-
随着我国工业化水平以及人民生活水平的日益提高,恶臭气体带来的污染逐渐引起了全社会的重视. 我国《恶臭污染物排放标准》(GB 14554-93)中限制排放的恶臭污染物质包括硫化氢、甲硫醚、二甲二硫、甲硫醇、氨、二硫化碳、苯乙烯和三甲胺. 其中甲硫醇来源广泛,污水处理废气[1-2]、污泥处置废气[2-3]、垃圾填埋场废气[4-5]、制药厂废气[6]、造纸厂废气[7]、餐厨垃圾废气[8-9]、炼油废气[10-11]等主要恶臭成分中均含有甲硫醇. 其中,污水处理厂、垃圾填埋场、餐厨垃圾废气中的甲硫醇主要是由于蛋氨酸、甲硫氨酸、胱氨酸、半胱氨酸等含硫氨基酸的降解、巯基的转移以及含硫化合物的甲基化产生的. 甲硫醇是臭味最强的气体之一,其阈值仅为0.045 mg·m−3,且具有毒性和腐蚀性[12-13]. 此外,甲硫醇还属于含硫挥发性有机物,不但具有恶臭污染物的特征,而且具有挥发性有机物的特征[14],能够参与雾霾、光化学烟雾、二次气溶胶等环境污染事件的形成[15]. 因此,甲硫醇气体污染物的治理对我国经济高质量发展以及优美人居环境创建具有重要意义.
目前,广泛研究的甲硫醇治理技术主要包括吸附法[16-17]、生物法[18-19]、氧化法[12, 20]、等离子体法[21-22]、光催化法[23-24]、催化氧化法[13-14]和催化降解法[25-27]等. 其中氧化法和催化氧化法受到了人们的广泛关注,但由于甲硫醇氧化降解过程中生成的含硫化合物易使催化剂失活[13],实际应用过程中氧化法因其设备简单(喷淋洗涤塔、接触反应塔等)、氧化剂选择性多(臭氧、过氧化氢、次氯酸钠、高锰酸钾等)等特点而使用较为普遍,其中臭氧氧化法具有臭氧制备方便、均相反应效率高、脱臭效果好等优点而被广泛应用[28]. 虽然臭氧对甲硫醇臭气具有较好的脱臭效果,但关于臭氧投加剂量、反应条件等因素对甲硫醇去除效果的影响以及甲硫醇的臭氧氧化降解产物的研究鲜见报道. 因此,本文通过对臭氧投加量、接触反应时间、反应温度、湿度等因素的考察,并结合原位红外光谱、气相色谱-质谱联用和离子色谱技术研究了臭氧对甲硫醇的氧化降解性能及主要降解产物,以期为臭氧氧化法在甲硫醇脱臭净化领域的应用提供技术参考.
-
甲硫醇标准气体(214.3 mg·m−3,大连大特气体有限公司);高纯氮气(纯度99.999%,郑州泽中科技有限公司);臭氧发生器(HK-10,郑州中仁臭氧技术服务有限公司);制氧机(IRC5PO2AW,Invacare,美国);加热磁力搅拌器(MR Hei-Tec,Heidolph,德国);所用其他试剂均为分析纯.
傅里叶变换红外光谱仪(Invenio S,Bruker,德国);气相色谱仪(GC2010,Shimadzu,日本);气体进样器(Acrichi OGS-11,北京聚芯追风科技有限公司);烟气分析仪(Testo 350,Testo,德国);总有机碳/总氮分析仪(Multi N/C 2100S,Analytikjena,德国);大气采样预浓缩进样系统(Entech7200,Entech,美国);气相色谱-质谱联用仪(7890B-7000D,Agilent,美国);臭氧浓度分析仪(UVOZ-1200:0—200 mg·L−1, 山东智普测控技术有限公司);质量流量控制器(CS200,北京七星华创电子股份有限公司);离子色谱仪(930,Metrohm,瑞士).
-
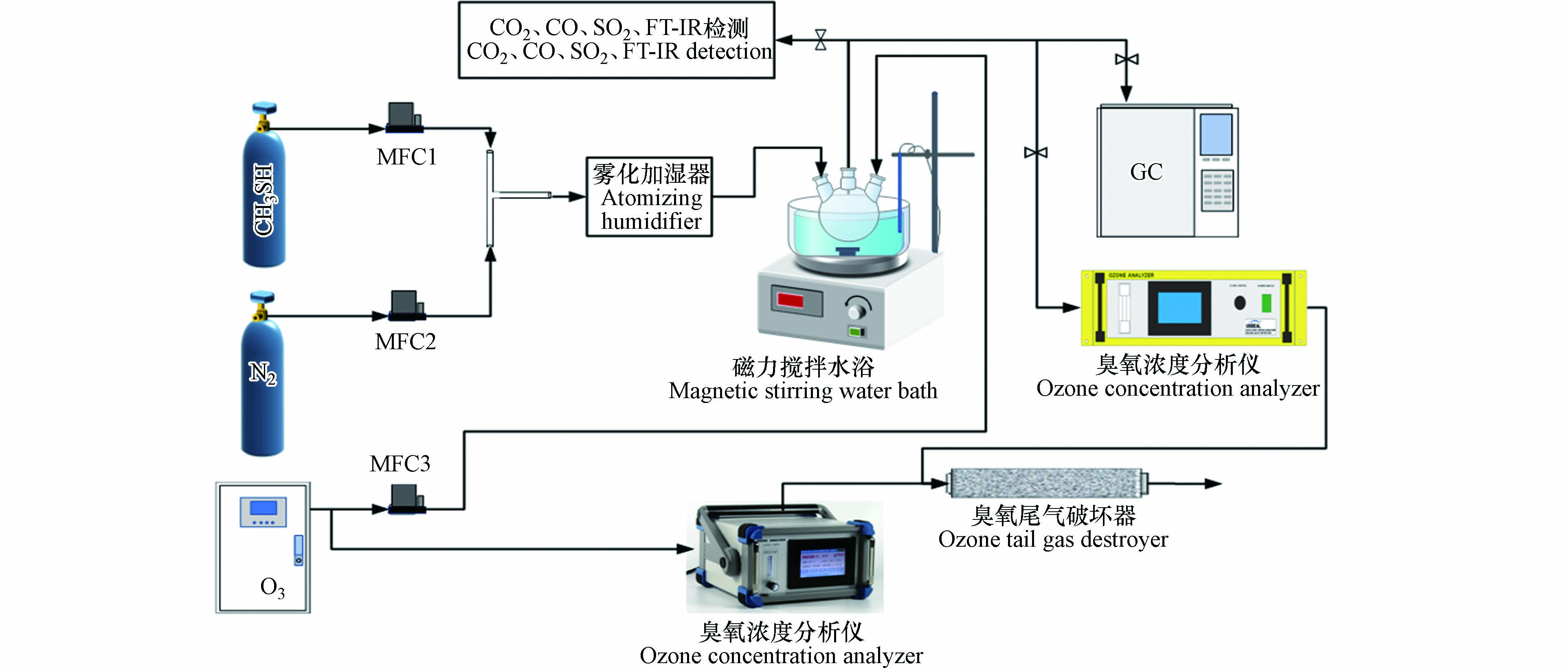
为了研究臭氧对甲硫醇的氧化降解性能,设计实验装置及工艺流程如图1所示. 根据所需甲硫醇和臭氧浓度计算甲硫醇、高纯N2和臭氧流量并通过质量流量控制器(MFC)进行调节控制,然后将磁力搅拌水浴调至需要的温度,臭氧与甲硫醇分别从两侧通入150 mL三颈烧瓶底部,并在三颈烧瓶中进行氧化反应,采用配备火焰光度检测器(FPD)的气相色谱仪进行定时采样监测甲硫醇、硫化氢、甲硫醚和二甲二硫浓度,每个条件采样测试3次并取平均值;同时,每次气体流程稳定后在线或使用聚四氟气体采样袋采样后进行SO2、CO2、CO浓度监测. 根据测得数据,分别按照式(1)和(2)计算甲硫醇去除率和二氧化碳选择性.
式中,η为甲硫醇去除率,%;C0为反应前气体中甲硫醇浓度,mg·m−3;C1为反应后气体中甲硫醇浓度,mg·m−3;
$ {S_{{\text{C}}{{\text{O}}_{\text{2}}}}} $ 为二氧化碳选择性,%;$ {C_{{\text{C}}{{\text{O}}_{\text{2}}}}} $ 为反应后气体中二氧化碳浓度,mg·m−3;1.091为甲硫醇与二氧化碳的相比分子质量比值. -
甲硫醇、二甲二硫、硫化氢等含硫化合物分析测试参照国标《空气质量硫化氢、甲硫醇、甲硫醚和二甲二硫的测定 气相色谱法》(GB/T 14678-93)方法并调整后测试,操作条件如下:岛津Rt-XLSulfur微填充柱,载气(N2)流速0.95 mL·min−1,进样量0.5 mL,气化室温度150 ℃,柱箱升温程序65—270 ℃(升温速率15 ℃·min−1,270 ℃保持10 min),FPD检测器温度280 ℃(H2:40 mL·min−1,空气:60 mL·min−1).
为了研究臭氧氧化降解甲硫醇过程中组分的变化,采用原位红外光谱分析技术对反应过程进行监测. 具体步骤为:(1)设置红外光谱仪为自动扫描模式,扫描间隔为1 min,红外光谱仪单次扫描条件为:扫描波数范围:4000—400 cm−1,分辨率4 cm−1,扫描次数32次;(2)采用高纯N2对硒化锌气体池(100 mL)进行置换;(3)用甲硫醇标准气体对硒化锌气体池中的高纯N2进行转换;(4)将气体池一端阀门打开,采用玻璃注射器从另一端阀门注入一定体积的臭氧,立即用红外光谱仪进行扫描测试.
参照国家环境保护标准《固定污染源废气甲硫醇等8种含硫有机化合物的测定 气袋采样-预浓缩/气相色谱-质谱法》(HJ 1078-2019)对甲硫醇的氧化降解产物进行分析,分析条件为:取样体积50.0 mL. 一级冷阱(空管):捕集温度:−30 ℃;捕集流速:80 mL·min−1;解析温度:10 ℃;阀温:120 ℃;烘烤温度:150 ℃;烘烤时间:10 min. 二级冷阱(TENAX管):捕集温度:−80 ℃;转移预热温度:−60 ℃;捕集流速:10 mL·min−1;解析温度:10 ℃;阀温:120 ℃;烘烤温度:190 ℃;烘烤时间:10 min. 三级冷阱(硅烷化不锈钢毛细管):聚焦温度:−170 ℃;解析时间:2 min;解析温度:50—70 ℃;烘烤时间:3 min. 色谱柱:安捷伦VF-1ms毛细管柱;柱箱升温程序:起始温度−35 ℃,保持 3 min 后以7.5 ℃·min−1 速度升温至180 ℃,保持3 min;进样口温度:100 ℃;溶剂延迟时间:2.7 min;载气流速:初始流速 2.5 mL·min−1,保持 4 min 后以 2 mL·min−1保持30 min;分流比:20:1. 质谱接口温度:250 ℃;离子源温度:230 ℃;离子化能量:70 eV;扫描方式:全扫描(SCAN);扫描范围:35—300 amu.
采用总有机碳/总氮分析仪进行二氧化碳浓度检测:气体六通阀进样,定量环体积40 mL,根据测得的碳含量计算二氧化碳浓度.
参照国家环境保护标准《环境空气总烃、甲烷和非甲烷总烃的测定 直接进样-气相色谱法》(HJ 604-2017)分析净化气中甲烷含量,分析条件为:GDX-502填充柱(3 m×3 mm),进样口温度:100 ℃,柱温80 ℃,柱流量10 mL·min−1,FID检测器(H2:30 mL·min−1,空气:300 mL·min−1).
参照文献[29]测试净化气中硫氧化物含量,具体步骤如下:移取0.01 mol· L−1 NaOH溶液10 mL于多孔玻板吸收管中,将净化气持续通入多孔玻板吸收管中,直到总吸收气体体积达到2 L,停止通气,移出吸收液,采用离子色谱仪进行分析测试.
-
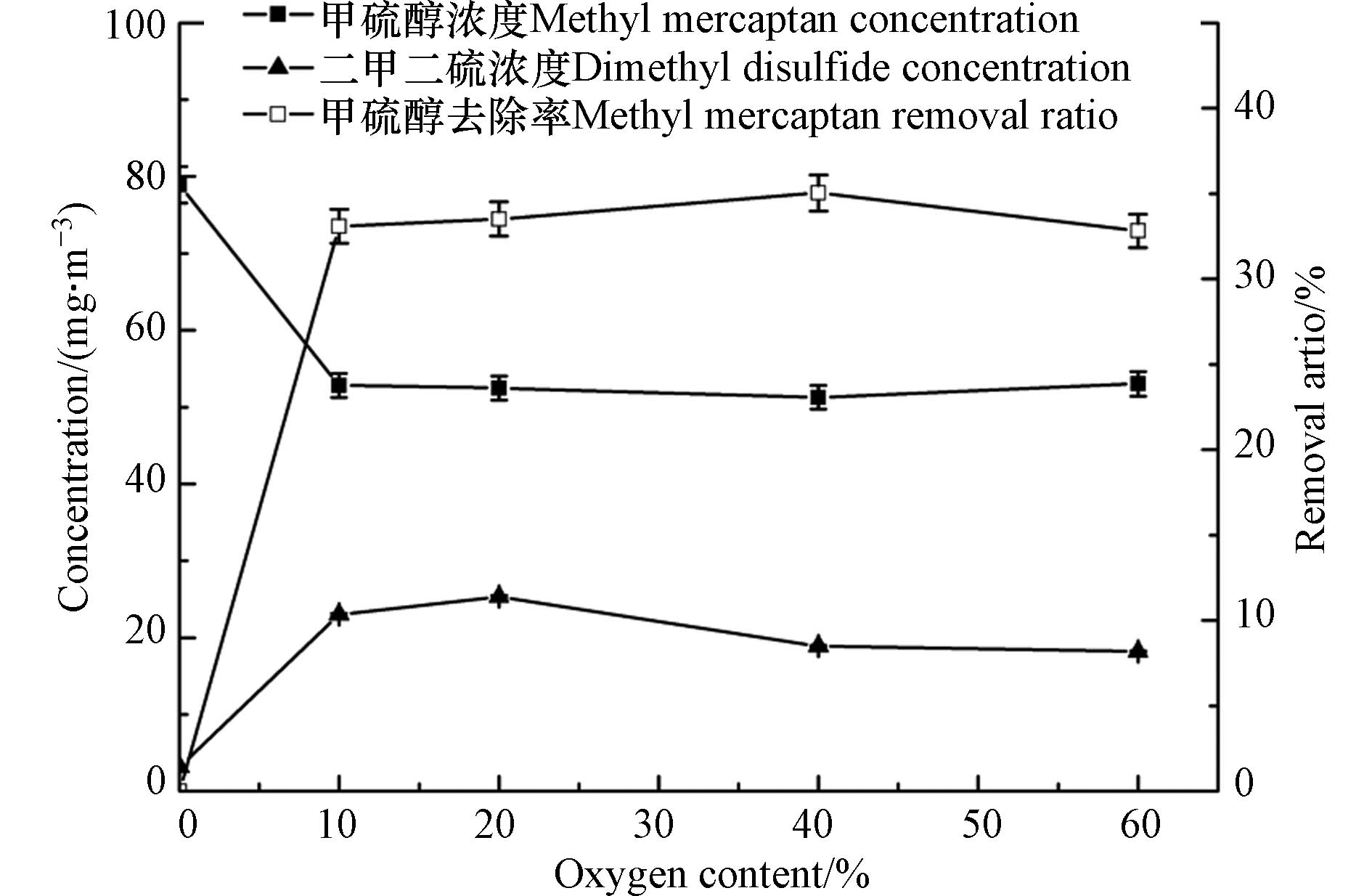
甲硫醇为还原性含硫化合物,浓度较高时会被氧气氧化,为了更客观地研究并评价臭氧对甲硫醇的氧化降解性能,该研究首先考察了氧气对甲硫醇的氧化性能,结果见图2. 由图2可以看出,对于初始浓度为78.9 mg·m−3的甲硫醇气体,氧气可将甲硫醇部分氧化去除,其主要氧化产物为二甲二硫,但其氧化效果有限,甲硫醇的去除率最高仅为28%,且当氧含量高于10%时,甲硫醇的去除率不再随氧含量的增大而变化;由于实际应用场景尤其是污水处理厂、垃圾填埋场等恶臭气体排放场景中均为空气背景,其氧含量为21%左右,因此,本研究在后续实验中均将氧含量调至21%左右.
-
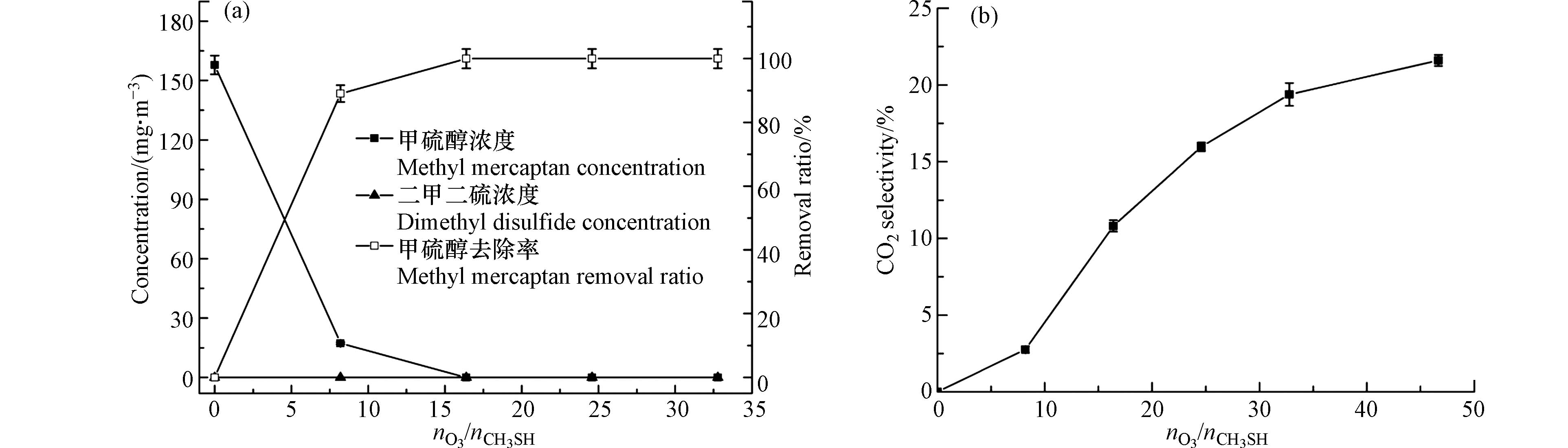
在臭氧对甲硫醇的氧化反应中,臭氧作为反应底物之一,其投加量对反应有较大的影响,且在实际应用中臭氧的投加量与能耗直接相关,因此,研究臭氧投加量的影响对于评价臭氧氧化降解甲硫醇工艺的技术经济性具有重要意义. 实验在室温((30±2)℃)条件下考察了臭氧投加量对甲硫醇去除率和二氧化碳选择性的影响,结果见图3. 由图3可知,对于初始浓度为157.9 mg·m−3的甲硫醇气体,反应时间为1.5 min时,甲硫醇去除率随臭氧投加量的增加而增大,当臭氧与甲硫醇的摩尔比为8.2时,甲硫醇的去除率达到91.3%;增加臭氧与甲硫醇的摩尔比至16.4,甲硫醇实现完全去除;臭氧对甲硫醇的氧化反应中二氧化碳选择性随臭氧投加量的增加呈现先快再缓的趋势,但整体看来其二氧化碳选择性较差,当臭氧与甲硫醇的摩尔比提高到46.7时,二氧化碳选择性仅为21.6%,这说明臭氧虽能将甲硫醇氧化去除,但将其完全矿化的难度较大. 通过对反应过程中二甲二硫浓度的监测发现,整个反应过程中二甲二硫被完全氧化,说明虽然氧气可将甲硫醇氧化为二甲二硫,但氧化性更强的臭氧对二甲二硫表现出更强的选择性,能够快速将二甲二硫氧化去除.
-
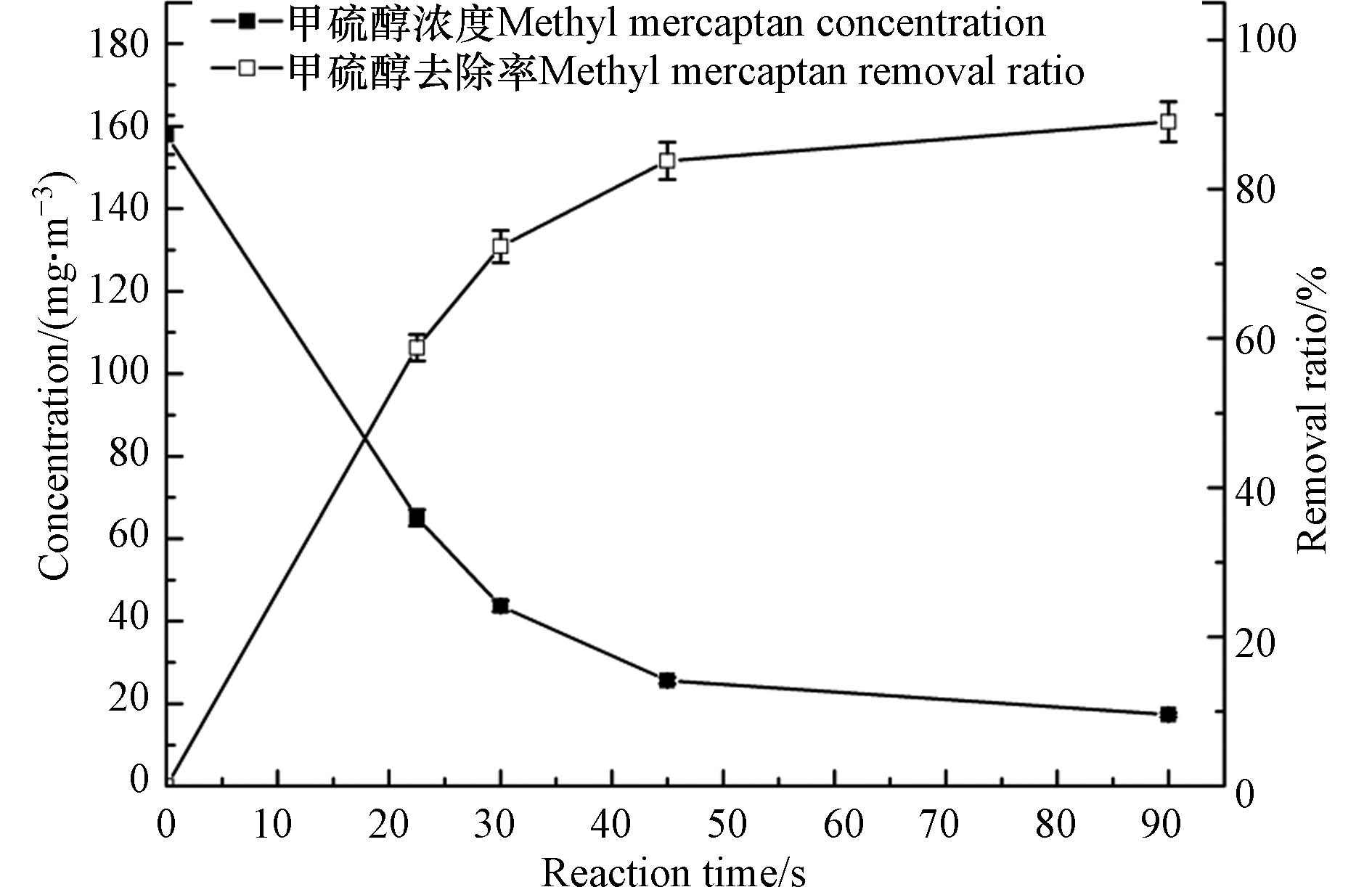
在臭氧对甲硫醇的氧化反应中,虽然臭氧具有强氧化性且其对甲硫醇的氧化反应属于均相反应,但反应时间对氧化性能的影响依然较大,研究反应时间的影响对于实际应用中接触反应器的设计具有重要意义. 实验通过调节反应气体流量研究了反应时间对臭氧氧化甲硫醇的影响,实验中甲硫醇初始浓度设定为157.9 mg·m−3,结果见图4. 由图4可以看出,甲硫醇的去除率随反应时间的延长而增大,当反应时间增加到45 s时,去除率达到81.8%,而将反应时间继续延长至90 s时,甲硫醇的去除率仅增加9.5%. 这主要是由于随着反应的进行气体中甲硫醇浓度降低,即反应底物浓度降低,反应速率降低.
为了更好地研究臭氧对甲硫醇的氧化降解动力学性能,参考文献[30]和[31]中臭氧氧化降解有机物的动力学特征进行研究. 由于臭氧投加量高于臭氧氧化甲硫醇的化学计量比,反应过程中臭氧浓度变化较小,分别采用伪一级反应动力学方程(式3)和伪二级反应动力学方程(式4)对臭氧氧化降解甲硫醇的动力学过程进行拟合,伪一级反应动力学模型拟合得到k1和R2分别为0.03995 s−1和0.9857,伪二级反应动力学模型拟合得到k2和R2分别为0.0005901 m3·mg−1·s−1和0.9639,可以看出两种动力学模型的拟合相关系数均较高. 分别采用式(5)和(6)所示的伪一级反应和伪二级反应半衰期计算公式对氧化动力学过程进行计算分析,计算得到伪一级反应和伪二级反应半衰期分别为17.35 s和10.74 s,而实验数据显示反应时间22.5 s时,甲硫醇的去除率58.77%,显然,17.35 s更接近实际数据. 因此,臭氧对气体中甲硫醇的氧化降解过程符合伪一级反应动力学模型.
式中,y为甲硫醇的去除率;C0为气体中甲硫醇的初始浓度,mg·m−3;Ct为反应时刻t气体中甲硫醇的浓度,mg·m−3;k1为伪一级反应速率常数,s−1;k2为伪二级反应速率常数,m3·mg−1·s−1;t 为反应时间,s.
-
反应温度是臭氧氧化反应的一个重要参数,其直接影响反应效率和能耗. 因此,实验通过调节水浴温度来实现反应温度的调节,从而研究了反应温度对臭氧氧化甲硫醇性能的影响,实验中甲硫醇初始浓度设定为157.9 mg·m−3,结果见图5. 由图5可以看出,在反应温度低于50 ℃时,甲硫醇的去除率随反应温度升高而增大,而随着反应温度升高至50 ℃以上,臭氧对甲硫醇的氧化去除率不再随反应温度的变化而变化,结合能耗综合考虑,臭氧对甲硫醇的最佳反应温度宜为50 ℃. 但由于反应温度由20 ℃到50 ℃,甲硫醇的去除率仅增加不到13%,且实际应用过程中气体温度常在30 ℃左右,因此,结合能耗综合考虑实际应用时可不采取升温措施.
-
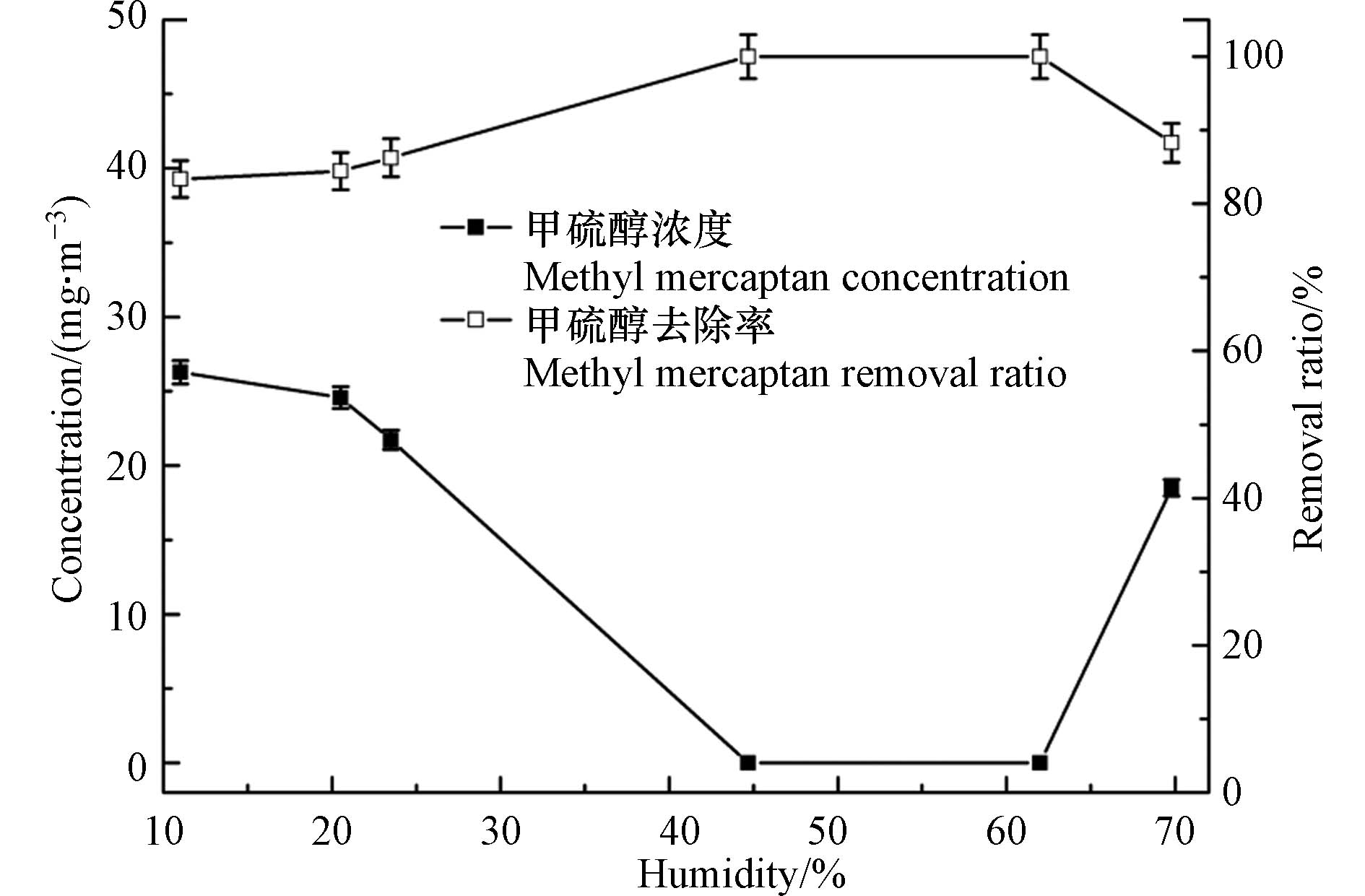
气体的湿度对甲硫醇的极化状态、臭氧的稳定性、反应产物的存在形态等均有较大的影响. 因此,实验通过超声雾化加湿装置对反应气体湿度进行了调节,从而研究了气体湿度对臭氧氧化甲硫醇性能的影响,实验中甲硫醇初始浓度设定为157.9 mg·m−3,结果见图6. 由图6可以看出,湿度对于臭氧氧化甲硫醇影响较大,过高和过低的湿度均不利于反应的进行,这主要是由于适当的湿度可促使臭氧生成氧化性更强的羟基自由基,进而实现甲硫醇去除率的提高[32];过高的湿度则会存在水汽的凝结形成雾滴,而雾滴会对气体的传质造成影响,且由于甲硫醇不溶于水,溶解于雾滴中的臭氧很难通过非均相反应氧化甲硫醇,致使有效臭氧浓度降低,从而影响臭氧对甲硫醇的去除率. 因此,在实际应用中应将气体湿度调节至45%—62%,以获得最佳的甲硫醇去除效果.
-
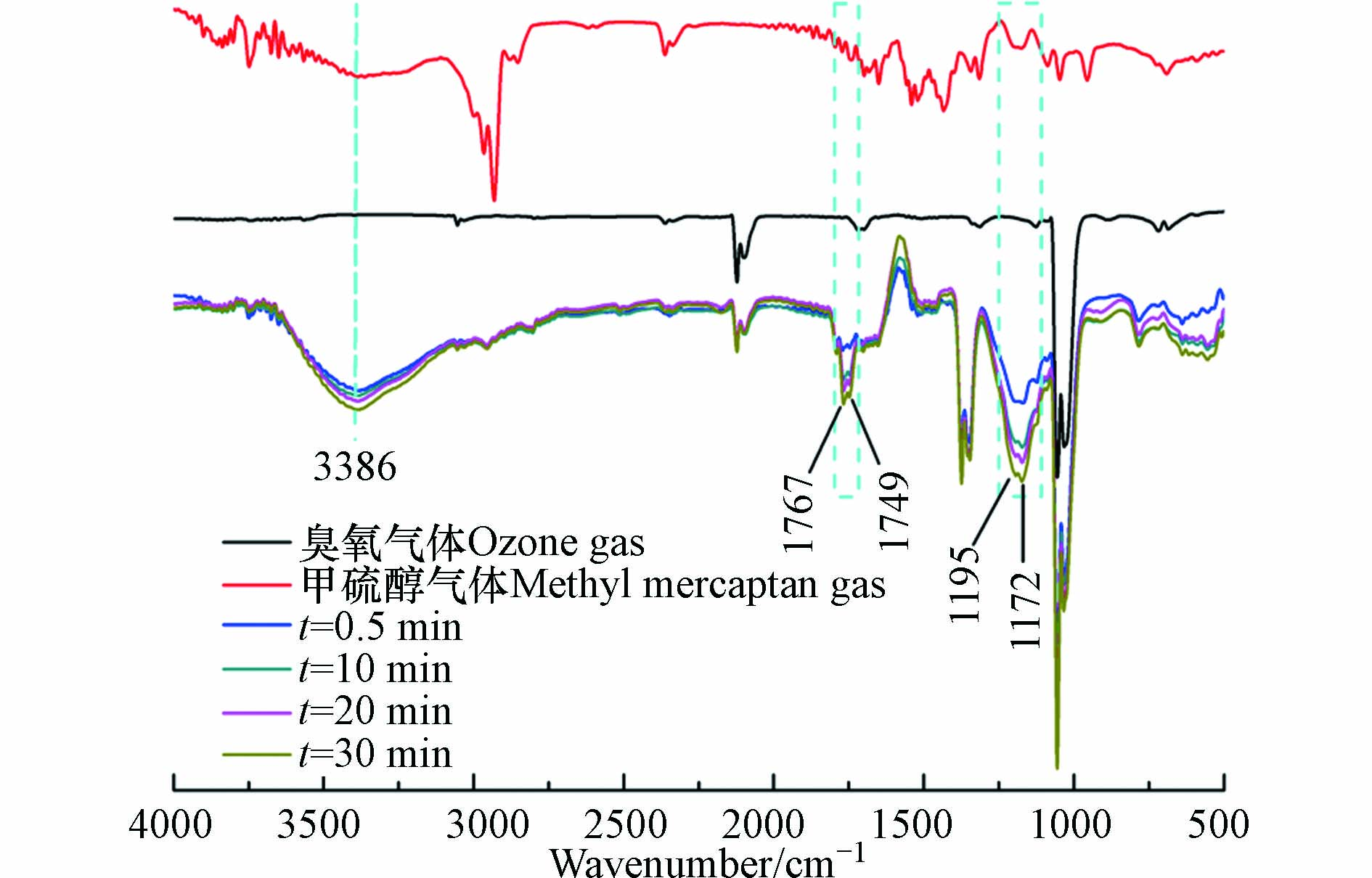
为了进一步研究臭氧对气体中甲硫醇的氧化降解过程,采用原位红外光谱和离子色谱技术对甲硫醇的氧化降解产物进行研究. 按照1.3节所述方法测得臭氧对气体中甲硫醇的氧化降解原位红外光谱如图7所示. 由图7可知,氧化过程中3386 cm−1处的O—H(H2O)伸缩振动吸收峰随反应时间的延长而增强,说明氧化过程伴随有水的生成;1767 cm−1和1749 cm−1处的醛和酮C=O伸缩振动吸收峰随反应的进行而增强,说明氧化过程中有醛和酮的生成;1172 cm−1和1195 cm−1是臭氧吸收峰的一部分,随着氧化反应的进行,该吸收峰逐渐增强,这主要是由于反应生成的化合物中C—H振动吸收峰与臭氧吸收峰叠加引起的[33].
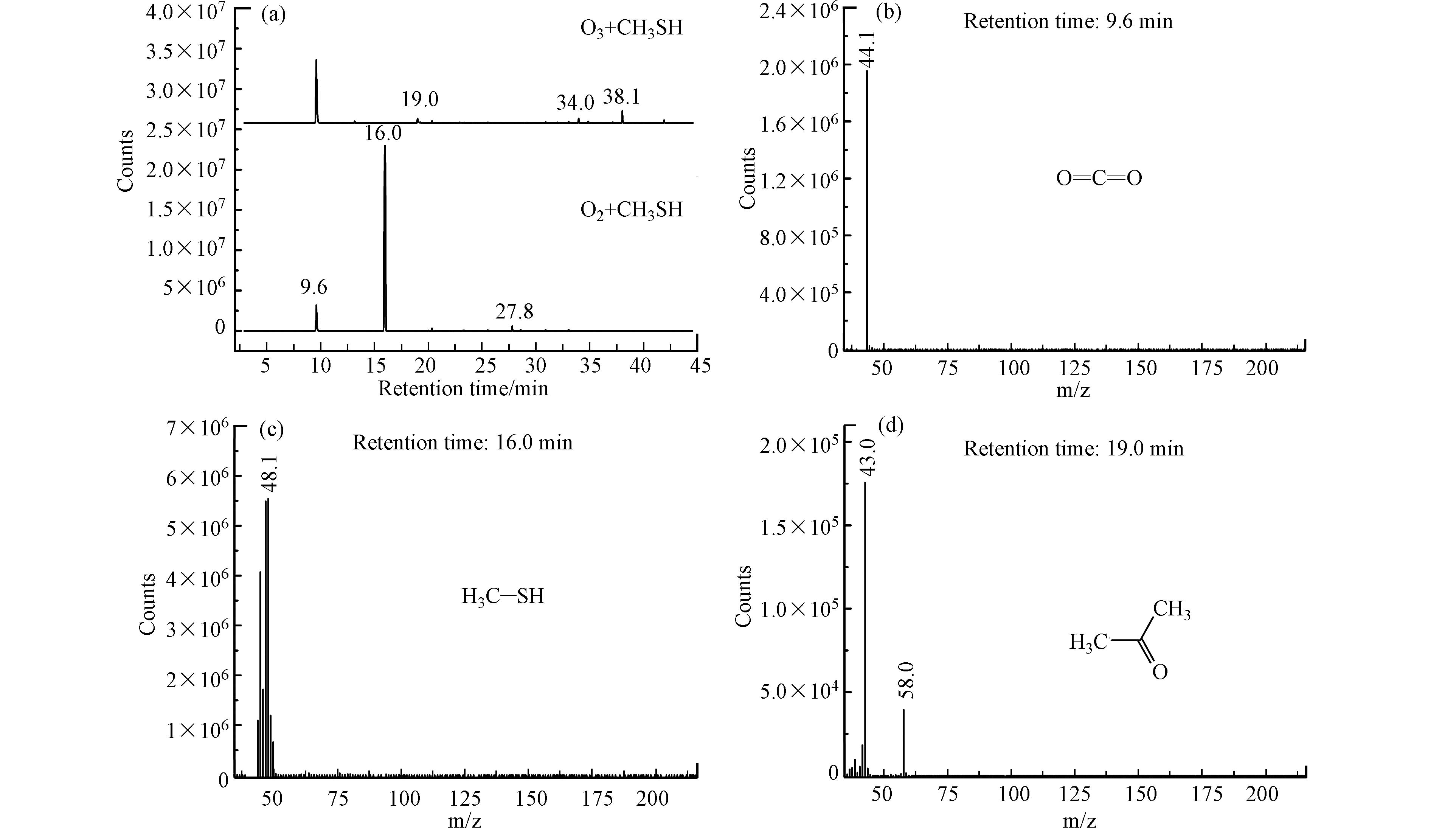
按照1.3节所述测试方法对反应后气体中甲烷含量进行分析,结果表明,反应后气体中无甲烷生成. 采用气袋采样-预浓缩/气相色谱-质谱法(GC-MS)对氧气甲硫醇混合气和臭氧甲硫醇混合气进行分析,结果如图8所示.
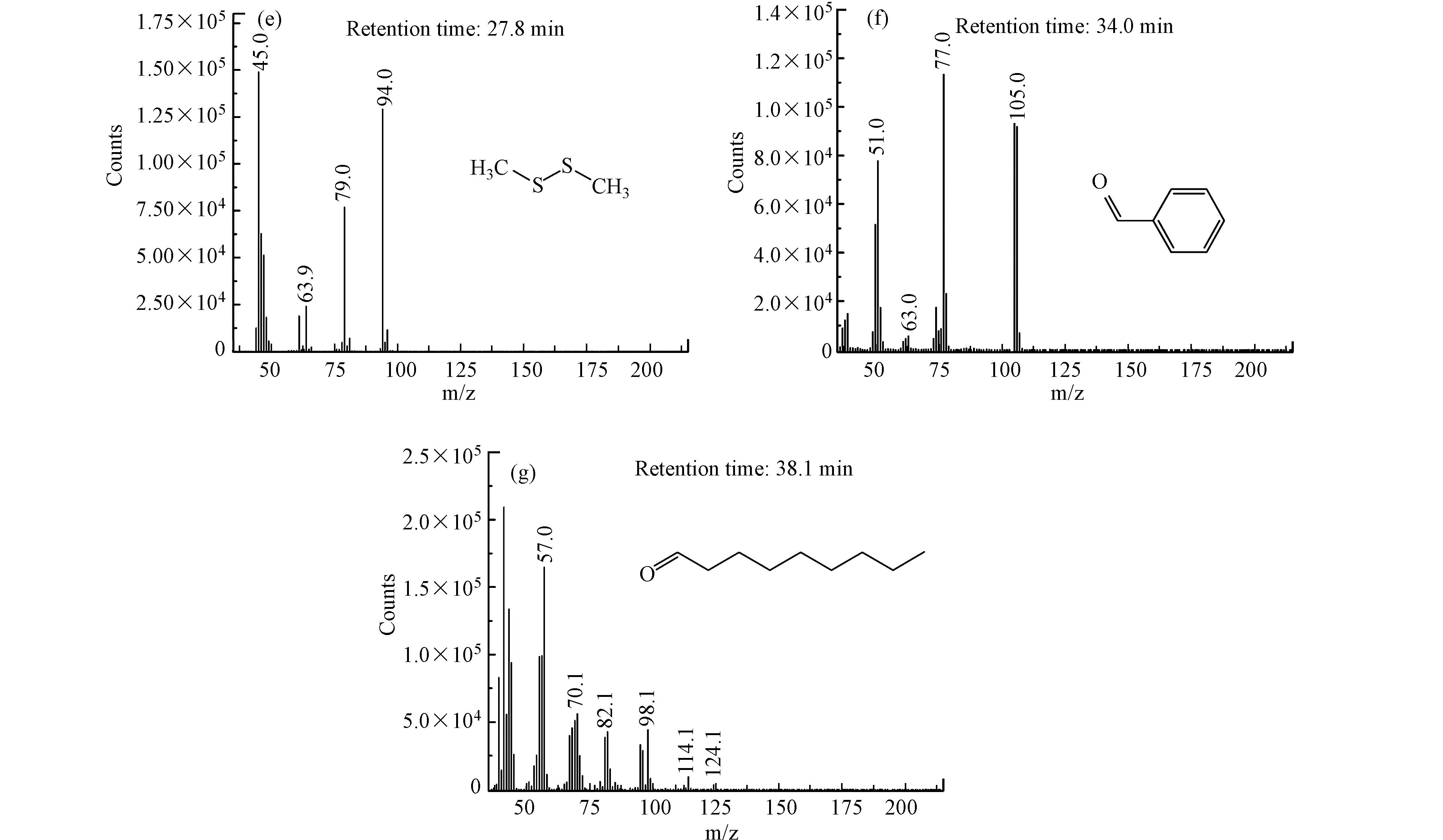
图8中(a)为GC-MS总离子流色谱图,图8(b)—(g)为不同保留时间出峰物质的质谱图. 由图8可以看出,由于采用制氧机所制氧气为氧源,气体中含有一定量的二氧化碳;在氧气存在下,甲硫醇被部分氧化为二甲二硫,该现象在2.1节已被证实;臭氧引入后,气体中二氧化碳浓度升高,说明臭氧可将部分甲硫醇矿化为二氧化碳,该现象与2.2节结果保持一致;臭氧净化气体中未检测到含硫有机化合物,说明甲硫醇被氧化降解为无机硫化物或单质硫;净化气体中检测到少量丙酮、苯甲醛和壬醛等醛酮类物质,且GC-FID未检测到甲烷的生成,说明甲硫醇的初步氧化降解产物可能为小分子烯烃(由于其分子量较小,GC-MS无法检测),小分子烯烃一部分被氧化降解为二氧化碳,少量烯烃在臭氧、自由基等作用下引发加成反应,并在水存在下生成少量醛酮化合物,这与红外光谱表征结果相一致.
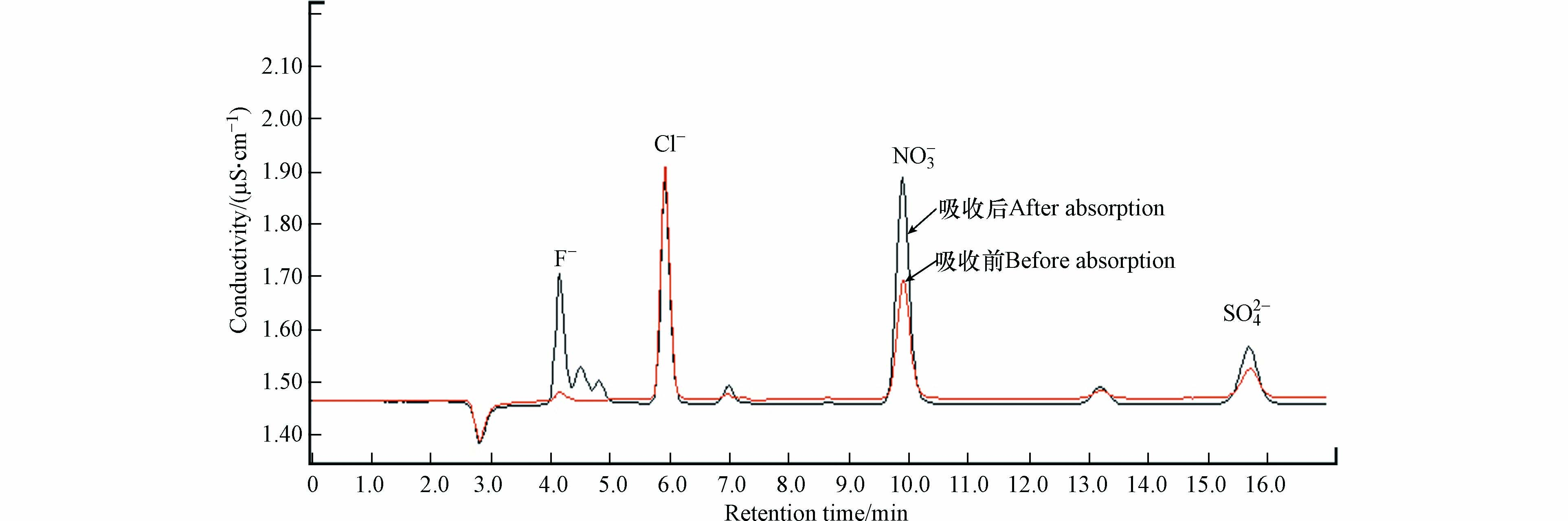
为了获得甲硫醇中硫的去向,按照1.3节所述测试方法对臭氧氧化降解甲硫醇过程中可能生成的硫氧化物进行测定,测试过程中臭氧氧化甲硫醇的反应条件为:甲硫醇初始浓度为157.9 mg·m−3,臭氧与甲硫醇摩尔比为32.8,反应时间为90 s,反应温度为30 ℃,湿度为46.5%. 吸收前后吸收液的离子色谱分析结果见图9和表1.
结果表明,吸收后吸收液中F−、NO3−浓度升高,其中NO3−浓度的升高主要是由于制氧机制备得到的氧气中含有少量氮气,当其进入臭氧发生器时经电晕放电生成二氧化氮,进而被吸收液吸收后生成NO3−;F−浓度升高主要是由于臭氧发生器及各种管路中均采用了大量的含氟制品引起的. 吸收后吸收液中SO42-浓度也有所升高,且未检测到SO32-等其他含硫阴离子,这说明甲硫醇被臭氧氧化生成的硫氧化物均为三氧化硫. 利用测得数据换算得到气体中三氧化硫的浓度为4.4 mg·m−3,其硫含量仅占甲硫醇气体中硫含量的1.69%,证明硫氧化物不是甲硫醇中硫的主要去向,且采用GC-FPD也未检测到H2S和含硫有机物的生成. 因此,推测甲硫醇中硫的主要氧化产物为硫单质,这与文献[34]中甲硫醇的催化氧化产物为甲磺酸的报道是不一致的,这也说明以氧气为氧化剂的催化氧化和O3对甲硫醇的直接氧化机理是不一致的.
-
(1)氧气可将甲硫醇氧化为二甲二硫,但远低于臭氧对甲硫醇的氧化去除性能.
(2)甲硫醇去除率随臭氧投加量的增加而增大,当臭氧与甲硫醇的摩尔比为8.2时,甲硫醇的去除率达到91.3%,增加臭氧与甲硫醇的摩尔比至16.4,甲硫醇可实现完全去除.
(3)甲硫醇的去除率随反应时间的延长而增大,臭氧对气体中甲硫醇的氧化降解过程符合伪一级动力学反应模型,其反应速率常数为0.03995 s−1,甲硫醇的半衰期为17.35 s.
(4)甲硫醇的去除率随反应温度升高而增大,而随着反应温度升高至50 ℃以上,臭氧对甲硫醇的氧化去除率不再随反应温度的变化而变化,结合能耗综合考虑,其最佳反应温度宜为50 ℃.
(5)过高和过低的湿度均不利于反应的进行,其最佳反应相对湿度为45%—62%.
(6)臭氧氧化降解甲硫醇的最佳反应条件为:臭氧与甲硫醇摩尔比16.4,反应时间45 s,反应温度50 ℃,反应相对湿度45%—62%.
(7)采用原位红外光谱、气相色谱-质谱联用和离子色谱技术对臭氧氧化降解甲硫醇过程的研究证明,考察条件下,臭氧不能将全部甲硫醇矿化为二氧化碳和水,而是生成了部分醛类和酮类中间产物,且甲硫醇中硫的主要氧化产物为硫单质.
(8)综合考虑臭氧氧化甲硫醇的最佳反应条件和降解产物,该工艺存在能耗高、易造成二次污染等不足,在实际应用中不建议采用臭氧直接氧化工艺对含甲硫醇臭气进行净化.
臭氧对气体中甲硫醇的氧化降解
Oxidative degradation of gaseous methyl mercaptan by ozone
-
摘要: 甲硫醇是主要恶臭污染物之一,研究甲硫醇的降解技术对于恶臭异味的治理具有重要意义. 为了获得臭氧对气体中甲硫醇的氧化降解性能,实验研究了臭氧对气体中甲硫醇的均相氧化过程,考察了氧含量、臭氧投加量、反应时间、温度和湿度等因素的影响. 结果表明,氧气可将甲硫醇部分氧化为二甲二硫. 甲硫醇去除率随臭氧投加量、反应时间的增加而升高,且臭氧对气体中甲硫醇的氧化降解过程符合伪一级动力学反应模型. 甲硫醇的去除率随温度升高而增大,该趋势在温度高于50 ℃时不再明显;氧化反应的最佳相对湿度为45%—62%. 采用原位红外光谱、气相色谱-质谱联用和离子色谱技术对甲硫醇氧化降解过程的研究证明,考察条件下,臭氧不能将全部甲硫醇矿化为二氧化碳和水,而是生成了部分醛类和酮类中间产物,且甲硫醇中硫的主要氧化产物是硫单质. 综合考虑臭氧氧化甲硫醇的最佳反应条件和降解产物,该工艺存在能耗高、易造成二次污染等不足,在实际应用中不建议采用臭氧直接氧化工艺对含甲硫醇臭气进行净化.Abstract: Methyl mercaptan is one of the main odor pollutants, and the research on degradation of methyl mercaptan is of great significance for the treatment of odor pollution. In this study, the homogeneous ozonation of gaseous methyl mercaptan was studied to obtain the performance of the oxidative degradation of gaseous methyl mercaptan by ozone. The effects of oxygen content, ozone dosage, reaction time, temperature and humidity were investigated. The results showed that oxygen can partially oxidize methyl mercaptan to dimethyl disulfide. The removal of methyl mercaptan increased with the increase of ozone dosage and reaction time, and the oxidative degradation process fitted well with the pseudo first-order kinetic reaction model. Moreover, the removal of methyl mercaptan increased firstly with the increase of temperature, and then tended to a constant value above 50 ℃. The optimum relative humidity was 45%—62% for the oxidation reaction. Further study by in-situ infrared spectroscopy, gas chromatography-mass spectrometry and ion chromatography showed that under the investigated conditions, ozone cannot mineralize all methyl mercaptan into carbon dioxide and water. Some aldehydes and ketones intermediates were produced, and the main oxidation product of sulfur in methyl mercaptan was elemental sulfur. Considering the optimal reaction conditions of the ozonation of methyl mercaptan and its degradation products, the process has the disadvantages of high energy consumption and potential secondary pollution. In practical application, direct ozonation process is not recommended to purify the odor containing methyl mercaptan.
-
Key words:
- methyl mercaptan /
- ozone /
- oxidation /
- odor.
-

-
表 1 吸收前后吸收液中阴离子浓度
Table 1. Anion concentration in absorption solution before and after absorption
吸收液
Absorption solutionF− /(mg·L−1) Cl−/(mg·L−1) NO3− /(mg·L−1) SO42− /(mg·L−1) 吸收前 0.42 3.55 4.96 1.62 吸收后 1.29 3.49 8.54 2.69 -
[1] 桂红艳, 盛彦清, 陈繁忠, 等. 污水处理厂恶臭含硫化合物的研究[J]. 给水排水, 2006, 32(S1): 133-137. GUI H Y, SHENG Y Q, CHEN F Z, et al. Study on odorous sulfur compounds emitted from the wastewater treatment plants[J]. Water & Wastewater Engineering, 2006, 32(Sup 1): 133-137(in Chinese).
[2] 申翰彰. 城市污水处理厂污泥处理过程中恶臭气体排放特征和净化研究[D]. 北京: 北京林业大学, 2020. SHEN H Z. Study on emission characteristics and purification of malodorous gases during sludge treatment in urban sewage treatment plants[D]. Beijing: Beijing Forestry University, 2020(in Chinese).
[3] 杨伟华, 邹克华, 李伟芳, 等. 污水处理厂臭气浓度预测方法及愉悦度评价 [J]. 环境污染与防治, 2018, 40(11): 1306-1309. YANG W H, ZOU K H, LI W F, et al. Odor concentration prediction method and hedonic tone evaluation for sewage treatment plant [J]. Environmental Pollution & Control, 2018, 40(11): 1306-1309(in Chinese).
[4] ORTA de VELASQUEZ M T, MONJE-RAMIREZ I. Combined pre-treatment of coagulation-ozonation for saline-stabilized landfill leachates [J]. Ozone:Science & Engineering, 2006, 28(5): 309-316. [5] WANG Q, ZUO X R, XIA M, et al. Field investigation of temporal variation of volatile organic compounds at a landfill in Hangzhou, China [J]. Environmental Science and Pollution Research, 2019, 26(18): 18162-18180. doi: 10.1007/s11356-019-04917-5 [6] 陶丽霞, 王成端, 李钧. 生物净化器处理制药恶臭废气工程实例 [J]. 中国给水排水, 2007, 23(20): 67-69. TAO L X, WANG C D, LI J. Engineering case of pharmaceutical odor gas treatment by biological purifier [J]. China Water & Wastewater, 2007, 23(20): 67-69(in Chinese).
[7] MOUAMMINE A, OJALA S, PIRAULT-ROY L, et al. Catalytic partial oxidation of methanol and methyl mercaptan: Studies on the selectivity of TiO2 and CeO2 supported V2O5 catalysts [J]. Topics in Catalysis, 2013, 56(9): 650-657. [8] 张妍, 王元刚, 卢志强, 等. 我国餐厨废物生化处理设施恶臭排放特征分析 [J]. 环境科学, 2015, 36(10): 3603-3610. ZHANG Y, WANG Y G, LU Z Q, et al. Odor emission characteristics from biochemical treatment facilities of kichen waste in China [J]. Environmental Science, 2015, 36(10): 3603-3610(in Chinese).
[9] 任连海, 郝艳, 王攀. 改性沸石对餐厨垃圾释放的恶臭气体吸附研究 [J]. 环境科学与技术, 2014, 37(7): 137-140. REN L H, HAO Y, WANG P. Absorption of modified zeolite to odorous gases released from kitchen waste [J]. Environmental Science & Technology, 2014, 37(7): 137-140(in Chinese).
[10] 廖定满, 谭伟红. 炼油厂液化气脱硫醇副产二硫化物废液的利用 [J]. 广州化工, 2014, 42(18): 203-205. doi: 10.3969/j.issn.1001-9677.2014.18.077 LIAO D M, TAN W H. Usage of LPG sweetening disulphide waste liquid [J]. Guangzhou Chemical Industry, 2014, 42(18): 203-205(in Chinese). doi: 10.3969/j.issn.1001-9677.2014.18.077
[11] FENG J J, GAO S, FU Q Y, et al. Indirect source apportionment of methyl mercaptan using CMB and PMF models: A case study near a refining and petrochemical plant [J]. Environmental Science and Pollution Research, 2019, 26(23): 24305-24312. doi: 10.1007/s11356-019-05728-4 [12] YANG S Y, LI Y, WANG L L, et al. Use of peroxymonosulfate in wet scrubbing process for efficient odor control [J]. Separation and Purification Technology, 2016, 158: 80-86. doi: 10.1016/j.seppur.2015.12.010 [13] HE H, HU L L, ZENG J W, et al. Activation of persulfate by CuO-sludge-derived carbon dispersed on silicon carbide foams for odorous methyl mercaptan elimination: Identification of reactive oxygen species [J]. Environmental Science and Pollution Research, 2020, 27(2): 1224-1233. doi: 10.1007/s11356-018-3038-3 [14] ZHAO Y T, HE D D, CHEN D K, et al. Investigating the support effect for catalytic elimination of methyl mercaptan: Role of hydroxyl groups over Cr-based catalysts [J]. Catalysis Letters, 2020, 150(10): 2763-2773. doi: 10.1007/s10562-020-03178-z [15] KAMAL M S, RAZZAK S A, HOSSAIN M M. Catalytic oxidation of volatile organic compounds (VOCs) - A review [J]. Atmospheric Environment, 2016, 140: 117-134. doi: 10.1016/j.atmosenv.2016.05.031 [16] VELLINGIRI K, KIM K H, KWON E E, et al. Insights into the adsorption capacity and breakthrough properties of a synthetic zeolite against a mixture of various sulfur species at low ppb levels [J]. Journal of Environmental Management, 2016, 166: 484-492. [17] YANG C, WANG J, FAN H L. Three-dimensionally ordered macroporous metal oxide-silica composite for removal of mercaptan [J]. Research on Chemical Intermediates, 2017, 43(7): 3847-3858. doi: 10.1007/s11164-016-2853-8 [18] van LEERDAM R C, van den BOSCH P L F, LENS P N L, et al. Reactions between methanethiol and biologically produced sulfur particles [J]. Environmental Science & Technology, 2011, 45(4): 1320-1326. [19] LIU B, ZHAO Y J, WU W F, et al. Biodegradation of methanethiol-laden waste gas stream using a combined bioreactor system [J]. Environmental Engineering Science, 2010, 27(5): 397-402. doi: 10.1089/ees.2009.0304 [20] PETRE C F, PICHÉ S, NORMANDIN A, et al. Advances in chemical oxidation of total reduced sulfur from kraft Mills atmospheric effluents [J]. International Journal of Chemical Reactor Engineering, 2007, 5(1): 1-31. [21] YAN N Q, QU Z, JIA J P, et al. Removal characteristics of gaseous sulfur-containing compounds by pulsed Corona plasma [J]. Industrial & Engineering Chemistry Research, 2006, 45(19): 6420-6427. [22] CUI T, SHEN C Y, XU A L, et al. Use of a novel coupled-oxidation tubular reactor (COTR)/NTP-DBD catalytic plasma in a synergistic electro-catalysis system for odorous mercaptans degradation [J]. Chemosphere, 2019, 216: 533-544. doi: 10.1016/j.chemosphere.2018.10.170 [23] CAI W M, LU G H, HE J, et al. The adsorption feature and photocatalytic oxidation activity of K1−2xMxTiNbO5 (M = Mn, Ni) for methyl mercaptan in methane [J]. Ceramics International, 2012, 38(4): 3167-3174. doi: 10.1016/j.ceramint.2011.12.020 [24] LIU T X, LI X Z, LI F B. AgNO3-lnduced photocatalytic degradation of odorous methyl mercaptan in gaseous phase: Mechanism of chemisorption and photocatalytic reaction [J]. Environmental Science & Technology, 2008, 42(12): 4540-4545. [25] CAO X H, LU J C, ZHENG X Q, et al. Regulation of the reaction pathway to design the high sulfur/coke-tolerant Ce-based catalysts for decomposing sulfur-containing VOCs [J]. Chemical Engineering Journal, 2022, 429: 132473. doi: 10.1016/j.cej.2021.132473 [26] LU J C, LIU J P, ZHAO Y T, et al. The identification of active chromium species to enhance catalytic behaviors of alumina-based catalysts for sulfur-containing VOC abatement [J]. Journal of Hazardous Materials, 2020, 384: 121289. doi: 10.1016/j.jhazmat.2019.121289 [27] LU J C, HAO H S, ZHANG L M, et al. The investigation of the role of basic lanthanum (La) species on the improvement of catalytic activity and stability of HZSM-5 material for eliminating methanethiol-(CH3SH) [J]. Applied Catalysis B:Environmental, 2018, 237: 185-197. doi: 10.1016/j.apcatb.2018.05.063 [28] 方晶晶, 江璐, 徐新宏, 等. 电解式臭氧除臭机对密闭舱室含硫恶臭气体净化效果研究[C]. 2020中国环境科学学会科学技术年会论文集(第一卷). 南京, 2020: 1390-1394.
FANG J J, JIANG L, XU X H, et al . Study on the purification of electrolytic ozone deodorizer on sulfur-containing odorous gases in closed compartments [C]. Proceedings of the 2020 Annual Conference of Science and Technology of the Chinese Society of Environmental Sciences (Volume I). Nanjing, 2020: 1390-1394.[29] 林瑜凡. 离子色谱法同时测定环境空气中的二氧化硫和氮氧化物 [J]. 环境与发展, 2017, 29(3): 205-206. doi: 10.16647/j.cnki.cn15-1369/X.2017.03.111 LIN Y F. Ion chromatography and determination of the sulfur dioxide and nitrogen oxides in air environment [J]. Environment and Development, 2017, 29(3): 205-206(in Chinese). doi: 10.16647/j.cnki.cn15-1369/X.2017.03.111
[30] 宋江燕, 李方鸿, 吴根义, 等. 氯咪巴唑在臭氧降解过程中的影响因素及其降解产物 [J]. 环境科学研究, 2022, 35(2): 478-487. SONG J Y, LI F H, WU G Y, et al. Degradation of climbazole by ozonation: Influencing factors and degradation products [J]. Research of Environmental Sciences, 2022, 35(2): 478-487(in Chinese).
[31] 张佳丽, 魏健, 任越中, 等. 臭氧氧化降解水中青霉素G特性和动力学特征 [J]. 环境科学研究, 2019, 32(7): 1231-1238. ZHANG J L, WEI J, REN Y Z, et al. Degradation characteristics and kinetics of penicillin G in water by ozone oxidation [J]. Research of Environmental Sciences, 2019, 32(7): 1231-1238(in Chinese).
[32] 姜宏斌. 催化臭氧氧化工艺处理芳香族VOCs废气的研究[D]. 大连: 大连理工大学, 2021. JIANG H B. Study on the treatment of aromatic VOCs waste gas by catalytic ozonation process[D]. Dalian: Dalian University of Technology, 2021(in Chinese).
[33] 朱自莹, 顾仁敖, 陆天虹. 拉曼光谱在化学中的应用[M]. 沈阳: 东北大学出版社, 1998. ZHU Z Y, GU R A, LU T H. Application of Raman spectroscopy in chemistry[M]. Shenyang: Northeast University Press, 1998(in Chinese)
[34] LIU Q, KE M, YU P, et al. High performance removal of methyl mercaptan on metal modified activated carbon [J]. Korean Journal of Chemical Engineering, 2018, 35(1): 137-146. doi: 10.1007/s11814-017-0272-1 -



 下载:
下载: