-
挥发性有机化合物(volatile organic compounds,VOCs)是一种广泛存在于环境中的有毒物质[1],根据世界卫生组织(WHO)的定义常温下沸点为50—260 ℃的各种有机物,主要来自于石油、表面涂层和电子制造等工业生产[2],长时间处在含有VOCs的环境中会对人体存在极大的危害. 目前VOCs的处理技术主要有吸附回收技术、生物法、等离子体技术、蓄热燃烧和紫外光催化氧化降解技术[3-9]. 其中紫外光催化氧化技术因其技术成本低、绿色环保等优点吸引着大量学者的关注[10-13]. 紫外光催化氧化技术的原理是在单独紫外光照射或紫外光协同催化剂的条件下,利用紫外光波段的光子轰击空气中的H2O和O2,使其解离成羟基自由基和游离的氧原子等活性氧基团,与有机物反应并将有机物降解为无毒无害的小分子[14]. 研究表明,相对湿度是紫外光催化氧化降解有机物的重要因素之一[12,15-17],存在较佳的反应相对湿度,在此相对湿度下有机物的直接转化率最高,能达到90%以上[18-19]. 这些研究中大多只使用有机物的直接转化率作为有机物降解程度的判断标准,但在最佳直接转化率的工况下有机物的矿化率并不一定最大,且其数值往往低于20%[11,15],这说明有机物经紫外光催化氧化降解后并没有完成转化为CO2和H2O,所以仅使用直接转化率作为有机物降解效果的判断依据是不全面的.
不同相对湿度条件下紫外光催化氧化降解有机物分子的程度不一,导致降解后的尾气中还存在着相当复杂的中间有机物. 而且从有机物降解产物的成分分析来看,在最佳转化率工况下有机物的降解方向也并不完全朝着生成小分子方向进行,这也是近年来紫外光催化氧化技术发展的瓶颈问题[11, 15]. 因此,确定紫外光催化氧化技术最佳的工艺条件,需要结合直接转化率、矿化率以及尾气成分来综合分析. 然而有机物种类众多,产物成分与降解路径复杂,需要进行大量的实验研究. 量子化学密度泛函理论(density functional theory,DFT)是一种主要以电子密度分布函数为基础的电子基态的结构理论,可以从分子水平上研究通过实验方法难以阐释的反应机制. 通过量子化学计算获得不同相对湿度条件下的有机物的降解路径,对于理解光催化氧化降解有机物、确定较佳的操作工况具有积极的指导意义.
目前理论计算研究还主要集中在对有机物氧化降解机理的猜测上[12, 17],针对不同相对湿度条件下的反应路径的研究报道较少. 本文在紫外光氧化反应实验系统中开展了有机物苯的降解实验,研究了相对湿度对苯的直接转化效率、矿化率以及对应的尾气成分的影响. 并基于量子化学密度泛函理论对不同相对湿度条件下中间有机物的生成机理进行理论计算,提出较为合理的降解路径.
-
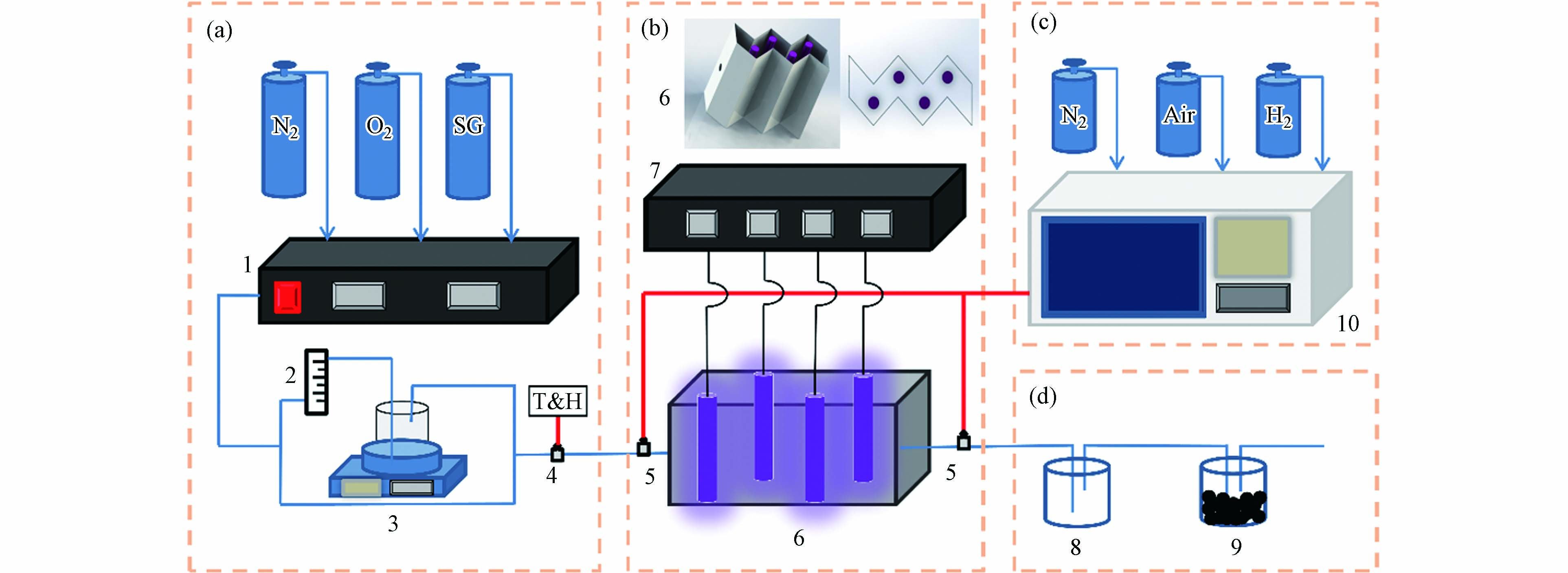
紫外光氧化降解实验系统示意图如图1所示,主要包括(a)配气系统、(b)紫外光反应系统(c)检测系统和(d)尾气处理系统四大部分组成. 紫外光反应器为自制不锈钢折流板式,其目的是为了增大有机分子与壁面的接触面积,且反应器的内壁敷设有活性炭纤维布用来增加对有机物苯分子的吸附能力,通过与其他没有在反应器内部敷设活性炭纤维吸附材料的学者研究对比发现,添加活性炭纤维布对后续反应路径的量子化学研究不存在影响. 反应器的高度为250 mm,截面宽度为68 mm,有效体积为3.27 L,4只紫外灯管(雪莱特)均匀置于反应器中,紫外灯管发出波长为(185+254)nm的紫外光,功率均为8 W,发光强度约为28 μW·cm−2.
芳香烃作为VOCs中的一类有机物,苯可以作为其代表物质,同时大多芳香烃在完全氧化降解的过程中都会经历到苯环的开环氧化过程. 本实验以苯作为目标污染物,采用苯的标气(氮气填充)、N2和O2,通过气体质量流量计控制柜1调节3种气体的比例配制苯初始浓度为100 mg·m−3,氧气含量为21%的混合气体. 混合后的气体分为两路,一路经过玻璃转子流量计2和恒温水浴箱3并与另一路气体混合,通过调节两路气流的比例以及恒温水浴箱的温度来调节实验气体的温湿度从而控制混合气体的相对湿度,进入反应器的气体温湿度由温湿度仪4实时检测. 气相色谱仪10可以对反应器进出口气体中的有机物连续检测,有机溶剂8和活性炭颗粒9是为了对反应后尾气中残留的有机物和臭氧等二次污染物进行处理.
-
所采集的气体样品采用GC-MS联用仪(2010 PLUS,日本岛津)测定,采用的色谱柱为DB-WAX色谱柱. 柱温首先在50 ℃下保持1 min,然后以5 ℃·min−1的升温速率加热到200 ℃,再以10 ℃·min−1的升温速率加热到280 ℃并维持10 min. 质谱离子源温度为200 ℃,扫描方式为全扫描,质量数范围为35—450. 分析结果通过VOCs图谱与NIST05数据库进行对比确定最终产物.
式中,ηD 为苯的直接转化效率,C0为苯进气口浓度(mg.m−3),Ct为苯经过紫外光反应器处理一定时间后的浓度(mg·m−3).
式中,ηM 为苯的矿化效率,C1理论上苯转化后全部矿化出口CO2浓度(mg·m−3),C2实际测得的出口CO2浓度(mg·m−3).
为了从理论上研究二者的竞争氧化过程,本研究采用密度泛函(DFT)的方法进行理论计算,所有的量子化学计算均使用Gaussian16[20]程序进行. 对于几何构型优化及过渡态搜索,均采用M062X/6-311+G(d,p)[18, 21],并且确认所有过渡态只有一个虚频,在此虚频的振动方向连接着反应物与产物,其他分子均没有虚频. 得到过渡态构型后,继续进行内禀反应坐标(IRC)计算,沿能量降低方向找寻过渡态所对应的反应势能面的能量最低点,确认两端对应着反应物与产物,同时对每一步反应的反应物、过渡态和产物进行频率计算得到各自的热力学能,再对其作差得到每一步反应的能垒.
-
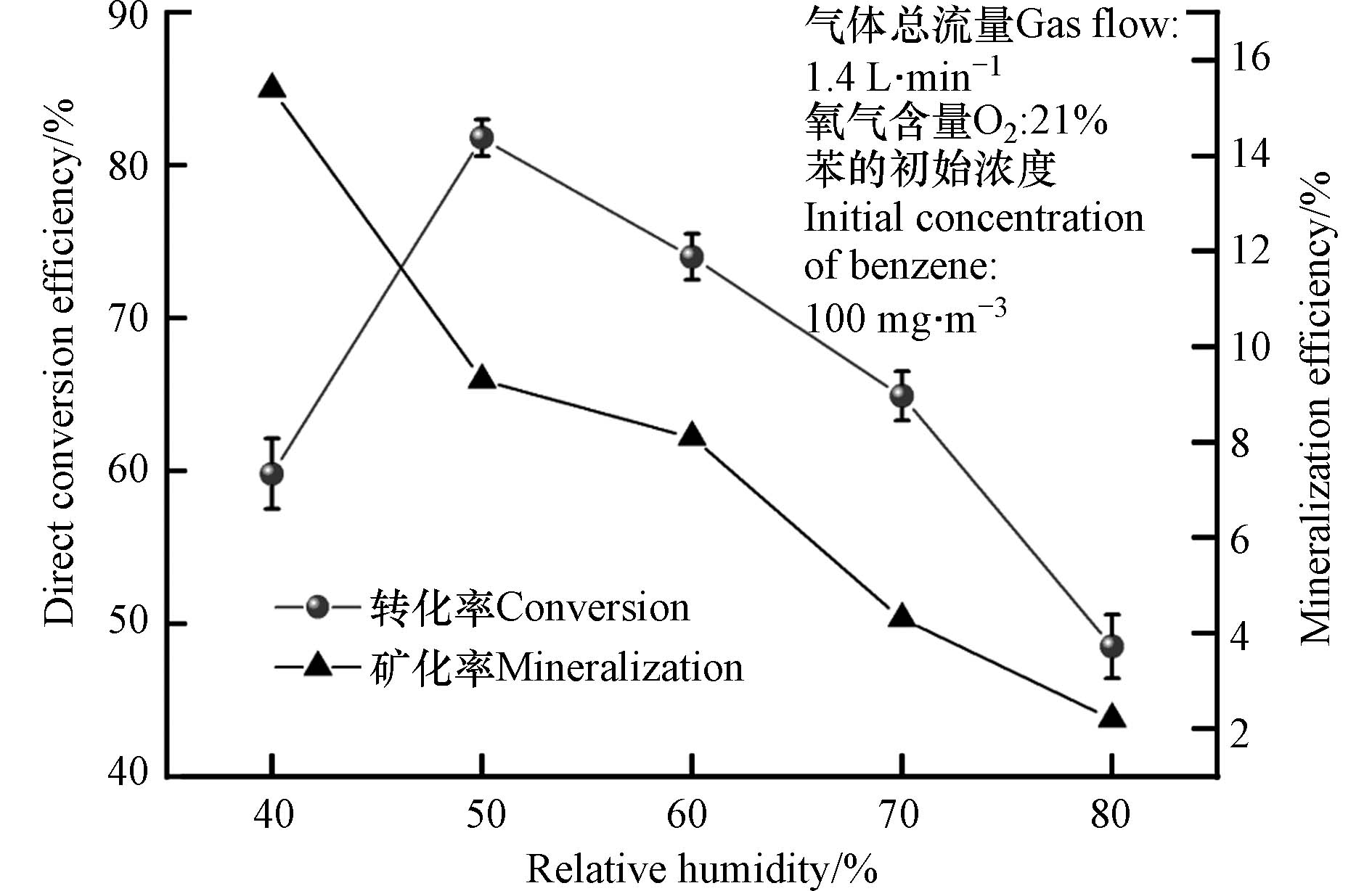
图2为相对湿度对有机物苯的直接转化效率和矿化率的影响. 由图2可见,随着相对湿度从40%增加到50%,苯的直接转化效率由60%增加到82%,这主要是因为随着相对湿度的增加,体系的H2O分子逐渐增加,产生的羟基自由基也不断增加,因此氧化效率增强;当相对湿度由50%增加到80%,苯的直接转化效率由82%显著下降到48%,这主要是因为体系的光子量恒定,随着相对湿度的继续增加,过多的水分子会和苯分子一起竞争吸收光子,从而导致苯的直接转化率开始降低. 然而,当体系的相对湿度从40%增加到80%,苯的矿化率由15%急剧降低至2%,较低的矿化率表明苯并没有完全转化为CO2和H2O,这与Kang等[11]和陈越平[15]的研究结果相同. 尤其特别的是,当相对湿度为50%时的矿化率并不是最大值,且相比相对湿度为40%,矿化率显著下降了40%. 因此,对相对湿度的选择需要继续结合尾气成分进行分析.
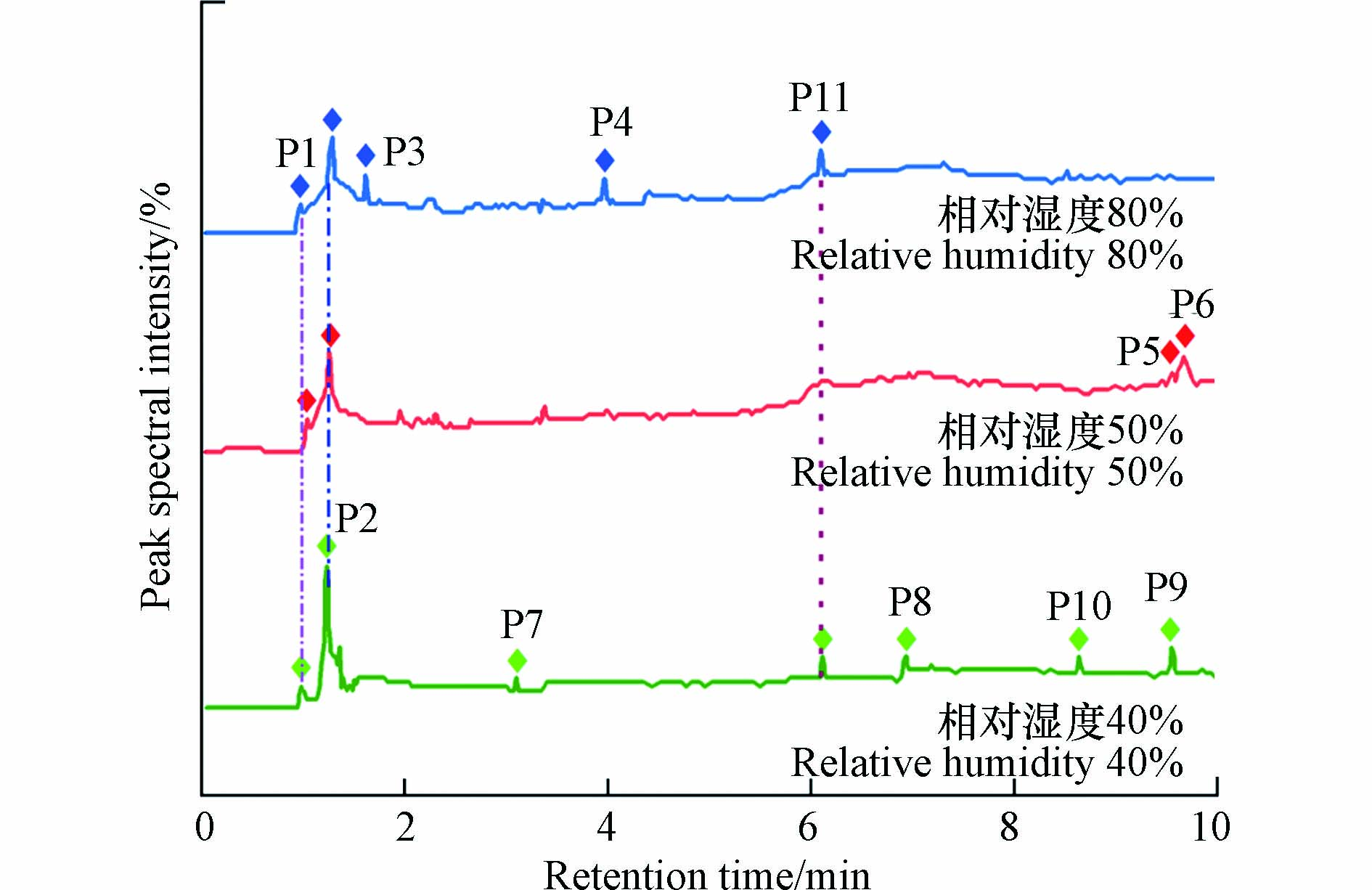
图3为3个相对湿度(40%、50%和80%)条件下的尾气经GC-MS检测后的谱图,每条谱线上的信号峰与标准数据库对比分析后所得到的有机物如表1所示.
本文对GC-MS谱图进行分析遵循两个原则:一是对峰的筛选以峰的强度为标准,因为强度较大的峰可以说明该有机物的含量较多,而含量多的有机物更能说明是苯分子氧化降解的主要产物;二是根据每一个峰与标准库对照后的可信度,本文只对可信度SI在90—95及以上的峰进行指认. 由图3可见,3种不同相对湿度条件下尾气的成分中都含有P1(二氧化碳),说明在本次紫外光氧化降解的实验中有一部分的苯分子可以被完全降解;尾气中都生成了P2(丙酮),丙酮的峰面积相较于其它几种有机物更大,说明丙酮是本研究中主要的降解产物,这与王子东[22]的研究结果是一样的. 除此之外,不同相对湿度条件下尾气中所含有机物的种类差异较大,其中低相对湿度条件下有机物的种类和数量最为复杂,包括降解生成的P3(丙酮醇)、芳香类有机物P11(苯甲醛)和P10(苯乙酮),碳原子的个数分别为六、八、九的P7(己醛)、P8(辛醛)和P9(壬醛),这与张宇飞[12]的研究结果相似. 高相对湿度条件下尾气中有机物成分还包括P11(苯甲醛)以及非苯环类的环状有机物P4(2,2,3-三甲基-3-氧杂环丁醇).
当体系中的相对湿度达到50%时,苯的直接转化效率最大,经反应后尾气中有机物的种类最少,但是尾气中却存在更大相对分子质量且毒性更大的有机物P5(1-甲基萘)和P6(2-甲基萘). 这表明较高的苯直接转化率所对应的工况并不一定是最好的工艺条件.
-
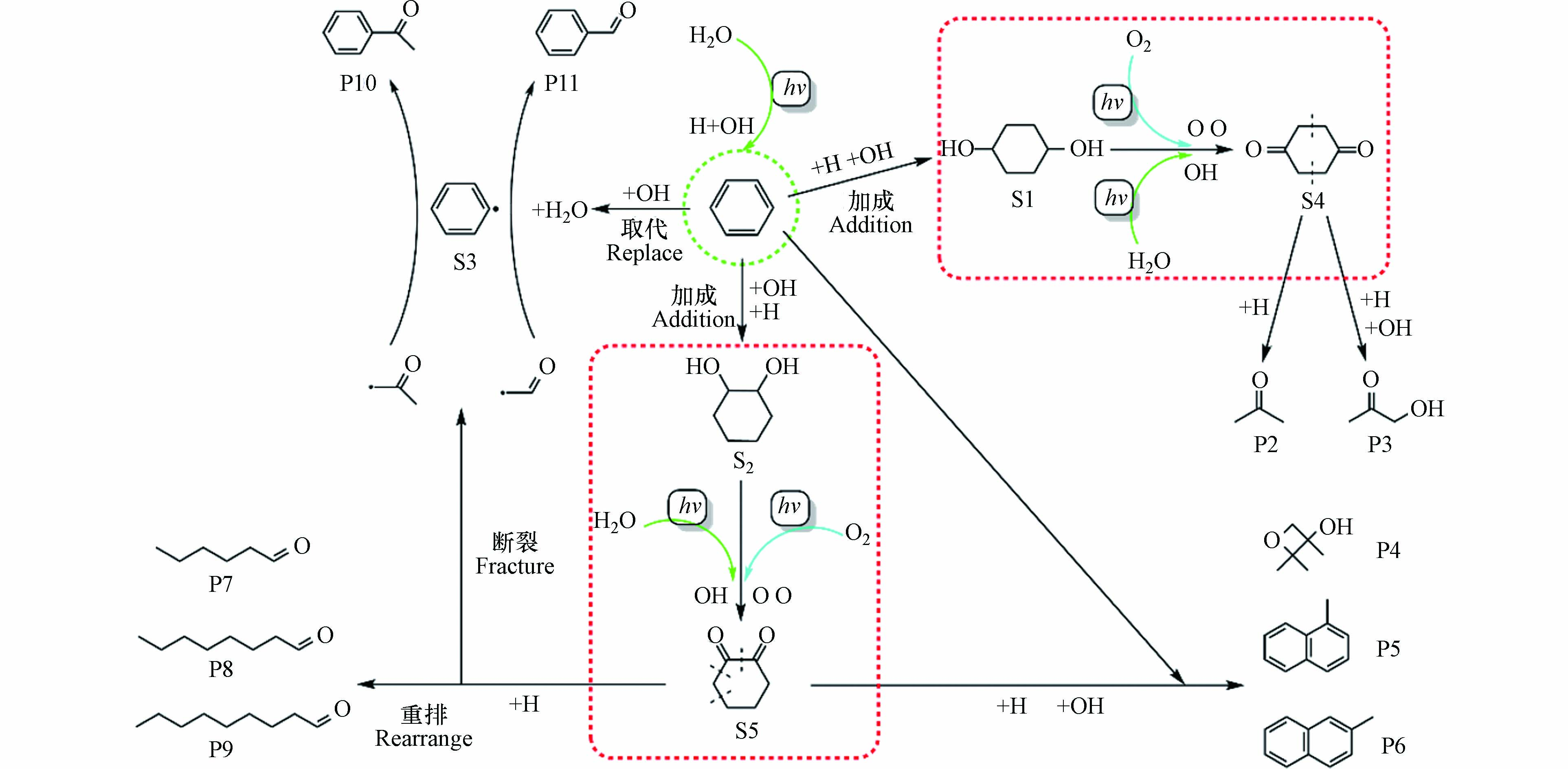
苯环平面上的π电子云是富电子基团,类似烯烃,可与缺电子的基团(亲电试剂)发生亲电取代反应. 在本研究中,亲电试剂是来自于水分子和氧气分子经紫外光照射后分解产生的游离H、OH和O自由基[10]. 苯分子的降解可能路径如图4所示,一部分苯与体系中游离的H和OH自由基发生完全亲电加成反应,这是因为在紫外光的照射条件下,苯的加成反应是在不饱和键上同时进行的[23],本文选择加成产物S1(1,4-环己二醇)和S2(1,2-环己二醇)作为中间物的代表[24-25]. 另一部分苯分子会与游离的OH自由基发生亲电取代反应,OH自由基与苯分子上的一个H原子反应后生成游离的中间物苯自由基S3和一个水分子.
中间物S1两端的羟基会被体系中的活性氧自由基O和OH继续氧化为羰基生成S4(1,4-环己二酮),S4的结构不稳定,会从对称轴处断裂形成两分子相同的基团—CH2COCH2—,该基团会与体系中的H以及OH自由基结合生成尾气中的产物P2(丙酮)或 P3(丙酮醇). 同理,中间物S2上的两个羟基也会被体系中的活性氧自由基O和OH继续氧化为羰基生成S5(1,2-环己二酮). 由于两个邻位羰基的作用使得S5很不稳定,所以会在体系其它原子的作用下四分五裂为小片段的游离基,其中一部分会与未参加反应的苯分子以及游离H、O和OH自由基作用生成P4(2,2,3-三甲基-3-氧杂环丁醇)、P5(1-甲基萘)和P6(2-甲基萘),另一部分会加氢后重排形成长链醛类有机物P7(己醛)、P8(辛醛)和P9(壬醛). 同时生成的游离乙酮基(—COCH3)和甲醛基(—COH)会与上述产生的中间物苯自由基S3结合形成产物P10(苯乙酮)和P11(苯甲醛). 由于在整个苯分子降解的过程中,中间物S1继续氧化为S4以及S2继续氧化为S5是最关键同时也是最为复杂的中间过程,在这一过程中,起到氧化作用的自由基主要来自空气中的氧气分子裂解而成的游离氧原子与不同湿度条件下产生的羟基自由基.
-
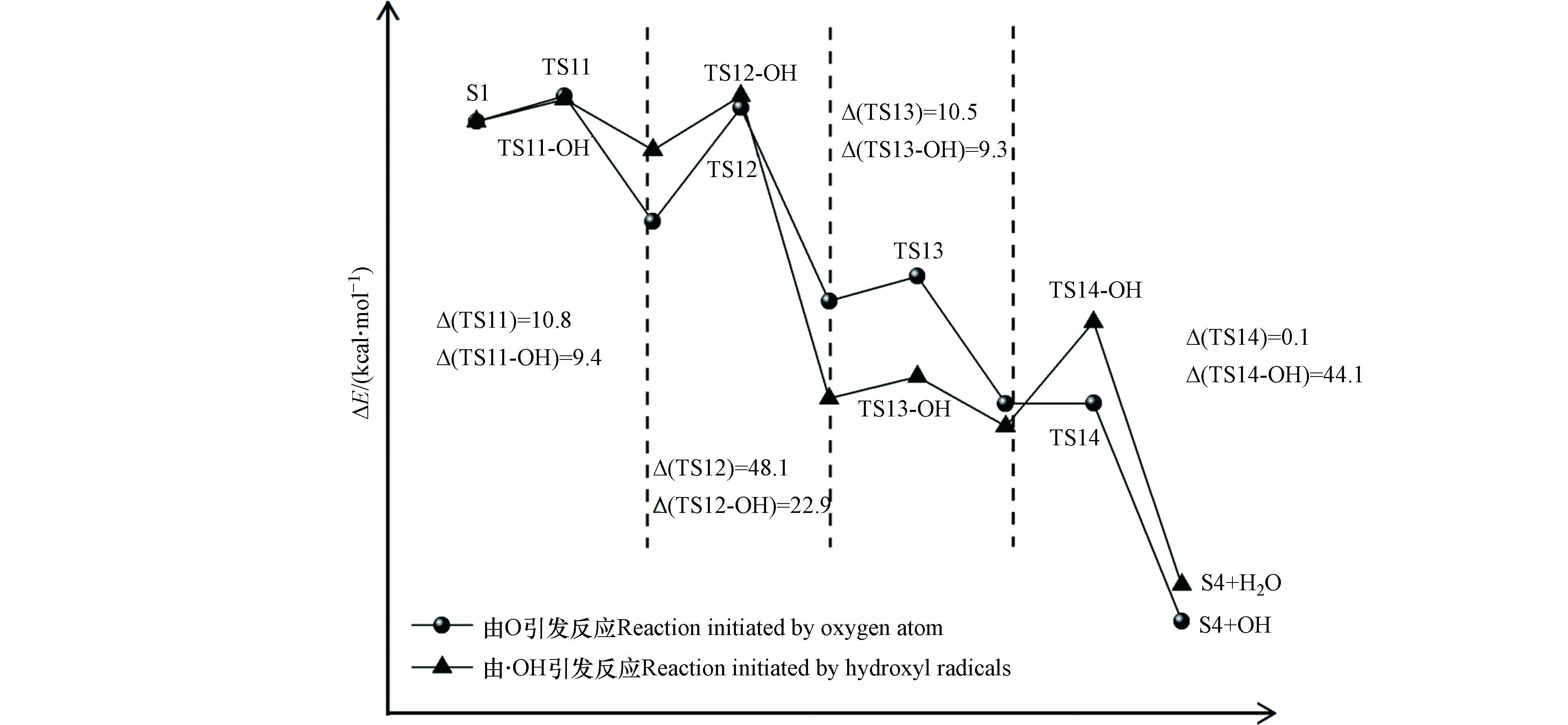
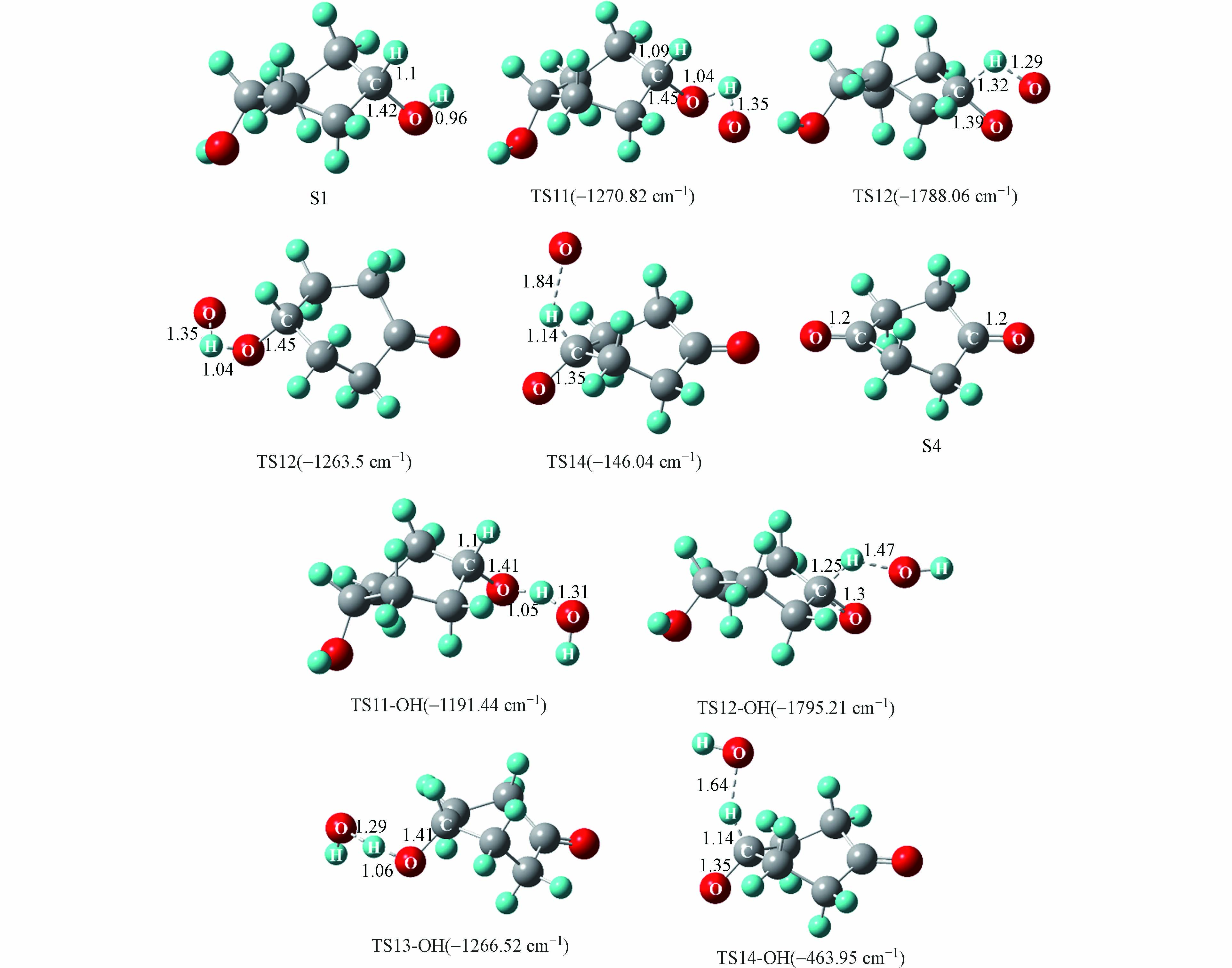
游离氧原子与羟基自由基引发的S1氧化为S4反应能量变化如图5所示,S1氧化反应主要分为四步,分别是羟基上的氢原子以及与羟基相连碳上的氢原子依次被氧化脱除,相对应的过渡态结构以及虚频值如图6所示.
实验中混合气体的氧气含量为21%,氧气分子含量多于由相对湿度带来的水分子含量,所以在波长为(185、254)nm的紫外光照射下,S1发生氧化反应过程中起主导作用的活性氧基团为游离的氧原子. 如图5,由氧原子引发的S1氧化为S4的四步反应能垒值依次为10.8、48.1、10.5、0.1 kcal·mol−1, 其中能垒最大的为第二步反应,说明该步反应对整个氧化过程起着关键作用. 由羟基自由基引发的S1氧化为S4四步反应的能垒值依次为9.4、22.9、9.3、44.1 kcal·mol−1,可以看到羟基自由基的引入能明显的降低S1氧化反应中的反应能垒,其中第一步到第三步反应的能垒分别降低了13.0%、11.4%和52.4%,这说明随着体系相对湿度的增加,有利于S1的氧化进行. 图3中也可以看到,相对湿度为80%的条件下出现了相对湿度为40%和50%没有的中间产物P3(丙酮醇),结合图4可以知道P3(丙酮醇)是中间物S4的后续反应形成的,同时P3(丙酮醇)的形成相较于P2(丙酮)需要更多的羟基自由基,这些都说明了,随着相对湿度的增加,羟基自由基的增加促进了S1的氧化.
-
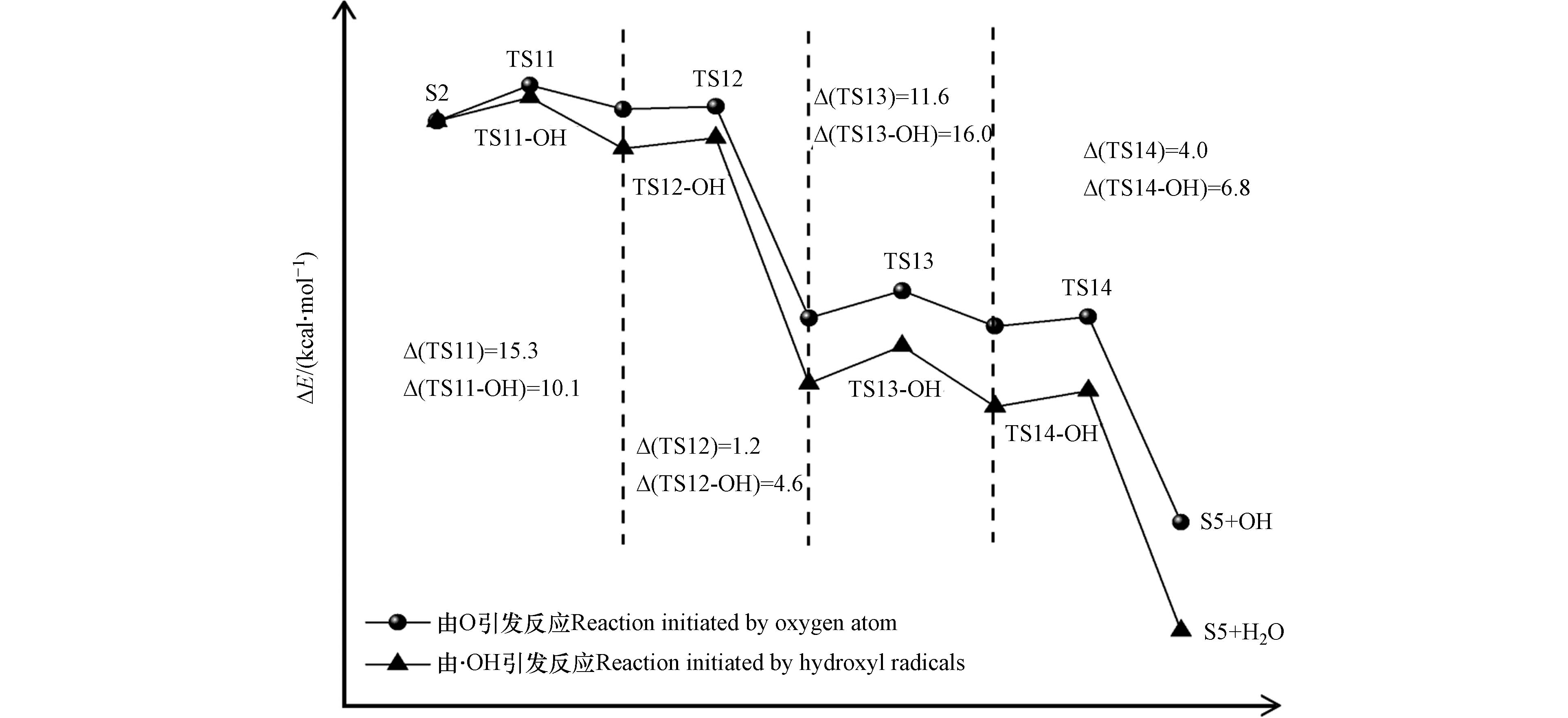
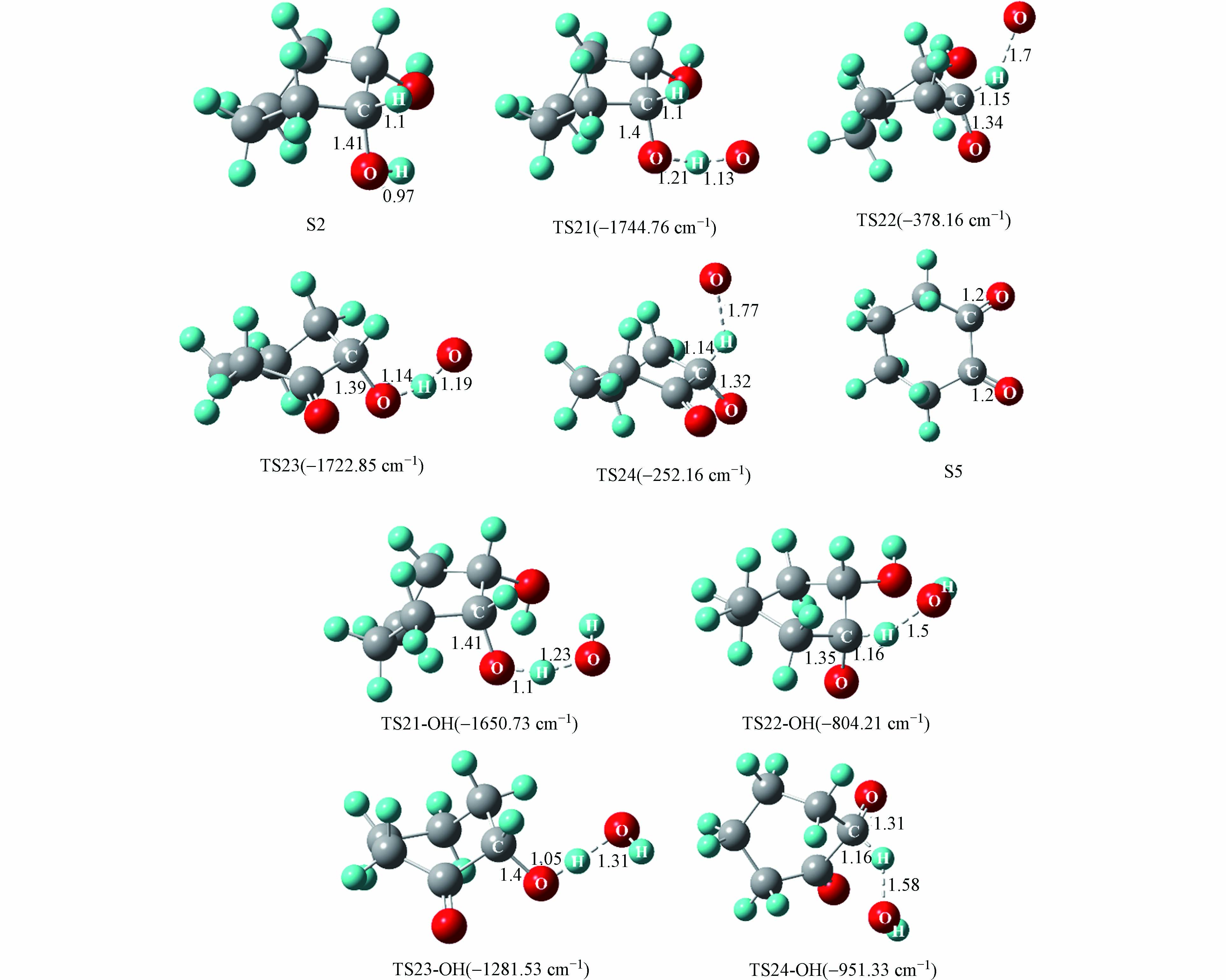
与S1到S4氧化过程类似,氧原子与羟基自由基竞争作用下的S2到S5氧化过程中起主导作用的活性氧基团为游离的氧原子. 游离氧原子与羟基自由基引发的S2氧化为S5反应能量变化如图7所示,S2氧化反应主要分为四步,分别是羟基上的氢原子以及与羟基相连的碳上面的氢原子依次被氧化,相对应的过渡态结构以及虚频值如图8所示.
由氧原子引发的S2氧化为S5四步反应的能垒值依次为15.3、1.2、11.6、4.0 kcal·mol−1,其中能垒最大的为第三步反应,说明该步反应对整个氧化过程起着关键作用. 由羟基自由基引发的S2氧化为S5四步反应的能垒值依次为10.1、4.6、16.0、6.8 kcal·mol−1. 可以看到,羟基自由基的引入仅在第一步反应中降低了34.0%的能垒值,而对于后三步的反应均有不同程度的增加,这表明随着体系相对湿度的增加,羟基自由基的引入也是有利于S2的氧化.
仅由氧原子引发的S1氧化为S4以及S2氧化为S5的四步反应中能垒最大值分别为48.1 kcal·mol−1和15.3 kcal·mol−1,所以在相对湿度较低的条件下,苯的主要氧化路径是S2氧化为S5. 结合图3和图4也可以看到,当体系的相对湿度为40%时,尾气中出现产物P7(己醛)、P8(辛醛)和P9(壬醛)、P10(苯乙酮)和P11(苯甲醛),这与所猜测的苯分子降解路径完全一致. 此时苯的直接转化率与矿化率分别为60%和15%,虽然此时苯降解的矿化效果最好,但是较低的直接转化率以及尾气中生成比苯分子质量更大的有机物.
羟基自由基的引入使得S1与S2氧化的四步反应中能垒最大值从之前的48.1 、15.3 kcal·mol−1分别下降到22.9 、11.6 kcal·mol−1,大大缩减了S1氧化为S4以及S2氧化为S5的难易程度差距,使得在较高相对湿度的条件下,苯的氧化路径是由S1氧化为S4以及S2氧化为S5共同决定的. 随着相对湿度的增加,尾气中出现产物P3(丙酮醇)、P4 (2,2,3-三甲基-3-氧杂环丁醇)、P5 (1-甲基萘)和P6 (2-甲基萘). 当体系的相对湿度为80%时,苯的直接转化率48%和矿化率2%均为最低,但此时尾气中的产物成分较少.
根据以上的分析表明,羟基自由基的引入对S1氧化为S4以及S2氧化为S5的过程都有积极的作用,同时改变了苯分子的降解路径,从而促进苯分子的氧化降解. 苯的氧化降解应结合直接转化率、矿化率以及尾气成分来综合分析,在相对湿度较高与较低的条件下,均出现了各自的利弊,所以对于特定的工艺氧化降解路径还需要谨慎的考虑相对湿度的调节.
-
(1)苯的直接转化率随着相对湿度的增加出现先升高后降低的趋势,在相对湿度为50%时,直接转化率达到最大值82%,但矿化率随相对湿度增加由15%急剧降低至2%.
(2)在相对湿度为40%的条件下,苯分子的氧化降解路径主要是1,2-环己二醇氧化为1,2-环己二酮,路径的四步反应中最大的能垒值为15.3 kcal·mol−1,苯降解的矿化效果最好,直接转化效率最低,尾气中出现分子质量更大的有机物. 随着相对湿度的增加,苯的氧化路径是由1,4-环己二醇氧化为1,4-环己二酮与1,2-环己二醇氧化为1,2-环己二酮共同决定,羟基自由基的引入使得1,4-环己二醇与1,2-环己二醇氧化的四步反应中能垒最大值从48.1 kcal·mol−1和15.3 kcal·mol−1分别下降到22.9 kcal·mol−1和11.6 kcal·mol−1,苯的矿化率和直接转化率急剧降低,尾气中的产物成分减少.
羟基自由基与游离氧原子竞争作用下的光氧化降解苯反应路径
Study on the reaction path of photo-oxidative degradation of benzene under the competition of hydroxyl radicals and free oxygen atoms
-
摘要: 本文实验研究了相对湿度对光氧化降解苯的直接转化效率、矿化率以及对应的尾气成分的影响,并利用量子化学DFT(Density functional theory)的方法讨论了不同相对湿度条件下羟基自由基与游离氧原子竞争作用下的光氧化降解苯反应路径. 结果表明,苯的直接转化效率随着体系相对湿度的增加出现先升高后降低的趋势,相对湿度为50%时直接转化效率最高,为82%,矿化率随相对湿度的增加显著降低,最低可达到2%,且不同相对湿度条件下的尾气成分差异较大. 在相对湿度为40%的条件下,苯分子的氧化降解路径主要是1, 2-环己二醇氧化为1,2-环己二酮,此时最大的反应能垒值为15.3 kcal·mol−1,有利于提高苯降解的矿化效果,生成了P10苯乙酮和P11苯甲醛等分子质量更大的有机物;随着相对湿度由50%提高到80%,最大的反应能垒值由48.1 kcal·mol−1降低到22.9 kcal·mol−1,此时苯的氧化路径由1,4-环己二醇氧化为1,4-环己二酮与1,2-环己二醇氧化为1,2-环己二酮共同决定,羟基自由基的引入大大降低了1,4-环己二醇与1,2-环己二醇氧化的难度,苯的矿化率和直接转化率急剧降低. 确定最佳的相对湿度需要综合分析直接转化效率、矿化率以及尾气成分.Abstract: The effects of relative humidity on the direct conversion efficiency, mineralization rate and components of waste gas from benzene photooxidation degradation were studied. Quantum chemical DFT method was used to analyze the reaction path of benzene photooxidation degradation under the competition of hydroxyl radical and oxygen atoms at different relative humidity. The results showed that the direct conversion efficiency of benzene increased first and then decreased with increasing relative humidity. The maximum direct conversion efficiency was 82% appearing at relative humidity of 50%. The mineralization rate decreased significantly at higher relative humidity, and the smallest value is 2%. The components of waste gas varied much at different relative humidity. At relative humidity of 40%, the oxidative degradation path of benzene was determined by oxidation of 1,2-cyclohexanediol to 1,2-cyclohexanedione, and the maximum reaction energy barrier is 15.3 kcal·mol−1, which is beneficial to improving the mineralization effect and generating some organic compounds with higher molecular weight such as P10 acetophenone and P11 benzaldehyde; As the relative humidity increased from 50% to 80%, the maximum reaction energy barrier decreased from 48.1 kcal·mol−1 to 22.9 kcal·mol−1. At this time, oxidative degradation path of benzene was determined by oxidation of 1, 4-cyclohexanediol to 1,4-cyclohexanedione and oxidation of 1,2-cyclohexanediol to 1,2-cyclohexanedione. The introduction of hydroxyl radicals greatly decreased the oxidation difficulty of 1,4-cyclohexanediol and 1,2-cyclohexanediol, resulting in sharply decreasing mineralization rate and direct conversion efficiency. To determine the optimal relative humidity, it is necessary to comprehensively analyze the direct conversion efficiency, mineralization rate, and components of waste gas.
-
Key words:
- photooxidation /
- benzene /
- relative humidity /
- quantum chemistry /
- DFT
-

-
表 1 不同相对湿度下尾气GC-MS检测主要成分
Table 1. Detection of main components in exhaust gas by GC-MS under different relative humidity
编号
Serial number名称
NameCAS 分子式
Molecular formula保留时间/min
Retention timeP1 二氧化碳 124-38-9 CO2 0.975 P2 丙酮 67-64-1 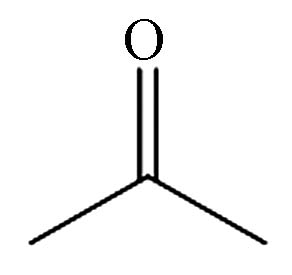
1.233 P3 丙酮醇 116-06-6 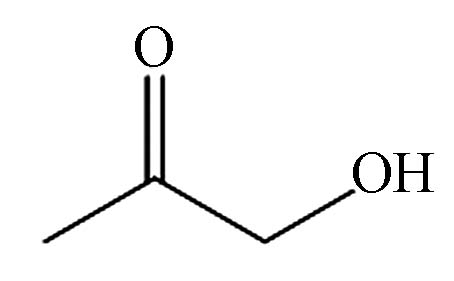
1.325 P4 2,2,3-三甲基-3-氧杂环丁醇 25910-96-7 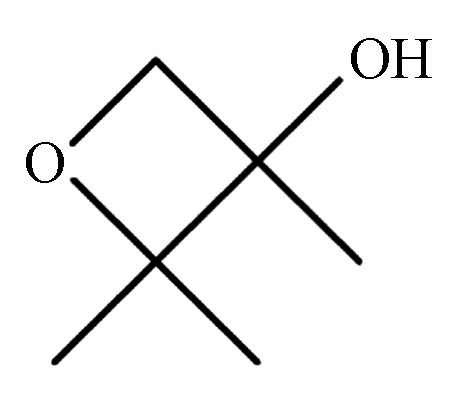
1.617 P5 1-甲基萘 90-12-0 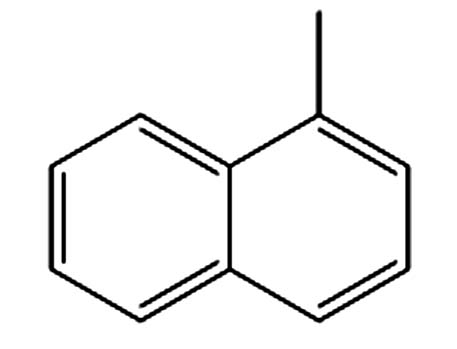
9.675 P6 2-甲基萘 91-12-0 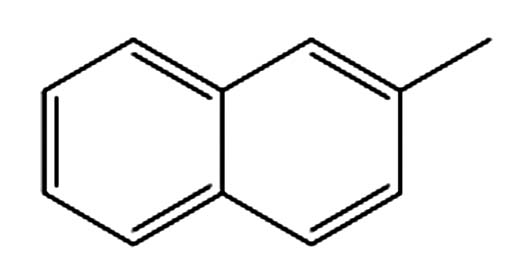
9.675 P7 己醛 66-25-1 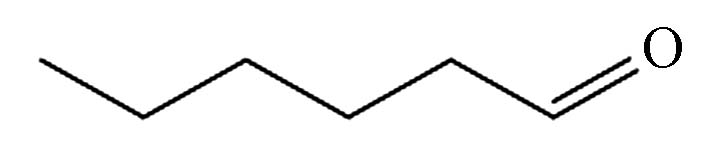
3.100 P8 辛醛 124-13-0 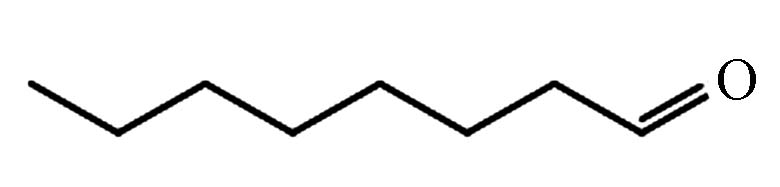
7.075 P9 壬醛 124-19-6 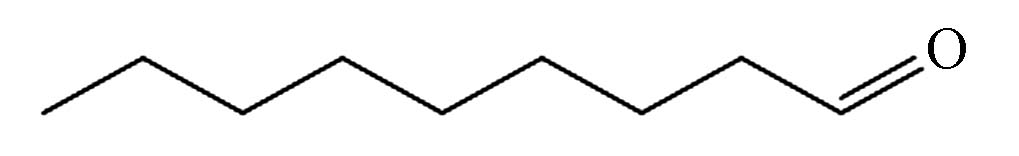
9.558 P10 苯乙酮 98-86-2 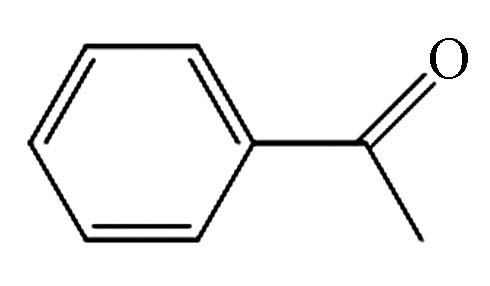
8.642 P11 苯甲醛 100-52-7 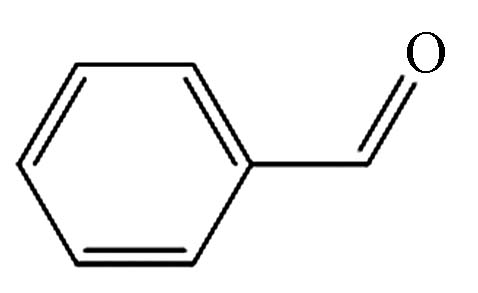
6.117 -
[1] 吴健, 高松, 陈曦, 等. 涂料制造行业挥发性有机物排放成分谱及影响 [J]. 环境科学, 2020, 41(4): 1582-1588. WU J, GAO S, CHEN X, et al. Source profiles and impact of volatile organic compounds in the coating manufacturing industry [J]. Environmental Science, 2020, 41(4): 1582-1588(in Chinese).
[2] PATERSON C A, SHARPE R A, TAYLOR T, et al. Indoor PM2.5, VOCs and asthma outcomes: A systematic review in adults and their home environments [J]. Environmental Research, 2021, 202: 111631. doi: 10.1016/j.envres.2021.111631 [3] 廖正祝, 田红. 煤化工VOCs吸附处理技术研究进展及展望 [J]. 洁净煤技术, 2021, 27(1): 155-168. LIAO Z Z, TIAN H. Research progress and prospect of coal chemical VOCs adsorption treatment technology [J]. Clean Coal Technology, 2021, 27(1): 155-168(in Chinese).
[4] DU Z H, LIN X. Research progress in treatment of VOCs by dielectric barrier plasma cooperating catalyst [J]. IOP Conference Series:Earth and Environmental Science, 2020, 508(1): 012132. doi: 10.1088/1755-1315/508/1/012132 [5] 郭海倩, 缪晶晶, 姜理英, 等. 低温等离子体-生物耦合系统对复合CVOCs的降解 [J]. 环境科学, 2018, 39(2): 640-647. GUO H Q, MIAO J J, JIANG L Y, et al. Composite CVOCs removal in a combined system of nonthermal plasma and a biotrickling filter [J]. Environmental Science, 2018, 39(2): 640-647(in Chinese).
[6] 孙昕, 史路肖, 张燚, 等. 真空紫外/过二硫酸盐去除饮用水中嗅味物质 [J]. 环境科学, 2018, 39(5): 2195-2201. SUN X, SHI L X, ZHANG Y, et al. Removal of odorants in drinking water using VUV/persulfate [J]. Environmental Science, 2018, 39(5): 2195-2201(in Chinese).
[7] 庄媛, 刘杰民, 曲琛, 等. 芬顿催化氧化VOCs过程中的传质增强及协同作用研究进展 [J]. 环境化学, 2021, 40(11): 3307-3315. doi: 10.7524/j.issn.0254-6108.2021033108 ZHUANG Y, LIU J M, QU C, et al. Mass transfer enhancement and synergistic effect during VOCs removal by Fenton oxidation [J]. Environmental Chemistry, 2021, 40(11): 3307-3315(in Chinese). doi: 10.7524/j.issn.0254-6108.2021033108
[8] 党小庆, 王琪, 曹利, 等. 吸附法净化工业VOCs的研究进展 [J]. 环境工程学报, 2021, 15(11): 3479-3492. doi: 10.12030/j.cjee.202011052 DANG X Q, WANG Q, CAO L, et al. Research progress on purification of VOCs in industrial gas by adsorption [J]. Chinese Journal of Environmental Engineering, 2021, 15(11): 3479-3492(in Chinese). doi: 10.12030/j.cjee.202011052
[9] 梁文俊, 李坚, 李依丽, 等. 低温等离子体法去除苯和甲苯废气性能研究 [J]. 环境污染治理技术与设备, 2005(5): 51-55. LIANG W J, LI J, LI Y L, et al. Degradation of benzene and toluene with cold plasma [J]. Techniques and Equipment for Environmental Pollution Control, 2005(5): 51-55(in Chinese).
[10] 黄雪燕, 庄晶晶, 胡学靖, 等. 室内VOCs光催化法处理研究进展 [J]. 环境保护与循环经济, 2021, 41(3): 29-32. HUANG X Y, ZHUANG J J, HU X J, et al. Research progress in photocatalytic treatment of indoor VOCs [J]. Environmental Protection and Circular Economy, 2021, 41(3): 29-32(in Chinese).
[11] KANG I S, XI J Y, HU H Y. Photolysis and photooxidation of typical gaseous VOCs by UV Irradiation: Removal performance and mechanisms[J]. 中国环境科学与工程前沿: 英文版, 2018(3): 107-120. KANG I S, XI J Y, HU H Y. Photolysis and photooxidation of typical gaseous VOCs by UV Irradiation: Removal performance and mechanisms [J]. Frontiers of Environmental Science & Engineering, 2018(3): 107-120.
[12] 张宇飞, 朱燕群, 王树荣, 等. 甲苯的光氧化降解试验研究 [J]. 环境科学学报, 2015, 35(9): 2759-2765. ZHANG Y F, ZHU Y Q, WANG S R, et al. Experimental study on the degradation of toluene by photo-oxidation [J]. Acta Scientiae Circumstantiae, 2015, 35(9): 2759-2765(in Chinese).
[13] de LUIS A M, LOMBRAÑA J I, MENÉNDEZ A, et al. Analysis of the toxicity of phenol solutions treated with H2O2/UV and H2O2/Fe oxidative systems [J]. Industrial & Engineering Chemistry Research, 2011, 50(4): 1928-1937. [14] BRASLAVSKY S, ACUNA A U, ADAM W, et al. Glossary of terms used in photochemistry, 3rd edition (IUPAC Recommendations 2006) [J]. Pure & Applied Chemistry, 2007, 79(3): 293-465. [15] 陈越平. H2O2强化紫外光催化降解低浓度二甲苯废气的研究[D]. 杭州: 浙江工业大学, 2016. CHEN Y P. Research on low concentration xylene degradation by H2O2 enhanced UV photocatalysis[D]. Hangzhou: Zhejiang University of Technology, 2016(in Chinese).
[16] 周灵浚, 卜岩枫, 成卓韦, 等. 真空紫外光解乙苯废气的工艺特性及转化机制研究 [J]. 环境污染与防治, 2014, 36(6): 13-19. ZHOU L J, BU Y F, CHENG Z W, et al. Conversion characteristics and mechanism analysis of gaseous ethylbenzene degraded by vacuum ultraviolet photodecomposition [J]. Environmental Pollution & Control, 2014, 36(6): 13-19(in Chinese).
[17] 张春洋, 马永亮. UV254nm+185nm光照降解气态甲苯的实验研究 [J]. 中国环境科学, 2011, 31(6): 898-903. ZHANG C Y, MA Y L. Experimental study on UV254nm+185nm photodegradation of gaseous toluene [J]. China Environmental Science, 2011, 31(6): 898-903(in Chinese).
[18] CHEN J Y, HE Z G, JI Y M, et al. OH radicals determined photocatalytic degradation mechanisms of gaseous styrene in TiO2 system under 254 nm versus 185 nm irradiation: Combined experimental and theoretical studies [J]. Applied Catalysis B:Environmental, 2019, 257: 117912. doi: 10.1016/j.apcatb.2019.117912 [19] DANESHVAR N, BEHNAJADY M A, MOHAMMADI M K A, et al. UV/H2O2 treatment of Rhodamine B in aqueous solution: Influence of operational parameters and kinetic modeling [J]. Desalination, 2008, 230(1/2/3): 16-26. [20] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 16 Rev. B. 01 [M]. Wallingford, CT. 2016. [21] BICZYSKO M, PANEK P, SCALMANI G, et al. Harmonic and anharmonic vibrational frequency calculations with the double-hybrid B2PLYP method: Analytic second derivatives and benchmark studies [J]. Journal of Chemical Theory and Computation, 2010, 6(7): 2115-2125. doi: 10.1021/ct100212p [22] 王子东, 马永亮. 利用UVC去除低浓度苯的实验研究 [J]. 环境工程学报, 2009, 3(7): 1284-1288. WANG Z D, MA Y L. Experimental study on removal of low concentration benzene with UVC [J]. Chinese Journal of Environmental Engineering, 2009, 3(7): 1284-1288(in Chinese).
[23] 周政. 基础有机化学[M]. 北京: 高等教育出版社, 1990.263-264. ZHOU Z. Basic organic chemistry [M]. Beijing: Higher Education Press, 1990.263-264(in Chinese).
[24] ZHANG W P, LI G Y, LIU H L, et al. Photocatalytic degradation mechanism of gaseous styrene over Au/TiO2@CNTs: Relevance of superficial state with deactivation mechanism [J]. Applied Catalysis B:Environmental, 2020, 272: 118969. doi: 10.1016/j.apcatb.2020.118969 [25] ZHANG W P, LI G Y, WANG W J, et al. Enhanced photocatalytic mechanism of Ag3PO4 nano-sheets using MS2 (M = Mo, W)/rGO hybrids as co-catalysts for 4-nitrophenol degradation in water [J]. Applied Catalysis B:Environmental, 2018, 232: 11-18. doi: 10.1016/j.apcatb.2018.03.006 -



 下载:
下载: