-
纳米技术的应用几乎触及了现代生活的方方面面,在消费品、化学和能源等领域,纳米材料的使用越来越广泛[1]. 在生产、运输和使用过程中,纳米材料不可避免地释放到水环境中[2],对水生生物造成潜在的危害[3—5]. 研究表明,纳米材料可以被水环境中的单细胞生物吞噬,使纳米材料从低营养向高营养水平转移[6],而纳米材料沿食物链的传递最终会威胁人类健康. 因此,这些单细胞生物被认为是纳米材料进入食物网的重要“入口”之一[7]. 例如嗜热四膜虫(四膜虫),它是一种在水环境广泛存在的处于底层营养级的水生单细胞真核原生生物[8]. 已有研究证明,四膜虫可以直接吞噬纳米和微米级颗粒[9—10],并将其传递到更高营养级的生物[11—12],为纳米材料在微生物环和经典食物网之间的传递提供了重要的联系[13]. 四膜虫还具有繁殖周期短[14]、易于在实验室中培养和保存[15]、对外源物质敏感性强[16]等优点,是良好的生态毒理学研究模式生物[17]. 研究纳米材料对四膜虫的生物效应,对理解纳米材料在水环境中进入食物网的起始环节,探讨纳米材料的环境风险有重要意义.
生物累积是研究纳米材料生物效应的重要基础,而暴露浓度是影响纳米材料生物累积的重要因素之一[18—21]. 相关研究表明纳米材料在环境中的浓度约为pg·mL−1至μg·mL−1[22—23],然而,目前大多数关于纳米材料生物累积的研究都是在比实际环境浓度高几个数量级的暴露浓度条件下进行的[24],这些研究结果不能完全准确地反映真实环境的暴露情况. 此外,单细胞生物群体中,细胞个体吸收纳米颗粒的能力有较大差异[25—26],基于群体平均值和高检出限的研究方法可能导致具有不同生理或吸收特性的稀有细胞群体被忽略[27—28]. 由于受到检测技术的限制,目前在单细胞水平研究环境相关剂量下纳米材料的生物累积特征和毒性效应还具有一定的挑战性.
质谱流式细胞术(CyTOF)是一种以电感耦合等离子体(ICP)与飞行时间质谱(TOF-MS)为检测器的检测技术,具有高通量、低检出限的特点[29],可以在单细胞水平同步进行金属定量和生理指标的检测[30]. 金纳米颗粒(AuNPs)作为一种重要的典型金属纳米颗粒,化学稳定性高,在自然环境或生物体内短时间内不易降解,常用作研究纳米颗粒生物效应的模型颗粒[31—33]. 结合CyTOF可以在单细胞水平同时检测多种金属元素和检出限低的优势[34—35],以及AuNPs稳定性高的特点,本研究以四膜虫作为研究对象,在低剂量暴露下研究了四膜虫对AuNPs的摄取与外排特征以及在单细胞水平上的异质性. 考虑到金属元素在水环境中以多种形态存在[36],本研究还比较了Au(Ⅲ)与AuNPs对四膜虫细胞的单细胞生物效应的差异.
-
本研究所用四膜虫株系为Tetrahymena thermophila(B2086),由中国科学院水生生物研究所缪伟教授赠送. 所用聚乙烯吡咯烷酮(PVP)修饰的AuNPs(直径5 nm)购自nanoComposix(美国). Au(Ⅲ)标准溶液购自国标(北京)检验认证有限公司. AuNPs储备液在4 °C下储存,并在使用前进行充分超声处理,使用灭菌的去离子水将储备液逐级稀释为工作液. Au(Ⅲ)溶液在4 °C下储存,使用前充分涡旋. 其他试剂如未注明均购自富鲁达公司(美国).
-
四膜虫培养于28 °C的灭菌培养基中,培养基包含2%蛋白胨(Becton,Dickinson and Company,美国)、0.1%酵母提取物(OXOID,Thermo Fisher Scientific,美国)、0.2%葡萄糖(Sigma-Aldrich,中国)和0.003%柠檬酸铁(Sigma-Aldrich,中国). 培养过程中,使用体积比为1%青霉素-链霉素溶液(HyClone,美国)预防细菌或真菌感染,并在135 r·min−1振荡条件下培养.
-
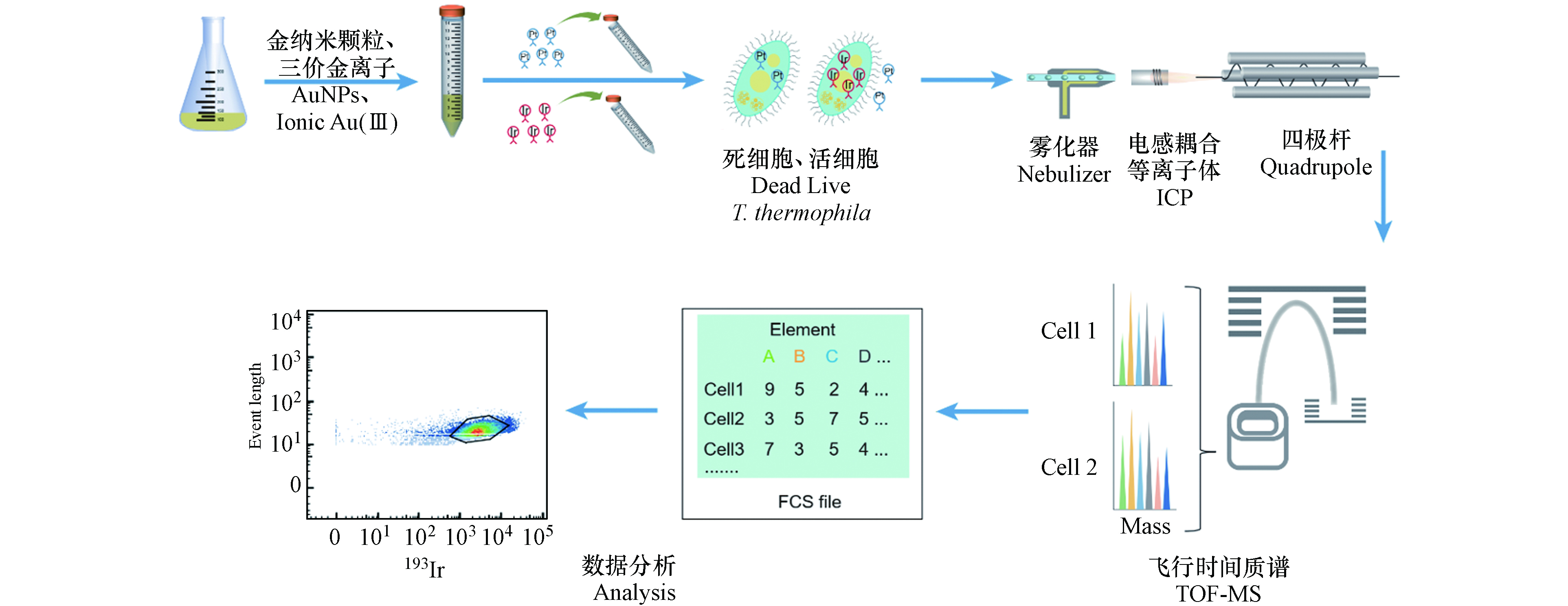
四膜虫复苏后在培养基中培养至细胞密度约1.2×105 cells·mL−1,取2—5 mL四膜虫悬浮液,分别暴露于直径为5 nm的AuNPs和Au(Ⅲ)溶液中,暴露浓度为1 ng·mL−1,分别于0 、0.5 、6 、24 、48 h后收集细胞. 暴露0 h即未暴露于AuNPs和Au(Ⅲ)的对照组,每组实验设置3个平行. 暴露48 h后进行离心(300 g,5 min,4 ℃)洗涤3次去除暴露液,将洗涤后的四膜虫在不含AuNPs和Au(Ⅲ)的培养基中培养,并分别在60 h、72 h和 96 h收集细胞.
暴露结束后,离心收集细胞(300 g,10 min,4 ℃),在相同的离心条件下使用PBS缓冲液洗涤3次,去除残余的暴露液或培养基. 洗涤后的四膜虫细胞重悬于1 mL PBS缓冲液并将细胞密度调整为1×106—3×106 cells·mL−1. 在样品中加入终浓度为2.5 μmol·L−1顺-二氯二氨合铂(cisPt,作为细胞膜通透性指示剂),室温孵育5 min,而后加入1 mL细胞染色缓冲液(CSB)终止cisPt与细胞的反应,最后离心去除上清液(500 g,5 min,4 ℃). 使用细胞固定和透膜缓冲液配制浓度为125 nmol·L−1的Ir工作液(含铱嵌入剂,含有191Ir和193Ir),Ir工作液可以标记核酸,因此根据193Ir信号可以识别细胞. 边涡旋样品边加入1 mL Ir工作液,使其与细胞混匀,然后将样品置于4 ℃冰箱过夜,使细胞的核酸被Ir标记. Ir标记后的样品加入1 mL CSB洗涤1次,再用超纯水洗涤3次以上,以去除未反应的Ir,将四膜虫重悬至超纯水中并调整细胞密度为6×105—1×106 cells·mL−1后,再进行后续的检测分析.
-
在样品上机检测前加入体积比为10%—20%的四元素校准微珠,四元素校准微珠包含多种已知金属同位素(140Ce、142Ce、151Eu、153Eu、165Ho、175Lu和176Lu),用于校正CyTOF的检测结果. 样品检测时收集197Au、193Ir和195Pt元素信号以及四元素校准微珠包含的元素信号,其中193Ir信号用于判断金属信号是否来自于四膜虫细胞,195Pt信号用于指示四膜虫细胞膜的通透性.
CyTOF主要由流动上样和雾化模块、ICP分析模块、TOF-MS模块、计算机与分析模块组成. 具体检测流程如下:样品以单细胞液滴形式雾化后进入ICP,在氩等离子体内被离子化形成离子云,通过四极杆筛选出一定的质荷比的离子,过滤其他离子;然后通过TOF-MS检测被电场加速后的离子到达检测器的时间[37],这些时间序列信号首先被还原为元素的质量数,然后被转换为细胞表面或内部的信号分子数据,数据可以通过多种软件进行分析[35](图1).
-
CyTOF检测所得数据使用flowjo 13.0软件进行分析. 高分辨率透射电子显微镜(HRTEM,JEM-2100F,日本)检测所得图像由image j软件进行处理和统计分析. 所有获得的数据均为3个重复实验所得,并由平均值±标准差表示. 使用SPSS Statistics 26和Origin 2021软件进行统计分析. 采用独立样本t检验和单因素方差分析(ANOVA)对各组数据均值进行差异性检验,当P < 0.05时具有显著性差异。
-
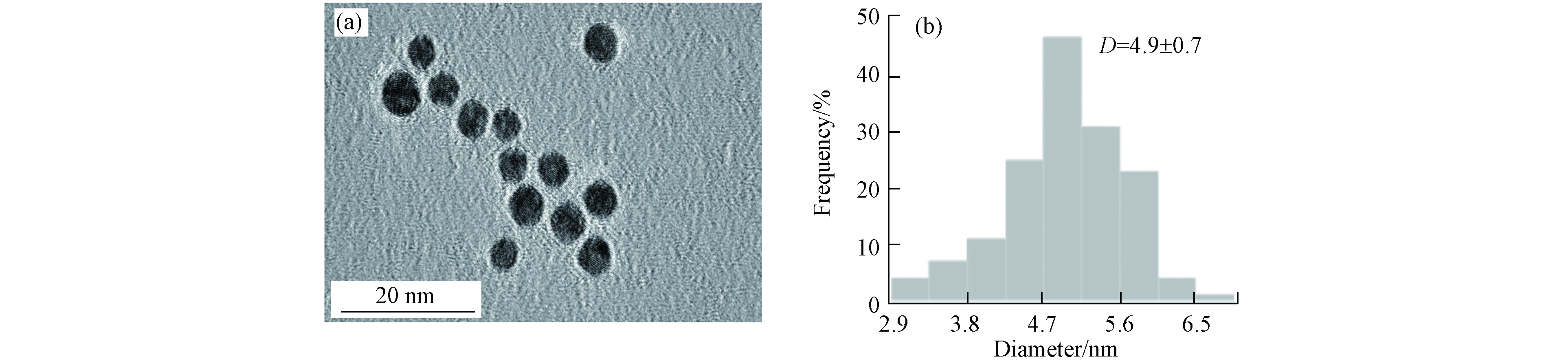
本实验采用了PVP修饰的AuNPs,高分辨率透射电子显微镜(HRTEM)表征结果如图2(a),AuNPs均为较规则的球状且分散性良好,粒径大小集中在(4.9 ± 0.7)nm左右(图2(b)).
-
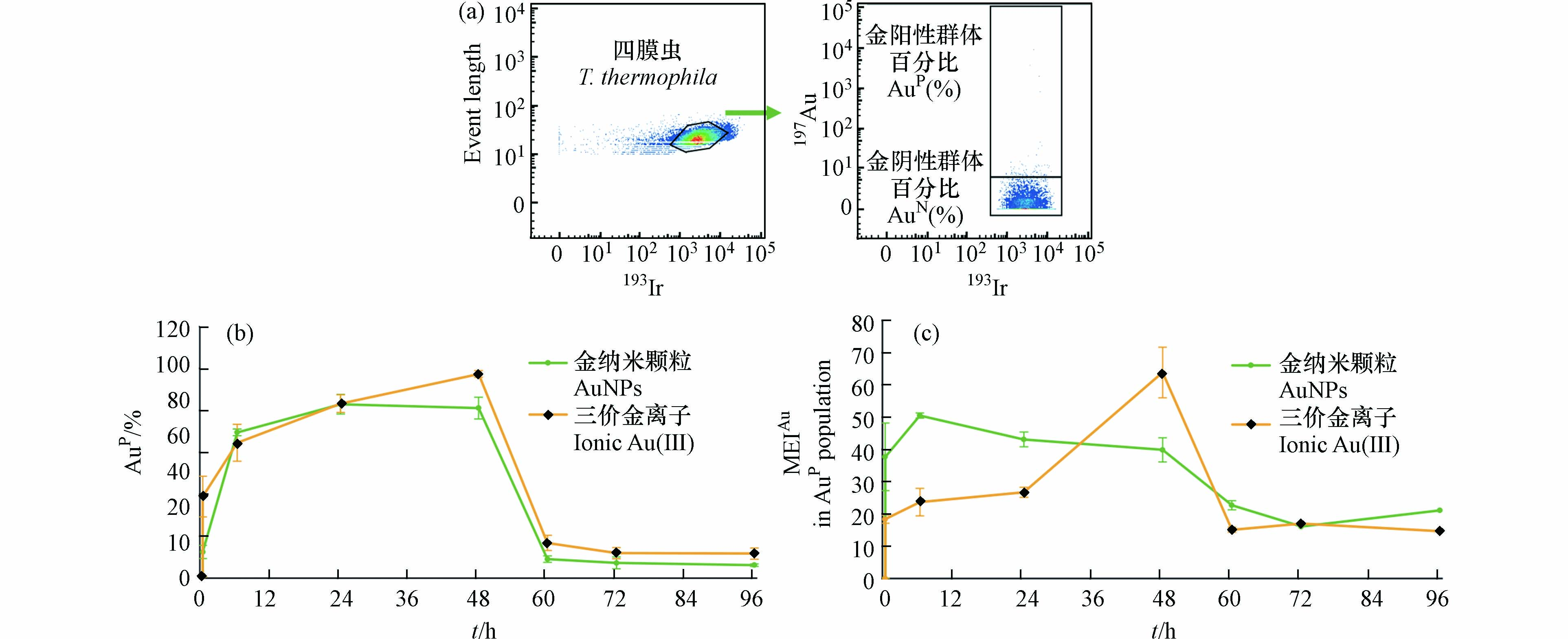
顺铂(195Pt)是一种广泛使用的铂基化学治疗剂[38],可以进入膜稳定性差的细胞,与蛋白质亲核试剂如R-SH或RS-CH3快速反应,形成共价铂-硫或巯基键,从而在细胞内非特异性标记蛋白质,而不易进入膜完整的细胞[39—40]. 因此,195Pt 的信号强弱可用于评估细胞的膜完整性.本文在检测四膜虫细胞内 Au 含量的同时也监测了195Pt 的信号,以分析 AuNPs 及 Au(Ⅲ)对四膜虫细胞膜通透性的影响(图3)。
如图3(a)所示,使用flowjo软件对数据进行初步的圈门分析,胞膜通透的四膜虫细胞吸收了较多的Pt,EIPt值均较高,并以EIPt值102为界与正常细胞形成明显的细胞分群,因此将EIPt > 102的个体定义为195Pt阳性(PtP),代表细胞膜通透的细胞(死细胞群,PtP),EIPt ≤ 102的个体定义为195Pt阴性(PtN),代表细胞膜正常的细胞(活细胞群,PtN).
结果如图3(b)所示,暴露组中正常状态的四膜虫占比均大于90%,与对照组(0 h)相比无显著性差异(P > 0.05),说明1 ng·mL−1的暴露剂量下AuNPs和Au(Ⅲ)对四膜虫的细胞膜通透性都没有明显的影响. 这与先前报道的低剂量暴露下AuNPs对四膜虫的膜通透性没有明显影响的结果类似[34].
-
通过对持续暴露阶段(0—48 h)和撤除暴露阶段(48—96 h),四膜虫群体中AuP细胞所占比例(%AuP)以及四膜虫个体的平均Au元素强度(MEIAu)变化的分析,研究了四膜虫摄取和外排AuNPs和Au(Ⅲ)的差异. 如图4(a)所示,在对照组中99.5%的四膜虫细胞EIAu ≤ 6.0,0.5%的四膜虫细胞EIAu > 6.0,因此将EIAu > 6.0的四膜虫个体定义为阳性细胞(AuP),将EIAu ≤ 6.0定义为阴性细胞(AuN).
研究结果表明,随着暴露时间增加,四膜虫的MEIAu和%AuP均升高. 在暴露24 h后,四膜虫摄取AuNPs的%AuP达到最大值(82.93%),MEIAu也达到最大值(40.70),而四膜虫摄取Au(Ⅲ)的%AuP和MEIAu在48 h撤除暴露时仍处于上升趋势,说明与AuNPs相比四膜虫累积Au(Ⅲ)有一定的滞后性(图4(b),(c)). 与之类似,有相关研究通过对淡水藻类进行AuNPs和Au(Ⅲ)的暴露实验证明了藻类细胞摄取AuNPs比摄取Au(Ⅲ)的速度更快[26].
暴露24 h后,AuNPs组中四膜虫内的MEIAu开始有所下降(图4(c)),Au(Ⅲ)组的MEIAu仍然呈上升趋势,这可能是因为四膜虫累积AuNPs是吸收和外排AuNPs的动态过程,部分已经吸收AuNPs达到饱和的四膜虫对AuNPs的外排大于吸收而导致整体MEIAu有所降低. 撤除暴露后(48 h后)AuNPs和Au(Ⅲ)组中四膜虫个体内的Au都快速降低,AuNPs组四膜虫个体内的MEIAu降低了89%,Au(Ⅲ)组下降94%,表明撤除暴露后四膜虫个体外排Au(Ⅲ)的速率高于AuNPs. 撤除暴露后四膜虫可以快速排出AuNPs和Au(Ⅲ),这可能是由于四膜虫具有胞肛,四膜虫的食物泡中没有消化的内容物可以通过胞肛被迅速排出[41—42]. 在72 h后,四膜虫内的Au趋于稳定,表明尽管四膜虫可以很快排出AuNPs和Au(Ⅲ),但是仍然有AuNPs和Au(Ⅲ)不能被排出,有一定的环境风险.
-
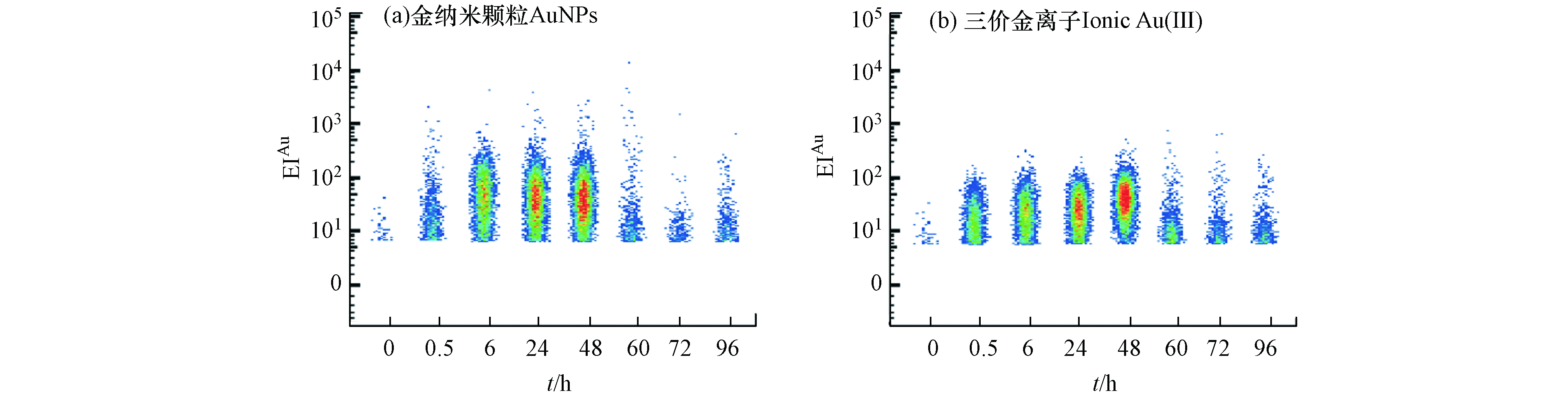
单细胞生物体之间存在显著的异质性,群体细胞的研究结果只能得到细胞群体的平均值,通常会掩盖个体间的差异信息[43]。研究了暴露0 、0.5 、6、24、48 h后,单个四膜虫细胞累积AuNPs和Au(Ⅲ)的Au信号强度分布(EIAu)。结果表明,四膜虫摄取AuNPs和Au(Ⅲ)都有较高的异质性,如图5所示,0—48 h的实验过程中,四膜虫个体累积的Au信号强度体现出较大的跨度范围,因此以往的检测手段所得的单个细胞中的平均含量可能并不能代表整体的结果。将EIAu > 6.0的四膜虫个体定义为AuP,由表1可知,撤除暴露(0—48 h)前,单个四膜虫摄取AuNPs的EIAu范围分别为6—107.08、6—247.58、6—361.54和6—271.46,而单个四膜虫摄取Au(Ⅲ)的EIAu范围分别为6—66.15、6—94.73、6—102.98和6—214.10,进一步表明四膜虫摄取AuNPs和Au(Ⅲ)均具有较高的异质性,且四膜虫个体摄取AuNPs的信号跨度范围比Au(Ⅲ)组更大,表现出更高的异质性特点。与之类似,有研究发现淡水藻类(Cyptomonas ovate)在摄取AuNPs和Au(Ⅲ)时,只有部分C. ovate细胞摄取了AuNPs和Au(Ⅲ),而有一些C. ovate细胞没有摄取AuNPs和Au(Ⅲ),表明淡水藻类细胞个体之间累积AuNPs和Au(Ⅲ)也存在较大差异[26]。
在0—48 h持续暴露过程中,如表1所示,AuNPs暴露组单个四膜虫可以达到的最大EIAu的范围为107.08—361.54,Au(Ⅲ)暴露组单个四膜虫可以达到的最大EIAu的范围为66.15—214.10,在相同的暴露剂量下,AuNPs组中单个四膜虫累积EIAu的最大值始终高于Au(Ⅲ)组,这可能是四膜虫摄取两种不同形态Au的途径不同导致的,其机制需要进一步研究.
撤除暴露后(60—96 h),如表1所示,单个四膜虫摄取AuNPs的EIAu范围分别为6—76.90、6—30.91和6—38.74,单个四膜虫摄取Au(Ⅲ) 的EIAu范围分别为6—65.09、6—63.23和6—46.00,表明四膜虫在外排AuNPs和Au(Ⅲ) 的过程中具有较高的异质性,四膜虫外排AuNPs和Au(Ⅲ)体现出的异质性也说明有一些小部分个体(分别为6.72%、11.83%)能够累积较多的AuNPs和Au(Ⅲ),这些吞噬能力较强的“少数个体”有可能进入食物链/网对高营养级生物产生影响. 因此,当环境受到低浓度AuNPs的污染时,这些对AuNPs吞噬能力较高的个体能够累积较多的AuNPs,成为“危险载体”. 低剂量的AuNPs的暴露极有可能通过这些“危险载体”进而影响整个种群.
四膜虫群体中存在“少数个体”对AuNPs具有较强的吞噬能力,在吞噬其他典型纳米材料时是否存在这些“少数个体”则需要进一步的实验验证. 同时这些吞噬能力较强的“少数个体”对AuNPs的高摄取能力的机制尚不明确,可以结合CyTOF在单细胞水平同时检测多种金属元素的优势,利用合适的稳定同位素作为特定生物分子的标记物,进一步研究这些“少数群体”在单细胞水平的作用机制.
-
本文利用质谱流式技术在低剂量暴露条件下,研究了嗜热四膜虫个体对AuNPs和Au(Ⅲ) 的摄取与外排特征,评估了AuNPs和Au(Ⅲ) 对四膜虫的毒性效应以及四膜虫对AuNPs和Au(Ⅲ) 在单细胞水平的累积特征. 研究发现AuNPs和Au(Ⅲ) 对四膜虫的细胞膜通透性均没有明显的影响,与Au(Ⅲ) 相比四膜虫能更快速的累积AuNPs,并且四膜虫累积AuNPs和Au(Ⅲ) 具有较高的异质性,少数四膜虫个体可以累积较多的AuNPs和Au(Ⅲ). 本文的研究结果为低剂量暴露下利用质谱流式技术研究单细胞生物对金属纳米颗粒和金属离子的摄取与外排特征及单细胞累积异质性研究提供了基础经验.
四膜虫摄取与外排AuNPs和Au(Ⅲ)的差异及单细胞异质性分析
Uptake and efflux of AuNPs and ionic Au(Ⅲ) by Tetrahymena thermophila and the single-cell heterogeneity
-
摘要: 纳米材料的广泛应用导致其不可避免地对水环境造成污染. 单细胞水生生物体内累积的纳米材料可能会沿着食物链/网向上传递,造成一定的环境风险. 为了探究真实环境中的金属纳米颗粒及其溶解态金属离子对水生生物的影响,本文研究了在低剂量暴露下,单细胞水生生物嗜热四膜虫(四膜虫)对金纳米颗粒(AuNPs)和三价金离子(Au(Ⅲ))的累积特征. 结果表明,1 ng·mL−1暴露剂量下AuNPs和Au(Ⅲ) 对四膜虫的膜通透性未产生明显影响;在持续暴露实验中,发现四膜虫累积Au(Ⅲ)与AuNPs相比有一定的滞后性;撤除暴露(48 h)后,四膜虫细胞内的AuNPs和Au(Ⅲ)会快速外排,但仍有部分AuNPs和Au(Ⅲ)未被排出. 在整个实验过程中,四膜虫累积AuNPs和Au(Ⅲ)在单细胞水平具有较高的异质性,有一小部分个体(分别为6.72%和11.83%)能够累积较多的AuNPs和Au(Ⅲ),而这小部分个体有可能进入食物链/网对高营养级生物产生影响. 借助质谱流式技术能够在单细胞水平同时检测多个金属元素的优势,可以通过适当的金属同位素生物分子标记物,进一步探究部分四膜虫细胞个体Au的异质性累积的分子机制.Abstract: The wide application of nanomaterials inevitably results in the environmental water contamination. Nanoparticles accumulated in unicellular aquatic organisms could be transported to higher trophic levels through the food chain/web, causing certain environmental risks. To explore the effects of metal nanoparticles and metal ions on aquatic organisms in real environments, we investigated the characteristics of gold nanoparticles(AuNPs)and ionic Au(Ⅲ) accumulation by the unicellular aquatic organism Tetrahymena thermophila under low-dose exposure. The results showed that AuNPs and ionic Au(Ⅲ) had no significant effect on the membrane permeability of T. thermophila at 1 ng·mL−1. In the exposure experiment, we found that the accumulation of ionic Au(Ⅲ) by T. thermophila was slower than that of AuNPs. After the exposure was removed(48 h), AuNPs and ionic Au(Ⅲ) in T. thermophila were rapidly excreted, however, some AuNPs and ionic Au(Ⅲ) could not be eliminated. In this study, the heterogeneity of the accumulation of AuNPs and ionic Au(Ⅲ) in T. thermophila was revealed at the single-cell level. A few T. thermophila individuals(6.72% and 11.83%, respectively)could accumulate more AuNPs and ionic Au(Ⅲ) than other T. thermophila cells, and through these individuals, Au may affect the organisms at the higher trophic level. Since CyTOF allows multielement detection of a single cell, the mechanisms underlying different abilities of uptake of Au by T. thermophila cells could be further studied by utilizing proper stable metal isotope bio-markers.
-
Key words:
- single-cell analysis /
- bioaccumulation /
- metal nanoparticles /
- mass cytometry /
- cellular heterogeneity.
-

-
图 3 (a)细胞膜正常的四膜虫(PtN)与细胞膜通透的四膜虫(PtP)圈门示意图;(b)不同暴露时间点细胞膜完整的四膜虫比例
Figure 3. (a)Gating scheme of T. thermophila with intact cell membrane(Living cells,PtN)and T. thermophila with permeable cell membrane(Dead cells,PtP);(b)Proportion of T. thermophila cells with intact cell membranes at different exposure times
表 1 不同暴露时间四膜虫细胞中EIAu的范围和平均值
Table 1. Range and mean value of EIAu in T. thermophila cells at different exposure times
暴露时间
Time金纳米颗粒暴露组
AuNPs exposure group三价金离子暴露组
Ionic Au(Ⅲ) exposure group0.5 h 6.0—107.08(4.81) 6.0—66.15(7.79) 6 h 6.0—247.58(35.37) 6.0—94.73(15.97) 24 h 6.0—361.54(40.70) 6.0—102.98(22.60) 48 h 6.0—271.46(32.77) 6.0—214.10(54.93) 60 h 6.0—76.90(3.62) 6.0—65.09(3.28) 72 h 6.0—30.91(1.52) 6.0—63.23(2.67) 96 h 6.0—38.74(1.68) 6.0—46.00(2.31) -
[1] HANSEN S F, HANSEN O F H, NIELSEN M B. Advances and challenges towards consumerization of nanomaterials [J]. Nature Nanotechnology, 2020, 15(12): 964-965. doi: 10.1038/s41565-020-00819-7 [2] WIGGER H, KÄGI R, WIESNER M, et al. Exposure and possible risks of engineered nanomaterials in the environment—current knowledge and directions for the future [J]. Reviews of Geophysics, 2020, 58(4): e2020RG000710. [3] BATLEY G E, KIRBY J K, MCLAUGHLIN M J. Fate and risks of nanomaterials in aquatic and terrestrial environments [J]. Accounts of Chemical Research, 2013, 46(3): 854-862. doi: 10.1021/ar2003368 [4] ABBAS Q, YOUSAF B, AMINA, et al. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review [J]. Environment International, 2020, 138: 105646. doi: 10.1016/j.envint.2020.105646 [5] MAHANA A, GULIY O I, MEHTA S K. Accumulation and cellular toxicity of engineered metallic nanoparticle in freshwater microalgae: Current status and future challenges [J]. Ecotoxicology and Environmental Safety, 2021, 208: 111662. doi: 10.1016/j.ecoenv.2020.111662 [6] YANG W W, WANG Y, HUANG B, et al. TiO2 nanoparticles act as a carrier of Cd bioaccumulation in the ciliate Tetrahymena thermophila [J]. Environmental Science & Technology, 2014, 48(13): 7568-7575. [7] KAHRU A, DUBOURGUIER H C. From ecotoxicology to nanoecotoxicology [J]. Toxicology, 2010, 269(2/3): 105-119. [8] ZINGEL P, NÕGES T. Seasonal and annual population dynamics of ciliates in a shallow eutrophic lake [J]. Fundamental and Applied Limnology, 2010, 176(2): 133-143. doi: 10.1127/1863-9135/2010/0176-0133 [9] WU Q, YAO L L, ZHAO X C, et al. Cellular uptake of few-layered black phosphorus and the toxicity to an aquatic unicellular organism [J]. Environmental Science & Technology, 2020, 54(3): 1583-1592. [10] WERLIN R, PRIESTER J H, MIELKE R E, et al. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain [J]. Nature Nanotechnology, 2011, 6(1): 65-71. doi: 10.1038/nnano.2010.251 [11] MAURYA R, PANDEY A K. Importance of protozoa Tetrahymena in toxicological studies: A review [J]. Science of the Total Environment, 2020, 741: 140058. doi: 10.1016/j.scitotenv.2020.140058 [12] MORTIMER M, PETERSEN E J, BUCHHOLZ B A, et al. Bioaccumulation of multiwall carbon nanotubes in Tetrahymena thermophila by direct feeding or trophic transfer [J]. Environmental Science & Technology, 2016, 50(16): 8876-8885. [13] MORTIMER M, GOGOS A, BARTOLOMÉ N, et al. Potential of hyperspectral imaging microscopy for semi-quantitative analysis of nanoparticle uptake by protozoa [J]. Environmental Science & Technology, 2014, 48(15): 8760-8767. [14] LYNN D H, DOERDER F P. The life and times of Tetrahymena [J]. Methods in Cell Biology, 2012, 109: 9-27. [15] CASSIDY-HANLEY D M. Tetrahymena in the laboratory: Strain resources, methods for culture, maintenance, and storage [J]. Methods in Cell Biology, 2012, 109: 237-276. [16] GOMIERO A, DAGNINO A, NASCI C, et al. The use of protozoa in ecotoxicology: Application of multiple endpoint tests of the ciliate E. crassus for the evaluation of sediment quality in coastal marine ecosystems [J]. Science of the Total Environment, 2013, 442: 534-544. doi: 10.1016/j.scitotenv.2012.10.023 [17] 傅诚杰, 俞婷, 缪炜, 等. 四膜虫: 毒理学与生态毒理学研究中的优良模式生物 [J]. 动物学杂志, 2005, 40(1): 108-113. doi: 10.3969/j.issn.0250-3263.2005.01.021 FU C J, YU T, MIAO W, et al. Tetrahymena: a good model organism for toxicology and ecotoxicology [J]. Chinese Journal of Zoology, 2005, 40(1): 108-113(in Chinese). doi: 10.3969/j.issn.0250-3263.2005.01.021
[18] GARCÍA-TORRA V, CANO A, ESPINA M, et al. State of the art on toxicological mechanisms of metal and metal oxide nanoparticles and strategies to reduce toxicological risks [J]. Toxics, 2021, 9(8): 195. doi: 10.3390/toxics9080195 [19] ASHRAF S, HASSAN SAID A, HARTMANN R, et al. Quantitative particle uptake by cells as analyzed by different methods [J]. Angewandte Chemie (International Ed. in English), 2020, 59(14): 5438-5453. doi: 10.1002/anie.201906303 [20] ROMA J, MATOS A R, VINAGRE C, et al. Engineered metal nanoparticles in the marine environment: A review of the effects on marine fauna [J]. Marine Environmental Research, 2020, 161: 105110. doi: 10.1016/j.marenvres.2020.105110 [21] QU G B, XIA T, ZHOU W H, et al. Property-activity relationship of black phosphorus at the nano-bio interface: From molecules to organisms [J]. Chemical Reviews, 2020, 120(4): 2288-2346. doi: 10.1021/acs.chemrev.9b00445 [22] WESTERHOFF P, ATKINSON A, FORTNER J, et al. Low risk posed by engineered and incidental nanoparticles in drinking water [J]. Nature Nanotechnology, 2018, 13(8): 661-669. doi: 10.1038/s41565-018-0217-9 [23] PETERS R J B, van BEMMEL G, MILANI N B L, et al. Detection of nanoparticles in Dutch surface waters [J]. Science of the Total Environment, 2018, 621: 210-218. doi: 10.1016/j.scitotenv.2017.11.238 [24] HOLDEN P A, KLAESSIG F, TURCO R F, et al. Evaluation of exposure concentrations used in assessing manufactured nanomaterial environmental hazards: Are they relevant? [J]. Environmental Science & Technology, 2014, 48(18): 10541-10551. [25] SHI J B, JI X M, WU Q, et al. Tracking mercury in individual Tetrahymena using a capillary single-cell inductively coupled plasma mass spectrometry online system [J]. Analytical Chemistry, 2020, 92(1): 622-627. doi: 10.1021/acs.analchem.9b03719 [26] MERRIFIELD R C, STEPHAN C, LEAD J R. Quantification of Au nanoparticle biouptake and distribution to freshwater algae using single cell - ICP-MS [J]. Environmental Science & Technology, 2018, 52(4): 2271-2277. [27] IVASK A, MITCHELL A J, HOPE C M, et al. Single cell level quantification of nanoparticle-cell interactions using mass cytometry [J]. Analytical Chemistry, 2017, 89(16): 8228-8232. doi: 10.1021/acs.analchem.7b01006 [28] HSIAO I L, BIERKANDT F S, REICHARDT P, et al. Quantification and visualization of cellular uptake of TiO2 and Ag nanoparticles: Comparison of different ICP-MS techniques [J]. Journal of Nanobiotechnology, 2016, 14(1): 50. doi: 10.1186/s12951-016-0203-z [29] 谭思源, 李曼莉, 傅博强, 等. 单细胞质谱分析方法研究进展 [J]. 计量科学与技术, 2021, 65(5): 20-29,13. doi: 10.12338/j.issn.2096-9015.2020.9021 TAN S Y, LI M L, FU B Q, et al. Recent advances in single-cell mass spectrometry methods [J]. Metrology Science and Technology, 2021, 65(5): 20-29,13(in Chinese). doi: 10.12338/j.issn.2096-9015.2020.9021
[30] NEWELL E W, CHENG Y. Mass cytometry: Blessed with the curse of dimensionality [J]. Nature Immunology, 2016, 17(8): 890-895. doi: 10.1038/ni.3485 [31] ZHANG L, LI Y, LI D W, et al. Single gold nanoparticles as real-time optical probes for the detection of NADH-dependent intracellular metabolic enzymatic pathways [J]. Angewandte Chemie International Edition, 2011, 50(30): 6789-6792. doi: 10.1002/anie.201102151 [32] DREADEN E C, ALKILANY A M, HUANG X H, et al. The golden age: Gold nanoparticles for biomedicine [J]. Chemical Society Reviews, 2012, 41(7): 2740-2779. doi: 10.1039/C1CS15237H [33] WANG M, ZHENG L N, WANG B, et al. Quantitative analysis of gold nanoparticles in single cells by laser ablation inductively coupled plasma-mass spectrometry [J]. Analytical Chemistry, 2014, 86(20): 10252-10256. doi: 10.1021/ac502438n [34] WU Q, SHI J B, JI X M, et al. Heterogenous internalization of nanoparticles at ultra-trace concentration in environmental individual unicellular organisms unveiled by single-cell mass cytometry [J]. ACS Nano, 2020, 14(10): 12828-12839. doi: 10.1021/acsnano.0c03587 [35] SPITZER M H, NOLAN G P. Mass cytometry: Single cells, many features [J]. Cell, 2016, 165(4): 780-791. doi: 10.1016/j.cell.2016.04.019 [36] CAMPBELL P G C, ERRÉCALDE O, FORTIN C, et al. Metal bioavailability to phytoplankton—applicability of the biotic ligand model [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2002, 133(1/2): 189-206. [37] BENDALL S C, NOLAN G P, ROEDERER M, et al. A deep profiler's guide to cytometry [J]. Trends in Immunology, 2012, 33(7): 323-332. doi: 10.1016/j.it.2012.02.010 [38] WANG D, LIPPARD S J. Cellular processing of platinum anticancer drugs [J]. Nature Reviews Drug Discovery, 2005, 4(4): 307-320. doi: 10.1038/nrd1691 [39] GUO Y T, BAUMGART S, STÄRK H J, et al. Mass cytometry for detection of silver at the bacterial single cell level [J]. Frontiers in Microbiology, 2017, 8: 1326. doi: 10.3389/fmicb.2017.01326 [40] SIDDIK Z H. Cisplatin: mode of cytotoxic action and molecular basis of resistance [J]. Oncogene, 2003, 22(47): 7265-7279. doi: 10.1038/sj.onc.1206933 [41] FOISSNER W. The cytopyge of Ciliata. I. its function, regeneration and morphogenesis in Uronema parduczi [J]. Acta Biologica Academiae Scientiarum Hungaricae, 1972, 23(2): 161-174. [42] FOISSNER W. SCHIFFMANN H. The cytopyge of Ciliata. IV. An experimental study of the ingestion, digestion and defaecation in Oxytricha Fallax [J]. Acta Biologica Academiae Scientiarum Hungaricae, 1974, 25(1/2): 61-74. [43] 赵苑, 董逸, 李海波, 等. 流式细胞术检测海洋浮游异养细菌异质性的研究进展 [J]. 海洋通报, 2020, 39(1): 12-23. ZHAO Y, DONG Y, LI H B, et al. Composition heterogeneity of marine heterotrophic bacterioplankton analyzed by flow cytometry [J]. Marine Science Bulletin, 2020, 39(1): 12-23(in Chinese).
-



 下载:
下载: