-
Cu2O是一种p型半导体,理论直接带隙为2.1 eV[1],具有较强的催化活性,高导热性,出色的抗菌活性. 近年来,Cu2O纳米粒子及其复合材料在气敏、太阳能电池、抗菌剂、催化、吸附等领域广泛应用[2-6]. Mao等用Cu2O掺杂其他金属吸附去除水中的碘化物 [7];Liu等制成Cu2O/活性炭纤维复合材料,Cu2O在其中发挥协同吸附光催化去除甲基橙的作用[8]. 上述实例均采用溶剂热法制得Cu2O,其他常用合成方法,如恒电位沉积、微波合成、液相合成、晶种法合成和微乳液,需使用苛刻且昂贵的化学药品,并不具有生物相容性或产生二次污染.
绿色合成能有效打破上述局限,利用生物提取物和生物废料来绿色合成各纳米颗粒,如使用皂甙、多酚、萜类化合物和碳水化合物已被不少文献报道[9-11]. Xiao等利用茶多酚合成纳米铁颗粒选择性去除阳离子染料[12],田利强等用黑茶绿色合成零价铁纳米颗粒[13]. Gopalakrishnan等以斐林试剂为前驱体与羽芒菊叶提取物合成了了六方和立方形的Cu2O[14]. 椰枣果肉的提取物在水性介质中可作为形成Cu2O的还原剂[11]. 研究发现合成的无规则Cu2O纳米颗粒对于污水中砷(Ⅲ)离子等污染物的吸收效果特别优异. Qin等研究表明稳定剂的存在能提升去污材料的稳定性和去污率[15-16],但Cu2O纳米颗粒由于其尺寸较小,易于团聚不利于回收重复利用,因此需要选择合适的载体进行固定. 无机纳米颗粒在纤维素基质中的吸附是目前研究的热点. Errokh等室温下在pH为10的水溶液中分别使用羟胺和肼作为还原剂,在棉织物上获得了晶粒尺寸分别为30 nm和45 nm的Cu2O[17]. Sedighi等在棉织物上合成了Cu2O,NaOH和氨作还原剂,40 ℃ 的温度下得到Cu2O微晶尺寸为21 nm[18]. Svetlichnyi等通过脉冲激光烧蚀制备Cu2O纳米粒子[19],反复浸渍得到纳米Cu2O/织物复合材料.
本文利用水浸法提取无毒绿色的茶多酚作为还原剂及稳定剂,在不使用强碱条件下,通过沉积法获得氧化亚铜/无纺布(Cu2O/Non-woven fabrics,Cu2O/NF)复合材料,符合清洁生产理念,减少了对环境的潜在有害影响. 通过XRD、EDS、SEM等对样品进行表征,分析复合材料结构和成分特征的变化. 此外,制备的Cu2O/NF对阴离子染料表现出独特的选择性去除能力. 通过对复合材料的动力学、热力学模型进行测试,探讨可能的选择性吸附机理,有助于促进绿色合成技术在废水处理中的实际应用.
-
金橙Ⅱ、甲基橙、罗丹明B、刚果红、乙酸乙酯、NaHCO3、CCl4、乙酸钠、乙酸购自国药集团化学试剂有限公司,Cu(NO3)2、钼酸铵购自阿拉丁,普洱、绿茶龙井、黑茶、白茶、红茶购自云南霸茶茶叶有限公司等.
傅里叶变换红外光谱仪(FTIR)(FTIR-8400s,日本shimadzu公司),扫描电镜(SEM)(JSM-7500,日本JEOL 公司),X射线粉末衍射仪(XRD)(D/max 2500,日本Rigaku 公司),X射线电子能谱仪(XPS)(K-Alpha,美国Thermo Scoemtific 公司),比表面积和孔径测定(BET)(SSA-4000,北京彼奥德电子技术有限公司),紫外可见分光光度计(UV-vis)(UV-2600,日本岛津公司).
-
本文采用水浸法提取茶多酚分别对绿茶、红茶、白茶、普洱、黑茶提取物进行了还原性比较. 探讨提取次数(1—3)、时间(40—60 min)、温度(60—80 ℃)、固液比(1:20—1:30)的4个因素对提取效率的影响,通过正交实验确定最佳提取条件. 采用钼酸铵分光光度法测定茶多酚的浓度.
将0.20 g的NaHCO3与35 mL茶多酚溶液混合,调整pH值至7,加入0.20 g的Cu(NO3)2后放入经预处理的NF. 将两份混合溶液磁力搅拌1.5 h,充分反应后将混合溶液移入烘箱,120 ℃下反应3 h. 样品取出后用乙醇和去离子水洗涤,在60 ℃下干燥,得到Cu2O/NF复合材料. 将所得Cu2O/NF复合材料进行多次负载实验.
-
称取0.03gCu2O/NF加入到50 mL水溶液中,并设置染料(金橙Ⅱ(orange Ⅱ)、刚果红(congo red,CR)、甲基橙(Methyl orange,MO)、亚甲基蓝(methylene blue, MB)、罗丹明B(rhodamine B, RB)、结晶紫(crystal violet, CV))相同初始浓度50.0 mg∙L−1. 以150 r·min−1的速度在298 K下振荡240 min完成吸附实验. 通过检测溶液中染料浓度变化计算Cu2O/NF对染料的去除率,使用紫外-可见分光光度计(UV-2600,岛津)测定染料浓度. 去除率(η,%)根据以下公式计算:
其中,η为去除率;C0为染料的初始浓度,mg∙L−1;Ce为染料反应后的浓度,mg∙L−1.
-
取一定量Cu2O/NF加入到50 mL浓度为50.0 mg∙L−1不同染料中,在常温常压条件下初始10 min每隔10 min用紫外-可见光分光光度计测定溶液的吸光度,计算出染料的浓度,绘制吸附动力学曲线并拟合. 伪一级动力学模型线性形式可以表示为:
其中,qe和qt(mg∙g−1)分别是在平衡点和在时间t吸附的被吸附物的量. k1是伪一级速率常数.
伪二级动力学模型线性形式可以表示为:
其中,qe和qt(mg∙g−1)分别是在平衡点和在时间t吸附的被吸附物的量. k2是伪二阶速率常数.
-
不同染料初始浓度下,分别取50 mL置于锥形瓶中,加入一定量的Cu2O/NF,在25 ℃下达到吸附平衡,用紫外-可见光分光光度计测定溶液的吸光度,计算出染料的浓度,绘制吸附等温线并拟合.
Langmuir吸附等温方程:
Freundlich吸附等温方程:
式中,qe是单位质量吸附剂吸附污染物质的质量,mg∙g−1;Qmax是吸附剂的饱和吸附量,mg∙g−1;kL是Langmuir吸附常数,与吸附剂的单层饱和吸附量有关;Ce吸附平衡浓度,mg∙L−1;kF为Freundlich方程中与吸附能有关的常数;n为与表面覆盖度有关的经验常数.
-
用 L9(34) 正交表设计实验,实验因素为A:温度,B:时间,C:提取次数,D:固液比,以茶多酚的浸提率为目标,确定最优提取方案,实验结果见表1.
根据R值,影响茶多酚提取的因素排列为D>A>C>B,即固液比影响最大,时间影响最小. 最佳提取工艺为A3B3C2D2,即温度80 ℃,固液比1:25,浸提60 min,提取次数2次. 在此条件下,通过3个平行实验,平均提取率为24.43%. 高于正交实验结果,且重现性好,达到了优化的目的.
5种茶叶的茶多酚含量测试结果如图1所示,绿茶(龙井)的茶多酚含量最高,其次是白茶,红茶的茶多酚含量最低.
-
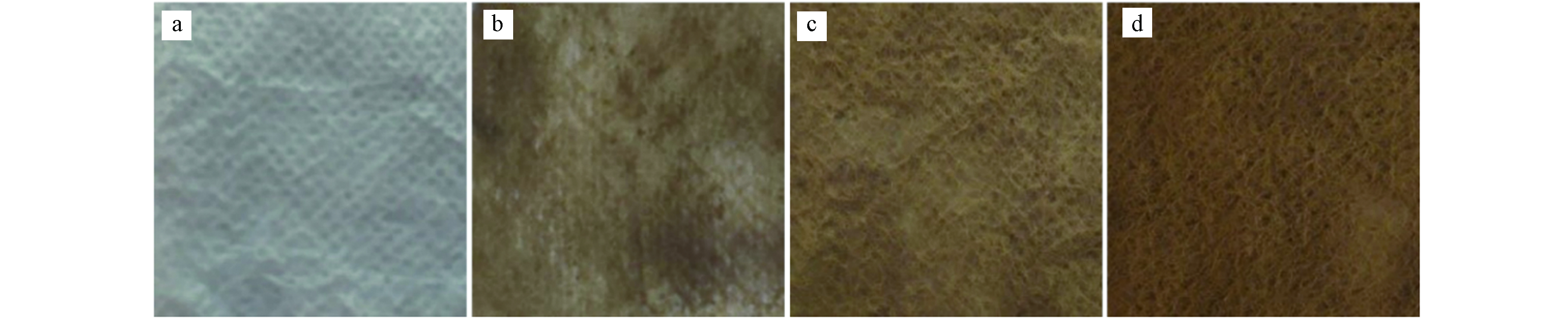
图2可以看出,负载Cu2O的无纺布仍保持其原结构和样貌. 样品颜色随负载次数的变化而变化. 图2(a)不含Cu2O的无纺布为原色,(b)、(c)、(d)分别为第一、二、三次负载. 随着Cu2O负载量增加(从a到d),Cu2O/NF颜色逐渐变深,呈现棕褐色.
-
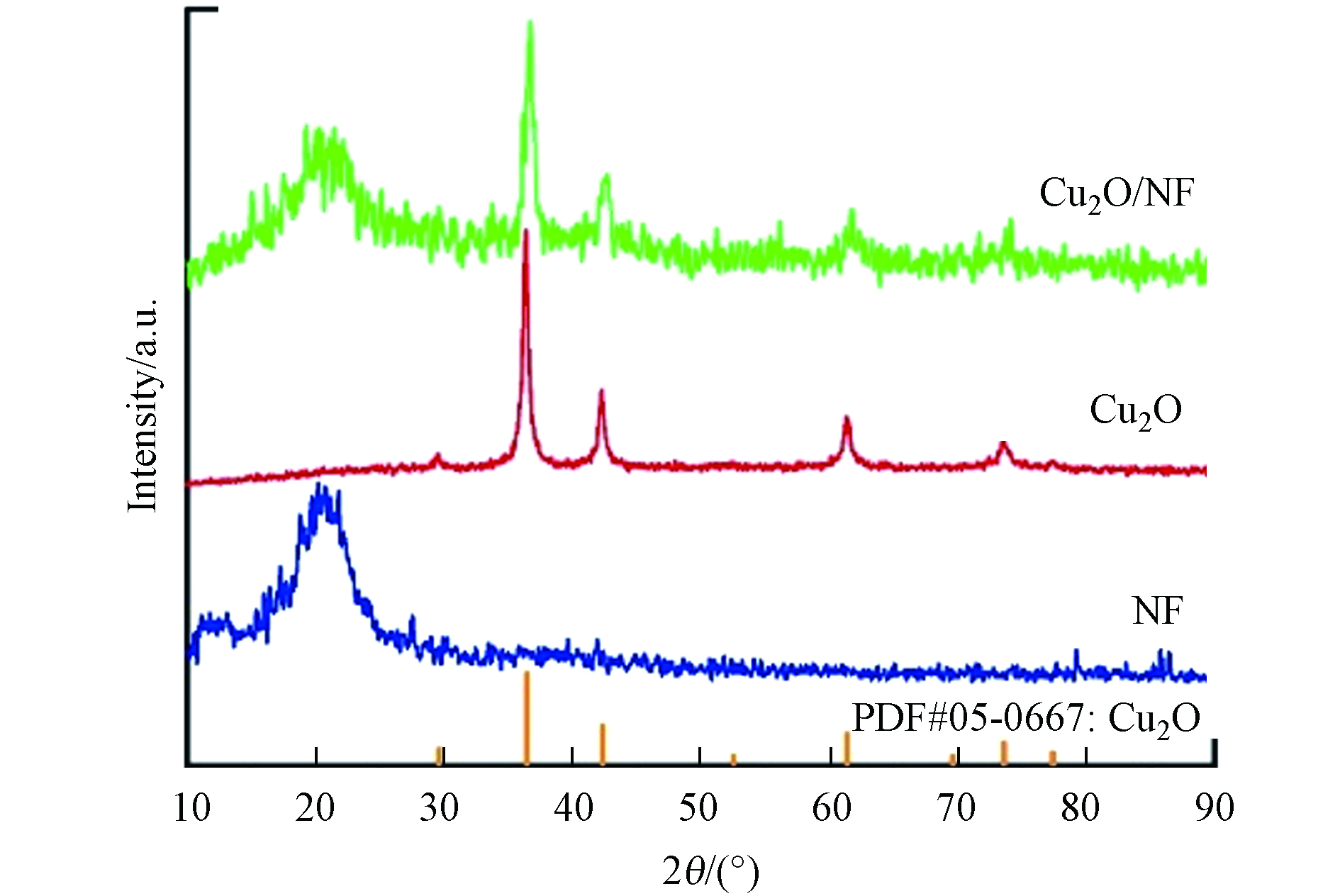
图3为NF、Cu2O和Cu2O/NF复合材料的XRD图谱. Cu2O和Cu2O/NF分别在29.6°、36.4°、42.2°、61.6°和73.9°处均有明显的特征峰,分别对应于Cu2O晶体(JCPDS No.05-0667)的(110)、(111)、(200)、(220)和(311)晶面. Cu2O/NF图谱在20.3°处的峰对应NF的特征峰,由于无纺布由非结晶性聚合物纤维构成,因此只有1个无定型的宽峰[20];Cu2O/NF复合材料包含NF和Cu2O颗粒的主要特征峰,证实了Cu2O颗粒在NF表面原位生长.
-
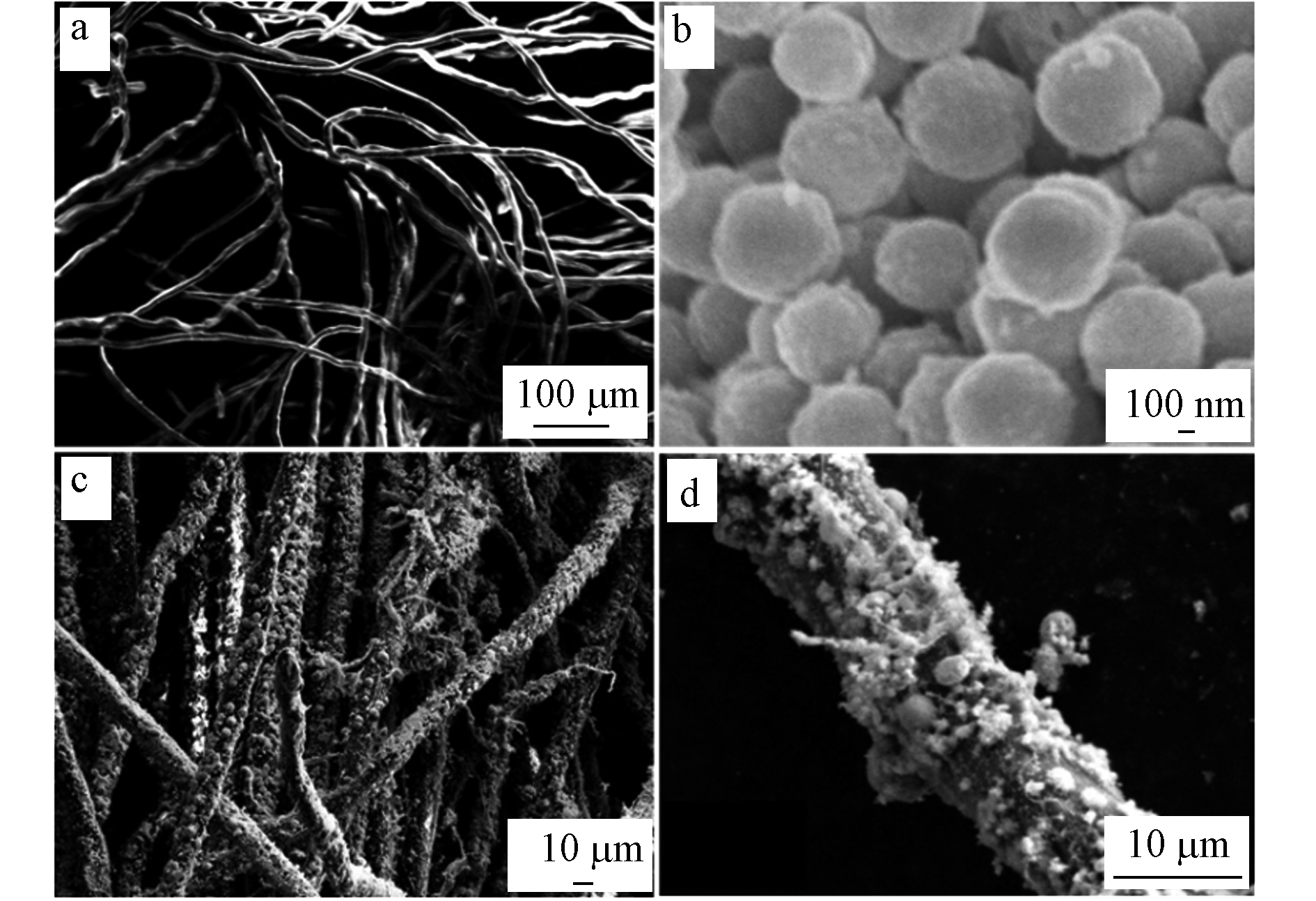
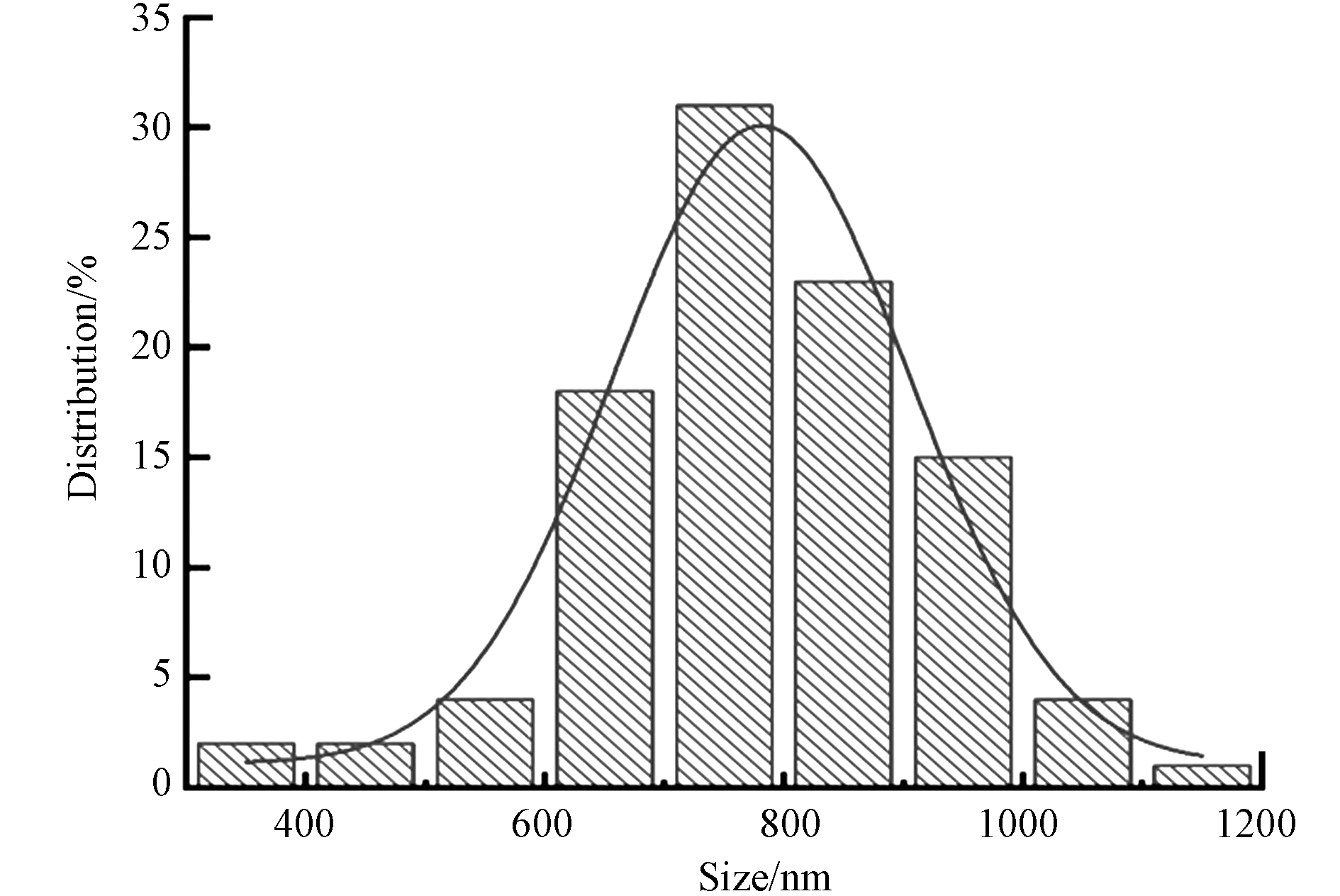
图4(a)是经过预处理的NF扫描图,NF呈现出表面较为光滑的线条状,纤维相互交织;图4(b)Cu2O颗粒呈现规则球形. 图4(c、d)是Cu2O/NF复合材料的SEM图,可以清晰地看到NF的纤维表面包覆大量规则球状的Cu2O颗粒,颗粒分布较为均匀,少部分出现团聚. 图5为制备Cu2O的粒径直方图,在96% 置信水平下,Cu2O的平均直径为(780.11±8.89)nm,最大粒径1.12 μm,最小粒径为350 nm.
-
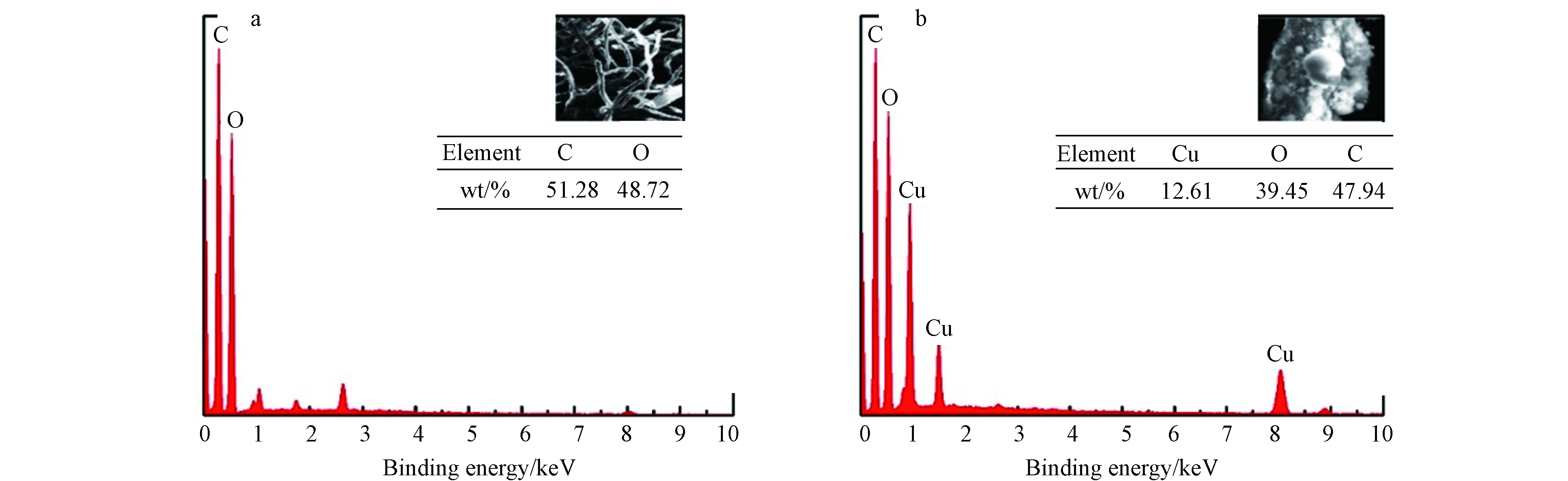
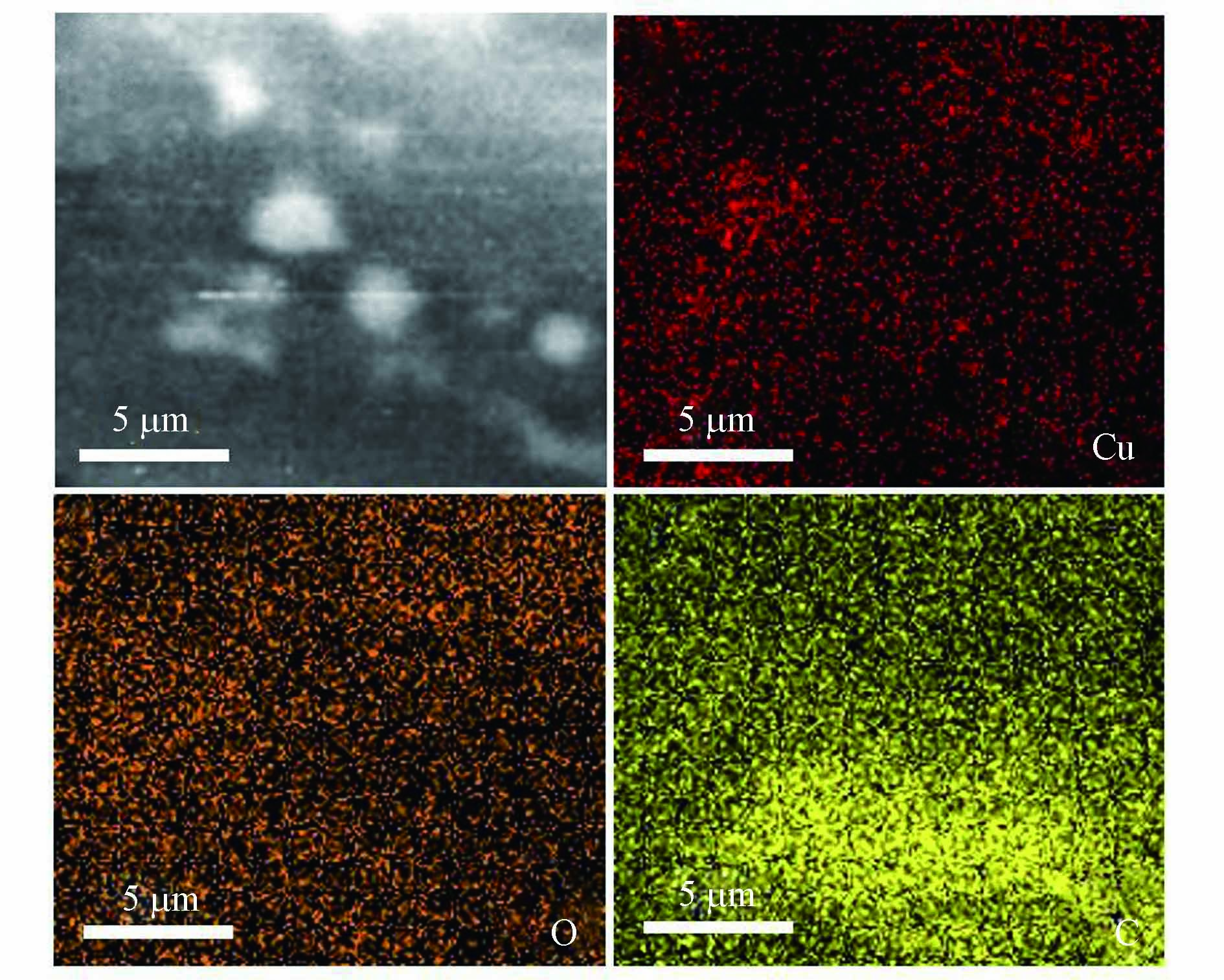
利用能谱仪分析Cu2O/NF复合材料的元素组成. 图6(a)NF的EDS图只存在C、O两种元素,说明预处理过的NF无杂质;图6(b)Cu2O/NF的EDS图显示了复合材料主要由Cu(12.61%)、C(39.45%)、O(47.94%)3种元素组成,Cu元素较为均匀地分布在NF上. 图7 Cu2O/NF的EDS面扫图显示Cu元素较为均匀地分布在NF上.
-
通过FTIR光谱研究样品官能团,见图8.
样品谱图在3415 cm−1处出现一个宽峰,对应于O—H拉伸,在1618 cm−1的和1637 cm−1处的附加谱带与羟基的弯曲和拉伸振动有关[21];Cu2O和Cu2O/NF的FTIR谱图在617 cm−1处均出现了1个强的吸收峰,对应于Cu—O振动模式,证实Cu2O成功负载到NF上.
-
利用氮吸附-解吸等温线测量了NF、Cu2O/NF的比表面积和孔隙率特性. 图9(a、b)分别为NF、Cu2O/NF复合材料的氮气吸附脱附等温曲线图. 该等温线被划分为Ⅳ型等温线,H3型磁滞回线,由孔径分布曲线可知孔尺寸集中分布在2—50 nm,为介孔材料的特性. 插图是NF、Cu2O/NF的孔径分布曲线,经计算得到NF的比表面积约为162.277 m2∙g−1,Cu2O/NF复合材料的比表面积约为420.113 m2∙g−1,较大的比表面积能够有效地促进材料吸附性能.
-
本文对金橙Ⅱ(Orange Ⅱ)、刚果红(CR)、甲基橙(MO)、亚甲基蓝(MB),结晶紫(CV),罗丹明B(RB)6种染料的脱色进行了测定,以了解Cu2O/NF的吸附性能. 首先,考察了复合材料Cu2O/NF中各组分对染料的吸附作用,见图10.
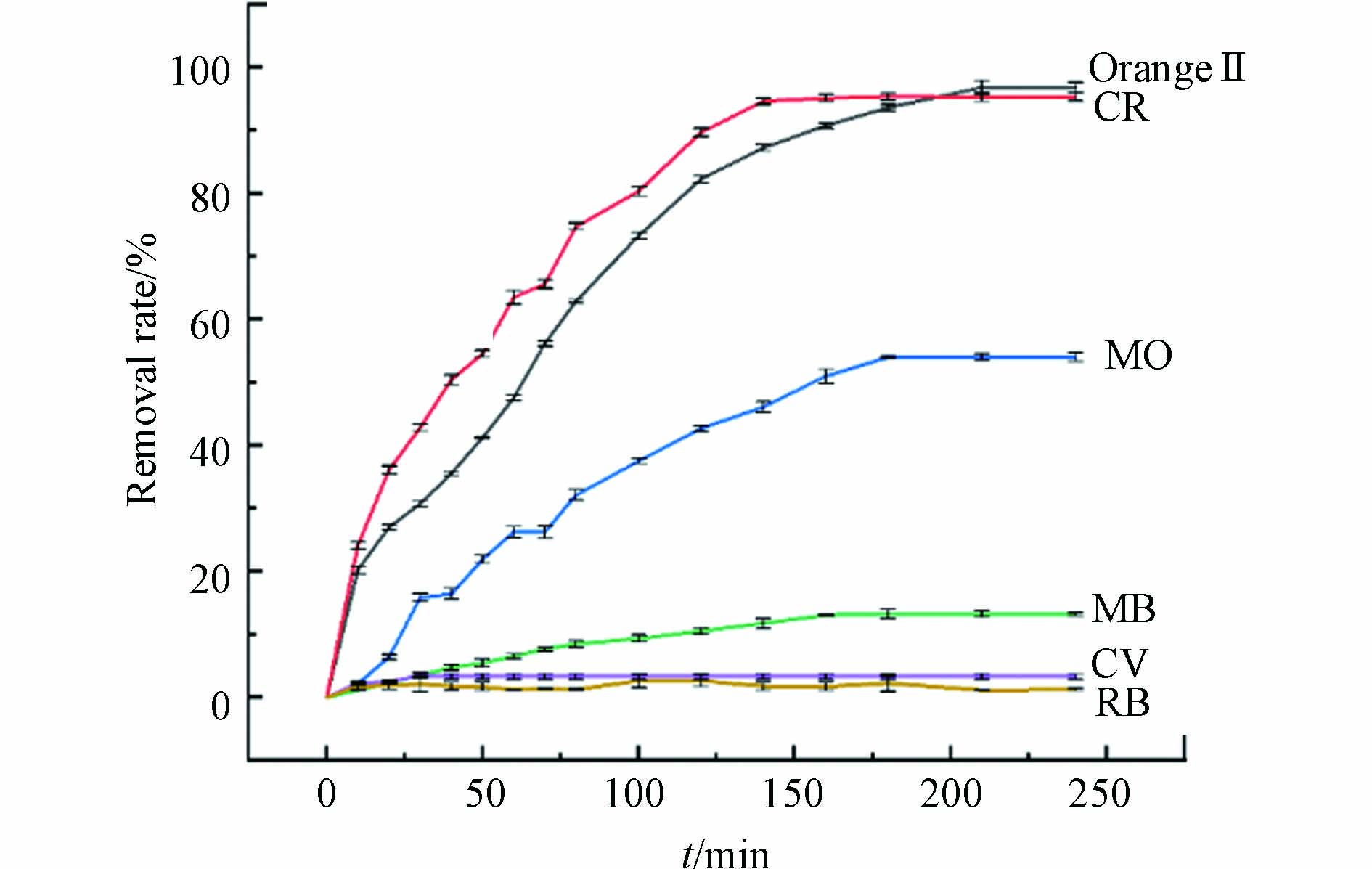
图中显示360 min NF对Orange Ⅱ的去除率仅为3.76%,复合材料中对染料起主要吸附作用的为Cu2O,Cu2O/NF对Orange Ⅱ去除率达96.26%. 其次,图11表明Cu2O/NF对不同染料的去除速率和能力存在明显差异. 对于阴离子染料Orange Ⅱ、CR和MO,最初的快速去除发生在 0—60 min 阶段,然后逐渐增加,在大约150 min 内达到平衡,去除率分别达到96.74%、95.47%和55.43%,另一方面,MB、CV、RB的去除率在240 min分别达到13.23%、3.34%和0.32%. 由此总结Cu2O/NF对阴离子染料具有选择性去除性能.
-
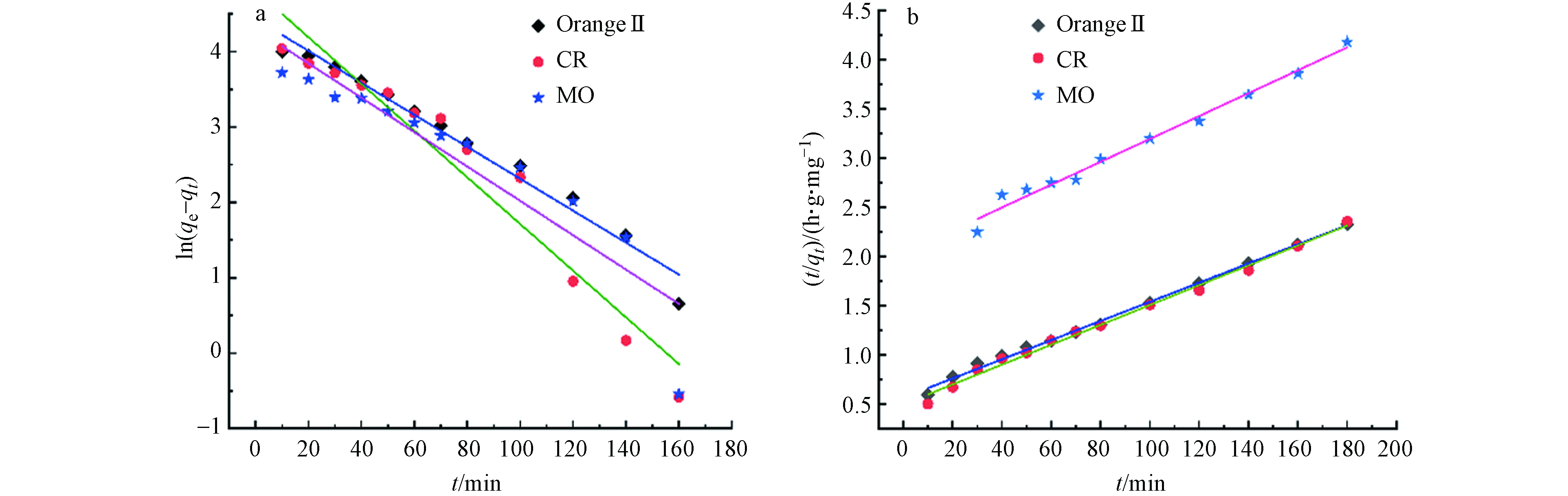
为了验证Cu2O/NF对阴离子染料上的去除机理,应用伪一级和伪二级动力学模型检验实验数据,表2给出了动力学参数(qe,k1,k2)和通过线性回归确定的相关系数(R2)。图12可以得出结论,Cu2O/NF对Orange Ⅱ,CR和MO的吸附更适合伪二级动力学模型,属于化学吸附类型,Cu2O和染料颗粒相互作用过程中化学相互作用是反应进行的限制步骤,其相关系数R2> 0.98。
-
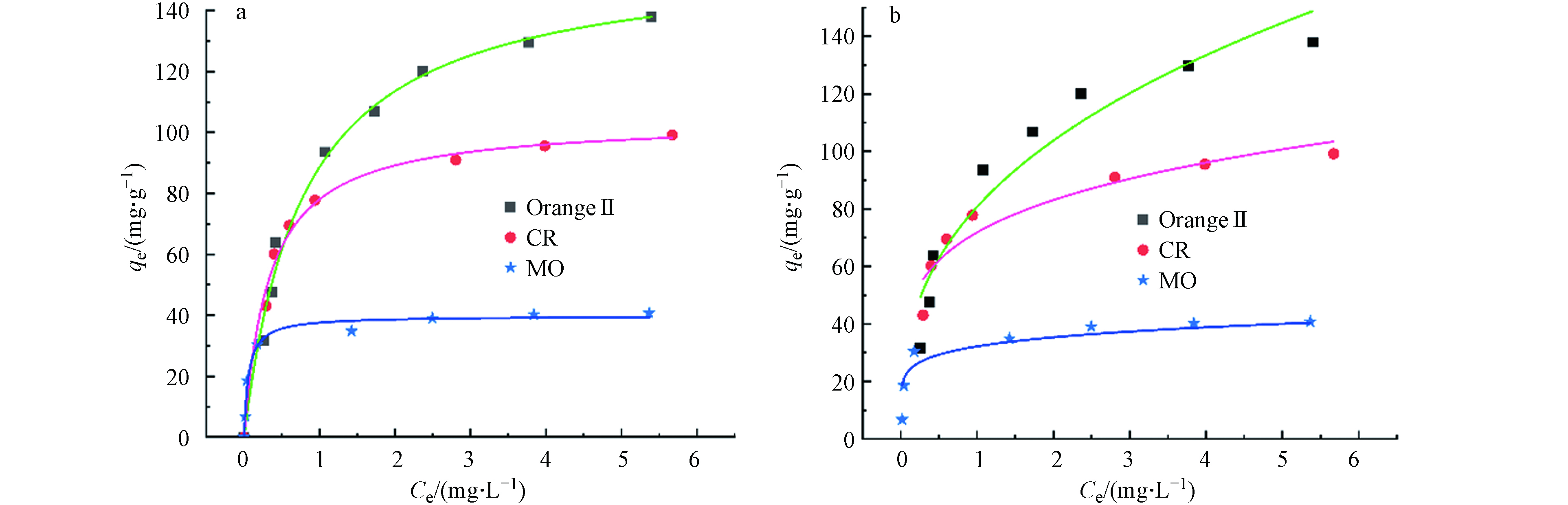
为研究Cu2O/NF表面吸附量和介质中溶质平衡浓度之间的关系,应用Langmuir和Freundlich吸附等温方程对实验数据进行拟合. 图13分别为 Cu2O/NF 吸附Orange Ⅱ、CR 和 MO 的Langmuir和Freundlich等温曲线拟合模型曲线,参数以及线性系数如表3所示. 结果表明,Langmuir 模型可以更好地描述吸附过程,相关系数R2>0.98,这说明 Cu2O/NF的表面上具有均匀的反应位点,吸附是单分子层吸附.
-
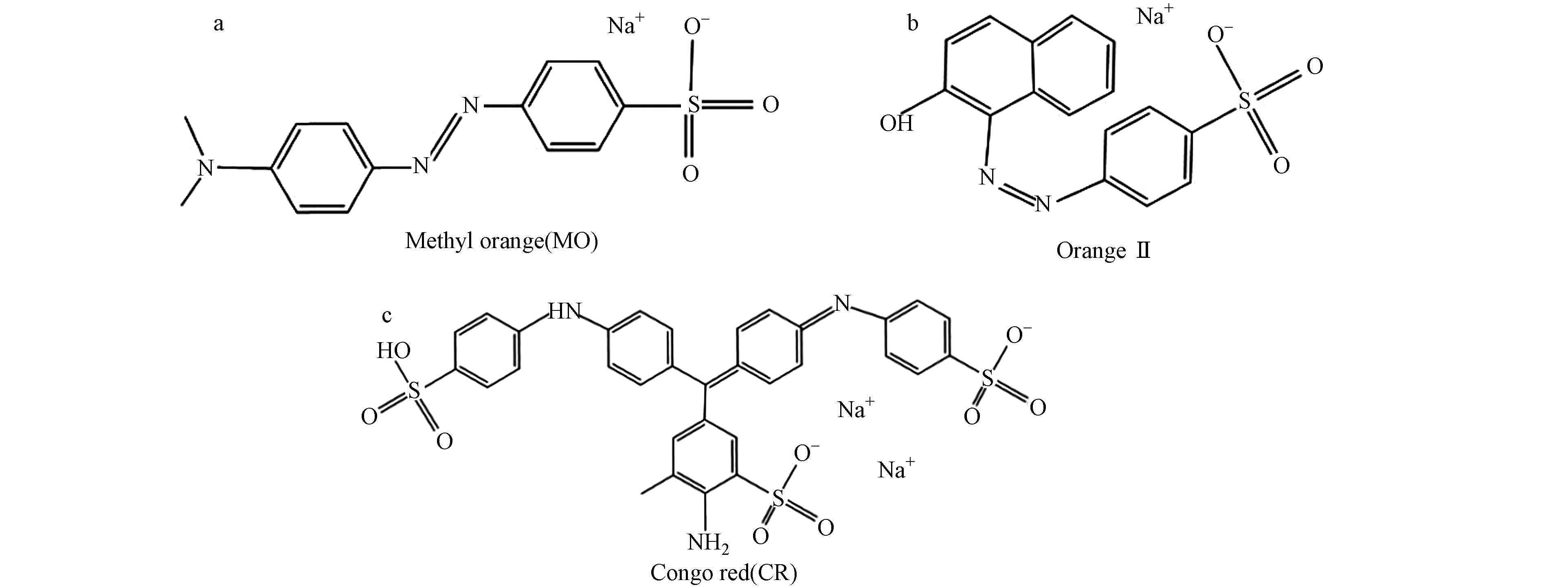
综上所述,Cu2O/NF对阴离子染料具有优异的去除性能. 图14显示了带负电的MO、Orange Ⅱ和CR的结构式.
通过紫外-可见光谱变化说明去除过程中Orange Ⅱ,CR和MO分子结构与特征的变化. 图15显示了3种染料在180 min内的吸收光谱. 图15(a)显示Orange Ⅱ在可见光区的主吸收峰位于484 nm对应于腙形式;位于230 nm和310 nm的紫外区的两个谱带分别属于Orange Ⅱ的苯环和萘环,在反应过程中吸收峰的逐渐下降表明吸附剂对Orange Ⅱ具有良好的脱色效果[22]. 对于CR,图13(b)的吸收光谱出现3个明显的特征峰,497 nm处的吸收峰位于可见光区域,与强发色基团偶氮双键相关,237 nm和345 nm处的紫外光区域的吸收峰分别对应苯环和萘环,这3处吸收峰的下降说明CR在吸附过程中被有效去除. 图15(c)为MO的吸收光谱,在465 nm处的强吸收峰是由MO分子中偶氮结构的n-π*跃迁引起的,属于MO的偶氮键特征峰;在271 nm处的吸收峰是由苯环共轭体系的π-π*跃迁引起的,属于MO分子中苯环的特征吸收峰[23],峰强度的不断减弱表明MO被Cu2O/NF有效吸附. Cu2O粒子带有正电荷,有利于通过静电场力吸附带负电荷的污染物. 在水溶液中阴离子染料分子与Cu2O吸附剂表面紧密接触,而对于阳离子染料分子,在静电排斥的作用下不利于吸附[24]. 因此,制备的Cu2O/NF材料对于带负电荷的染料具有选择吸附性,可潜在地应用于废水的初次分离或某些化学物质的富集.
-
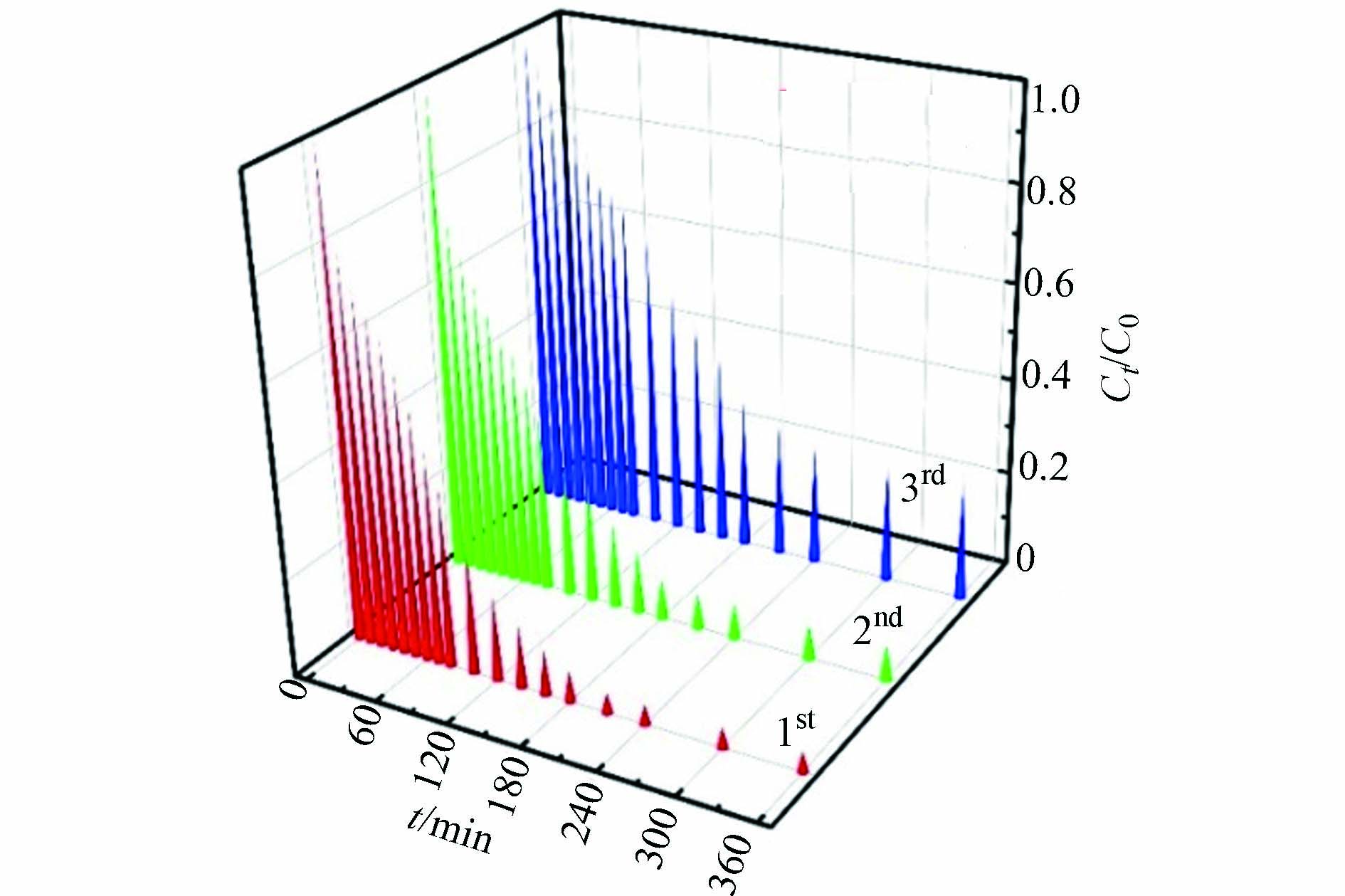
如图16所示,3次循环吸附实验,测得Cu2O/NF对Orange Ⅱ 最终去除率分别为96.54%、91.67%和75.41%.
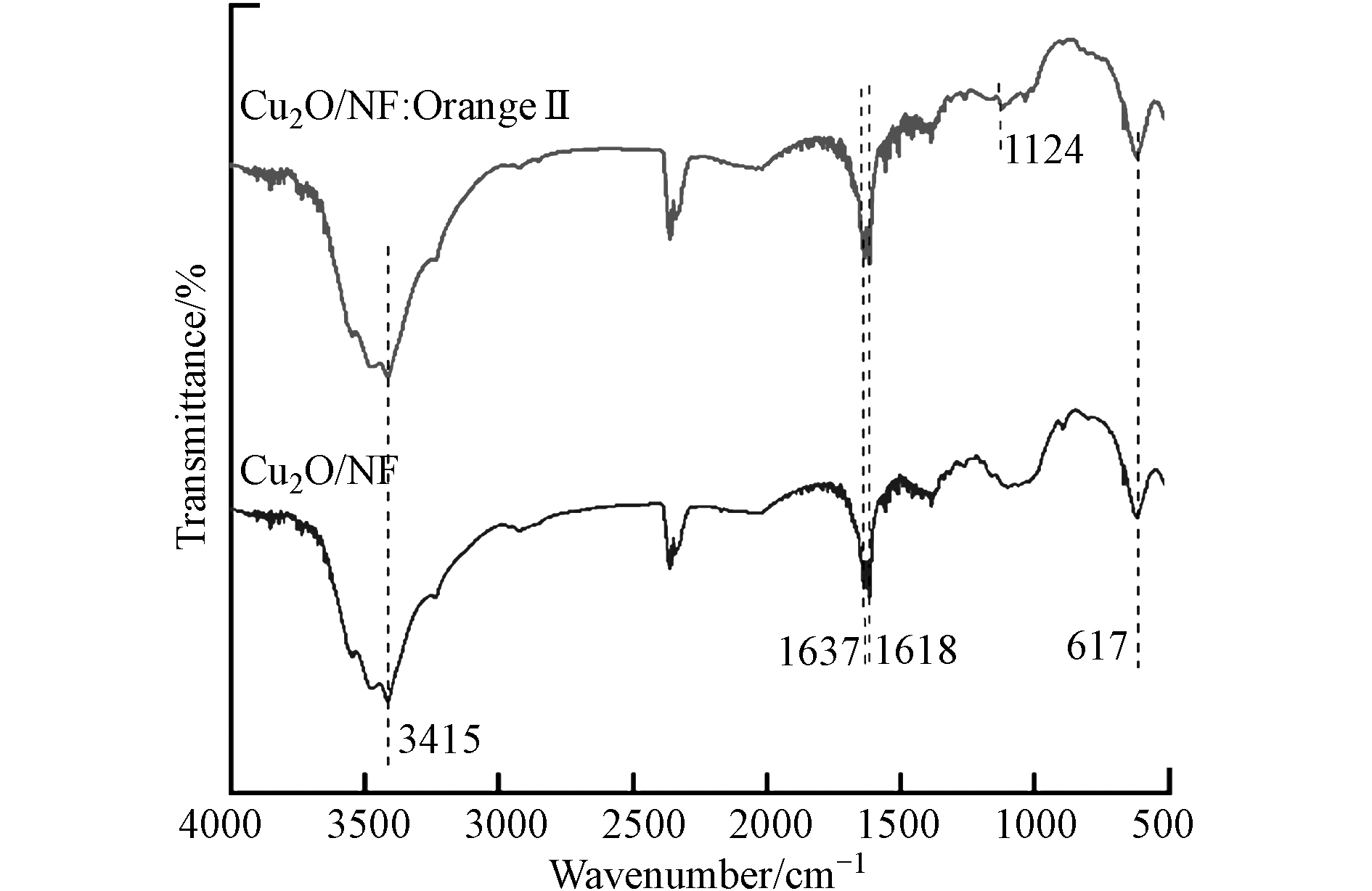
图17为Cu2O/NF吸附Orange Ⅱ反应前后的FTIR图,反应后1124 cm−1处对应Orange Ⅱ的特征峰,表明Cu2O/NF对Orange Ⅱ有吸附作用,并且在吸附实验结束后,Cu2O/NF复合材料的官能团及结构特征(3415 cm−1、1618 cm−1和1637 cm−1、617cm−1处)未发生变化,这表明所制得的Cu2O/NF复合材料的稳定性较好,可以实现多次快捷回收利用. 为进一步提高Cu2O/NF的利用率,可将吸附饱和后的吸附剂在真空状态下加热(80—150 ℃,1—8 h)进行再生[25],或采用新兴的生物再生法,利用微生物对污染物的降解作用来实现吸附剂的脱附再生,操作简单且不产生污染.
-
利用绿茶(龙井)提取物作为还原剂绿色合成Cu2O/NF复合材料,Cu2O颗粒呈规则的球形,其表面被茶提取物中的有机物质包围,有利于其分散性和稳定性. Cu2O/NF比表面积约为420.113 m2∙g−1,对金橙Ⅱ、刚果红和甲基橙等阴离子染料具有较好的选择去除性能,去除率分别达到 96.74%、95.47%和55.43%. 吸附动力学分析表明,Cu2O/NF对阴离子染料的去除符合伪二级动力学;吸附热力学符合Langmuir等温吸附模型. 复合材料3次循环利用后对染料去除率仍可达75.41%. 文中制备的Cu2O/NF是一种环境友好材料,可实现对阴离子染料的高效去除.
绿色合成氧化亚铜/无纺布选择性去除阴离子染料
Green synthesis of Cu2O/non-woven fabrics for selective removal of anionic dyes
-
摘要: 以茶多酚为还原剂绿色合成氧化亚铜/无纺布复合材料,应用于染料的选择性去除. 通过XRD、EDS、SEM、FTIR、BET、XPS等对复合材料进行表征分析,结果表明,Cu2O颗粒呈较为规则的球形并均匀地分布于无纺布上;该复合材料对阴离子染料(金橙Ⅱ、刚果红、甲基橙)有良好的选择去除效果;其中,氧化亚铜/无纺布对金橙Ⅱ的去除率可达96.26%,去除过程符合伪二级动力学模型和Langmuir吸附等温模型;复合材料在3次循环去除染料后,去除率仍保持在75.41%,稳定性较高且能实现快捷回收.Abstract: The composite materials of Cu2O/non-woven fabrics (Cu2O/NF) were green synthesized by using tea polyphenol as the reducing agent, and utilized for selective removal of dyes. The morphological and structural properties of Cu2O/NF were analyzed with XRD, EDS, SEM, FTIR, BET and XPS, and found that Cu2O particles were somewhat ordered spherical shaped and evenly distributed on the non-woven fabrics. The Cu2O/NF presented good capacity of selective removal towards anionic dyes such as OrangeⅡ, Congo Red and Methyl Orange. Amongst the removal rate of Orange Ⅱ reached 96.26%. The removal processes were well fitted as pseudo second-order dynamic and Langmuir adsorption isotherm models. Furthermore, the removal rate remained at 75.41% after three cycled use, suggesting good stability and reusability of the prepared Cu2O/NF.
-
Key words:
- green synthesis /
- tea polyphenols /
- Cu2O /
- non-woven fabrics /
- selective removal.
-

-
表 1 茶多酚含量测定正交实验
Table 1. Orthogonal experiment of tea polyphenols content determination
实验号
NumberA温度/℃
A TemperatureB时间/min
B TimeC提取次数
C Extraction timesD固液比(g∙mL−1)
D Solid-liquid ratio浸提率/%
Extraction rate1 1(60℃) 1(40) 1(1) 1(1:20) 18.37% 2 1 2(50) 2(2) 2(1:25) 24.15% 3 1 3(60) 3(3) 3(1:30) 21.55% 4 2(70 ℃) 1 2 3 20.62% 5 2 2 3 1 19.54% 6 2 3 1 2 21.45% 7 3(80 ℃) 1 3 2 24.32% 8 3 2 1 3 21.48% 9 3 3 2 1 23.58% K1 64.23 63.55 61.85 61.46 K2 61.88 65.17 68.49 70.23 K3 69.54 67.19 65.26 64.12 R 7.53 3.39 6.49 8.87 影响度 D>A>C>B 最优方案 A3B3C2D2 表 2 Cu2O/NF吸附染料动力学参数表
Table 2. Kinetic parameters of Cu2O/NF removing dyes
染料
Dyes平衡吸附量/(mg∙g−1)
qe,exp伪一级动力学
Pseudo second-order kinetic model伪二级动力学
Pseudo second-order kinetic modelK1/min−1 相关系数R2 K2/min−1 相关系数R2 Orange Ⅱ 77 0.0213 0.9756 0.00321 0.9963 CR 76 0.0310 0.9421 0.00333 0.9921 MO 40 0.0230 0.8473 0.00383 0.9836 表 3 Cu2O/NF吸附染料Langmuir和Freundlich相关参数
Table 3. Langmuir and Freundlich parameters of Cu2O/NF removing dyes
染料
DyesLangmuir Freundlich qe,exp/(mg∙g−1) KL/(L∙mg−1) R2 KF 1/n R2 Orange Ⅱ 158 1.2770 0.9905 80.6702 0.3630 0.9129 CR 104 3.0000 0.9914 71.4934 0.2111 0.8820 MO 40 17.1242 0.9824 31.8179 0.1850 0.8355 -
[1] NISHI Y, MIYATA T, MINAMI T. Electrochemically deposited Cu2O thin films on thermally oxidized Cu2O sheets for solar cell applications [J]. Solar Energy Materials and Solar Cells, 2016, 155: 405-410. doi: 10.1016/j.solmat.2016.06.013 [2] PENG L P, WEI H N, TIAN L, et al. Phospholipid/protein co-mediated assembly of Cu2O nanoparticles for specific inhibition of growth and biofilm formation of pathogenic fungi [J]. Science China Materials, 2021, 64(3): 759-768. doi: 10.1007/s40843-020-1457-1 [3] YANAGIDA S, YAJIMA T, TAKEI T, et al. Removal of hexavalent chromium from water by Z-scheme photocatalysis using TiO2 (rutile) nanorods loaded with Au core-Cu2O shell particles [J]. Journal of Environmental Sciences, 2022, 115: 173-189. doi: 10.1016/j.jes.2021.05.025 [4] ZHANG X, FENG Y C, GAO D C, et al. Functionalization of cellulosic hydrogels with Cu2O@CuO nanospheres: Toward antifouling applications [J]. Carbohydrate Polymers, 2022, 282: 119136. doi: 10.1016/j.carbpol.2022.119136 [5] SCHRODER S, ABABII N, LUPAN O, et al. Sensing performance of CuO/Cu2O/ZnO: Fe heterostructure coated with thermally stable ultrathin hydrophobic PV3D3 polymer layer for battery application [J]. Materials Today Chemistry, 2022, 23: 100642. doi: 10.1016/j.mtchem.2021.100642 [6] 杨开, 徐云兰, 钟登杰. C-TiO2/Ti-Cu2O/Cu光催化燃料电池的性能 [J]. 环境化学, 2018, 37(1): 108-114. doi: 10.7524/j.issn.0254-6108.2017071302 YANG K, XU Y L, ZHONG D J. Performance of C-TiO2/Ti-Cu2O/Cu photocatalytic fuel cell [J]. Environmental Chemistry, 2018, 37(1): 108-114(in Chinese). doi: 10.7524/j.issn.0254-6108.2017071302
[7] MAO P, LIU Y, LIU X, et al. Bimetallic AgCu/Cu2O hybrid for the synergetic adsorption of iodide from solution [J]. Chemosphere, 2017, 180: 317-325. doi: 10.1016/j.chemosphere.2017.04.038 [8] LIU F, LIU Q, LIU Y, et al. Synthesis and photocatalytic activity of cubic cuprous oxide supported on activated carbon fibers [J]. Chemical Physics Letters, 2019, 718: 54-62. doi: 10.1016/j.cplett.2019.01.011 [9] BORAH R, SAIKIA E, BORA S J, et al. On-water synthesis of phenols using biogenic Cu2O nanoparticles without using H2O2 [J]. RSC Advances, 2016, 6(102): 100443-100447. doi: 10.1039/C6RA22972G [10] ARAM K, NALLAL M, CHOHYE Y, et al. MOF-derived Cu@Cu2O nanocatalyst for oxygen reduction reaction and cycloaddition reaction [J]. Nanomaterials, 2018, 8(3): 138. doi: 10.3390/nano8030138 [11] AMRANI M A, SRIKANTH V V S S, LABHSETWAR N K, et al. Phoenix dactylifera mediated green synthesis of Cu2O particles for arsenite uptake from water [J]. Science and Technology of Advanced Materials, 2016, 17(1): 760-768. doi: 10.1080/14686996.2016.1244472 [12] XIAO C Y, LI H Y, ZHAO Y, et al. Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes [J]. Journal of Environmental Management, 2020, 275: 111262. doi: 10.1016/j.jenvman.2020.111262 [13] 田利强, 龙康, 陈秀清. 绿色合成膨胀石墨负载纳米零价铁去除水中Cd(Ⅱ) [J]. 环境化学, 2021, 40(12): 3909-3918. doi: 10.7524/j.issn.0254-6108.2020081104 TIAN L Q, LONG K, CHEN X Q. Research on removal of cadmium(Ⅱ) by green synthesized nanoscale zero-valent iron supported on expanded graphite [J]. Environmental Chemistry, 2021, 40(12): 3909-3918(in Chinese). doi: 10.7524/j.issn.0254-6108.2020081104
[14] GOPALAKRISHNAN K, RAMESH C, RAGUNATHAN V, et al. Antibacterial activity of Cu2O nanoparticles on E. coli synthesized from tridax procumbens leaf extract and surface coating with polyaniline [J]. Digest Journal of Nanomaterials & Biostructures, 2012, 7(2): 833-839. [15] QIN C Y, ZHAO Y S, LI L L, et al. Mechanisms of surfactant-enhanced air sparging in different media [J]. Journal of Environmental Science and Health. Part A-Toxic/Hazardous Substances & Environmental Engineering, 2013, 48(9): 1047-1055. [16] SU Y, ZHAO Y S, LI L L, et al. Transport characteristics of nanoscale zero-valent iron carried by three different “vehicles” in porous media [J]. Journal of Environmental Science and Health, Part A-Toxic/Hazardous Substances & Environmental Engineering, 2014, 49(14): 1639-1652. [17] ERROKH A, FERRARIA A M, Conceição D S, et al. Controlled growth of Cu2O nanoparticles bound to cotton fibres [J]. Carbohydrate Polymers, 2016, 141: 229-237. doi: 10.1016/j.carbpol.2016.01.019 [18] SEDIGHI A, MONTAZER M, SAMADI N. Synthesis of nano Cu2O on cotton: morphological, physical, biological and optical sensing characterizations [J]. Carbohydr Polym, 2014, 110: 489-498. doi: 10.1016/j.carbpol.2014.04.030 [19] SVETLICHNYI V A, GONCHAROVA D A, SHABALINA A V, et al. Cu2O water dispersions and nano-Cu2O/fabric composite: preparation by pulsed laser ablation, characterization and antibacterial properties [J]. Nano Hybrids and Composites, 2017, 13: 75-81. doi: 10.4028/www.scientific.net/NHC.13.75 [20] 李奇, 田杜, 陈韶云, 等. 银纳米片在无纺布纤维上的可控组装及其SERS效应 [J]. 高等学校化学学报, 2021, 42(3): 736-744. LI Q, TIAN D, CHEN S Y, et al. Controllable assembling of silver nanosheets on non-woven fabric fibers and its SERS effect [J]. Chemical Journal of Chinese Universities, 2021, 42(3): 736-744(in Chinese).
[21] PANHWAR R, SAHITO I A, KHATRI A, et al. Improved photocatalytic activity of nonwoven fabric coated with graphene by a novel elevated temperature padding method [J]. Materials Chemistry and Physics, 2021, 262: 124294. doi: 10.1016/j.matchemphys.2021.124294 [22] 刘馨钰, 张永丽, 张怡, 等. 铁酸铋可见光催化过一硫酸盐去除金橙Ⅱ [J]. 化学研究与应用, 2019, 31(5): 887-893. LIU X Y, ZHANG Y L, ZHANG Y, et al. BiFeO3 visible-light photocatalysis peroxymonosulfate for removal of orange Ⅱ [J]. Chemical Research and Application, 2019, 31(5): 887-893(in Chinese).
[23] 吴正雷, 彭文博, 董凯, 等. Ni基催化剂处理甲基橙废水动力学及机理研究 [J]. 当代化工, 2020, 49(5): 850-854. WU Z L, PENG W B, DONG K, et al. Research on kinetics and mechanism of degradation of methyl orange wastewater catalyzed by Ni-based catalysts [J]. Contemporary Chemical Industry, 2020, 49(5): 850-854(in Chinese).
[24] LIU J, GAO Z, HAN H, et al. Mesoporous Cu2O submicro-spheres, facile synthesis and the selective adsorption properties [J]. Chemical Engineering Journal, 2012, 185-186: 151-159. doi: 10.1016/j.cej.2012.01.064 [25] 刘晓勤, 孙林兵, 蒋文娟, 等. 一种负载型氧化亚铜吸附剂、制备方法、应用及再生方法: CN103007874B[P].2014-12-31. LIU X Q, SUN L B, JIANG W J, et al. Supported cuprous oxide adsorbent as well as preparation method, application and regeneration method: CN103007874B[P]. 2014-12-31 (in Chinese).
-




 下载:
下载: