-
烷烃和多环芳烃(polycyclic aromatic hydrocarbons,PAHs)是大气环境中重要的污染物,与大气细粒子(fine particle,PM2.5)和臭氧(O3)的形成和演化密切相关[1-2],其中以苯并(a)芘为代表的PAHs具有强致癌性[3],严重影响人体健康;根据饱和蒸气压浓度,可将其划分为挥发性有机污染物(volatile organic compounds,VOCs)和半/中等挥发性有机物(semi-/intermediate-volatile organic compounds,S/IVOCs)[4]. VOCs是二次有机气溶胶(secondary organic aerosol,SOA)的重要前体物,但并不能很好地解释城市站点中观测到有机气溶胶的总量[5-6]. 烟雾箱研究表明,S/IVOCs对SOA的生成有重要贡献,但其形成机制尚不明确且严重被低估[7-9],近年来越来越受到重视[10-12]. 机动车尾气是我国城市大气中烷烃和PAHs的重要来源[13],现阶段机动车尾气中烷烃和PAHs的研究多为颗粒态 [14-17],而气态烷烃和PAHs的相关报道较少,且以VOCs为主[18]. 因此完善机动车尾气中气态烷烃和PAHs、尤其S/IVOCs的成分谱和排放因子,对SOA来源解析的研究具有重要意义.
目前我国针对废气中PAHs的检测制定的标准方法较少,主要包括气质联用法(HJ646—2013)[19]和高效液相色谱法(HJ647—2013)[20],但分析的PAHs组分及苯环数均较少;此外采样复杂、易污染、目标物易发生反应等问题有待解决[21];而对于废气中烷烃的检测,目前还没制定相应的标准方法. 吸附管采样-热脱附/气相色谱质谱法有采样过程简单、目标物被填料富集后不易反应、吸附管可重复使用等优势;且样品分析无需复杂前处理,可检测沸点高、碳数多的化合物[22].
为了探索机动车尾气中烷烃和PAHs的检测方法,同时尽可能准确分析更多的S/IVOCs物种,本研究借鉴环境气溶胶中烷烃和PAHs检测方法的最新研究成果[23],以吸附管采样,采用热脱附-气相色谱-质谱联用系统(thermal desorption-gas chromatography-mass spectrometry,TD-GC-MS),建立了适用于机动车废气样品中30种正构烷烃(C7—C36)、2种支链烷烃(植烷、姥鲛烷)和19种PAHs(2—6环)的检测方法;其中C12—C36烷烃及PAHs属S/IVOCs[4];该法具有检出限低、稳定性好、精密度高、定量准确等特点,可满足机动车尾气中烷烃和PAHs的检测需求.
-
7890B/5977气相色谱-质谱联用仪(美国Agilent公司);TD100-xr热脱附仪(配U-T9TNX-2S聚焦冷阱)(英国Marks公司);Marks C1-AAXX-5003不锈钢吸附管(填料:200 ng Tenax-TA 35/60,89 mm×6.4 mm)(英国Marks公司);CSLR标样制备装置(英国Marks公司);BTH-10型活化仪(北京踏实德研仪器公司);ZC-QL便携式大气(恒流)采样器(浙江恒达仪器仪表公司);SEMTECH-MPS等比例稀释系统(美国Sensors公司);采样过滤器(孔径0.22 μm,天津津腾公司);微量注射器(50、100、250、500 μL,瑞士Hamilton公司).
混合标准品:30种正构烷烃(C7—C36)混合标准溶液(1000 mg·L−1于正己烷,上海安谱公司)、19种PAHs混合标准溶液(500 mg·L−1于二氯甲烷,上海安谱公司);单标:植烷标准溶液(100 mg·L−1于正己烷,上海安谱公司),姥鲛烷标准溶液(1000 mg·L−1于正己烷,上海安谱公司);内标物:氘代二十四烷(1000 mg·L−1于正己烷,美国Aldrich公司),氘代荧蒽(2000 mg·L−1于二氯甲烷,北京BePure公司). 试剂:甲醇(LC/MS纯,上海麦克林公司)、正己烷(HPLC纯,美国Fisher chemical公司)、二氯甲烷(GC/MS纯,美国Fisher chemical公司). 气体:高纯氦气、高纯氮气(纯度>99.999%,广州广气公司).
-
分别取适量的正构烷烃、植烷和姥鲛烷、PAHs标准溶液,用甲醇/二氯甲烷(体积比2:1)混合溶剂定容至5 mL,配制成浓度为:1、5、10、20、50 μg·mL−1的51种目标物混合标准品使用液;取适量的内标母液,以甲醇/二氯甲烷(体积比2:1)为溶剂定容至5 mL,配置成浓度为25 μg·mL−1的内标使用液;均于4 ℃保存,待用. 用微量注射器依次取1 μL混合标准品使用液和1 μL内标使用液注入标样制备装置,以流速为80 mL·min−1的高纯氮气吹扫5 min,迅速取下吸附管,制备成目标物含量为1—50 ng的标准样品管(内标物含量为25 ng),标准样品管需现配现用. 微量注射器在使用前后均依次用甲醇、二氯甲烷、正己烷各清洗30次,进样前再用样品清洗3次;玻璃器皿使用前用重铬酸钾浓硫酸洗液浸泡24 h,用纯水清洗干净后,依次用纯水、二氯甲烷、正己烷超声清洗15 min,最后使用甲醇:二氯甲烷:正己烷1:1:1的溶液清洗,晾干待用.
-
吸附管在采样前使用老化仪,在氮气流量50 mL·min−1、温度320 ℃下老化1 h,以除去杂质干扰. 老化后的吸附管两端应立即密封,用铝箔纸包裹放入密实袋中、置于装有活性炭的干燥器中4 ℃避光保存,待用.
采用车载尾气采样系统采集样品:机动车尾气从排气筒排出后,进入稀释器(稀释气为高纯氮气,稀释比为1:30)稀释,经过滤器、干燥器除去颗粒物和水汽,再通过采样器(采样流量为0.1 L·min−1,采样时间为15 min)将稀释废气采集到吸附管中;详细的采样系统构成、采样方法和方法质控参见文献[24]. 采样结束后立刻取下吸附管,用铝箔纸包裹放入密实袋中、置于装有活性炭的干燥器中4 ℃避光保存,7 d内分析完毕.
-
样品采集后,将吸附管置于热脱附仪中进行二级热脱附,脱附气体经气相色谱分离后用质谱检测,根据保留时间、质谱图和特征离子定性,内标法定量.
-
传输线温度:250 ℃,吸附管初始温度:35 ℃,聚焦冷阱初始温度:35 ℃;吸附管解吸温度:300 ℃,解吸时间:15 min,解吸流量:50 mL·min−1,解吸分流量:100 mL·min−1;聚焦冷阱捕集方式:不分流,吹扫时间:4 min,吹扫流量:50 mL·min−1;聚焦冷阱捕集温度:-20 ℃;聚焦冷阱脱附温度:300 ℃,脱附时间:8 min,脱附分流流量:5 mL·min−1;聚焦冷阱加热速率:100 ℃·s−1;干吹流量:50 mL·min−1,干吹时间:5 min;GC循环时间:60 min.
-
进样口温度:200 ℃,载气:高纯氦气,进样模式:不分流;色谱柱:Agilent HP-5毛细管柱(30 m×0.25 mm×0.25 μm);恒流:1.2 mL·min−1;升温程序:初始温度50 ℃,保持2 min;以20 ℃·min−1升至150 ℃,保持2 min;再以10 ℃·min−1升至310 ℃,保持25 min;后运行温度320 ℃,保持2 min.
-
离子源:EI源;调谐文件:BFB;溶剂延迟时间:4.2 min;接口温度:230 ℃;离子源温度:230 ℃;四极杆温度:150 ℃;传输线温度:230 ℃;电子能量:70 eV;扫描方式:定性分析采用全扫描(SCAN),扫描范围:30—500 amu;定量采用选择性离子监测(selected ion monitoring, SIM),扫描参数见表1.
-
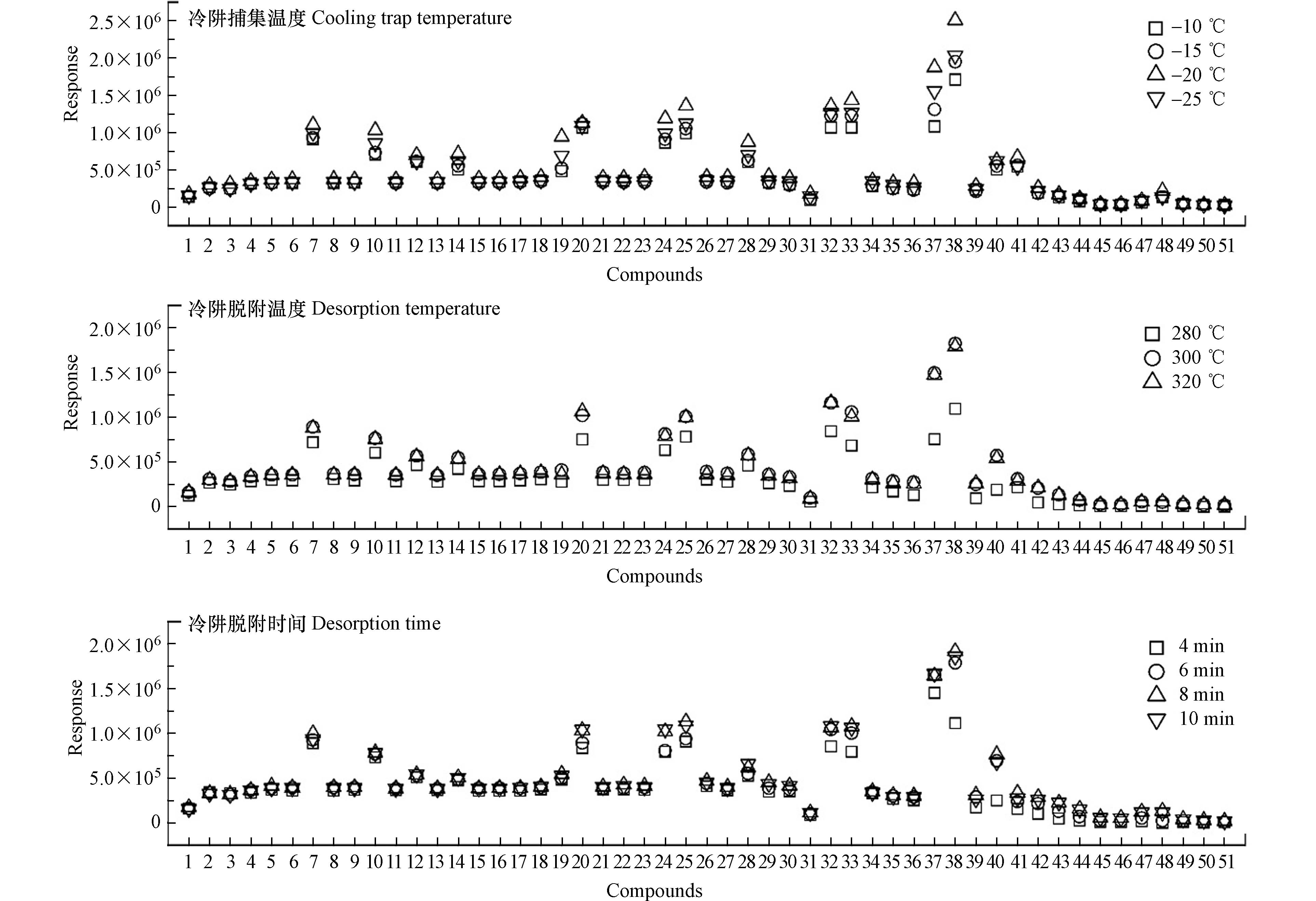
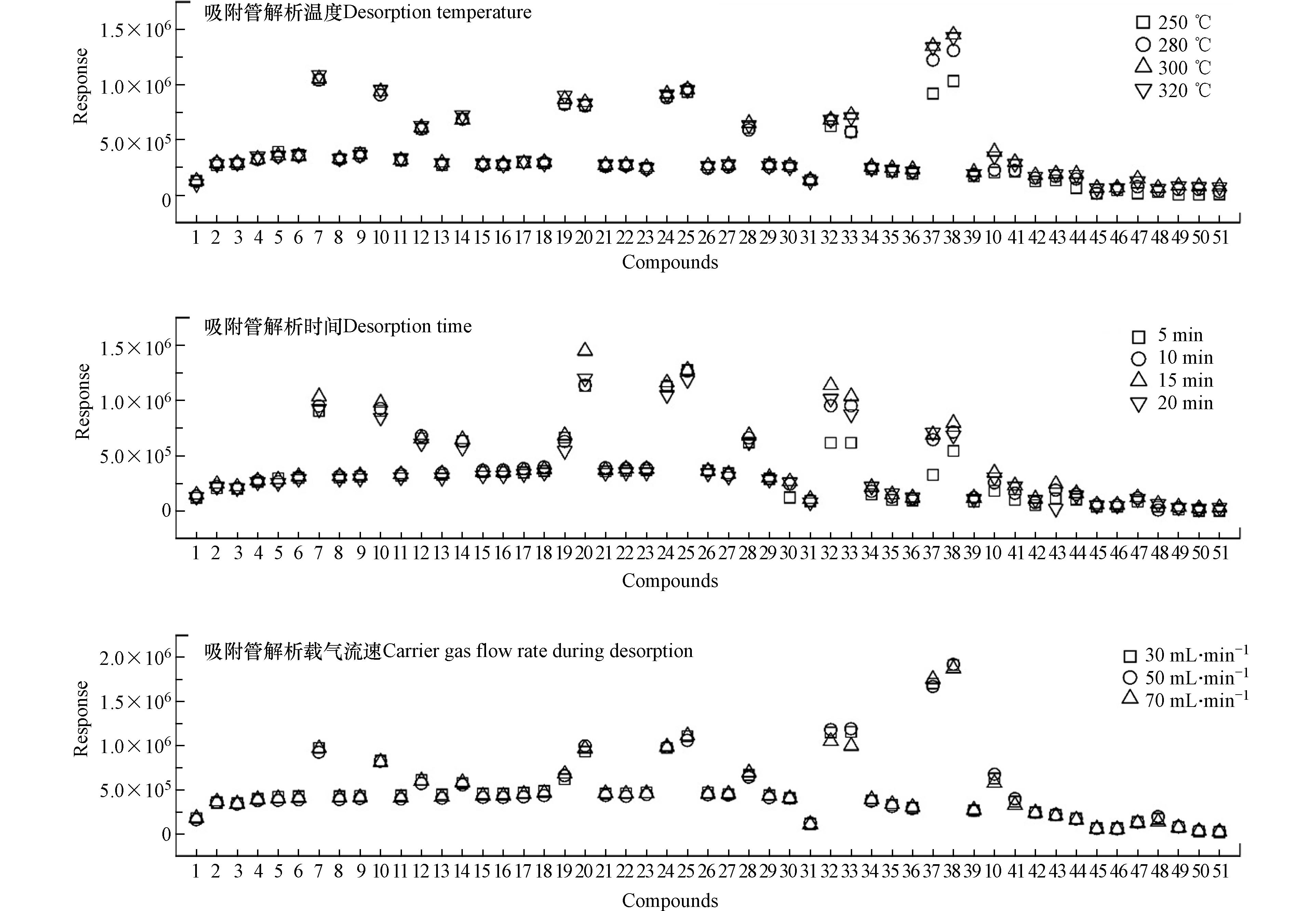
热脱附条件的设定直接影响方法的灵敏度和精准度,其中吸附管条件(解吸温度、解吸时间和解吸流速)和冷阱条件(捕集温度、解吸温度和解吸时间)尤为关键,故采用目标物含量为10 ng的标准品管对其进行优化.
-
吸附管解吸温度、解吸时间及载气流速主要影响目标物的解吸效率[25-27]. 首先优化解吸温度,将解吸温度分别设定为250、280、300、320 ℃,其他方法参数设定为初始值[23],考察各目标物的响应变化,结果(图1)显示,中、低沸点目标物的响应在不同解吸温度之间差异较小;高沸点目标物的响应随解吸温度的升高而增加,至300 ℃时响应达到高值,继续升温响应无明显变化;此外Tenax填料在300 ℃以上长时间使用会降低吸附效率,因此吸附管解吸温度设定为300 ℃.
采用同样的优化方式,依次设定不同的解吸时间(5—20 min)和载气流速(30—70 mL·min−1),结果(图1)显示,烷烃的响应在不同解吸时间之间差异较小,PAHs的响应随着解吸时间的增加而提高,至15 min时响应达到高值;继续增加时长,部分目标物的响应反而降低,表明时间过长导致部分目标物在冷阱Tenax填料中被载气带走而损失. 目标物的响应在不同的载气流速之间的差异较小,整体上50 mL·min−1时响应最高. 因此将脱附管解吸时间和载气流速分别设定为15 min和50 mL·min−1.
-
冷阱捕集温度主要影响目标物的捕集效率、冷阱脱附温度和脱附时间主要影响目标物的脱附效率[25-27]. 脱附管条件设定为优化值,采用2.1.1节的优化方式,依次设定不同的捕集温度(−10—−25 ℃)、脱附温度(280—320 ℃)和脱附时间(4—10 min). 结果(图2)显示,烷烃的响应几乎不受捕集温度变化的影响,而PAHs的响应随着捕集温度的降低而增加,在-20 ℃时响应值最高;继续降温,PAHs的响应均有不同程度的下降,表明当实际捕集温度过低会导致PAHs的捕集效率降低. 脱附温度为300 ℃时所有目标物的响应均高于脱附温度为280 ℃时的响应,PAHs表现尤其显著;继续升高温度,目标物的响应值无显著变化. 目标物的响应随着脱附时间的增加而提高,中、高沸点目标物尤为显著,在8 min时响应最高;继续增加时长,目标物的响应无显著变化. 因此将冷阱捕集温度、脱附温度和脱附时间分别设定为-20 ℃、300 ℃和8 min.
-
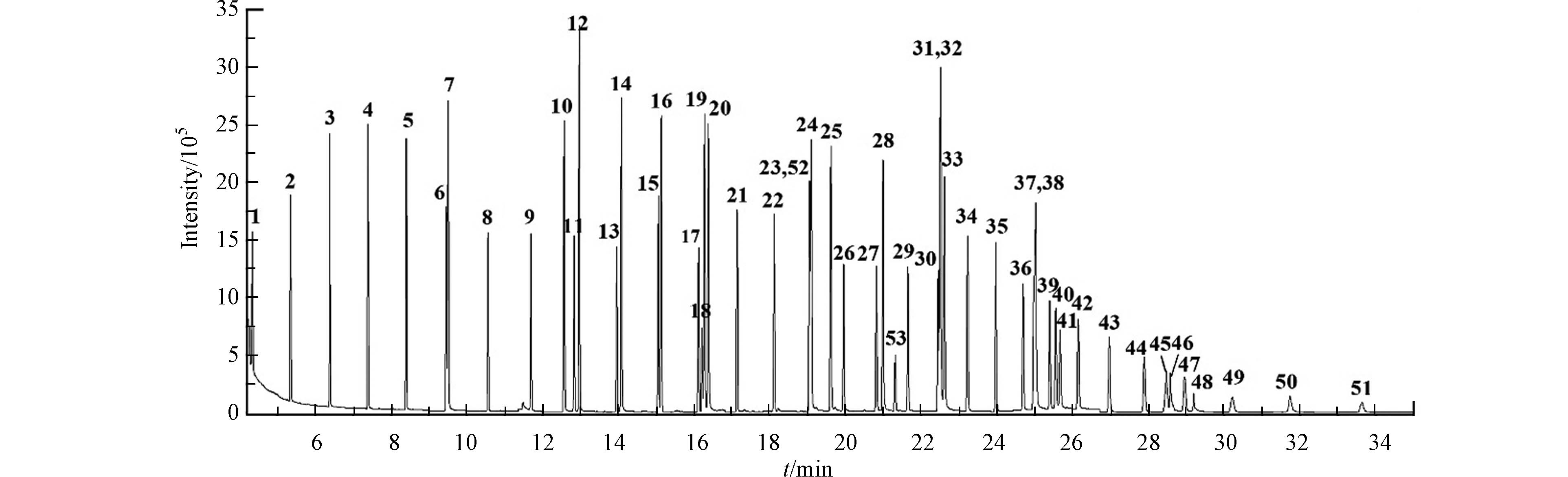
已有研究表明,烷烃及PAHs组分在中等极性色谱柱HP-5(30 m×0.25 mm×0.25 μm)色谱柱上均有较好的分离度和峰形[23],故本研究采用HP-5色谱柱对样品进行分离. 由于目标物数量多、沸点跨度(98—536 ℃)大,为得到最佳的目标物响应、分离度和峰形,本研究采用程序升温及2.1.1节的优化方式,使用目标物含量为10 ng的标准品管对柱初温、柱终温、升温速率、柱流速、梯度温度、恒温时间等重要参数进行逐一优化,最终结果见1.4.2节. 使用目标物含量为10 ng的标准品管在SCAN扫描下采集目标物的质谱图,根据Nist14谱库检索及保留时间对51种目标物及2种内标物进行定性分析并找出合适的定性定量离子(表1);为提高方法灵敏度、降低方法检出限,MS采用SIM扫描对51种目标物进行定量分析,总离子流图见图3. 由图3可知51种目标物在较短的时间(34 min)内完全出峰,基线平稳、峰形良好,所有目标物均得到有效分离.
-
分别抽取1 μL浓度分别为1、5、10、20、50 μg·mL−1的混合标准品使用液,注入老化好的吸附管中,同时加入1 μL内标使用液(25 μg·mL−1),制成标准品管,对应校正点目标物含量分别为1、5、10、20、50 ng,内标含量为25 ng,在优化条件下测定,每个校正点重复3次. 各目标物以外标与内标的定量离子峰面积之比(y)对浓度之比(x)绘制标准曲线. 结果显示,51种目标物在1—50 ng范围内线性良好,相关性系数(R2)为0.994—1.000. 配置目标物含量为0.2 ng的标准品管,重复测定7次,按照HJ168—2020[28]方法计算得51种目标物的检出限(MDL)为0.05—0.48 μg·m−3,定量下限(LOQ)为0.20—1.90 μg·m−3.
-
对老化后的空吸附管分别进行2、25、45 ng水平的加标实验,重复测定7次;计算得51种目标物在3个加标水平下的回收率分别为:91.3%—119.9%,86.7%—105.2%,90.2%—109.3%;相对标准偏差RSD(n=7)分别为:1.0%—5.2%,1.9%—5.7%,1.7%—5.3%.
上述质控指标均优于HJ646—2013[19]、HJ647—2013[20]要求,满足机动车尾气中烷烃和PAHs含量的测定需求,详情见表2.
-
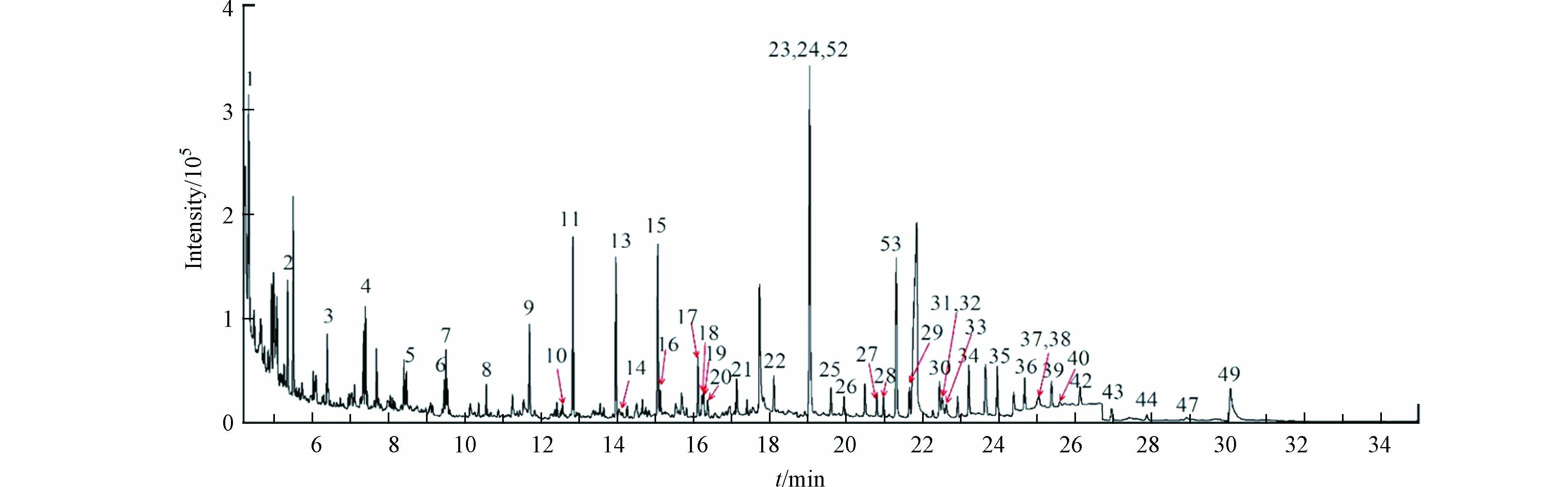
按照上述方法,采集1辆国Ⅳ轻型柴油车在匀速行驶状态下的尾气并进行检测,以验证该方法测定实际样品的可行性. 结果显示实际样品的峰形良好(图4);共检测出44种目标物,各目标物浓度均在方法线性范围内,总含量为7096.74 μg·m−3,其中包括28种正构烷烃(总含量为6467.61 μg·m−3)、2种支链烷烃(总含量为211.07 μg·m−3)和14种PAHs(总含量为418.05 μg·m−3).
由表3可知,该辆国Ⅳ轻型柴油车尾气中正十五烷、正十六烷、正庚烷、正十七烷和正十四烷等为主要污染物,含量依次为933.98、792.22、681.15、642.17、346.03 μg·m−3;萘的含量为74.25 μg·m−3,为首要PAHs组分;此外,S/IVOCs(包括C12—C36正构烷烃、姥鲛烷、植烷、19种PAHs)总含量为5828.60 μg·m−3,占烷烃和PAHs总含量的82.1%.
相较于以往文献中柴油车尾气的检测结果[29-30],该方法检测出更多的烷烃和PAHs组分,且低浓度目标物也具有更好的响应和峰形. 主要因为该方法经过优化后,扩充了更多的目标物(包括支链烷烃、高碳数正构烷烃和部分PAHs),其次该方法灵敏度较高、检出限较低,使得原本一些高沸点、低浓度、难识别的目标物被检出(例如正三十三烷、苯并(e)芘等);提供了更为丰富的污染源信息.
-
本研究采用吸附管采样,基于热脱附/气相色谱-质谱联用系统,建立了一种测定机动车尾气中30种正构烷烃(C7—C36)、2种支链烷烃(植烷、姥鲛烷)和19种PAHs(2—6环)的分析方法,该方法简便快速、稳定灵敏、准确度高,满足机动车尾气中烷烃和PAHs的检测需求,有助于完善机动车尾气污染物的成分谱及排放因子,为进一步厘清环境大气中SOA的来源奠定了坚实可靠的基础.
热脱附-气相色谱-质谱法测定机动车尾气中气相烷烃和多环芳烃含量
Determination of gas phase alkanes and polycyclic aromatic hydrocarbons in vehicle exhaust by thermal desorption-gas chromatography-mass spectrometry
-
摘要: 采用热脱附结合气相色谱质谱联用仪(TD-GC-MS),建立了机动车尾气中30种正构烷烃(C7—C36)、2种支链烷烃(植烷、姥鲛烷)和19种多环芳烃(PAHs)的分析方法. 利用Tenax-TA吸附管采集机动车尾气样品,加入定量的内标物;热解吸后经HP-5色谱柱分离、在选择性离子监测(SIM)模式下用GC-MS检测,内标法定量. 为提高方法的灵敏度、精准度等指标,优化了脱附管解吸温度、解吸时间,冷阱捕集温度、脱附温度、脱附时间和GC-MS分析条件. 结果表明,51种目标物在1—50 ng范围内线性良好,相关系数(R2)均大于0.994,方法检出限为0.05—0.48 μg.m−3,定量下限为0.20—1.90 μg.m−3,在低、中、高的3个加标水平下的回收率为86.7%—119.9%,相对标准偏差(RSD,n=7)为1.0%—5.7%,将其应用于轻型柴油车尾气样品的测定,共检测出44种目标物,包括28种正构烷烃、2种支链烷烃、14种PAHs. 该方法简便快速、稳定灵敏、准确度高,可满足机动车尾气中烷烃和PAHs的测定需求.Abstract: An analytical method was established for the determination of 30 n-alkanes (C7—C36), 2 branched alkanes (phytane, pristane) and 19 polycyclic aromatic hydrocarbons (PAHs) in vehicle emission by thermal desorption-gas chromatography-mass spectrometry (TD-GC-MS). Samples were collected by Tenax-TA adsorption tube, and the internal standard was added. After desorbed by thermal desorption, the analytes were determined by GC-MS in selected ion monitor (SIM) mode after separation by HP-5 column, and quantified by internal standard method. In order to improve the sensitivity and precision of method, various parameters such as desorption temperature and time of adsorption tube / cold trap, capture temperature of cold trap, and the analysis conditions of GC-MS were optimized. Results showed that the calibration curves for 51 compounds exhibited good linearity in the concentration range of 1-50 ng, with all the correlation coefficients (R2) morn than 0.994. The limits of detection and the limits of quantification were in the ranges of 0.05—0.48 μg·m−3 and 0.20—1.90 μg·m−3, espectively. The average recoveries at low, medium and high spiked levels ranged from 86.7% to 119.9% with the relative standard deviations (RSDs, n = 7) of 1.0%—5.7%. The method was applied to the determination of alkane and PAHs in the exhaust of light diesel vehicle. A total of 44 targets were detected, including 28 n-alkanes, 14 PAHs and 2 branched alkanes. The method was simple, rapid, stable, sensitive and accurate, and was suitable for the determination of alkane and PAHs in motor vehicle emissions.
-

-
表 1 51种目标化合物的CAS号、保留时间、定性及定量离子
Table 1. CAS No, retention time, qualitative and quantitative ions of 51 target compounds
序号
No.中文名称
Chinese name英文名称
English nameCAS号
CAS No.保留时间/min
Retention time定量离子(m/z)
Quantitative ion定性离子(m/z)
Qualitative ion目标物 Analytes 1 正庚烷 Heptane 142-82-5 4.338 43 41,71 2 正辛烷 Octane 111-65-9 5.355 43 57,29 3 正壬烷 Nonane 111-84-2 6.387 43 57,85 4 正癸烷 Decane 124-18-5 7.397 57 43,71 5 正十一烷 Undecane 1120-21-4 8.408 57 43,41 6 正十二烷 Dodecane 112-40-3 9.458 57 43,71 7 萘 Naphthalene 91-20-3 9.506 128 127,129 8 正十三烷 Tridecane 629-50-5 10.568 57 43,71 9 正十四烷 Tetradecane 629-59-4 11.7 57 43,71 10 苊烯 Acenaphthylene 208-96-8 12.574 152 153,151 11 正十五烷 Pentadecane 629-62-9 12.839 57 43,71 12 苊 Acenaphthene 83-32-9 12.976 153 154,152 13 正十六烷 Hexadecane 544-76-3 13.963 57 43,71 14 芴 Fluorene 86-73-7 14.092 166 165,167 15 正十七烷 Heptadecane 629-78-7 15.061 57 43,71 16 姥鲛烷 Pristane 1921-70-6 15.131 57 43,71 17 正十八烷 Octadecane 593-45-3 16.119 57 43,71 18 植烷 Phytane 638-36-8 16.218 57 43,71 19 菲 Phenanthrene ‘85-01-8 16.271 178 176,179 20 蒽 Anthracene 120-12-7 16.372 178 176,179 21 正十九烷 Nonadecane 629-92-5 17.132 57 43,71 22 正二十烷 Eicosane 112-95-8 18.108 57 71,43 23 正二十一烷 Heneicosane 629-94-7 19.046 58 71,44 24 荧蒽 Fluoranthene 206-44-0 19.088 202 203,200 25 芘 Pyrene 129-00-0 19.61 202 203,200 26 正二十二烷 Docosane 629-97-0 19.946 57 71,43 27 正二十三烷 Tricosane 638-67-5 20.809 57 71,43 28 1-甲基芘 1-Methylpyrene 2381-21-7 20.984 216 215,217 29 正二十四烷 Tetracosane 646-31-1 21.641 57 71,43 30 正二十五烷 Pentacosane 629-99-2 22.446 57 71,43 31 环戊烯(c,d)芘 Cyclopenta(c,d)pyrene 27208-37-3 22.502 226 227,224 32 苯并(a)蒽 Benzo(a)anthracene 56-55-3 22.509 228 226,229 33 䓛 Chrysene 218-01-9 22.607 228 226,229 34 正二十六烷 Hexacosane 630-01-3 23.22 57 71,43 35 正二十七烷 Heptacosane 593-49-7 23.967 57 71,43 36 正二十八烷 Octacosane 630-02-4 24.681 57 71,43 37 苯并(b)荧蒽 Benzo(b)fluorathene 205-99-2 25.018 252 253,250 38 苯并(k)荧蒽 Benzo(k)fluoranthene 207-08-9 25.038 252 250,253 39 正二十九烷 Nonacosane 630-03-5 25.385 57 71,43 40 苯并(e)芘 Benzo(e)pyrene 192-97-2 25.55 252 253,250 41 苯并(a)芘 Benzo(a)pyrene 50-32-8 25.655 252 253,250 42 正三十烷 Triacontane 638-68-6 26.135 57 71,43 43 正三十一烷 Hentriacontane 630-04-6 26.961 57 43,71 44 正三十二烷 Dotriacontane 544-85-4 27.885 57 71,43 45 茚并(1,2,3-cd)芘 Indeno(1,2,3-c,d)pyrene 193-39-5 28.477 276 277,274 46 二苯并(a,h)蒽 Dibenz(a,h)anthtacene 53-70-3 28.571 278 279,139 47 正三十三烷 Tritriacontane 630-05-7 28.942 57 71,43 48 苯并(g,h,i)苝 Benzo(g,h,i)perylene 191-24-2 29.196 276 277,138 49 正三十四烷 Tetratriacontane 14167-59-0 30.204 57 71,43 50 正三十五烷 Pentatriacontane 630-07-9 31.742 57 71,43 51 正三十六烷 Hexatriacontane 630-06-8 33.634 57 71,43 内标物Internal standards 52 氘代荧蒽 Fluoranthene-d10 93951-69-0 19.045 212 57 53 氘代二十四烷 n-Tetracosane-d50 204244-81-5 21.304 66 82 表 2 51种目标物的线性范围、相关系数、回收率、相对标准偏差(n=7)、检出限及定量下限
Table 2. Linear ranges, correlation coefficient(R2), recoveries,RSDs(n=7), MDLs and LOQs of 51 target compounds
序号
No.化合物
Compound线性方程
Linear equation相关系数R2 检出限/
(μg·m−3)
MDL定量下限/
(μg·m−3)
LOQ回收率/%
Recovery相对标准偏差/%
RSD1 正庚烷 Heptane y=0.26x 0.999 0.07 0.29 102.3, 86.7, 95.8 2.4, 4.5, 2.8 2 正辛烷 Octane y=0.64x+0.03 0.999 0.05 0.21 102.9, 96.2, 94.7 1.3, 4.7, 3.5 3 正壬烷 Nonane y=0.62x+0.03 1.000 0.11 0.45 100.4, 94.8, 95.7 1.3, 3.1, 3.6 4 正癸烷 Decane y=0.70x+0.04 1.000 0.10 0.42 100.6, 93.9, 96.6 1.8, 4.0, 2.8 5 正十一烷 Undecane y=0.73x+0.05 1.000 0.15 0.59 103.8, 94.3, 97.3 2.4, 3.9, 3.0 6 正十二烷 Dodecane y=0.77x+0.03 1.000 0.14 0.55 101.5, 94.3, 95.6 2.8, 3.5, 2.8 7 萘 Naphthalene y=0.92x+0.04 0.999 0.39 1.56 92.6, 99.0, 90.2 2.2, 3.8, 4.8 8 正十三烷 Tridecane y=0.75x+0.02 1.000 0.17 0.68 98.5, 93.5, 97.7 3.6, 3.6, 2.8 9 正十四烷 Tetradecane y=0.76x+0.06 0.999 0.16 0.66 100.8, 93.9, 98.0 3.9, 3.6, 2.4 10 苊烯 Acenaphthylene y=0.92x-0.01 0.999 0.40 1.62 91.6, 98.8, 98.1 3.1, 3.4, 3.7 11 正十五烷 Pentadecane y=0.78x 0.999 0.24 0.95 103.9,93.3, 98.8 3.1, 2.8, 1.9 12 苊 Acenaphthene y=0.55x+0.01 0.999 0.46 1.85 97.0, 101.3, 97.1 2.9, 1.9, 2.1 13 正十六烷 Hexadecane y=0.78x 0.999 0.20 0.81 105.8, 92.7, 98.9 4.1, 2.0, 1.7 14 芴 Fluorene y=0.64x 0.999 0.23 0.93 111.2, 96.8, 92.8 5.1, 3.9, 4.4 15 正十七烷 Heptadecane y=0.74x 0.999 0.33 1.31 107.2, 92.4, 99.0 4.7, 2.8, 2.2 16 姥鲛烷 Pristane y=0.68x+0.01 1.000 0.23 0.93 98.7, 95.2, 93.1 2.3, 2.5, 1.7 17 正十八烷 Octadecane y=0.76x 1.000 0.23 0.94 103.0, 90.8, 100.5 4.7, 4.1, 2.6 18 植烷 Phytane y=0.70x+0.01 1.000 0.16 0.66 99.5, 96.2, 96.2 2.2, 1.6, 1.9 19 菲 Phenanthrene y=0.86x 0.999 0.18 0.71 95.2, 91.3, 99.5 1.4, 2.8, 4.4 20 蒽 Anthracene y=0.87x-0.03 0.999 0.47 1.86 119.7, 93.3, 92.2 2.2, 4.9, 4.9 21 正十九烷 Nonadecane y=0.73x 0.999 0.33 1.31 106.2, 89.7, 99.3 4.0, 4.9, 2.4 22 正二十烷 Eicosane y=0.72x 0.999 0.46 1.84 108.2, 91.4, 99.1 4.7, 2.3, 2.3 23 正二十一烷 Heneicosane y=0.69x-0.01 0.999 0.33 1.34 105.9, 91.4, 98.9 5.2, 2.1, 2,7 24 荧蒽 Fluoranthene y=0.89x+0.01 0.999 0.41 1.62 96.4, 94.9, 90.7 1.5, 3.4, 5.0 25 芘 Pyrene y=0.94x+0.01 0.999 0.37 1.47 91.3, 96.3, 97.7 4.9, 2.6, 2.9 26 正二十二烷 Docosane y=0.75x-0.01 0.999 0.34 1.36 102.6, 90.6, 98.8 4.8, 1.9, 3.1 27 正二十三烷 Tricosane y=0.76x-0.01 0.999 0.27 1.06 105.6, 93.7, 103.1 3.8, 2.4, 3.5 28 1-甲基芘
1-Methylpyreney=0.63x-0.01 0.999 0.48 1.90 118.7, 96.3, 94.9 2.0, 3.1, 4.1 29 正二十四烷 Tetracosane y=0.75x 0.998 0.25 1.00 115.9, 92.0, 97.4 4.5, 3.0, 4.5 30 正二十五烷 Pentacosane y=0.73x-0.01 0.999 0.37 1.48 114.5, 93.9, 98.0 5.1, 3.9, 2.5 31 环戊烯(c,d)芘 Cyclopenta(c,d)pyrene y=0.16x-0.01 0.999 0.17 0.70 107.3, 92.4, 101.8 3.0, 2.5, 4.3 32 苯并(a)蒽 Benzo(a)anthracene y=0.65x-0.01 0.999 0.20 0.78 104.5, 102.1, 96.2 3.6, 4.1, 3.0 33 䓛 Chrysene y=0.67x+0.01 0.999 0.32 1.27 100.7, 94.2, 95.2 2.8, 4.8, 4.2 34 正二十六烷 Hexacosane y=0.67x 0.999 0.39 1.55 104.7, 93.6, 97.9 3.2, 3.8, 3.2 35 正二十七烷 Heptacosane y=0.63x 0.999 0.18 0.72 106.8, 93.2, 98.9 3.2, 3.5, 3.7 36 正二十八烷 Octacosane y=0.61x 0.999 0.42 1.68 117.2, 90.4, 98.5 1.9, 3.2, 4.1 37 苯并(b)荧蒽 Benzo(b)fluorathene y=1.38x+0.02 0.998 0.24 0.96 111.6, 97.1, 97.8 4.5, 3.2, 2.4 38 苯并(k)荧蒽 Benzo(k)fluoranthene y=1.37x+0.04 0.998 0.28 1.12 111.9, 102.1, 105.7 3.1, 3.0, 4.8 39 正二十九烷 Nonacosane y=0.57x-0.01 0.998 0.37 1.48 101.8, 92.1, 99.6 3.9, 2.4, 4.8 40 苯并(e)芘 Benzo(e)pyrene y=0.40x+0.02 0.999 0.44 1.75 103.3, 99.4, 101.9 1.7, 3.1, 3.1 41 苯并(a)芘 Benzo(a)pyrene y=0.33x+0.01 0.998 0.39 1.55 108.6, 101.5, 98.2 3.6, 4.2, 3.9 42 正三十烷 Triacontane y=0.50x-0.02 0.998 0.45 1.82 111.6, 105.2, 103.9 4.4, 5.7, 4.8 43 正三十一烷 Hentriacontane y=0.36x 0.999 0.39 1.55 116.0, 105.2, 103.9 3.2, 2.7, 2.7 44 正三十二烷 Dotriacontane y=0.33x-0.01 0.999 0.43 1.74 115.9, 99.4, 106.0 2.7, 5.3, 4.2 45 茚并(1,2,3-cd)芘 Indeno(1,2,3-c,d)pyrene y=0.07x+ 0.02 0.995 0.32 1.28 119.9, 101,8, 106.4 1.0, 4.3, 4.0 46 二苯并(a,h)蒽 Dibenz(a,h)anthtacene y=0.03x+0.02 0.996 0.16 0.64 105.9, 104.3, 97.8 4.4, 3.1, 2.5 47 正三十三烷 Tritriacontane y=0.24x 0.997 0.36 1.44 109.3, 94.6, 105.5 3.3, 3.4, 2.9 48 苯并(g,h,i)苝 Benzo(g,h,i)perylene y=0.05x+0.06 0.994 0.12 0.47 119.6, 102.2, 100.6 1.0, 3.9, 5.3 49 正三十四烷 Tetratriacontane y=0.15x+0.13 0.998 0.09 0.35 116.8, 100.5, 97.0 1.6, 4.3, 2.5 50 正三十五烷 Pentatriacontane y=0.06x+0.02 0.999 0.07 0.27 113.9, 103.2, 109.3 1.7, 5.5, 4.4 51 正三十六烷 Hexatriacontane y=0.03x+0.04 0.996 0.05 0.20 106.1, 101.2, 104.5 1.3, 4.3, 3.5 表 3 轻型柴油车尾气中烷烃和PAHs含量(μg·m−3)
Table 3. Concentration of alkanes and PAHs in exhaust gas from light diesel vehicle (μg·m−3)
序号
No.化合物
Compound浓度
Concentration序号
No.化合物
Compound浓度
Concentration1 正庚烷 Heptane 681.15 24 荧蒽 Fluoranthene 28.06 2 正辛烷 Octane 244.41 25 芘 Pyrene 27.01 3 正壬烷 Nonane 94.87 26 正二十二烷 Docosane 74.54 4 正癸烷 Decane 145.65 27 正二十三烷 Tricosane 91.89 5 正十一烷 Undecane 102.06 28 1-甲基芘 1-Methylpyrene 33.73 6 正十二烷 Dodecane 219.51 29 正二十四烷 Tetracosane 119.11 7 萘 Naphthalene 74.25 30 正二十五烷 Pentacosane 228.38 8 正十三烷 Tridecane 120.93 31 环戊烯(c,d)芘 Cyclopenta(c,d)pyrene 56.61 9 正十四烷 Tetradecane 346.03 32 苯并(a)蒽 Benzo(a)anthracene 24.01 10 苊烯 Acenaphthylene 10.47 33 䓛 Chrysene 45.17 11 正十五烷 Pentadecane 933.98 34 正二十六烷 Hexacosane 179.61 13 正十六烷 Hexadecane 792.22 35 正二十七烷 Heptacosane 168.57 14 芴 Fluorene 5.34 36 正二十八烷 Octacosane 139.84 15 正十七烷 Heptadecane 642.17 37 苯并(b)荧蒽 Benzo(b)fluorathene 7.82 16 姥鲛烷 Pristane 92.19 38 苯并(k)荧蒽 Benzo(k)fluoranthene 12.23 17 正十八烷 Octadecane 312.47 39 正二十九烷 Nonacosane 151.16 18 植烷 Phytane 118.88 40 苯并(e)芘 Benzo(e)pyrene 9.74 19 菲 Phenanthrene 34.23 42 正三十烷 Triacontane 140.27 20 蒽 Anthracene 49.39 43 正三十一烷 Hentriacontane 98.18 21 正十九烷 Nonadecane 92.15 44 正三十二烷 Dotriacontane 76.14 22 正二十烷 Eicosane 103.48 47 正三十三烷 Tritriacontane 25.42 23 正二十一烷 Heneicosane 77.21 49 正三十四烷 Tetratriacontane 66.21 -
[1] LEE S C, CHIU M Y, HO K F, et al. Volatile organic compounds (VOCs) in urban atmosphere of Hong Kong [J]. Chemosphere, 2002, 48(3): 375-382. doi: 10.1016/S0045-6535(02)00040-1 [2] LI Y J, REN B N, QIAO Z, et al. Characteristics of atmospheric intermediate volatility organic compounds (IVOCs) in winter and summer under different air pollution levels [J]. Atmospheric Environment, 2019, 210: 58-65. doi: 10.1016/j.atmosenv.2019.04.041 [3] YU Q Q, GAO B, LI G H, et al. Attributing risk burden of PM2.5-bound polycyclic aromatic hydrocarbons to major emission sources: Case study in Guangzhou, South China [J]. Atmospheric Environment, 2016, 142: 313-323. doi: 10.1016/j.atmosenv.2016.08.009 [4] ROBINSON A L, DONAHUE N M, SHRIVASTAVA M K, et al. Rethinking organic aerosols: semivolatile emissions and photochemical aging [J]. Science, 2007, 315(5816): 1259-1262. doi: 10.1126/science.1133061 [5] HUNTER J F, DAY D A, PALM B B, et al. Comprehensive characterization of atmospheric organic carbon at a forested site [J]. Nature Geoscience, 2017, 10(10): 748-753. doi: 10.1038/ngeo3018 [6] HENZE D K, SEINFELD J H, NG N L, et al. Global modeling of secondary organic aerosol formation from aromatic hydrocarbons: high- vs. low-yield pathways [J]. Atmospheric Chemistry and Physics, 2008, 8(9): 2405-2420. doi: 10.5194/acp-8-2405-2008 [7] 谢绍东, 田晓雪. 挥发性和半挥发性有机物向二次有机气溶胶转化的机制 [J]. 化学进展, 2010, 22(4): 727-733. XIE S D, TIAN X X. Formation mechanism of secondary organic aerosols from the reaction of volatile and semi-volatile compounds [J]. Progress in Chemistry, 2010, 22(4): 727-733(in Chinese).
[8] LIU X X, DAY D A, KRECHMER J E, et al. Direct measurements of semi-volatile organic compound dynamics show near-unity mass accommodation coefficients for diverse aerosols [J]. Communications Chemistry, 2019, 2: 98. doi: 10.1038/s42004-019-0200-x [9] PYE H O T, SEINFELD J H. A global perspective on aerosol from low-volatility organic compounds [J]. Atmospheric Chemistry and Physics, 2010, 10(9): 4377-4401. doi: 10.5194/acp-10-4377-2010 [10] ZHAO Y L, HENNIGAN C J, MAY A A, et al. Intermediate-volatility organic compounds: A large source of secondary organic aerosol [J]. Environmental Science & Technology, 2014, 48(23): 13743-13750. [11] 唐荣志, 王辉, 刘莹, 等. 大气半/中等挥发性有机物的组成及其对有机气溶胶贡献 [J]. 化学进展, 2019, 31(1): 180-190. TANG R Z, WANG H, LIU Y, et al. Constituents of atmospheric semi-volatile and intermediate volatility organic compounds and their contribution to organic aerosol [J]. Progress in Chemistry, 2019, 31(1): 180-190(in Chinese).
[12] LI J, HAN Z W, LI J W, et al. The formation and evolution of secondary organic aerosol during haze events in Beijing in wintertime [J]. Science of the Total Environment, 2020, 703: 134937. doi: 10.1016/j.scitotenv.2019.134937 [13] FANG H, HUANG X Q, ZHANG Y L, et al. Measurement report: Emissions of intermediate-volatility organic compounds from vehicles under real-world driving conditions in an urban tunnel [J]. Atmospheric Chemistry and Physics, 2021, 21(13): 10005-10013. doi: 10.5194/acp-21-10005-2021 [14] 马英歌, 孙谦, 李莉, 等. 热脱附结合GC-MS测定大气总悬浮颗粒物中的半挥发性有机物 [J]. 环境化学, 2017, 36(6): 1424-1427. MA Y G, SUN Q, LI L, et al. Determination of semi-volatile organic pollutants from atmospheric using thermal desorption system with gas chromatography-mass spectrometer [J]. Environmental Chemistry, 2017, 36(6): 1424-1427(in Chinese).
[15] 解迎双, 周围. 双燃料汽车与汽油车尾气中有机物成份对比分析 [J]. 科技导报, 2010, 28(6): 93-97. XIE Y S, ZHOU W. Comparative analysis of the organic composition in the exhaust fumes between dual fuel autombiles and gasoline vehicles [J]. Science & Technology Review, 2010, 28(6): 93-97(in Chinese).
[16] JUNG S, MUN S, CHUNG T, et al. Emission characteristics of regulated and unregulated air pollutants from heavy duty diesel trucks and buses [J]. Aerosol and Air Quality Research, 2019, 19(2): 431-442. doi: 10.4209/aaqr.2018.05.0195 [17] PERRONE M G, CARBONE C, FAEDO D, et al. Exhaust emissions of polycyclic aromatic hydrocarbons, n-alkanes and phenols from vehicles coming within different European classes [J]. Atmospheric Environment, 2014, 82: 391-400. doi: 10.1016/j.atmosenv.2013.10.040 [18] 陆思华, 白郁华, 张广山, 等. 机动车排放及汽油中VOCs成分谱特征的研究 [J]. 北京大学学报(自然科学版), 2003, 39(4): 507-511. doi: 10.13209/j.0479-8023.2003.077 LU S H, BAI Y H, ZHANG G S, et al. Study on the characteristics of VOCs source profiles of vehicle exhaust and gasoline emission [J]. Acta Scicentiarum Naturalium Universitatis Pekinensis, 2003, 39(4): 507-511(in Chinese). doi: 10.13209/j.0479-8023.2003.077
[19] 中华人民共和国环境保护部. 环境空气和废气 气相和颗粒物中多环芳烃的测定 气相色谱-质谱法: HJ 646—2013[S]. 北京: 中国环境科学出版社, 2013. Ministry of Environmental Protection of the People's Republic of China. Ambient air and stationary source emissions. Determination of gas and particle-phase polycyclic aromatic hydrocarbons with gas chromatography/mass spe: HJ 646—2013[S]. Beijing: China Environment Science Press, 2013(in Chinese).
[20] 中华人民共和国环境保护部. 环境空气和废气 气相和颗粒物中多环芳烃的测定 高效液相色谱法: HJ 647—2013[S]. 北京: 中国环境科学出版社, 2013. Ministry of Environmental Protection of the People's Republic of China. Ambient air and stationary source emissions. Determination of gas and particle-phase polycyclic aromatic hydrocarbons. High performance liquid chromat: HJ 647—2013[S]. Beijing: China Environment Science Press, 2013(in Chinese).
[21] 谭鑫, 袁斌, 王超敏, 等. 环境大气中半/中等挥发性有机物(S/IVOCs)的测量技术进展 [J]. 中国环境科学, 2020, 40(10): 4224-4236. doi: 10.3969/j.issn.1000-6923.2020.10.005 TAN X, YUAN B, WANG C M, et al. Progress in measurements of semi-/ intermediate-volatile organic compounds in ambient air [J]. China Environmental Science, 2020, 40(10): 4224-4236(in Chinese). doi: 10.3969/j.issn.1000-6923.2020.10.005
[22] U. S. Environmental Protection Agency. Determination of volatile organic compounds in ambient air using active sampling onto sorbent tubes (Method TO-17) [S]. Cincinnati: U. S. Environmental Protection Agency, 1999. [23] WANG M, HUANG R J, CAO J J, et al. Determination of n-alkanes, PAHs and hopanes in atmospheric aerosol: evaluation and comparison of thermal desorption GC-MS and solvent extraction GC-MS approaches [J]. Atmospheric Measurement Techniques, 2019, 12(9): 4779-4789. doi: 10.5194/amt-12-4779-2019 [24] 周文钦, 李成, 刘俊文, 等. 典型内燃叉车尾气挥发性有机物与正构烷烃的排放特征研究 [J]. 环境科学, 2022, 43(2): 735-742. ZHOU W Q, LI C, LIU J W, et al. Emission characterstics of VOCs and n-alkanes from diesel forklifts [J]. Environmental Science, 2022, 43(2): 735-742(in Chinese).
[25] 董瑞, 沈秀娥, 张琳, 等. 热脱附-GC/MS快速测定大气细颗粒物中支链烷烃和烷基环己烷 [J]. 质谱学报, 2018, 39(4): 433-441. doi: 10.7538/zpxb.2017.0138 DONG R, SHEN X E, ZHANG L, et al. Determination of branched alkane and alkylcyclohexane in atmospheric fine particulate matter using rapid direct thermal desorption-GC/MS [J]. Journal of Chinese Mass Spectrometry Society, 2018, 39(4): 433-441(in Chinese). doi: 10.7538/zpxb.2017.0138
[26] HO S S H, YU J Z. In-injection port thermal desorption and subsequent gas chromatography-mass spectrometric analysis of polycyclic aromatic hydrocarbons and n-alkanes in atmospheric aerosol samples [J]. Journal of Chromatography A, 2004, 1059(1/2): 121-129. [27] CACHIER H, BRÉMOND M P, BUAT-MÉNARD P. Thermal separation of soot carbon [J]. Aerosol Science and Technology, 1989, 10(2): 358-364. doi: 10.1080/02786828908959273 [28] 中华人民共和国生态环境部. 环境监测分析方法标准制订技术导则: HJ 168—2020[S]. 北京: 中国环境科学出版社, 2020. Ministry of Ecology and Environment of the People's Republic of China. Technical guideline for the development of environmental monitoring analytical method standards: HJ 168—2020[S]. Beijing: China Environment Science Press, 2020(in Chinese).
[29] 李登科. 机动车排气中SVOCs及颗粒物中SOF成分分析[D]. 天津: 天津大学, 2012. LI D K. Research of composition of semi volatile organic compounds and SOF in particulate matter in vehicle exhaust[D]. Tianjin: Tianjin University, 2012(in Chinese).
[30] SHAH S D, OGUNYOKU T A, MILLER J W, et al. On-road emission rates of PAH and n-alkane compounds from heavy-duty diesel vehicles [J]. Environmental Science & Technology, 2005, 39(14): 5276-5284. -



 下载:
下载: