物种及其分布比例. CuO与催化材料25%-CuO@CeO2、62%-CuO@CeO2的氢气程序升温还原H2-TPR谱图如图4所示. 一般而言,铜物种具有3个还原峰(表示为α、β和γ).α峰归属于铜-铈界面的相互作用,β和γ峰对应于高度分散的CuO和团聚的CuO大颗粒[30]. 从图4可以看到,CuO的还原峰经过拟合后,出现了两个还原峰(β和γ),而25%-CuO@CeO2与62%-CuO@CeO2样品中发现了3个还原峰(α、β和γ). 样品25%-CuO@CeO2同62%-CuO@CeO2相对比,还原峰逐渐向低温移动,其两种材料的α峰的面积分别占45.6%和11.8%,并且前者具有比β和γ峰更大的α峰面积,表明在铜-铈界面生成了更多的活性铜位[31]. 此外,由于铜-铈的强相互作用,还可以通过增强Ce4+/Ce3+和Cu2+/Cu+的电子转移而在界面上形成氧空位[32]. 据此推断, 样品25%-CuO@CeO2更能表现出较好的催化活性.
-
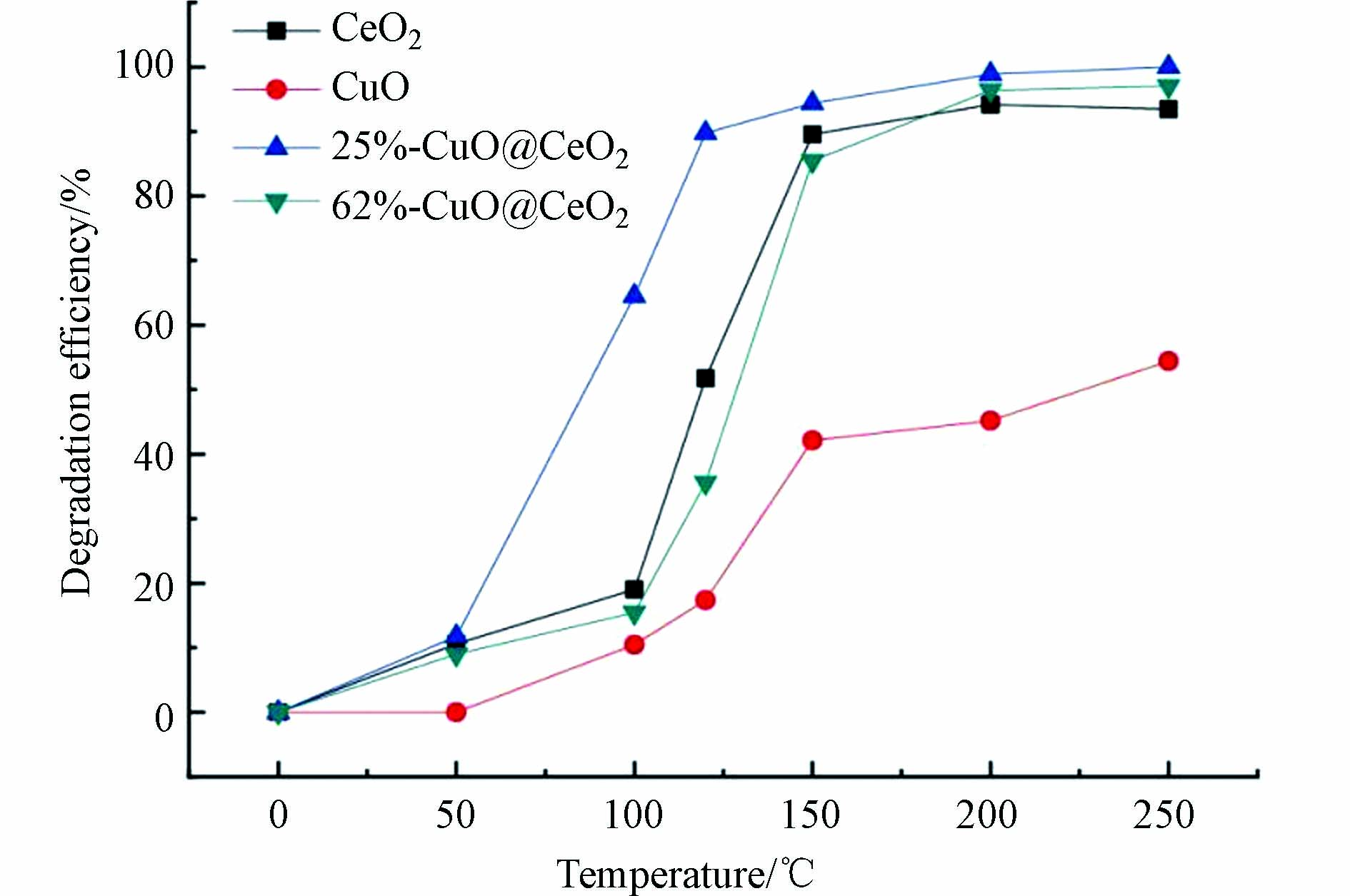
图5所示为不同反应温度下,1,2-二氯苯在CuO、CeO2及CuO@CeO2上的降解趋势. 总体来看,在不同催化材料上,1,2-二氯苯的降解率均随反应温度的上升而升高.在相同反应温度下,CeO2和CuO@CeO2对1,2-二氯苯的催化降解效率均高于CuO,四种材料的催化降解效率高低顺序为:25%-CuO@CeO2 > 62%-CuO@CeO2 ≈ CeO2 > CuO. 当反应温度为150 oC时,CeO2、25%-CuO@CeO2、62%-CuO@CeO2以及CuO对1,2-二氯苯催化降解效率分别为89.6%、94.4%、85.5%和42.2%. 由图5可见,在100 oC时,25%-CuO@CeO2对1,2-二氯苯就有很高的催化降解活性,其降解效率达到64.5 %,在200 oC时达到98.9%. 从结果可以看出,掺杂适量比例的CuO,可通过同CeO2的协同作用促进1,2-二氯苯的催化降解,掺杂过量比例的CuO,则会抑制1,2-二氯苯的催化降解.
结合BET、H2-TPR数据可以得到,较高的比表面积和较大的孔径及CuO与CeO2之间的相互作用强度是决定催化剂活性的重要因素. 介孔材料的孔体积和孔径会影响催化剂对目标物的吸附能力. 适量的铜铈比不但能够保证恰当的活性组分,而且可以使活性组分能够均匀分布在载体表面. 另外,过高的铜负载量可能会引起催化剂表面活性组分的堆积和团聚,从而堵塞了催化剂表面活性位点[33]. 由此可见,不同因素的共同影响促进了催化剂25%-CuO@CeO2对1,2-二氯苯的低温催化降解活性.
-
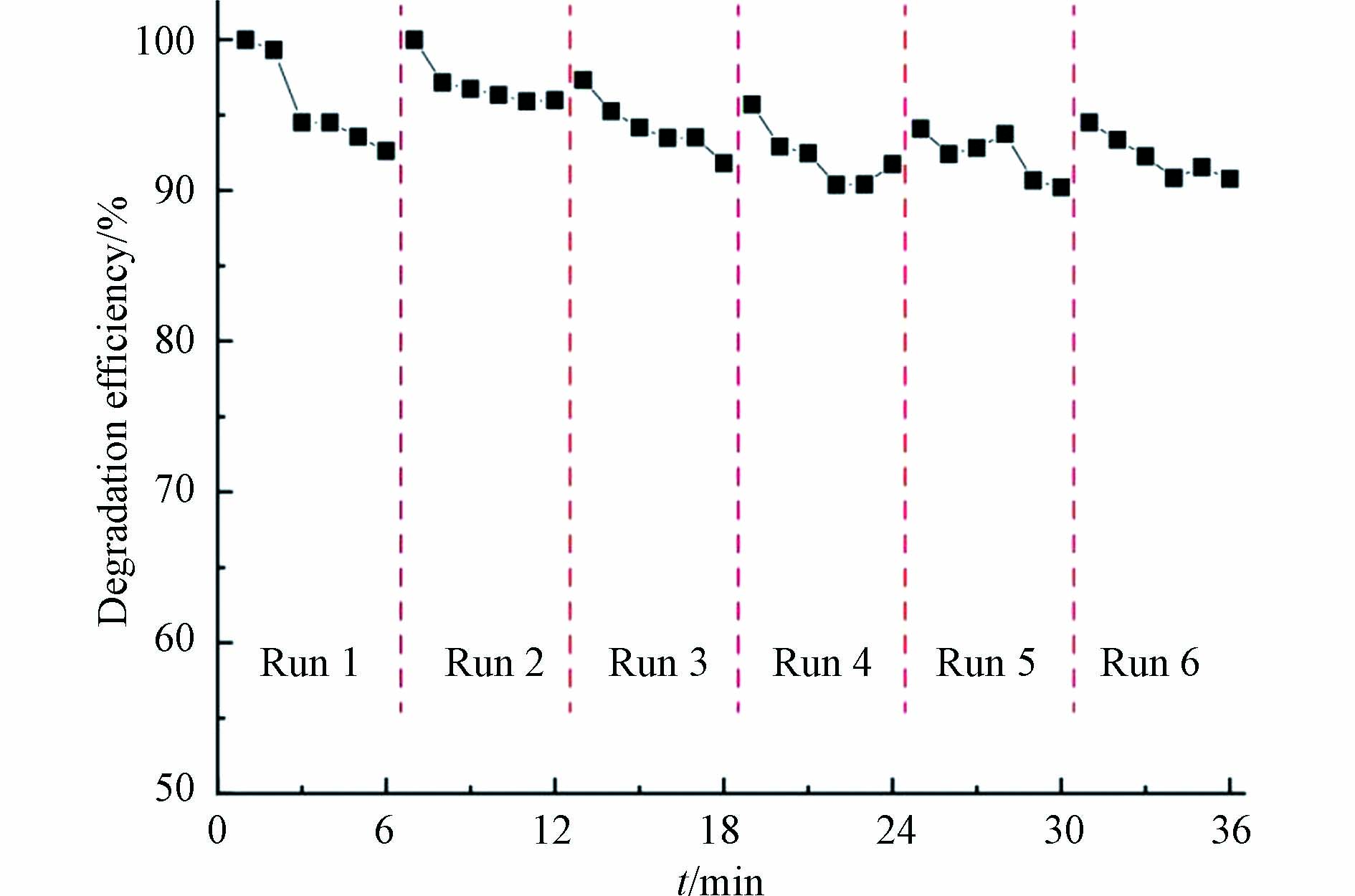
催化降解过程的经济性取决于催化剂在保持较高催化活性的前提下可重复使用的次数. 因此,选用催化效果较好的25%-CuO@CeO2催化剂,研究该催化剂在循环使用过程中催化活性的变化. 每经过6次催化降解反应,催化剂按照“1.5节”的步骤活化后,继续下一轮循环使用. 结果如图6所示,催化材料25%-CuO@CeO2在6次循环使用后对1,2-二氯苯的降解效率仍能保持在90%以上,表明此催化剂可重复使用并保持较高的催化活性.
-
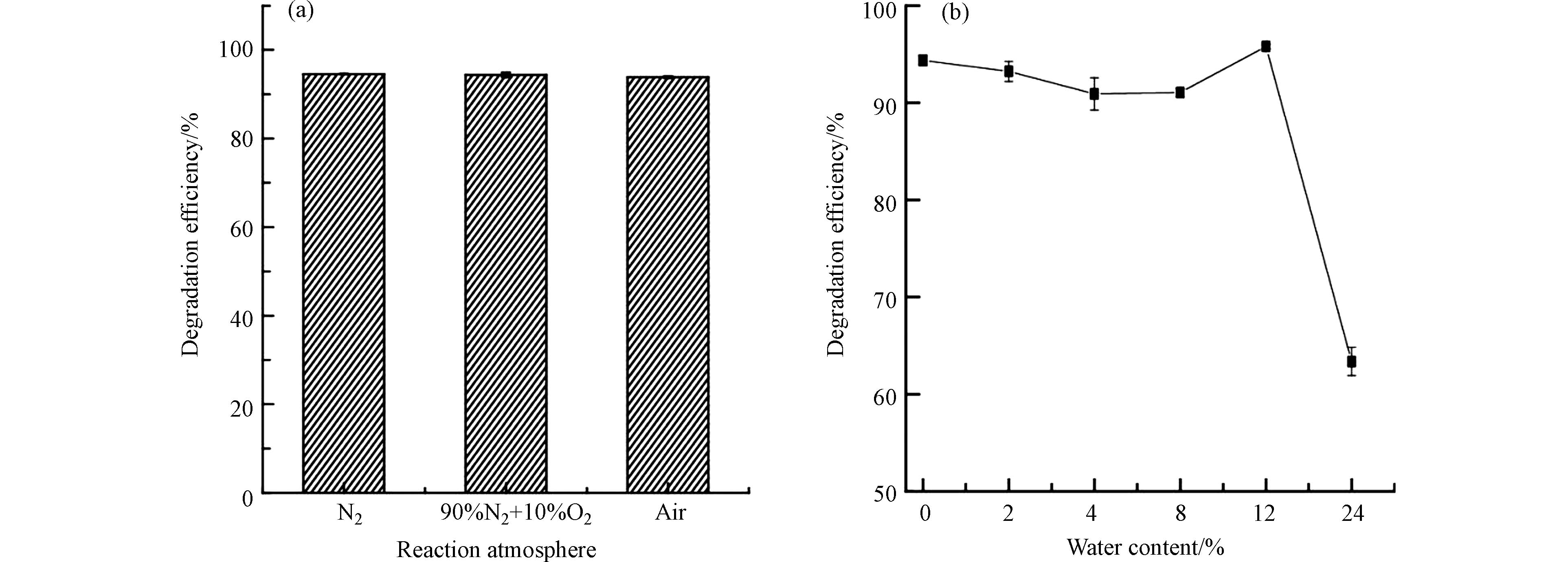
由于焚烧烟气中氧含量和水汽均可以影响催化材料对污染物的降解效果,因此,研究了3种不同氧含量气氛和不同含水量的情况下催化剂25%-CuO@CeO2对1,2-二氯苯催化降解效率的变化,以考察催化剂的稳定性和耐水性. 如图7(a)所示,当反应温度为150 oC,反应气氛分别为N2、90% N2 + 10% O2和空气的3种气氛时,该催化剂对1,2-二氯苯的降解效率变化不明显,分别为94.5%、94.4%和93.9%. 因此,当氧气含量低于20%时,其氧含量大小对降解效率影响不大. 如图7(b)所示,适量水(< 12.0% wt)的存在对催化剂的催化活性影响同样不大,1,2-二氯苯的降解效率维持在90%以上,当含水率达到12.0 wt%时,催化降解效率还略有升高,也说明了该催化剂具有一定的抗水性. 根据文献报道,H2O可通过以下反应促进催化剂表面Cl−的去除:Cl− + H2O → HCl + OH− [34]. 另一方面,H2O也可以通过竞争吸附机制阻断许多活性中心,从而抑制反应速率. 因此,当含水率继续增加到24.0% wt时,催化降解效率显著下降至70%以下.
-
本文以负载Cu2+的Ce-BTC为前驱体,通过煅烧制备出一种介孔双金属氧化物CuO@CeO2催化剂,并考察了该催化剂对CVOCs的低温催化降解效果. 结果表明,以MOFs为模板制备的金属氧化物具有较大比表面积和孔径,从而利于活性成分的分布和对目标污染物的吸附. 同时,研究还发现CuO同CeO2的协同作用,可以促进低温条件下催化剂的催化活性,提高对1,2-二氯苯的降解效率. 当CuO摩尔百分含量为25%时,CuO@CeO2催化材料具有较高催化活性,对1,2-二氯苯降解率在150 oC可达94.4%,且循环使用六次之后催化效率仍然可达90%以上. 催化剂25%-CuO@CeO2还表现出一定氧化活性和抗水性. 本研究以MOFs为牺牲模板制备介孔双金属氧化物催化剂,并将其应用到CVOCs低温催化降解反应中,不仅拓宽了MOFs材料的应用领域,而且为以后CVOCs低温催化剂的研究开拓了新的思路.
基于MOFs的CuO@CeO2双金属催化剂的制备及其对1,2-二氯苯的低温催化降解
CuO@CeO2 bimetallic catalyst derived from MOFs for the degradation of 1,2-dichlorobenzene at low temperature
-
摘要: 以负载铜离子的均苯三甲酸铈-金属有机骨架(Ce-BTC)为前驱体,通过煅烧制备得到CuO/CeO2双金属氧化物催化剂. 通过XRD、SEM、BET、H2-TPR等手段对所制得催化剂的组成、结构、形貌等进行了表征.以1,2-二氯苯为模型化合物,采用固定床微反应装置对催化剂低温催化降解含氯挥发性有机物(CVOCs)性能进行评价.以金属有机骨架(MOFs)为模板制备的金属氧化物为具有较大比表面积的介孔材料. 通过对比实验发现,25%-CuO@CeO2具有较好的低温催化降解1,2-二氯苯性能.当反应温度为100、150、200 oC时,对1,2-二氯苯降解效率分别为64.5 %、94.4%、98.9%. 同时,该催化剂还表现出较高的稳定性. 当反应温度为150 oC时,催化剂经过连续反应后通过煅烧活化,再循环使用六次之后其催化降解效率仍然可达90%以上. 该催化材料表现出一定氧化活性和抗水性,当氧含量低于20%或含水量小于15%时,该催化材料对1,2-二氯苯催化降解效率能保持在90%以上.Abstract: The CuO@CeO2 bimetallic catalysts were prepared by calcining the precursor of cerium based metal-organic frameworks (MOFs) Ce-BTC (BTC = 1,3,5-benzenetricarboxylic acid) loaded with copper ions. The composition, structure and morphology of the catalysts were characterized by XRD, SEM, BET and H2-TPR. A fixed bed micro reactor was employed to study the catalytic degradation of chlorinated volatile organic compounds (CVOCs) on catalysts at low temperature. The metal oxides derived from MOFs are mesoporous materials with large specific surface area. The catalyst of 25%-CuO@CeO2 showed better catalytic degradation performance for 1,2-dichlorobenzene at low temperature through comparative experiment. The degradation efficiencies of 1,2-dichlorobenzene on the catalyst of 25%-CuO@CeO2 were 64.5%, 94.4%, 98.9% at 100, 150 and 200 oC, respectively. The catalyst also showed high stability, and the degradation efficiencies of 1,2-dichlorobenzene on the catalyst activated by calcination still reached more than 90% at 150 oC after six cycles of reuse. The catalytic material exhibited certain oxidation activity and water resistance. When the oxygen content was less than 20% or the water content was less than 15%, the degradation efficiencies of 1,2-dichlorobenzene on 25%-CuO@CeO2 could be maintained above 90%.
-
Key words:
- Ce-BTC /
- 1,2-dichlorobenzene /
- CuO@CeO2 /
- catalytic degradation /
- VOC
-

-
表 1 制备所得催化材料的孔结构数据
Table 1. Pore structure data of prepared catalysts
催化材料
Catalysts比表面积/(m2·g−1)
Specific surface area总孔容/(cm3·g−1)
Total pore volume平均孔径/nm
Average pore sizeCeO2 19.1 0.079 2.77 25%-CuO@CeO2 18.8 0.063 4.54 62%-CuO@CeO2 1.99 0.008 2.77 CuO 0.342 0.001 2.65 -
[1] IIJIMA S, NAKAMURA M, YOKOI A, et al. Decomposition of dichloromethane and in situ alkali absorption of resulting halogenated products by a packed-bed non-thermal plasma reactor [J]. Journal of Material Cycles and Waste Management, 2011, 13(3): 206-212. doi: 10.1007/s10163-011-0022-0 [2] OJALA S, PITKÄAHO S, LAITINEN T, et al. Catalysis in VOC abatement [J]. Topics in Catalysis, 2011, 54(16/17/18): 1224-1256. [3] ZHANG C H, WANG C, ZHAN W C, et al. Catalytic oxidation of vinyl chloride emission over LaMnO3 and LaB0.2Mn0.8O3 (B = Co, Ni, Fe) catalysts [J]. Applied Catalysis B:Environmental, 2013, 129: 509-516. doi: 10.1016/j.apcatb.2012.09.056 [4] ŚRĘBOWATA A, BARAN R, ŁOMOT D, et al. Remarkable effect of postsynthesis preparation procedures on catalytic properties of Ni-loaded BEA zeolites in hydrodechlorination of 1, 2-dichloroethane [J]. Applied Catalysis B:Environmental, 2014, 147: 208-220. doi: 10.1016/j.apcatb.2013.08.040 [5] ZHAO H J, HAN W L, TANG Z C. Tailored design of high-stability CoMn1.5Ox@TiO2 double-wall nanocages derived from Prussian blue analogue for catalytic combustion of o-dichlorobenzene [J]. Applied Catalysis B:Environmental, 2020, 276: 119133. doi: 10.1016/j.apcatb.2020.119133 [6] HUANG B B, LEI C, WEI C H, et al. Chlorinated volatile organic compounds (Cl-VOCs) in environment—sources, potential human health impacts, and current remediation technologies [J]. Environment International, 2014, 71: 118-138. doi: 10.1016/j.envint.2014.06.013 [7] LIN C, TSENG Y H, HSIEH M K, et al. Co-treatment for chlorinated organic waste solvents and volatile organic compounds in a laboratory scale spouted bed incineration system[C]. Abstracts of Papers of The American Chemical Society, 2003 [8] GROSTERN A, EDWARDS E A. A 1, 1, 1-trichloroethane-degrading anaerobic mixed microbial culture enhances biotransformation of mixtures of chlorinated ethenes and ethanes [J]. Applied and Environmental Microbiology, 2006, 72(12): 7849-7856. doi: 10.1128/AEM.01269-06 [9] WEI Z S, SEO Y. Trichloroethylene (TCE) adsorption using sustainable organic mulch [J]. Journal of Hazardous Materials, 2010, 181(1/2/3): 147-153. [10] VEERAPANDIAN S, LEYS C, de GEYTER N, et al. Abatement of VOCs using packed bed non-thermal plasma reactors: A review [J]. Catalysts, 2017, 7(12): 113. doi: 10.3390/catal7040113 [11] ZHENG Y F, LIU Q L, SHAN C P, et al. Defective ultrafine MnOx nanoparticles confined within a carbon matrix for low-temperature oxidation of volatile organic compounds [J]. Environmental Science & Technology, 2021, 55(8): 5403-5411. [12] FINOCCHIO E, BUSCA G, NOTARO M. A review of catalytic processes for the destruction of PCDD and PCDF from waste gases [J]. Applied Catalysis B:Environmental, 2006, 62(1/2): 12-20. [13] DAI X X, WANG X W, LONG Y P, et al. Efficient elimination of chlorinated organics on a phosphoric acid modified CeO2 catalyst: A hydrolytic destruction route [J]. Environmental Science & Technology, 2019, 53(21): 12697-12705. [14] EVERAERT K, MATHIEU M, BAEYENS J, et al. Combustion of chlorinated hydrocarbons in catalyst-coated sintered metal fleece reactors [J]. Journal of Chemical Technology and Biotechnology, 2003, 78(2-3): 167-172. doi: 10.1002/jctb.725 [15] WENG X L, SUN P F, LONG Y, et al. Catalytic oxidation of chlorobenzene over MnxCe1-xO2/HZSM-5 catalysts: A study with practical implications [J]. Environmental Science & Technology, 2017, 51(14): 8057-8066. [16] SCIRÈ S, MINICÒ S, CRISAFULLI C. Pt catalysts supported on H-type zeolites for the catalytic combustion of chlorobenzene [J]. Applied Catalysis B:Environmental, 2003, 45(2): 117-125. doi: 10.1016/S0926-3373(03)00122-X [17] LI C L, ZHAO Y X, SONG H, et al. A review on recent advances in catalytic combustion of chlorinated volatile organic compounds [J]. Journal of Chemical Technology & Biotechnology, 2020, 95(8): 2069-2082. [18] SU Y, FU K X, ZHENG Y F, et al. Catalytic oxidation of dichloromethane over Pt-Co/HZSM-5 catalyst: Synergistic effect of single-atom Pt, Co3O4, and HZSM-5 [J]. Applied Catalysis B:Environmental, 2021, 288: 119980. doi: 10.1016/j.apcatb.2021.119980 [19] TROVARELLI A. Catalytic properties of ceria and CeO2-containing materials [J]. Catalysis Reviews, 1996, 38(4): 439-520. doi: 10.1080/01614949608006464 [20] GORTE R J. Ceria in catalysis: From automotive applications to the water-gas shift reaction [J]. AIChE Journal, 2010: NA. [21] HE C, YU Y K, SHEN Q, et al. Catalytic behavior and synergistic effect of nanostructured mesoporous CuO-MnOx-CeO2 catalysts for chlorobenzene destruction [J]. Applied Surface Science, 2014, 297: 59-69. doi: 10.1016/j.apsusc.2014.01.076 [22] TSONCHEVA T, ISSA G, BLASCO T, et al. Catalytic VOCs elimination over copper and cerium oxide modified mesoporous SBA-15 silica [J]. Applied Catalysis A:General, 2013, 453: 1-12. doi: 10.1016/j.apcata.2012.12.007 [23] LEE J, FARHA O K, ROBERTS J, et al. Metal-organic framework materials as catalysts [J]. Chemical Society Reviews, 2009, 38(5): 1450. doi: 10.1039/b807080f [24] JIANG H L, LIU B, AKITA T, et al. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework [J]. Journal of the American Chemical Society, 2009, 131(32): 11302-11303. doi: 10.1021/ja9047653 [25] HUANG Y B, LIANG J, WANG X S, et al. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions [J]. Chemical Society Reviews, 2017, 46(1): 126-157. doi: 10.1039/C6CS00250A [26] ZHENG Y F, ZHAO Q, SHAN C P, et al. Enhanced acetone oxidation over the CeO2/Co3O4 catalyst derived from metal–organic frameworks [J]. ACS Applied Materials & Interfaces, 2020, 12(25): 28139-28147. [27] MA X D, SUN Q, FENG X, et al. Catalytic oxidation of 1, 2-dichlorobenzene over CaCO3/α-Fe2O3 nanocomposite catalysts [J]. Applied Catalysis A:General, 2013, 450: 143-151. doi: 10.1016/j.apcata.2012.10.019 [28] LONG G Y, CHEN M X, LI Y J, et al. One-pot synthesis of monolithic Mn-Ce-Zr ternary mixed oxides catalyst for the catalytic combustion of chlorobenzene [J]. Chemical Engineering Journal, 2019, 360: 964-973. doi: 10.1016/j.cej.2018.07.091 [29] CHEN J, CHEN X, YAN D X, et al. A facile strategy of enhancing interaction between cerium and manganese oxides for catalytic removal of gaseous organic contaminants [J]. Applied Catalysis B:Environmental, 2019, 250: 396-407. doi: 10.1016/j.apcatb.2019.03.042 [30] XU D Y, CHENG F, LU Q Z, et al. Microwave enhanced catalytic degradation of methyl orange in aqueous solution over CuO/CeO2 catalyst in the absence and presence of H2O2 [J]. Industrial & Engineering Chemistry Research, 2014, 53(7): 2625-2632. [31] HOSSAIN S T, AZEEVA E, ZHANG K F, et al. A comparative study of CO oxidation over Cu-O-Ce solid solutions and CuO/CeO2 nanorods catalysts [J]. Applied Surface Science, 2018, 455: 132-143. doi: 10.1016/j.apsusc.2018.05.101 [32] DING J F, LI L P, LI H X, et al. Optimum preferential oxidation performance of CeO2-CuO x-RGO composites through interfacial regulation [J]. ACS Applied Materials & Interfaces, 2018, 10(9): 7935-7945. [33] LUO J K, ZHANG H, LI Z H. Highly efficient degradation of phenol from wastewater via an electro-catalytic oxidation approach with a CeO2–CuO cathode [J]. RSC Advances, 2018, 8(27): 15167-15172. doi: 10.1039/C8RA00983J [34] KRISHNAMOORTHY S, RIVAS J A, AMIRIDIS M D. Catalytic oxidation of 1, 2-dichlorobenzene over supported transition metal oxides [J]. Journal of Catalysis, 2000, 193(2): 264-272. doi: 10.1006/jcat.2000.2895 -



 下载:
下载: