-
全氟和多氟化合物(per- and polyfluoroalkyl substances,PFASs)是一类氢原子完全或部分被氟取代的人工合成有机化合物[1]. 由于其独特的物理化学性质,PFASs在工业和消费品中具有多种用途,例如纺织品、食品包装、化妆品、和消防泡沫等[2]. 然而,接触PFASs可能会造成激素分泌、生殖、免疫、代谢以及胎儿生长发育等方面的毒理作用[3]. 根据全球许多研究,PFASs已在地表水、土壤、沉积物、动物、空气、灰尘等基质中被发现[4],它们在各环境介质中的普遍存在引起了越来越多的关注. PFASs的环境持久性和生物累积性导致其最终在动物、植物和人体内蓄积. 太湖作为长三角地区的重要水源地,其污染状况一直备受关注. 太湖流域包括江苏、浙江等几个人口密集、工业发达的地区,PFASs生产及纺织处理、金属电镀、消防、半导体等相关产业分布密集[5-6],PFASs生产使用量及其环境排放潜力较大. 目前已经有少量研究报道了太湖地表水和沉积物中PFASs的污染特征[7-10],但其关注的目标PFASs化合物种类较少,尤其是关于一些新型PFASs还鲜有报道. 目前,国内外持久性有机污染物(POPs)履约行动力度逐渐加强,对一些库存PFASs例如全氟辛基磺酸(PFOS)等使用、生产的限制管控和替代不断强化,世界范围内的许多区域出现了PFOS等管控目标污染水平的逐年降低,也伴随着各种新型短链以及杂原子取代型PFASs环境污染水平的逐渐升高. 因此在环境监测中将更多的新型PFASs纳入分析目标更有利于对PFASs污染的全面准确认识.
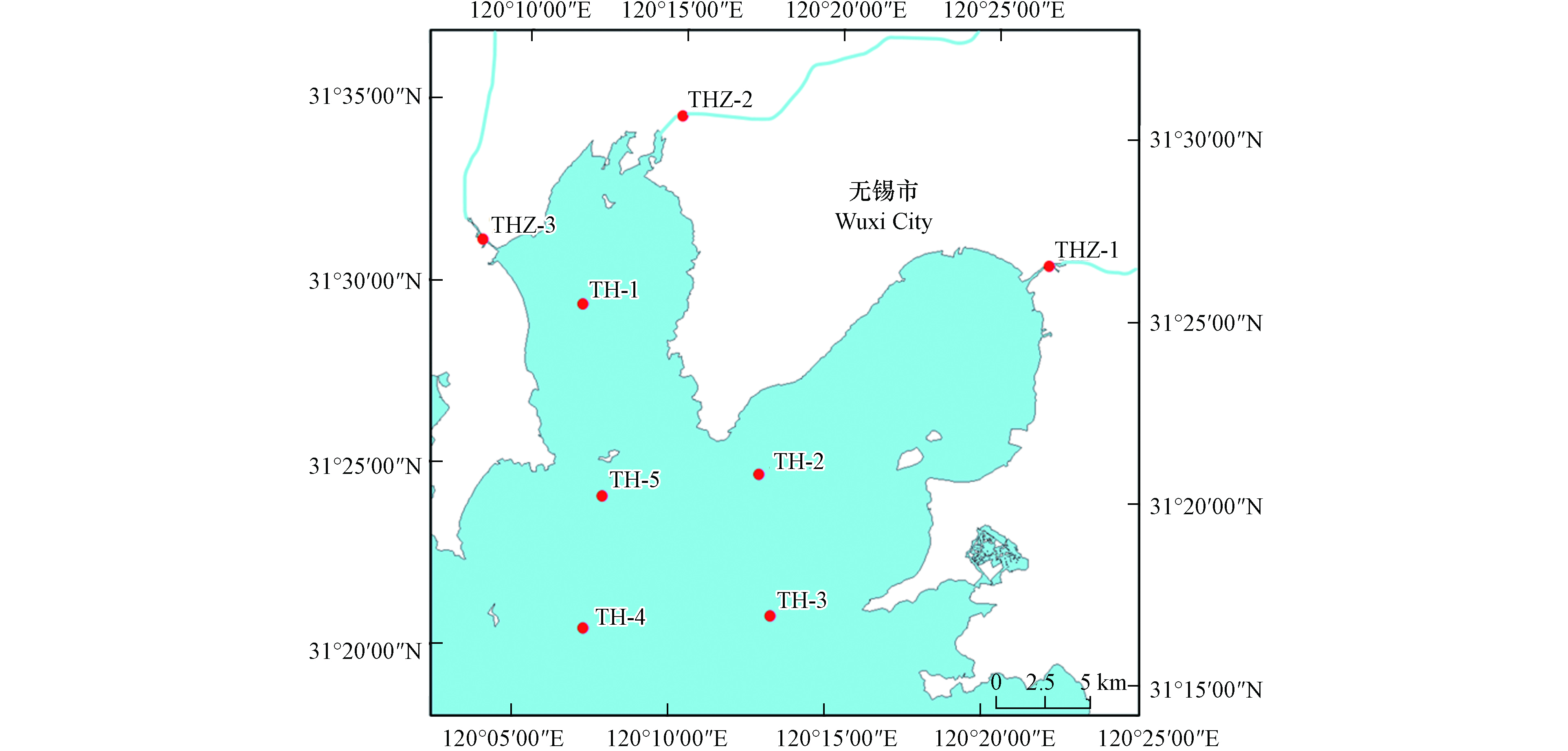
梅梁湾是太湖污染最严重的地区[9-10]. 因此,本研究选取靠近无锡市的太湖梅梁湾为研究区域,设置5个湖体采样点和3个河流入湖口采样点,采集地表水和沉积物样品,对其中的包括多种新型PFASs在内的32种PFASs目标物进行分析测定. 在此基础上研究PFASs组成特征和PFASs在地表水和沉积物两相之间的分配,并从生态和人体健康两方面对PFASs进行了风险评估,以期全面了解该区域PFASs的污染状况和风险,为污染预防管控和治理提供基础数据.
-
甲醇(色谱纯)购自德国Merck公司;乙酸铵(色谱纯)购自美国Thermo Fisher Scientific公司;氨水(色谱纯,~50% V/V)和冰醋酸(色谱纯,>99.8%)均购自美国Alfa Aesar公司;超纯水(电阻率>18.2 MΩ·cm)经Milli-Q(美国,Milipore公司)系统制得. 弱阴离子交换吸附剂填料的Oasis WAX固相萃取小柱(150 mg,6 cm3)购自美国Waters公司.
包含30种PFASs的PFAC30PAR标准品和10:2氯代多氟醚磺酸(10:2 Cl-PFESA)标准品以及质量标记的内标液MPFAC-MXA(包括13C4-PFBA、13C4-PFOA、13C2-PFDA、13C2-PFDoDA、18O2-PFHxS和13C4-PFOS)均购自惠灵顿实验室(Guelph,ON,Canada). 全氟壬烯氧基苯磺酸(OBS)标准品是从凯尔氟新材料有限公司(Kaierfu New Material Co.,Ltd.)的商业产品(95%,工业级)中纯化而来的. 32种PFASs混标储备液(100 ng·mL−1)采用甲醇配制,置于4 ℃的冰箱中保存.
-
2021年6月,在江苏省无锡市太湖梅梁湾布设了5个湖体采样点(TH1—5),以及3个无锡市主要河流入湖口采样点(THZ1—3,望虞河、梁溪河、直湖港河)(如图1所示),采集了地表水和沉积物样品. 水样使用不锈钢水桶采集,沉积物则采用不锈钢抓泥斗. 所有样品均收集在聚丙烯(PP)采样瓶中,寄到实验室前均暂存于冰箱,两天内运送到实验室,水样置于4 ℃的冰箱中冷藏保存,沉积物样品在−20 ℃下冷冻保存直至处理.
水样经0.7 μm玻璃纤维滤膜抽滤后,准确量取400 mL,加入2 ng的MPFAC-MXA,然后经固相萃取净化处理. 固相萃取小柱Oasis WAX依次用4 mL 1%的氨水-甲醇溶液、4 mL甲醇和8 mL超纯水进行活化. 之后上样,该过程流速控制在3 mL·min−1. 上样完成后依次加入4 mL 2.5 mmol·L−1的醋酸铵-冰醋酸缓冲液和8 mL超纯水清洗WAX小柱. 待清洗液流干后,WAX小柱真空泵抽干1.5 h,最后用4 mL甲醇和4 mL 0.1%的氨水-甲醇溶液洗脱待测物,将合并后的洗脱液氮吹定容至1 mL,以4500 r·min−1的转速离心10 min后,取上清液转移至进样小瓶待测.
沉积物经真空冷冻干燥后,研磨过筛. 准确称取0.5 g沉积物置于15 mL聚丙烯离心管,加入2 ng 的MPFAC-MXA,充分涡旋振荡混匀后老化30 min. 加入10 mL甲醇,50 ℃水浴超声提取30 min,混匀后用摇床以250 r·min−1速度振荡提取16 h,离心(4500 r·min−1)后收集上清液;残留部分加入5 mL甲醇进行二次提取,摇床30 min(250 r·min−1),离心10 min(4500 r·min−1),合并上清液. 氮吹至约1 mL,离心(4500 r·min−1)10 min,取上清液加超纯水稀释至50 mL,用活化好的WAX小柱富集净化(净化过程与水样一致),洗脱液氮吹定容至1 mL,离心(4500 r·min−1)后取上清液进样分析.
-
样品分析采用配有二元梯度泵、自动进样器和柱温箱的高效液相色谱HPLC(Ultimate 3000,Thermo Fisher Scientific)与配有ESI源的三重四极杆质谱(API 3200,Applied Biosystems/MDS SCIEX)串联仪器进行. 使用Acclaim 120 C18色谱柱(5 μm,4.6 mm×150 mm,Thermo Fisher Scientific,USA)分离PFASs,柱温25 ℃. 流动相A为甲醇,B为50 mmol·L−1乙酸铵,梯度洗脱程序为:0 min流动相A比例为72%,4 min内增加到95%,在此状态下保持3 min,最后在0.1 min内恢复到72%,并维持2.9 min,分析总时长为10 min. 流速为1 mL·min−1,进样量为10 μL. 质谱在ESI负离子模式下使用多反应监测(MRM)模式运行,气帘气压20 psi,碰撞气压6 psi,离子源喷雾电压−1000 V,温度350 ℃,雾化气60 psi,辅助雾化气40 psi,PFASs详细的质谱参数和离子对见表1.
-
在整个实验过程中,完全避免使用含PFASs材料制成的容器和试剂. 在实验前,对所有使用的实验用品和试剂进行分析,确保其浓度始终低于根据信噪比(S/N)为3确定的仪器检出限,方法检出限(LOD)按照400 mL水,0.5 g沉积物计算. 另外,在每10个样品中加入程序空白,检查背景污染;并在测定20个样品后测定标准质控,检查信号漂移. 使用内标法对目标物进行定量,标准曲线系列浓度为0.05、0.1、0.2、0.5、1.0、2.0、5.0、10、20、50 ng·mL−1. 使用1/x2加权回归,所有PFASs目标物的相关系数均>0.99. 分析过程中如果标准品测定值与其真实值的偏差超过±20%,须重新构建标准曲线. 进行加标回收实验以评估整个方法的准确性和精密度,将PFASs标准品加入到地表水和沉积物样品中并采用与实际样品相同的提取和净化步骤进行处理. 大部分PFASs的平均基质加标回收率(n=3)在80%至120%之间(表2).
-
沉积物-水分配系数(Kd,单位L·kg−1)常用于评估污染物在沉积物中的吸附能力[11],计算公式如下:
其中,Csediment代表沉积物中目标物浓度(ng·g−1干重),Cwater代表水样目标物浓度(ng·L−1).
进一步地,沉积物有机碳归一化分配系数(Koc,单位L·kg−1)计算如下[11]:
其中,foc为沉积物有机质含量(%),采用配备有固体附件SSM-5000A的TOC分析仪(Shimadzu,Japan)测定.
文献中常用用风险商(RQs)来评价化合物对水生生物的潜在影响[12-13],计算公式如下所示:
这里MEC是地表水中所测得的化合物的最大浓度(ng·L−1);PNEC是预测的化合物的无效应浓度(ng·L−1),其数值按下面公式进行计算:
其中,最低中位数有效浓度EC50或LC50(mg·L−1)基于模型操作(ECOSAR™软件,EPA)得出[13];f是安全系数,根据欧洲水框架指令, f一般取1000. RQ小于0.1表示化合物对水生生物风险较低,在0.1到1之间属于中等程度风险,大于1表示风险较高.
太湖作为重要的饮用水水源地,且采样点TH-2为无锡市自来水厂沙渚取水口,饮用水摄入是附近居民暴露PFASs的可能途径,参照先前报道[10, 12],以湖水PFASs浓度代替当地居民饮用水浓度,评估人群通过饮用水对PFASs的单位体重日摄入量(EDI)(以体重计,ng·(kg·d)−1),计算公式如下[12]:
其中,C为目标PFASs的平均浓度(ng·L−1),IR为每天饮用水摄入量(L·d−1),BW为平均体重(kg). IR和BW的成人和儿童数据引自中国人群暴露参数手册[14].
采用IBM PASW统计18.0(SPSS Inc.,1993—2007)进行统计分析,统计显著性阈值为P<0.05. 低于LOD的值被指定为LOD/2. 沉积物的浓度均为干重(dw)浓度.
-
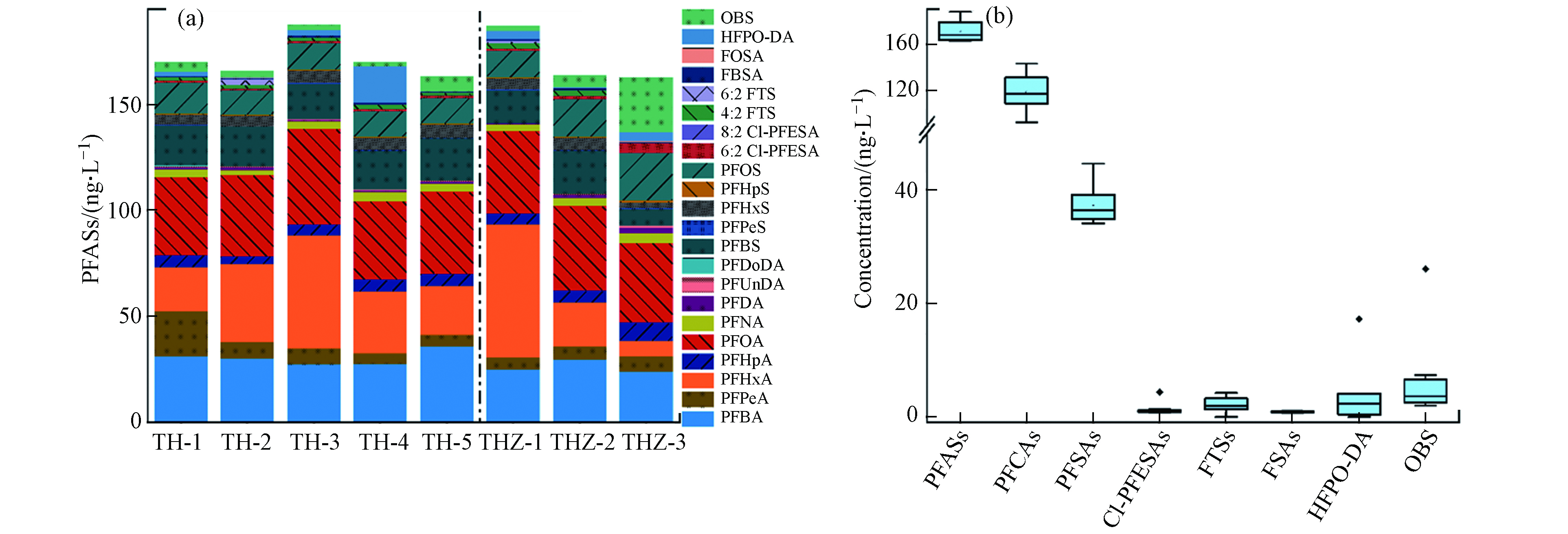
太湖梅梁湾地表水中共检出22种PFASs,其中15种PFASs的检出率为100%,主要包括C4—10 PFCAs、C4—8 PFSAs、6:2 Cl-PFESA、FBSA和OBS(图2a),说明PFASs应用的广泛性和污染的普遍性,尤其是新型PFASs的广泛检出值得进一步关注. 根据文献报道,全氟丁基磺酰胺(FBSA)是全氟丁基磺酸(PFBS)的前体物,它可能来自新的全氟丁基类替代品或其他短链全氟烷基类产品[15]. 然而,FBSA在环境中并不常见,此次在太湖梅梁湾地表水中检出浓度范围为0.65—1.05 ng·L−1(平均值0.82),可能与当地的食品包装、纺织、农药等[6]工业生产有关. 另外,OBS和Cl-PFESA是两种被忽视40余年的新型PFASs,在PFOS被公约限制后被认为是其主要替代品. 其中OBS在中国作为消防泡沫助剂和石油工业采油助剂被大量使用,其已经被证明具有与PFOS相似的急性毒性[16]和生物蓄积性[17]. 因此,这种新型PFAS在太湖梅梁湾中的普遍检出(2.01—26.06 ng·L−1,平均值6.67)需引起重视. 6:2 Cl-PFESA主要应用于金属电镀领域,虽然其检出浓度(范围0.73—4.25 ng·L−1,平均值1.38)并不高,但由于具有很强的生物蓄积性[18],所以其对水生生物和当地人群的影响不可忽略.
水样中∑PFASs范围为162.82—187.62 ng·L−1(平均值171.34,中位值167.82),此浓度与之前测得的∑PFASs:96.2—330 ng·L−1(平均值147)相当[7, 9]. 与其他湖泊相比,太湖梅梁湾∑PFASs要高于同位于华东地区的淡水湖—巢湖(平均值14.46 ng·L−1)[19],以及北京城区地表水(∑PFASs范围2.88—309.23 ng·L−1,平均值46.14)[13],同样高于北美五大湖[20],但远低于位于武汉氟化学工业生产基地附近的汤逊湖(∑PFASs范围4570—11890 ng·L−1,平均值9850)[21].
通过浓度组成分布(图2a)可以发现,地表水中以传统PFASs为主,主要是中短链PFCAs和PFSAs,其具有较高的水溶性和解离度,更容易赋存在水中,所以在地表水中占比大. 所有目标PFASs中,以平均值计算,太湖梅梁湾地表水中主要化合物浓度和贡献分别为:PFOA(39.13 ng·L−1,23%)、PFHxA(31.71 ng·L−1,19%)、PFBA(28.80 ng·L−1,17%)、PFBS(16.93 ng·L−1,10%)和PFOS(14.49 ng·L−1,8%). ∑PFCAs和∑PFSAs两者之和占比约为90%,而且∑PFCAs始终高于∑PFSAs浓度(图2b),说明PFCAs相比于PFSAs亲水性更强. 地表水中均以中短链PFASs为主,且C4-8 PFCAs是最主要的PFASs,这与前人研究一致[22]. 在测得的10种PFCAs同系物中,除PFHxA和PFOA外,其浓度大致随着碳链的增加而降低. 其中,PFBA、PFHxA、PFOA是最重要的PFCAs,这在一定程度上可能反映了短链PFASs对长链PFASs的替代结果.
由图1和图2a可知,太湖梅梁湾湖体和河流入湖口水样中检出的主要PFASs种类总体一致,说明这些物质可随水流迁移;其中TH-1点位PFASs组成特征与入湖口THZ-2和THZ-3相似,而TH-2和TH-3与入湖口THZ-1的组成特征相似,说明入湖河流对太湖梅梁湾湖体中PFASs污染有一定影响. 另外,采用Kruskal-Waillis检验对不同采样点之间的∑PFASs浓度差异进行统计学分析,显示入湖河流和湖体间没有显著性差异,这说明两者具有相似的污染来源;河流经过城市,纳污量大,这在一定程度上预示着人类活动对水产养殖可能存在影响. 该区域属于长三角地区,经济发达,工业密集,多种PFASs的检出与其周边完备的生产体系有很大联系. 根据以前的报道,太湖梅梁湾地区大多数PFASs直接来自于人类活动,如工业生产和家庭使用,而不是通过间接或远距离运输[23]. 另外,太湖梅梁湾本身较为封闭,所以PFASs浓度相对较高.
-
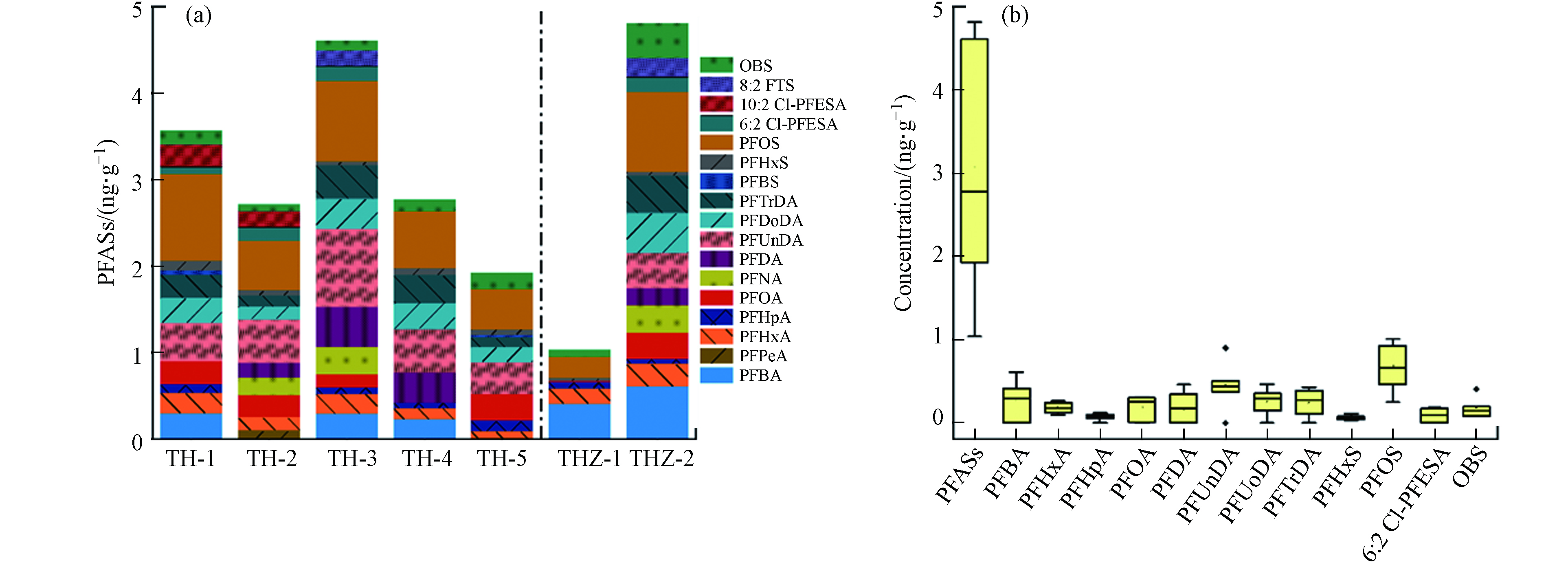
太湖梅梁湾地表水中的PFASs通过分配作用吸附在沉积物中,入湖河流与湖体沉积物中PFASs浓度水平与组成如图3a所示. 检出的17种PFASs中,只有PFHxA、PFHxS、PFOS和OBS的检出率在90%以上. 以平均值计算,太湖梅梁湾沉积物中主要化合物对∑PFASs贡献由大到小为:PFOS(21%),PFUnDA(14%),PFBA(9%),PFDoDA(8%),PFTrDA(7%),PFOA(6%),可以看出除PFBA外,其余均为中长链PFAAs,体现出其更高的亲脂性,更易于在沉积物中累积. 特别是PFDoDA和PFTrDA在地表水中未检出或浓度很低,说明相比于地表水来说,其更易吸附在沉积物中. 如图3b所示,PFOS作为沉积物中浓度最高的PFASs,其浓度范围在0.25—1.01 ng·g−1 dw(平均值0.69),与文献报道浓度相近[7]. 在太湖梅梁湾地表水中普遍检出的两种新型PFASs,OBS和6:2 Cl-PFESA在沉积物中的平均浓度分别为0.17 ng·g−1 dw和0.10 ng·g−1 dw.
总体来看,太湖梅梁湾沉积物中PFASs浓度处于较低水平(<5 ng·g−1 dw),∑PFASs浓度范围为1.04—4.81 ng·g−1 dw(平均值3.06),与之前测得太湖沉积物浓度相似:0.62—5.55 ng·g−1 dw(平均值2.15)[7],1.11—8.21 ng·g−1 dw(平均值2.42)[8]. 太湖梅梁湾∑PFASs浓度与渤海周边河流沉积物∑PFASs浓度(平均值3.34 ng·g−1 dw)持平,但高于渤海中海底沉积物∑PFASs浓度(范围0.37—4.18 ng·g−1 dw,平均值0.98 ng·g−1 dw)[11].
-
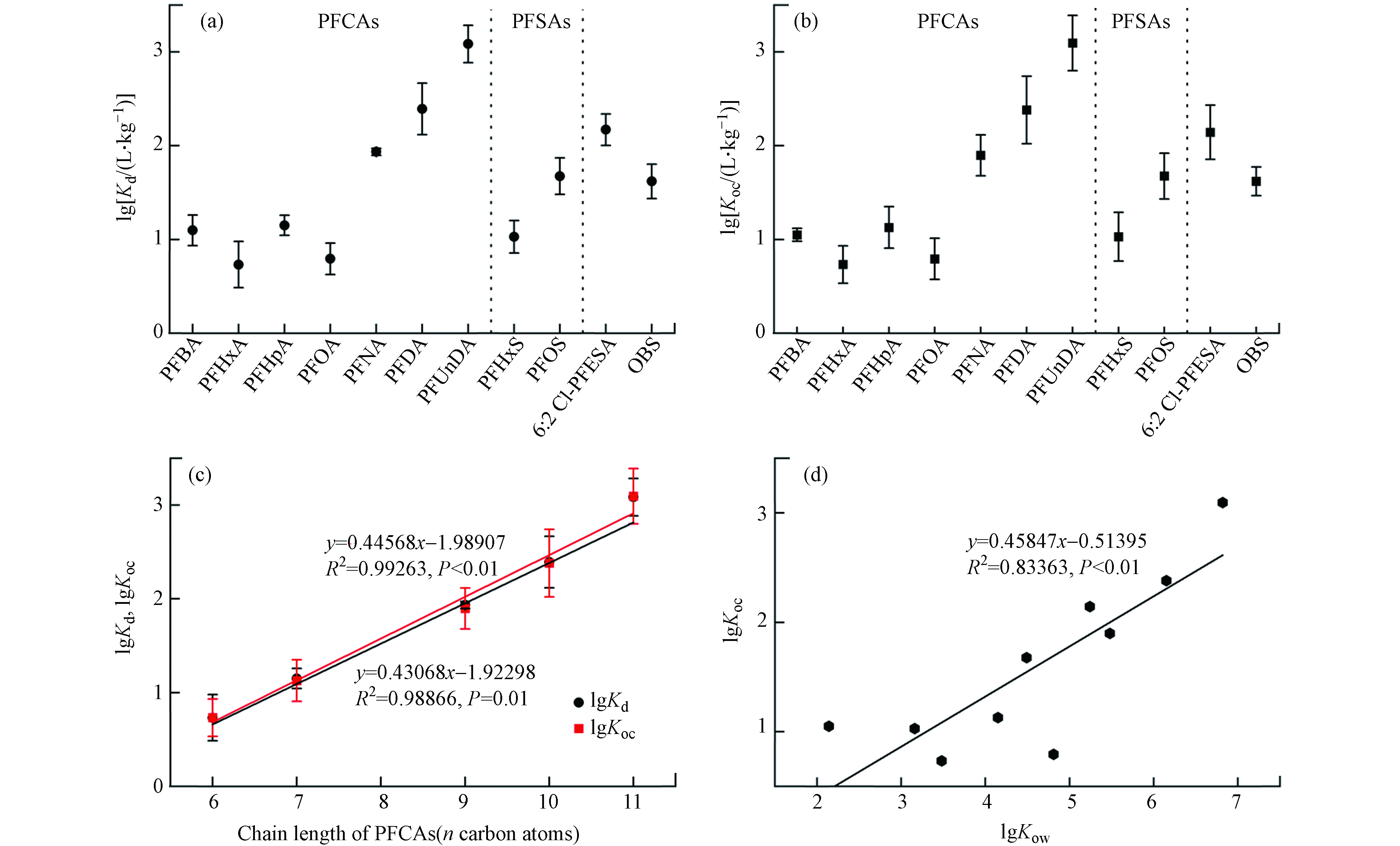
水和沉积物中PFASs的浓度低于LOD值的指定为LOD/2,计算了太湖梅梁湾两种介质中检出率高于40%的PFASs的沉积物-水分配系数(Kd)和沉积物有机碳归一化分配系数(Koc). 如图4a和b所示,太湖梅梁湾中PFOS的lg Kd(0.74—3.77)始终高于具有相同碳链长的PFOA(0.17—3.19),同样PFOS的lg Koc值也高于PFOA. 该结果与多数先前报道结果相似,即PFSAs的lg Kd值比具有相同碳数的PFCAs高0.71—0.76个对数单位[24]. 太湖梅梁湾中4种磺酸类化合物的lg Kd值顺序为:PFHxS(0.82—1.34,平均值:1.08)<OBS(1.42—1.85,1.66)<PFOS(1.29—1.86,1.70)<6:2 Cl-PFESA(1.56—2.32,2.05).
众所周知,疏水相互作用是PFASs分配到沉积物中的关键因素. 6:2 Cl-PFESA的醚键和更大的分子体积可以增加其疏水性[25],这是其Kd值高于PFOS的主要原因. 另外,OBS的Kd值与PFOS的Kd和Koc值非常接近,表明两种化合物在水和沉积物之间的分配特征相似. 相关研究表明,全氟烷基链长是影响PFASs沉积物吸附的主要因素,其lg Kd值通常随全氟碳链长度的增加而增加[26],这与图4c呈现的结果基本吻合. PFASs的Kd值在不同采样点之间的差异可能是因为Kd值受PFASs的理化性质以及多种环境因素(例如沉积物的有机碳含量、pH、水温和盐度)的影响[26]. 本研究中,PFASs的辛醇-水分配系数(Kow)来源于EPA EPIWEB 4.1,图4d中lg Koc与lg Kow呈正相关(R2 = 0.83363,P < 0.01),表明疏水性更高的PFASs能够更有效地从水相分配到沉积物中.
-
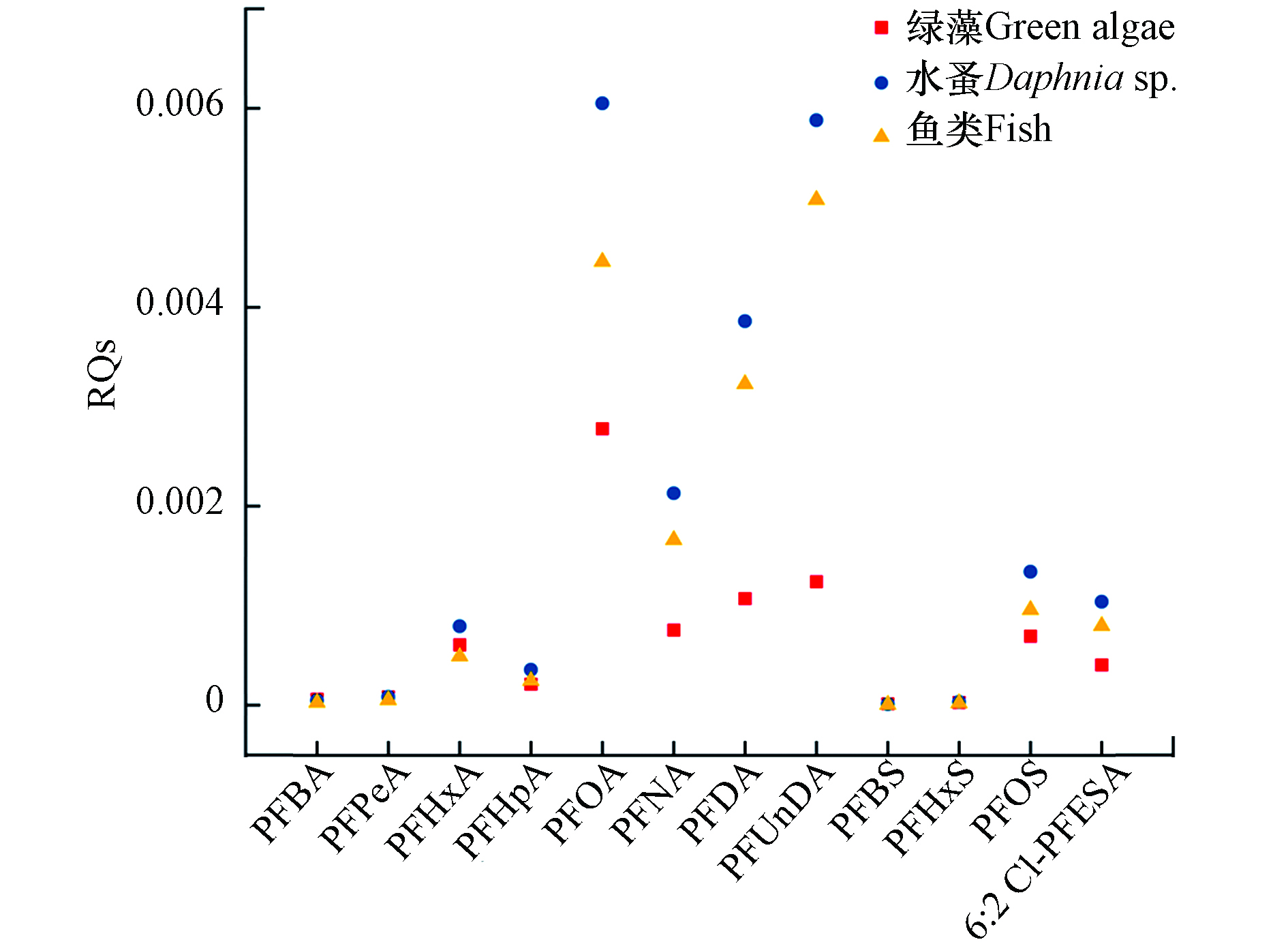
近年来大量毒理学研究报道了PFASs可对水生生物造成不良影响,本研究利用目前可获得的数据,对该区域水生环境中PFASs环境风险进行评估. 有研究提出了中国水环境中PFOA和PFOS的最大基准浓度(CMC)和持续基准浓度(CCC)[27]:CMC分别为45.54 mg·L−1和3.78 mg·L−1;CCC分别为3.52 mg·L−1和0.25 mg·L−1. 本研究中PFOA(36.80—45.05 ng·L−1)和PFOS(11.36—22.67 ng·L−1)的浓度均远低于上述值,说明太湖梅梁湾中PFOS和PFOA对水生生物风险较低. 另外,本文采用风险商(RQs)进行PFASs的生态风险评价. 选取地表水中检出率比较高的PFASs,计算了它们对于绿藻、水蚤和鱼类的RQs,发现其均低于0.1(图5),说明这些化合物对当地的水生生物风险较低. 但部分PFASs,如PFOA、PFUnDA等的RQs较高,其对水生生物的生物蓄积性和毒性仍需长期监控.
有文献[28]根据相对来源贡献推测出我国PFOA和PFOS的饮用水健康指导值分别为85 ng·L−1和47 ng·L−1;即将实施的《生活饮用水卫生标准(GB 5749—2022)》[29]规定了生活饮用水中PFOA和PFOS的限值分别为80 ng·L−1和40 ng·L−1,太湖梅梁湾水环境中PFOA和PFOS均低于上述健康指导值和国家标准. 然而,目前我国尚缺乏对许多新型PFASs的阈值和标准,因此进一步的系统研究很有必要.
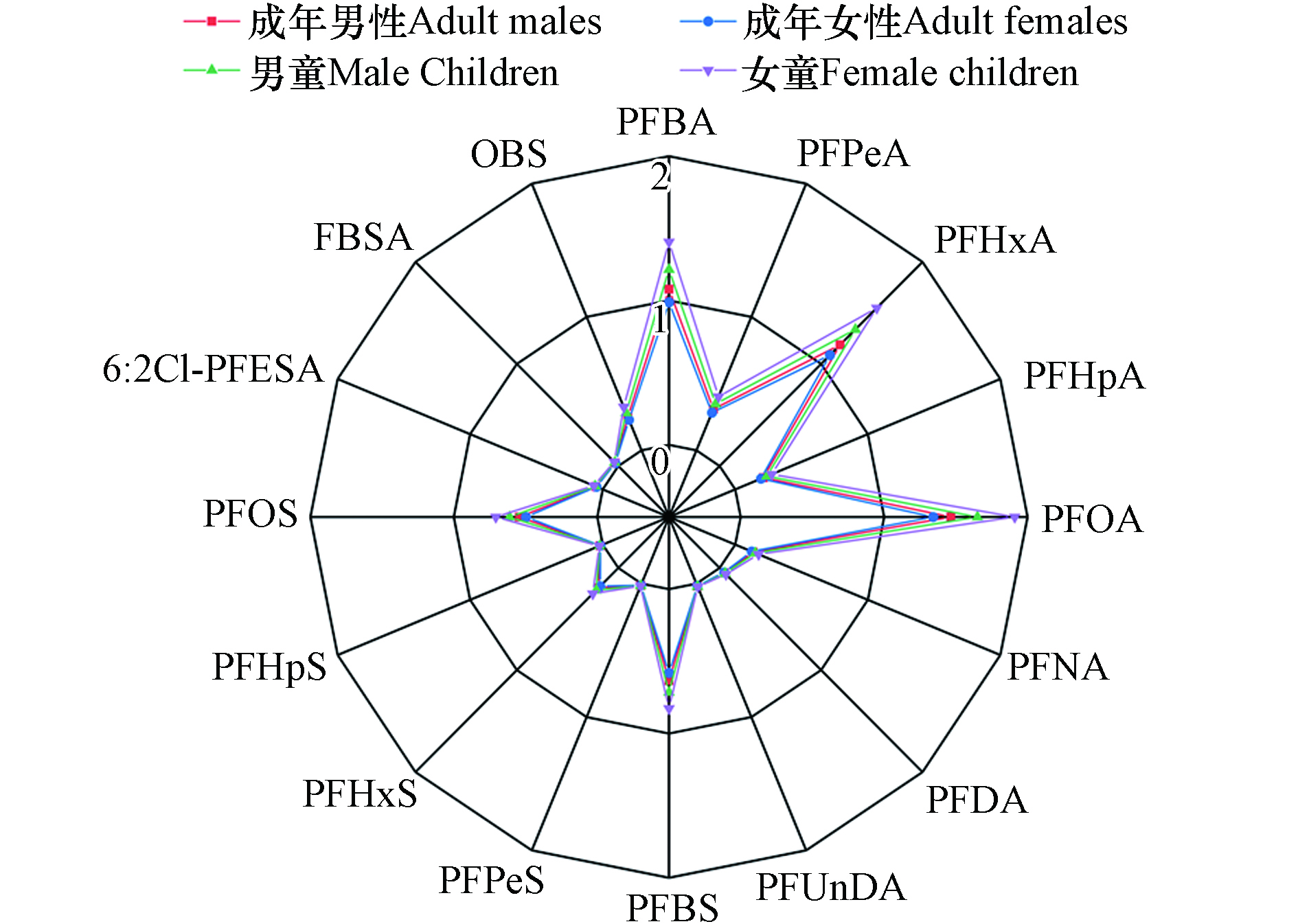
图6显示了通过饮用水估计的成年和儿童PFASs的每日摄入量(EDI). 成年男性、成年女性和男童、女童∑PFASs的EDIs分别为6.42、5.87 ng·(kg·d)−1和7.22、8.36 ng·(kg·d)−1,单个PFAS在不同人群中的EDI均小于2 ng·(kg·d)−1.
在成人群体中,男性的EDI值略高于女性;而女童的EDI高于男童,说明具有更高的暴露风险. 儿童的EDI高于成年人,主要是因为他们的单位体重耗水量较高,这表明摄入PFASs可能会对儿童造成更严重的危害[30]. PFOA的EDI对∑PFASs的EDI贡献最大,其次是PFBA、PFHxA、PFBA、PFBS和PFOS等. 该地区PFOA的EDI(1.34—1.91 ng·(kg·d)−1)和PFOS的EDI(0.50—0.71 ng·(kg·d)−1)高于其它水源地[30-31]. 成年人通过饮用水摄入PFOS的量低于通过太湖鲫鱼估计的EDI(16.4 ng·(kg·d)−1),但通过饮用水摄入PFOA的量高于通过太湖鲫鱼估计的EDI值(0.531 ng·(kg·d)−1)[32],可能会导致人类暴露PFOA的潜在健康风险. 本研究中PFOA和PFOS的EDI比欧洲食品安全局规定的每日可耐受摄入量(1500 ng·(kg·d)−1和150 ng·(kg·d)−1)[33]低很多,这意味着饮用水中的PFOA和PFOS对居民几乎无害. 然而,其他暴露途径(如食物或空气)未被考虑在内,由于PFASs的持久性、生物积累和长期健康影响,当地居民通过与水接触的累积不应被忽视. 因此,持续监测是必要的,以避免因接触PFASs而导致的不良健康效应.
-
PFASs在太湖梅梁湾地表水和沉积物样品中的普遍检出,表明了PFASs在该地区的广泛应用,一些新型PFASs在地表水和沉积物中的检出尤其应该引起重视. 地表水中∑PFASs为162.82—187.62 ng·L−1(平均值171.34),主要以C4—8 PFCAs、PFBS等中短链化合物为主;沉积物中∑PFASs为1.04—4.81 ng·g−1干重(平均值3.06),以中长链化合物为主,PFOS贡献升高. PFASs在地表水和沉积物之间的分配与其疏水性及碳链长度有很大关系,并可能受到环境因素的影响. 虽然生态风险和人体健康风险评估的结果表明PFASs具有较低风险,但作为当地饮用水源,长期暴露的危害值得进一步关注.
太湖梅梁湾水环境中全氟和多氟化合物的污染特征及风险评估
Pollution characteristics and risk assessment of per- and polyfluoroalkyl substances in waters of Meiliang Bay, Taihu Lake
-
摘要: 以太湖梅梁湾为研究区域,采集地表水和沉积物样品,分析和比较了32种PFASs目标物的污染特征. ∑PFASs在地表水和沉积物中的浓度分别为162.82—187.62 ng·L−1(平均值171.34 ng·L−1)和1.04—4.81 ng·g−1干重(平均值3.06 ng·g−1干重). 地表水中以PFOA(平均值39.13 ng·L−1)、PFHxA(平均值31.71 ng·L−1)、PFBA(平均值28.80 ng·L−1)、PFBS(平均值16.93 ng·L−1)、PFOS(平均值14.49 ng·L−1)等中短链化合物为主,沉积物中则以PFOS(平均值0.69 ng·g−1干重)等中长链化合物为主. 另外,在地表水和沉积物中检出了3种新型PFASs:全氟丁基磺酰胺(FBSA),全氟壬烯氧基苯磺酸(OBS)和6:2氯代多氟醚磺酸(6:2 Cl-PFESA). PFASs在地表水和沉积物之间的分配与其疏水性及碳链长度有关. 生态风险评估结果表明,PFASs对水生生物风险较低;人体健康风险评估表明,通过饮用水估计的PFASs摄入量低于相关标准,不存在健康风险,但PFASs对太湖流域生态环境和周边居民的长期风险仍需引起关注.Abstract: Surface water and sediment samples were collected from Meiliang Bay, Taihu Lake, for investigating the pollution characteristics of 32 per- and polyfluoroalkyl substances (PFASs). The concentrations of ∑PFASs in water and sediment ranged from 162.82—187.62 ng·L−1 and 1.04—4.81 ng·g−1 dry weight (dw) with the mean value at 171.34 ng·L−1 and 3.06 ng·g−1 dw, respectively. Short- and medium-chain PFASs, such as PFOA, PFHxA, PFBA, PFBS, and PFOS, were predominant compounds in water with the mean concentration at 39.13 ng·L−1, 31.71 ng·L−1, 28.80 ng·L−1, 16.93 ng·L−1, and 14.49 ng·L−1, respectively, while medium- and long-chain compounds such as PFOS (mean concentration: 0.69 ng·g−1 dw) were dominant in sediment. Additionally, three emerging PFASs, perfluorobutylsulphonamide (FBSA), p-perfluorous nonenoxybenzene sulfonate (OBS), and 6:2 chlorinated polyfluoroalkyl ether sulfonic acid (6:2 Cl-PFESA), were also detectable in water and sediment. The distribution of PFASs between water and sediment was strongly related to their hydrophobicity and carbon chain length. The risk assessment showed that PFASs in water display low risk to aquatic organisms. The estimated intake value of PFASs via drinking water was lower than the relevant health standard, indicating that PFASs posed no health risk for the residents. However, the long-term risk of PFASs to the ecological environment of Taihu Lake basin and the surrounding residents still needs to be concerned.
-

-
表 1 PFASs的离子对和质谱参数
Table 1. Mass transitions and parameters of mass spectrometry
化合物
Compound名称
Name母离子Q1 子离子Q3 解簇电压/ V
DP入口电压/ V
EP碰撞入口
电压/ V
CEP碰撞能量/ eV
CE碰撞出口
电压/ V
CXPPFCAs 全氟烷基羧酸 PFBA 全氟丁酸 212.8 168.8 −15.5 −10.0 −15.0 −16.2 −4.7 PFPeA 全氟戊酸 262.8 218.9 −21.5 −10.0 −19.2 −15.5 −5.8 PFHxA 全氟己酸 312.8 269.0 −18.7 −10.0 −19.5 −15.6 −7.8 PFHpA 全氟庚酸 362.8 319.0 −23.0 −10.0 −20.0 −13.0 −9.0 PFOA 全氟辛酸 412.8 369.0* −16.5 −10.0 −21.7 −15.2 −10.4 412.8 168.8 −20.6 −10.0 −40.0 −24.5 −4.4 PFNA 全氟壬酸 462.8 419.1 −31.4 −10.0 −43.2 −19.1 −12.1 PFDA 全氟癸酸 512.8 469.1 −24.4 −10.0 −37.0 −24.0 −7.4 PFUnDA 全氟十一酸 562.8 519.1 −23.4 −10.0 −50.2 −30.0 −8.5 PFDoDA 全氟十二酸 612.8 569.0 −24.0 −10.0 −40.3 −25.6 −9.2 PFTrDA 全氟十三酸 662.8 619.0 −33.4 −10.0 −40.7 −24.0 −10.3 PFTeDA 全氟十四酸 712.8 669.0 −52.9 −10.0 −38.1 −21.1 −11.2 PFSAs 全氟烷基磺酸 PFBS 全氟丁基磺酸 298.8 79.9* −71.2 −10.0 −29.8 −51.2 −3.5 298.8 99.0 −70.2 −10.0 −31.0 −40.0 −5.1 PFPeS 全氟戊基磺酸 348.8 79.9* −72.0 −10.0 −26.4 −63.0 −3.4 348.8 99.0 −72.8 −10.0 −93.3 −42.5 −5.0 PFHxS 全氟己基磺酸 398.8 79.9* −75.3 −10.0 −44.2 −66.0 −3.1 398.8 99.0 −72.6 −10.0 −30.2 −51.4 −2.0 PFHpS 全氟庚基磺酸 448.8 79.9* −81.0 −10.0 −46.0 −75.0 −3.6 448.8 99.0 −85.0 −10.0 −40.0 −56.1 −2.0 PFOS 全氟辛基磺酸 498.8 79.9* −89.1 −10.0 −34.0 −86.1 −3.4 498.8 99.0 −82.0 −10.0 −56.1 −62.7 −2.0 PFNS 全氟壬基磺酸 548.8 79.9* −90.6 −10.0 −34.0 −92.1 −3.3 548.8 99.0 −94.6 −10.0 −31.5 −75.8 −2.0 PFDS 全氟癸基磺酸 599.3 79.9* −97.0 −10.0 −21.3 −76.0 −3.6 599.3 99.0 −98.2 −10.0 −36.1 −81.0 −2.0 Cl-PFESAs 氯代多氟醚磺酸 6:2 Cl-PFESA 6:2氯代多氟醚磺酸 530.6 351.0* −66.0 −10.0 −40.0 −41.6 −9.8 530.6 83.0 −56.0 −10.0 −38.0 −50.6 −1.6 8:2 Cl-PFESA 8:2氯代多氟醚磺酸 631.0 451.2* −31.0 −10.0 −35.5 −35.0 −7.0 631.0 83.2 −75.0 −10.0 −52.9 −53.0 −4.3 10:2 Cl-PFESA 10:2氯代多氟醚磺酸 731.1 551.1 −90.1 −10.0 −32.5 −33.6 −8.6 731.1 83.1 −91.5 −10.0 −64.6 −70.7 −1.5 FTSs 氟调聚磺酸 4:2 FTS 4:2氟调聚磺酸 326.8 81.0 −50.1 −10.0 −31.8 −46.4 −4.0 326.8 307.0 −51.5 −10.0 −22.5 −32.7 −8.9 6:2 FTS 6:2氟调聚磺酸 426.8 81.0 −54.4 −10.0 −35.2 −61.0 −4.0 426.8 407.0 −55.2 −10.0 −28.2 −39.0 −6.1 8:2 FTS 8:2氟调聚磺酸 526.9 81.0 −65.9 −10.0 −37.9 −73.2 −4.0 526.9 506.9 −71.0 −10.0 −33.9 −43.2 −15.0 FSAs 全氟磺酰胺 FBSA 全氟丁基磺酰胺 297.8 77.9 −46.7 −10.0 −23.9 −39.8 −3.8 FHxSA 全氟己基磺酰胺 397.8 77.9 −60.0 −10.0 −31.8 −47.2 −4.3 FOSA 全氟辛基磺酰胺 497.8 77.9 −74.1 −10.0 −33.9 −60.0 −4.5 N-MeFOSAA N-甲基全氟-1-辛烷磺酰胺基乙酸 569.7 419.2 −50.5 −10.0 −31.2 −31.9 −6.4 569.7 511.8 −42.1 −10.0 −29.1 −32.0 −7.9 N-EtFOSAA N-乙基全氟-1-辛烷磺酰胺基乙酸 583.8 419.2 −47.2 −10.0 −34.0 −33.0 −5.3 583.8 525.9 −44.0 −10.0 −31.2 −32.2 −8.3 NaDONA 4,8-二氧杂-3-氢-全氟壬酸 377.0 85.0* −25.0 −10.0 −27.0 −48.9 −4.9 377.0 251.0 −21.0 −10.0 −26.0 −18.8 −7.0 HFPO-DA 六氟环氧丙烷二聚体 329.0 285.0 −11.0 −10.0 −18.5 −11.8 −7.9 OBS 全氟壬烯氧基苯磺酸 603.1 172.0* −87.1 −10.0 −49.5 −52.6 −4.2 602.5 108.0 −82.2 −10.0 −41.5 −85.9 −2.5 602.5 465.0 −101.9 −10.0 −32.2 −49.0 −6.3 IS 内标 13C4−PFBA 13C标记全氟丁酸 216.9 171.9 −24.0 −10.0 −12.6 −13.5 −4.8 13C4−PFOA 13C标记全氟辛酸 416.8 372.1 −25.4 −10.0 −20.7 −13.5 −5.5 13C2−PFDA 13C标记全氟癸酸 515.0 470.0 −22.3 −7.0 −26.2 −18.2 −13.2 13C2−PFDoDA 13C标记全氟十二酸 614.8 570.0 −25.6 −10.0 −33.1 −22.0 −9.4 18O2−PFHxS 18O标记全氟己基磺酸 403.0 102.9 −81.2 −9.0 −40.9 −48.0 −2.5 13C4−PFOS 13C标记全氟辛基磺酸 502.8 79.9 −91.8 −10.0 −44.4 −78.3 −3.6 *定量离子quantification ion. 表 2 PFASs的加标回收率和检出限(LODs)
Table 2. The spike recoveries and limits of detection (LODs) of PFASs
化合物
Compound地表水
Water沉积物
Sediment回收率/%
Recovery相对标准偏差/%
RSD方法检出限/(ng·L−1)
LOD回收率/%
Recovery相对标准偏差/%
RSD方法检出限/ (ng·g−1)
LODPFBA 92.9±6.9 7.4 0.23 98.3±4.4 4.5 0.09 PFPeA 101.0±8.5 8.4 0.14 92.9±7.8 8.3 0.06 PFHxA 101.7±9.5 9.3 0.09 101.3±9.3 9.1 0.03 PFHpA 110.5±8.5 7.6 0.12 98.3±8.1 8.2 0.05 PFOA 104.7±10.5 9.9 0.36 92.0±7.3 7.0 0.14 PFNA 103.4±7.7 7.4 0.18 100.1±10 10.0 0.07 PFDA 102.9±9.5 9.3 0.17 109.9±6.6 6.0 0.07 PFUnDA 92.2±7.2 7.6 0.18 91.5±7.4 8.1 0.07 PFDoDA 95.2±12.3 12.8 0.20 92.3±4.1 4.4 0.08 PFTrDA 97.5±9.3 9.4 0.28 96.5±10.9 11.3 0.11 PFTeDA 102.7±10.9 9.5 0.68 102.9±12.1 11.8 0.27 PFBS 105.1±12.3 11.6 0.07 102.6±4.6 4.4 0.03 PFPeS 109.6±9.1 8.3 0.09 98.3±3.0 3.0 0.04 PFHxS 99.0±9.6 9.7 0.08 93.7±4.5 4.8 0.03 PFHpS 99.1±8.7 8.7 0.06 95.1±3.9 4.1 0.03 PFOS 99.2±8.7 7.3 0.07 99.6±7.1 6.3 0.03 PFNS 89.1±7.7 8.4 0.07 98.4±4.8 4.9 0.03 PFDS 80.7±4.1 5.1 1.36 94.2±5.5 5.7 0.55 6:2 Cl-PFESA 86.9±6.1 7.0 0.20 96.8±4.5 4.5 0.08 8:2 Cl-PFESA 75.4±6.1 8.0 0.12 84.1±8.8 10.5 0.05 10:2 Cl-PFESA 78.3±7.5 9.5 0.27 77.3±4.6 5.6 0.11 4:2 FTS 154.3±11 6.9 0.79 94.1±8.5 7.3 0.32 6:2 FTS 124.7±15 11.7 0.50 119.9±8.5 6.9 0.20 8:2 FTS 115.3±3.4 2.9 0.28 112.6±6.0 5.1 0.11 FBSA 90.8±6.9 7.6 0.05 82.2±3.4 4.2 0.02 FHxSA 78.4±2.1 2.7 0.03 72.6±5.1 7.1 0.01 FOSA 97.0±8.5 8.8 0.03 78.7±3.1 3.9 0.01 NaDONA 104.6±7.5 7.2 0.19 96.0±4.3 4.5 0.08 HFPO-DA 107.9±7.8 7.3 1.58 70.9±3.7 4.4 0.63 N-MeFOSAA 92.0±9.0 9.8 0.68 108.8±7 6.4 0.27 N-EtFOSAA 100.3±10.6 10.5 0.71 104.8±2.0 1.9 0.29 OBS 100.9±4.5 4.4 0.36 131.0±4.9 3.7 0.14 -
[1] RAHMAN M F, PELDSZUS S, ANDERSON W B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review [J]. Water Research, 2014, 50: 318-340. [2] TEYMOURIAN T, TEYMOORIAN T, KOWSARI E, et al. A review of emerging PFAS contaminants: Sources, fate, health risks, and a comprehensive assortment of recent sorbents for PFAS treatment by evaluating their mechanism [J]. Research on Chemical Intermediates, 2021, 47(12): 4879-4914. doi: 10.1007/s11164-021-04603-7 [3] SUNDERLAND E M, HU X C, DASSUNCAO C, et al. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects [J]. Journal of Exposure Science & Environmental Epidemiology, 2019, 29(2): 131-147. [4] CHENG B, ALAPATY K, ZARTARIAN V, et al. Per- and polyfluoroalkyl substances exposure science: Current knowledge, information needs, future directions [J]. International Journal of Environmental Science and Technology, 2021, 3: 1-16. [5] LI L, ZHAI Z H, LIU J G, et al. Estimating industrial and domestic environmental releases of perfluorooctanoic acid and its salts in China from 2004 to 2012 [J]. Chemosphere, 2015, 129: 100-109. doi: 10.1016/j.chemosphere.2014.11.049 [6] XIE S W, WANG T Y, LIU S J, et al. Industrial source identification and emission estimation of perfluorooctane sulfonate in China [J]. Environment International, 2013, 52: 1-8. doi: 10.1016/j.envint.2012.11.004 [7] CHEN M, WANG Q, SHAN G Q, et al. Occurrence, partitioning and bioaccumulation of emerging and legacy per- and polyfluoroalkyl substances in Taihu Lake, China [J]. Science of the Total Environment, 2018, 634: 251-259. doi: 10.1016/j.scitotenv.2018.03.301 [8] GUO C S, ZHANG Y, ZHAO X, et al. Distribution, source characterization and inventory of perfluoroalkyl substances in Taihu Lake, China [J]. Chemosphere, 2015, 127: 201-207. doi: 10.1016/j.chemosphere.2015.01.053 [9] MA X X, SHAN G Q, CHEN M, et al. Riverine inputs and source tracing of perfluoroalkyl substances (PFASs) in Taihu Lake, China [J]. Science of the Total Environment, 2018, 612: 18-25. doi: 10.1016/j.scitotenv.2017.08.235 [10] PAN G, ZHOU Q, LUAN X, et al. Distribution of perfluorinated compounds in Lake Taihu (China): Impact to human health and water standards [J]. Science of the Total Environment, 2014, 487: 778-784. doi: 10.1016/j.scitotenv.2013.11.100 [11] MENG L Y, SONG B Y, ZHONG H F, et al. Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in the Bohai Sea and its inflow rivers [J]. Environment International, 2021, 156: 106735. doi: 10.1016/j.envint.2021.106735 [12] LENG Y F, XIAO H L, LI Z, et al. Occurrence and ecotoxicological risk assessment of perfluoroalkyl substances in water of lakes along the middle reach of Yangtze River, China [J]. Science of the Total Environment, 2021, 788: 147765. doi: 10.1016/j.scitotenv.2021.147765 [13] WANG Y, SHI Y L, CAI Y Q. Spatial distribution, seasonal variation and risks of legacy and emerging per- and polyfluoroalkyl substances in urban surface water in Beijing, China [J]. Science of the Total Environment, 2019, 673: 177-183. doi: 10.1016/j.scitotenv.2019.04.067 [14] 环境保护部. 中国人群暴露参数手册(成人卷/儿童卷)[M]. 北京: 中国环境出版社, 2013/2016. Ministry of Environmental Protection. Exposure factors handbook of Chinese population (adults/children)[M]. Beijing: China Environmental Press, 2013/2016(in Chinese).
[15] CHU S G, LETCHER R J, MCGOLDRICK D J, et al. A new fluorinated surfactant contaminant in biota: Perfluorobutane sulfonamide in several fish species [J]. Environmental Science & Technology, 2016, 50(2): 669-675. [16] XU L, SHI Y L, LI C X, et al. Discovery of a novel polyfluoroalkyl benzenesulfonic acid around oilfields in Northern China [J]. Environmental Science & Technology, 2017, 51(24): 14173-14181. [17] SHI Y L, SONG X W, JIN Q, et al. Tissue distribution and bioaccumulation of a novel polyfluoroalkyl benzenesulfonate in crucian carp [J]. Environment International, 2020, 135: 105418. doi: 10.1016/j.envint.2019.105418 [18] SHI Y L, VESTERGREN R, XU L, et al. Human exposure and elimination kinetics of chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs) [J]. Environmental Science & Technology, 2016, 50(5): 2396-2404. [19] LIU W X, HE W, QIN N, et al. Temporal-spatial distributions and ecological risks of perfluoroalkyl acids (PFAAs) in the surface water from the fifth-largest freshwater lake in China (Lake Chaohu) [J]. Environmental Pollution, 2015, 200: 24-34. doi: 10.1016/j.envpol.2015.01.028 [20] GEWURTZ S B, BRADLEY L E, BACKUS S, et al. Perfluoroalkyl acids in great lakes precipitation and surface water (2006-2018) indicate response to phase-outs, regulatory action, and variability in fate and transport processes [J]. Environmental Science & Technology, 2019, 53(15): 8543-8552. [21] ZHOU Z, LIANG Y, SHI Y L, et al. Occurrence and transport of perfluoroalkyl acids (PFAAs), including short-chain PFAAs in Tangxun Lake, China [J]. Environmental Science & Technology, 2013, 47(16): 9249-9257. [22] ZHOU Y Q, WANG T Y, LI Q F, et al. Spatial and vertical variations of perfluoroalkyl acids (PFAAs) in the Bohai and Yellow Seas: Bridging the gap between riverine sources and marine sinks [J]. Environmental Pollution, 2018, 238: 111-120. doi: 10.1016/j.envpol.2018.03.027 [23] YU N Y, SHI W, ZHANG B B, et al. Occurrence of perfluoroalkyl acids including perfluorooctane sulfonate isomers in Huai River Basin and Taihu Lake in Jiangsu Province, China [J]. Environmental Science & Technology, 2013, 47(2): 710-717. [24] AHRENS L, TANIYASU S, YEUNG L W Y, et al. Distribution of polyfluoroalkyl compounds in water, suspended particulate matter and sediment from Tokyo Bay, Japan [J]. Chemosphere, 2010, 79(3): 266-272. doi: 10.1016/j.chemosphere.2010.01.045 [25] SHI Y L, VESTERGREN R, ZHOU Z, et al. Tissue distribution and whole body burden of the chlorinated polyfluoroalkyl ether sulfonic acid F-53B in crucian carp (Carassius carassius): Evidence for a highly bioaccumulative contaminant of emerging concern [J]. Environmental Science & Technology, 2015, 49(24): 14156-14165. [26] HIGGINS C P, LUTHY R G. Sorption of perfluorinated surfactants on sediments [J]. Environmental Science & Technology, 2006, 40(23): 7251-7256. [27] YANG S W, XU F F, WU F C, et al. Development of PFOS and PFOA criteria for the protection of freshwater aquatic life in China [J]. Science of the Total Environment, 2014, 470/471: 677-683. doi: 10.1016/j.scitotenv.2013.09.094 [28] ZHANG S Y, KANG Q Y, PENG H, et al. Relationship between perfluorooctanoate and perfluorooctane sulfonate blood concentrations in the general population and routine drinking water exposure [J]. Environment International, 2019, 126: 54-60. doi: 10.1016/j.envint.2019.02.009 [29] 国家市场监督管理总局, 国家标准化管理委员会. 生活饮用水卫生标准: GB 5749—2022[S]. 北京: 中国标准出版社, 2022. State Administration for Market Regulation, Standardization Administration of the People's Republic of China. Standards for drinking water quality: GB 5749—2022[S]. Beijing: Standards Press of China, 2022(in Chinese).
[30] MENG J, LIU S F, ZHOU Y Q, et al. Are perfluoroalkyl substances in water and fish from drinking water source the major pathways towards human health risk? [J]. Ecotoxicology and Environmental Safety, 2019, 181: 194-201. doi: 10.1016/j.ecoenv.2019.06.010 [31] LI J, AI Y F, HU J R, et al. Polyfluoroalkyl substances in Danjiangkou Reservoir, China: Occurrence, composition, and source appointment [J]. Science of the Total Environment, 2020, 725: 138352. doi: 10.1016/j.scitotenv.2020.138352 [32] CHEN M, ZHU L Y, WANG Q, et al. Tissue distribution and bioaccumulation of legacy and emerging per-and polyfluoroalkyl substances (PFASs) in edible fishes from Taihu Lake, China [J]. Environmental Pollution, 2021, 268: 115887. doi: 10.1016/j.envpol.2020.115887 [33] European Food Safety Authority (EFSA). Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts Scientific Opinion of the Panel on Contaminants in the Food chain [J]. EFSA Journal, 2008, 6(7): 653. -



 下载:
下载: