-
氮是自然界植物和动物生长所必须的营养元素,硝酸盐是农作物的重要养分,并在维持水生生态系统中起着关键作用[1]. 然而,过量的硝酸盐进入水环境会导致水体富营养化,引起藻类和浮游植物大量生长,并导致水华和赤潮[2]. 此外,长期饮用含过量硝酸盐的水会对人类健康造成潜在影响,如糖尿病、自然流产、甲状腺疾病、直肠癌和胃癌等[3]. 世界卫生组织规定,水体中硝酸盐的允许浓度为50 mg∙L−1,饮用水中硝酸盐含量最高含量不超过10 mg∙L−1[4-5]. 人类生活、工农业生产排放导致水体中硝酸盐浓度持续增加,采取一系列技术手段进行氮净化刻不容缓.
水体硝酸盐污染的修复治理方法主要有化学法、物理法及生物法[6-8]. 相对于生物法,物理化学吸附具有效率高、操作和工艺简单的特点,适用于需要硝酸盐快速去除的场景. 为了提高吸附剂吸附性能,通常在吸附剂表面引入官能团或者金属离子等,以调节其物理化学性质[9]. 表面含有大量羟基的壳聚糖,可作为吸附剂用于去除水中硝酸盐,然而其在酸性环境中的稳定性较低,实际水处理应用受到限制;有研究通过在壳聚糖中引入功能性官能团以改善其结构性能,但制备过程较为复杂[10];氧化镁改性后的生物炭可以提高硝酸盐去除效率,然而处理成本高昂,因此不适合大规模应用[11]. 季铵盐作为一种阳离子表面活性剂,常被用于吸附剂表面修饰. 研究表明,季铵盐功能化吸附剂对硝酸盐具有较高亲和力[12-13],季铵盐改性蒙脱土对硝酸盐等阴离子表现出优异的吸附性能[14]. 壳聚糖珠与环氧丙基三甲基氯化铵交联得到的季铵化壳聚糖珠对1000 mg∙L−1硝酸盐溶液吸附能力达到67.5 mg∙g-1[15].
进行阴离子吸附剂的功能化改性时,季铵盐的选择尤为重要,例如十二烷基二甲基溴化铵改性蒙脱石用于去除水中硝酸盐时,尽管最大吸附量达到8.77 mg∙g−1,但烷基季铵盐中Br– 在离子交换过程中会释放到水中,造成二次污染,故在制备吸附剂时需要用FeCl4–进一步处理[16]. 活性炭经十四烷基三甲基溴化铵处理后在25 ℃,pH=6.6,投加量为1 g∙L−1条件下对100 mg∙L−1
${\rm{NO}}_3^{-} $ 的吸附量仅有1.94 mg∙g−1,将活性炭与碳纳米管进一步处理后,吸附量方可达到14.59 mg∙g−1[17]. 聚二烯丙基二甲基氯化铵(pDADMAC)为季铵盐的一种,在水体中极易电离带有正电荷,被广泛应用于废水和饮用水处理行业,具有絮凝藻类,细菌及有机物等物质的作用[18-21]. 相比于其他季铵盐类,pDADMAC毒性低且价格便宜. 有研究表明,pDADMAC改性活性炭颗粒可以增加水中磷酸根吸附,且改性方法简便,不会对水环境造成二次污染[22]. 活性炭具有高比表面积、丰富的孔隙率和良好的阳离子交换能力,被作为吸附剂广泛应用于气体净化、水处理、冶金和食品加工等领域. 目前,以活性炭为载体用于吸附硝酸盐的烷基季铵盐功能化吸附剂的研究还鲜见报道.本文用pDADMAC对活性炭进行改性,制备一种硝酸盐氮吸附剂. 通过对照实验,验证改性吸附剂的吸附能力;利用SEM和FT-IR表征改性前后材料的表面特性,以及吸附动力学和吸附等温线拟合分析,探讨其吸附机制;进一步地,根据重复性和干扰性实验,研究材料的重复利用性和抗阴离子干扰性能.
-
所用活性炭从市场购得. 硝酸钠(NaNO3)、磷酸二氢钠(NaH2PO4)、盐酸(HCl)、氢氧化钠(NaOH)、硫酸钠(Na2SO4)、氯化钠(NaCl)均购自国药集团上海化学试剂公司. 聚二烯丙基二甲基氯化铵(pDADMAC)购自山东优索化工科技有限公司.
所用仪器包括:UV-2000紫外分光光度计,尤尼柯(上海)仪器有限公司;RH-Q恒温振荡器,常州奥华仪器有限公司;磁力搅拌器,上海梅颖浦仪仪器制造公司;HACH HQ-40d pH计,美国哈希公司;Brookhaven NanoBrook 90 Plus PALS Zeta电位分析仪; Quanta-250环境扫描电子显微镜;傅立叶红外显微成像光谱仪,Thermo Fisher Scientific USA.
-
选用粒径0.5—1 mm活性炭颗粒(GAC),去离子水清洗后65 ℃烘干. 称取一定质量pDADMAC于塑料烧杯中,加入去离子水,磁力搅拌30 min得到混合均匀的pDADMAC溶液. 将5 g活性炭分别浸渍到64 mL 7.5—1000 g·L−1不同浓度的pDADMAC溶液中,室温搅拌24 h. 为去除非结合和松散结合的pDADMAC,用去离子水清洗数次,每次清洗30 min. 将清洗干净的活性炭于55 ℃烘箱内烘至恒重,即得到改性活性炭pDADMAC-GAC.
-
扫描电子显微镜(SEM)用于观察不同放大倍数(300倍和1000倍)下GAC和pDADMAC-GAC的表面形态和结构. 能量色散X射线光谱仪(DES)用于分析改性前后活性炭各元素含量. 用Brunauer- Emmett-Teller (BET) 法测量样品的比表面积和微孔尺寸分布. 傅立叶红外显微成像光谱仪(FT-IR)用于测定材料官能团. 使用Zeta电位分析仪测定材料表面Zeta电位. 紫外-可见光分光光度计用于测定溶液中硝酸盐的浓度.
-
称取不同质量硝酸钠,用去离子水配置不同质量浓度的
${\rm{NO}}_3^{-} $ 溶液(以N计). 取上述溶液0.2 L于锥形瓶中,投加适量吸附剂,摇床130 r∙min−1振荡,水样经0.45 μm滤膜过滤后用紫外分光光度计测定硝酸根浓度,不同条件下吸附剂对溶液中${\rm{NO}}_3^{-} $ 的平衡吸附量qe根据式(1)计算:式中,qe为平衡吸附量(mg∙g−1);C0为溶液中初始
${\rm{NO}}_3^{-} $ 浓度(mg∙L−1);Ce为吸附平衡时溶液中${\rm{NO}}_3^{-} $ 浓度(mg∙L−1);V为溶液体积(L);m为吸附剂质量(g). -
采用准一级动力学模型(2)、准二级动力学模型(3)和颗粒内扩散模型(4)对实验所得数据进行拟合处理,并对拟合参数进行分析以解释吸附机制[23-25]. 3种模型公式如下:
式中,qe和qt分别为吸附平衡时和t时刻
${\rm{NO}}_3^{-} $ 吸附量(mg∙g−1);k1为准一级吸附速率常数(min−1);k2为准二级吸附速率常数(g∙(mg∙min)−1);kp为扩散速率常数(mg∙(g∙min−0.5)−1). -
采用Langmuir(5)和Freundlich(6)两种等温线方程对不同温度下所得数据进行拟合[26]:
式中,qe为平衡吸附量(mg∙g−1);C0为溶液中初始
${\rm{NO}}_3^{-} $ 浓度(mg∙L−1);Ce为吸附平衡时溶液中${\rm{NO}}_3^{-} $ 浓度(mg∙L−1);qmax为理论最大吸附量(mg∙g−1);b为Langmuir常数(L∙mg−1);KF是Freundlich吸附平衡常数;n为浓度指数. -
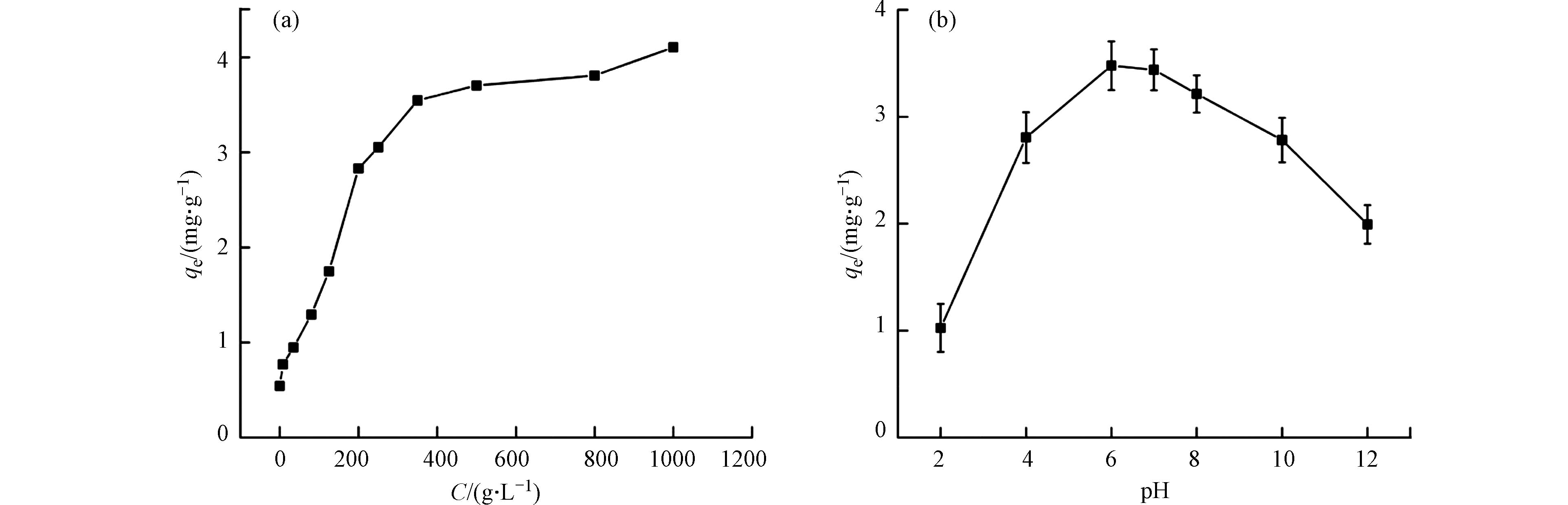
在0—1000 g∙L−1区间内设置11个pDADMAC改性浓度梯度,在pH=6、25 ℃、130 r∙min−1、0.2 g GAC条件下,对初始浓度为15 mg∙L−1的
${\rm{NO}}_3^{-} $ 进行吸附,改性活性炭对${\rm{NO}}_3^{-} $ 吸附量随pDADMAC浓度变化如图1(a)所示. 随着pDADMAC浓度的增加,改性吸附剂对${\rm{NO}}_3^{-} $ 的平衡吸附量增加;当改性浓度达到350 g∙L−1后,平衡吸附量增长缓慢. 这是由于pDADMAC浓度过高,GAC表面结合位点有限,不能充分利用溶液中的pDADMAC. 因此,后续实验选择350 g∙L−1 pDADMAC溶液对GAC进行改性. -
溶液pH值对包括硝酸盐在内的许多吸附质的吸附性能有显著影响. 为探究体系中不同初始pH对pDADMAC-GAC吸附
${\rm{NO}}_3^{-} $ 的影响,用0.1 mol∙L−1 HCl和NaOH两种溶液调整硝酸盐溶液的初始pH,分别向不同初始pH的硝酸盐溶液(0.2 L, 15 mg∙L−1)中投加0.2 g pDADMAC-GAC,结果如图1(b)所示. pH从2增加至6,吸附量持续增加,pH=6时增至最大(3.48 mg∙g−1). 这是由于pH较低时,溶液可以将${\rm{NO}}_3^{-} $ 离子质子化以HNO3的形式存在,削弱其与吸附剂之间的静电吸引作用[27]; pH逐渐增加,静电吸引力增强,吸附剂的活性位点有利于${\rm{NO}}_3^{-} $ 吸附,从而增强了${\rm{NO}}_3^{-} $ 吸附能力. 在pH=6—12范围内,随着pH增加,OH –与${\rm{NO}}_3^{-} $ 对pDADMAC-GAC表面吸附位点的竞争逐渐增强,不利于${\rm{NO}}_3^{-} $ 吸附,从而导致被pDADMAC-GAC吸附的${\rm{NO}}_3^{-} $ 逐渐下降[28]. 综上所述,最佳pH值为6,后续实验在此pH值下进行. -
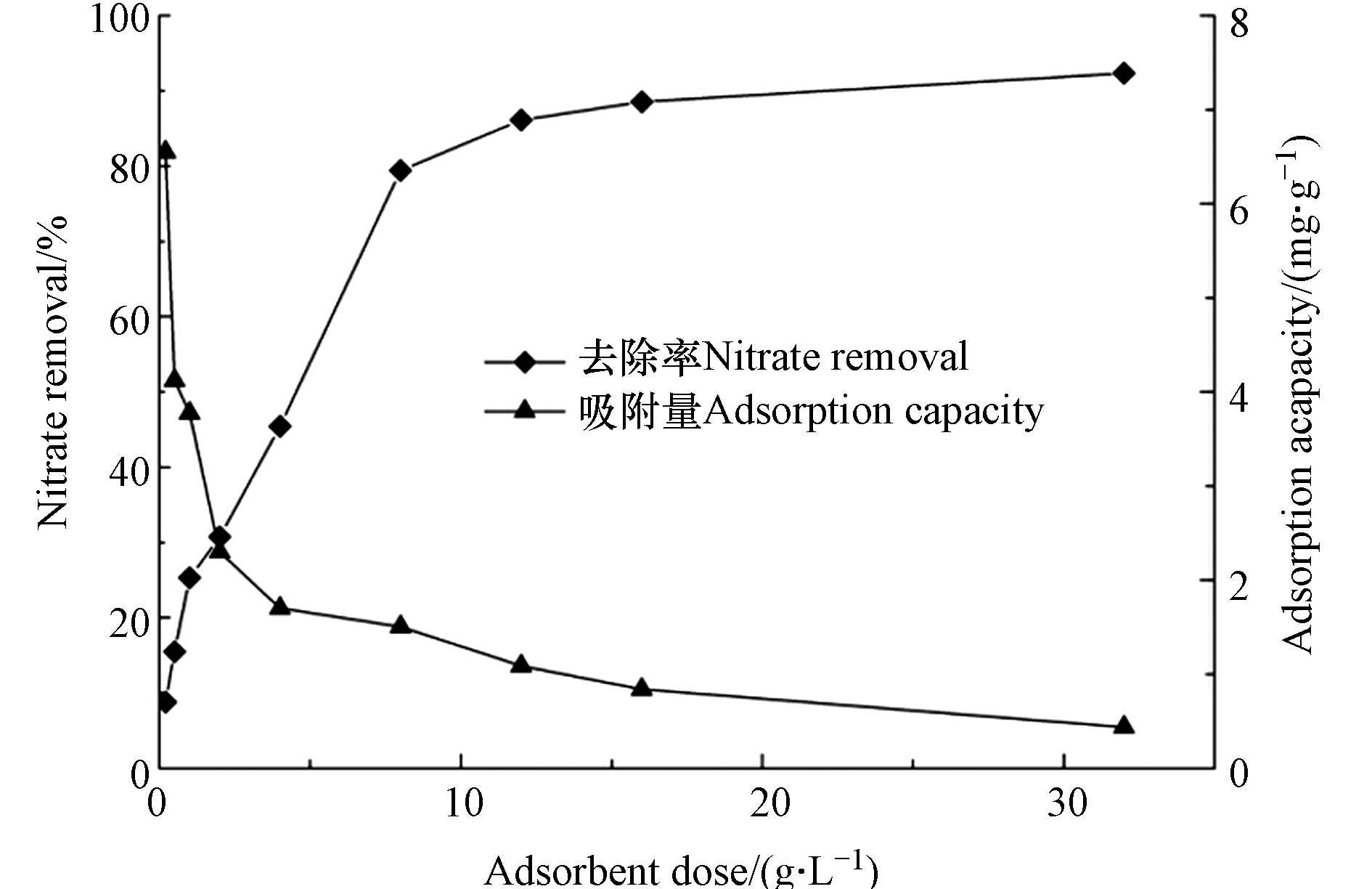
在350 g∙L−1 pDADMAC、pH=6、25 ℃条件下,分别向硝酸盐溶液(0.2 L, 15 mg∙L−1)中投加不同质量的pDADMAC-GAC,吸附效果见图2. 可见,当吸附剂投加量从0.2 g∙L−1增加至32 g∙L−1,
${\rm{NO}}_3^{-} $ 去除率从8.78%提高至92.37%. 去除率显著增加主要归因于引入更多的吸附点位来去除${\rm{NO}}_3^{-} $ . pDADMAC-GAC吸附能力从6.55 mg∙g−1急剧下降至0.44 mg∙g−1,主要是由于大量未被占用的吸附位点造成的吸附能力损失. 从去除率以及经济成本两方面考虑,确定pDADMAC-GAC对${\rm{NO}}_3^{-} $ 的最佳投加量为1 g∙L−1. -
利用SEM对GAC和pDADMAC-GAC(350 g∙L−1 pDADMAC、pH=6、25 ℃)的表面形态分析如图3. 改性前后活性炭均具有蜂窝状结构,但表面粗糙程度存在差异. 改性后活性炭表面更粗糙,凹凸不平. 该现象与BET结果一致,由表1,改性前后比表面积和孔容从948.12 m2∙g−1和0.41 cm2∙g−1下降至729.97 m2∙g−1和0.36 cm2∙g−1,分别减小23%和12%. 这是由于负载的pDADMAC占据了部分活性炭孔隙. EDS分析表明,改性前的吸附剂表面未检测到N元素,改性后有N元素出现,这是由pDADMAC的负载引入的. 进一步地,由图4 (a)可知,pDADMAC-GAC在1129 cm−1处有强吸收峰,这是胺类的C—N伸缩振动,这也验证了pDADMAC的成功负载.
图4表示改性前后不同pH条件下pDADMAC-GAC表面Zeta电位. 结果表明,GAC在pH=6—12范围内为负电位,与
${\rm{NO}}_3^{-} $ 之间存在静电斥力,这限制了其对硝酸盐的吸附. 而pDADMAC-GAC在很宽的pH范围内逆转了GAC的表面电位. pH=6时吸附剂表面Zeta电位达到最大值+40.19 mV;当pH高于6时,Zeta电位逐渐减小,吸附剂表面带有的正电荷减少. 这与溶液初始pH对吸附量影响的结果一致,pH由6增加至8时,Zeta电位从+40.19 mV降至+36.75 mV,减少3.44 mV,${\fbox{}}{\rm{NO}}_3^{-} $ 吸附量也相应减少7.59%. pDADMAC首先通过活性炭表面羧基和羟基的静电力和范德华力负载到活性炭表面,在此过程中只有一小部分胺基基团被活性炭表面负电荷中和,因此,pDADMAC对活性炭进行表面修饰时引入大量带正电荷的胺基基团,带负电荷的${\rm{NO}}_3^{-} $ 可以通过与吸附剂表面正电荷点位之间的静电引力和离子交换作用而被吸附[22, 29]. -
在前述获得的最佳改性浓度和吸附条件下,探究吸附剂对不同初始质量浓度
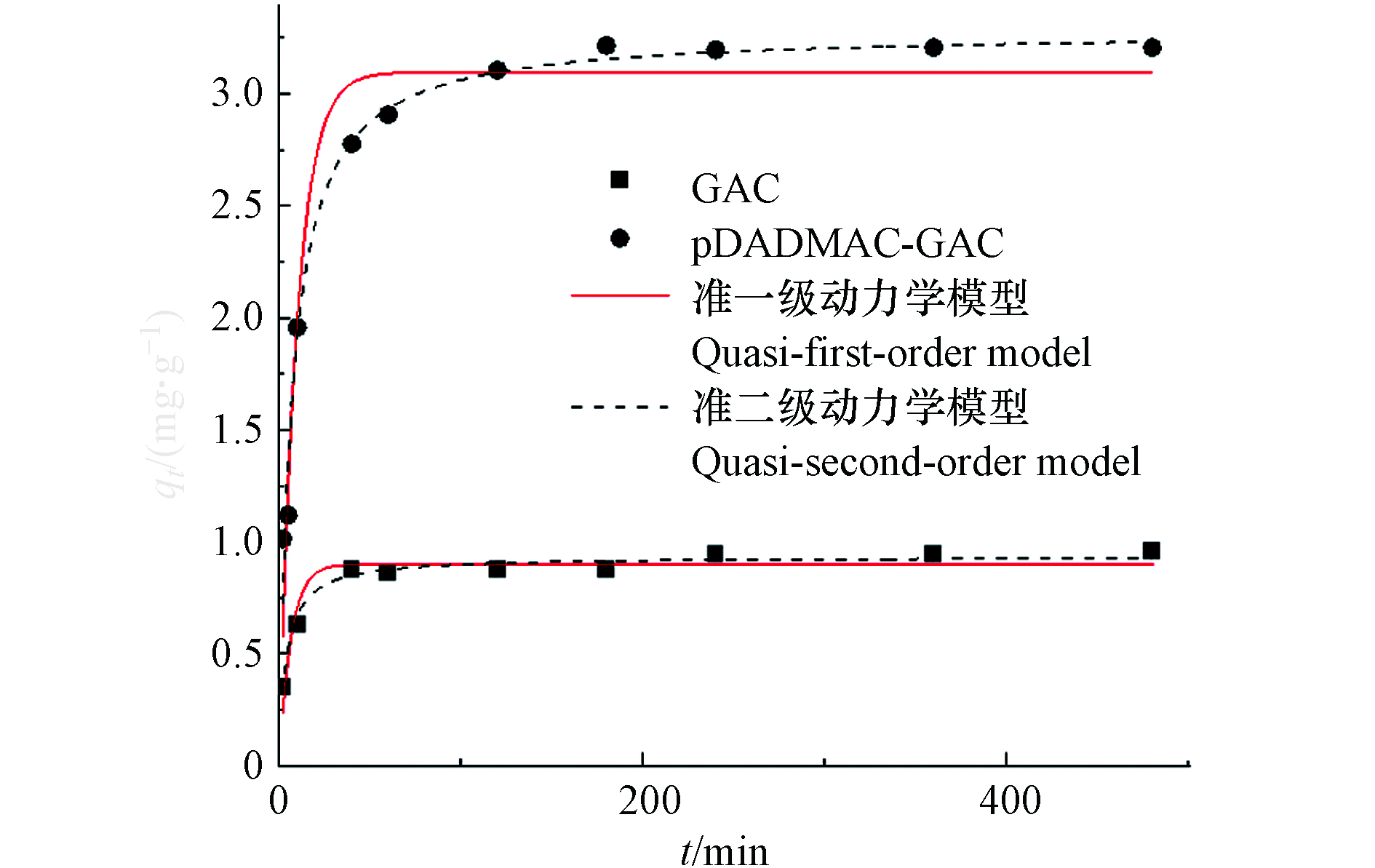
${\rm{NO}}_3^{-} $ (5、15、25 mg∙L−1)的吸附量随时间变化关系,采用准一级动力学、准二级动力学和颗粒内扩散模型对实验数据进行拟合.由图5可知,整个硝酸盐吸附动力学曲线大致包括3个阶段. 第一阶段(0—40 min)硝酸盐吸附量(qt)急剧增加,约占总吸附量的60.14%—86.35%;下一阶段(40—180 min)瞬时吸附速率逐渐降低,吸附剂对
${\rm{NO}}_3^{-} $ 的吸附量逐渐趋于平衡状态,说明吸附剂表面的官能团被充分利用;第三阶段(180 min以后),吸附达到平衡,改性使得吸附量提高了3.36—5.06倍. 这是由于pDADMAC改性引入了叔胺基(—NH2),使活性炭带正电荷,这与Xia[30]等的研究结果相似.动力学拟合参数见表2. 准一级动力学决定系数R2值为0.9407—0.9875,准二级动力学R2值为0.9948—0.9999. pDADMAC-GAC平衡吸附量更接近准二级动力学模型拟合的最大吸附量. 当硝酸盐初始浓度为15 mg∙L−1时,pDADMAC-GAC平衡吸附量为3.22 mg∙g−1,准二级动力学模型拟合的最大吸附量为3.26 mg∙g−1,理论值和实际值相差甚微. 因此,准二级动力学更适合描述
${\rm{NO}}_3^{-} $ 在pDADMAC-GAC上的吸附,这说明pDADMAC-GAC对${\rm{NO}}_3^{-} $ 的吸附是化学吸附[23].颗粒内扩散模型拟合结果(表2)表明,随着
${\rm{NO}}_3^{-} $ 浓度增加,颗粒内扩散速率常数kp增大,这说明${\rm{NO}}_3^{-} $ 初始浓度对吸附效果有影响. 由动力学数据可知,${\rm{NO}}_3^{-} $ 初始浓度增加,平衡吸附量也会相应增加,达到平衡需要的时间也会缩短,这与kp随${\rm{NO}}_3^{-} $ 浓度变化的趋势一致[25]. -
3种温度下两种吸附等温线拟合结果如表3. 可见,其决定系数(R2)均大于0.9,说明两种模型均可用于描述pDADMAC-GAC的等温吸附过程;同一温度下Langmuir模型的R2值大于Freundlich模型,表明改性活性炭表面吸附位点分布均匀,以单分子层吸附为主[26].
浓度指数n是与吸附强度有关的常数. 3种温度下n均大于1,说明即使当水体中硝酸盐浓度较低时,pDADMAC-GAC仍具有较好的吸附量,可用于去除地表水和饮用水中的硝酸盐,且pDADMAC-GAC对硝酸盐的吸附容量受温度影响较小.
-
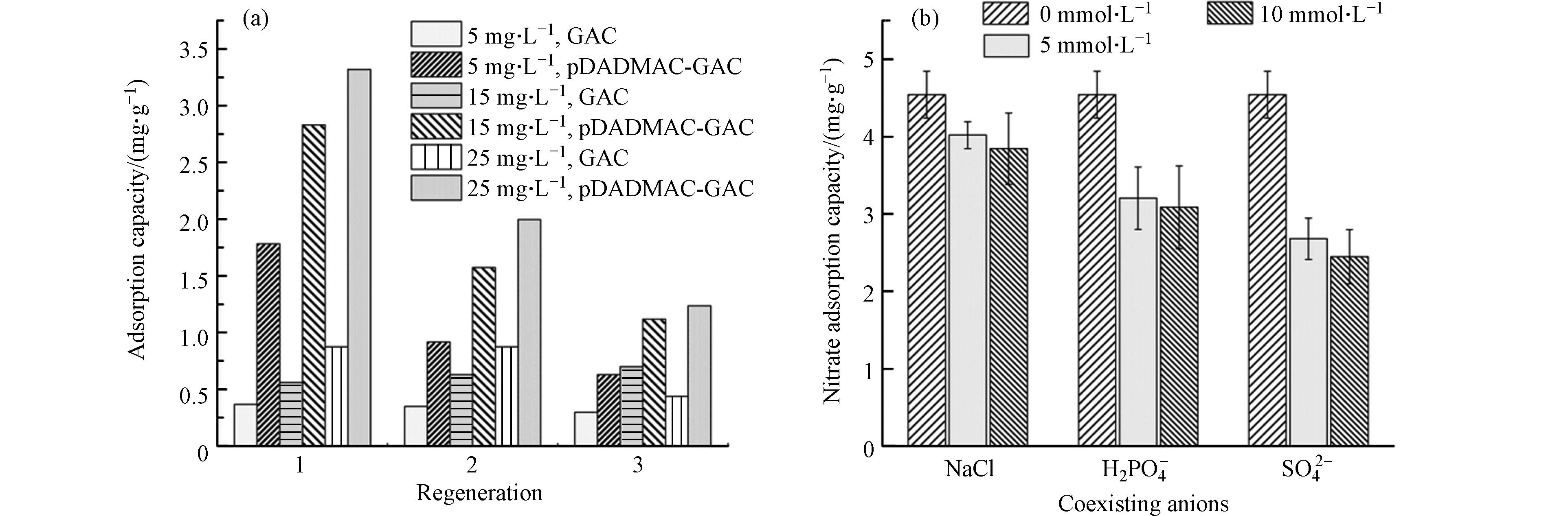
吸附材料的再生和重复利用性可以降低水污染修复的成本. 通过吸附解吸实验测试了pDADMAC-GAC的重复利用性. 在25 ℃,pH=6条件下,分别向初始浓度为5、15、25 mg∙L−1
${\rm{NO}}_3^{-} $ 溶液(0.2 L)中投加0.2 g 吸附剂,待吸附平衡后计算${\rm{NO}}_3^{-} $ 吸附量;然后以0.1 mmol∙L−1 NaCl溶液为淋洗液对吸附剂进行再生处理后进行再次吸附,结果如图6(a) . 吸附剂的吸附量随材料重用次数逐渐下降,第3次循环时吸附量较第2次损失约32.33%. 这意味着吸附位点不能完全被重新激活,部分${\rm{NO}}_3^{-} $ 仍和吸附剂上的吸附位点相结合. 这说明pDADMAC-GAC吸附硝酸盐的机制既包括静电吸附又包括离子交换. 通过静电吸附的${\rm{NO}}_3^{-} $ 可以从吸附剂解离,相应吸附位点得到再生;而通过离子键与pDADMAC-GAC结合的${\rm{NO}}_3^{-} $ 则难以被解离. 尽管如此,连续3个吸附-解吸循环后pDADMAC-GAC吸附量约为GAC的2.3倍,具有明显高于GAC的吸附能力. 因此,pDADMAC-GAC具有一定的重复利用性. -
天然水中除
${\rm{NO}}_3^{-} $ 外还有其它阴离子,与${\rm{NO}}_3^{-} $ 之间可能存在竞争吸附. 选择天然水中常见的3种阴离子(Cl–、${\rm{H}}_2 {\rm{PO}}_4^– $ 及${\rm{SO}}_4^{2-} $ ),探究阴离子对${\rm{NO}}_3^{-} $ 吸附的干扰. 在前述吸附条件下,向0.2 L,5 mmol∙L−1${\rm{NO}}_3^{-} $ 溶液中分别加入 NaCl、NaH2PO4、Na2SO4,各阴离子与${\rm{NO}}_3^{-} $ 摩尔比为1:1或2:1,待吸附平衡后计算吸附量. 需要注意的是,磷酸盐在pH=6条件下主要以H2PO4– 的形态存在,所以在探讨共存阴离子干扰作用时向溶液中投加的是NaH2PO4.由图6 (b)可知,当浓度由0增加至5 mmol∙L−1时,Cl–、
${\rm{H}}_2 {\rm{PO}}_4^– $ 及${\rm{SO}}_4^{2-} $ 均会对${\rm{NO}}_3^{-} $ 的吸附产生干扰(P<0.05);当3种阴离子浓度由5 mmol∙L−1提高至10 mmol∙L−1时,平衡吸附量无显著性差异(P>0.05),说明增大溶液中共存阴离子浓度,${\rm{NO}}_3^{-} $ 平衡吸附量变化不大. 若硝酸盐仅通过静电力作用被吸附剂吸附,则共存阴离子浓度会明显抑制${\rm{NO}}_3^{-} $ 吸附过程;若硝酸盐主要是通过离子交换被吸附剂吸附,则共存离子浓度对${\rm{NO}}_3^{-} $ 吸附过程无影响[31]. 因此,进一步说明pDADMAC-GAC吸附硝酸盐的机制包括静电吸引和离子交换作用. 3种阴离子对${\rm{NO}}_3^{-} $ 吸附的干扰作用大小为:Cl–<${\rm{H}}_2 {\rm{PO}}_4^– $ <${\rm{SO}}_4^{2-} $ . 由于Cl–、${\rm{H}}_2 {\rm{PO}}_4^– $ 与${\rm{NO}}_3^{-} $ 带有相同负电荷,存在对吸附位点的竞争,所以溶液中Cl–、${\rm{H}}_2 {\rm{PO}}_4^– $ 的存在会对${\rm{NO}}_3^{-} $ 吸附产生一定干扰. 电荷密度大的多价阴离子比单价阴离子吸附更迅速,${\rm{SO}}_4^{2-} $ 带有的负电荷比${\rm{NO}}_3^{-} $ 多[32]. 因此${\rm{SO}}_4^{2-} $ 更易被吸附剂通过静电作用吸附,从而对${\rm{NO}}_3^{-} $ 的吸附产生较大影响[33-34]. -
(1)pDADMAC-GAC对NO3– 的吸附去除效果明显. 最佳条件为硝酸盐氮初始质量浓度为15 mg∙L−1时,pDADMAC改性浓度为350 g·L−1,吸附剂投加量1 g∙L−1,pH=6,25 ℃,
${\rm{NO}}_3^{-} $ 平衡吸附量达到3.21 mg∙g−1.(2)FT-IR和Zeta结果表明pDADMAC负载到了活性炭表面,pDADMAC负载使得GAC表面的Zeta电位在pH=4—12范围内实现了由负到正的逆转. pDADMAC-GAC对硝酸根的吸附主要通过静电吸附和离子交换作用,本质上是一种单分子层化学吸附.
(3)pDADMAC-GAC连续3次吸附平衡-再生循环后吸附量约为GAC的2.3倍,具有可重复利用性;天然水体中的
${\rm{SO}}_4^{2-} $ 、${\rm{H}}_2 {\rm{PO}}_4^– $ 和Cl–会影响吸附剂对${\rm{NO}}_3^{-} $ 的吸附,共存阴离子对${\rm{NO}}_3^{-} $ 吸附的影响强弱依次为:${\rm{NO}}_3^{-} $ >${\rm{H}}_2 {\rm{PO}}_4^– $ > Cl–.(4)pDADMAC-GAC吸附剂制备过程简单,可以作为水处理过滤拦截材料,快速去除水中硝酸盐;还可用作生物反硝化处理区填料,将物理化学吸附与生物脱氮结合,提高硝酸盐去除效率.
pDADMAC改性活性炭对水中硝酸盐的吸附特性
Adsorption characteristics of nitrate in water by pDADMAC modified activated carbon
-
摘要: 相对于生化反硝化,吸附法对
${\fbox{}}{\rm{NO}}_3^{-} $ 的去除速率更快. 自然胶体对${\rm{NO}}_3^{-} $ 的吸附能力有限,研发高效、低成本、制备流程简便的${\rm{NO}}_3^{-} $ 吸附剂具有重要价值. 用聚二烯丙基二甲基氯化铵(pDADMAC)改性活性炭(GAC),提升其Zeta电位,实现对${\rm{NO}}_3^{-} $ 的高效吸附,并通过改性前后材料的形貌表征,探究其对水中${\rm{NO}}_3^{-} $ 的吸附机制. 结果表明,pDADMAC负载到了活性炭表面,pDADMAC-GAC表面Zeta电位在pH=4—12范围内都得到了提升,因此在较宽的pH范围内对${\rm{NO}}_3^{-} $ 都有良好的吸附效果. pDADMAC改性后的活性炭(pDADMAC-GAC)对${\rm{NO}}_3^{-} $ 的吸附量明显提高,约为改性前的3.36—5.06倍. pDADMAC-GAC对${\rm{NO}}_3^{-} $ 的吸附动力学过程符合准二级动力学,吸附过程以化学吸附为主;在25 ℃、 pH=6、初始${\rm{NO}}_3^{-} $ 浓度为15 mg∙L-1条件下,准二级动力学模型计算得到的pDADMAC-GAC对硝酸盐的最大吸附量为3.26 mg∙g-1. Langmuir吸附等温线拟合效果最好,表明其主要是单分子层吸附. 重复性和干扰性实验表明,pDADMAC-GAC具有可重复利用性,水中共存的${\rm{SO}}_4^{2-} $ 、${\rm{H}}_2 {\rm{PO}}_4^- $ 和Cl− 阴离子对${\rm{NO}}_3^{-} $ 吸附有一定干扰. 综上,pDADMAC-GAC具有作为优良的${\rm{NO}}_3^{-} $ 阴离子吸附剂的潜力.Abstract: Compared with biochemical denitrification, the adsorption process is more faster to the removal of${\rm{NO}}_3^{-} $ from water. As natural colloids, their adsorption capacity of${\rm{NO}}_3^{-} $ was limited, so, it is of great value to develop adsorbents with high efficiency, low cost and simple preparation process. In this study, activated carbon (GAC) was modified with polydiallyl dimethyl ammonium chloride (pDADMAC) to improve its Zeta potential and achieve the efficient adsorption capacity for${\rm{NO}}_3^{-} $ . The adsorption mechanism was investigated by the morphological characterization of the materials before and after modification. The results showed that pDADMAC was loaded on the surface of activated carbon, and the Zeta potential of pDADMAC-GAC surface increased in the range of pH 4—12, so it had a good adsorption performance on${\rm{NO}}_3^{-} $ in a wide range of pH. The adsorption capacity of pDADMAC-GAC to${\rm{NO}}_3^{-} $ was significantly increased, which was about 3.36—5.06 times of that before modification. The adsorption process of pDADMAC-GAC on${\rm{NO}}_3^{-} $ was corresponding with quasi-second-order kinetics model, which indicates the chemical adsorption is main. Under the conditions of 25 ℃, pH= 6 and initial${\rm{NO}}_3^{-} $ concentration of 15 mg∙L−1, the maximum adsorption capacity of pDADMAC-GAC on nitrate calculated by quasi-second-order kinetics model was 3.26 mg∙g−1. Langmuir model are better to correspond the adsorption isotherms, indicating that it is a monolayer adsorption. Repeatability and interference experiments showed that pDADMAC-GAC was reusable, and the coexistence of${\rm{SO}}_4^{2-} $ ,${\rm{H}}_2 {\rm{PO}}_4^- $ and Cl− anions in water interfered with${\rm{NO}}_3^{-} $ adsorption to a certain extent. In conclusion, pDADMAC-GAC has the potential to be an excellent${\rm{NO}}_3^{-} $ anionic adsorbent.-
Key words:
- nitrate adsorption /
- pDADMAC /
- modified activated carbon /
- kinetics /
- coexisting anions.
-

-
图 1 pDADMAC浓度(a)及溶液初始pH(b)对
${\rm{NO}}_3^{-} $ 吸附的影响(a) 0.2 g GAC, 0.2 L 15 mg∙L−1${\rm{NO}}_3^{-} $ , pH=6, 25 ℃; (b) 350 g∙L−1 pDADMAC, 0.2 L 15 mg∙L−1${\rm{NO}}_3^{-} $ , 0.2 g pDADMAC-GAC, 25 ℃Figure 1. Effect of pDADMAC concentration (a) and initial pH (b) of solution on
${\rm{NO}}_3^{-} $ adsorption表 1 改性前后吸附剂的元素含量和BET分析
Table 1. Elemental contents and BET analysis of activated carbon before and after modification
材料
Material质量百分比/%
Weight percentage原子百分比/%
Atomic percentage比表面积/(m2∙g−1)
Surface area孔容/ (cm2∙g−1)
Total pore volumeC N O Cl Ca C N O Cl Ca GAC 94.97 — 4.15 — 0.25 96.56 — 3.17 — 0.08 948.12 0.4099 pDADMAC-GAC 92.83 0.47 4.57 2 0.13 95.33 0.41 3.53 0.69 0.04 729.97 0.3606 表 2 吸附动力学模型拟合参数
Table 2. Fitting parameters of adsorption kinetic models
拟合方程
Kinetic models参数
Parameters初始条件
Initial conditions5 mg∙L−1 15 mg∙L−1 25 mg∙L−1 GAC pDADMAC-GAC GAC pDADMAC-GAC GAC pDADMAC-GAC qe实际值/(mg∙g−1) 0.4768 2.4114 0.9582 3.2152 1.0058 3.5443 准一级动力学 k1/min−1 6.69×10−3 1.75×10−2 0.50×10−1 0.35×10−1 0.12×10−1 0.48×10−1 qe理论值/(mg∙g−1) 0.4334 2.0281 0.6124 2.1542 0.7550 3.1402 R2 0.9790 0.9875 0.9079 0.9479 0.9758 0.9407 准二级动力学 k2/(g∙(mg∙min)−1) 0.31×10−1 1.95×10−2 1.36×10−1 4.87×10−2 4.97×10−2 4.24×10−2 qe理论值/(mg∙g−1) 0.5137 2.4443 0.9649 3.2609 1.0180 3.4777 R2 0.9805 0.9948 0.9991 0.9999 0.9948 0.9988 颗粒内扩散 kp/(mg∙(g∙min0.5)−1) 0.0289 0.2453 0.0807 0.3151 0.0711 0.6640 R2 0.9906 0.9836 0.9007 0.9337 0.9782 0.9683 表 3 不同温度下pDADMAC-GAC的吸附等温线拟合参数
Table 3. Fitting parameters of adsorption isotherms at different temperatures
模型
Models参数
Parameters温度/K
Temperature288 298 308 Langmuir qmax/(mg∙g−1) 80.4538 81.2759 84.0346 KL 8.58×10−4 6.41×10−4 6.51×10−4 R2 0.9258 0.9761 0.9789 Freundlich 1/n 0.7946 0.7936 0.7787 KF 0.1670 0.1359 0.1567 R2 0.9032 0.9666 0.9761 -
[1] SERIO F, MIGLIETTA P P, LAMASTRA L, et al. Groundwater nitrate contamination and agricultural land use: A grey water footprint perspective in Southern Apulia Region (Italy) [J]. Science of the Total Environment, 2018, 645: 1425-1431. doi: 10.1016/j.scitotenv.2018.07.241 [2] GLIBERT P M. Harmful algae at the complex Nexus of eutrophication and climate change [J]. Harmful Algae, 2020, 91: 101583. doi: 10.1016/j.hal.2019.03.001 [3] ZHANG Q Y, QIAN H, XU P P, et al. Effect of hydrogeological conditions on groundwater nitrate pollution and human health risk assessment of nitrate in Jiaokou Irrigation District [J]. Journal of Cleaner Production, 2021, 298: 126783. doi: 10.1016/j.jclepro.2021.126783 [4] ZHAO F, XIN J, YUAN M J, et al. A critical review of existing mechanisms and strategies to enhance N2 selectivity in groundwater nitrate reduction [J]. Water Research, 2022, 209: 117889. doi: 10.1016/j.watres.2021.117889 [5] WHO. Guidelines for Drinking-water Quality, Fourth ed [R]. World HealOrgan, 2011. [6] SONG N F, XU J, CAO Y P, et al. Chemical removal and selectivity reduction of nitrate from water by (nano) zero-valent iron/activated carbon micro-electrolysis [J]. Chemosphere, 2020, 248: 125986. doi: 10.1016/j.chemosphere.2020.125986 [7] CECCONET D, DEVECSERI M, CALLEGARI A, et al. Effects of process operating conditions on the autotrophic denitrification of nitrate-contaminated groundwater using bioelectrochemical systems [J]. Science of the Total Environment, 2018, 613/614: 663-671. doi: 10.1016/j.scitotenv.2017.09.149 [8] WU J L, YIN Y N, WANG J L. Hydrogen-based membrane biofilm reactors for nitrate removal from water and wastewater [J]. International Journal of Hydrogen Energy, 2018, 43(1): 1-15. doi: 10.1016/j.ijhydene.2017.10.178 [9] TONG D L, ZHUANG J, LEE J, et al. Concurrent transport and removal of nitrate, phosphate and pesticides in low-cost metal- and carbon-based materials [J]. Chemosphere, 2019, 230: 84-91. doi: 10.1016/j.chemosphere.2019.05.056 [10] BANU H A T, KARTHIKEYAN P, VIGNESHWARAN S, et al. Adsorptive performance of lanthanum encapsulated biopolymer chitosan-Kaolin clay hybrid composite for the recovery of nitrate and phosphate from water [J]. International Journal of Biological Macromolecules, 2020, 154: 188-197. doi: 10.1016/j.ijbiomac.2020.03.074 [11] LI R H, WANG J J, ZHOU B Y, et al. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment [J]. Journal of Cleaner Production, 2017, 147: 96-107. doi: 10.1016/j.jclepro.2017.01.069 [12] 郑雯婧, 林建伟, 詹艳慧, 等. 氯化十六烷基吡啶改性活性炭对水中硝酸盐的吸附作用 [J]. 环境科学, 2013, 34(11): 4325-4332. doi: 10.13227/j.hjkx.2013.11.036 ZHENG W J, LIN J W, ZHAN Y H, et al. Removal of nitrate from aqueous solution using cetylpyridinium chloride (CPC)-modified activated carbon as the adsorbent [J]. Environmental Science, 2013, 34(11): 4325-4332(in Chinese). doi: 10.13227/j.hjkx.2013.11.036
[13] 郑雯婧, 林建伟, 詹艳慧, 等. 锆-十六烷基三甲基氯化铵改性活性炭对水中硝酸盐和磷酸盐的吸附特性 [J]. 环境科学, 2015, 36(6): 2185-2194. doi: 10.13227/j.hjkx.2015.06.036 ZHENG W J, LIN J W, ZHAN Y H, et al. Adsorption characteristics of nitrate and phosphate from aqueous solution on zirconium-hexadecyltrimethylammonium chloride modified activated carbon [J]. Environmental Science, 2015, 36(6): 2185-2194(in Chinese). doi: 10.13227/j.hjkx.2015.06.036
[14] BAGHERIFAM S, KOMARNENI S, LAKZIAN A, et al. Highly selective removal of nitrate and perchlorate by organoclay [J]. Applied Clay Science, 2014, 95: 126-132. doi: 10.1016/j.clay.2014.03.021 [15] SOWMYA A, MEENAKSHI S. An efficient and regenerable quaternary amine modified chitosan beads for the removal of nitrate and phosphate anions [J]. Journal of Environmental Chemical Engineering, 2013, 1(4): 906-915. doi: 10.1016/j.jece.2013.07.031 [16] LUO W H, HUANG Q D, ZENG P, et al. Gemini surfactant-modified montmorillonite with tetrachloroferrate (FeCl4−) as a counterion simultaneously sequesters nitrate and phosphate from aqueous solution [J]. Journal of Hazardous Materials, 2021, 409: 124829. doi: 10.1016/j.jhazmat.2020.124829 [17] MENG X M, YAO L, JIANG W J, et al. In situ growth synthesis of the CNTs@AC hybrid material for efficient nitrate-nitrogen adsorption [J]. ACS Omega, 2021, 6(2): 1612-1622. doi: 10.1021/acsomega.0c05566 [18] PALTRINIERI L, REMMEN K, MÜLLER B, et al. Improved phosphoric acid recovery from sewage sludge ash using layer-by-layer modified membranes [J]. Journal of Membrane Science, 2019, 587: 117162. doi: 10.1016/j.memsci.2019.06.002 [19] RAY J R, SHABTAI I A, TEIXIDÓ M, et al. Polymer-clay composite geomedia for sorptive removal of trace organic compounds and metals in urban stormwater [J]. Water Research, 2019, 157: 454-462. doi: 10.1016/j.watres.2019.03.097 [20] 岳钦艳, 许鹏举, 李倩, 等. 聚二甲基二烯丙基氯化铵改性高炉渣的制备及其应用 [J]. 环境化学, 2006, 25(6): 735-738. doi: 10.3321/j.issn:0254-6108.2006.06.016 YUE Q Y, XU P J, LI Q, et al. Preparation and application of pdmdaac-bf slag [J]. Environmental Chemistry, 2006, 25(6): 735-738(in Chinese). doi: 10.3321/j.issn:0254-6108.2006.06.016
[21] 田秉晖, 潘纲, 栾兆坤. 阳离子聚电解质强化絮凝去除活性染料的研究 [J]. 环境化学, 2007, 26(1): 46-50. doi: 10.3321/j.issn:0254-6108.2007.01.011 TIAN B H, PAN G, LUAN Z K. Study on enhanced flocculation removal of reactive dyes by cationic polyelectrolyte [J]. Environmental Chemistry, 2007, 26(1): 46-50(in Chinese). doi: 10.3321/j.issn:0254-6108.2007.01.011
[22] WANG Z Y, BAKSHI S, LI C Y, et al. Modification of pyrogenic carbons for phosphate sorption through binding of a cationic polymer [J]. Journal of Colloid and Interface Science, 2020, 579: 258-268. doi: 10.1016/j.jcis.2020.06.054 [23] SHEPHERD J G, JOSEPH S, SOHI S P, et al. Biochar and enhanced phosphate capture: Mapping mechanisms to functional properties [J]. Chemosphere, 2017, 179: 57-74. doi: 10.1016/j.chemosphere.2017.02.123 [24] WANG J L, GUO X. Adsorption kinetic models: Physical meanings, applications, and solving methods [J]. Journal of Hazardous Materials, 2020, 390: 122156. doi: 10.1016/j.jhazmat.2020.122156 [25] MAZARJI M, AMINZADEH B, BAGHDADI M, et al. Removal of nitrate from aqueous solution using modified granular activated carbon [J]. Journal of Molecular Liquids, 2017, 233: 139-148. doi: 10.1016/j.molliq.2017.03.004 [26] GUO X, WANG J L. Comparison of linearization methods for modeling the Langmuir adsorption isotherm [J]. Journal of Molecular Liquids, 2019, 296: 111850. doi: 10.1016/j.molliq.2019.111850 [27] YAZDI F, ANBIA M, SALEHI S. Characterization of functionalized chitosan-clinoptilolite nanocomposites for nitrate removal from aqueous media [J]. International Journal of Biological Macromolecules, 2019, 130: 545-555. doi: 10.1016/j.ijbiomac.2019.02.127 [28] ISLAM M, PATEL R. Physicochemical characterization and adsorption behavior of Ca/Al chloride hydrotalcite-like compound towards removal of nitrate [J]. Journal of Hazardous Materials, 2011, 190(1/2/3): 659-668. [29] HSIEH H S, PIGNATELLO J J. Modified carbons for enhanced nucleophilic substitution reactions of adsorbed methyl bromide [J]. Applied Catalysis B:Environmental, 2018, 233: 281-288. doi: 10.1016/j.apcatb.2018.04.007 [30] XIA F, YANG H F, LI L, et al. Enhanced nitrate adsorption by using cetyltrimethylammonium chloride pre-loaded activated carbon [J]. Environmental Technology, 2020, 41(27): 3562-3572. doi: 10.1080/09593330.2019.1615133 [31] ZHANG G S, LIU H J, LIU R P, et al. Removal of phosphate from water by a Fe-Mn binary oxide adsorbent [J]. Journal of Colloid and Interface Science, 2009, 335(2): 168-174. doi: 10.1016/j.jcis.2009.03.019 [32] WAN D J, LIU H J, LIU R P, et al. Adsorption of nitrate and nitrite from aqueous solution onto calcined (Mg-Al) hydrotalcite of different Mg/Al ratio [J]. Chemical Engineering Journal, 2012, 195/196: 241-247. doi: 10.1016/j.cej.2012.04.088 [33] SOWMYA A, MEENAKSHI S. Removal of nitrate and phosphate anions from aqueous solutions using strong base anion exchange resin [J]. Desalination and Water Treatment, 2013, 51(37/38/39): 7145-7156. [34] LONG L, XUE Y W, HU X L, et al. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar [J]. Environmental Science and Pollution Research International, 2019, 26(3): 3065-3074. doi: 10.1007/s11356-018-3815-z -































 下载:
下载: