-
纳米金属氧化物(MeONPs)因具有良好的光学、磁性和电子性能,广泛应用于催化剂、传感器、光学材料、电气材料和磁性存储器等行业[1-4]. MeONPs可以长期漂浮在空气中,容易通过肺部吸入进入人体,并沉积在肺部深处,即肺泡区域[5-8]. 在肺泡区域,MeONPs可以直接与肺部表面活性物质(PS)接触[8-9]. PS由脂质(90%)和蛋白质(10%)组成,在哺乳动物中含量最多的是二棕榈酰磷脂酰胆碱(DPPC),约占PS总质量的40%[10-11]. PS在与MeONPs接触后,会吸附在MeONPs表面形成脂质冠. 已有研究表明,对于聚苯乙烯纳米粒子,随着其表面吸附PS的增加,扁平型肺泡Ⅱ型上皮细胞对纳米粒子的细胞摄取量也呈现增加趋势[12]. 此外也有研究表明,由于金红石型 TiO2( TiO2-R) 比锐钛矿型 TiO2 (TiO2-A)对脂质的吸附能力更强,使TiO2-R更易破坏小鼠巨噬细胞的溶酶体膜进而导致细胞调亡[13]. 因此,MeONPs对脂质的吸附量将会影响MeONPs的细胞摄取和毒性[5,12].
近年来,有关纳米粒子对脂质吸附能力影响因素的研究备受关注. Konduru等[14]的研究表明,由于CeO2纳米粒子的疏水性高于Si-CeO2、ZnO和BaSO4纳米粒子,使得其对小鼠PS吸附量高于其他3种纳米粒子. Luo等[15]通过分子动力学研究发现,随着石墨烯纳米片长度的增加,其对PS的吸附量呈线性增加. 此外,Luo等[16]还发现与立方体和球形碳纳米粒子相比,长方体和四面体碳纳米粒子吸附PS量显著增加,并导致PS层破裂. 因此,MeONPs的疏水性、尺寸、形状等理化性质都是影响脂质吸附能力的重要因素.
目前关于MeONPs对脂质吸附的定量研究十分匮乏,前人研究涉及的MeONPs种类非常有限,仅包括:TiO2-R 、TiO2-A 、CeO2、Si-CeO2、ZnO、Fe2O3[13-14,17-18]. 但由于合成MeONPs种类(化学组成、晶体构型和尺寸上具有差异)不断增多,逐一进行吸附实验测定成本高且耗时. 因此,需要建立一种定量预测MeONPs对脂质吸附量的模型,阐明MeONPs与脂质吸附的相互作用机制.
本研究将利用超声分散法制备的DPPC囊泡与25种不同晶型和粒径的MeONPs孵育,达到吸附平衡后,通过高效液相色谱串联质谱联用仪(LC-MS/MS)定量测定了DPPC的吸附量,分析了影响DPPC吸附的关键因素. 引入MeONPs对磷酸盐的吸附量作为描述符,并结合MeONPs实验测定理化性质及元素周期表描述符,构建了MeONPs对脂质吸附量的定量预测模型,揭示了影响MeONPs对脂质吸附量的关键因素.
-
25种不同晶型粒径的MeONPs包括:氧化钇(Y2O3,50 nm)、氧化钕(Nd2O3,40 nm)、氧化铁(Fe2O3,30 nm)、氧化镨(Pr6O11,50 nm)、氧化钐(Sm2O3,40 nm)、氧化镝(Dy2O3,40 nm)、氧化钆(Gd2O3,100 nm)、氧化钬(Ho2O3,100 nm)、氧化镍(NiO,30 nm)、氧化镍(NiO,50 nm)、氧化钴(Co3O4,50 nm)、氧化钴(Co3O4,100 nm)、氧化铟(In2O3,50 nm)、氧化铟(In2O3,100 nm)、氧化铝(Al2O3,10 nm)、氧化铝(Al2O3,20 nm)、氧化铝(Al2O3,30 nm)、氧化锌(ZnO,30 nm)、氧化锌(ZnO,50 nm)、氧化锌(ZnO,90 nm)、二氧化锡(SnO2,60 nm)、三氧化二铬(Cr2O3,100 nm)、二氧化钛(金红,TiO2-R,100 nm)、二氧化钛(锐钛,TiO2-A,10 nm)、二氧化钛(锐钛,TiO2-A,40 nm). 上述材料均纯度 > 99%,购买自上海阿拉丁生化科技股份有限公司. DPPC(纯度 ≥ 99%)购买自美国Avanti Polar Lipids公司. Hepes缓冲溶液购买自美国西格玛奥德里奇公司.
-
本研究采用超声分散法制备脂质囊泡,具体制备过程如下:取25 mg的DPPC溶于10 mL甲醇,充分涡旋后,制得2.5 mg·mL−1的DPPC储备液,于 −20 ℃下冷冻保存. 取400 µL DPPC储备液于玻璃容器中,加入适量甲醇并涡旋后,将溶液氮吹至干. 向玻璃容器中加入10 mL Hepes缓冲溶液(1 mmol·L−1 NaCl,pH 7.4)水化脂质膜,并加盖在室温下静置1 h后,涡旋形成乳白色的溶液. 将该溶液转移至50 mL圆底塑料管中,在冰浴条件下使用超声波细胞破碎仪(JY92-IIDN,中国)分散囊泡. 超声波细胞破碎仪的条件设置为温度20 ℃,有效超声时间为50%(超声5 s,间隙5 s),超声至溶液澄清,得到的溶液即为10 µg·mL−1的DPPC溶液,于4 ℃的冰箱保存[19].
-
取300 µL的DPPC溶液(10 µg·mL−1)和150 µL的MeONPs溶液(10 mg·L−1)于Hepes缓冲溶液中孵育,每种MeONPs设置3组平行实验. 同时设置空白组实验,孵育条件同上. 将所有孵育溶液置于25 ℃恒温振荡箱中振荡4 h.
-
4 h后将孵育溶液在14500 g的条件下离心50 min后,取100 µL上清液加入装有900 µL甲醇的液相小瓶中,加入20 µL 2.5 mg·L−1的油酰磷脂酰胆碱(POPC,母离子:760.8 m/z;子离子:598.7 m/z)作为内标,利用高效液相色谱串联质谱联用仪(LC-MS/MS)(UPLC I-Class-Waters Xevo TQS,美国)定量测定DPPC(母离子:734.7 m/z;子离子:184.1 m/z). LC-MS/MS的条件如下:流动相A:水,流动相B:甲醇;流动相A和B各包含0.01%甲酸和5 mmol·L−1乙酸铵;流动相A : B = 2 : 98;流速:0.3 mL·min−1;进样体积:5 µL;离子源:电喷雾电离(正离子模式);驻留时间:0.003 s;锥孔电压:50 V;碰撞能量:30 V. MeONPs对DPPC吸附量的计算公式为:
式中,Qm为MeONPs对DPPC的吸附量,Q0为空白组上清液中DPPC含量,Q为MeONPs与DPPC孵育溶液上清液中DPPC含量.
-
MeONPs(80 mg·L−1)与磷酸二氢钾(85 mg·L−1),在4 mL水溶液中孵育24 h,将孵育后的溶液经0.45 µm的尼龙膜(聚醚砜,津腾)过滤后,取1 mL溶液于具塞比色管中,加入去离子水定容至50 mL. 每种MeONPs设置3组平行实验,并设置3组空白实验.
向所有具塞比色管中加入1 mL 10%抗坏血酸溶液并混匀,30 s后加入2 mL钼酸盐溶液充分混匀,静置15 min. 使用紫外/可见光谱仪(Agilent, Cary 100, Malaysia),在700 nm的波长下测定溶液吸光度. MeONPs对磷酸盐吸附量的计算公式为:
式中,
$Q'_{{\rm{m}}} $ 为MeONPs对磷酸盐的吸附量,Q′0为空白组中磷酸盐含量,$Q' $ 为MeONPs与磷酸盐反应溶液中磷酸盐的含量. -
利用透射电子显微镜(TEM)(FEI, Tecnai G2F30 STWIN, 美国)测定MeONPs的初始粒径. 结合TEM图像,对每个MeONPs随机计数300个粒子计算平均粒径. 以Hepes缓冲溶液为溶剂配制50 µg·mL−1 MeONPs溶液,使用马尔文纳米粒度仪(Malvern,Nano-ZS90,英国)测定MeONPs的水动力学直径和Zeta电势,每种材料测量3次取平均值. 使用接触角测量仪(KINO,SL 200KB,美国)测量MeONPs接触角,每种材料测量3次计平均值.
-
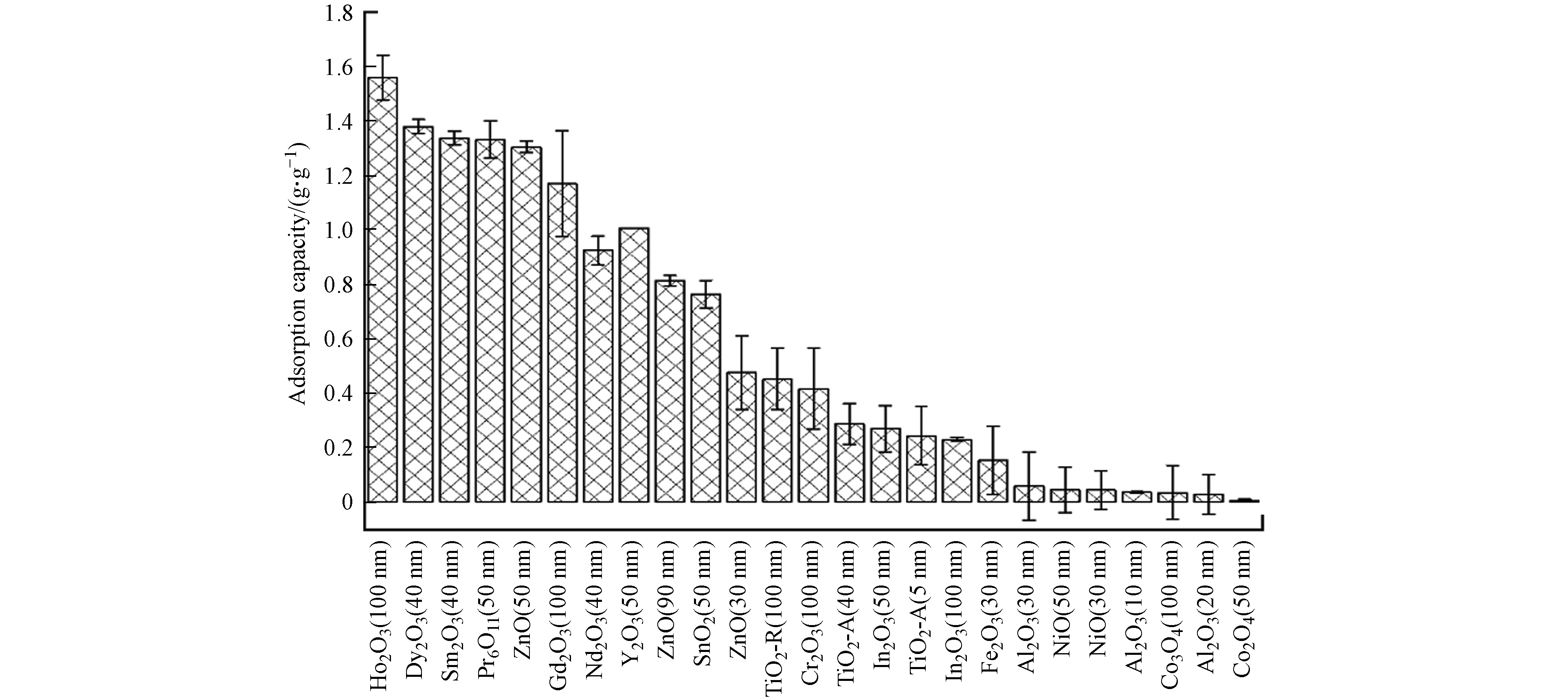
本文定量测定了25种不同晶型和粒径的MeONPs对DPPC的吸附量,以每克MeONPs吸附的DPPC量作为评价MeONPs对DPPC吸附能力的指标.
-
本研究选取每克MeONPs对磷酸盐的吸附量、MeONPs理化性质和元素周期表描述符,构建预测模型. 其中4个理化性质描述符分别为:MeONPs的初始粒径、水动力学直径、Zeta电势和接触角. 4个元素周期表描述符分别为:MeONPs的分子量、阳离子电荷、金属元素电负性以及金属元素质量百分比.
-
将数据集按4∶1随机分为训练集和验证集,训练集包含20个数据点,验证集包含5个数据点. 使用SIMCA 13.0软件中的偏最小二乘(PLS)回归建立预测MeONPs对脂质吸附量的模型[20].
模型拟合能力和稳健性通过调整后的决定系数(
$ R^2_{\rm{adj}} $ )、均方根误差(RMSE)以及去一法计算得到的交叉验证系数($ Q^2_{\rm{cv}} $ )进行评价[21-23]. 外部预测能力采用验证集的均方根误差(RMSEext)和外部验证系数($ Q^2_{\rm{ext}} $ )评价[21-23].采用基于标准残差(δ)和杠杆值(hi)绘制的Williams图表征模型的应用域,δ和hi的计算公式如下所示:
式中,yi 和 y分别为第i个化合物活性数据的实测值和预测值,n为训练集化合物个数,m为模型中描述符的个数.
式中,xi 和 xT i分别为描述符向量及其转置,X为描述符矩阵,XT是X的转置,h*为杠杆警戒值,m为模型中描述符的个数,n为训练集中化合物的个数. 当化合物|δ|>3.0 时被视为模型离群点. 当化合物 |δ|<3.0 且 hi<h* 时,表明模型预测效果较好. 当化合物 |δ|<3.0 且 hi>h*时,若出现在训练集,表明在模型数据集中该类化合物较少,但该化合物提高了模型的准确性和稳定性;若该结果出现在验证集中,说明模型具有一定的外推能力[24-25].
-
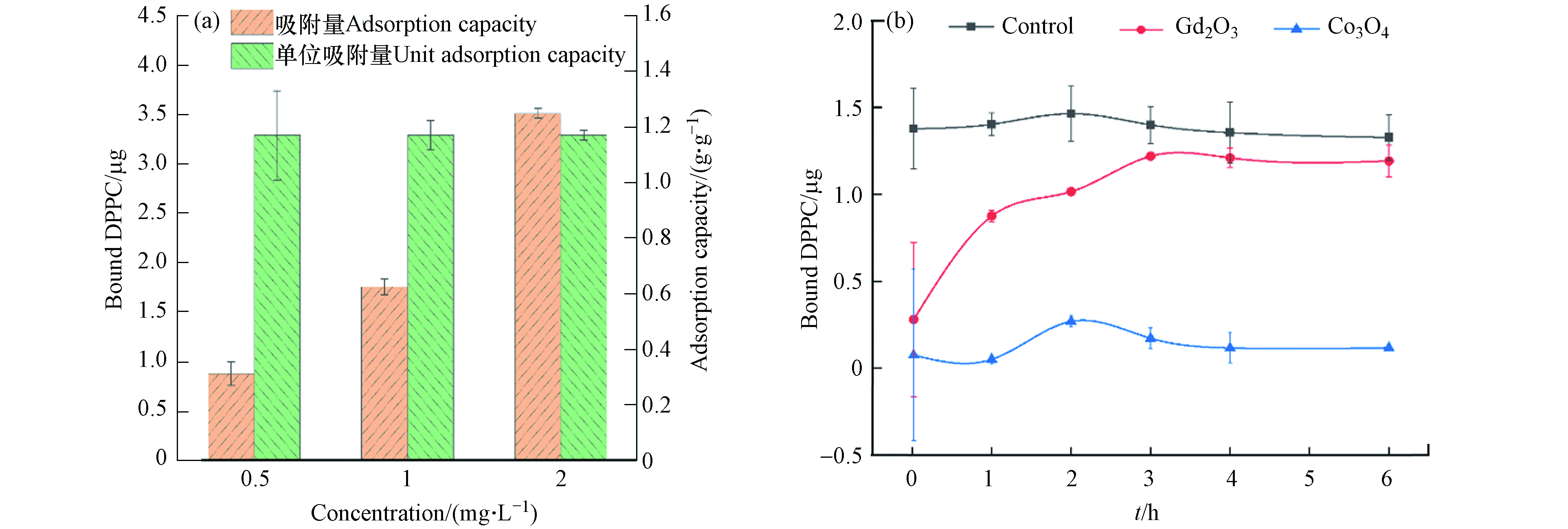
本研究以Gd2O3例,测定纳米颗粒在3种孵育浓度下(0.5、1、2 mg·L−1)对DPPC(2 mg·L−1)的吸附量,发现3种孵育浓度下Gd2O3对DPPC的吸附量均为1.17 g·g−1(图1a),表明这3种体系中DPPC分子的数量相较于Gd2O3的吸附位点来说是足够的.
因此,本研究后续实验中DPPC的孵育浓度设为:2 mg·L−1;同时为确保吸附能力强于Gd2O3的纳米颗粒能够达到饱和吸附且区分不同MeONPs对DPPC的吸附能力,将MeONPs的孵育浓度设为:1 mg·L−1. 此外,基于预实验结果,本研究以吸附量相对较多的Gd2O3和吸附量相对较少的Co3O4为例,进行吸附动力学实验(图1b). 通过测定Gd2O3和Co3O4在不同孵育时间对DPPC的吸附量,发现孵育4 h后,两种MeONPs对DPPC的吸附量趋于平衡,因此本研究设置孵育时间为4 h.
-
本研究测定的10 nm和40 nm TiO2-A对DPPC 的吸附量分别为0.24 g·g−1和0.28 g·g−1(图2 ). Yu等[13]测定了平均粒径为27—28 nm的TiO2-A对磷脂的吸附量(0.4 g·g−1),该研究的磷脂中DPPC占比50%,表明TiO2-A对DPPC的吸附量约为0.2 g·g−1,与本研究测定的TiO2-A的吸附结果相似.
25种不同晶型和粒径的MeONPs对DPPC吸附量的定量结果如图2所示. 与本研究其他MeONPs相比,稀土金属氧化物(Ho2O3、Dy2O3、Sm2O3、Pr6O11、Gd2O3、Nd2O3、Y2O3)对DPPC有相对较高的吸附量(>1 g·g−1),说明稀土金属元素有利于MeONPs对DPPC的吸附.
本研究中涉及的TiO2-A、In2O3、Al2O3、NiO和Co3O4的粒径在10—100 nm范围内变化. 这些纳米材料对DPPC的吸附量不随粒径的变化而改变(P>0.05),表明粒径不是影响DPPC吸附的关键因素. Thorley等[12]的研究也表明对于50 nm和100 nm的聚苯乙烯纳米粒子,其对人支气管肺泡灌洗液的吸附量无显著性差异. 由此可知,粒径在10—100 nm范围内的纳米颗粒,无论对于吸附PS还是DPPC,粒径并不是影响吸附的关键因素.
本研究中50 nm ZnO对DPPC的吸附量显著高于 90 nm和30 nm ZnO(P<0.05). 这可能是由于本研究中50 nm ZnO的疏水性(接触角17°)强于90 nm (接触角7.62°)和30 nm ZnO(接触角6.27°),使其更易与DPPC疏水的尾部发生吸附. Raesch等[8]的研究也表明,与聚乳酸-羟基乙酸共聚物磁性纳米粒子和脂质包裹的磁铁矿纳米粒子相比,较为亲水的聚乙二醇磁性纳米粒子吸附较少的猪PS. 上述结果表明,接触角大的强疏水性纳米颗粒更易与磷脂的尾部发生吸附.
-
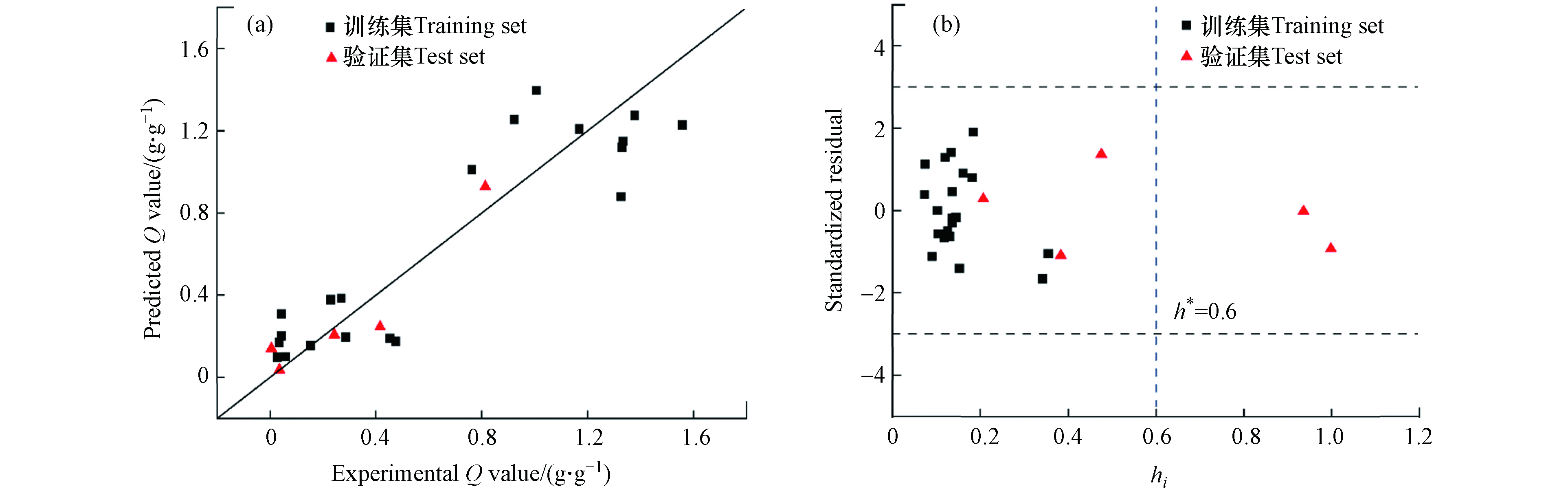
将数据集按4:1随机分为训练集和验证集,其中训练集包含20个数据点,验证集包含5个数据点. 基于PLS回归建立了预测MeONPs对脂质吸附量的模型,表达式如下:
式中, Q为每克MeONPs对DPPC的吸附量,
$Q' $ 为每克MeONPs对磷酸盐的吸附量,$\,\chi_{\rm{me}} $ 为金属元素电负性,M(%)为金属元素质量百分比.模型性能评价结果表明,该模型具有良好的拟合度 (
$ R^2_{\rm{adj}} $ = 0.79,RMSEtra = 0.23)、稳健性 ($ Q^2 _{\rm{cv}} $ = 0.74) 和预测能力 ($Q^2_{\rm{ext}} $ = 0.86). MeONPs对DPPC吸附能力的实测值和模型预测值的拟合结果见图3a. 采用 Williams 图对模型的应用域进行表征(图3b). 从图中可以看出,所有MeONPs的 |δ| < 3.0,表明模型没有离群点. 验证集中有两种MeONPs的杠杆值大于杠杆警戒值(hi > h*),说明这两种MeONPs是对模型有显著影响的化合物. 且这两种MeONPs的|δ| < 3.0,说明模型对此类远离描述符空间中心的化合物具有较好的泛化能力. 综上,本研究构建的模型可以用于预测MeONPs对DPPC的吸附量. -
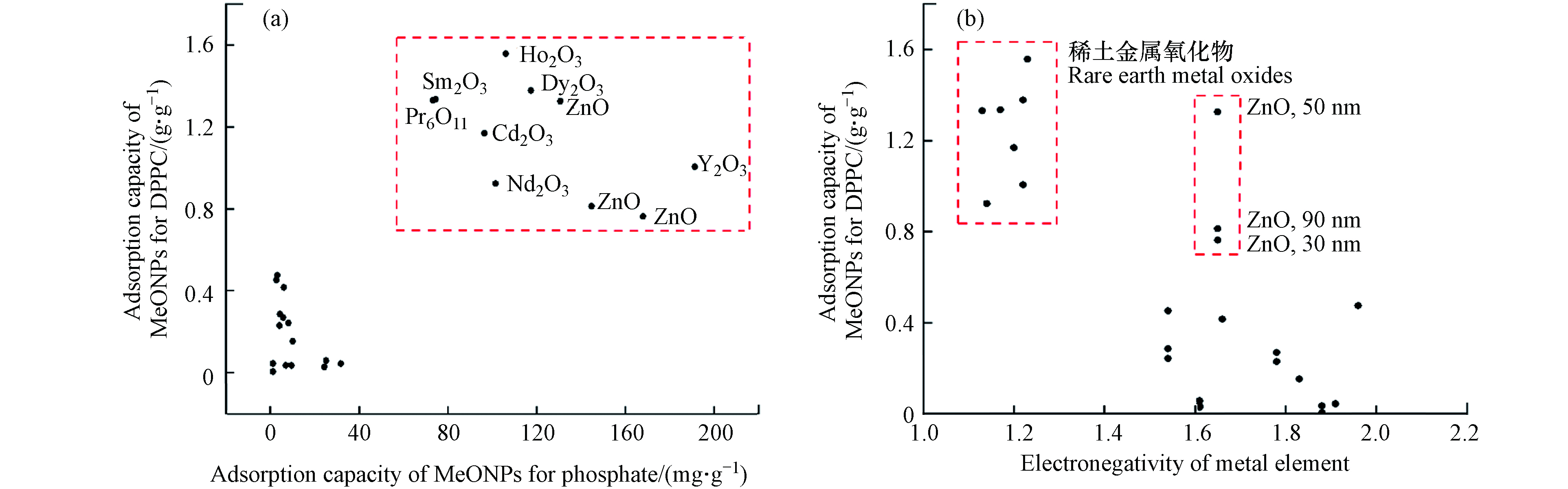
模型结果表明,MeONPs吸附磷酸盐的能力是影响MeONPs对DPPC吸附的关键因素. 如图4a所示,对于DPPC吸附量相对较高的MeONPs(> 0.7 g·g−1),其对磷酸盐也有较高的吸附量(> 73 mg·g−1). 在这些吸附量较高的MeONPs中,除3种不同粒径的ZnO外,均为稀土金属氧化物. 该结果表明,DPPC头部的磷酸基团是稀土金属氧化物对DPPC吸附的重要位点.
MeONPs金属元素电负性也是DPPC的吸附重要影响因素. 如图4b所示,当MeONPs金属元素电负性较低(< 1.22)时,更易与DPPC发生吸附. 这可能是由于电负性较低的金属元素均为稀土金属,与稀土金属氧化物发生吸附的DPPC头部磷酸基团的电负性为2.87,更易与电负性较小的金属原子发生作用. 因此,当MeONPs元素电负性较高时(> 1.22),相对不利于其对磷酸基团的吸附.
此外,模型中金属元素质量百分比的回归系数为正,这反映MeONPs的金属元素质量百分比越大,其对DPPC的吸附量越大. 本研究中3种不同粒径的ZnO,其Zn元素的电负性(1.65)较大,但它们对DPPC的吸附量却相对较高(图4b),这可能与ZnO的金属元素质量百分比较大有关. ZnO的金属元素质量百分比为80.34,其值大于其他非稀土金属氧化物的金属元素质量百分比(52.92—78.77). 这进一步说明了金属元素质量百分比较大的纳米颗粒更有利于DPPC的吸附.
-
本研究以25种不同晶型和粒径的MeONPs为研究对象,将DPPC囊泡与其孵育4 h后,利用LC-MS/MS定量测定了其对DPPC的吸附量,并建立了可以快速、低成本地预测MeONPs对DPPC吸附量的模型. 吸附实验结果显示,本研究中的稀土金属氧化物(Ho2O3、Dy2O3、Sm2O3、Pr6O11、Gd2O3、Nd2O3、Y2O3)对DPPC有相对较高的吸附量(> 1 g·g−1),说明稀土金属元素促进了MeONPs对DPPC的吸附. 对于10—100 nm范围内具有不同粒径的MeONPs(包括TiO2-A、In2O3、Al2O3、NiO和Co3O4),粒径不是影响这些纳米颗粒对DPPC吸附的关键因素. 针对3种不同粒径ZnO的吸附量与其接触角的分析发现,接触角大的强疏水性纳米颗粒更易与磷脂的尾部发生吸附. 模型结果显示,对DPPC吸附量相对较高的MeONPs,其对磷酸盐的吸附量也较高,且这些纳米颗粒大部分为稀土金属氧化物,表明DPPC头部的磷酸基团是稀土金属氧化物对DPPC的重要吸附位点. 金属元素电负性及金属元素质量百分比共同影响MeONPs对DPPC的吸附,当MeONPs金属元素电负性较低(< 1.22)时,更易与DPPC发生吸附. 本研究为纳米颗粒对脂质吸附量的定量评价提供了关键数据,并有助于拓展对纳米颗粒与脂质发生吸附作用机制的理解.
纳米金属氧化物对脂质吸附能力的定量预测模型
Prediction model of the adsorption capacity of metal oxide nanoparticles for lipids
-
摘要: 纳米金属氧化物(MeONPs)对肺部表面活性物质(PS)的主要成分脂质的吸附量,是影响呼吸暴露生物效应的重要因素. 目前关于MeONPs对脂质吸附的定量研究十分有限. MeONPs种类繁多,有必要构建MeONPs对脂质吸附量的预测模型. 本研究利用二棕榈酰磷脂酰胆碱(DPPC)囊泡模拟PS,测定25种MeONPs对DPPC的吸附量,建立了可预测MeONPs对DPPC吸附量的模型. 结果显示,预测模型具有良好的拟合度、稳健性和预测能力(
$ R^2_{\rm{adj}} $ = 0.79,$ Q^2_{\rm{cv}} $ = 0.74,$ Q^2_{\rm{ext}} $ = 0.86). 机理解释表明,DPPC头部的磷酸基团是稀土金属氧化物吸附DPPC的重要位点,且MeONPs的金属元素电负性和金属元素质量百分比共同影响DPPC的吸附. 本研究建立的预测模型不仅为MeONPs对脂质吸附能力评价提供了基础数据,还拓展了对脂质吸附机制的理解.Abstract: Adsorption capacity of metal oxide nanoparticles (MeONPs) for lipids that are the main components of pulmonary surfactant (PS), is an important factor affecting the biological effect of nanoparticles entering the human body through the respiratory tract. At present, studies on the adsorption capacity of MeONPs for lipids are very limited. Due to the increasing variety of synthetic MeONPs, it is necessary to establish a model to predict the adsorption capacity of MeONPs for lipids. In this study, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) vesicles were used to simulate PS, and 25 kinds of MeONPs were incubated with DPPC to determine the adsorption capacity of MeONPs for DPPC. The model for predicting the adsorption capacity of MeONPs for DPPC was established based on the experimental data. The results show that the prediction model exhibits satisfactory goodness of fit, robustness and predictive ability ($R^2_{\rm{adj}} $ = 0.79,$ Q^2_{\rm{cv}} $ = 0.74,$ Q^2_{\rm{ext}} $ = 0.86). The explanation of the mechanism suggests that the phosphoric groups in the head of DPPC are important adsorption sites of rare earth metal oxides for DPPC, and the electronegativity of metal elements and the mass percentage of metal elements jointly affect the adsorption capacity of MeONPs for DPPC. The prediction model established in this study not only provides basic data for the evaluation of the adsorption capacity of MeONPs for lipids, but also expands the understanding of the adsorption mechanism of MeONPs for lipids.-
Key words:
- metal oxide nanoparticles /
- lipids /
- pulmonary surfactant /
- adsorption /
- prediction model.
-

-
-
[1] BASANT N, GUPTA S. Multi-target QSTR modeling for simultaneous prediction of multiple toxicity endpoints of nano-metal oxides [J]. Nanotoxicology, 2017, 11(3): 339-350. doi: 10.1080/17435390.2017.1302612 [2] KIM T, HYEON T. Applications of inorganic nanoparticles as therapeutic agents [J]. Nanotechnology, 2014, 25(1): 012001. doi: 10.1088/0957-4484/25/1/012001 [3] LI Y, YU S L, YUAN T Z, et al. Rational design of metal oxide nanocomposite anodes for advanced lithium ion batteries [J]. Journal of Power Sources, 2015, 282: 1-8. doi: 10.1016/j.jpowsour.2015.02.016 [4] ZHAO Z H, TIAN J, SANG Y H, et al. Structure, synthesis, and applications of TiO2 nanobelts [J]. Advanced Materials, 2015, 27(16): 2557-2582. doi: 10.1002/adma.201405589 [5] KAPRALOV A A, FENG W H, AMOSCATO A A, et al. Adsorption of surfactant lipids by single-walled carbon nanotubes in mouse lung upon pharyngeal aspiration [J]. ACS Nano, 2012, 6(5): 4147-4156. doi: 10.1021/nn300626q [6] ZHAO Q, LI Y J, CHAI X L, et al. Interaction of pulmonary surfactant with silica and polycyclic aromatic hydrocarbons: Implications for respiratory health [J]. Chemosphere, 2019, 222: 603-610. doi: 10.1016/j.chemosphere.2019.02.002 [7] KIM K H, KABIR E, KABIR S. A review on the human health impact of airborne particulate matter [J]. Environment International, 2015, 74: 136-143. doi: 10.1016/j.envint.2014.10.005 [8] RAESCH S S, TENZER S, STORCK W, et al. Proteomic and lipidomic analysis of nanoparticle Corona upon contact with lung surfactant reveals differences in protein, but not lipid composition [J]. ACS Nano, 2015, 9(12): 11872-11885. doi: 10.1021/acsnano.5b04215 [9] MONOPOLI M P, ÅBERG C, SALVATI A, et al. Biomolecular coronas provide the biological identity of nanosized materials [J]. Nature Nanotechnology, 2012, 7(12): 779-786. doi: 10.1038/nnano.2012.207 [10] PARRA E, PÉREZ-GIL J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films [J]. Chemistry and Physics of Lipids, 2015, 185: 153-175. doi: 10.1016/j.chemphyslip.2014.09.002 [11] GUAGLIARDO R, PÉREZ-GIL J, de SMEDT S, et al. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins [J]. Journal of Controlled Release, 2018, 291: 116-126. doi: 10.1016/j.jconrel.2018.10.012 [12] THORLEY A J, RUENRAROENGSAK P, POTTER T E, et al. Critical determinants of uptake and translocation of nanoparticles by the human pulmonary alveolar epithelium [J]. ACS Nano, 2014, 8(11): 11778-11789. doi: 10.1021/nn505399e [13] YU Q L, WANG H G, PENG Q, et al. Different toxicity of anatase and rutile TiO2 nanoparticles on macrophages: Involvement of difference in affinity to proteins and phospholipids [J]. Journal of Hazardous Materials, 2017, 335: 125-134. doi: 10.1016/j.jhazmat.2017.04.026 [14] KONDURU N V, DAMIANI F, STOILOVA-MCPHIE S, et al. Nanoparticle wettability influences nanoparticle-phospholipid interactions [J]. Langmuir, 2018, 34(22): 6454-6461. doi: 10.1021/acs.langmuir.7b03741 [15] LUO Z, LI S X, XU Y, et al. Extracting pulmonary surfactants to form inverse micelles on suspended graphene nanosheets [J]. Environmental Science:Nano, 2018, 5(1): 130-140. doi: 10.1039/C7EN00843K [16] LUO Z, LI S X, XU Y, et al. The role of nanoparticle shape in translocation across the pulmonary surfactant layer revealed by molecular dynamics simulations [J]. Environmental Science:Nano, 2018, 5(8): 1921-1932. doi: 10.1039/C8EN00521D [17] CHHODEN T, CLAUSEN P A, LARSEN S T, et al. Interactions between nanoparticles and lung surfactant investigated by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry [J]. Rapid Communications in Mass Spectrometry, 2015, 29(11): 1080-1086. doi: 10.1002/rcm.7199 [18] WANG F, LIU J W. Liposome supported metal oxide nanoparticles: Interaction mechanism, light controlled content release, and intracellular delivery [J]. Small, 2014, 10(19): 3927-3931. doi: 10.1002/smll.201400850 [19] ZHANG X, PANDIAKUMAR A K, HAMERS R J, et al. Quantification of lipid Corona formation on colloidal nanoparticles from lipid vesicles [J]. Analytical Chemistry, 2018, 90(24): 14387-14394. doi: 10.1021/acs.analchem.8b03911 [20] BYLESJÖ M, RANTALAINEN M, CLOAREC O, et al. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification [J]. Journal of Chemometrics, 2006, 20(8/9/10): 341-351. [21] SCHÜÜRMANN G, EBERT R U, CHEN J W, et al. External validation and prediction employing the predictive squared correlation coefficient test set activity mean vs training set activity mean [J]. Journal of Chemical Information and Modeling, 2008, 48(11): 2140-2145. doi: 10.1021/ci800253u [22] FATEMI M, GHORBANNEZHAD Z. Estimation of the volume of distribution of some pharmacologically important compounds from their structural descriptors [J]. Journal of the Serbian Chemical Society, 2011, 76(7): 1003-1014. doi: 10.2298/JSC101104091F [23] HUANG Y, LI X H, XU S J, et al. Quantitative structure-activity relationship models for predicting inflammatory potential of metal oxide nanoparticles [J]. Environmental Health Perspectives, 2020, 128(6): 67010. doi: 10.1289/EHP6508 [24] GRAMATICA P. Principles of QSAR models validation: Internal and external [J]. QSAR & Combinatorial Science, 2007, 26(5): 694-701. [25] 祁晓娟, 李雪花, 黄杨, 等. 预测农药植物角质层-水分配系数的LSER模型 [J]. 农药学学报, 2020, 22(2): 249-255. doi: 10.16801/j.issn.1008-7303.2020.0053 QI X J, LI X H, HUANG Y, et al. LSER model for predicting cuticle-water partition coefficients of pesticides [J]. Chinese Journal of Pesticide Science, 2020, 22(2): 249-255(in Chinese). doi: 10.16801/j.issn.1008-7303.2020.0053
-









 下载:
下载: