-
我国每年皮革产量折合标准皮约为7000万张,制革过程中原皮质量的30%变成铬屑[1],铬屑中有3%—5%的铬含量[2],90%以上为胶原纤维,目前,主要通过酸法、碱法和酶法等方法[3—5]将铬屑水解转化为工业蛋白或工业明胶,并已实现产业化[6—7]。但由于铬屑水解过程中溶解性胶原分子量分布的不可控[8],导致工业明胶和蛋白中残余铬达不到产品质量要求[9],成为革屑资源化的技术瓶颈。目前有关铬屑脱铬过程中铬残留问题,人们都通过水解方式及工艺参数控制加以解决[10—11],对水解脱铬过程中铬的形态变化与结合方式没有给予关注。
鞣制化学理论表明,胶原分子内由3条肽链以平行,右手螺旋形式缠绕形成三螺旋结构,胶原分子在三螺旋结构基础上通过共价键交联形成稳定的胶原微纤维,并逐步聚集成束形成胶原亚纤维、胶原原纤维最终组成胶原纤维[12]。制革鞣制过程中,铬通过渗透达到不同层级的胶原纤维表面,再与胶原纤维表面相邻分子链上离解羧基配合形成铬鞣革。胶原分子肽链中每3个氨基酸残基就有一个要经过此三螺旋结构中央区,残基沿轴向距离为0.29 nm[13],分布于胶原纤维相邻分子链上离解羧基之间间距约1.4 nm,铬一般通过多核配位来实现架桥络合[14]。从渗透阻力和胶原分子结构等方面来说,鞣制过程中铬分布在胶原分子内部的可能性极小,甚至进入胶原微纤维层次的比例也较少。但是在水解过程中,由于水解工艺对铬屑胶原结构的破坏作用,使铬在胶原纤维内部和水解液中铬的分布发生显著变化,由此导致铬从固相脱除至水解液中的脱除过程并非单纯的铬鞣的逆过程。目前水解过程中胶原与铬分离不彻底与铬在胶原间分布有关关系目前并不明确,有机酸等强络合剂浸提后仍无法从胶原上彻底脱除铬的原因也不清晰,在当前含铬危废资源化利用要求越来越严格的背景下,相关方面的研究也越来越迫切。
本实验以碱水解协同酸淋洗对铬屑脱铬的实际生产过程进行模拟,探讨了不同水解程度下铬屑的脱铬效果和水解液中铬与水解胶原的分布规律,以Ⅰ型胶原为铬屑皮胶原模拟物采用荧光光谱法解释了柠檬酸及柠檬酸-铬络合物与胶原的相互作用,为铬屑资源化提供参考依据。
-
铬屑来自辛集市梅花集团,生羊皮用石灰、硫化碱、氯粉、硝酸和铬粉等进行去油、去脂、脱毛和铬鞣处理后的蓝湿革,其中水分14.5%、灰分6.3%、铬含量3.414%和pH 3.93。实验前进行预处理粉碎成大小均为5 cm×4 mm左右的废屑。
-
(1) 铬屑水解过程残余铬的测定
取5 g绝干铬屑于烧杯中,分别加入4%、8%、12%、16%、20%的NaOH(以绝干铬屑计),加入固液比1:20的去离子水,放入恒温振荡培养箱中保持30 ℃振荡24 h。分别在2、4、6、8、10、12 h取上清液保存,测定总铬、TOC和游离氨基含量。振荡结束后将剩余固体分离,在剩余固体中加入100 mL去离子水继续振荡10 min,水洗两次后将水解液定容,收集样品,测定总铬含量。根据铬屑总铬含量可计算得出残余铬含量。
将经过碱处理后的铬屑加入150 mL 3%稀硫酸,放入恒温振荡培养箱中保持30 ℃振荡1 h,分别在10、20、30、40、50、60 min取上清液保存,测定总铬、TOC和游离氨基含量。振荡结束后将剩余固体分离,在剩余固体中加入一定量去离子水继续振荡10 min,水洗两次后将水解液定容,收集酸水解液,测定总铬含量。根据铬屑总铬含量可计算得出残余铬含量。
(2) 水解液中水解胶原分子量分布及铬含量的测定
将12%、16%和20%浓度碱处理后的铬屑水解液分为两组,其中一组调pH为8.5,沉淀1 h后,同时进行微滤和超滤膜过滤,微滤膜采用孔径为0.45 μm(截留分子量约为1000 kDA)和0.1 μm(截留分子量约为250 kDA),超滤膜采用截留分子量为100 kDA和50 kDA。将水解液按照分子量由大到小分别分级过滤,且每级过滤两次更换两次滤膜,收集样品,测定溶液中总铬和TOC含量。
-
经上述酸淋洗处理后的铬屑中分别加入100 mL浓度为0.2、0.5、0.7、0.9、1.1 mol·L−1 的柠檬酸钠保证固体完全浸没,放入恒温振荡培养箱振荡1 h。将剩余固体分离,在剩余固体中加入一定量去离子水继续振荡10 min,水洗两次后将固体冷冻干燥保存,处理后的铬屑消解测定固相中铬含量。
-
“柠檬酸-铬”溶液配制:分别配置浓度为0.0016、0.0048、0.0064、0.008 mol·L−1的柠檬酸溶液,以柠檬酸和Cr摩尔比10:1加入Cr2(SO4)3·6H2O,反应24 h后调pH8.5过滤后保存待用。
以Ⅰ型胶原蛋白为铬屑中胶原蛋白模拟物,用磷酸缓冲液配制25 mL浓度为0.001 mol·L−1的胶原蛋白溶液,分别加入25 mL浓度为0.0016、0.0032、0.008 mol·L−1的柠檬酸钠和浓度0.0016、0.0048、0.0064、0.008 mol·L−1“柠檬酸-铬”,测定其荧光光谱。激发波长277 nm[15],响应时间0.1 s,狭缝宽度Ex:4 nm,Em:3.5 nm。实验流程图如图1所示。
-
(1) 溶液中总铬含量的测定:采用国标二苯碳酰二肼分紫外光光度法(GB 7466—87)测定。铬浸出率W的计算公式(1)。
式(1)中,N为浸出液的稀释倍数;Cj为稀释后浸出液中Cr浓度,mg∙L−1;V为稀释后浸出液取样体积,mL;mz为铬屑中Cr含量,mg∙g−1;M为铬屑样品质量,g。
(2) 铬屑中总铬含量的测定:将固相铬屑进行预处理消解成溶液后,采用国标二苯碳酰二肼分紫外光光度法(GB 7466—87)测定。铬屑消解方法采用三酸消解法,具体操作步骤如下:称取含革铬屑0.1000 g于聚四氟乙烯坩埚中,加入2 mL超纯水润湿;加入5 mL HNO3和2 mL 氢氟酸(HF),于200 ℃下加热至近干,取下冷却;再加入5 mL HF,同样于200 ℃下加热至近干,取下冷却;进一步加入3—4 mL 混酸(HNO3:HClO4=1:1),继续于160 ℃下加热至近干,取下冷却。用5% HNO3加热溶解处理后的样品,过滤,定容至50 mL。
(3) 溶液中TOC含量的测定:利用德国Elementar公司生产的Liqui TOC II型测定仪测定,具体测定参数参考文献[16]。
(4) 水解液中游离氨基浓度测定:采用OPA法测定游离氨基浓度[17]
(5) 铬屑水解度的测定:电子分析天平(ME204E)称量试验前后铬屑的质量,水解度参照公式(2)。
式(2)中,M0为未处理铬屑质量,g;M1为水解后残余铬屑质量,g;
(6) 铬屑的形态表征:通过高分辨场发射扫描电镜(FEI Verios 460)观察铬屑处理前后的微观变化,以及样品的前后表观变化。
-
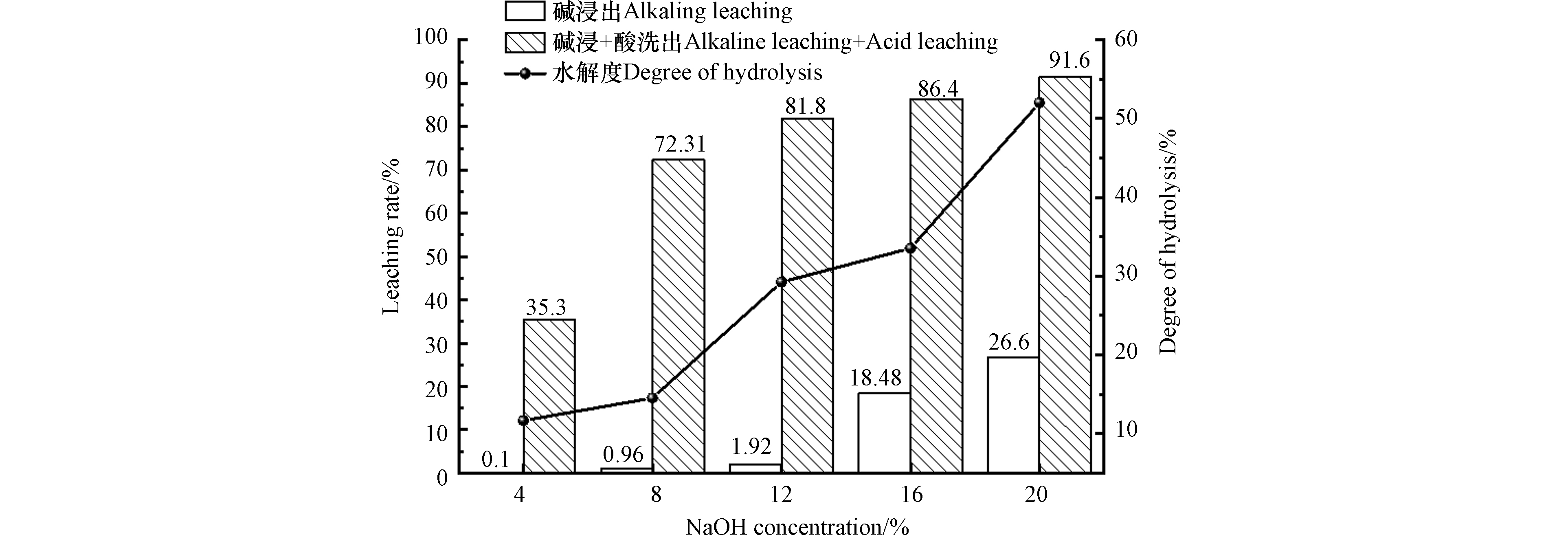
在不同浓度碱水解情况下,考察对无机酸脱铬效果的影响,结果如图2所示。通过不同浓度的碱处理,铬屑脱铬最高浸出率达到26.6%,在低于16%浓度碱浸出实验中,浸出率均低于2%,几乎没有浸出效果,但水解度上升,说明在体系中主要发生了皮胶原水解反应,并没有过多生成Cr(OH)4-或有机结合态铬。浓度由12%提高到16%,浸出率从1.92%提升到18.48%,这说明该浓度下铬屑中肽键断裂达到较大程度,水解度有了显著升高,肽键断裂使得更多的铬鞣分子暴露,铬鞣分子与胶原的氢键被破坏,交联强度降低,但胶原羧基与铬之间依然以配位键相结合,最终与胶原短链一同浸出,这与罗艳华等[18]研究碱用量在12%后肽键断裂程度显著上升结论一致。碱浓度达到20%时浸出率上升到26.6%,相对16%浓度的浸出率提高并不明显,并且水解度有了明显上升,说明碱浓度的增加导致铬屑脱铬不再以初步肽键断裂铬鞣分子暴露为主,而是直接将皮胶原以不同长短的肽链水解为主,而非单独铬的脱除。
将碱浸后的铬屑统一进行酸淋洗,收集酸水解液(pH约为0.6),测定总铬含量发现浸出率有了显著提高,最高达到91.6%。经过4%和8%碱浸后铬屑通过酸淋洗浸出率有明显差别,这说明碱浸过程对水解胶原的破坏有助于酸淋洗脱铬,破坏程度越大,脱铬效果越好。12%碱浓度处理后酸淋洗浸出率效果达到最大化,淋洗过程脱除约80%的铬,而碱浓度增至16%,淋洗过程脱除约68%,淋洗效果出现下降。这可能是因为残存在铬屑中的铬交联强度过大,虽然在酸性条件下能够破坏铬分子间氧配聚作用,但不能完全解开铬与胶原羧基的配位作用,同时存在铬鞣分子与胶原氨基的氢键作用且铬分子间的配聚作用,使得络合反应不能反向进行导致脱铬不利。当碱浓度达到20%时,酸淋洗脱铬达到65%,依然在下降,说明酸淋洗最高极限浸出率约为80%,铬屑中剩余残存铬无法通过无机酸淋洗脱除。
水解液中总铬与水解胶原含量随时间变化如图3所示。碱水解过程中,总铬含量在2 h后已达到稳定,这说明脱铬过程已经达到平衡,在2 h后TOC和游离氨基含量逐步增加,说明水解过程仍然进行,更多的胶原被水解进入液相,由于总铬含量不变,TOC和游离氨基浓度增加,说明水解脱除铬可能与皮胶原发生再络合的情况,在退鞣过程和再络合过程中达到动态平衡。酸水解过程中,总铬含量再20 min后达到稳定,在40 min时略有降低,这说明脱铬过程已经达到平衡,在20 min后TOC和游离氨基含量增高,过程中持续发生胶原水解反应。
通过上述实验数据分析得出,碱浸过程以破坏皮胶原结构为主,利于后续无机酸能够完全渗入皮屑内部发生反应,达到高效脱铬的目的。
-
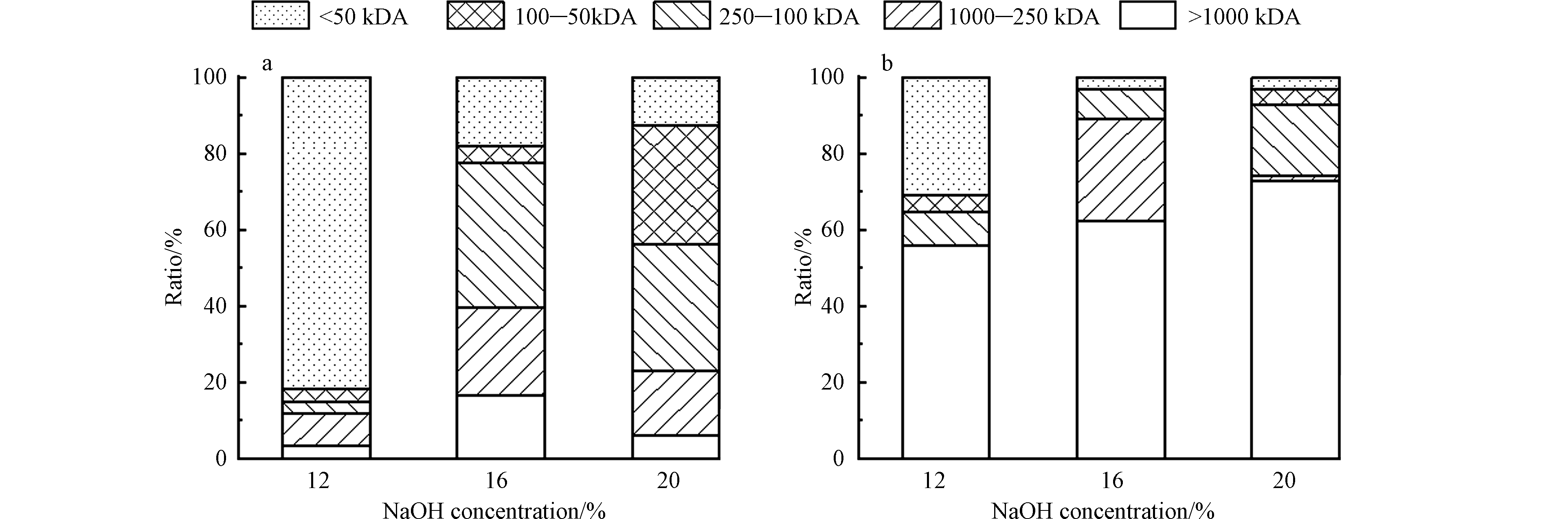
根据文献资料显示[13],工业明胶平均分子量分布为100—50 kDA;<50 kDA区间为多肽混合物;胶原分子量约300 kDA,250—100 kDA区间为胶原分子和明胶类混合物;将水解液经过不同孔径的微滤、超滤膜分级过滤,考察水解液中水解胶原与铬的分布规律,结果如图4所示。
随着碱浓度的增加,水解液中250—100 kDA区间原胶原分子和明胶类混合物含量逐渐增加,且<50 kDA区间的多肽混合物含量占比逐渐减少,这说明铬屑水解随碱浓度递增,水解液中以胶原分子逐渐以为主,16%和20%碱浓度水解液中以大分子胶原为主,12%浓度碱水解铬屑后以多肽混合物为主,其原因是12%碱强度低并未对皮胶原过多水解,整体皮胶原结构完整,更易于将液相中水解胶原向小分子转化,水解液中以小分子水解胶原为主。
12%、16%和20%浓度碱水解液中总铬分布均集中在>1000 kDA区间,占比达到50%以上。随着碱浓度增加,在<1000 kDA区间的总铬占比均出现递减,而>1000 kDA区间总铬逐级增加。这说明铬屑水解程度越大,脱出的铬50%以上为大分子水解胶原络合态或铬沉淀。
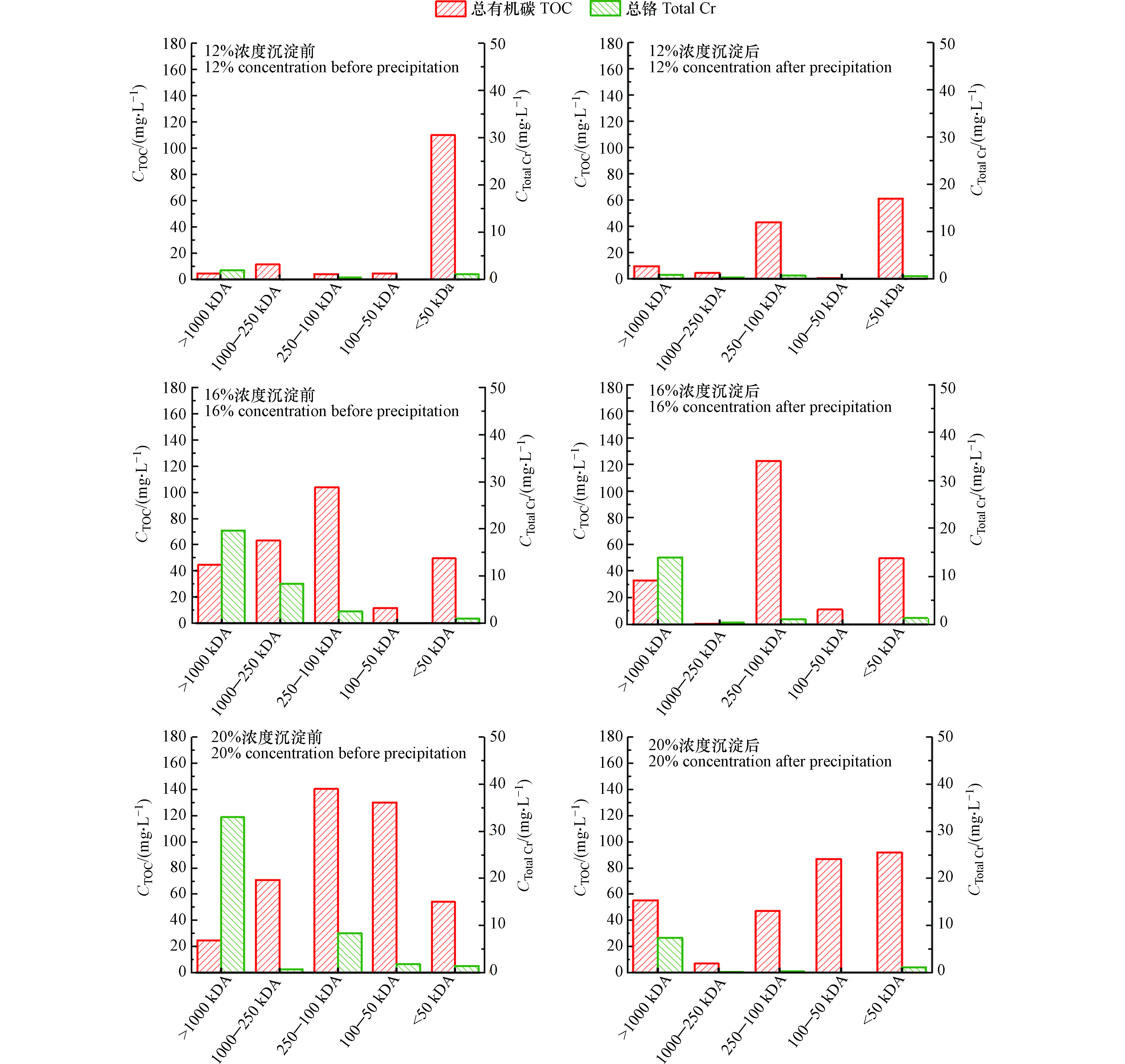
将12%、16%和20%浓度碱水解液经沉淀后分级过膜过滤,结果如图5所示。经12%浓度碱水解后,未沉淀水解液中总铬含量随水解胶原分子量减小出现先减少后增加再减少情况,在1000—250 kDA分子量较大的胶原分子混合物中已不含铬,进一步水解为分子量250—100 kDA胶原分子和明胶混合物中含铬。说明水解过程已脱除的游离态铬与小分子胶原或明胶发生再络合。沉淀后在250—100 kDA区间原胶原分子和明胶类混合物含量明显增加,这可能是pH值由12到8.5过程中,导致胶原分子或明胶之间形成更多氢键而发生团聚[19],本应通过膜部分的胶原蛋白被截留。
经16%浓度碱水解后,未沉淀水解液中总铬含量随水解胶原的分子量减小而减少,当水解到分子量为明胶时,总铬基本为0。沉淀后1000—250 kDA区间的水解胶原和总铬一同析出,铬稳定性较差;而<50 kDA区间的多肽混合物和总铬含量基本不变,稳定性较好。
经20%浓度碱水解后,未沉淀水解液中总铬含量随水解胶原分子量变化与12%浓度碱水解情况相同,再次表明水解过程中已经脱除的铬与胶原分子片段或明胶发生再络合,结合铬含量增加。沉淀后在>1000 kDA区间的大分子胶原含量增加,同样说明pH改变导致大分子胶原发生团聚。在<50 kDA区间也同样出现多肽混合物含量增加,可能是由于过滤过程较慢,水解反应持续进行将部分大分子胶原或明胶分解为多肽混合物。沉淀后在>50 kDA区间总铬含量有明显减少,而<50 kDA区间总铬含量基本不变,稳定性较好。
值得注意的是,不论水解液浓度大小或者沉淀前后变化,<50 kDA区间的总铬浓度基本恒定,不随多肽混合物含量的变化而变化。并且在12%和16%浓度碱水解液中100—50 kDA区间的工业明胶已经不含铬,那么继续水解到分子量更小的多肽混合物上也应该不含铬,以上结果说明<50 kDA区间的总铬与多肽混合物无关,以无机态铬存在,并不与多肽混合物络合。在实际生产中水解液都是经过沉淀和过滤处理的,真正被利用的部分是分子量在<1000 kDA区间,影响水解液中总铬含量的是<50 kDA中的无机态铬和部分稳定络合在胶原分子和明胶混合物上的铬,其中无机态铬含量稳定在0.5—1.2 mg·L−1,而络合在胶原分子和明胶混合物上的铬含量为0.2—1 mg·L−1。根据图5和上述分析结果得出,水解液沉淀前总铬分布在>1000 kDA区间上占比55.63%—72.66%;总铬分布在1000—250 kDA区间上占比0—26.53%;总铬分布在胶原分子和明胶混合物上占比7.92%—18.53%;总铬分布在工业明胶上占比0—4.6%;总铬分布在多肽混合物上占比0。
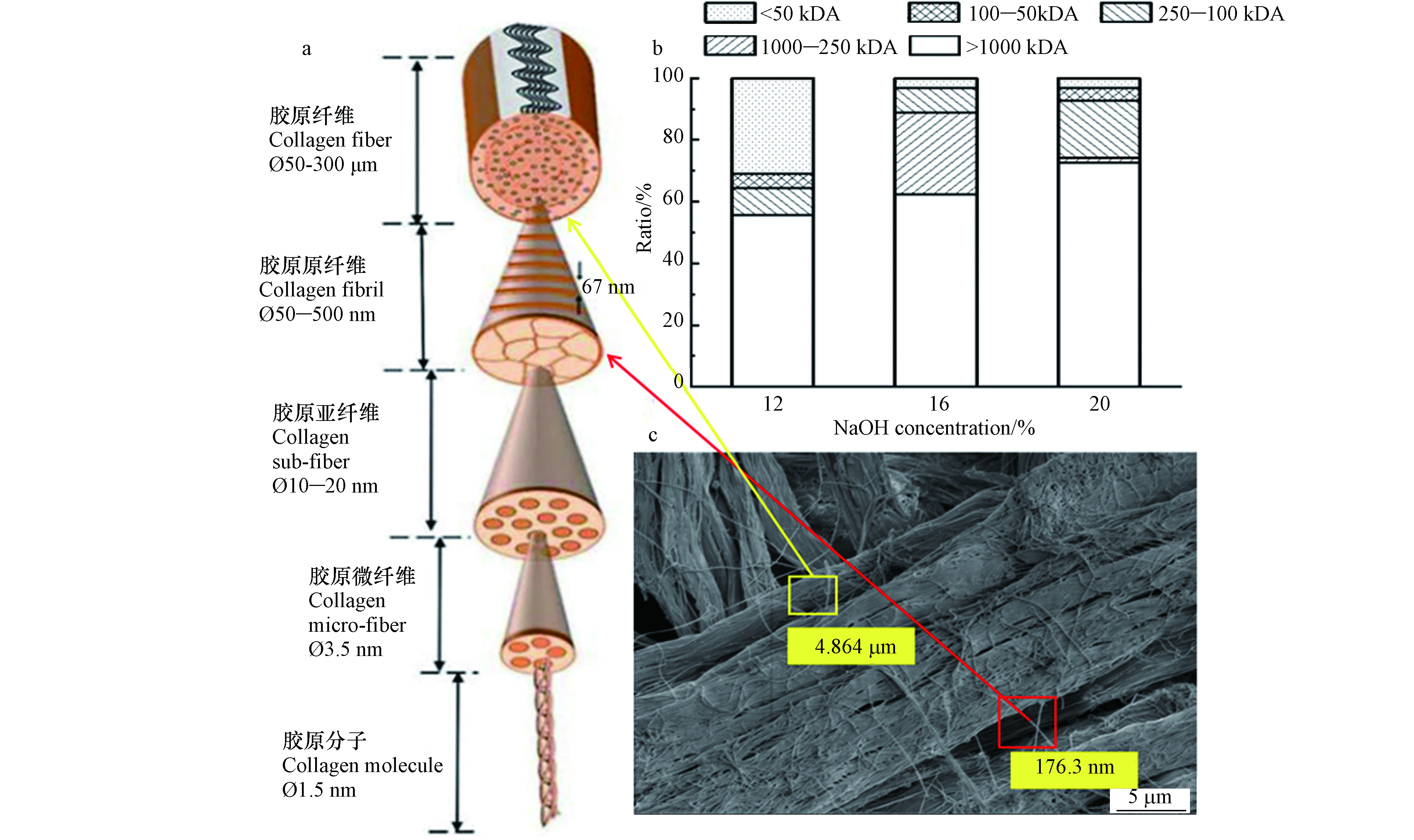
结合水解胶原和总铬的分布以及胶原多层结构图,如图6所示。胶原分子分子量约300 kDA,由三条肽链组成三股螺旋,且肽链中每三个氨基酸残基中有一个要经过此三股螺旋中央区,三螺旋内部空间十分狭窄。但水解液中<250 kDA区间总铬占比最高达20%左右,这与胶原分子内部结构形成矛盾。这说明水解液中<250 kDA区间总铬并不是皮革铬鞣过程中进入胶原分子三螺旋结构内部,而是水解过程已经脱掉的铬与小分子胶原片段或明胶发生了再络合。
根据水解液中>250 kDA区间总铬占比约55.63%—88.89%,该部分铬并不来自胶原分子中,而是比胶原分子更大的胶原微纤维或胶原亚纤维中,这说明皮革铬鞣过程中铬可能主要分布在胶原微纤维或胶原亚纤维层次结构。
-
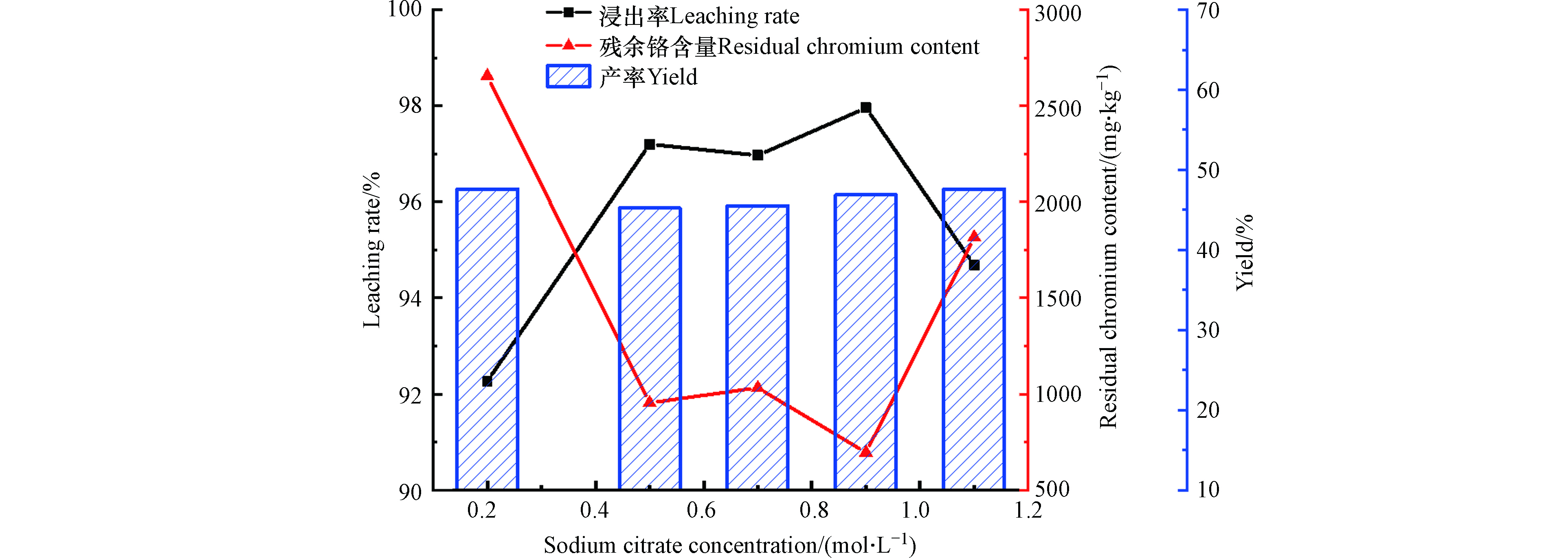
将碱浸和酸淋洗后的铬屑加入有机酸深度脱铬,考察碱对无机酸-有机酸协同脱铬效果的影响,结果如图7所示。
经过碱水解后,配合无机酸-有机酸协同处理脱铬率均在92%以上,最高达到97.96%,产率稳定在45.24%—47.63%之间。在柠檬酸钠浓度超过0.5 mol·L−1后浸出率出现波动,甚至在1.1 mol·L−1时浸出率出现了明显下降,这说明有机酸浓度在0.5 mol·L−1浸出脱铬已经到达浸出极限,浓度过高对有机酸的浸出有不利影响,残存在铬屑中的铬仍占2.04%。这可能是柠檬酸钠直接与皮胶原交联阻碍了铬的脱除或者已经脱除的“柠檬酸-铬”与皮胶原又发生交联。为了验证这种情况,以Ⅰ型胶原为皮胶原模拟物分别与柠檬酸钠和“柠檬酸-铬”进行荧光淬灭试验。
-
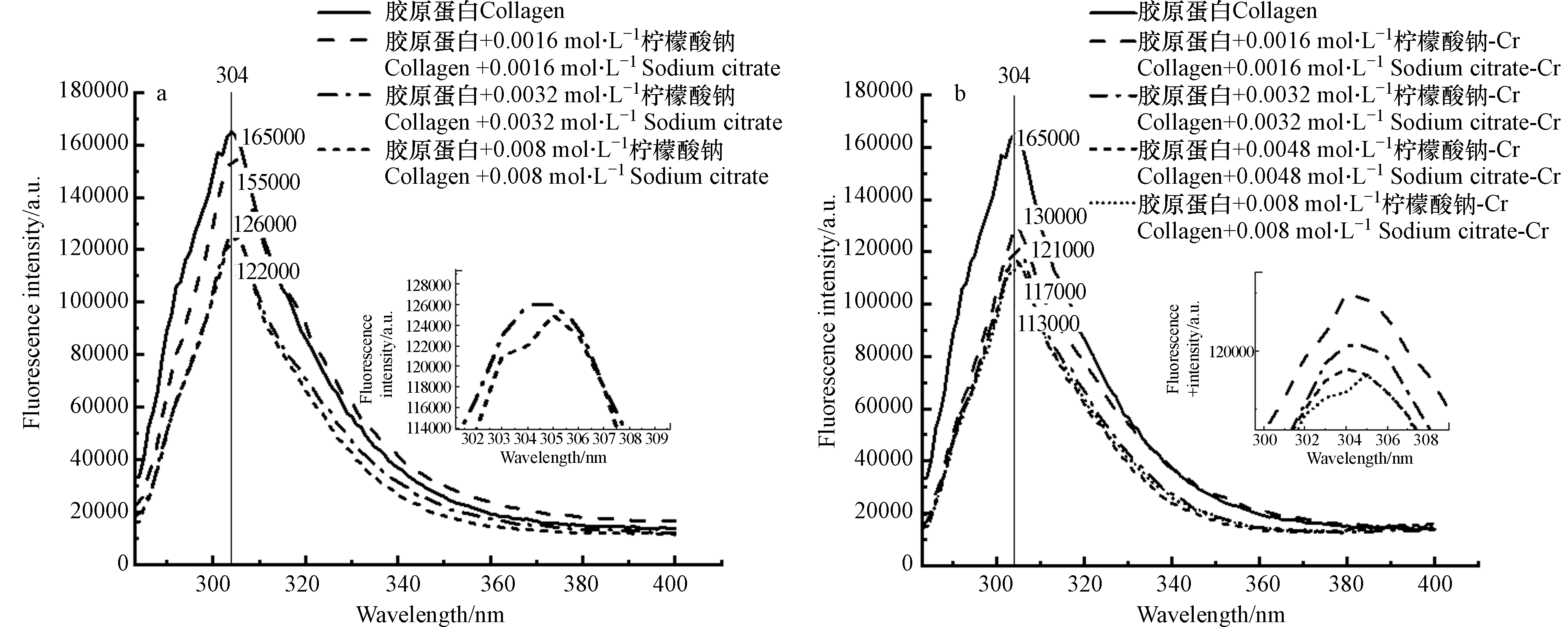
皮胶原中胶原蛋白主要成分为Ⅰ型胶原蛋白[20],以Ⅰ型胶原蛋白为铬屑中胶原蛋白模拟物,分别加入不同浓度的柠檬酸钠和“柠檬酸-铬”,测定其荧光光谱,结果如图8所示。
由图8可见,Ⅰ型胶原蛋白发射峰在304 nm处,该处主要是酪氨酸类蛋白区[21—22]。分别加入不同浓度的柠檬酸钠和“柠檬酸-铬”,蛋白质荧光强度均不同程度降低。这一结果与安辉等[23]发现酪氨酸类蛋白与铬的结合起重要作用结论一致。蛋白质的荧光强度下降能说明与柠檬酸钠和“柠檬酸-铬”发生了结合或者相互作用,这种现象称为荧光淬灭。
在使用柠檬酸钠脱铬过程中不仅是柠檬酸与铬的络合反应,同时存在柠檬酸和“柠檬酸-铬”与皮胶原的结合作用,阻碍了铬与皮胶原的分离,所以铬屑中总会由残余铬无法脱除。
-
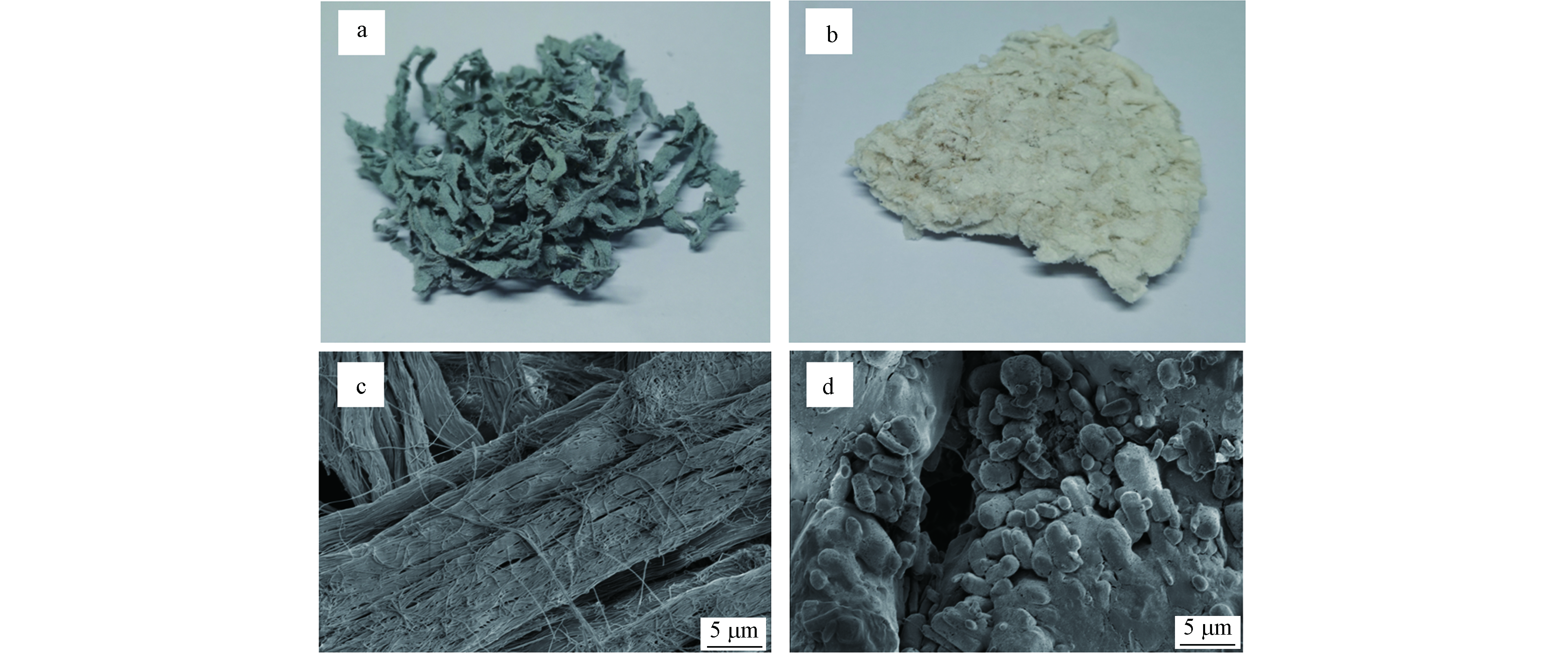
使用电子显微镜观察铬屑处理前后的微观变化,以及样品变化,结果如图9所示。从图9可以看出,从宏观角度分析未处理的铬屑颜色是蓝色,经过脱铬处理后变成白色。由于铬的脱除导致皮胶原的柔性降低,材料较脆。从微观角度分析未处理铬屑的纤维结构明显,结构紧密有序,经处理后的铬屑微纤维结构消失,表面发生胶化局部光滑,这是由于铬的浸出和酸碱的共同作用破坏了胶原纤维结构[24],处理前后的铬屑存在较大差异。
-
(1) 碱水解协同无机酸脱铬,水解度到达50%时,最高脱铬率91.6%。协同无机酸—有机酸脱铬最高脱铬率达到97.96%。
(2) 碱水解过程导致铬从固相脱除至水解液中的脱除过程并非单纯的铬鞣的逆过程,从铬屑中脱除的铬会与小分子胶原片段发生了再络合。残存的铬除大量[Cr(H2O)6]3+外,部分以络合态与水解胶原结合。追踪水解液中>250 kDA区间总铬占比约55.63%—88.89%,可以认为制革鞣制过程中铬经渗透后主要结合在胶原微纤维或亚纤维层次结构上。
(3) 残余铬屑中铬无法彻底脱除是因为柠檬酸钠和“柠檬酸-铬”均能和皮胶原发生结合,铬在脱除的过程中又结合皮胶原,直接导致铬与皮胶原无法彻底分离。
铬屑碱解过程中铬与胶原分子量分布特征
Dechromation and molecular weight distribution of hydrolyzed collagen from chromium—containing leather during alkaline
-
摘要: 酸碱法对含铬革脱铬时,由于胶原水解过程中胶原分子分布的不可控性,导致资源化利用生产的工业明胶和蛋白中残余铬达不到产品要求。通过实际生产过程模拟,探讨了不同碱解程度下铬屑水解液中胶原分子量分布与铬的分布规律。研究结果表明,随着碱浓度增加,铬屑脱铬率逐渐上升。碱水解24 h协同硫酸淋洗1 h的脱铬率为91.60%,加入柠檬酸可促进了铬屑上铬的溶解和分离,脱铬率达到97.96%。不同碱解液中溶解性胶原分子量分布表现出显著差异,而铬与水解胶原分子量则存在着明显的非均匀性分布,其中55.63%—72.66%的总铬分布在>1000 kDA区间;0—26.53%的总铬与1000—250 kDA区间的水解胶原结合;7.92%—18.53%的总铬与胶原分子和明胶混合物结合;0—4.60%的总铬与工业明胶结合;<50 kDA的多肽混合物上未结合铬。上述残存的铬除大量[Cr(H2O)6]3+外,部分以络合态与水解胶原结合。结合的铬含量占比最高达到20%,水解过程中从铬屑中脱除的铬与小分子胶原片段发生了再络合。追踪水解液中>250 kDA区间总铬占比约55.63%—88.89%,可以认为制革鞣制过程中铬经渗透后主要结合在胶原微纤维或亚纤维层次结构上。加入的柠檬酸在促进了铬从固相中分离出铬的同时,柠檬酸及柠檬酸-铬络合物可与皮胶原形成复合物,铬脱除的过程中以上两种结合皮胶原的方式,是铬与皮胶原无法彻底分离的主要原因。Abstract: The uncontrollable distribution of collagen molecules during collagen hydrolysis in the acid-base method for chromium-containing leather results in the production of gelatin and protein with residual chromium that does not meet industrial product requirements. In this study, the distribution patterns of collagen molecular weight and chromium in chromium scrap hydrolysate under different alkali concentrations were investigated by simulating the actual production process. The results showed that the rate of chromium removal from chromium chips gradually increased with the increase of alkali concentration. The chrome removal rate of alkali hydrolysis for 24 h in cooperation with sulfuric acid drenching for 1 h was 91.60%, while the addition of citric acid promoted the dissolution and separation of chromium on the chips, and the chrome removal rate reached 97.96%. The chromium and hydrolyzed collagen molecular weight showed a significant non-uniform distribution, where 55.63%—72.66% of total chromium was distributed in the >1000 kDA interval; 0—26.53% of total chromium was bound to 1000—250 kDA interval; 7.92%—18.53% of the total chromium was bound to the collagen molecules and gelatin mixture; 0—4.60% of the total chromium was bound to the industrial gelatin; and no chromium was bound to the peptide mixture of <50 kDA. The above residual chromium was partially bound to hydrolyzed collagen as complex, except for a significant amount of [Cr(H2O)6]3+. The bound chromium content accounted for up to 20%, and the chromium removed from the chromium chips during hydrolysis underwent re-complexation with small molecule collagen fragments. The experimental data confirmed that the percentage of total chromium in the >250 kDA interval in the hydrolysate was about 55.63%—88.89%, which can be assumed that chromium was mainly bound to the collagen microfibrils or sub-fibrillar hierarchy after penetration during the tanning process. While the added citric acid facilitated the separation of chromium from the solid phase, citric acid and citric acid-chromium complexes can reform complexes with collagen, and the above two ways of binding collagen in the process of chromium removal are the main reasons why chromium and collagen cannot be completely separated by acid-base method.
-

-
图 6 胶原多层结构与铬分布图(a)胶原多层结构图[12](b)不同浓度碱水解液中总铬占比图(c)铬屑SEM图
Figure 6. Collagen multilayer structure and chromium distribution map (a) collagen multilayer structure (b) proportion of total chromium in different concentrations of alkaline hydrolysis solution (c) SEM images of chromium-containing leather shavings
-
[1] 马宏瑞. 制革工业清洁生产和污染控制技术[M]. 北京: 化学工业出版社, 2004: 2-3. MA H R. Cleaner production and pollution control technology in leather industry[M]. Beijing: Chemical Industry Press, 2004: 2-3(in Chinese).
[2] ZHAO L, MU S D, WANG W X, et al. Toxicity evaluation of collagen hydrolysates from chrome shavings and their potential use in the preparation of amino acid fertilizer for crop growth [J]. Journal of Leather Science and Engineering, 2022, 4(1): 2. doi: 10.1186/s42825-021-00072-1 [3] FERREIRA M J, ALMEIDA M F, PINHO S C, et al. Finished leather waste chromium acid extraction and anaerobic biodegradation of the products [J]. Waste Management, 2010, 30(6): 1091-1100. doi: 10.1016/j.wasman.2009.12.006 [4] MALEK A, HACHEMI M, DIDIER V. New approach of depollution of solid chromium leather waste by the use of organic chelates [J]. Journal of Hazardous Materials, 2009, 170(1): 156-162. doi: 10.1016/j.jhazmat.2009.04.118 [5] WANG L, LI J, JIN Y, et al. Study on the removal of chromium(III) from leather waste by a two-step method [J]. Journal of Industrial and Engineering Chemistry, 2019, 79: 172-180. doi: 10.1016/j.jiec.2019.06.030 [6] PICCIN J S, FERIS L A, COOPER M, et al. Dye adsorption by leather waste: Mechanism diffusion, nature studies, and thermodynamic data [J]. Journal of Chemical & Engineering Data, 2013, 58(4): 873-882. [7] DING X L, SHAN Z H, LONG Z Z, et al. Utilization of collagen protein extracted from chrome leather scraps as a set retarders in gypsum [J]. Construction and Building Materials, 2020, 237: 117584. doi: 10.1016/j.conbuildmat.2019.117584 [8] DING W, XUEPIN L, ZHANG W H, et al. Dechroming of chromium-containing leather waste with low hydrolysis degree of collagen [J]. Journal- Society of Leather Technologists and Chemists, 2015, 99(3): 129-133. [9] MU C D, LIN W, ZHANG M R, et al. Towards zero discharge of chromium-containing leather waste through improved alkali hydrolysis [J]. Waste Management, 2003, 23(9): 835-843. doi: 10.1016/S0956-053X(03)00040-0 [10] 丁凡, 刘通, 王全杰. 碱法水解含铬革屑制备胶原蛋白粉的研究 [J]. 皮革与化工, 2020, 37(1): 14-20. doi: 10.3969/j.issn.1674-0939.2020.01.003 DING F, LIU T, WANG Q J. Study on preparation of collagen powder by alkaline hydrolysis of chromiumcontaining leather shavings [J]. Leather and Chemicals, 2020, 37(1): 14-20(in Chinese). doi: 10.3969/j.issn.1674-0939.2020.01.003
[11] 周健. 利用含铬革屑制备工业明胶的工艺探究[D]. 烟台: 烟台大学, 2020. ZHOU J. Study on the technology of preparing industrial gelatin with chrome-containing leather shavings[D]. Yantai: Yantai University, 2020(in Chinese).
[12] ZHAO C X, XIAO Y L, LING S J, et al. Structure of collagen [J]. Methods in Molecular Biology (Clifton, N. J. ), 2021, 2347: 17-25. [13] 蒋挺大. 胶原与胶原蛋白[M]. 北京: 化学工业出版社, 2006: 9-14. JIANG T D. Collagen and collagen[M]. Beijing: Chemical Industry Press, 2006: 9-14(in Chinese).
[14] 陈武勇, 李国英. 鞣制化学[M]. 3版. 北京: 中国轻工业出版社, 2011: 28-29. CHEN W Y, LI G Y. Tanning chemistry[M]. 3rd ed. Beijing: China Light Industry Press, 2011: 28-29(in Chinese).
[15] 俞园园. 荧光光谱法研究类人胶原蛋白与Zn(Ⅱ)的相互作用 [J]. 化学工程, 2013, 41(10): 9-12,16. doi: 10.3969/j.issn.1005-9954.2013.10.003 YU Y Y. Interaction between human-like collagen and zinc(Ⅱ) by fluorescence [J]. Chemical Engineering (China), 2013, 41(10): 9-12,16(in Chinese). doi: 10.3969/j.issn.1005-9954.2013.10.003
[16] 金鹏康, 孔茜, 金鑫. 二级出水中溶解性有机物的分级表征特性 [J]. 环境化学, 2015, 34(9): 1649-1653. doi: 10.7524/j.issn.0254-6108.2015.09.2015020405 JIN P K, KONG Q, JIN X. Characterization of dissolved organic matter in the secondary effluent of urban waste water treatment plant [J]. Environmental Chemistry, 2015, 34(9): 1649-1653(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.09.2015020405
[17] 罗艳华, 王全杰, 陈沛海, 等. 蛋白水解物水解度测定方法的研究 [J]. 皮革与化工, 2017, 34(2): 26-31. doi: 10.3969/j.issn.1674-0939.2017.02.007 LUO Y H, WANG Q J, CHEN P H, et al. Study on determination method for hydrolyzed de gree of protein hydrolysate [J]. Leather and Chemicals, 2017, 34(2): 26-31(in Chinese). doi: 10.3969/j.issn.1674-0939.2017.02.007
[18] 罗艳华, 王全杰, 陈沛海. CaO、NaOH对铬革屑水解效果的对比 [J]. 皮革与化工, 2017, 34(3): 1-12. doi: 10.3969/j.issn.1674-0939.2017.03.001 LUO Y H, WANG Q J, CHEN P H. Comparison for hydrolysis effect of chrome shaving between CaO and NaOH [J]. Leather and Chemicals, 2017, 34(3): 1-12(in Chinese). doi: 10.3969/j.issn.1674-0939.2017.03.001
[19] 吴坤远. 马面鲀鱼皮胶原结构、功能及流变性能的研究[D]. 福州: 福建农林大学, 2020. WU K Y. Study on skin collagen structure, function and rheological properties of navodon septentrionalis[D]. Fuzhou: Fujian Agriculture and Forestry University, 2020(in Chinese).
[20] ANDONEGI M, de la CABA K, GUERRERO P. Effect of citric acid on collagen sheets processed by compression [J]. Food Hydrocolloids, 2020, 100: 105427. doi: 10.1016/j.foodhyd.2019.105427 [21] WANG Z P, ZHANG T. Characterization of soluble microbial products (SMP) under stressful conditions [J]. Water Research, 2010, 44(18): 5499-5509. doi: 10.1016/j.watres.2010.06.067 [22] 陈继芬, 贺子欣, 党丽慧, 等. 利用光谱分析法表征银川市湿地水体中溶解性有机质的特征 [J]. 环境化学, 2021, 40(8): 2524-2534. doi: 10.7524/j.issn.0254-6108.2020040702 CHEN J F, HE Z X, DANG L H, et al. Characterization of dissolved organic matter in the surface water of the Yinchuan Wetlands using spectroscopic analysis [J]. Environmental Chemistry, 2021, 40(8): 2524-2534(in Chinese). doi: 10.7524/j.issn.0254-6108.2020040702
[23] 安辉, 王梓婷, 金若菲, 等. Shewanella putrefaciens CN32非饱和生物膜对环境压力的响应以及与铬的作用 [J]. 环境化学, 2022, 41(6): 1880-1889. doi: 10.7524/j.issn.0254-6108.2021020803 AN H, WANG Z T, JIN R F, et al. Responses of Shewanella putrefaciens CN32 unsaturated biofilms to environmental stress and interaction with chromium [J]. Environmental Chemistry, 2022, 41(6): 1880-1889(in Chinese). doi: 10.7524/j.issn.0254-6108.2021020803
[24] NOGUEIRA F G E, DO PRADO N T, OLIVEIRA L C A, et al. Incorporation of mineral phosphorus and potassium on leather waste (collagen): A new NcollagenPK-fertilizer with slow liberation [J]. Journal of Hazardous Materials, 2010, 176(1/2/3): 374-380. -



 下载:
下载: