-
精神活性物质是指在摄入后能够影响思维、情感和意志行为的物质[1]. 这些物质经人体代谢后,随污水管网系统进入污水处理厂,由于现有污水处理工艺并不能完全将其去除,导致残留的药物最终被排入地表水中[2]. 随着使用量的增加,精神活性物质正逐渐成为水环境中一类新兴污染物[3].
暴露于精神活性物质会对水生生物,特别是鱼类产生不利影响[4]. 精神活性物质最主要的功能在于调节神经递质等神经化学物质,从而影响生物体的行为,因此有大量研究关注了精神活性物质对鱼类的神经毒性. 例如,安非他酮增加了杂交条纹鲈鱼的大脑内多巴胺(dopamine,DA)水平[5];而文拉法辛暴露则导致杂交条纹鲈鱼大脑内5-羟色胺(serotonin,5-HT)含量降低[6];此外,卡马西平会抑制斑马鱼体内谷氨酸(L-Glutamic acid,Glu)水平[7],安非他明会诱导斑马鱼体内DA信号蛋白表达[8]. 但现有的研究所关注的神经化学物质的种类相对较少,对于其它神经化学物质的影响还甚少涉及,因此关于精神活性物质对鱼类的神经毒性,我们还缺乏全面认识.
根据组成的化学物质不同,神经化学物质可分为肾上腺素能神经传递系统、谷氨酸能神经传递系统、羟色胺能神经传递系统、儿茶酚胺能神经传递系统、胆碱能神经传递系统以及其它神经传递系统. 这些系统对生物体具有重要的调节作用,肾上腺素能神经传递系统主要参与生物压力应对、应激反应、焦虑、抑郁等[9] ,同时也在生物体内对新信号识别和记忆起着至关重要的作用[10];谷氨酸能神经传递系统参与许多重要的生命活动的调节,如记忆和学习、性行为、攻击行为等[11],也参与一些疾病的调节,如强迫症、抑郁症、情感障碍和阿尔茨海默病、帕金森病、焦虑和精神分裂症等[12];动物体内的羟色胺能神经传递系统与各种行为和疾病有关,包括帕金森症、亨廷顿舞蹈病、肌张力障碍和家族性震颤等[13],其中5-HT在动物的运动活动、昼夜节律、进食、社会互动和攻击性、焦虑、情绪、学习和记忆等行为中都扮演着重要的角色,它能增强或减轻对大多数行为进行微调所必需的神经元反应[14]. 儿茶酚胺能神经传递系统主要在应激诱导中起着重要的作用,在紧急情况下会导致其释放增加[15]. 胆碱能神经传递系统中的乙酰胆碱也可能通过烟碱或毒蕈碱受体在恐惧和焦虑相关行为中发挥作用[16]. 在神经毒性研究中,尽可能地覆盖更多的神经化学物质,有助于准确和全面地评估污染物的神经毒性效应及其机制.
可待因是一种常见的阿片类药物,常用作基础镇咳药 [17]和急性镇痛剂[18]. 在临床上,可待因还具有抗应激和止泻的作用[19]. 可待因可与大脑中的阿片受体结合,增强大脑和身体其它部位的信号传递过程[20]. 此外,在人体内,少量的可待因在代谢过程中可转化为吗啡[20]. 直到现在,可待因的确切作用机制尚不完全清楚. 随着使用量的增加,可待因在地表水中的被广泛检出. 现阶段,在地表水中检测到的可待因浓度可高达μg·L−1的水平[21]. 可待因在欧洲地表水中检出较多,如意大利波河和兰布罗河的检出水平分别为1.8 ng·L−1和12 ng·L-1[22],西班牙地表水则检出8.9、14、26.8、231.8 ng·L−1不等[23],荷兰地表水中检出7 ng·L−1[24]. 在中国境内也频繁在地表水中检测到可待因,在台湾各河流中检测到可待因浓度在12—341 ng·L−1不等[25-26],而在北京、昆明地表水中可待因浓度分别在5.6、2.3 ng·L−1左右[27-28],在黄河和珠江中可待因浓度范围为0.5—2.1 ng·L−1[29]. 然而,目前有关可待因对鱼类的神经毒性的研究还鲜有报道.
鲫鱼是一种在亚洲地区广泛分布的物种,对水生态系统具有重要的功能和意义,也常被用于水体污染物的毒性测试[30]. 本研究利用鲫鱼幼鱼作为受试生物,探究了环境浓度暴露下可待因在鲫鱼体内的蓄积和对不同神经递质系统中24种神经化学物质的影响,以此表征可待因对鱼类的神经毒性效应,为评估其水生态风险提供依据.
-
可待因及其同位素内标(纯度> 97%)均购自美国Cerilliant公司. 24种神经化学物质和对应的11种同位素内标均购自中国百灵威公司,其详细信息如表1所示. 所有标准品均用乙腈或含一定比例超纯水的乙腈溶液配制成浓度为1 g·L−1的储备液,配制低浓度单标或混标溶液时用含有5%乙腈的水溶液逐步进行稀释. 实验所需甲酸,中性Al2O3购自中国百灵威公司,乙腈和甲醇均购自上海麦克林公司. C18购自美国Welch公司,吸附剂PSA(Primary Secondary Amine)购自美国Agilent Technologies公司,无水MgSO4、NaCl均购自国药集团化学试剂有限公司. 无水MgSO4和NaCl使用前在450 ℃下烘烤4 h以去除其中的有机物,中性Al2O3在180 ℃烘烤12 h以活化. 实验所用溶剂均为LC-MS级。
-
2月左右的鲫鱼幼鱼((0.3 ± 0.1)g,(27.8 ± 2.5)mm)购自中国南方某渔场. 实验前,鱼在脱氯(曝气48 h)自来水中驯化两周. 驯化期间,每天保持12 h光照和12 h黑暗,喂食1次小型鱼通用饲料. 根据可待因的水环境残留水平,暴露实验的暴露浓度设置为 5 ng·L−1 (低浓度)和 500 ng·L−1(高浓度). 每个暴露组包含 16条鱼,平均放置在两个水缸中(每个水缸中8 条鱼,水量V=10 L). 以等量5%乙腈水溶液(乙腈终浓度0.0001%)为对照组. 根据之前的研究[31-32],暴露时间设置为 7 d. 暴露完成后,从每个水缸中随机选取 4 条鱼进行可待因残留和神经化学物质分析. 将剩余的鱼留在原水缸中,在干净的脱氯水中进行 7 d的恢复. 在恢复期结束后对剩余个体进行相同可待因残留和神经化学物质分析. 在暴露期和恢复期,每 24 小时更换水缸中一半的水,并在暴露期加入相应量的暴露化合物,以保持暴露浓度不变. 整个实验过程中,每天测量环境温度、湿度、pH值、溶解氧(DO)和水温,分别为(25.3 ± 1.5) ℃、45% ± 8.7%、(7.22 ± 0.17) mg·L−1、(7.06 ± 0.78) mg·L−1和(22.8 ± 0.6) ℃. 涉及鱼类暴露和处理的实验均经过浙江大学实验动物伦理委员会批准.
-
依据本课题组已有的方法[33]进行样品预处理:将鱼在-20 ℃快速冷冻麻醉,然后切断颈部脊柱处死. 每条鱼称重后加体重两倍比例的超纯水(如0.5 g鱼加1 mL超纯水)进行充分匀浆,并将所有匀浆液置于2 mL离心管中. 取200 µL匀浆液于10 mL离心管中,加入含3 mL 0.1%甲酸的乙腈溶液、20 μL可待因内标(浓度为100 μg·L−1)和20 μL神经化学物质同位素内标混合液(各内标浓度均为100 μg·L−1),超声萃取10 min. 离心管内加入NaCl和无水MgSO4各100 mg,涡旋1 min后离心5 min(5000 r·min−1). 取上清液转移至另一10 mL离心管,加入60 mg PSA、30 mg C18、30 mg 中性Al2O3和20 mg无水MgSO4,涡旋1 min后离心5 min(5000 r·min−1),取上清液至另一10 mL离心管,剩余部分加入2 mL含 0.1%甲酸的乙腈溶液后润洗,再次离心5 min后合并两次上清液,并用氮吹仪吹至近干,再用200 μL 5%乙腈水溶液复溶,将提取液通过0.22 μm滤膜以去除可能含有的颗粒物,样品进超高效液相色谱-三重四极杆串联质谱仪(Xevo TQ-S,Waters,美国)进行分析.
-
可待因:液相分离色谱柱为BEH C18柱(100 mm×2.1 mm,1.7 μm,Waters,美国). 进样量为10 μL,BEH C18柱温为40 ℃. 流动相采用二元洗脱液,A相乙腈,B相水,均添加0.1%的甲酸,梯度洗脱程序如下:0 min 5% A,2 min 25% A,6 min 75% A,7 min 95% A,8 min 5% A,流速为0.3 mL·min−1.
神经化学物质:液相分离色谱柱为Synergi Polar-RP 100 Å column(100 mm×2 mm,2.5 μm,Phenomenex,Torrance,美国). 进样量为10 μL,色谱柱柱温为20 ℃. 流动相用二元洗脱液,A相甲醇,B相水,均添加0.1%的甲酸,梯度洗脱程序为:0 min 5% A,2 min 5% A,4 min 25% A,7 min 95% A ,8 min 5% A,流速为0.2 mL·min−1.
可待因、神经化学物质及其同位素内标的质谱分析参数、方法学参数参见已有的研究[33].
-
可待因残留浓度和神经化学物质浓度均用平均值±标准误(SEM)表示. 可待因的残留浓度以鱼体湿重计算. 在SPSS 26.0(SPSS, Chicago, IL, 美国)中,运用单因素方差分析进行多重比较. 当P < 0.05时,认为组间存在显著差异. 鱼体中可待因残留浓度以及神经化学物质含量的计算公式如公示(1)所示:
其中,
$ {\mathit{C}}^{\mathit{\text{'}}} $ 表示鱼体内可待因或神经化学物质含量(ng·g−1湿重);C为鱼体样品最终提取液中可待因或神经化学物质浓度(ng·mL−1);V表示鱼匀浆液总体积(mL);m表示鱼的质量(g). 详细参数参照实验室以往方法[33]. -
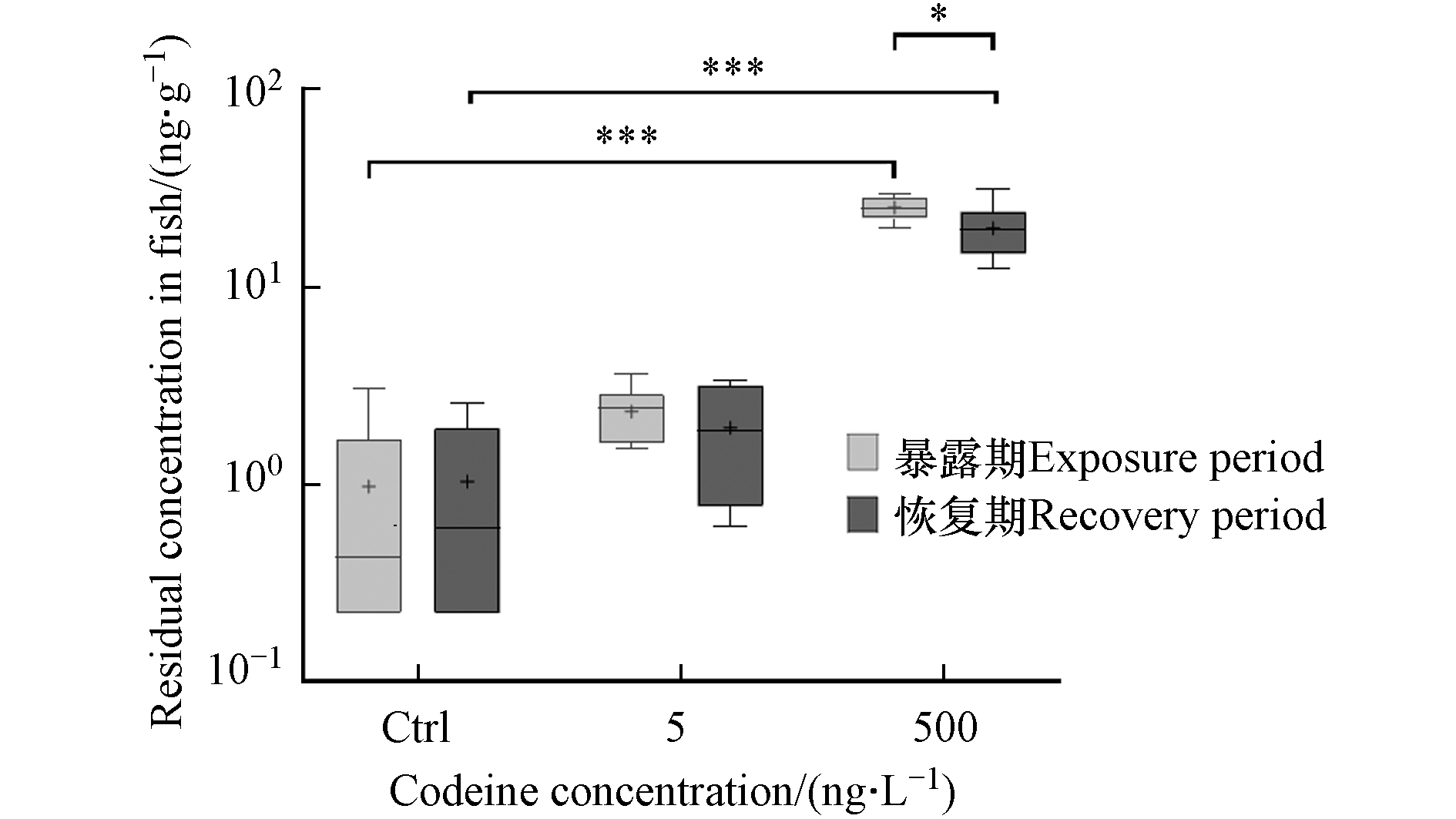
可待因在鲫鱼体内蓄积情况如图1所示. 实验鱼中均检测到可待因. 在低、高浓度可待因暴露后,鱼体内可待因平均残留浓度分别为2.35 ng·g−1和25.30 ng·g−1. 可待因的生物蓄积系数(BCF,即鱼体内可待因浓度(ng·kg−1)与水中可待因浓度(ng·L−1)的比值)分别为471和51,表明COD在持续暴露的情况下可在鱼体内蓄积,并且蓄积系数随暴露浓度增加而降低. 恢复期结束后,两个暴露组鱼体内可待因的平均水平分别下降17.3%和21.7%,降至1.95 ng·g−1和19.82 ng·g−1,但仍高于对照组,表明可待因在鱼体内的蓄积具有一定的持久性.
可待因在人体内的降解/排泄速度快,半衰期仅为(1.47 ± 0.32)h[34]. 虽然可待因在鱼体中的半衰期研究很少,但我们的研究表明,它在鱼体中的半衰期似乎比在人体中要长得多. 此前的一项研究证明,另两种精神活性物质甲基苯丙胺和氯胺酮在斑马鱼体内的半衰期在0.18—6.98 d之间[35],而两种药物在人体内的半衰期仅为11 h [36] 和2.5 h [37]. 这些结果表明,精神活性物质的半衰期在人体内和鱼体内可能大不相同,在鱼体内更长的半衰期意味着它们对鱼类的影响可能更加持久.
-
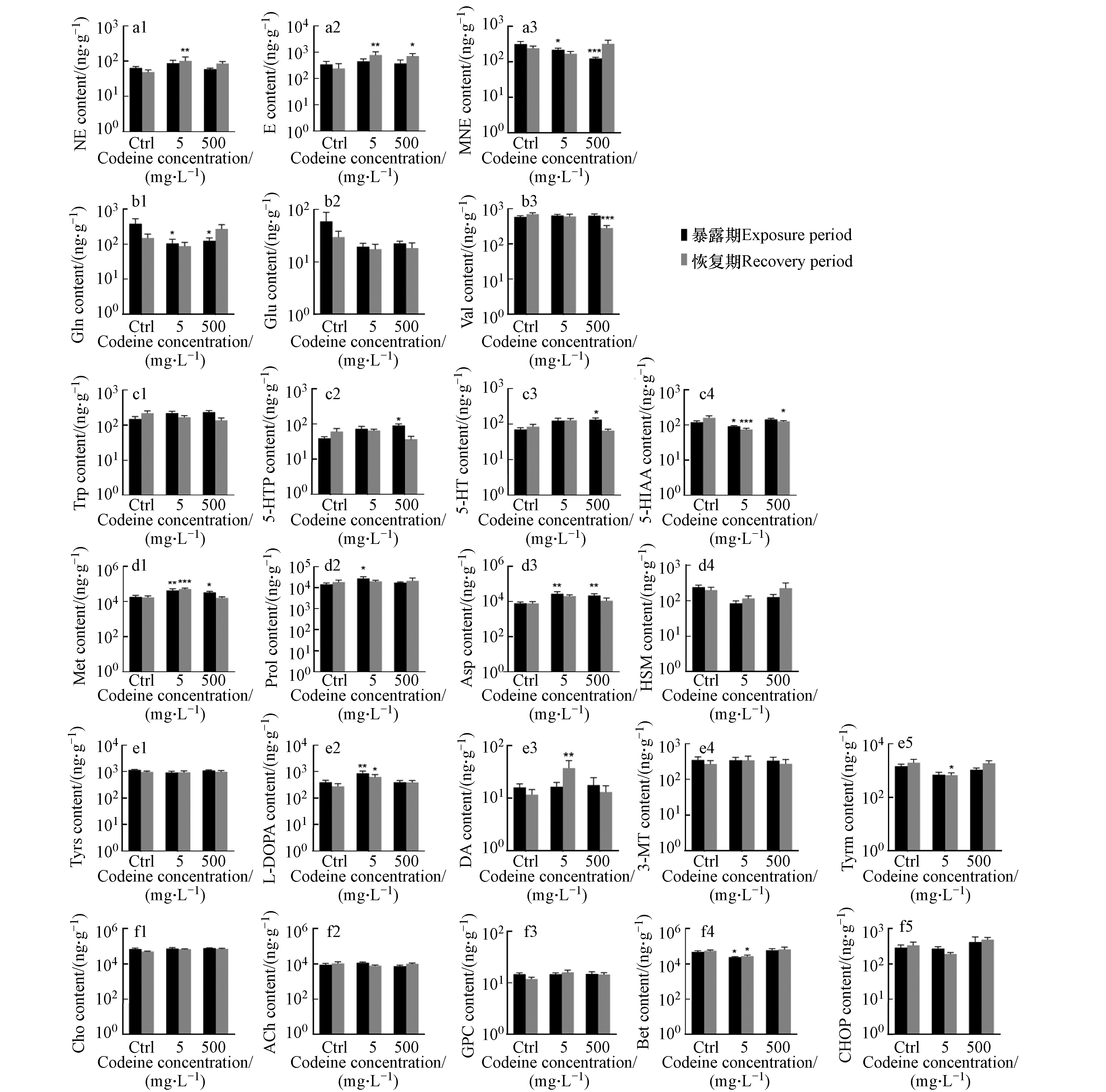
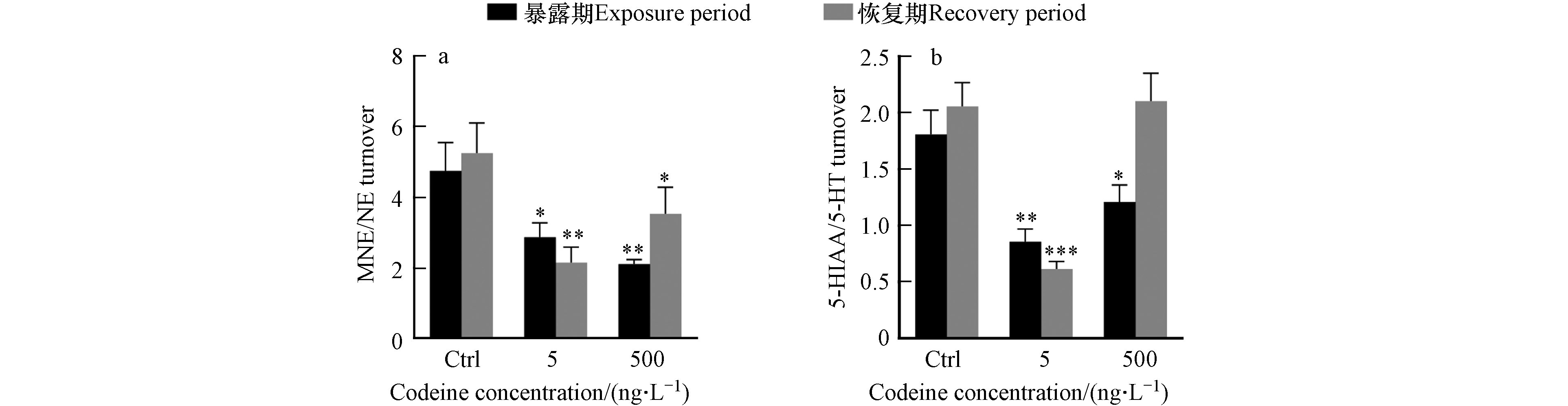
可待因对来自不同神经递质系统的神经化学物质的影响如图2所示. 对于肾上腺素能系统的神经化学物质(图2a1—a3),暴露结束后,可待因没有显著影响NE水平,但低浓度下增加了NE浓度,这种增加在恢复期后表现得更加明显,使 NE含量显著增加了37%(图2a1),呈现出延迟效应. 此外,在两种暴露浓度下,均观察到可待因暴露对E的延迟效应,7 d恢复期后, NE含量分别较对照组显著增加2.2倍和2.0倍(图2a2). MNE含量在两种暴露浓度下分别被显著抑制了31%和61%,但该抑制在恢复期后消失(图2a3),表明这种影响是可逆的. NE在生物转化过程中主要转化为MNE和E[38-39]. 本研究中,NE水平增加,其代谢产物E水平增加而MNE水平降低,推测可待因暴露可能促进鱼体内NE向E的转化,同时抑制NE向MNE的转化,但机制尚不明确. 此外,通常用MNE/NE的比值来代表肾上腺素能系统中神经化学物质的周转[40]. 本研究中,两种暴露浓度可待因均显著抑制该比率,并在7 d恢复期结束后不可逆(图3a),这与可待因降低NE向MNE转换的假设一致.
在谷氨酸能系统中(图2b1—b3),在两种暴露浓度下,Gln含量分别显著降低72%和67%,但在恢复期结束时恢复正常(图2b1). 虽然Glu含量在暴露结束后也分别降低67%和62%,恢复后仍有41%和38%下降,但均无显著性差异(图2b2). 在生物体中,Gln通过特异性转运蛋白被转运到细胞中,并通过谷氨酰胺酶转化为Glu[41]. 在本研究中,Gln和Glu含量均受到抑制,但对Gln的抑制作用更为明显,推测可待因暴露可能促进了两种神经化学物质之间的转化.
在羟色胺能系统中(图2c1—c4),高浓度可待因显著诱导5-HT,使其含量增加87%,暴露解除后影响消失(图2c3). 作为5-HT的代谢产物[42],5-HIAA含量在低浓度可待因暴露后受到显著抑制,含量下降24%,恢复后抑制程度达到54%,表明影响不可逆(图2c4). 至于5-HTP,作为5-HT的前体[43],高浓度可待因使其含量增加1.3倍(图2c2). 5-HTP和5-HT的变化相似,但5-HTP含量增加幅度更大,同时5-HIAA水平随5-HT含量的增加而降低,可能是可待因抑制了5-HT向5-HIAA的转化所致. 之前的一项研究发现,暴露于奥沙西泮(7 μg·L−1)28 d后,野生斑马鱼体内5-HT含量增加,5-HT向5- HIAA的转化受到抑制,5-HIAA/5-HT比例降低. 该比值通常用于示5-HT的周转情况,反应生物在压力下的反应情况[44]. 本研究同样发现,可待因暴露导致5-HIAA/5-HT比例下降,而且在低浓度下更加明显,而且这种下降还具有一定的持久性(图3b).
在儿茶酚胺能系统中(图2,e1—e5),低浓度可待因显著诱导L-DOPA含量,且该影响在7 d恢复期后不可逆(图2,e2). 低浓度可待因在暴露期间对DA水平有轻微诱导作用,在恢复期结束后变为显著诱导,表明有可待因对DA含量存在持续影响(图2,e3). 据报道,杂交条纹鲈鱼暴露于50 μg·L−1和更高浓度的安非他酮后,DA水平也表现为增加[5]. L-DOPA是DA的前体[45],本研究中,低浓度可待因在暴露期显著诱导了L-DOPA水平,而对DA含量无明显影响,但在恢复期后两者均被诱导,表明可待因可能抑制了L-DOPA向DA的转化,但抑制在恢复期后消失. 本研究中,低浓度可待因对L-DOPA有显著诱导作用,但这种作用在高浓度暴露下消失,呈现非线性剂量关系,这种现象在精神活性物质对鱼类的神经毒性研究中较为普遍. 如在本实验室最近的一项研究中,雄性稀有鮈鲫体内L-DOPA在低浓度(10 ng·L−1)可待因暴露后被显著抑制,但在更高浓度(100 ng·L−1和1000 ng·L−1)下这种抑制作用消失[33]. 在另一项研究中也发现,中剂量安非他酮能够显著提升杂交条纹鲈体内的Tyrs,DA及其代谢物的水平,而高剂量暴露下这种影响消失[5]. 现阶段关于精神活性物质对L-DOPA的影响还较少研究,但我们的研究结果表明这种影响可能是非线性剂量关系的,其作用机制尚不明确.
在胆碱能传递系统中(图2,f1—f5),低浓度可待因在暴露期和恢复期结束后对Bet的抑制率分别为51%和50%,有显著性且影响不可逆(图2,f4). 对该系统中的其它神经化学物质无明显影响. 与 L-DOPA相似,低浓度可待因对Bet含量有显著抑制作用,但高浓度下影响消失,结果和实验室最近的一项研究结果相似[33],由此推测精神活性物质对Bet的影响可能也呈非线性剂量关系.
对于来自其它系统的神经化学物质(图2,d1—d4),两种浓度可待因暴露均能显著诱导Met和Asp的水平,Met在两种浓度下含量分别增加133%和82%,而Asp则增加260%和 177% (图2,d 1和d3). 同时,低浓度可待因使Prol含量显著增加了92%(图2,d2). 恢复期结束后,Met的水平仍然升高,表明该影响不可逆. 而其它3种神经化学物质在恢复期与对照组相比均无显著变化.
以上研究结果表明,可待因暴露可导致可待因在鲫鱼体内的蓄积,而且这种蓄积是持久性的. 环境浓度的可待因暴露对鲫鱼幼鱼的神经传递系统产生了一定影响,能够增加或减少部分神经化学物质的含量,其中一些影响在很大程度上表现为不可逆或存在持续性影响. 这些神经化学物质的变化可能改变鲫鱼幼鱼的行为,从而影响鲫鱼的生长、繁殖、存活甚至种群结构. 因此,可待因暴露引起的包括行为学改变在内的后果将是接下来可待因对鱼类神经毒性研究的重点方向.
-
本研究表明,暴露于环境相关浓度的可待因会导致其在鲫鱼体内持久性的蓄积,并引起神经化学物质在一定时间内不可逆的变化,从而可能产生神经毒性,并影响其生命活动.
可待因对鲫鱼体内神经化学物质的影响
Effects of codeine on neurochemicals in crucian carp
-
摘要: 随着使用量的增加,可待因在水环境中被频繁检出. 然而,可待因对水生生物,尤其是鱼类的不利影响尚不清楚. 本文研究了环境浓度暴露下可待因对鲫鱼幼鱼体内神经化学物质的影响. 结果表明,可待因(5 ng·L−1和500 ng·L−1)暴露7 d后,导致可待因在鲫鱼体内的蓄积,并在7 d的恢复期内表现出持久性. 神经化学物质分析显示,鲫鱼体内去甲肾上腺素(NE)、左旋多巴(L-DOPA)、多巴胺 (DA)、5-羟色胺(5-HT)、5-羟色氨酸(5-HTP)、蛋氨酸(Met)、天冬氨酸(Asp)和脯氨酸(Prol)水平在暴露后升高,而去甲变肾上腺素(MNE)、谷氨酰胺(Gln)、谷氨酸(Glu)、5-羟基吲哚乙酸 (5-HIAA)和甜菜碱(Bet)水平则降低,并且可待因对大多数神经化学物质的影响在7 d的恢复期内具有不可逆性或持续性. 环境浓度的可待因暴露能够导致其在鲫鱼体内的蓄积和神经化学物质的变化,由此而产生的神经毒性及其后果值得进一步关注.Abstract: With the increasing application of codeine (COD), the psychoactive substance has been frequently detected in aquatic environment. However, the adverse effects of COD on aquatic organisms remain unclear, especially on fish. In this study, the effects of COD on neurochemicals were investigated in larve crucian carp at environmentally relevant concentrations. The results revealed that exposure to COD (5 and 500 ng·L−1) for 7 days resulted in a persistent accumulation in fish. Analysis on neurochemicals showed that the levels of norepinephrine (NE), 3,4-L- dihydroxyphenylalanine (L-DOPA), dopamine (DA), 5-hydroxy-L-tryptophan (5-HTP), serotonin (5-HT), L-methionine (Met), L-aspartic acid (Asp) and L-proline (Prol) increased while the levels of norepinephrine (MNE), L-glutamine (Gln), L-glutamic acid (Glu), 5-hydroxyindoleacetic acid (5-HIAA) and betaine (Bet) decreased in the fish. Most of such changes of neurochemicals were irreversible or persistent in a 7-day recovery period. The bioaccumulation of COD and alterations of neurochemcials in crucian carp exposed to COD at environmentally relevant concentrations suggest that the neurotoxicity of COD and related adverse outcomes should receive more attentions.
-
Key words:
- codeine /
- neurochemical /
- neurtoxicity /
- bioaccumulation /
- environmentally relevant concentration /
- crucian carp.
-

-
表 1 24种神经化学物及其11种同位素内标详细信息
Table 1. The detailed information of 24 neurochemicals and 11 isotope internal standards
分类
Category化合物
Compound英文全称
English name简称
Abbreviation纯度/%
Purity肾上腺素能神经传递系统 去甲肾上腺素 Norepinephrine NE 98 肾上腺素 Epinephrine E 100 去甲变肾上腺素 Normetanephrine MNE 99 谷氨酸能神经传递系统 L-谷氨酰胺 L-Glutamine Gln 99 L-谷氨酸 L-Glutamic acid Glu 99 L-缬氨酸 L-Valine Val 99 羟色胺能神经传递系统 色氨酸 Tryptophan Trp 99 5-羟基色氨酸 5-Hydroxy-L-tryptophan 5-HTP 98 5-羟色胺 Serotonin 5-HT 99 5-羟基吲哚-3-乙酸 5-Hydroxyindoleacetic acid 5-HIAA 99 儿茶酚胺能神经传递系统 酪氨酸 Tyrosine Tyrs 99 左旋多巴 3,4-L-dihydroxyphenylalanine L-DOPA 99 多巴胺 Dopamine DA 98 3-甲氧基酪胺 3-Methoxytyramine 3-MT 99 酪胺 Tyramine Tyrm 97 胆碱能神经传递系统 胆碱 Choline Cho 98 磷酸胆碱 Phosphocholine CHOP 99 乙酰胆碱 Acetylcholine ACh 98 甜菜碱 Betaine Bet 98 甘油磷酰胆碱 Glycerophosphocholine GPC 99 其他神经传递系统相关物质 L-蛋氨酸 L-Methionine Met 99 L-脯氨酸 L-Proline Prol 99 L-天冬氨酸 L-Aspartic acid Asp 98 组织胺 Histamine HSM 97 神经化学物质同位素内标 去甲变肾上腺素-d3 Normetanephrine-d3 MNE-d3 99 L-缬氨酸-13C L-Valine-13C Val-13C 99 5-羟色胺-d4 Serotonin-d4 5-HT-d4 99 5-羟基吲哚-3-乙酸-d5 5-Hydroxyindoleacetic acid-d5 5-HIAA-d5 99 酪氨酸-13C Tyrosine -13C Tyrs-13C 99 左旋多巴-d3 3,4-L-dihydroxyphenylalanine-d3 L-DOPA-d3 99 多巴胺-d4 Dopamine-d5 DA-d4 99 胆碱-d4 Choline-d4 Cho-d4 99 乙酰胆碱-d4 Acetylcholine-d4 Ach-d4 99 甜菜碱-d3 Betaine-d3 Bet-d3 99 脯氨酸-13C5,15N L-Proline-13C5,15N Prol-13C5,15N 99 -
[1] WANG Z Y, GAO S Y, DAI Q Y, et al. Occurrence and risk assessment of psychoactive substances in tap water from China [J]. Environmental Pollution, 2020, 261: 114163. doi: 10.1016/j.envpol.2020.114163 [2] YADAV M K, SHORT M D, GERBER C, et al. Removal of emerging drugs of addiction by wastewater treatment and water recycling processes and impacts on effluent-associated environmental risk [J]. Science of the Total Environment, 2019, 680: 13-22. doi: 10.1016/j.scitotenv.2019.05.068 [3] BURKINA V, ZLABEK V, ZAMARATSKAIA G. Effects of pharmaceuticals present in aquatic environment on Phase I metabolism in fish [J]. Environmental Toxicology and Pharmacology, 2015, 40(2): 430-444. doi: 10.1016/j.etap.2015.07.016 [4] PAROLINI M, PEDRIALI A, RIVA C, et al. Sub-lethal effects caused by the cocaine metabolite benzoylecgonine to the freshwater mussel Dreissena polymorpha [J]. Science of the Total Environment, 2013, 444: 43-50. doi: 10.1016/j.scitotenv.2012.11.076 [5] SWEET L E, BISESI JR J H, LEI E N Y, et al. The effects of bupropion on hybrid striped bass brain chemistry and predatory behavior [J]. Environmental Toxicology and Chemistry, 2016, 35(8): 2058-2065. doi: 10.1002/etc.3350 [6] BISESI J H Jr, BRIDGES W, KLAINE S J. Reprint of: Effects of the antidepressant venlafaxine on fish brain serotonin and predation behavior [J]. Aquatic Toxicology, 2014, 151: 88-96. doi: 10.1016/j.aquatox.2014.02.015 [7] CHEN H H, YANG H T, ZHAO Y Y, et al. Development and molecular investigation into the effects of carbamazepine exposure in the zebrafish (Danio rerio) [J]. International Journal of Environmental Research and Public Health, 2020, 17(23): 8882. doi: 10.3390/ijerph17238882 [8] SERPA B J, BULLARD J D, MENDIOLA V C, et al. D-amphetamine exposure differentially disrupts signaling across ontogeny in the zebrafish [J]. Bioelectricity, 2019, 1(2): 85-104. doi: 10.1089/bioe.2019.0006 [9] FRIEDMAN J I, ADLER D N, DAVIS K L. The role of norepinephrine in the pathophysiology of cognitive disorders: Potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer's disease [J]. Biological Psychiatry, 1999, 46(9): 1243-1252. doi: 10.1016/S0006-3223(99)00232-2 [10] TITULAER J, BJÖRKHOLM C, FELTMANN K, et al. The importance of ventral hippocampal dopamine and norepinephrine in recognition memory [J]. Frontiers in Behavioral Neuroscience, 2021, 15: 667244. doi: 10.3389/fnbeh.2021.667244 [11] FRANCIS P T. Glutamatergic approaches to the treatment of cognitive and behavioural symptoms of Alzheimer's disease [J]. Neuro-Degenerative Diseases, 2008, 5(3/4): 241-243. [12] SANACORA G, ZARATE C A , KRYSTAL J H, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders [J]. Nature Reviews Drug Discovery, 2008, 7(5): 426-437. [13] KAWASHIMA T. The role of the serotonergic system in motor control [J]. Neuroscience Research, 2018, 129: 32-39. doi: 10.1016/j.neures.2017.07.005 [14] BACQUÉ-CAZENAVE J, BHARATIYA R, BARRIÈRE G, et al. Serotonin in animal cognition and behavior [J]. International Journal of Molecular Sciences, 2020, 21(5): 1649. doi: 10.3390/ijms21051649 [15] QIN S Z, COUSIJN H, RIJPKEMA M, et al. The effect of moderate acute psychological stress on working memory-related neural activity is modulated by a genetic variation in catecholaminergic function in humans [J]. Frontiers in Integrative Neuroscience, 2012, 6: 16. [16] KUTLU M G, GOULD T J. Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders [J]. Biochemical Pharmacology, 2015, 97(4): 498-511. doi: 10.1016/j.bcp.2015.07.029 [17] KARTTUNEN P, SILVASTI M, SAANO V, et al. Effect of codeine on rat and Guinea pig tracheal ciliary beat frequency [J]. Arzneimittel-Forschung, 1991, 41(10): 1095-1097. [18] LIVINGSTONE M J, GROENEWALD C B, RABBITTS J A, et al. Codeine use among children in the United States: A nationally representative study from 1996 to 2013 [J]. Pediatric Anesthesia, 2017, 27(1): 19-27. doi: 10.1111/pan.13033 [19] TREMLETT M, ANDERSON B J, WOLF A. Pro-con debate: Is codeine a drug that still has a useful role in pediatric practice? [J]. Pediatric Anesthesia, 2010, 20(2): 183-194. doi: 10.1111/j.1460-9592.2009.03234.x [20] PRATIWI R, NOVIANA E, FAUZIATI R, et al. A review of analytical methods for codeine determination [J]. Molecules (Basel, Switzerland), 2021, 26(4): 800. doi: 10.3390/molecules26040800 [21] MUTIYAR P K, GUPTA S K, MITTAL A K. Fate of pharmaceutical active compounds (PhACs) from River Yamuna, India: An ecotoxicological risk assessment approach [J]. Ecotoxicology and Environmental Safety, 2018, 150: 297-304. doi: 10.1016/j.ecoenv.2017.12.041 [22] ZUCCATO E, CASTIGLIONI S, BAGNATI R, et al. Illicit drugs, a novel group of environmental contaminants [J]. Water Research, 2008, 42(4/5): 961-968. [23] BOLEDA M R, GALCERAN M T, VENTURA F. Monitoring of opiates, cannabinoids and their metabolites in wastewater, surface water and finished water in Catalonia, Spain [J]. Water Research, 2009, 43(4): 1126-1136. doi: 10.1016/j.watres.2008.11.056 [24] van der AA M, BIJLSMA L, EMKE E, et al. Risk assessment for drugs of abuse in the Dutch watercycle [J]. Water Research, 2013, 47(5): 1848-1857. doi: 10.1016/j.watres.2013.01.013 [25] JIANG J J, LEE C L, FANG M D, et al. Source apportionment and risk assessment of emerging contaminants: An approach of pharmaco-signature in water systems [J]. PLoS One, 2015, 10(4): e0122813. doi: 10.1371/journal.pone.0122813 [26] LIN A Y C, WANG X H, LIN C F. Impact of wastewaters and hospital effluents on the occurrence of controlled substances in surface waters [J]. Chemosphere, 2010, 81(5): 562-570. doi: 10.1016/j.chemosphere.2010.08.051 [27] HU P, GUO C S, ZHANG Y, et al. Occurrence, distribution and risk assessment of abused drugs and their metabolites in a typical urban river in North China [J]. Frontiers of Environmental Science & Engineering, 2019, 13(4): 1-11. [28] LI K Y, DU P, XU Z Q, et al. Occurrence of illicit drugs in surface waters in China [J]. Environmental Pollution, 2016, 213: 395-402. doi: 10.1016/j.envpol.2016.02.036 [29] KHAN U, van NUIJS A L N, LI J, et al. Application of a sewage-based approach to assess the use of ten illicit drugs in four Chinese megacities [J]. Science of the Total Environment, 2014, 487: 710-721. doi: 10.1016/j.scitotenv.2014.01.043 [30] SONG W T, LU G H, WANG C, et al. Study on environmental estrogen pollution in Yangtze River (Nanjing section) by an in vivo bioassay [J]. Bulletin of Environmental Contamination and Toxicology, 2010, 84(4): 406-412. doi: 10.1007/s00128-010-9944-9 [31] OVERTURF C L, OVERTURF M D, HUGGETT D B. Bioconcentration and endocrine disruption effects of diazepam in channel catfish, Ictalurus punctatus [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2016, 183/184: 46-52. [32] NKOOM M, LU G H, LIU J C, et al. Biological uptake, depuration and biochemical effects of diclofenac and carbamazepine in Carassius carassius [J]. Ecotoxicology and Environmental Safety, 2020, 205: 111106. doi: 10.1016/j.ecoenv.2020.111106 [33] GAO S Y, YANG F X. Behavioral changes and neurochemical responses in Chinese rare minnow exposed to four psychoactive substances [J]. Science of the Total Environment, 2022, 808: 152100. doi: 10.1016/j.scitotenv.2021.152100 [34] VREE T B, WISSEN C P W G M V V. Pharmacokinetics and metabolism of codeine in humans [J]. Biopharmaceutics & Drug Disposition, 1992, 13(6): 445-460. [35] YIN X X, GUO C S, DENG Y H, et al. Tissue-specific accumulation, elimination, and toxicokinetics of illicit drugs in adult zebrafish (Danio rerio) [J]. Science of the Total Environment, 2021, 792: 148153. doi: 10.1016/j.scitotenv.2021.148153 [36] HARRIS D S, BOXENBAUM H, EVERHART E T, et al. The bioavailability of intranasal and smoked methamphetamine [J]. Clinical Pharmacology & Therapeutics, 2003, 74(5): 475-486. [37] VROEGOP M P, van DONGEN R T M, VANTROYEN B, et al. Ketamine as a party drug [J]. Nederlands Tijdschrift Voor Geneeskunde, 2007, 151(37): 2039-2042. [38] GONZALEZ-LOPEZ E, VRANA K E. Dopamine beta-hydroxylase and its genetic variants in human health and disease [J]. Journal of Neurochemistry, 2020, 152(2): 157-181. doi: 10.1111/jnc.14893 [39] TELLA S H, JHA A, TAÏEB D, et al. Comprehensive review of evaluation and management of cardiac paragangliomas [J]. Heart (British Cardiac Society), 2020, 106(16): 1202-1210. [40] XU M M, YANG F X. Integrated gender-related effects of profenofos and paclobutrazol on neurotransmitters in mouse [J]. Ecotoxicology and Environmental Safety, 2020, 190: 110085. doi: 10.1016/j.ecoenv.2019.110085 [41] CHEN L, CUI H M. Targeting glutamine induces apoptosis: A cancer therapy approach [J]. International Journal of Molecular Sciences, 2015, 16(9): 22830-22855. doi: 10.3390/ijms160922830 [42] DU C K, ZHAN D Y, AKIYAMA T, et al. Myocardial interstitial serotonin and its major metabolite, 5-hydroxyindole acetic acid levels determined by microdialysis technique in rat heart [J]. Life Sciences, 2014, 117(1): 33-39. doi: 10.1016/j.lfs.2014.09.019 [43] NAKAMURA K, HASEGAWA H. Production and peripheral roles of 5-HTP, a precursor of serotonin [J]. International Journal of Tryptophan Research:IJTR, 2009, 2: 37-43. [44] VOSSEN L E, CERVENY D, ÖSTERKRANS M, et al. Chronic exposure to oxazepam pollution produces tolerance to anxiolytic effects in zebrafish (Danio rerio) [J]. Environmental Science & Technology, 2020, 54(3): 1760-1769. [45] WINBERG S, NILSSON G E. Induction of social dominance by L-dopa treatment in Arctic charr [J]. Neuroreport, 1992, 3(3): 243-246. doi: 10.1097/00001756-199203000-00006 -



 下载:
下载: