-
汞(Hg)是一种毒性很强的重金属污染物,具有高迁移性和生物积累性,极易渗透进入食物链,且难以进行有效控制[1-2]. 汞向环境中排放实际上是一个不可逆过程,因此,即便是长期低剂量的汞排放也可能会导致严重的环境问题和健康后果[3]. 人为排放是环境中汞的最主要来源,我国每年汞排放量约为500—1000 t之间,1976—2000年期间农田土壤中的平均汞浓度为0.25(0.01—1.65)mg·kg−1, 2006—2010年期间上升至0.47(0.0009—71.09)mg·kg−1,2011—2016年略有下降[4]. 《全国土壤污染状况调查公报》[5]显示我国土壤汞点位超标率达1.6%,其中汞矿区和工业区土壤受污染程度最为严重,其总汞含量远高于我国土壤汞含量的最大限制值1.5 mg·kg−1(GB 15618—2018). 汞的毒性与其化学形态密切相关,无机汞对人体的毒性相对较弱,但其在特殊环境条件下能转化成毒性更强的甲基汞(MeHg). MeHg是一种毒性极强的有机汞化合物,通过食物链富集进入生物体内,会对中枢神经系统造成极大危害. 国际上普遍认为MeHg暴露的主要途径为食用水产品[6],有研究揭示汞矿等污染区域人群存在食用稻米造成的MeHg暴露健康风险问题[7],这一发现打破了原有认知. 水稻是世界上最重要的粮食作物之一,稻田由于季节性淹水灌溉,为汞甲基化提供了有利条件[8-9],土壤有机质、pH以及微生物均有可能影响土壤中汞的甲基化. 水稻MeHg污染问题已经引起国际社会的高度关注. 据调查发现[10],我国受汞污染农田达3.2万 hm2,每年生产的汞超标大米约有1.85亿 kg,对居民健康构成潜在威胁.
溶解性有机质(DOM)[11]主要来自于动植物残体分解,其结构复杂,含有多种官能团. DOM作为自然界中重要的活性组分,能通过吸附、络合、离子交换等方式影响汞在土壤中的迁移转化[12-13],尤其是汞向MeHg的转变. 此外,DOM也会改变沉积物的Eh、pH[14]、微生物群落结构,从而影响汞的转移和生物可利用性. 目前,关于DOM对汞甲基化影响研究尚存分歧. 有研究表明DOM能显著降低汞的生物有效性和甲基化过程[15]. DOM含有丰富的金属结合位点,易与Hg2+结合,能增强光化学反应中Hg2+转化为Hg0的过程[16-17],降低生物可利用的汞浓度,从而抑制MeHg产生[2]. 也有部分研究表明,DOM能促进汞甲基化. 水体中DOM含量增加,为汞甲基化过程提供充足的甲基供体,促进汞甲基化发生. 另一方面,DOM与汞的络合产物在一定程度上也更易被微生物同化利用,进而促进MeHg的形成[18]. 此外,何杉杉等[19]研究发现,DOM的腐殖化程度会影响汞的甲基化,长期进行渔业养殖活动的水体DOM腐殖化程度更高[20],MeHg含量及甲基化速率普遍高于养殖时间较短的水体. 陈春羽等[21]提取3种不同来源DOM,研究其对土壤中汞的吸附行为影响. 结果表明,不同来源的DOM对土壤中汞的吸附行为抑制作用有明显差异. 何鑫龙等[22]对渔业养殖区不同来源有机物对水体中汞甲基化影响展开研究,证实了不同来源和组成的DOM对汞甲基化影响不同.
受国家倡导绿色发展理念以及全面推行化肥减施宏观战略的影响,不难预见绿肥、厩肥、圈肥等有机肥的需求将大幅增加[23]. 满江红[24] (Azolla imbricata )是一种常见的水生蕨类植物,常见于水田、池塘等地,具有生长快、产量高、能与蓝藻共生固氮、富钾等生理特点,我国浙江、贵州、四川等南方省份常将其用作稻田绿肥. 已有研究表明,满江红在修复水体和资源化利用等方面存在诸多优势[25]. Cohen-Shoel等[26]采用含Sr2+溶液的模型研究满江红与重金属离子结合交换的机制,发现满江红细胞壁中的阳离子结合基团如羧基、磷酸基团等对重金属离子具有较高的亲和力,解释了满江红对重金属的吸附机理. 无生命的干燥满江红已被证明能从水溶液中结合重金属[27],包括Cs、Sr、Ce等,其重金属结合积累能力是活体满江红的7倍左右. 综上,现有的研究主要集中在满江红的生态环境修复价值以及其开发利用[28-29],而对于满江红腐解后的残体对重金属有效性的影响研究较为鲜见,其对汞甲基化是否存在促进或者抑制效应也未见报道. 从理论上说,满江红作为水生植物,当其进入衰亡期后,大量植物残体通过分解、淋溶进入水体,势必产生大量的DOM,对一些重金属污染物的环境化学行为产生影响. 因此,有必要对汞污染区域满江红腐解过程中DOM的化学行为特征进行系统的研究. 在有机质腐解过程中,其结构与组成不断发生改变,因此从更微观的角度切入探究DOM对汞甲基化的影响更具现实意义.
本研究以稻田满江红DOM为研究对象,探究在稻田体系中满江红DOM对汞甲基化的影响,同时结合光谱分析与亚组分分离技术,从微观角度探究满江红DOM影响汞甲基化的作用机制及其贡献度,以期为提高汞污染农田生产安全性提供理论依据.
-
供试材料新鲜满江红购买自浙江省金华市江东镇竹文园艺经营部. 用去离子水清洗去除杂质,沥干后备用. 分别称取400 g新鲜满江红至6个2 L烧杯中,每个烧杯中加入1 L超纯水,放入厌氧培养箱中恒温培养,模拟稻田活体满江红腐解过程.
分别在腐解开始后的0 、15 、30、60 、90 、120 d取出样品,冷冻干燥后充分研磨. 采用水浸提法[30-31]提取满江红DOM,将1 g满江红与30 mL超纯水混合后,在25 ℃恒温培养箱中振荡24 h,18000 r·min−1高速离心30 min,取上层清液过0.45 μm滤膜,过滤后液体即为满江红DOM,放置于4 ℃冰箱中保存备用.
-
三维荧光光谱测定采用日立F-7000型荧光分光光度计,激发光源为150 W氙灯,PMT电压为700 V,激发波长扫描范围230—450 nm,发射波长扫描范围250—620 nm,狭缝宽度和增量均设为5 nm,响应速度为自动,扫描速度设为2400 nm·min−1. 以超纯水为空白,扣除拉曼和瑞利散射,测定过程中保持温度恒定.
-
紫外光谱测定采用岛津紫外可见分光光度计UV-2600,扫描波长为230—800 nm,扫描间隔为1 nm,将254 nm处的吸光强度与DOC的比值记为SUVA254,S275—295和S350—400分别代表275—295 nm和350—400 nm波长范围内的曲线斜率,将S275—295/S350—400的比值计为SR.
-
本研究采用Amberlite XAD-8、XAD-4大孔树脂、BIO-RAD AG-MP-50饱和阳离子交换树脂、DUOLITE A-7阴离子交换树脂,根据DOM在不同树脂上吸附能力的差异,将其分离成疏水碱性物(HOB)、疏水酸性物(HOA)、疏水中性物(HON)、亲水碱性物(HIB)、亲水酸性物(HIA)、亲水中性物(HIN)6种有机组分.
-
用3倍树脂体积(约200 mL)的95%乙醇分别清洗Amberlite XAD-8、XAD-4大孔树脂,后用超纯水淋洗至流出液无醇味. 用50 ℃的超纯水反复浸洗阳离子和阴离子树脂,洗至浸洗液无色后将树脂装入树脂柱,用浓度为5%的氢氧化钠(NaOH)和盐酸(HCl)进行二次清洗,以酸-水-碱-水-酸的顺序清洗阳离子交换树脂,以碱-水-酸-水-碱的顺序清洗阴离子交换树脂,最后用超纯水清洗至中性,处理后的树脂密封保存.
-
组分分离参照Leenheer等[32-33]方法并加以改进,通过蠕动进样泵将DOM加入XAD-8/XAD-4树脂柱中,用0.01 mol·L−1和0.10 mol·L−1的HCl反洗XAD-8树脂柱得到组分HOB,XAD-8/XAD-4流出废水酸化至pH=2,然后通过柱回收,将XAD-8树脂取出自然风干,用甲醇索式提取得到HON. 将脱附混合物进一步过柱,用0.10 mol·L−1 NaOH反洗XAD-8树脂后获得HOA. 由于几乎所有的亲水小分子都被吸附在XAD-4上,因此使用NaOH脱附有机质,调节pH后泵入阴离子交换树脂,用0.10 mol·L−1氨水(NH4OH)反洗得到HIB,流出物泵入阳离子交换树脂,用3.00 mol·L−1的NH4OH反洗得到HIA,HIN通过用纯水淋洗阳离子交换树脂后获得. 所有获得的亚组分用旋转蒸发仪在40 ℃下干燥[34].
-
测定不同腐解时间(0、15 、30、60、90、120 d)DOM及其亚组分的DOC浓度,稀释至相同浓度2.5 mg·L−1,配制1000 ng·L−1的硝酸汞(Hg(NO3)2)溶液,在100 mL离心管中加入50 mL的DOM或亚组分溶液和25 mL Hg2+溶液,在常温黑暗条件下培养24 h.
采用蒋红梅等[35]建立的方法(蒸馏-乙基化结合气相色谱-冷原子荧光,CVAFS法)在BROOKS RAND测汞仪上测定MeHg质量浓度(方法回收率为88.2%—108.4%,方法空白为(0.045±0.003)ng·L−1,最低检出限为0.009 ng·L−1). 取培养好的样品于聚四氟乙烯蒸馏瓶中,125 ℃蒸馏,使MeHg富集于接收瓶中. 将蒸馏液转移到气泡瓶中并定容至80 mL,加入乙酸-乙酸钠(HAc-NaAc)缓冲溶液和四乙基硼酸钠(NaBEt4)试剂,密闭反应一段时间通入氮气,使MeHg富集在Tenax管上,测定样品中的MeHg含量. 本试验测定的重复样品的相对标准偏差<9%.
-
光谱数据分析采用MATLAB R2020b软件,由SPSS 26进行数据统计分析,P<0.05表示各组处理之间存在显著差异,数据处理采用Excel 2019,图表制作采用Origin 2019.
-
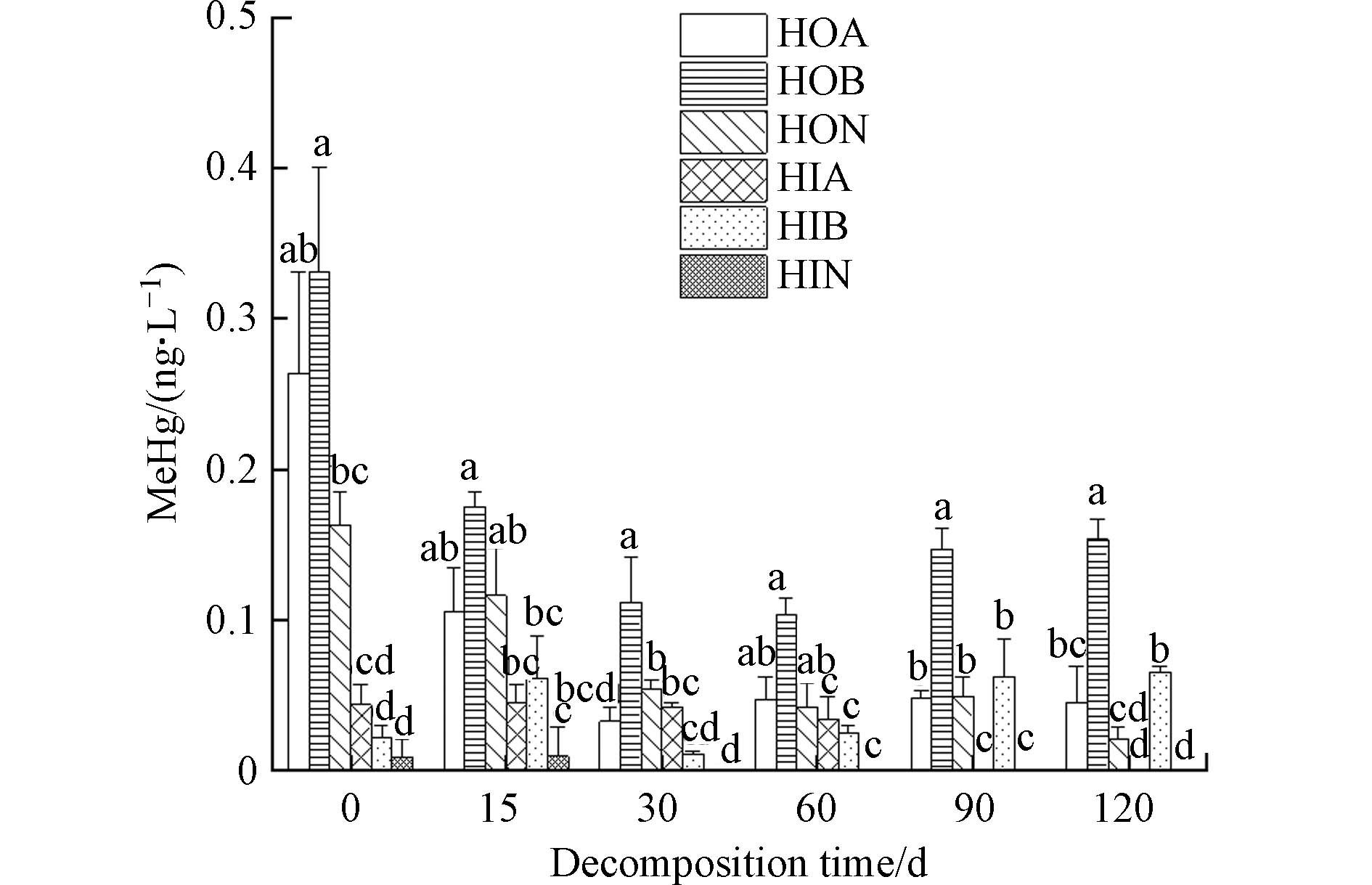
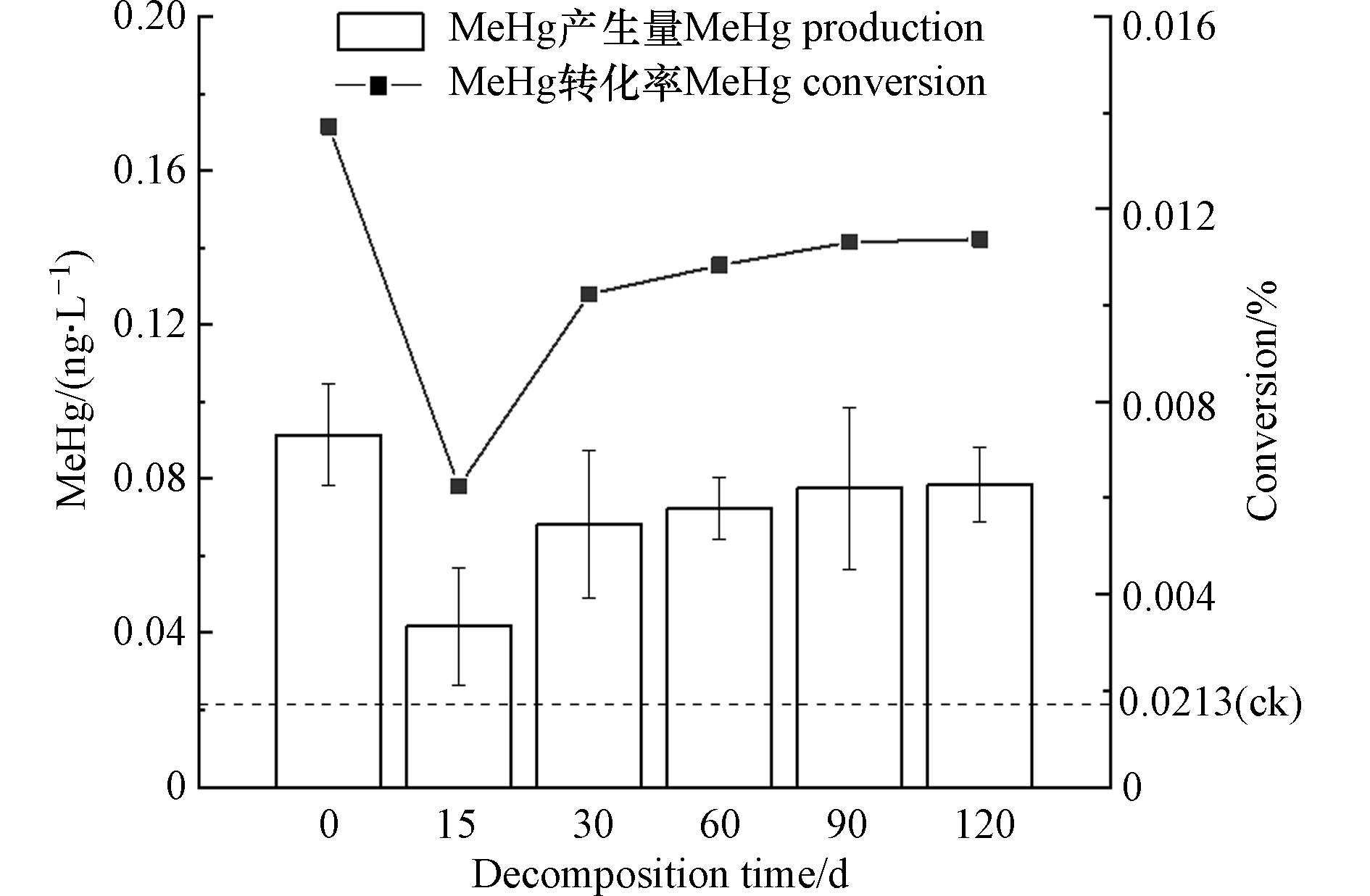
腐解过程满江红DOM可溶性有机碳(DOC)浓度量呈现不规则变化(表1). 腐解初始时(0 d)的满江红DOC值最大. 腐解前15 d,DOC有明显的快速下降趋势. 15—30 d逐渐增大,随后DOC整体呈下降趋势. 图1为不同腐解时间满江红DOM对汞甲基化影响结果. 未添加DOM对照组MeHg产生量为0.0213 ng·L−1,添加DOM实验组MeHg产生量高于对照组,说明不同腐解时间满江红DOM均能促进汞甲基化. 其中未腐解满江红DOM促汞甲基化能力最强,溶液MeHg为0.09 ng·L−1,随腐解进行,MeHg产生量出现先降低后升高后逐渐平稳的趋势. 腐解第15 d MeHg产生量及转化率最低,随腐解时间增加而增加,总体在0.072%—0.078%左右. 图2为不同腐解时间满江红DOM亚组分对汞甲基化影响结果,可以发现不同腐解时间生成的DOM亚组分影响汞甲基化能力不同. 6个组分中HOB对汞甲基化促进效果最强,显著促进了MeHg的产生,其中0 d HOB 促进MeHg生成的效果最为明显,产生的MeHg高达0.32 ng·L−1,而后随着腐解时间呈现先下降后上升的趋势. HOA与HON促汞甲基化能力相近,腐解初期MeHg产生量较高,随腐解进行不断下降,腐解后期基本不变. 相比而言HIA、HIB、HIN 3个亲水性组分促汞甲基化能力较弱,HIB对汞甲基化影响呈现不规则波动,HIA和HIN前期对汞甲基化影响基本保持稳定,腐解第30 天后HIN、第90 天后的HIA未能检测到MeHg生产,说明其促汞甲基化能力消失.
-
显色溶解性有机质(CDOM)是DOM中的可吸光部分,在水体光场的测定中发挥着重要作用[36-37],常用吸收系数a(355)来表征CDOM的浓度,吸收系数越大,CDOM含量越高. 表2可以看出随腐解进行满江红DOM中CDOM处于动态变化,腐解前期CDOM含量显著下降,后期逐渐增大. 这可能是由于腐解前期受到微生物降解、金属离子共沉淀、水解等去除作用导致其浓度降低. 腐解后期降解速率减慢,CDOM逐渐累积. 光谱斜率比值(SR)常用来评估DOM结构变化[38],SR值与分子量呈反比. 随腐解进行,DOM的SR值先增大后减小,即腐解过程中前期以小分子DOM为主,后期大分子DOM居多. SUVA254为254 nm处吸收系数与DOC浓度的比值,用于表征DOM芳香性大小,SUVA254值越大表明DOM的芳香化程度越高,其苯环结构数量更多且更加复杂.
根据表2将整个腐解过程大致分为3个阶段,第一阶段(0—15 d)属于组分的净消耗阶段,这一阶段中微生物大量繁殖,同时大量消耗氨基酸、单糖、多糖等物质. 0 d 满江红DOM的SUVA254最高,后续随实验进行经历了一个骤降缓升的过程. 腐解初期碳水化合物初始含量较低时,芳香性化合物如木质素分解产物等也可作为微生物的能量和碳源被消耗,导致SUVA254降低. He等[39]研究微生物降解大型植物中DOM的浸出过程,也发现腐解初期微生物种群数量增幅最大. Chen等[40]发现,腐解过程物质代谢沿不同生化途径进行,不同阶段腐解产物存在差异,腐解初期产生的芳香性物质主要为直链脂肪酯类,结构较为简单,能被作为底物消耗[41],而带有较为复杂的芳环结构的难降解芳香族化合物才是腐解后期DOM中的优势组分,难以被微生物利用. 第二阶段(15—60 d)属于快速分解期,SUVA254随腐解时间逐渐增大,芳香性组分富集,小分子量DOM增多. 此阶段中纤维素、木质素等被微生物分解成大量单糖、寡糖等小分子物质以及大量难降解的芳香族化合物,Dilling等[42]研究证实,木质素分解会产生大量芳香性物质,降解产物相对富集,芳香性物质逐渐占据主导地位. 第三阶段(60 d以后)属于准平衡阶段,分子量和SUVA254整体增大,这一阶段腐解速率减慢,高生物可利用性的组分例如碳水化合物,有机酸等逐步被微生物消耗,难降解的芳香性和大分子物质被保留[43]. 这与Almendros等[44]研究土壤衍生腐殖酸生物降解的发现一致,由于形成的芳香性物质结构趋向复杂,生物可降解性降低,其含量的总体变化趋势为逐渐增加.
-
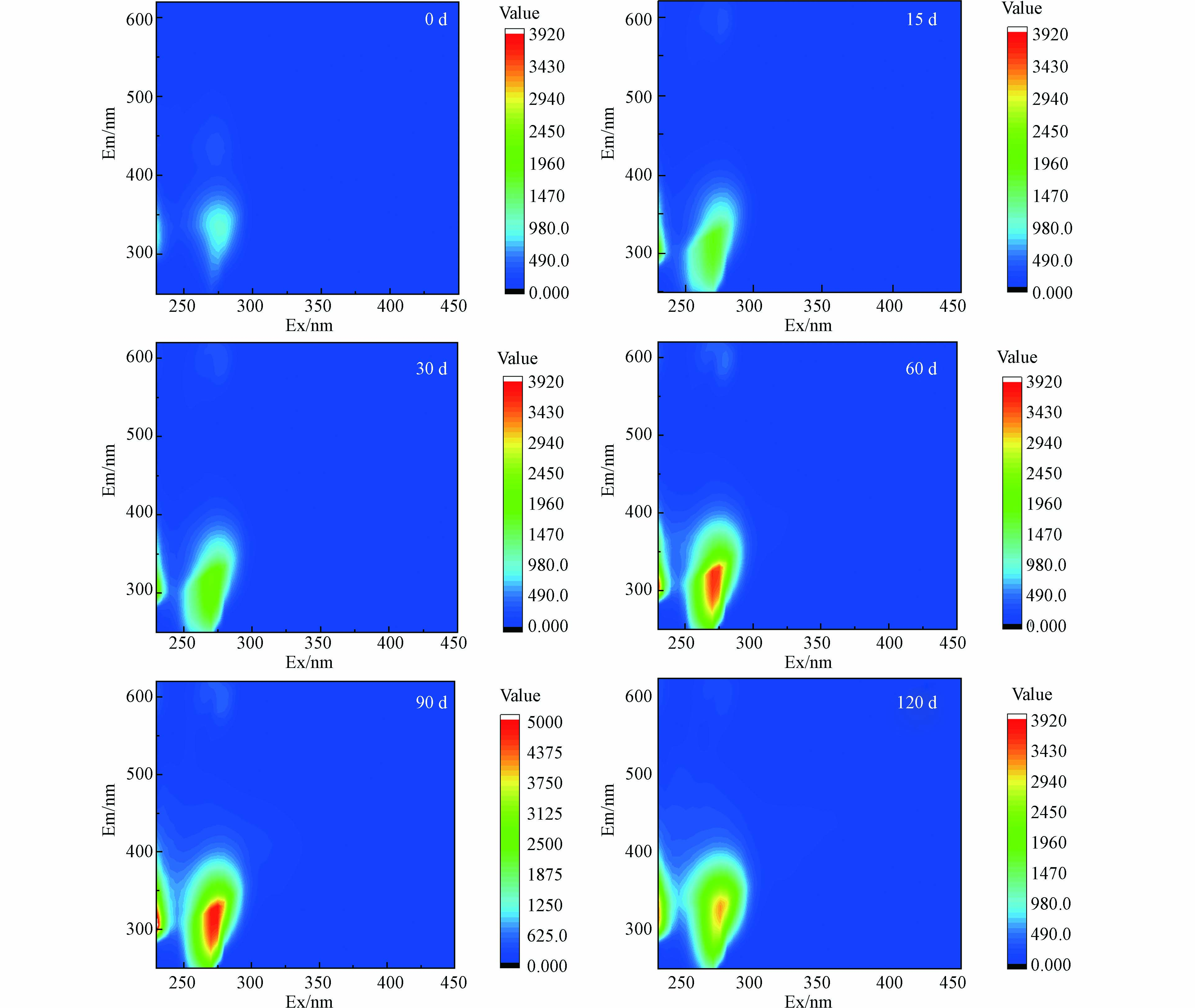
三维荧光光谱(3D-EEM)技术常用于对溶解性有机质进行表征,不同来源的DOM荧光峰和强度存在差异[45]. 图3为不同腐解时期满江红DOM的三维荧光光谱图,本研究共识别出5个荧光峰,荧光峰A(Ex/Em=260 nm/380 nm)为紫外光区类腐殖质荧光峰;荧光峰B(Ex/Em=275 nm/320 nm)为类酪氨酸荧光峰;荧光峰C(Ex/Em=335 nm/420 nm)为可见光区类腐殖质荧光峰;荧光峰M(Ex/Em=320 nm/406 nm)为微生物源类腐殖质荧光峰;荧光峰T(Ex/Em=275 nm/330 nm)为类色氨酸荧光峰. 根据表3荧光光谱参数所示,类蛋白组分荧光强度较大,说明腐解过程中类蛋白质物质占主导地位,随着腐解时间增加,荧光峰数量和位置保持不变,但峰强度在前90 天随着时间的增长而增大,120 d峰强度降低.
荧光指数(FI)指的是当Ex=370 nm时,Em=470 nm与Em=520 nm的荧光强度的比值. 该指标用于指示DOM中类腐殖质来源,外源和内源DOM的两个端源FI值分别为1.4和1.9[46]. 当FI>1.9时,DOM主要源于细菌和藻类活动,自生源特征明显. FI<1.4时,主要是外源(陆源)输入为主,异生源特征明显. 不同腐解时间满江红DOM的FI均接近或大于1.9,表明其DOM中的类腐殖质主要是内源分解产生.
生物指数(BIX)指的是当Ex=310 nm时,Em=380 nm与Em=430 nm处的荧光强度的比值[47-48]. 该指标可以反映某一微域生物活动强度、DOM来源等,当BIX>1时,DOM主要以生物源为主,包括动植物残体降解产物;当BIX值较低时,DOM主要以陆源输入为主. 如表3所示,未腐解的BIX为0.54,代表其主要以陆源输入为主,自生源贡献较少,腐解后满江红DOM的BIX大于1,表明DOM主要以自生源为主,且多为最新产生.
腐殖化指数(HIX)为当Ex=254 nm时,Em=435—480 nm范围内的积分面积与Em=300—345 nm范围内的积分面积之比,是应用于表征有机质腐殖化程度的指标,也常用来衡量DOM分子的复杂性[49]. Ohno[30]的研究显示HIX值在0—1间变动,其值越大表明样品腐殖化程度越高,0 d满江红DOM的腐殖化程度最大,随着腐解时间腐殖化程度先下降后不断增大.
尽管三维荧光光谱能提供一些DOM组分信息,但仍无法定量判别DOM荧光特性,因此,采用区域体积积分法(FRI)对不同腐解时间满江红DOM的荧光特性进行定量表征. 根据Chen等[50]提出的FRI法,通过不同的激发和发射波长,将DOM划分成5个荧光区域,其中I区、II区和IV区的荧光物质与类蛋白物质有关,III区和V区的荧光物质与类腐殖质有关. 对比表4不同区域荧光峰体积可以发现,腐解初期,类蛋白物质含量显著增量,在90 d达到最大,随后有少量减少. 而类腐殖质物质在腐解前期略有下降,后随着腐解时间增长不断增多. 有研究者[51]认为,腐解前期类腐殖质含量下降,可能是由于水中仍含有部分氧,导致其在氧化条件下更易被氧化降解,转变为分子量更小的物质,为微生物的生长提供碳源. 随着腐解的进行,水体中溶解氧含量急剧下降,微生物代谢对DOM的降解作用减缓[39],类腐殖质逐渐累积[52].
结合紫外与三维荧光光谱对不同腐解时期满江红DOM及其亚组分对汞甲基化的影响进行分析. 结果表明,不同腐解时期满江红DOM的DOC浓度大小与其汞甲基化能力无相关性. 0 d满江红DOM的芳香性最大,溶液MeHg产生量也显著高于腐解后的,腐解0—15 d,由于芳香性物质大量减少,DOM对汞甲基化的促进作用也显著减弱,腐解中后期芳香性物质不断增加,此期间满江红DOM对汞甲基化的促进作用也随之增大,之后基本保持稳定. Lavoie等[53]研究证实,DOC浓度会影响水体中MeHg含量,但并非MeHg产生的唯一影响因素,相较于DOC浓度,Burns等[54]通过SUVA254测量发现,芳香性物质是影响汞甲基化的重要因素[55],高芳香性物质含有丰富的还原性硫结合位点,有利于稳定HgS纳米颗粒,促进汞甲基化. 同时分子量大小也会影响DOM促汞甲基化能力[56]. Hisamitus等[57]研究发现不同分子量的DOM促汞甲基化能力不同,分子量较小的DOM具有更高的活性,在汞甲基化过程中起主要作用. 本实验中DOM分子量随腐解时间表现出先减小后增大的趋势,腐解30—60 d小分子量DOM较多,对汞甲基化促进作用较强. 满江红腐解过程中类蛋白质组分占主导地位,类腐殖质物质在腐解过程中呈现先减少后增多的趋势,类腐殖质作为重要的甲基供体,对促进汞的非生物甲基化过程有重要作用,腐解15 d时,类腐殖质物质含量相对较少,溶液MeHg产生量也相对较少. 随腐解时间类腐殖质物质逐步积累,DOM促汞甲基化能力也有所增强. 文献[58-60]表明,DOM中的类蛋白质物质是Hg的强配位体之一,类蛋白质组分更易与Hg2+结合,继而促进甲基汞生成,也有研究发现部分类腐殖质物质能够直接参与汞的非生物甲基化. 未腐解满江红DOM类蛋白质组分低于腐解后的DOM,但其芳香性和腐殖化程度显著高于腐解后满江红DOM,这一定程度促进了MeHg的产生.
亚组分促汞甲基化试验研究表明,疏水性组分促汞甲基化的能力强于亲水性组分. 这主要是由于不同亚组分间差异,导致其与汞结合能力不同,研究[61]表明芳香性物质几乎全部存在于疏水组分中,使得DOM更易与Hg2+之间发生电子迁移反应,进而促进汞甲基化反应进行[62]. Guggenberger等[63]的研究发现,亲水性酸性物质对金属离子有较强的络合能力,是疏水性性物质的2—8倍. 相比于疏水组分,亲水组分更易与金属离子发生聚集和沉淀[64-65],表现出胶体颗粒特征,进一步影响Hg的迁移转化. 在本研究中亲水性有机组分也显示出相同的情况,对汞甲基化的促进效果弱于疏水性有机组分.
-
(1)满江红DOM对汞甲基化具有促进作用. 未腐解满江红DOM促汞甲基化能力最强,达到0.09 ng·L−1,随腐解时间表现出先降低后增加的趋势,腐解15 d MeHg产生量及转化率均为最低,腐解后期基本稳定. 6种组分中HOB对汞甲基化促进效果最强,亲水性组分对汞甲基化促进效果较弱,腐解后期HIA、HIN等组分对汞甲基化无促进作用.
(2)腐解过程分为三阶段,第一阶段(0—15 d)微生物大量繁殖,碳水化合物及简单芳香性物质被消耗. 第二阶段(15—60 d)纤维素、木质素等被微生物分解成小分子物质以及大量难降解芳香族化合物,芳香性物质逐渐富集. 第三阶段(60 —120 d)碳水化合物、有机酸等逐步被微生物消耗,难降解芳香性物质和大分子物质保留. DOM中类蛋白质物质占比较大,随腐解时间不断增多,90 d后有少量下降,类腐殖质物质含量呈现先降低后升高的趋势.
(3)满江红DOM促汞甲基化能力受芳香性、分子量大小、类蛋白质组分含量等因素影响,高芳香性、小分子量DOM促进MeHg产生. 疏水性组分含有大量芳香性物质,使得其对汞甲基化的促进作用显著高于亲水性组分. 亲水性组分的强络合能力使金属离子更易发生聚集和沉淀,促汞甲基化能力较弱.
稻田满江红溶解性有机质光谱特征及其对汞甲基化影响
Spectral characteristics of dissolved organic matter derived from Azolla imbricata in rice paddy and its effect on mercury methylation
-
摘要: 满江红作为稻田重要的有机质输入来源,研究其溶解性有机质(DOM)对稻田土壤中汞形态及转化机理具有重要意义. 以稻田满江红DOM为研究对象,运用紫外吸收光谱(UV-Vis)和三维荧光光谱(3D-EEM)技术对不同腐解时间满江红DOM进行表征;此外,通过树脂串联技术分离得到疏水酸性物(HOA)、疏水碱性物(HOB)、疏水中性物(HON)、亲水酸性物(HIA)、亲水碱性物(HIB)、亲水中性物(HIN)等6种DOM亚组分,将不同腐解时间提取的满江红DOM及其亚组分与Hg2+溶液混合培养,分析其对汞甲基化过程的影响,以期为提高汞污染农田生产安全性提供理论依据. 结果表明,腐解初始时满江红DOM的芳香性最大,随腐解进行呈骤降缓升趋势. 腐解中期小分子量DOM较多. 类蛋白质组分始终占据主导地位,腐解后期含量缓慢减少,类腐殖质组分含量则呈现先低后高的趋势. 甲基化实验表明,不同腐解时间满江红DOM对汞的甲基化反应均具有促进作用,当DOM芳香性较高,分子量较小时,其促进汞甲基化能力增强,腐解初始时DOM促进汞甲基化作用最强,随腐解时间呈现先降低后升高逐渐平稳的趋势. 疏水性组分对汞甲基化促进作用显著高于亲水性组分,其中HOB促汞甲基化能力最强. 腐解后期HIA、HIN对汞甲基化无促进作用.Abstract: The dissolved organic matter (DOM) derived from Azolla imbricata, one of major organic matter contributors in the rice field, plays an important role in the morphology and transformation mechanism of mercury in paddy soil. In this study, taking DOM of A. imbricata from paddy field as research object, such advanced instruments as UV Absorption Spectroscopy and 3D Fluorescence Spectroscopy were employed to characterize the DOM derived from A. imbricata which was sampled at different intervals during the decomposition of A. imbricata biomass. In addition, six sub-fractions of the DOM (HOA, HOB, HON, HIA, HIB, HIN) were obtained via a tandem resin setup. The DOM and its subfractions sampled from the different interval during decomposition of A. imbricata were mixed with Hg2+ solution respectively, in an attempt to reveal their effects on the mercury methylation process and thereafter provide theoretical basis for improving the production safety of mercury-contaminated farmland. The results showed that the highest SUVA254 value of the DOM was observed at the initial stage (0 day) of the decomposition experiment, followed by a sudden decline and slow rise as the decay of A. imbricata biomass lasted. Small molecular weight DOM increases in the middle stage of decomposition. In accordance with spectral of fluorescence spectroscopy, the protein-like component dominated the whole decomposition process, with a slight decline at the late stage. The humus-like component decreased at the early stage and then increased gradually. The DOM derived form A. imbricata could promote the methylation of mercury at different decomposition intervals. And DOM with high aromaticity and small molecular weight has a stronger ability to promote MeHg production. It was at the initial time of decomposition that DOM exhibited the strongest promoting effect on the production of methylmercury, and followed by a trend of decreasing first and then increasing gradually. The hydrophobic component showed a significantly higher capacity than the hydrophilic ones in term of promoting the methylation reaction of mercury. Among six subfractions, HOB seemed to be the greatest contributor toward the promotion of the methylation of mercury while the contribution from hydrophilic components such as HIA and HIN was negligible, especially in the later stage of decomposition.
-
Key words:
- mercury /
- methylation /
- dissolved organic matter /
- spectral analysis /
- sub-fraction.
-

-
表 1 不同腐解时间满江红DOM的DOC变化情况
Table 1. Variation of the DOM derived from A. imbricata in different decomposition time
腐解时间/d
Decomposition time0 15 30 60 90 120 DOCDOM/(mg·L−1) 2010 1548 1724 1362 1016 946 表 2 不同腐解时间满江红DOM紫外吸收光谱参数
Table 2. Spectral parameters of UV absorption of the DOM derived from A. imbricata at different decomposition time
腐解时间/d
Decomposition timeS275—295 S350—400 SR SUVA254 a(355)/m−1 0 0.0111 0.0242 0.4584 35.9422 10.8441 15 0.0328 0.0512 0.6409 6.9077 1.6118 30 0.0413 0.0319 1.2935 8.7498 0.6908 60 0.0380 0.0267 1.4260 10.3616 1.6118 90 0.0181 0.0184 0.9841 15.8439 3.6403 120 0.0108 0.0245 0.4415 13.8922 4.2214 表 3 不同腐解时间满江红DOM荧光光谱参数
Table 3. Fluorescence spectral parameters of the DOM derived from A. imbricata at different decomposition time
腐解时间/d
Decomposition time0 15 30 60 90 120 荧光峰强度
Fluorescence intensityB 761.44 1604.17 2009.44 3404.49 4652.30 2980.78 T 930.53 1221.73 1473.73 2529.73 3686.73 2976.73 A 192.98 195.24 205.64 351.54 502.74 512.44 M 312.49 397.19 431.59 704.79 986.69 952.49 C 81.03 22.40 24.44 67.47 169.00 111.34 荧光参数
Fluorescence parameterFI 2.40 1.86 2.43 2.42 2.42 2.48 BIX 0.54 1.67 1.51 1.61 0.96 1.06 HIX 0.47 0.07 0.08 0.10 0.15 0.23 表 4 不同腐解时间满江红DOM三维荧光光谱区域积分值(×104 au·nm2)
Table 4. FRI of three-dimensional fluorescence spectrum of the DOM derived from A. imbricata at different decomposition time
腐解时间/d
Decomposition time0 15 30 60 90 120 Ⅰ(芳香族蛋白类Ⅰ)
Aromatic ProteinⅠ52.62 149.35 178.204 216.85 348.50 226.01 Ⅱ(芳香族蛋白类Ⅱ)
Aromatic ProteinⅡ96.10 111.44 131.38 223.63 397.14 355.77 Ⅲ(富里酸类)
Fulvic acid-like23.51 21.82 25.722 40.80 75.99 74.85 Ⅳ(溶解性微生物副产物类)
Soluble microbial by-product-like43.43 87.24 98.89 168.75 216.473 156.29 Ⅴ(腐殖酸类)
Humic acid-like11.05 8.46 9.78 15.322 26.30 22.92 -
[1] LIU S J, WANG X D, GUO G L, et al. Status and environmental management of soil mercury pollution in China: A review [J]. Journal of Environmental Management, 2021, 27: 111442. [2] 孟博, 胡海燕, 李平, 等. 稻田生态系统汞的形态转化及同位素分馏 [J]. 矿物岩石地球化学通报, 2020, 39(1): 12-23,3. MENG B, HU H Y, LI P, et al. Transformation and stable isotope fractionation of mercury in the rice paddy ecosystem [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2020, 39(1): 12-23,3(in Chinese).
[3] SIMPSON V R, STUART N C, MUNRO R, et al. Poisoning of dairy heifers by mercurous chloride [J]. The Veterinary Record, 1997, 140(21): 549-552. doi: 10.1136/vr.140.21.549 [4] LI X Y, ZHANG J R, GONG Y W, et al. Status of mercury accumulation in agricultural soils across China (1976-2016) [J]. Ecotoxicology and Environmental Safety, 2020, 197: 110564. doi: 10.1016/j.ecoenv.2020.110564 [5] 陈能场, 郑煜基, 何晓峰, 等. 《全国土壤污染状况调查公报》探析 [J]. 农业环境科学学报, 2017, 36(9): 1689-1692. CHEN N C, ZHENG Y J, HE X F, et al. Analysis of the Report on the national general survey of soil contamination [J]. Journal of Agro-Environment Science, 2017, 36(9): 1689-1692(in Chinese).
[6] LIANG P, WU S C, ZHANG C, et al. The role of antibiotics in mercury methylation in marine sediments [J]. Journal of Hazardous Materials, 2018, 360: 1-5. doi: 10.1016/j.jhazmat.2018.07.096 [7] 李平, 陈敏, 王波. 中国居民甲基汞暴露的来源和健康风险 [J]. 矿物岩石地球化学通报, 2019, 38(4): 725-728,661. LI P, CHEN M, WANG B. Sources and health risks of methylmercury exposure in Chinese residents [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2019, 38(4): 725-728,661(in Chinese).
[8] ZHAO L, ANDERSON C W N, QIU G L, et al. Mercury methylation in paddy soil: Source and distribution of mercury species at a Hg mining area, Guizhou Province, China [J]. Biogeosciences, 2016, 13(8): 2429-2440. doi: 10.5194/bg-13-2429-2016 [9] TANNER K C, WINDHAM-MYERS L, MARVIN-DIPASQUALE M, et al. Alternate wetting and drying decreases methylmercury in flooded rice (Oryza sativa) systems [J]. Soil Science Society of America Journal, 2018, 82(1): 115-125. doi: 10.2136/sssaj2017.05.0158 [10] 牟树森, 青长乐. 环境土壤学[M]. 北京: 农业出版社, 1993. MOU S S, QING C L. Environmental soil science[M]. Beijing: China Agriculture Press, 1993 (in Chinese).
[11] ZHAO C, GAO S J, ZHOU L, et al. Dissolved organic matter in urban forestland soil and its interactions with typical heavy metals: A case of Daxing District, Beijing [J]. Environmental Science and Pollution Research International, 2019, 26(3): 2960-2973. doi: 10.1007/s11356-018-3860-7 [12] OLE K B, PETER E H, BJARNE W S. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review [J]. Geoderma, 2019, 343: 235-246. doi: 10.1016/j.geoderma.2019.02.041 [13] LIU P, PTACEK C J, BLOWES D W. Mercury complexation with dissolved organic matter released from thirty-six types of biochar [J]. Bulletin of Environmental Contamination and Toxicology, 2019, 103(1): 175-180. doi: 10.1007/s00128-018-2397-2 [14] SCHARTUP A T, BALCOM P H, MASON R P. Sediment-porewater partitioning, total sulfur, and methylmercury production in estuaries [J]. Environmental Science & Technology, 2014, 48(2): 954-960. [15] LOUX N T. An assessment of mercury-species-dependent binding with natural organic carbon [J]. Chemical Speciation & Bioavailability, 1998, 10(4): 127-136. [16] ALLARD B, ARSENIE I. Abiotic reduction of mercury by humic substances in aquatic system—an important process for the mercury cycle [J]. Water Air & Soil Pollution, 1991, 56(1): 457-464. [17] LIANG P, LI Y C, ZHANG C, et al. Effects of salinity and humic acid on the sorption of Hg on Fe and Mn hydroxides [J]. Journal of Hazardous Materials, 2013, 244/245: 322-328. doi: 10.1016/j.jhazmat.2012.11.050 [18] GU B H, BIAN Y R, L MILLER C L, et al. Mercury reduction and complexation by natural organic matter in anoxic environments [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(4): 1479-1483. doi: 10.1073/pnas.1008747108 [19] 何杉杉, 尤琼智, 吴胜春, 等. 渔业养殖区水环境中汞的形态分布 [J]. 环境化学, 2015, 34(10): 1911-1917. HE S S, YOU Q Z, WU S C, et al. Distribution of mercury species in aquatic environment in marine aquaculture zones [J]. Environmental Chemistry, 2015, 34(10): 1911-1917(in Chinese).
[20] XU Z T, WU S C, CHRISTIE P, et al. Impacts of estuarine dissolved organic matter and suspended particles from fish farming on the biogeochemical cycling of mercury in Zhoushan island, eastern China Sea [J]. Science of the Total Environment, 2020, 705(C): 135921. [21] 陈春羽, 王定勇. 水溶性有机质对土壤及底泥中汞吸附行为的影响 [J]. 环境科学学报, 2009, 29(2): 312-317. CHEN C Y, WANG D Y. Effect of dissolved organic matter on adsorption of mercury by soils and sediment [J]. Acta Scientiae Circumstantiae, 2009, 29(2): 312-317(in Chinese).
[22] 何鑫龙, 裴福云, 吴胜春, 等. 渔业养殖区溶解性有机质的来源、组成及其对汞甲基化的影响 [J]. 环境科学学报, 2021, 41(10): 4097-4106. HE X L, PEI F Y, WU S C, et al. The sources, composition and influence of dissolved organic matter on mercury methylation in fish aquaculture area [J]. Acta Scientiae Circumstantiae, 2021, 41(10): 4097-4106(in Chinese).
[23] WANG P C, PENG H, LIU J L, et al. Effects of exogenous dissolved organic matter on the adsorption-desorption behaviors and bioavailabilities of Cd and Hg in a plant-soil system [J]. Science of the Total Environment, 2020, 728: 138252. doi: 10.1016/j.scitotenv.2020.138252 [24] 万合锋, 龙朝波, 兰晨, 等. 满江红资源化利用及对环境修复作用的研究进展 [J]. 福建农业学报, 2015, 30(11): 1120-1126. WAN H F, LONG C B, LAN C, et al. Research progress on utilization of Azolla and Its potential contribution to environmental restoration [J]. Fujian Journal of Agricultural Sciences, 2015, 30(11): 1120-1126(in Chinese).
[25] 周兵. 伴生蕨类植物满江红提取物对水稻生长的影响 [J]. 热带作物学报, 2011, 32(7): 1229-1234. ZHOU B. Effects of companion fern Azolla imbricata extracts on rice growth [J]. Chinese Journal of Tropical Crops, 2011, 32(7): 1229-1234(in Chinese).
[26] COHEN-SHOEL N, LLZYCER D, GILATH I, et al. The involvement of pectin in Sr2+ biosorption by azolla [J]. Water, Air, and Soil Pollution, 2002, 135(1-4): 195-205. [27] MAHSA H, RAHMAT Z, ROGHAYEH S. Uptake and accumulation of heavy metals by water body and Azolla filiculoides in the Anzali wetland [J]. Applied Water Science, 2021, 11(6): 91. doi: 10.1007/s13201-021-01428-y [28] ARSHADI M, ABDOLMALEKI M K, MOUSAVINIA F, et al. Nano modification of NZVI with an aquatic plant Azolla filiculoides to remove Pb(II) and Hg(II) from water: Aging time and mechanism study [J]. Journal of Colloid And Interface Science, 2017, 486: 296-308. doi: 10.1016/j.jcis.2016.10.002 [29] NAGHIPOUR D, ASHRAFI S D, GHOLAMZADEH M, et al. Phytoremediation of heavy metals (Ni, Cd, Pb) by Azolla filiculoides from aqueous solution: A dataset [J]. Data in Brief, 2018, 21: 1409-1414. doi: 10.1016/j.dib.2018.10.111 [30] OHNO T, BRO R. Dissolved organic matter characterization using multiway spectral decomposition of fluorescence landscapes [J]. Soil Science Society of America Journal, 2006, 70(6): 2028-2037. doi: 10.2136/sssaj2006.0005 [31] RENNERT T, GOCKEL K F, MANSFELDT T. Extraction of water-soluble organic matter from mineral horizons of forest soils [J]. Journal of Plant Nutrition and Soil Science, 2007, 170(4): 514-521. doi: 10.1002/jpln.200625099 [32] LEENHEER J A. Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters [J]. Environmental Science & Technology, 1981, 15(5): 578-587. [33] 杨淇茹, 吴胜春, 李雨桐, 等. 应用XAD大孔树脂与阴阳离子交换树脂串联技术探究不同腐解时期鱼粪溶解性有机质组分差异 [J]. 江苏农业科学, 2019, 47(22): 303-307. YANG Q R, WU S C, LI Y T, et al. Study on distribution differences of various components of fish manure dissolved organic matter in different decomposingperiods based on XAD macroporous resin and anion-cation exchange resin series technology [J]. Jiangsu Agricultural Sciences, 2019, 47(22): 303-307(in Chinese).
[34] ŚWIETLIK J, DĄBROWSKA A, RACZYK-STANISŁAWIAK U, et al. Reactivity of natural organic matter fractions with chlorine dioxide and ozone [J]. Water Research, 2004, 38(3): 547-558. doi: 10.1016/j.watres.2003.10.034 [35] 蒋红梅, 冯新斌, 梁琏, 等. 蒸馏-乙基化GC-CVAFS法测定天然水体中的甲基汞 [J]. 中国环境科学, 2004, 24(5): 568-571. JIANG H M, FENG X B, LIANG L, et al. Determination of methyl mercury in waters by distillation-GC-CVAFS technique [J]. China Environmental Science, 2004, 24(5): 568-571(in Chinese).
[36] 朱伟健, 沈芳, 洪官林. 长江口及邻近海域有色溶解有机物(CDOM)的光学特性 [J]. 环境科学, 2010, 31(10): 2292-2298. ZHU W J, SHEN F, HONG G L. Optical characteristics of colored dissolved organic material(CDOM) in Yangtze Estuary [J]. Environmental Science, 2010, 31(10): 2292-2298(in Chinese).
[37] TWARDOWSKI M S, BOSS E, SULLIVAN J M, et al. Modeling the spectral shape of absorption by chromophoric dissolved organic matter [J]. Marine Chemistry, 2004, 89(1/2/3/4): 69-88. [38] HELMS J R, STUBBINS A, RITCHIE J D, et al. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter [J]. Limnology and Oceanography, 2008, 53(3): 955-969. doi: 10.4319/lo.2008.53.3.0955 [39] HE Y H, SONG N, JIANG H L. Effects of dissolved organic matter leaching from macrophyte litter on black water events in shallow lakes [J]. Environmental Science and Pollution Research International, 2018, 25(10): 9928-9939. doi: 10.1007/s11356-018-1267-0 [40] CHEN H L, ZHOU J M. Characterization of dissolved organic matter derived from rice straw at different decay stages[M]. Molecular Environmental Soil Science at the Interfaces in the Earth’s Critical Zone. Berlin, Heidelberg: Springer Berlin Heidelberg, 2010: 35-37. [41] 梁俭, 江韬, 卢松, 等. 淹水条件下三峡库区典型消落带土壤释放DOM的光谱特征: 紫外-可见吸收光谱 [J]. 环境科学, 2016, 37(7): 2496-2505. LIANG J, JIANG T, LU S, et al. Spectral characteristics of dissolved organic matter(DOM) releases from soils of typical water-level fluctuation zones of Three Gorges reservoir areas: UV-vis spectrum [J]. Environmental Science, 2016, 37(7): 2496-2505(in Chinese).
[42] DILLING J, KAISER K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry [J]. Water Research, 2002, 36(20): 5037-5044. doi: 10.1016/S0043-1354(02)00365-2 [43] DON A, KALBITZ K. Amounts and degradability of dissolved organic carbon from foliar litter at different decomposition stages [J]. Soil Biology and Biochemistry, 2005, 37(12): 2171-2179. doi: 10.1016/j.soilbio.2005.03.019 [44] ALMENDROS G, DORADO J. Molecular characteristics related to the biodegradability of humic acid preparations [J]. European Journal of Soil Science, 1999, 50(2): 227-236. doi: 10.1046/j.1365-2389.1999.00240.x [45] COBLE P G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy [J]. Marine Chemistry, 1996, 51(4): 325-346. doi: 10.1016/0304-4203(95)00062-3 [46] MCKNIGHT D M, BOYER E W, WESTERHOFF P K, et al. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity [J]. Limnology and Oceanography, 2001, 46(1): 38-48. doi: 10.4319/lo.2001.46.1.0038 [47] HUGUET A, VACHER L, RELEXANS S, et al. Properties of fluorescent dissolved organic matter in the Gironde Estuary [J]. Organic Geochemistry, 2009, 40(6): 706-719. doi: 10.1016/j.orggeochem.2009.03.002 [48] BIRDWELL J E, VALSARAJ K T. Characterization of dissolved organic matter in fogwater by excitation-emission matrix fluorescence spectroscopy [J]. Atmospheric Environment, 2010, 44(27): 3246-3253. doi: 10.1016/j.atmosenv.2010.05.055 [49] KALBITZ K, SCHWESIG D, SCHMERWITZ J, et al. Changes in properties of soil-derived dissolved organic matter induced by biodegradation [J]. Soil Biology and Biochemistry, 2003, 35(8): 1129-1142. doi: 10.1016/S0038-0717(03)00165-2 [50] CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter [J]. Environmental Science & Technology, 2003, 37(24): 5701-5710. [51] 朱维晃, 黄廷林, 张亚宁. 氧化还原条件变化对上覆水体中溶解有机质的三维荧光光谱特征影响 [J]. 光谱学与光谱分析, 2010, 30(12): 3272-3276. ZHU W H, HUANG T L, ZHANG Y N. The influence of the redox conditions on the three-dimensional excitation-emission matrix (3DEEM) fluorescence spectroscopy of the dissolved organic matter (DOM) in the overlying water [J]. Spectroscopy and Spectral Analysis, 2010, 30(12): 3272-3276(in Chinese).
[52] ŚWIETLIK J, SIKORSKA E. Application of fluorescence spectroscopy in the studies of natural organic matter fractions reactivity with chlorine dioxide and ozone [J]. Water Research, 2004, 38(17): 3791-3799. doi: 10.1016/j.watres.2004.06.010 [53] LAVOIE R A, AMYOT M, LAPIERRE J F. Global meta-analysis on the relationship between mercury and dissolved organic carbon in freshwater environments [J]. Journal of Geophysical Research:Biogeosciences, 2019, 124(6): 1580-1523. [54] BURNS D A, AIKEN G R, BRADLEY P M, et al. Specific ultra-violet absorbance as an indicator of mercury sources in an Adirondack River Basin [J]. Biogeochemistry, 2013, 113(1/2/3): 451-466. [55] MANGAL V, LAM W Y, HUANG H, et al. Molecular correlations of dissolved organic matter with inorganic mercury and methylmercury in Canadian boreal streams [J]. Biogeochemistry, 2022, 160(1): 127-144. doi: 10.1007/s10533-022-00944-6 [56] WANG Y Q, LIU J, VAN L N, et al. Binding strength of mercury (II) to different dissolved organic matter: The roles of DOM properties and sources [J]. The Science of the total environment, 2021, 807(P3): 150979. [57] NAGASE H, OSE Y, SATO T, et al. Methylation of mercury by humic substances in an aquatic environment [J]. Science of the Total Environment, 1982, 25(2): 133-142. doi: 10.1016/0048-9697(82)90082-1 [58] 何小松, 席北斗, 魏自民, 等. 三维荧光光谱研究垃圾渗滤液水溶性有机物与汞相互作用 [J]. 分析化学, 2010, 38(10): 1417-1422. HE X S, XI B D, WEI Z M, et al. Three-dimensional excitation emission matrix fluorescence spectroscopic characterization of complexation between mercury(Ⅱ) and dissolved organic matter extracted from landfill leachate [J]. Chinese Journal of Analytical Chemistry, 2010, 38(10): 1417-1422(in Chinese).
[59] YIN Y J, ALIEN H E, HUANG C P, et al. Interaction of Hg (II) with soil-derived humic substances [J]. Analytica Chimica Acta, 1997, 341(1): 73-82. doi: 10.1016/S0003-2670(96)00509-0 [60] SHANLEY J B, TAYLOR V F, RYAN K A, et al. Using dissolved organic matter fluorescence to predict total mercury and methylmercury in forested headwater streams, Sleepers River, Vermont USA [J]. Hydrological Processes, 2022, 36(5): 14572. [61] SARDANA A, COTTRELL B, SOULSBY D, et al. Dissolved organic matter processing and photoreactivity in a wastewater treatment constructed wetland [J]. Science of the Total Environment, 2019, 648: 923-934. doi: 10.1016/j.scitotenv.2018.08.138 [62] 阴永光, 李雁宾, 马旭, 等. 天然有机质介导的汞生物地球化学循环: 结合作用与分子转化 [J]. 化学进展, 2013, 25(12): 2169-2177. YIN Y G, LI Y B, MA X, et al. Role of natural organic matter in the biogeochemical cycle of mercury: Binding and molecular transformation [J]. Progress in Chemistry, 2013, 25(12): 2169-2177(in Chinese).
[63] GUGGENBERGER G, ZECH W. Composition and dynamics of dissolved carbohydrates and lignin-degradation products in two coniferous forests, N. E. Bavaria, Germany [J]. Soil Biology and Biochemistry, 1994, 26(1): 19-27. doi: 10.1016/0038-0717(94)90191-0 [64] ALEKSANDRA B, PATRYK O, RYSZARD D. Adsorption and desorption of heavy metals by the sewage sludge and biochar-amended soil [J]. Environmental geochemistry and health, 2019, 41(4): 1663-1674. doi: 10.1007/s10653-017-0036-1 [65] HE Y, MEN B, YANG X F, et al. Relationship between heavy metals and dissolved organic matter released from sediment by bioturbation/bioirrigation [J]. Journal of Environmental Sciences, 2018, 75: 216-223. -



 下载:
下载: