-
铅作为一种重金属,对人体和环境有着巨大的危害[1],可进入人体的中枢神经系统和重要器官,通过一系列生理反应造成不良影响甚至致死[2]. 去除含铅废水中的铅离子对于人类的生存发展具有重要的现实意义. 对于溶液中重金属离子的去除,常用方法有离子交换法[3]、化学沉淀法[4]、电化学法[5]和吸附法等. 吸附法相对于其他方法而言,不需要向体系中引入其他化学试剂,不会造成二次污染.
我国作为茶叶的主要生产国和消费国,每年产生大量废弃的工业茶渣[6],这些茶渣很少得到有效利用. 茶渣具有多孔性结构和大量活性基团,结合壳聚糖富有氨基和羟基可以与重金属离子螯合的特点[7],可制备出高效的重金属离子吸附剂,对废弃茶渣进行改性处理,以实现其效用价值最大化. 使用生物材料和壳聚糖制备复合吸附材料吸附重金属离子的研究一直以来受到国内外许多学者的重视. 如王威振等[8]探究了以梧桐树产生的不同废弃物制备出的生物质炭对Cd2+的吸附效果,并通过多种表征手段结合实验结果提出吸附剂的吸附机理为化学沉淀及物理吸附. 刘爽等[9]利用茶渣制备生物炭并用不同浓度的磷酸进行活化处理. 结果表明,在磷酸浓度为50%时,吸附剂对铅离子的吸附效果最佳,吸附机理为表面络合和沉淀. 此外,壳聚糖这种来源广泛的天然多糖,可由自然界大量存在的甲壳素制备而成,具有可降解性、抑菌及无毒等优点[10],近年来也被应用于重金属吸附材料的制备中. 如刘珊等[11]以壳聚糖、纳米铁和聚乙二醇为原料,采用包埋法制备出用于吸附铜离子的凝胶球,该吸附材料具有较好的吸附效果,饱和吸附量可达133.4 mg·g−1. Prerana等[12]利用戊二醛交联壳聚糖和氨基丙基硅烷氧化石墨烯,制备出可在Cu2+、Ni2+、Pb2+和Cr3+等多种混合重金属离子中选择性吸附Pb2+的吸附剂,探究了温度、pH、吸附质浓度等因素对吸附效果的影响,并通过拟合吸附过程符合Langmuir和Freundlich等温吸附模型. 有研究表明[13],壳聚糖中加入聚乙烯醇可以增强混合物中的氢键数量和强度,可作为提高吸附剂机械强度和化学性能的一种方法.
本研究以茶渣、壳聚糖、聚乙烯醇等为原料,通过改变原料配比,制备出4种铅离子吸附膜,探究了溶液pH、Pb2+初始浓度、温度和吸附时间对吸附效果的影响,并对吸附膜进行扫描电子显微镜测试(SEM)、能量色散X射线光谱测试(EDAX)、红外光谱测试(IR). 本实验所制备的膜型吸附剂吸附效果良好、耐酸性和机械强度强、比表面积大、材料利用率高,且制备时不需要冷冻干燥,操作更为简便,可应用于对含铅废水的处理.
-
双光束紫外可见分光光度计(UV-1780),恒温摇床(TS-100B),磁力搅拌器,电热恒温鼓风干燥箱,真空冷冻干燥机,pH计,标准筛(100目,孔径0.15 mm),圆形培养皿(外径为70 mm),扫描电子显微镜(赛默飞 Apreo S),傅立叶变换红外光谱仪(NEXUS 670).
安徽省六安市某茶叶企业所售的六安瓜片(无芽无梗,大叶绿茶);壳聚糖(脱乙酰度≥95%,上海阿拉丁生化科技股份有限公司),聚乙烯醇(2088型),氢氧化钠(上海阿拉丁生化科技股份有限公司),硝酸(上海阿拉丁生化科技股份有限公司),硝酸铅(购上海阿拉丁生化科技股份有限公司),二甲酚橙(上海麦克林生化科技股份有限公司),丙酮(上海阿拉丁生化科技股份有限公司),醋酸钠(上海阿拉丁生化科技股份有限公司),醋酸(上海麦克林生化科技股份有限公司),以上试剂均为分析纯试剂;蒸馏水(实验室自制).
-
由于本实验所制备的吸附剂为圆形的膜型吸附剂,吸附发生在膜的表面,以面积定量比质量定量更为合理,故将吸附膜的最小使用单位定为1/8圆. 以下是具体的制备流程.
-
取10 g干燥后的茶叶于100 mL的1 g·L−1氢氧化钠溶液中超声1 h,过滤、洗涤、干燥至恒重,粉碎研磨后过100目的标准筛,即得茶渣粉末.
-
以壳聚糖、PVA(聚乙烯醇2088型)、茶渣粉末质量比为3:3:5制备混合溶液(在壳聚糖溶液中加入PVA,再加入茶渣粉末,超声至茶渣粉末均匀分散于体系中),再将其用注射器滴于1 mol·L−1氢氧化钠溶液中,得到球形颗粒,用蒸馏水洗涤至中性后,冷冻干燥. 将冷冻干燥后的球形颗粒研磨后,过100目的标准筛即得改性茶渣粉末.
-
将1 g壳聚糖溶于100 mL的体积分数为5%的HOAc溶液中,再加入2 g茶渣粉末配制混合溶液,搅拌,超声10 min,取5 mL溶液铺于圆形培养皿中制备膜,在70℃下烘干至恒重,用1 mol·L−1氢氧化钠溶液浸泡,将膜剥离,再用蒸馏水反复冲洗至中性,将膜平均分为8份,干燥后,记录每一个1/8圆的膜的平均质量为0.0191 g.
-
将1 g壳聚糖溶于100 mL的体积分数为5%的HOAc溶液,将1 g PVA溶于5 mL的90℃的热水中,再加入壳聚糖溶液中,然后加入2 g茶渣粉末配制成混合溶液,搅拌,超声10 min,取5 mL溶液铺于圆形培养皿中制备膜,在70℃下烘干至恒重,用1 mol·L−1氢氧化钠溶液浸泡,将膜剥离,再用蒸馏水反复冲洗至中性,将膜平均分为8份,干燥后,记录每一个1/8圆的膜的平均质量为0.0236 g.
-
将1 g壳聚糖溶于100 mL的体积分数为5%的HOAc溶液,再加入2 g改性后的茶渣粉末配制混合溶液,搅拌,超声10 min,取5 mL溶液铺于圆形培养皿中制备膜,在70℃下烘干至恒重,用1 mol·L−1氢氧化钠溶液浸泡,将膜剥离,再用蒸馏水反复冲洗至中性,将膜平均分为8份,干燥后,记录每一个1/8圆的膜的平均质量为0.0210 g.
-
将1 g壳聚糖溶于100 mL的体积分数为5%的HOAc溶液,1 g PVA溶于5 mL的90℃的热水中,再加入壳聚糖溶液中,然后加入2 g改性后的茶渣粉末配制成混合溶液,搅拌,超声10 min,取5 mL溶液铺于圆形培养皿中制备膜,在70℃下烘干至恒重,用1 mol·L−1氢氧化钠溶液浸泡,将膜剥离,再用蒸馏水反复冲洗至中性,膜平均分为8份,干燥后,记录每个1/8圆的膜的平均质量为0.0240 g.
-
以二甲酚橙为显色剂,邻菲啰啉为掩蔽剂,采用双光束紫外可见分光光度法测定溶液中铅离子含量,最大吸收波长位于534 nm处,溶液中铅离子含量在0.05—4.0 mg·L−1内遵循朗伯-比尔定律[14]. 吸附实验中表示吸附效果的吸附量q(mg·g−1)和吸附率r的计算公式见公式(1—2), 其中,C0(mg·L−1)表示铅离子初始浓度,Ce(mg·L−1)表示吸附平衡时溶液中剩余铅离子的浓度,m(g)为吸附剂的质量,V(L)为含铅溶液的体积,M(g·mol−1)为铅的原子质量.
-
取1/8圆的吸附膜,分别加入25.00 mL的pH值为3.0、4.0、5.0、6.0、7.0(用0.01 mg·L−1硝酸/0.001 mg·L−1氢氧化钠微调)的含有铅离子浓度为100 mg·L−1的硝酸铅溶液和无铅参比溶液中, 在30℃的恒温摇床中振荡6 h,每次测定取出样品和相应参比,经离心、过滤,得到10 mL含铅溶液和10 mL无铅参比溶液,用双光束紫外可见分光光度法检测铅离子浓度.
-
取1/8圆的吸附膜,加入25.00 mL的pH为“1.3.1”中确定的最佳值(CT、PCT、CM、PCM膜吸附时的pH分别为6.0、6.0、5.0、5.0),铅离子浓度分别为20、40、60、80、100、200、400 mg·L−1的硝酸铅溶液,恒定温度为30℃,摇床恒温振荡6 h,将试样离心、过滤后检测铅离子浓度.
-
取1/8圆的吸附膜,加入25.00 mL的pH为“1.3.1”中确定的最佳值(CT、PCT、CM、PCM膜吸附时的pH分别为6.0、6.0、5.0、5.0)的含有铅离子浓度为100 mg·L−1的硝酸铅溶液和无铅参比溶液中,恒定温度分别为30、35、40℃,摇床恒温摇荡6 h,将试样离心、过滤后检测铅离子浓度.
-
取1/8圆的膜,加入25.00 mL的pH为“1.3.1”中确定的最佳值(CT、PCT、CM、PCM膜吸附时的pH分别为6.0、6.0、5.0、5.0)的含有铅离子浓度为100 mg·L−1的硝酸铅溶液中,恒定温度30℃,平行设置12个实验组和12个无铅参比溶液,在15、30、45、60、75、90、105、120、180、240、360、720 min时分别取出1个实验组和1个参比溶液,经过离心、过滤,检测铅离子含量,其他组继续吸附,直至最后一组吸附完毕.
-
在pH为0—14的范围内(pH值每隔0.05检测1次)对4种吸附膜进行耐酸性检测,每次调节pH后将吸附膜在溶液中浸泡1 h,实验过程中通过加入硝酸和氢氧化钠调节溶液的pH值,使用pH计进行测定酸度.
-
对4种不同的吸附膜在吸附前和吸附后分别用扫描电子显微镜(SEM)进行表征. 对吸附后的吸附膜进行EDAX(Energy dispersive X-ray spectrometer)测试,以探究其表面结晶的元素组成.
-
将最佳pH条件(CT、PCT、CM、PCM膜吸附时的pH分别为6.0、6.0、5.0、5.0),恒定温度为30℃,Pb2+初始浓度为100 mg·L−1吸附6 h后的4种吸附膜用蒸馏水清洗晾干,置于25 mL的0.1 mol·L−1的EDTA中脱附10 h,完成吸附剂的再生. 再生后的吸附膜再在相同吸附条件下吸附6 h,检测吸附后溶液中铅离子含量,该循环再生实验实施2次.
-
通过扫描电子显微镜对吸附前的4种膜进行形貌表征. 从图1可见,CM膜和PCM膜的表面可以观察到分布均匀的茶渣粉末颗粒;PCT膜表面无明显凸起,最为平整;CT膜表面有少量凸起. 这种差异性的主要原因是在将固体粉末和壳聚糖溶液进行混合时,四者的粉末的质量和壳聚糖的质量比是相同的,但是制备CM膜和PCM膜所加入的改性茶渣粉末密度小于茶渣粉末,故二者中的粉末颗粒的体积占比更高,更容易显现在表面. 而吸附以后的4种膜,表面均出现了一种大小相近、形状规律的圆片状固体,查阅文献[15]后发现,这种固体应当是吸附的铅离子形成的含铅结晶,为进一步验证,对吸附后样品进行EDAX测试,结果如表1,可见吸附后产生的圆片状结晶确为含铅的结晶. 由于各个膜的表面状态以及在溶液中所处环境不同,所产生的结晶的大小也不同.
-
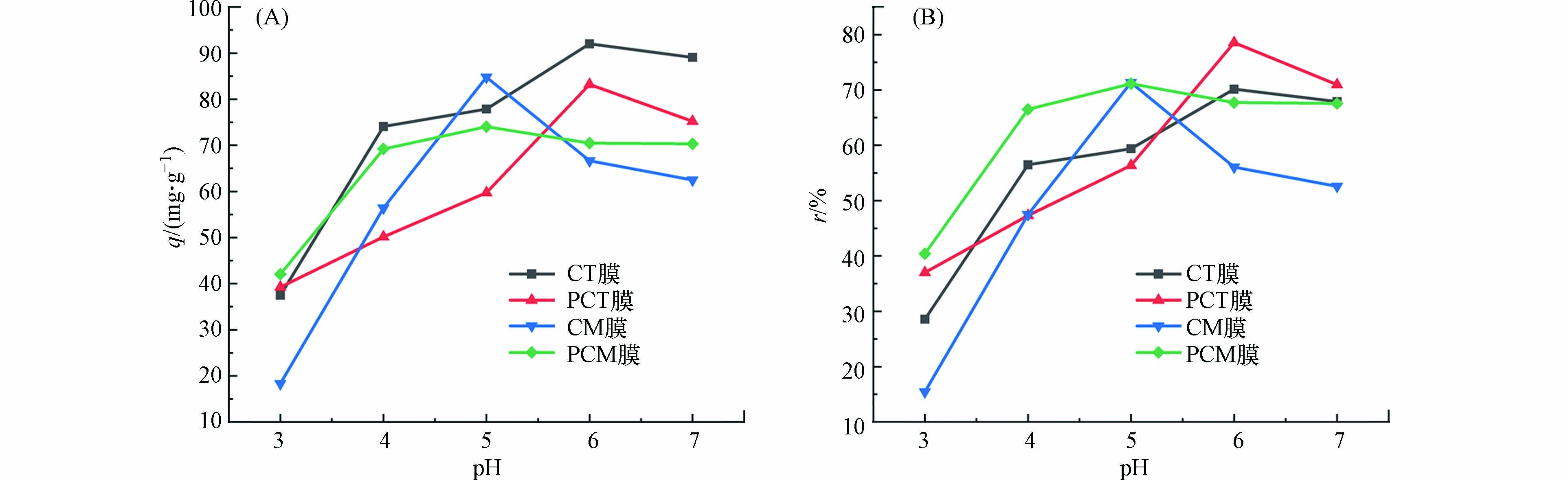
pH对4种吸附膜的影响不尽相同,当pH值为3—7时,CT膜在pH6.0时吸附效果最佳,吸附量为92.0 mg·g−1,吸附率为70.1%;PCT膜在pH6.0时吸附效果最佳,其吸附量为83.2 mg·g−1,吸附率为78.5%;CM膜在pH5.0时吸附效果最佳,其吸附量为84.8 mg·g−1,吸附率为71.4%;PCM膜在pH5.0吸附效果最佳,其吸附量为74.0 mg·g−1,吸附率为71.1%. 从图2(A)可见,在最佳pH条件下,4种膜的吸附量的大小顺序依次为:CT膜>CM膜>PCT膜>PCM膜;从图2(B)可见,在最佳pH条件下,4种膜的吸附率的大小顺序依次为:PCT膜>CM膜>PCM膜>CT膜. 为了更好地进行比较,4种膜在最佳pH条件下的吸附量和吸附率汇总于表2.
-
由图3(A)可见,每一种吸附膜的吸附量均随着Pb2+初始浓度的增大而增大,并且增大的幅度逐渐减小. 从图3(B)可见,吸附率随Pb2+初始浓度的增大总体上呈现减小的趋势,但是由于吸附量增大的同时伴随着Pb2+初始浓度的增大,在二者的共同作用下,折线出现了部分“驻点”,即部分数据点同时高于或同时低于其左右的数据点. 4种吸附膜在各自的最佳pH条件下,CT膜的吸附量在Pb2+初始浓度为20—200 mg·L−1的范围内高于其他3种膜;在Pb2+初始浓度为400 mg·L−1时CM膜的吸附量最大;当Pb2+初始浓度为80、100 mg·L−1时,PCT膜的吸附率明显高于其他吸附膜.
-
由图4可见,在温度范围为30—40℃时,4种吸附膜的吸附量和吸附率均随着温度的增加而增大. 其中在各个温度下,CT膜的吸附量均高于其他3种膜;PCT膜的吸附率在4种膜中最高.
-
从图5可见,随着吸附时间的增长,4种吸附膜的吸附量和吸附率均不断增大,变化的幅度不断减小. 360 min时,吸附量和吸附率趋于最大值,此后数值只有微小变化,可认为在360 min时,吸附已经达到平衡.
-
由于吸附膜的组分中含有可溶于酸的壳聚糖,故吸附膜的耐酸性是衡量其质量的重要指标之一. 各种吸附膜的在不同pH值时的溶解情况汇总于表3. 其中CT膜组成成分除了壳聚糖外还有茶渣粉末的存在,茶渣粉末的引入一定程度上加强了吸附膜中高分子链的交联程度,可以充当网状结构的连接点,进而增强耐酸性,使其在pH大于1.75时,在调节溶液pH后1 h内无明显溶解现象;PCT膜在CT膜是基础上加入了PVA,进一步增强其耐酸性,使得在所检测的pH为0—14的范围中1 h内均不溶解,在4种膜中耐酸性最好;CM膜在pH 1.5时浸泡1 h,开始有明显的溶解现象(吸附膜有明显破损),增大至pH2.0浸泡1 h,吸附膜无明显溶解现象;PCM膜在pH大于1.75浸泡1 h,吸附膜无明显溶解现象. 因此,PCT膜的酸度应用范围最广,可适用于常见的含铅废水的处理. 4种吸附膜的组成成分不同,PVA、壳聚糖和茶渣对耐酸性影响各不相同,而茶渣粉末和改性茶渣粉末也由于粉末颗粒的表面和内部结构不同,和高分子链连接的结合位点数量和位置不同,形成的网状结构也不相同,这种微观上的差别最终导致在宏观上表现出的耐酸性有所不同.
-
热力学参数
ΔG(吉布斯自由能变)、ΔH(焓变)、ΔS(熵变)可用来描述吸附反应的热力学过程,利用公式(3—4),可得到ΔG,通过公式(3—5)[12]可以得到公式(6),再用lnKc对1/T进行线性拟合,线性拟合结果见图6. 由拟合出的直线的斜率和截距算出 ΔH、ΔS. 其中,qe(mg·g−1)表示吸附平衡时的吸附量,ΔG(kJ·mol−1)为吉布斯自由能变,ΔH(kJ·mol−1)为焓变,ΔS(J·mol−1·K−1)为熵变,Kc为吸附平衡常数,R(8.314 J·mol−1·K−1)为摩尔气体常数,T(K)为开氏温度. 由表4可知,吉布斯自由能变ΔG均是负值,说明吸附过程是自发进行的热力学过程;焓变ΔH均为正值,说明吸附过程是吸热的,当温度升高时,平衡吸附量增大;熵变ΔS均为正值,说明吸附过程为熵增过程,混乱程度增大.
-
Langmuir吸附等温线模型和Freundlich吸附等温线模型是最常见的两种热力学模型. 二者的吸附等温线方程分别为公式(7)和公式(8). 其中Freundlich吸附等温线方程中qm(mg·g−1)表示最大吸附量,b(mg·L−1)为Langmuir键;Freundlich吸附等温线方程中KF(mg·L−1)为Freundlich亲和系数,n为Freundlich线性系数. 此外,定义一个无量纲的分离因子RL来预测吸附反应是否为有利过程,若RL的数值为0,则吸附过程为不可逆吸附;若RL的数值在0—1,则吸附过程为有利吸附;若RL的数值为1,则吸附过程为线性吸附;若RL的数值大于1,则吸附过程为不利吸附,其计算公式为公式(9),其中C0(mg·L−1)为铅离子初始浓度.
通过Ce/qe对Ce的线性拟合得到4种膜的Langmuir吸附等温线模型拟合结果;通过lnqe对lnCe的线性拟合得到4种膜的Freundlich吸附等温线模型拟合结果. 将模型拟合得到的模型参数和拟合的相关系数汇总于表5. 从表5可知,对于CT膜、PCT膜和PCM膜而言,其Langmuir吸附等温线模型拟合的相关系数R2比Freundlich吸附等温线模型拟合的相关系数R2更大,说明更符合Langmuir吸附等温线模型,吸附过程为单层吸附;对于CM膜而言,其Freundlich吸附等温线模型拟合的相关系数R2更大,故更符合Freundlich吸附等温线模型,吸附过程为多层吸附.
通过计算C0在20—400 mg·L−1范围内各吸附膜的分离因子RL的数值可知,CT膜、PCT膜、CM膜和PCM膜的RL的数值范围分别为0.06—0.56、0.07—0.62、0.09—0.66和0.09—0.65,均位于0—1范围内,故4种膜的吸附过程均为有利吸附. 通过拟合,还可以得到4种膜的理论饱和吸附量分别为202.8、173.3、223.7、172.4 mg·g−1.
-
为了研究吸附过程存在的速率控制步骤,常采用准一级吸附动力学模型和准二级吸附动力学模型对数据进行处理,其方程分别为公式(10)(11),公式中qt(mg·g−1)为t时刻的吸附量,k1(min−1)为准一级速率常数,k2(g·(mg·min)−1)为准二级速率常数. 准一级吸附动力学模型假定吸附速率受扩散步骤的控制;准二级吸附动力学模型则假定吸附速率受化学吸附的控制,吸附剂和吸附质之间存在电子共用或转移.
两个动力学模型的相关参数汇总于表6,对于模型的符合程度的评价,由2个评价指标组成,其中首先考虑相关系数R2,在相关系数相近的情况下再通过比较平衡吸附量理论值qe和平衡吸附量实验值qe,实验(mg·g−1)的差值判断所符合的模型.
从结果可见,CT膜的准一级吸附动力学模型的相关系数R2更大,PCM膜的两个模型的相关系数R2相近,而其准一级吸附动力学模型的平衡吸附量理论值和平衡吸附量实验值的差值更小,故认为二者更符合准一级吸附动力学模型,吸附主要受扩散步骤控制. PCT膜从2个评价指标考虑,均是更符合准二级吸附动力学模型,CM膜的两个模型的相关系数R2相近,而其准二级吸附动力学模型的平衡吸附量理论值和平衡吸附量实验值的差值更小. 因此,二者更符合准二级吸附动力学模型,吸附主要受化学吸附控制.
-
实验所制备的吸附膜中含有大量的羟基和氨基,二者均可以对Pb2+进行螯合[16],从而实现化学吸附,而这种螯合作用很容易受到溶液pH的影响,当溶液pH值较低时,吸附膜中的-NH2容易结合H+生成-NH3+,而-NH3+没有多余的电子与Pb2+进行配位. 由于PCT膜的耐酸性最好,将其分别浸泡在pH6.0(即其最佳pH条件)和pH1.0的溶液中,晾干后进行红外光谱测试,结果如图7所示,当溶液pH值过低时,3300 cm−1附近所出的-NH2的峰强度明显降低,说明-NH2的数量减少,对Pb2+的螯合作用减弱,宏观上使得吸附量和吸附率减小.
因此,溶液pH值太低会使得吸附膜的吸附效果降低;当溶液的pH值太高时,Pb2+在水中的存在形式会发生改变,生成不同形态的水合铅离子,甚至形成沉淀析出,也会对吸附造成不利的影响. 故而,每种吸附膜均有一个最佳pH条件. 在实验中,CT、PCT、CM和PCM膜分别在溶液pH值为6.0、6.0、5.0和5.0时吸附效果最佳. 此外,由于吸附膜中的茶渣具有多孔性结构,也会对Pb2+形成物理吸附[17].
-
对4种吸附膜在完成首次吸附(即循环次数为0)后再进行2次再生实验(即循环次数分别为1和2),结果如图8所示,4种吸附膜的吸附量和吸附率均随循环次数的增加而减小,但即使进行了2次循环吸附脱附后,吸附膜仍具有较好的吸附效果.
-
(1)实验制备和检测了4种以茶渣为基体的铅离子吸附膜,4种吸附膜在各自的最佳吸附条件下均能表现出优异的吸附效果.
(2)吸附热力学研究结果表明,CT、PCT、PCM膜更符合Langmuir吸附等温线模型,吸附过程为单层吸附;CM膜更符合Freundlich吸附等温线模型,吸附过程为多层吸附.
(3)吸附动力学研究结果表明,CT、PCM膜的吸附动力学过程符合准一级吸附动力学模型,速率主要受扩散过程控制;PCT、CM膜的吸附动力学过程符合准二级吸附动力学模型,速率主要受化学吸附控制.
(4)吸附膜主要是通过羟基和氨基的化学作用实现对铅离子的吸附,此外,具有多孔性结构的茶渣基体也为铅离子提供大量的吸附位点.
(5)吸附膜的适用范围广,在常见的废水酸度范围内均可发挥作用,使用时相对于粉末型吸附剂更为简单方便,应用前景广阔. 本实验可以为含铅废水的处理提供新思路,具有重要的参考价值.
壳聚糖功能化的茶基水凝胶膜材料的制备及其在Pb2+吸附中的应用
Preparation of chitosan-functionalized tea-based hydrogel membrane materials and their application in Pb2+ adsorption
-
摘要: 为充分发挥茶渣的利用价值,以茶渣为原料,制备了4种不同性能的铅离子吸附剂. 通过改变溶液pH、Pb2+初始浓度、温度和吸附时间等因素探究其最佳吸附条件,并对吸附过程进行吸附热力学和吸附动力学研究. 结果表明,Chitosan/Tea 膜(CT膜)、PVA/Chitosan/Tea 膜(PCT膜)、Chitosan/Modified tea 膜(CM膜)和PVA/Chitosan/Modified tea 膜(PCM膜)的最佳pH值分别为6.0、6.0、5.0和5.0;Pb2+初始浓度为400 mg·L−1时吸附量最大,分别为185.8、160.7、197.0、153.9 mg·g−1. Pb2+初始浓度为20 mg·L−1时吸附率最大,分别为90.1%、90.9%、91.1%和83.8%. 在360 min内,吸附均可达到平衡. 吸附热力学结果显示,CT膜、PCT膜和PCM膜的吸附过程符合Langmuir吸附等温线模型,属于单层吸附;CM膜的吸附过程符合Freundlich吸附等温线模型,属于多层吸附,4种膜的吸附过程均为吸热、熵增、有利的自发过程. 四者的饱和吸附量分别为202.8、173.3、223.7、172.4 mg·g-1. 吸附动力学结果显示,CT膜和PCM膜吸附速率主要受扩散过程控制,符合准一级吸附动力学模型;PCT膜和CM膜吸附速率主要受化学吸附控制,符合准二级吸附动力学模型. 4种吸附膜对Pb2+均具有良好的吸附能力,并且适用的酸度范围较广,使用时操作简单,回收时处理方便,具有重要的参考作用和应用价值.Abstract: This study aimed at preparing four different performance adsorbents for removal of Pb2+ from water in order to give full play to the utilization value of tea waste. And their optimal adsorption conditions were explored by changing the pH of the solution, the initial concentration of Pb2+, the temperature and the time of adsorption. The adsorption thermodynamics and adsorption kinetics of the adsorption process were also investigated. The results show that the optimal pH values of Chitosan/Tea (CT), PVA/Chitosan/Tea (PCT), Chitosan/Modified tea (CM) and PVA/Chitosan/Modified tea (PCM) adsorption films are 6.0, 6.0, 5.0 and 5.0, respectively. The adsorption capacities were the largest at the initial concentration of 400 mg·L−1, which were 185.8, 160.7, 197.0 and 153.9 mg·g−1. And the adsorption rates were the largest at the initial concentration of 20 mg·L−1, which were 90.1%, 90.9%, 91.1% and 83.8%, respectively. The adsorption can reach equilibrium within 360 min. The adsorption thermodynamic results show that the adsorption processes of CT, PCT and PCM adsorption films are in accordance with the Langmuir adsorption isotherm model and belong to monolayer adsorption; while the adsorption processes of CM adsorption film is in accordance with the Freundlich adsorption isotherm model and belongs to multilayer adsorption, the adsorption processes of all four adsorption films are heat-absorbing, entropy-increasing and favorable spontaneous processes. The saturation adsorption capacities of 202.8, 173.3, 223.7 and 172.4 mg·g-1 were obtained for the four adsorption films. Besides, the adsorption kinetic results show that the adsorption rates of CT and PCM adsorption films are mainly controlled by the diffusion process and are consistent with the pseudo-first order kinetic model; the adsorption rates of PCT and CM adsorption films are mainly controlled by chemisorption and are consistent with the pseudo-second order kinetic model. All four adsorption films have good adsorption capacity for Pb2+, and have a wide range of applicable acidity, simple operation in use, easy handling in recovery, large reference role and high application value.
-
Key words:
- tea waste /
- chitosan /
- polyvinyl alcohol /
- Pb2+ /
- adsorption
-

-
表 1 吸附膜的EDAX测试结果
Table 1. EDAX test results of adsorption films
膜的类型
Types of adsorption films元素
Element质量百分比/%
Weight原子百分比/%
AtomicCT膜 C 44.1 56.9 O 44.3 42.6 Mo 0.5 0.1 Pb 11.1 0.8 PCT膜 C 50.9 60.3 O 44.2 39.3 Mo 0.1 0.0 Pb 4.9 0.3 CM膜 C 35.0 56.4 O 33.6 40.6 Mo 0.4 0.1 Pb 30.9 2.9 PCM膜 C 31.2 55.2 O 30.8 40.9 Mo 0.1 0.0 Pb 37.9 3.9 表 2 吸附膜在最佳pH条件下的吸附量和吸附率
Table 2. Adsorption capacity and adsorption rate of adsorption films at optimal pH conditions
膜的类型
Types of adsorption films最佳pH条件
Optimal pH conditionsq/(mg·g−1) r/% CT膜 6.0 92.0 70.1 PCT膜 6.0 83.2 78.5 CM膜 5.0 84.8 71.4 PCM膜 5.0 74.0 74.0 表 3 吸附膜在不同pH条件下的溶解情况
Table 3. Dissolution of adsorption films under different pH conditions
膜的类型
Types of adsorption films在不同酸性条件下的溶解情况
Dissolution under different acidic conditionsCT膜 pH>1.75时不溶解 PCT膜 pH在0—14范围内不溶解 CM膜 pH=1.5时较快溶解,pH>2时不溶解 PCM膜 pH>1.75时不溶解 表 4 吸附膜的热力学参数
Table 4. Thermodynamic parameters of adsorption films
膜的类型
Types of adsorption filmsT/K ΔG/(kJ·mol−1) ΔH/(kJ·mol−1) ΔS/(J·mol−1·K−1) CT膜 303.15 −2.8 9.2 39.7 308.15 −3.1 313.15 −3.2 PCT膜 303.15 −3.4 13.5 55.9 308.15 −3.7 313.15 −4.0 CM膜 303.15 −2.7 7.7 34.3 308.15 −2.9 313.15 −3.1 PCM膜 303.15 −1.8 7.6 30.9 308.15 −2.0 313.15 −2.1 表 5 吸附膜的吸附等温线模型参数
Table 5. Adsorption isotherm model parameters of adsorption films
膜的类型
Types of
adsorption filmsLangmuir吸附等温线模型
Langmuir adsorption isotherm modelFreundlich吸附等温线模型
Freundlich adsorption isotherm model符合的模型
Compliant modelsqm/(mg·g−1) b/(L·mg−1) R2 n KF/(mg·L−1) R2 CT膜 202.8 0.0390 0.993 2.34 20.65 0.969 Langmuir吸附等温线模型 PCT膜 173.3 0.0311 0.964 2.35 16.35 0.936 Langmuir吸附等温线模型 CM膜 223.7 0.0256 0.973 2.18 16.89 0.995 Freundlich吸附等温线模型 PCM膜 172.4 0.0267 0.987 2.09 12.55 0.960 Langmuir吸附等温线模型 表 6 吸附膜的吸附动力学模型参数
Table 6. Adsorption kinetics model parameters of adsorption films
膜的类型
Types of
adsorption filmsqe,实验/
(mg·g−1)准一级吸附动力学模型拟合
Fitting of Pseudo-first order model准二级吸附动力学模型拟合
Fitting of Pseudo-second order model符合的模型
Compliant modelsqe/(mg·g−1) k1/(min−1) R2 qe/(mg·g−1) k2/[g·(mg·min)−1] R2 CT膜 92.0 134.2 0.0143 0.986 119.3 5.60×10−5 0.920 准一级吸附动力学方程 PCT膜 83.2 96.7 0.0163 0.987 90.3 2.41×10−4 0.996 准二级吸附动力学方程 CM膜 79.8 102.9 0.0131 0.993 98.0 1.21×10−4 0.989 准二级吸附动力学方程 PCM膜 69.2 78.9 0.0136 0.993 78.3 1.83×10−4 0.992 准一级吸附动力学方程 -
[1] MANNA K, DEBNATH B, SINGH W. Sources and toxicological effects of lead on human health [J]. Indian Journal of Medical Specialities, 2019, 10(2): 66. doi: 10.4103/INJMS.INJMS_30_18 [2] 连灵君, 徐立红. 氧化损伤与铅毒性研究进展 [J]. 环境与职业医学, 2007, 24(4): 435-439. doi: 10.3969/j.issn.1006-3617.2007.04.023 LIAN L J, XU L H. A review of studies on oxidative damage and lead toxicity [J]. Journal of Environmental & Occupational Medicine, 2007, 24(4): 435-439(in Chinese). doi: 10.3969/j.issn.1006-3617.2007.04.023
[3] DHARMAPRIYA T N, LI D Y, CHUNG Y C, et al. Green synthesis of reusable adsorbents for the removal of heavy metal ions [J]. ACS Omega, 2021, 6(45): 30478-30487. doi: 10.1021/acsomega.1c03879 [4] TEH C Y, BUDIMAN P M, SHAK K P Y, et al. Recent advancement of coagulation-flocculation and its application in wastewater treatment [J]. Industrial & Engineering Chemistry Research, 2016, 55(16): 4363-4389. [5] LIU C, WU T, HSU P C, et al. Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode [J]. ACS Nano, 2019, 13(6): 6431-6437. doi: 10.1021/acsnano.8b09301 [6] 黄峥, 黄泽界, 杨莹. 茶叶机械化生产加工现状与思考 [J]. 农业工程与装备, 2021, 48(5): 10-12,26. doi: 10.3969/j.issn.1007-8320.2021.05.003 HUANG Z, HUANG Z J, YANG Y. Current situation and consideration of tea mechanization production and processing [J]. Agricultural Engineering and Equipment, 2021, 48(5): 10-12,26(in Chinese). doi: 10.3969/j.issn.1007-8320.2021.05.003
[7] PERUMAL S, ATCHUDAN R, YOON D H, et al. Spherical chitosan/gelatin hydrogel particles for removal of multiple heavy metal ions from wastewater [J]. Industrial & Engineering Chemistry Research, 2019, 58(23): 9900-9907. [8] 王威振, 陈颢明, 闵芳芳, 等. 不同部位梧桐生物质炭对水溶液中镉吸附的机理 [J]. 环境化学, 2022, 41(1): 327-339. doi: 10.7524/j.issn.0254-6108.2020082701 WANG W Z, CHEN H M, MIN F F, et al. Mechanism of cadmium adsorption by biochar from different parts of platanus acerifolia in aqueous solution [J]. Environmental Chemistry, 2022, 41(1): 327-339(in Chinese). doi: 10.7524/j.issn.0254-6108.2020082701
[9] 刘爽, 汪东风, 徐莹. 磷酸活化茶渣生物炭对铅的吸附性能影响和吸附机理研究 [J]. 中国海洋大学学报(自然科学版), 2022, 52(1): 56-64. doi: 10.16441/j.cnki.hdxb.20210070 LIU S, WANG D F, XU Y. Studies on lead adsorption performance of phosphoric acid activated tea residue biochar and associating mechanism [J]. Periodical of Ocean University of China, 2022, 52(1): 56-64(in Chinese). doi: 10.16441/j.cnki.hdxb.20210070
[10] 焦天宇, 王宇宁, 向巧灵, 等. 左氧氟沙星/壳聚糖/聚乙烯醇纳米纤维膜的制备与抑菌性能研究 [J]. 化学与粘合, 2022, 44(1): 32-38. doi: 10.3969/j.issn.1001-0017.2022.01.009 JIAO T Y, WANG Y N, XIANG Q L, et al. Preparation and antibacterial properties of levofloxacin/chitosan/polyvinyl alcohol nanofibrous membrane [J]. Chemistry and Adhesion, 2022, 44(1): 32-38(in Chinese). doi: 10.3969/j.issn.1001-0017.2022.01.009
[11] 刘珊, 赵春朋, 李涛, 等. 改性壳聚糖凝胶球对Cu(Ⅱ)的吸附 [J]. 环境化学, 2020, 39(7): 2013-2021. doi: 10.7524/j.issn.0254-6108.2019051303 LIU S, ZHAO C P, LI T, et al. Adsorption of Cu(Ⅱ) by modified chitosan gel ball [J]. Environmental Chemistry, 2020, 39(7): 2013-2021(in Chinese). doi: 10.7524/j.issn.0254-6108.2019051303
[12] SHARMA P, SINGH A K, SHAHI V K. Selective adsorption of Pb(II) from aqueous medium by cross-linked chitosan-functionalized graphene oxide adsorbent [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1427-1436. [13] JIN L, BAI R B. Mechanisms of lead adsorption on chitosan/PVA hydrogel beads [J]. Langmuir, 2002, 18(25): 9765-9770. doi: 10.1021/la025917l [14] 何春晓. 分光光度法测定水中铅的含量 [J]. 山东化工, 2018, 47(18): 62-63,67. doi: 10.3969/j.issn.1008-021X.2018.18.027 HE C X. Determination of lead in water by spectrophotometry [J]. Shandong Chemical Industry, 2018, 47(18): 62-63,67(in Chinese). doi: 10.3969/j.issn.1008-021X.2018.18.027
[15] 刘晶. 金属离子在典型铁氧化物表面的吸附、氧化/还原及结晶生长研究[D]. 广州: 中国科学院大学(中国科学院广州地球化学研究所), 2019. LIU J. The ad/desorption, redox, and crystallization of metals on typical iron (oxyhydr) oxides[D]. Guangzhou: Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, 2019 (in Chinese).
[16] 李婷婷. 壳聚糖衍生材料制备及其对水中铅和酸性橙7的吸附机理研究[D]. 长沙: 湖南大学, 2016. LI T T. Synthesis of chitosan derived materials and their adsorption mechanisms for Pb(Ⅱ) and acid orange 7 from aqueous solutions[D]. Changsha: Hunan University, 2016 (in Chinese).
[17] 龚新怀, 李明春, 杨坤, 等. 纳米Fe3O4@茶渣/海藻酸钙磁性复合材料制备及其对亚甲基蓝的吸附性能与吸附机制 [J]. 复合材料学报, 2021, 38(2): 424-438. GONG X H, LI M C, YANG K, et al. Preparation of nano-Fe3O4@tea waste/calcium alginate magnetic composited bead and it’s adsorption characteristics and mechanisms for methylene blue from aqueous solution [J]. Acta Materiae Compositae Sinica, 2021, 38(2): 424-438(in Chinese).
-




 下载:
下载: